Abstract
Depression is now recognised as a common complication of pregnancy and the postpartum period. If untreated this mental illness has implications for maternal morbidity and foetal, infant and child outcomes. Most treatment guidelines recommend for moderate to severe depression the consideration of pharmacological treatment and this includes guidelines developed for the perinatal period. This chapter will provide an overview of depression in pregnancy, risks and benefits of antidepressant treatment in pregnancy and suggestions for management should pharmacological treatment be instigated or maintained in pregnancy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Major depression
- Prevalence
- EPDS
- Assessment
- Treatment
- Antidepressants
- SSRI
- Malformations
- Miscarriage
- Obstetric complications
- Risk benefit analysis
- Poor neonatal adaptation syndrome
- Persistent pulmonary hypertension of the newborn
6.1 Introduction
Major depression is a common and distressing mental disorder that carries with it considerable disease burden (Murray et al. 2012), making it a major public health issue. Its particular relevance in perinatal mental health is that major depression is more common among women than men and has its highest prevalence levels during the childbearing years (Kessler et al. 2003; Slade et al. 2009) making it likely that women could be depressed prior to conception or over the course of their pregnancy. There is also building evidence that maternal depression can have adverse effects on infant development making it critically important to recognise and treat depression over the perinatal period (Chaudron 2013).
There has been interest in an association between childbirth and mental illness since antiquity, with the recognition that severe mental illness, leading to hospital admission or even suicide, can arise following childbirth. A series of studies linking obstetric and psychiatric data demonstrated the elevated risk of the onset of psychosis, especially puerperal psychosis (a variant of bipolar disorder) following childbirth (Kendell et al. 1981, 1987) with 1–2 women per 1,000 confinements having such an illness (Boyce and Barriball 2010). Severe depression following childbirth also occurred, but the rate was considered to be low based upon hospital admissions (Tod 1964). These studies also found that the risk of psychosis onset and admission to hospital was low during pregnancy with suggestions that pregnancy had a protective effect. The idea that pregnancy was protective probably stalled efforts into examining mental disorders, particularly depression, occurring during pregnancy.
It was not until community-based studies were conducted that there was a recognition that there were high rates of depression arising following childbirth (Kumar and Robson 1984; Pitt 1968; O’Hara et al. 1984; Watson et al. 1984). These studies, examining the prevalence of postnatal depression and its risk factors, highlighted that many women with postnatal depression often did not have their illness recognised and thus went without treatment (Boyce and Stubbs 1994). Major efforts were then made to develop instruments to screen for, and identify, postnatal depression (Buist et al. 2002) so that women could access treatment and minimise against any possible adverse effects of depression upon infant development (Murray 1992; Boyce and Stubbs 1994). In response to the potential adverse effects of postnatal depression, there was an increased effort to identify at-risk women earlier, ideally during pregnancy. A number of risk factors are known to predict postnatal depression (Boyce and Hickey 2005; O’Hara and Swain 1996) and scales developed to assess them (Appleby et al. 1994; Austin et al. 2005); however, one of the best predictors of postnatal depression was found to be depressive symptoms during pregnancy (O’Hara and Swain 1996). Identifying depressive symptoms in pregnancy then seemed to be a strategy to identify women at risk of developing postnatal depression. When screening tools, such as the Beck Depression Inventory (BDI) (Beck et al. 1961) and the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al. 1987), were used during pregnancy high levels of depressive symptoms were found to be present. Not only that, using cut-off scores to identify major depression (generally developed for postnatal samples) the rates of depression were found to be much higher than previously thought. This was clearly demonstrated in the influential Avon longitudinal study (Evans et al. 2001). Here 14,541 pregnant women completed the EPDS at 18 and 32 weeks gestation (and at 8 weeks and 8 months postpartum). The women’s scores on the EPDS were significantly higher at 18 weeks (6.62) and 32 weeks (6.72) gestation than the postpartum scores of 5.84 and 5.25. Using the standard cut-off score on the EPDS of greater than 12, 13.5 % of women scored above the threshold for probable depression at 32 weeks of pregnancy compared to only 9.1 % at 8 weeks postpartum.
Subsequent studies confirmed the high levels of depressive symptoms and depression in pregnancy; these were reviewed by Bennett et al. (2004b). They examined findings from studies that used self-report measures; mainly the EPDS and the BDI. Studies that used structured diagnostic instruments were also reviewed, where the rates were much lower than those found using questionnaires. The highest rates of depression were found when the BDI was used (see Fig. 6.1), especially in the first trimester, whereas the highest rates of depression were found in the third trimester when the EPDS was used as the case finding measure. The EPDS was developed specifically for assessing postnatal depression and did not focus on the somatic symptoms associated with depression. This would account for the lower rates of depression identified using the EPDS rather than the BDI which includes the common somatic symptoms of depression that could be accounted for by pregnancy itself.
Prevalence of depression during pregnancy assessed by questionnaire or interview. Notes: Edinburgh Postnatal Depression Scale (EPDS) cut-off score greater than or equal to 1.0 Beck Depression Inventory (BDI) cut-off score greater than or equal to 9. Adapted from Bennett et al. (2004b)
Structured clinical instruments had lower rates of depression, with the highest rates in the second (9.1 %) and third trimesters (8.9 %), a rate not much higher than the rate of depression among women identified in community epidemiological studies and consistent with the findings of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) in which data from 14,549 women were examined using a validated structured interview (Vesga-Lopez et al. 2008). The prevalence rate of major depression for non-pregnant women was 8.1 % and similar to the rate of depression among women who had been pregnant in the past year (8.4 %), whereas the rate of major depression was significantly higher for postpartum women (9.3 %).
Women with recurrent episodes of depression (recurrent unipolar disorder or bipolar disorder) are of particular interest in that they are at high risk of having a relapse of the illness postpartum. Viguera et al. assessed episodes of illness, using DSM-IV criteria, during pregnancy or up to 6 months postpartum in a cohort of women who had been diagnosed with bipolar I, bipolar II or unipolar depressive disorder (Viguera et al. 2011). An episode of major depressive disorder occurred in 2.7 % of the 1,132 women with unipolar disorder during pregnancy (1.89 % reported an episode of anxiety or panic), with the rate of depression being 16.1 % in the first 6 months postpartum.
6.2 Impact of Depression
These studies make it clear that major depression occurs during pregnancy with a prevalence rate of between 3 and 9 %. They also demonstrate that a significant proportion of women will report high levels of depressive symptoms. Major depression and depressive symptoms may cause significant distress and impairment for women over an important life transition, as well as potentially having an adverse effect on pregnancy outcomes and the developing foetus. Maternal depression has been associated with increased risk of premature delivery, low birth weight, gestational hypertension (Grote et al. 2010a; Grigoriadis et al. 2013c) and perinatal death (Howard et al. 2007). It is not clear, however, whether this is a direct result of the depression or behaviours associated with depression such as poor diet and smoking and greater use of prescription drugs such as hypnotics, anti-emetics and opioid analgesics (Newport et al. 2012). It is also associated with a low uptake of breastfeeding (Grote et al. 2010a; Grigoriadis et al. 2013c), which could have an adverse impact on infant development and well-being.
Depression in pregnancy may have an adverse impact on infant emotional and cognitive development (Deave et al. 2008; Field 2011; Chaudron 2013). It has been proposed that the mechanism of that is the result of the biological substrates of depression, such as activation of the HPA axis that primes the foetus itself to depression in later life, and that activation of the maternal HPA axis leading to placental hypersecretion of corticotropin-releasing factor with adverse effects of the foetus and on labour (Field 2011; Chaudron 2013). Such findings emphasise the importance of recognising, and treating, depression in pregnancy.
6.3 Relapse Risk of Major Depression in Pregnancy
Relapse rates have been examined in those women who continue or cease antidepressants during pregnancy. Cohen et al. (2006) found that patients with major depression who ceased antidepressant treatment during pregnancy showed significantly higher relapse rates than those who remained on their preconception dose of medication throughout pregnancy (68 % vs. 26 %) (Cohen et al. 2006). However, a more recent study by Yonkers et al. (2011) found no difference at all. Significant methodological differences, which include patient population and severity of illness factors, limit our ability to form firm conclusions regarding risk (Yonkers et al. 2011). However, an earlier study of women who abruptly discontinued antidepressants on becoming pregnant found 31 % developed suicidal ideation (Einarson et al. 2001). This is of particular concern given the high rates of cessation found in community samples when pregnancy is diagnosed (Ververs et al. 2006).
The biological mechanisms of exposure to antidepressant medication are further discussed in Chap. 4 and the literature regarding untreated maternal mental illness, including depression, in Chap. 5.
6.4 Assessment of Depression in Pregnancy
The key to successful management of depression in pregnancy is a careful assessment. It is important to distinguish between women with distressing depressive symptoms and those that meet the full diagnostic criteria for a major depressive disorder. That is not to say that these symptoms should be ignored; their origin should be explored, the relevant stressors identified and their worries dealt with through supportive counselling. Making a diagnosis of major depressive disorder in pregnancy is complicated by the fact that there are overlapping symptoms between major depression and pregnancy-related symptoms. Yonkers et al. (2009a) found that some of the typical somatic depressive symptoms such as appetite, sleep and energy level disturbance did not differentiate well between depressed and non-depressed pregnant women. By contrast, the more cognitive symptoms of depression, such as feeling guilty or worthless and having trouble concentrating, were much more discriminatory. The assessment process needs to take this into account. The same has been found using questionnaires such as the EPDS, as a diagnostic tool (it was not designed to be used as a diagnostic tool but as a screening tool). Scores on the EPDS are often inflated on the first administration (especially if it is administered at the first antenatal visit when women are naturally apprehensive) and it is recommended that women who score high should complete the instrument a week or two later to give a more valid result (Matthey and Ross-Hamid 2012). Asking about the attribution of endorsed symptoms on the EPDS will also alter the rate of depression, (identified using a structured interview) when symptoms that were attributable to the normal physical changes of pregnancy are excluded (Matthey and Ross-Hamid 2011).
When assessing women in pregnancy it is necessary to enquire about the woman’s attribution for the symptom and not merely use a “checkbox” approach to make a diagnosis; otherwise there is a risk of making a spurious diagnosis and leading to overpathologising.
The woman’s previous history of depression should also be elicited as well as her treatment response as this will give an indication of what will be beneficial for her current episode of depression. The possibility of bipolar depression should also be considered, so asking about possible episodes of hypomania or mania is essential, especially when there is a family history of bipolar disorder.
The assessment of depression should include an assessment of the severity and type of depression. The treatment approach for a more severe, melancholic type of depression will be very different from the treatment that is offered to a woman with mild depression arising as a consequence of psychosocial difficulties in which psychosocial intervention is preferable.
6.5 Treatment of Depression in Pregnancy
Antidepressants are a mainstay for the treatment of moderate to severe depression in adults. Guidelines which are specific to the perinatal period suggest the consideration of their use where there has been a failure of response to psychosocial interventions or significant and debilitating symptoms of depression (Yonkers et al. 2009c; Austin et al. 2011). There is evidence from studies in the US, Canada and Denmark of the increasing use of antidepressants in pregnancy with rates between 3.2 % and 13.4 % shown in these studies (Cooper et al. 2007; Jimenez-Solem et al. 2013; Andrade et al. 2008; Oberlander et al. 2006), although studies from both the Netherlands and Australia show lower rates of use of around 2 % (Lewis et al. 2012; Ververs et al. 2006).
There is an absence of specific evidence examining the effectiveness of antidepressant medication for depression in pregnancy. The absence of evidence regarding the efficacy of antidepressants in pregnancy is not surprising as pregnant women are routinely excluded from clinical trials (Coverdale et al. 2008). However, given the absence of evidence it is reasonable to make use of the treatment approaches for depression in general as the clinical presentation of depression in pregnancy is not different from other forms of depression (Yonkers et al. 2009b; Bennett et al. 2004a). Broadly speaking this would mean that women with mild-to-moderate depression be preferentially offered psychosocial treatments such as interpersonal therapy (Spinelli 1997) or cognitive behaviour therapy; however, there is a lack of evidence to recommend their use.
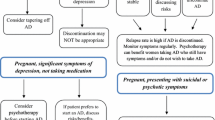
What is essential, especially if antidepressant medication is being considered, is to conduct a careful risk:benefit analysis. The essence of this is shown in the figure below (Fig. 6.2).
First, no antidepressant medications are used because of concerns about the possible adverse effects on the developing foetus. Many women will not contemplate taking medication during pregnancy because of fears that it could harm the developing foetus and a significant number stop their antidepressant in the first trimester of pregnancy (Ververs et al. 2006). The consequence of this is that the depression will persist and that the depression could have an adverse effect on pregnancy outcome. The foetus, meanwhile, will not be exposed to the antidepressant, but will be exposed to any adverse consequences of the woman’s depression.
If, however, the woman takes an antidepressant her depression will remit (though not in all cases) but she may experience significant side effects from the medication, and effects of the medication on obstetric outcome, and a possible risk of gestational hypertension (Toh et al. 2009). Care, therefore, has to be taken to provide an antidepressant that is both efficacious and well tolerated (Malhi et al. 2013). When an antidepressant is used, the foetus will be exposed to the medication and potential harmful effects. These have been reviewed and there have now been eight meta-analyses examining this (Galbally 2013), which show a small increased risk of foetal abnormality. The major adverse effects of antidepressant medications in pregnancy include foetal malformations, persistent pulmonary hypertension and poor neonatal adaptation syndrome.
6.6 Pregnancy and Neonatal Complications of Antidepressant Medication
6.6.1 Malformations
There has been considerable debate as a result of conflicting findings for an association with SSRI antidepressant exposure and a range of malformations. In addition to numerous conflicting studies there have now been eight meta-analyses in an attempt to resolve this question; however, these too have produced varied results (Rahimi et al. 2006; Addis and Koren 2000; Einarson and Einarson 2005; O’Brien et al. 2008; Myles et al. 2013). Of the eight meta-analyses four have found no association between antidepressant exposure and malformation and three have found an association between paroxetine and heart defects (Wurst et al. 2010; Bar-Oz et al. 2007). The two most recent meta-analyses also found an association between fluoxetine and an increased malformation risk (Grigoriadis et al. 2013b; Myles et al. 2013). Both tricyclic antidepressants (TCAs) and the newer antidepressants such as Selective Noradrenaline Reuptake Inhibitors (SNRIs) have not been associated with malformation, although the literature is far more limited (Lennestal and Kallen 2007; Simon et al. 2002).
The challenge with the original research and with the subsequent meta-analyses is the considerable variation in methodology. The larger studies which have examined this question have tended to be retrospective studies based on population-based registries or reviews of medical files with variables analysed which were not originally collected for research projects. Many of these studies have unverified data on exposure, such as measuring exposure as the provision of a prescription, not adequately accounting for key confounding variables and a lack of measures of maternal mental illness that in itself has the potential to be a teratogen.
The issue of confounding variables is important, given that studies such as Malm et al. and Lewis et al. both show higher alcohol and cigarette exposure, especially the latter as well as other prescribed medications in women taking antidepressants (Malm et al. 2011; Lewis et al. 2012). Given that women with mental illness, including depression, have higher rates of vitamin deficiencies, such as vitamin D, poorer nutrition, obesity and unplanned pregnancy (Warner et al. 1996)' the latter is significant given the importance of folate, ideally preconception, and early pregnancy, to prevent malformations such as neural tube defects, but also potentially for cardiac malformation (Scanlon et al. 1998; Leanza et al. 2013). Another important potential confounder that has been mostly unexamined is maternal obesity. Depression is associated with a higher incidence of co-morbid obesity (Onyike et al. 2003) and in turn maternal obesity is associated with an increased risk of malformations (Stothard et al. 2009). What has not been adequately investigated is whether specific maternal mental illnesses are associated with increased risk of malformations. Other maternal illnesses such as Rubella, Diabetes Mellitus and Gestational Diabetes are regarded as teratogenic (Balsells et al. 2012). There is now a volume of research particularly in maternal anxiety which has shown an association with effects on specific foetal outcomes as well as longer term child development (Van den Bergh et al. 2005). Whether either depression or anxiety, are in themselves, teratogenic, and therefore associated with structural or neurodevelopmental teratogenicity, is yet to be explored.
The basic premise of teratology is that exposure during early pregnancy to a specific agent results in a specific malformation. This risk inherently must be above the baseline risk for malformation of 2–3 %. While there is concerning data about a potential small increase in malformation risk from exposure to antidepressants in pregnancy, particularly specific SSRIs such as paroxetine, there have not yet been data that are conclusive as to this risk. Future studies, which clearly account for important key confounding variables, accurately document exposure and maternal mental illness and are needed in order to elucidate any association between antidepressant exposure and malformation.
6.6.2 Miscarriage
Two previous meta-analyses that examined the rate of spontaneous abortion and antidepressant exposure both found an increased risk (Hemels et al. 2005; Rahimi et al. 2006). However, the authors could not account for maternal depression as a variable across the identified studies. Interestingly, a more recent meta-analysis found no association between spontaneous abortion and antidepressant exposure (Ross et al. 2013).
6.6.3 Delivery Complications: Birth Weight, Preterm Delivery and Neonatal Adaptation
Around the time of delivery two major issues have been raised for infants exposed to antidepressants; one is that of potential growth effects and the other of neonatal adaption. The latter in particular is relevant to the clinical management of the mother and whether reducing the medication prior to delivery reduces negative effects in the infant and how this is weighed up against the risk of mother’s depression recurring.
Prematurity and low birth weight, even when adjusted for gestation, were noted in early evaluations of the first use of SSRI in pregnancy. In Kallen’s meta-analysis (2004) he estimated the risk of this to be increased two- to threefold (Kallen 2004). Our study (Galbally et al. 2009; Lewis et al. 2010) of a prospective case-controlled comparison of women treated and not treated with antidepressants showed the former group to be significantly more likely to have infants with a low birth weight (BW), be shorter and have a decreased head circumference. Davidson et al. (2009) had similar findings and proposed a correlation to lower cortisol, higher TSH and increased placental I GF-1 receptor expression. Wisner et al. (2009) found similar rates of prematurity between those on SSRIs (21 %) and those depressed but not on SSRIs (23 %) compared to only 6 % of those women with neither, but found no differences between these three groups with respect to length, weight and head circumference (Wisner et al. 2009).
Wisner’s comparison groups highlight one of the key areas of difficulties in this research, being a lack in many studies of an adequate or appropriate control group, as well as taking into account maternal symptoms and confounding variables. Grote et al. (2010) concluded that women with depression were more likely to have poor obstetric care, be isolated, smoke, have poor nutrition and use illicit substances, all of which may affect foetal growth as well as neonatal adaption (Grote et al. 2010). They found a 39 % increased risk of preterm birth associated with maternal depression, a 49 % increase in low birth weight and a 45 % increase in intrauterine growth retardation. They proposed possible mechanisms of deregulation of the HPA axis, increased uterine artery resistance with placental hyperperfusion secondary to maternal stress or an inflammatory response.
Wisner et al. (2013) in an attempt to resolve the issue of depression versus SSRI causation completed a detailed prospective study of 97 women with no SSRI exposure, 46 women with SSRI exposure and 31 women with depression but no SSRI exposure, from 20 weeks gestation to 52 weeks postpartum (Wisner et al. 2013). They found no significant association between either of the study groups and birth weight, length or head circumference. Prematurity was associated with SSRI exposure compared to no SSRIs, but not when compared to the depressed group. These findings may relate to the relatively small depressed study group, but anxiety was also calculated for and the infant ratings were blind, strengthening these results and certainly suggesting that there is not a good evidence base to advise women to stop taking medication in order to prevent these outcomes.
After delivery these infants have been noted to have an increased risk of poor neonatal adaption (PNAS), characterised by jitteriness, decreased muscle tone and cry, respiratory distress, hypoglycaemia, low APGAR and seizures (Koren et al. 2005). These authors concluded in their review of six studies that the absolute risk was up to 30 % (compared to 6–9 % in those not exposed or exposed early in pregnancy) and a 2–10-fold increase if the exposure was around the time of delivery. Warburton et al. (2010) in a large population data study found that neonates with exposure in the previous 14 days had higher rates of respiratory distress compared to those with earlier exposures, but not when confounding variables were controlled for (Warburton et al. 2010). They concluded that unlike previous assumptions of it being a toxicity or withdrawal, the phenomena may not be an acute pharmacological issue.
Byatt et al, in a literature review found PNAS in up to 30 % of infants, but criticised the data because of a lack of systematic infant evaluation and blind raters, as well as the inappropriate control groups and lack of consideration of maternal variables (Byatt et al. 2013). As for the possible mechanisms, besides serotonin toxicity or overstimulation, infant genotype was also considered. Given the lack of clear associations, and the relatively minor self-limiting nature of the symptoms, and the risk to the mother of relapse, and to the infant through untreated depression (antenatal and postnatal), recommendations for management tend towards keeping the mother well and treating the infant conservatively, but with observation and intervention best suited to a specialised neonatal unit. Koren et al. (2005) comment that earlier recommendations to taper may in fact be ill-conceived and even potentially dangerous. A recent meta-analysis identified 12 studies that met their inclusion criteria and found evidence for an increased risk of PNAS in neonates exposed to antidepressants in pregnancy (Grigoriadis et al. 2013a).
6.6.4 Persistent Pulmonary Hypertension of the Newborn
Persistent Pulmonary Hypertension of the Newborn (PPHN) occurs in 1–2 per 1,000 babies. It represents the failure to transition to newborn circulatory functioning during delivery. PPHN is associated with increased rates of morbidity and mortality and hence is of significant concern. There have been seven studies investigating the potential association between SSRI exposure in pregnancy and PPHN. Of these, four, have found an association of variable magnitude and three have not found any association (Galbally et al. 2012a).
The concerns, until the two most recent studies (Wilson et al. 2011; Kieler et al. 2012) were the considerable methodological variation between studies and the failure to account for known confounding variables such as caesarean delivery (Galbally et al. 2012a). These two recent studies have had conflicting findings with Wilson et al. finding no association and Kieler et al. finding a small association (Kieler et al. 2012). The latter was a combined study of the Scandinavian national health registers and this allowed an adequately powered study to investigate a relatively rare condition. They found a general incidence of PPHN of 1.2 per 1,000, and with exposure to any of the SSRIs this increased to 3 per 1,000. While this is an increased risk this is far lower than previously reported.
Kieler et al. also examined a range of other antidepressants including tricyclic antidepressants (TCAs) and Selective Noradrenaline Reuptake Inhibitors (SNRIs) and found that exposure in late pregnancy was also associated with an elevated risk. A recent meta-analysis found evidence for a small increase in PPHN with exposure to antidepressants in pregnancy (Grigoriadis et al. 2014).
6.6.5 Longer Term Complications
There are only a limited number of studies that have examined child development outcomes following antidepressant exposure in utero and none have followed children beyond early childhood (Gentile and Galbally 2010; Galbally et al. 2012b). These studies have predominantly been done on SSRIs with some including TCAs. Studies have predominantly examined children under 12 months of age using measures with low predictive validity (Gentile and Galbally 2010). There have been seven studies that have used more comprehensive measures of development such as the Bayleys Scales of Infant Development or the Wechsler measures in children over 12 months of age. The majority of these studies have not found any association with poorer child development. However, three found poorer motor development (Galbally et al. 2011; Casper et al. 2003, 2011). Whether this represents a ‘real’ finding and why antidepressants could potentially effect the developing motor system are still unanswered questions. What is known is that serotonin acts as a developmental signal during neural development (Whitaker-Azmitia et al. 1996; Whitaker-Azmitia 1999). Animal models such as Jacob’s studies on cats have shown that the serotonergic system does regulate motor activity (Jacobs and Fornal 1997).
Like most of the published research on the effects of exposure to antidepressants in pregnancy the studies on child development are hampered by variable methodology, verification of exposure and adequate measures of key confounding variables including maternal mental illness. The reassuring finding is that in the 21 published studies there have been none that have found an effect on global cognition (Galbally et al. 2012b).
6.7 Specific Clinical Considerations and Recommended Monitoring
Given the high risk of unrecognised physical comorbidity in women who suffer from major depression it is recommended that a baseline organic screen be performed as a matter of course. Investigations should include full blood examination, renal, thyroid and hepatic function testing, estimations of iron, vitamin B12, folate and vitamin D, fasting glucose and serum lipids, in addition to the usual obstetric investigations. Consideration should also be given to ECG examination if a patient is taking a medication that could compromise the QT interval (e.g. TCA’s and citalopram/escitalopram in high dose).
Antidepressants vary in their placental passage (in part due to molecular size and degree of protein binding) and extent of breast milk excretion. Placental passage studies for the SSRIs, in which cord blood estimations were measured, have found variable concentrations; however, all were much lower than maternal levels (Hendrick et al. 2003). Such data should influence the choice of antidepressant used, as well as the knowledge base regarding its potential to cause teratogenicity or adverse neonatal outcome.
Changes in gastric emptying, increased volume of distribution, decreased gastrointestinal motility, decreased drug-binding capacity and increased hepatic metabolism during pregnancy frequently alter the therapeutic dose of antidepressants. Increased frequency of psychiatric review is essential and medication doses may need to be increased in order to maintain efficacy (especially during the third trimester).
Consideration should be given to the administration of folate at a dose of 0.5–5 mg daily depending on a woman’s risk for neural tube defect, preferably from 3 months before conception and throughout pregnancy, as it may reduce the risk of various birth defects. Similarly, given the high rates of inadequate nutrition and self-care in this patient population, multivitamins specifically formulated for pregnancy may also be recommended.
Given that both depression and its pharmacological treatment have been (in some studies) associated with preterm labour, growth restriction and intrauterine growth retardation, adequate obstetric monitoring above and beyond usual care may be necessary.
A written individualised Perinatal Mental Health Care Plan should be prepared for each mother and baby and placed in a prominent position within the case file. It should outline the current treatment team, all pharmacological and other treatments, a plan for mode of infant feeding, recommendations for support, minimum length of stay, plans for regular psychiatric and paediatric review and a comprehensive discharge plan that ideally includes support for the mother, partner, mother–infant relationship, early parenting skills and establishment of pathways to care should relapse occur.
6.8 Recommendations for the Treatment of Women with Major Depression in Pregnancy
-
1.
Careful and considered diagnostic assessment utilising a bio-psycho-social-cultural model.
-
2.
Organise a baseline organic screen.
-
3.
Wherever possible, taking into account the potential for pregnancy, preconception consideration should be given to the most appropriate form of treatment in women who suffer from depression. Illness of mild severity should be treated first and foremost utilising psychosocial interventions with established efficacy. Pharmacotherapy should be reserved for moderately to severely depressed women and those not responsive to psychotherapeutic interventions alone.
-
4.
Optimise the therapeutic alliance and non-pharmacological treatments.
-
5.
Address lifestyle factors such as exercise, diet, sleep, sunlight exposure, stress, smoking, substance abuse and support structures.
-
6.
Make an active, rather than passive, decision regarding the continuation of established antidepressant treatment during pregnancy following consideration of personal risk factors for relapse.
-
7.
Should antidepressant medication be initiated in pregnancy consider the data regarding placental passage, breast milk excretion, teratogenic risk, obstetric risks and the potential for adverse neonatal or longer term outcomes.
-
8.
Aim for monotherapy wherever possible.
-
9.
Use the lowest effective dose of any antidepressant utilised; however, the emphasis needs to be on effective rather than lowest as partial treatment exposes mother and the foetus to both the risks of treatment and illness.
-
10.
Consider prescribing folate daily from 3 months preconception and throughout pregnancy as well as multivitamins.
-
11.
Ensure that a process of obtaining informed consent is followed in which all available information regarding risks and benefits of treatment and non-treatment in the perinatal setting are detailed.
-
12.
Establish a close liaison relationship between all disciplines involved: psychiatry, obstetrics, paediatrics, general practice, midwifery, social work and maternal and child health-care nursing.
-
13.
Ensure that adequate monitoring throughout pregnancy occurs of foetal development, obstetric physiology and maternal mental state.
-
14.
At delivery commence observation for evidence of neonatal withdrawal, toxicity, sedation, persistent pulmonary hypertension or other adverse effects and ensure that a careful morphological examination is undertaken.
-
15.
Create and implement a Mental Health Care Plan for the post-delivery maternity setting that encourages a close liaison between all health-care providers and allows an extended maternity stay in which observations can be made for any neonatal compromise secondary to exposure to antidepressant medications in utero.
-
16.
Establish early warning signs for relapse and pathways to care should this occur.
-
17.
When treating all women of childbearing age for depression consideration should be paid to the fact that she could conceive. If an antidepressant is prescribed ensure she is aware of the potential risks and advised to have a medical review, especially when planning to conceive.
6.9 Conclusion
Given maternal depression is now recognised as one of the most common complications of childbirth understanding the risks and benefits of specific treatments for this condition is relevant to a range of clinical disciplines. There is evidence of increasing use of antidepressant medications, and given that SSRIs are one of the most commonly prescribed psychotropic medication, this is of no surprise. The challenge to researchers is to clarify the mechanisms by which maternal depression may impact on child development. In clarifying this it may be clearer which treatment options are most effective at ameliorating this risk for offspring of women with depression. Given the potential for treatments to both improve maternal morbidity and prevent poorer child outcomes this is an important future area for public health research (Lewis et al. 2014).
References
Addis A, Koren G. Safety of fluoxetine during the first trimester of pregnancy: a meta-analytical review of epidemiological studies. Psychol Med. 2000;30(1):89–94.
Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–5. doi:10.1016/j.ajog.2007.07.036. S0002-9378(07)00915-5 [pii].
Appleby L, Gregoire A, Platz C, Prince M, Kumar R. Screening women for high risk of postnatal depression. J Psychosom Res. 1994;38(6):539–45.
Austin MP, Hadzi-Pavlovic D, Saint K, Parker G. Antenatal screening for the prediction of postnatal depression: validation of a psychosocial Pregnancy Risk Questionnaire. Acta Psychiatr Scand. 2005;112(4):310–7. doi:10.1111/j.1600-0447.2005.00594.x.
Austin M, Highet N, GEA Committee. The beyondblue clinical practice guidelines for depression and related disorders – anxiety, bipolar disorder and puerperal psychosis – in the perinatal period. A guideline for primary care health professionals providing care in the perinatal period. Melbourne: beyondblue: the national depression initiative; 2011.
Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Major congenital malformations in women with gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(3):252–7.
Bar-Oz B, Einarson T, Einarson A, Boskovic R, O’Brien L, Malm H, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918–26. doi:10.1016/j.clinthera.2007.05.003. S0149-2918(07)00121-X [pii].
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Depression during pregnancy: overview of clinical factors. Clin Drug Investig. 2004a;24(3):157–79. doi:2434 [pii].
Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004b;103(4):698–709. doi:10.1097/01.AOG.0000116689.75396.5f. 103/4/698 [pii].
Boyce P, Barriball E. Puerperal psychosis. Arch Womens Ment Health. 2010;13(1):45–7. doi:10.1007/s00737-009-0117-y.
Boyce P, Hickey A. Psychosocial risk factors to major depression after childbirth. Soc Psychiatry Psychiatr Epidemiol. 2005;40(8):605–12.
Boyce PM, Stubbs JM. The importance of postnatal depression. Med J Aust. 1994;161(8):471–2.
Buist AE, Barnett BEW, Milgrom J, Pope S, Condon JT, Ellwood DA, et al. To screen or not to screen–that is the question in perinatal depression. Med J Aust. 2002;177(Suppl):S101–5.
Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand. 2013;127(2):94–114. doi:10.1111/acps.12042.
Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142(4):402–8. doi:10.1067/mpd.2003.139.
Casper RC, Gilles AA, Fleisher BE, Baran J, Enns G, Lazzeroni LC. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology (Berl). 2011;217:211–9.
Chaudron LH. Complex challenges in treating depression during pregnancy. Am J Psychiatry. 2013;170(1):12–20. doi:10.1176/appi.ajp.2012.12040440.
Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi:10.1001/jama.295.5.499. 295/5/499.
Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1–5. doi:10.1016/j.ajog.2007.01.033. S0002-9378(07)00144-5 [pii].
Coverdale JH, McCullough LB, Chervenak FA. The ethics of randomized placebo-controlled trials of antidepressants with pregnant women: a systematic review. Obstet Gynecol. 2008;112(6):1361–8. doi:10.1097/AOG.0b013e31818c2a27.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6.
Davidson S, Prokonov D, Taler M, Maayan R, Harell D, Gil-Ad I, et al. Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes. Pediatr Res. 2009;65(2):236–41.
Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043–51. doi:10.1111/j.1471-0528.2008.01752.x.
Einarson TR, Einarson A. Newer antidepressants in pregnancy and rates of major malformations: a meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf. 2005;14(12):823–7. doi:10.1002/pds.1084.
Einarson A, Selby P, Koren G. Discontinuing antidepressants and benzodiazepines upon becoming pregnant. Beware of the risks of abrupt discontinuation. Can Fam Physician. 2001;47:489–90.
Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–60.
Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14. doi:10.1016/j.infbeh.2010.09.008.
Galbally M. Teratology: more than malformations. Aust N Z J Psychiatry. 2013;47(11):1082–4. doi:10.1177/0004867413495931.
Galbally M, Lewis AJ, Lum J, Buist A. Serotonin discontinuation syndrome following in utero exposure to antidepressant medication: prospective controlled study. Aust N Z J Psychiatry. 2009;43(9):846–54. doi:10.1080/00048670903107583. 913775190 [pii].
Galbally M, Lewis AJ, Buist A. Developmental outcomes of children exposed to antidepressants in pregnancy. Aust N Z J Psychiatry. 2011;45(5):393–9. doi:10.3109/00048674.2010.549995.
Galbally M, Gentile S, Lewis AJ. Further findings linking SSRIs during pregnancy and persistent pulmonary hypertension of the newborn. CNS Drugs. 2012a;26(10):813–22.
Galbally M, Lewis AJ, Gentile S, Buist A, Walker S. The biology of fetal exposure to serotonin reuptake inhibitors: implications for neurodevelopment. In: Migne LJ, Post JW, editors. Antidepressants: pharmacology, health effects and controversy. New York: Nova; 2012b. p. 1–26.
Gentile S, Galbally M. Prenatal exposure to antidepressant medications and neurodevelopmental outcomes: a systematic review. J Affect Disord. 2010;128(1–2):1–9. doi:10.1016/j.jad.2010.02.125. S0165-0327(10)00262-4 [pii].
Grigoriadis S, VonderPorten EH, Mamisashvili L, Eady A, Tomlinson G, Dennis CL, et al. The effect of prenatal antidepressant exposure on neonatal adaptation: a systematic review and meta-analysis. J Clin Psychiatry. 2013a;74(4):e309–20. doi:10.4088/JCP.12r07967.
Grigoriadis S, VonderPorten EH, Mamisashvili L, Roerecke M, Rehm J, Dennis CL, et al. Antidepressant exposure during pregnancy and congenital malformations: is there an association? A systematic review and meta-analysis of the best evidence. J Clin Psychiatry. 2013b;74(4):e293–308. doi:10.4088/JCP.12r07966.
Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013c;74(4):e321–41. doi:10.4088/JCP.12r07968.
Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. BMJ. 2014;348:f6932.
Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–24. doi:10.1001/archgenpsychiatry.2010.111.
Hemels ME, Einarson A, Koren G, Lanctot KL, Einarson TR. Antidepressant use during pregnancy and the rates of spontaneous abortions: a meta-analysis. Ann Pharmacother. 2005;39(5):803–9. doi:10.1345/aph.1E547. aph.1E547.
Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160(5):993–6.
Howard LM, Kirkwood G, Latinovic R. Sudden infant death syndrome and maternal depression. J Clin Psychiatry. 2007;68(8):1279–83.
Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7(6):820–5.
Jimenez-Solem E, Andersen JT, Petersen M, Broedbaek K, Andersen NL, Torp-Pedersen C, et al. Prevalence of antidepressant use during pregnancy in Denmark, a nation-wide cohort study. PLoS One. 2013;8(4):e63034.
Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312–6. doi:10.1001/archpedi.158.4.312. 158/4/312 [pii].
Kendell RE, Rennie D, Clarke JA, Dean C. The social and obstetric correlates of psychiatric admission in the puerperium. Psychol Med. 1981;11:341–50.
Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–73.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105. doi:10.1001/jama.289.23.3095.
Kieler H, Artama M, Engeland A, Ericsson O, Furu K, Gissler M, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012.
Koren G, Matsui D, Einarson A, Knoppert D, Steiner M. Is maternal use of selective serotonin reuptake inhibitors in the third trimester of pregnancy harmful to neonates? CMAJ. 2005;172(11):1457–9. doi:10.1503/cmaj.1041100. 172/11/1457.
Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35–47.
Leanza V, Stracquadanio M, Ciotta L, Pafumi C, Giannone T, Giunta M, et al. Folates and prevention of neural-tube diseases. Science. 2013;2(2):47–51.
Lennestal R, Kallen B. Delivery outcome in relation to maternal use of some recently introduced antidepressants. J Clin Psychopharmacol. 2007;27(6):607–13. doi:10.1097/jcp.0b013e31815ac4d2. 00004714-200712000-00009 [pii].
Lewis AJ, Galbally M, Opie G, Buist A. Neonatal growth outcomes at birth and one month postpartum following in utero exposure to antidepressant medication. Aust N Z J Psychiatry. 2010;44(5):482–7. doi:10.3109/00048670903559593.
Lewis A, Galbally M, Bailey C. Perinatal mental health, antidepressants and neonatal outcomes: findings from the Longitudinal Study of Australian Children. Neonatal Paediatr Child Health Nurs. 2012;15(3):22–8.
Lewis A, Galbally M, Gannon T, Symeonides C. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med. 2014;12(1):33.
Malhi GS, Hitching R, Berk M, Boyce P, Porter R, Fritz K. Pharmacological management of unipolar depression. Acta Psychiatr Scand Suppl. 2013;443:6–23. doi:10.1111/acps.12122.
Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol. 2011;118(1):111.
Matthey S, Ross-Hamid C. The validity of DSM symptoms for depression and anxiety disorders during pregnancy. J Affect Disord. 2011;133(3):546–52. doi:10.1016/j.jad.2011.05.004.
Matthey S, Ross-Hamid C. Repeat testing on the Edinburgh Depression Scale and the HADS-A in pregnancy: differentiating between transient and enduring distress. J Affect Disord. 2012;141(2–3):213–21. doi:10.1016/j.jad.2012.02.037.
Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry. 1992;33(3):543–61.
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi:10.1016/S0140-6736(12)61689-4.
Myles N, Newall H, Ward H, Large M. Systematic meta-analysis of individual selective serotonin reuptake inhibitor medications and congenital malformations. Aust N Z J Psychiatry. 2013;47(11):1002–12. doi:10.1177/0004867413492219. 0004867413492219 [pii].
Newport DJ, Ji S, Long Q, Knight BT, Zach EB, Smith EN, et al. Maternal depression and anxiety differentially impact fetal exposures during pregnancy. J Clin Psychiatry. 2012;73(2):247–51. doi:10.4088/JCP.10m06783.
O’Hara MW, Swain AM. Rates and risk of postpartum depression – a meta-analysis. Int Rev Psychiatry. 1996;8:37–54.
O’Hara MW, Neunaber J, Zekoski EM. Prospective study of postpartum depression: prevalence, course and predictive factors. J Abnorm Psychol. 1984;93:158–71.
Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898–906. doi:10.1001/archpsyc.63.8.898. 63/8/898 [pii].
O’Brien L, Einarson TR, Sarkar M, Einarson A, Koren G. Does paroxetine cause cardiac malformations? J Obstet Gynaecol Can. 2008;30(8):696–701.
Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(12):1139–47.
Pitt B. ‘Atypical’ depression following childbirth. Br J Psychiatry. 1968;114:1325–35.
Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22(4):571–5. doi:10.1016/j.reprotox.2006.03.019. S0890-6238(06)00099-2 [pii].
Ross LE, Grigoriadis S, Mamisashvili L, VonderPorten EH, Roerecke M, Rehm J, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis outcomes after antidepressant use in pregnancy. JAMA Psychiatry. 2013;70:1–8.
Scanlon KS, Ferencz C, Loffredo CA, Wilson PD, Correa-Villaseñor A, Khoury MJ, et al. Preconceptional folate intake and malformations of the cardiac outflow tract. Epidemiology. 1998;9(1):95–8.
Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159(12):2055–61.
Slade T, Johnston A, Oakley Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: methods and key findings. Aust N Z J Psychiatry. 2009;43(7):594–605. doi:10.1080/00048670902970882.
Spinelli MG. Interpersonal psychotherapy for depressed antepartum women: a pilot study. Am J Psychiatry. 1997;154(7):1028–30.
Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies. JAMA. 2009;301(6):636–50.
Tod ED. Puerperal depression. A prospective epidemiological study. Lancet. 1964;2(7372):1264–6.
Toh S, Mitchell AA, Louik C, Werler MM, Chambers CD, Hernandez-Diaz S. Selective serotonin reuptake inhibitor use and risk of gestational hypertension. Am J Psychiatry. 2009;166(3):320–8. doi:10.1176/appi.ajp.2008.08060817.
Van den Bergh BRH, Mulder EJH, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29(2):237–58.
Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong-van den Berg L, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62(10):863–70. doi:10.1007/s00228-006-0177-0.
Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805–15. doi:10.1001/archpsyc.65.7.805.
Viguera AC, Tondo L, Koukopoulos AE, Reginaldi D, Lepri B, Baldessarini RJ. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. Am J Psychiatry. 2011;168(11):1179–85. doi:10.1176/appi.ajp.2011.11010148.
Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–9. doi:10.1111/j.1600-0447.2009.01490.x. ACP1490 [pii].
Warner R, Appleby LS, Whitton A, Faragher BA. Demographic and obstetric risk factors for postnatal psychiatric morbidity. Br J Psychiatry. 1996;168(5):607–11.
Watson JP, Elliott SA, Rugg AJ, Brough DI. Psychiatric disorder in pregnancy and the first postnatal year. Br J Psychiatry. 1984;144:453–62.
Whitaker-Azmitia PM. The discovery of serotonin and its role in neuroscience. Neuropsychopharmacology. 1999;21(2 Suppl):2S.
Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73(1–2):19.
Wilson KL, Zelig CM, Harvey JP, Cunningham BS, Dolinsky BM, Napolitano PG. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19.
Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557–66. doi:10.1176/appi.ajp.2008.08081170. appi.ajp.2008.08081170 [pii].
Wisner KL, Bogen DL, Sit D, McShea M, Hughes C, Rizzo D, et al. Does fetal exposure to SSRIs or maternal depression impact infant growth? Am J Psychiatry. 2013;170(5):485–93. doi:10.1176/appi.ajp.2012.11121873. 1669748.
Wurst KE, Poole C, Ephross SA, Olshan AF. First trimester paroxetine use and the prevalence of congenital, specifically cardiac, defects: a meta-analysis of epidemiological studies. Birth Defects Res A Clin Mol Teratol. 2010;88(3):159–70. doi:10.1002/bdra.20627.
Yonkers KA, Smith MV, Gotman N, Belanger K. Typical somatic symptoms of pregnancy and their impact on a diagnosis of major depressive disorder. Gen Hosp Psychiatry. 2009a;31(4):327–33. doi:10.1016/j.genhosppsych.2009.03.005. S0163-8343(09)00050-4 [pii].
Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009b;31(5):403–13. doi:10.1016/j.genhosppsych.2009.04.003.
Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009c;114(3):703–13. doi:10.1097/AOG.0b013e3181ba0632. 00006250-200909000-00044 [pii].
Yonkers KA, Gotman N, Smith MV, Forray A, Belanger K, Brunetto WL, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848–54. doi:10.1097/EDE.0b013e3182306847.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Boyce, P., Galbally, M., Snellen, M., Buist, A. (2014). Pharmacological Management of Major Depression in Pregnancy. In: Galbally, M., Snellen, M., Lewis, A. (eds) Psychopharmacology and Pregnancy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54562-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-54562-7_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54561-0
Online ISBN: 978-3-642-54562-7
eBook Packages: MedicineMedicine (R0)