Abstract
Near-infrared (NIR) fluorescence imaging using indocyanine green (ICG) has the potential to improve the sentinel lymph node (SLN) procedure by facilitating percutaneous and intraoperative identification of lymphatic channels and SLNs. Previous studies suggested that a dose of 0.62 mg (1.6 mL of 0.5 mM) ICG is optimal for SLN mapping in breast cancer. The aim of this study was to evaluate the diagnostic accuracy of NIR fluorescence for SLN mapping in breast cancer patients when used in conjunction with conventional techniques. Study subjects were 95 breast cancer patients planning to undergo SLN procedure at either the Dana-Farber/Harvard Cancer Center (Boston, MA, USA) or the Leiden University Medical Center (Leiden, the Netherlands) between July 2010 and January 2013. Subjects underwent the standard-of-care SLN procedure at each institution using 99Technetium-colloid in all subjects and patent blue in 27 (28 %) of the subjects. NIR fluorescence-guided SLN detection was performed using the Mini-FLARE imaging system. SLN identification was successful in 94 of 95 subjects (99 %) using NIR fluorescence imaging or a combination of both NIR fluorescence imaging and radioactive guidance. In 2 of 95 subjects, radioactive guidance was necessary for initial in vivo identification of SLNs. In 1 of 95 subjects, NIR fluorescence was necessary for initial in vivo identification of SLNs. A total of 177 SLNs (mean 1.9, range 1–5) were resected: 100 % NIR fluorescent, 88 % radioactive, and 78 % (of 40 nodes) blue. In 2 of 95 subjects (2.1 %), SLNs-containing macrometastases were found only by NIR fluorescence, and in one patient this led to upstaging to N1. This study demonstrates the safe and accurate application of NIR fluorescence imaging for the identification of SLNs in breast cancer patients, but calls into question what technique should be used as the gold standard in future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sentinel lymph node (SLN) biopsy is regarded as standard-of-care in staging the axilla in breast cancer patients with clinically negative lymph nodes [1]. Three methods of mapping the SLN are currently standard-of-care: (1) radioactive tracer alone, (2) blue dye, and (3) a combination of both. The combination of both radioactive tracer and blue dye currently report the highest identification rates (>95 %) and lowest false-negative rates (<10 %) [2–6]. However, both modalities have certain disadvantages. For example, blue dyes cannot be seen through skin and fatty tissue, blue staining results in tattooing of the breast lasting for several months, skin necrosis can occur with subdermal injections, and allergic reactions with rare anaphylaxis have been reported [7, 8]. Radioactive colloids are expensive, require involvement of a nuclear physician, and do not provide real-time visual guidance. Moreover, many hospitals worldwide are limited to the use of blue dye only to perform SLN biopsy because of the lack of access to radioactive isotopes or sufficient funding, although blue dye alone is associated with higher false negative rates [9].
As a result of these issues, near-infrared (NIR) fluorescence (700–900 nm) imaging has recently been introduced for SLN mapping and tested in several cancer centers worldwide [10–23]. NIR fluorescence imaging has several characteristics that are advantageous for the SLN procedure, which include a relatively high penetration into living tissue (up to 5 mm) and real-time, high-resolution optical guidance [24, 25]. Indocyanine green (ICG) is currently the only FDA and EMEA approved NIR fluorescent probe that can be used (off-label) in clinical trials as a lymphatic tracer. This tracer has outperformed blue dye staining for SLN identification in four clinical trials [10, 19, 22, 26]. Previous studies indicated that a dose of 0.5 mM (1.6 mL) ICG is optimal for SLN mapping in breast cancer [19, 21, 22].
A significant advantage of NIR imaging is that it can provide real-time guidance. However, to enable the surgeon to work under direct image guidance, navigation in relation to the surgical anatomy is obligatory. In contrast to most camera systems used worldwide, the system used in this study is capable of displaying NIR fluorescence signal simultaneously with surgical anatomy, using a hands-free design. Moreover, the real-time, high-resolution images allow clear detection of fluorescent lymphatic channels, which has shown to be beneficial in the visualization of the lymphatic drainage pathway and position of the SLN [21, 27].
The primary objective of this multicenter experience was to validate the diagnostic accuracy of fluorescence-guided SLN biopsy when used with conventional techniques. The secondary objective was to evaluate the safety of intraoperative NIR fluorescence SLN mapping using 0.5 mM (1.6 mL) ICG.
Methods
Preparation of ICG
ICG (25 mg vials) was purchased from Pulsion Medical Systems (Munich, Germany) or Akorn (Decatur, IL, USA). Directly before surgery, it was resuspended in 10 cc of supplied diluent (sterile water) for injection to yield a 2.5 mg/mL (3.2 mM) stock solution. To obtain the desired 0.5 mM (0.39 mg/mL) dilution of ICG, 7.8 mL of the 3.2 mM ICG solution was diluted in 42.8 mL of sterile water (NL) or 9.2 mL of the stock solution was diluted in 50 mL of sterile saline (US). In previous studies, we determined that the optimal dose of ICG lies between 1.6 mL of 0.4 and 0.8 mM; therefore, a dose of 0.5 mM was chosen [21, 22].
Intraoperative NIR imaging system
SLN mapping was performed using two identical versions of the Mini-Fluorescence-Assisted Resection and Exploration (Mini-FLARE™) [21] image-guided surgery system at each institution. In brief, the system consists of two wavelength-isolated light sources: a “white” light source, generating 26,600 lx of 400–650 nm light, and a “near-infrared” light source, generating 7.7 mW/cm2 of 760-nm light. Color video and NIR fluorescence images are simultaneously acquired and displayed in real time using custom optics and software that separate the color video and NIR fluorescence images. A pseudo-colored (lime green) merged image of the color video and NIR fluorescence images is also displayed in real time. The imaging head is attached to a flexible gooseneck arm, which permits positioning of the imaging head at extreme angles virtually anywhere over the surgical field. For intraoperative use, the imaging head and imaging system pole stand are wrapped in a sterile shield and drape (Medical Technique Inc., Tucson, AZ, USA).
Clinical trial
The data in this paper are combined from two Phase 2 trials approved separately by the Medical Ethics Committees of the Dana-Farber/Harvard Cancer Center (Boston, MA, USA) and the Leiden University Medical Center (Leiden, the Netherlands) and performed in accordance with the ethical standards of the Helsinki Declaration of 1975. All patients planning to undergo a SLN procedure for invasive breast cancer or high-risk carcinoma in situ were eligible for participation in the trials between July 2010 and January 2013. Study subjects had clinically negative axillary nodes as assessed by palpation and ultrasonography. Exclusion criteria were pregnancy, lactation, or an allergy to iodine, shellfish, or ICG. All subjects gave informed consent and were anonymized. Subjects received the standard-of-care sentinel node procedure without interference from the Mini-FLARE imaging system. In the Netherlands, this implied the periareolarly administration of approximately 100 MBq 99mTechnetium-nanocolloid the day before surgery, followed by a lymphoscintigraphy 15 and 180 min after injection. In addition, in 27 subjects, 1 mL total of patent blue (Bleu Patenté V, Guerbet, Brussels, Belgium) was injected intradermally and periareolarly in multiple deposits directly before the start of surgery. Usage of patent blue was performed in concordance with the Dutch guidelines. However, a randomized controlled trial published during this study showed no benefit of using patent blue; therefore, it was omitted in the latter part of the current trial [19]. In the United States this implied the subareolar injection of approximately 0.8 mCi 99mTechnetium-sulfur colloid 1–3 h before surgery.
Immediately before surgery, the attending surgeon injected 1.6 mL total of 0.5 mM ICG at multiple sites periareolarly (NL) or intradermally and peritumorally (US). Afterward, the injection site was massaged to assess lymphatic drainage. After surgical scrub and sterile draping of the operative field, NIR fluorescence imaging was performed using the Mini-FLARE camera system at approximately 30 cm distance to the surgical field. The surgical field was also illuminated using the white light source of the Mini-FLARE imaging system. NIR camera exposure times were between 5 and 500 ms.
Before incision, the surgical field was inspected for percutaneous lymphatic channels and potential SLNs using NIR fluorescence. With respect to detection of the SLN, surgeons had direct access to both the gamma probe and the NIR fluorescence images. The “hands-free” design of the Mini-FLARE permitted continuous image acquisition during the SLN procedure. The method used for first detection of the all SLNs was noted. Whether a SLN was deemed fluorescent or radioactive was dependent on the signal-to-background ratio (SBR) or radioactive counts, respectively. A region of interest (ROI) from adjacent skin, identical in size and shape to that over the SLN, was chosen as background. A SLN exhibiting a SBR ≥1.1 in situ was considered positive by NIR fluorescence. The NIR fluorescence and radioactive signatures of all SLNs were also inspected ex vivo using Mini-FLARE and a handheld gamma probe, respectively. When multiple SLNs were found, a SLN was deemed radioactive when the radioactive counts were more than 10 % of the SLN with the highest radioactive counts.
All subjects underwent routine histopathological analysis of the SLNs according to the Dutch and US guidelines.
Statistical analysis
For statistical analysis, SPSS statistical software package (Version 20.0, Chicago, IL, USA) was used. Correlation between values was determined using the Spearman’s rank correlation coefficient, in case of nonparametric values. To compare BMI between subject groups, the independent-sample t test was used. To test differences between identification time in BMI subgroups, the Kruskal–Wallis one-way analysis of variance test and the Dunn’s Multiple Comparison Test (only computed if overall P < 0.05) were used. P < 0.05 was considered significant.
Results
Subject and tumor characteristics
Ninety-five breast cancer patients who underwent SLN mapping using both radioactive and fluorescence guidance (Table 1) were included in the study. Median age of study subjects was 57 years (range 30–75) and median BMI was 25 kg/m2 (range 19–47). Tumor characteristics are shown in Table 1. The majority of subjects underwent a wide local excision (90 %); the remainder underwent a mastectomy or SLN biopsy only. Nine subjects were treated with neoadjuvant hormonal or chemotherapy. The average time between injection of ICG and the skin incision was 19 ± 7.1 min. No adverse reactions associated with ICG or the Mini-FLARE imaging system occurred.
SLN detection
In 94 of 95 subjects, at least one SLN was identified using NIR fluorescence or radioactivity (identification rate = 99 %; Figs. 1, 2a). A total of 177 SLNs (mean 1.9, range 1–5) were resected: 155 (88 %) were radioactive and 177 (100 %) were fluorescent (Fig. 2b). In one subject, all SLNs were only NIR fluorescent and not radioactive. In two subjects, the gamma probe was necessary for initial localization of SLNs (2/95 = 2.1 %, Figs. 2a, 3). However, after detection, these nodes were found to be NIR fluorescent in vivo (mean SBR = 2.5). Of note, the BMI of these subjects was higher compared to the other subjects (mean BMI = 35.5 vs. 27.3; t = 1.72, P = 0.09; Fig. 3). In four subjects (Fig. 3), additional SLNs could be located using the gamma probe after initial identification of one or more NIR fluorescent SLNs. Again, these nodes were fluorescent in vivo (n = 6, mean SBR = 6.27). The mean BMI of these subjects was not significantly different from the rest of the subjects (mean BMI = 25.0 vs. 27.3; t = 0.75, P = 0.45). In one of these four subjects, an additional SLN located by radioactivity (but also NIR fluorescent in vivo at the time of resection) contained a micrometastasis. The BMI of this subject was 23. In summary, in 6 of 95 subjects (6.3 %), radioactive guidance was necessary for the initial or additional localization of SLNs, though 100 % of those nodes were NIR fluorescent at the time of resection.
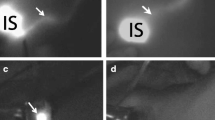
NIR fluorescence-guided sentinel lymph node (SLN) mapping. Top row percutaneous NIR identification of afferent lymphatic channels flowing away from the injection site (Inj.). The planned incision site, based on the presumed location of the SLN, is shown as a dashed line. Middle row real-time fluorescence identification of the SLN directly after incision. Bottom row ex vivo image of the SLN. Scale bars 1 cm. Camera exposure times were 150 ms (upper row), 55 ms (middle row), and 50 ms (bottom row)
Method of identification of initial SLN and SLN sources of contrast. a The method of initial identification of the SLN in all patients (n = 95) is shown as a percentage of total. b The source(s) of contrast for all SLNs (left; N = 177) and tumor-positive SLNs (right; N = 22) after in vivo localization by fluorescence and/or radioactivity. F Fluorescence, R radioactivity
SLN identification in study subjects. Figure shows the effect of Body Mass Index (BMI) (abscissa) on skin incision to SLN identification time (ordinate) in the context of SLN detectability and study site. Points represent individual patients. Red circles represent patients enrolled at Dana-Farber/Harvard Cancer Center (Boston); blue circles represent patients enrolled at the Leiden University Medical Center (Leiden). Magenta rims indicate patients in which radioactivity was necessary for initial identification of SLNs. Green rim represents the single patient in which the SLN was only NIR fluorescent. Dotted green rims indicate patients with tumor-positive SLNs that could only be found using NIR fluorescence. In one patient (BMI = 29) the SLN could be identified after 42 min using NIR fluorescence (outside table limit). Dotted line represents the median for each group
Lymph node involvement was found in 22 (12 %) of the resected lymph nodes including 18 nodes with macrometastases and 4 nodes with micrometastases. Isolated tumor cells were found in 7 nodes (4 %). All tumor-positive SLNs were fluorescent, however, only 20 (91 %) were also radioactive. In two subjects (2.1 %), macrometastases were found in SLNs that could only be found using NIR fluorescence (Table 2; Fig. 3). Even during ex vivo inspection with the gamma probe, radioactivity counts of those nodes were very low (less than 5 % of other nodes). One of those two subjects also had other fluorescent/radioactive nodes that were tumor positive. More importantly, in the other patient, tumor-positive SLNs could only be detected using NIR fluorescence, and resulted in upstaging to N1. Furthermore, in 1 other patient isolated tumor cells were found in a SLN that could only be detected using fluorescence.
In the subgroup that also received patent blue, 31 out of 40 SLNs (78 %) were blue. In subjects that received patent blue, only 3 out of 5 tumor-positive SLNs were stained blue.
NIR fluorescence enabled visualization of the percutaneous lymphatic channels in 81 % of subjects, which was inversely correlated to BMI (R = −0.36; P < 0.001). In 42 % of subjects, the complete lymphatic channel could be followed percutaneously from injection site to area in which the SLN was identified. In addition, percutaneous NIR fluorescence revealing the location of the SLN could be observed in 31 % of subjects. Average brightness of exposed SLNs, expressed as SBR, was 10.5 ± 6.7. Average time between skin incision and SLN identification was 7 ± 6 min and was also inversely correlated to BMI (R = 0.46; P < 0.001). Time between skin incision and SLN identification was significantly higher in subjects with BMI >35 compared to subjects with a BMI ≤25 (P < 0.001). In one subject, the interval between skin incision and SLN detection was 42 min. This subject received neoadjuvant chemotherapy, and the SLN was located in the area next to the latissimus dorsi muscle.
Estimation of diagnostic accuracy
There are two key performance metrics for SLN mapping: (1) identification of all SLN(s) and (2) identification of tumor-positive SLN(s) that will change patient management. Using both NIR fluorescence and radioactivity, the false negative rate for SLN mapping was only 1 %. Using radioscintigraphy as the gold standard, the sensitivity of NIR fluorescence for initial localization of SLNs in all subjects was 98 %. After initial localization, though, the sensitivity of NIR fluorescence was 100 % whereas radioscintigraphy was only 88 %. With respect to identifying the 22 tumor-positive nodes found in this study, the sensitivity of NIR fluorescence was 96 % compared to 91 % for radioscintigraphy.
Discussion
The primary endpoint of this study was measurement of the effectiveness of intraoperative NIR fluorescence imaging using ICG for SLN mapping in breast cancer. Based on our data and those from several other large trials (Table 3), it appears that NIR fluorescence is, at the least, complementary to radioactivity, but might also lead to a new standard-of-care in the future.
The use of NIR fluorescence for SLN mapping has several advantages over conventional modalities, such as superior depth penetration compared to blue dyes, real-time visual guidance, as well as broad availability compared to radioactive tracers. Table 3 summarizes all NIR fluorescence breast cancer SLN clinical studies with 50 or more study subjects each. To the best of our knowledge, all large studies performed worldwide have used the Photo Dynamic Eye (PDE; Hamamatsu, Japan) camera system [10–18]. This hand-held fluorescence camera system is easy to use and commercially available; however, no color overlay is possible. Moreover, most studies only compared fluorescence to patent blue and only one study made a large (i.e., n > 50) comparison between radioactive and fluorescence guidance [15]. In that study, Wishart et al. [15] observed that fluorescence imaging using ICG was not associated with harvesting of an excessive number of nodes compared to radioactive guidance, which is concordant with our data. In addition, the sensitivity of fluorescence and blue dye was 95 % in their study. This suggests that a combination of blue dye and fluorescence is a potential alternative to radioactivity, although the present study, as well as previous studies, suggests that blue dye is itself unnecessary [19].
As shown in Table 3, most large clinical studies on this topic have utilized a dose of ICG almost tenfold higher than that used in our study. In a series of controlled trials, we demonstrated that the concentration and volume of ICG was of considerable importance. Counterintuitively, higher injected ICG concentrations actually lead to worse detectability because of fluorophore “quenching.” That is, at too high a concentration, photons emitted by ICG are reabsorbed and are therefore not detectable [28]. Even 0.5 mM (used in our study) is a concentration that exhibits quenching, but by the time the ICG is diluted in lymph fluid, the final concentration in the SLN is such that NIR fluorescence is maximal. A high concentration can also lead to increased flow to second-tier nodes, as reflected in the higher number of “SLNs” seen with higher injected doses (Table 3). Careful attention to concentration and dilution is one factor that enables improved performance of the technology, especially because identification of a higher number of SLNs does not increase patient survival and harbors the risk of increased morbidity. Retrospective analysis of 1,530 consecutive patients with negative SLNs (detected using radioactivity and blue dye) showed an axillary recurrence of only 0.26 % [29].
The second factor is the imaging system used. Our study employed hands-free imaging using the Mini-FLARE imaging system, which is capable of displaying NIR fluorescence images simultaneously with surgical anatomy. This enabled the surgeon to perform surgery under direct image guidance. In addition, a significant advantage of fluorescence-guided SLN mapping is the introduction of real-time visualization of lymphatics and, in some cases, also lymph nodes through the skin. In the current study, we were able to visualize percutaneous lymphatic channels in 81 % and axillary lymph nodes in 31 % of cases. Even when the SLN itself is not visible through the skin, its location can still be inferred with reasonable accuracy by projecting downward and slightly away from the point at which the lymphatic channel dives deep and becomes invisible. Exploiting this feature is an important part of the learning curve for the technique. Pre-incision SLN detection, when it occurs, is a major advantage because it can minimize both surgical incision length and the exploration time needed to find the SLN.
No adverse events related to fluorescence imaging were reported in this study, which confirms safe application of NIR fluorescence and ICG. Moreover, the estimated incidence of adverse events using ICG is considerably lower (<1:10,000 cases) than that of Patent Blue (~1:150 cases) [30–32].
It should also be pointed out that the hydrodynamic diameters of ICG (≤1 nm) and radioactive colloid (≥50 nm) are vastly different and can have a major impact on performance [33]. ICG flows much faster (seconds to minutes) and potentially has access to smaller lymphatic channels, but can also pass through the SLN into second-tier nodes. Colloid requires hours to flow but has excellent retention in the SLN. It is unclear what role hydrodynamic diameter and timing played for the SLNs found only using NIR fluorescence. Another parameter not addressed in this study is the optimal formulation of ICG, which is known to be unstable over time in aqueous environments [34].
Our study calls into question what should be considered the “gold standard” in SLN mapping. By one metric, initial SLN identification, NIR fluorescence identified the SLN in 1 patient that could not be detected using radioactivity. However, radioactivity was necessary for initial SLN identification in two cases. In NIR failures, BMI did not appear to be a discriminating factor. It is unclear at present if NIR fluorescence failures could have been avoided by improved intraoperative techniques and greater experience, or whether those failures would have resulted in a clinically poorer outcome. By understanding the limitations of NIR fluorescence (e.g., ≈5 mm penetration depth), it might be possible to modify exploratory techniques to improve detectability. It is equally possible, however, that the superior depth of radioactive detection will be required in some cases. A well-designed, properly powered, randomized controlled clinical trial could answer this critical question.
By a second metric, though, identification of tumor-positive SLNs, NIR fluorescence identified N+ SLNs with macrometastases in two patients that could not be identified using radioactivity. It could be argued that this is a more important metric because upstaging changes patient management, as it did in one study subject. This also calls into question whether radioactivity can be safely omitted from SLN mapping of breast cancer under certain conditions. Definitive conclusions await a much larger clinical trial.
A key aspect of NIR fluorescence SLN mapping is that it can be readily translated to breast cancer centers worldwide. Aspects supporting implementation of NIR fluorescence SLN mapping include: (1) it utilizes a contrast agent already FDA approved for other indications, (2) multiple imaging systems are now commercially available, and (3) unlike radioactive tracers, no extensive infrastructure is needed to implement the technology.
In conclusion, this multi-center experience validates the safe and accurate application of NIR fluorescence imaging for the identification of SLN in breast cancer patients using 1.6 mL of 0.5 mM ICG and the Mini-FLARE camera system. In the context of finding tumor-positive SLNs, NIR fluorescence outperformed both radioactivity and blue dye staining. We believe that fluorescence-guided SLN mapping using ICG is a safe and accurate method that can be used in combination with radioactivity and/or blue dye, but would be of special value in breast cancer centers that currently have access to only blue dye.
References
Cox CE, Pendas S, Cox JM, Joseph E, Shons AR, Yeatman T, Ku NN, Lyman GH, Berman C, Haddad F, Reintgen DS (1998) Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg 227:645–651
Goyal A, Newcombe RG, Chhabra A, Mansel RE (2006) Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer: results of the ALMANAC validation phase. Breast Cancer Res Treat 99:203–208
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Mammolito DM, McCready DR, Mamounas EP, Costantino JP, Wolmark N (2007) Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 8:881–888
Zavagno G, De Salvo GL, Scalco G, Bozza F, Barutta L, Del BP, Renier M, Racano C, Carraro P, Nitti D (2008) A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg 247:207–213
Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, Duez N, Cataliotti L, Klinkenbijl JH, Westenberg HA, van der Mijle H, Snoj M, Hurkmans C, Rutgers EJ (2010) Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol 17:1854–1861
Mansel RE, MacNeill F, Horgan K, Goyal A, Britten A, Townson J, Clarke D, Newcombe RG, Keshtgar M, Kissin M, Layer G, Hilson A, Ell P, Wishart G, Brown D, West N (2013) Results of a national training programme in sentinel lymph node biopsy for breast cancer. Br J Surg 100:654–661
Reyes F, Noelck M, Valentino C, Grasso-Lebeau L, Lang J (2010) Complications of methylene blue dye in breast surgery: case reports and review of the literature. J Cancer 2:20–25
Zakaria S, Hoskin TL, Degnim AC (2008) Safety and technical success of methylene blue dye for lymphatic mapping in breast cancer. Am J Surg 196:228–233
Pesek S, Ashikaga T, Krag LE, Krag D (2012) The false-negative rate of sentinel node biopsy in patients with breast cancer: a meta-analysis. World J Surg 36:2239–2251
Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T (2010) Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast 19:210–213
Tagaya N, Aoyagi H, Nakagawa A, Abe A, Iwasaki Y, Tachibana M, Kubota K (2011) A novel approach for sentinel lymph node identification using fluorescence imaging and image overlay navigation surgery in patients with breast cancer. World J Surg 35:154–158
Sugie T, Sawada T, Tagaya N, Kinoshita T, Yamagami K, Suwa H, Ikeda T, Yoshimura K, Niimi M, Shimizu A, Toi M (2013) Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol 20:2213–2218
Takeuchi M, Sugie T, Abdelazeem K, Kato H, Shinkura N, Takada M, Yamashiro H, Ueno T, Toi M (2012) Lymphatic mapping with fluorescence navigation using indocyanine green and axillary surgery in patients with primary breast cancer. Breast J 18:535–541
Hirano A, Kamimura M, Ogura K, Kim N, Hattori A, Setoguchi Y, Okubo F, Inoue H, Miyamoto R, Kinoshita J, Fujibayashi M, Shimizu T (2012) A comparison of indocyanine green fluorescence imaging plus blue dye and blue dye alone for sentinel node navigation surgery in breast cancer patients. Ann Surg Oncol 19:4112–4116
Wishart GC, Loh SW, Jones L, Benson JR (2012) A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol 38:651–656
Aoyama K, Kamio T, Ohchi T, Nishizawa M, Kameoka S (2011) Sentinel lymph node biopsy for breast cancer patients using fluorescence navigation with indocyanine green. World J Surg Oncol 9:157
Abe H, Mori T, Umeda T, Tanaka M, Kawai Y, Shimizu T, Cho H, Kubota Y, Kurumi Y, Tani T (2011) Indocyanine green fluorescence imaging system for sentinel lymph node biopsies in early breast cancer patients. Surg Today 41:197–202
Kitai T, Kawashima M (2011) Transcutaneous detection and direct approach to the sentinel node using axillary compression technique in ICG fluorescence-navigated sentinel node biopsy for breast cancer. Breast Cancer 19:343–348
van der Vorst JR, Schaafsma BE, Verbeek FP, Hutteman M, Mieog JS, Lowik CW, Liefers GJ, Frangioni JV, van de Velde CJ, Vahrmeijer AL (2012) Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99mTechnetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol 19:4104–4111
Schaafsma BE, Verbeek FP, Rietbergen DD, van der Hiel B, van der Vorst JR, Liefers GJ, Frangioni JV, van de Velde CJ, van Leeuwen FW, Vahrmeijer AL (2013) Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg 100:1037–1044
Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, Gioux S, Kuppen PJ, Ashitate Y, Lowik CW, Smit VT, Oketokoun R, Ngo LH, van de Velde CJ, Frangioni JV, Vahrmeijer AL (2011) Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol 18:2483–2491
Hutteman M, Mieog JS, van der Vorst JR, Liefers GJ, Putter H, Lowik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL (2011) Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat 127:163–170
Hirche C, Murawa D, Mohr Z, Kneif S, Hunerbein M (2010) ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat 121:373–378
Frangioni JV (2003) In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7:626–634
Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV (2013) Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol 10(9):507–518
Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F (2012) Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J Surg Oncol 106:41–45
Blum KS, Proulx ST, Luciani P, Leroux JC, Detmar M (2013) Dynamics of lymphatic regeneration and flow patterns after lymph node dissection. Breast Cancer Res Treat 139:81–86
Gioux S, Choi HS, Frangioni JV (2010) Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging 9:237–255
Kiluk JV, Ly QP, Santillan AA, Meade T, Ramos D, Reintgen DS, Dessureault S, Davis M, Shamehdi C, Cox CE (2010) Erratum to: axillary recurrence rate following negative sentinel node biopsy for invasive breast cancer: long-term follow-up. Ann Surg Oncol 17:552–557
Benya R, Quintana J, Brundage B (1989) Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn 17:231–233
Bezu C, Coutant C, Salengro A, Darai E, Rouzier R, Uzan S (2011) Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol 20:e55–e59
Alford R, Simpson HM, Duberman J, Hill GC, Ogawa M, Regino C, Kobayashi H, Choyke PL (2009) Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging 8:341–354
Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV (2005) Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging 4:172–181
Rajagopalan R, Uetrecht P, Bugaj JE, Achilefu SA, Dorshow RB (2000) Stabilization of the optical tracer agent indocyanine green using noncovalent interactions. Photochem Photobiol 71:347–350
Tagaya N, Nakagawa A, Abe A, Iwasaki Y, Kubota K (2010) Non-invasive identification of sentinel lymph nodes using indocyanine green fluorescence imaging in patients with breast cancer. Open Surg Oncol J 2:71–74
Acknowledgments
The authors thank Alan Stockdale, Dr. Vivek Venugopal, Florin Neacsu, Frank W. Kettenring, Dr. Yoshitomo Ashitate, Dr. Summer Gibbs, and Yang (Allison) Xie for operation of the Mini-FLARE imaging system, and David J. Burrington, Jr. for editing. F.P.R. Verbeek’s travel to Boston for completion of the trials was sponsored by the Leiden University Fund/Piso Kuperus. J.S.D. Mieog is a MD-medical research trainee funded by The Netherlands Organization for Health Research and Development (Grant No. 92003526). This study was performed within the framework of the Centre for Translational Molecular Medicine, project MUSIS (grant 03O-202), and was funded by the Nuts Ohra Fund, Dutch Cancer Society grant UL2010-4732 and National Institutes of Health grants R21-CA-130297 and R01-CA-115296. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
Floris P.R. Verbeek, Susan L. Troyan, J. Sven D. Mieog, Gerrit-Jan Liefers, Lorissa A. Moffitt, Mireille Rosenberg, Judith Hirshfield-Bartek, Sylvain Gioux, Cornelis J.H. van de Velde, Alexander L. Vahrmeijer: none. John V. Frangioni: FLARE™ technology is owned by the Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. Dr. Frangioni has started three for-profit companies, Curadel, Curadel ResVet Imaging, and Curadel Surgical Innovations, which has optioned FLARE™ technology for potential licensing from Beth Israel Deaconess Medical Center.
Ethical standards
This study was approved separately by the Medical Ethics Committees of the Dana-Farber/Harvard Cancer Center (Boston, MA, USA) and the Leiden University Medical Center (Leiden, The Netherlands) and performed in accordance with the ethical standards of the Helsinki Declaration of 1975. All subjects gave informed consent and were anonymized.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical Trial Registrations: National Clinical Trial NCT01468649 and the Netherlands Trial Register NTR2084.
Floris P.R. Verbeek and Susan L. Troyan contributed equally to the study and share first authorship.
Rights and permissions
About this article
Cite this article
Verbeek, F.P.R., Troyan, S.L., Mieog, J.S.D. et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res Treat 143, 333–342 (2014). https://doi.org/10.1007/s10549-013-2802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2802-9