Abstract
Objectives
This study evaluated the question whether length of in utero exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants might affect neonatal outcome and psychomotor development in infancy.
Methods
Birth outcome was determined in the offspring of 55 women with major depressive disorder who used SSRI medication for different durations during their pregnancies. At an average age of 14 months, children underwent a pediatric examination and an evaluation with the Bayley Scales of Infant Development (BSID-II).
Results
Duration of in utero exposure to SSRIs was negatively associated with total Apgar scores, specifically the activity subscale. Odds ratios for a low score (<2) on this scale were 3.8 and 6.0 at 1 and 5 min, respectively. Newborns with longer exposure were more often admitted to the Neonatal Intensive Care Unit (p < .03). Mental Development Index scores of the infants were not associated with the length of gestational exposure to SSRIs. A longer duration of exposure increased the risk for lower Psychomotor Developmental Index and Behavioral Rating Scale scores in infancy (p = 0.012 and p = 0.007, respectively) on the BSID-II.
Conclusions
The findings provide evidence that the length of prenatal SSRI antidepressant use can affect neonatal adjustment and can have an effect on psychomotor test scores in infancy. Importantly, the children’s mental development and motor function by neurological examination were within the normal range. Timing of exposure to SSRIs during susceptible periods of fetal development and variations in the severity of maternal depression may have contributed to the associations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relative safety of the selective serotonin reuptake inhibitors (SSRI) antidepressant medications has led to their widespread use to treat depressive disorders in women of childbearing age. Antidepressant use during pregnancy has been on the increase in the past decade (Bakker et al. 2008), even though knowledge regarding the risks of prenatal exposure to SSRI medications remains far from complete.
SSRI medications cross the placental barrier (Hendrick et al. 2003; Stowe et al. 2003) and can be measured in amniotic fluid (Loughhead et al. 2006). Early human studies have found no detectable effects of SSRI exposure on overall rates of major congenital malformations (Chambers et al. 1996; Pastuszak et al. 1993; Simon et al. 2002) the exception being a Danish cohort study by Wogelius et al. (2006) who reported an increased risk of congenital malformations after exposure to SSRIs. Several population-based studies suggest a small increase in congenital cardiac defects (Berard et al. 2007; Diav-Citrin et al. 2008; Louik et al. 2007; Merlob et al. 2009; Pedersen et al. 2009), albeit Alwan et al. (2007) found no increased risk of congenital cardiac malformations. In newborns, maternal use of SSRI medication, especially during the last trimester, has been linked to an increased risk of persistent pulmonary hypertension (Chambers et al. 2006), premature births (Simon et al. 2002; Suri et al. 2007; Toh et al. 2009; Wisner et al. 2009), and alterations in regulatory processes reflected in changes in arousal, rapid eye movement sleep, and pain responses (Laine et al. 2003; Oberlander et al. 2002; Zeskind and Stephens 2004). Studies on the neurodevelopment of children exposed prenatally to SSRIs have shown variable results, from normal mental and psychomotor development (Misri et al. 2006; Nulman et al. 1997; Oberlander et al. 2005) to evidence for a slight delay in motor development (Casper et al. 2003; Mattson et al. 2004; Mortensen et al. 2003; Pedersen et al. 2010). So far, surprisingly little has been published as to whether length of gestational drug exposure bears on birth outcome. Analyzing population-based health data, Oberlander et al. (2008) reported with increasing duration of in utero exposure to SSRIs an increased risk for adverse birth outcomes. The present study addresses the potential influence of the duration of SSRI antidepressant use during pregnancy on birth outcome and on postnatal infant development in a clinical cohort.
Subjects and methods
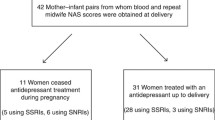
Participants (N = 55) consisted of the offspring of women who took SSRI medication for major depressive disorder (MDD) in pregnancy for different lengths of time. Women were recruited consecutively from the Women’s Clinic at Stanford University (38 of the women were recruited before or early in pregnancy; 17 directly postpartum). All participants, who started the protocol, completed the study and the follow-up. Women who had a physical diagnosis that required treatment were excluded. All received supportive psychotherapy. The study was approved by the Panel on Human Subjects in Medical Research at Stanford University. Maternal written informed consent was obtained with separate assent forms signed for the participating child.
Maternal diagnostic assessment
The women in this study were interviewed in person using the Structured Clinical Interview for DSM—IV Axis I Disorders (First et al. 1997) to confirm the diagnosis of MDD. Each woman completed a questionnaire on sociodemographic information, a medical, family, and psychiatric history and information about the index pregnancy. Data regarding the delivery and the neonatal and postnatal course were collected from obstetric and neonatal medical records. Psychological functioning was recorded during the pregnancy and then again at follow-up. Women rated levels of depression using the Beck Depression Inventory (Beck et al. 1961) and an analog scale after each trimester. At the follow-up examination, women were interviewed again (DSM 1994), and maternal mood was interview-rated using the Hamilton Depression Scale (Hamilton 1960) and self-rated using the Beck Depression Inventory (Beck 1972) and the Center for Epidemiologic Studies—Depression Scale (CES-D; Radloff 1977).
Antidepressant medication use and dose and substance use during pregnancy
The information about the type and dose of medication was listed per day for each month. Twenty women took sertraline, 18 used fluoxetine, 15 took paroxetine, and 2 took citalopram. To adjust for the higher therapeutic doses of sertraline, an average for each drug dose per day was calculated: (low = 1: <20 mg fluoxetine/paroxetine/citalopram/day or <100 mg sertraline/day; medium = 2: 20 mg fluoxetine/paroxetine/citalopram/day or 100 mg sertraline/day; high = 3: >20 mg fluoxetine/paroxetine/citalopram/day or >100 mg sertraline/day). Smoking was recorded as number of cigarettes per day. Alcohol use was defined as having more than five drinks during the pregnancy. One drink was defined as one glass of wine, one bottle of beer, or one mixed drink on any day while pregnant.
Infant evaluation at follow-up
All children, ranging in age from 12 to 40 months, underwent a pediatric neurological examination performed by pediatricians certified in a standardized evaluation method for neurological functioning (NIH Neonatal Network). The pediatricians who examined the children as newborns and as infants and the psychologists who conducted outcome evaluations had no knowledge of the mothers’ medication status. A pediatric geneticist conducted the dysmorphology examinations for major malformations and completed a 130-item checklist to record minor anomalies. Preterm status was defined as delivery at <37 weeks gestation. Two developmental psychologists assessed levels of mental and motor development using the Bayley Scales of Infant Development—Second Edition (BSID-II; Bayley 1993). Both were reliability certified on the BSID-II annually as part of a NIH-funded collaborative neonatal outcome study. The BSID-II consists of three scales: the Mental Development Index (MDI), the Psychomotor Development Index (PDI), and the Behavioral Rating Scale (BRS). The Mental and Motor Scales assess the child’s level of cognitive, language, personal–social, and fine and gross motor development. The Behavioral Rating Scale assesses qualitative aspects of the child’s behavior during the testing situation on a 5-point scale with 12 items for orientation/engagement, ten items for emotional regulation, and eight items for motor quality (Thompson et al. 1996). The data are expressed as standard scores based on samples for each age group consisting of equal numbers of males and females.
Statistical analysis
Maternal and qualitative infant characteristics were compared among exposure groups. ANOVA was used for continuous variables and χ 2 analyses for categorical variables. If a variable showed significant differences among groups, post hoc analyses using t tests or Fisher’s exact tests were utilized.
Primary outcome variables were analyzed as follows: Apgar scores and subscale scores were converted into binary variables to avoid concerns about the effects of outliers when only one or a few infants had the lowest score. Apgar scores were defined to be low if they were less than 8. Subscales scores were defined to be low if they were less than 2, except for skin coloration. Skin coloration was defined to be low if it was a value of 0. Motor quality factor items were converted to binary variables and defined to be low if they were less than 5. Logistic regression was used to analyze the Apgar scores and motor quality scores with weeks of in utero exposure as the independent variable. Linear regression was used to analyze Bayley II mental and motor development scores and behavioral rating scales with weeks of in utero exposure as the independent variable. Odds ratios and SSRI effects in the logistic and linear regression models were standardized to reflect the effects of 23 weeks of exposure, which was the median duration of exposure in our sample. Estimated odds ratios for logistic regression analyses and correlations for linear regression analyses are reported. The women and their children were divided into three comparison groups based on SSRI medication use: trimester 1 (N = 14), trimesters 2 and 3 (N = 11) and trimester 3 (N = 7), and trimesters 1, 2, and 3 (N = 23). Analyses focusing on timing of SSRI exposure rather than duration used linear regression with exposure group (trimester pattern treated as a three-level factor variable) as the independent variable. Trimester and duration models were compared in terms of the log-likelihood. All hypothesis tests were two-sided. Tests and confidence intervals used 0.05 significance levels and 95% confidence levels, respectively.
Results
Maternal characteristics and SSRI exposure
Maternal characteristics were similar among women in the three groups separated by trimester (Table 1). Ninety percent of the women were Caucasian; all received early and regular prenatal care. Age at delivery, education, regular exercise, prenatal vitamin use, miscarriages, gravidity, parity, weight gain during pregnancy, and numbers of cesarean section did not differ between the three groups. The proportion of prospectively/retrospectively recruited women was similar in the three groups.
Depression
Numbers of depressive episodes experienced prior to the index pregnancy were similar in the three comparison groups. Depressive symptoms measured on the Beck Depression Inventory (Beck et al. 1961) at their most severe during the pregnancy did not differ significantly. Women who took SSRI antidepressants throughout pregnancy rated themselves as less depressed on the analog scale during the first 1–3 (p < 0.02) and 4–6 months of pregnancy (p < 0.05) than women in either other group. The groups did not differ with regard to occasional use of analgesics, antacids, or decongestants, except for more frequent occasional use of anti-anxiety medication in the early-exposure group (29%, 7%, and 5%, respectively; χ 2 = 4.7; p < 0.02).
Antidepressant medication use and dose and substance use (Table 1)
Among the three groups, mean doses of SSRI medication were in the medium range and were not significantly different. As would be expected, the groups differed significantly in length of time SSRI medications were taken. Women who used SSRIs throughout pregnancy spent more hours in labor (p < 0.05) than women in the first trimester group, but time in labor was not longer when compared to the second/third trimester group.
Substance use
Based on self-report, one woman smoked cigarettes (during the first 4 months of her pregnancy) and five women used alcohol, defined as more than five drinks during pregnancy (n.s.). Four women reported between 23 and 36 drinks, and one woman reported 56 drinks.
Newborn characteristics and birth outcome
The three groups had similar sex distributions and similar percentages of first-born children (Table 2). Three preterm births (at 34, 35, and 36 weeks) were recorded in the continuously exposed group as compared to none in the first trimester exposure group (p < 0.05) and one (36 weeks) in the second/third trimester exposure group. Birth weights and lengths were not different between the three groups. Newborns with second/third trimester and continuous exposure to SSRIs were more often admitted to neonatal intensive care (NICU). NICU admissions were not significantly associated with duration of exposure. Admitted newborns did not differ from not-admitted newborns in drug dose. Reasons for admission included respiratory distress (in six newborns) and meconium aspiration (in four) as well as infection, hypoglycemia, low birth weight, and a cardiac murmur. Findings from the dysmorphology examination listing major and minor malformations are displayed in Table 2.
Apgar scores
Increased length of SSRI exposure significantly increased the odds of low Apgar scores (<8) at 1 and 5 min (p = 0.022 and p = 0.048, respectively). Odds ratios for a low Apgar score were 3.0 (CI 1.2, 7.8) and 5.2 (CI 1.0, 26.8), respectively. Analysis of Apgar subscale scores revealed that only the activity/muscle tone subscale score was significantly decreased by longer SSRI use. Odds ratios for a low score (<2) on this scale were 3.8 (CI 1.3, 10.6, p = 0.0121) and 6.0 (CI 1.3, 27.8, p = 0.0221) at 1 and 5 min, respectively. No significant relationship was found for the other subscales.
Figure 1 illustrates how the probability of a low Apgar score (<8) depends on the weeks of exposure. For the 1-min Apgar, the estimated probability increases from 17% for 1 week of exposure to 58% for 40 weeks of exposure. For the 5-min Apgar, the estimated probability increases from 2% for 1 week of exposure to 29% for 40 weeks duration of exposure.
Follow-up examinations
Maternal characteristics
At follow-up examination, 64% of women who had taken SSRIs in the first trimester, 94% of the women in the second/third trimester group, and 87% of women who took SSRIs throughout pregnancy took SSRI medication (χ 2 = 5.6; p < 0.06). Maternal depressive symptom levels ranged from normal to mildly depressed mood. There were no significant differences between the groups for severity of depression as rated by the Hamilton Depression scale (F = 0.46; p = 0.6), the Beck Depression Inventory (F = 0.57; p = 0.6), the Beck Anxiety Scale (F = 1.1; p = 0.3), or the CES-D (F = 0.68; p = 0.52).
Infant characteristics
At follow-up (see Table 2), the infants in the three groups did not differ in chronological age, body weight, height, or fronto-occipital head circumference. Results from the pediatric/neurological examinations revealed no between group differences. One child in the first trimester exposure group was noted to have slight hypotonia and one had a slight tremor. In the two to three trimester exposure group, one child was noted to have slight hypotonia and another “gross motor delay,” whereas in the continuous exposure group, two children showed gross motor delay, one had hypotonia, one had a broad based gait and one child a slightly increased tone at the hips.
Duration of in utero exposure to SSRIs and physical and developmental outcome
Length of in utero exposure to SSRIs was not significantly related to physical growth as reflected in birth weight, birth length, in follow-up weight, height, or head circumference. MDI scores of the infants were not associated with duration of SSRI exposure. Significant negative correlations of the Psychomotor Development Index with length of exposure were observed (p = 0.012, R 2 = 0.11, r = −0.34; Fig. 2).There was a significant negative correlation between the Behavior Rating Scale and duration of exposure (p = 0.007, R 2 = 0.13, r = −0.36; Fig. 3). Length of exposure was also significantly negatively correlated with the BRS factor scales: orientation/engagement (p = 0.001, R 2 = 0.21, r = −0.46), emotional regulation (p = 0.004, R 2 = 0.18, r = −0.42) and motor quality (p = 0.024, R 2 = 0.09, r = −0.31). In the trimester model, infants with early exposure were rated significantly higher on the PDI than infants with continuous exposure (p = 0.048) and on the BRS (p = 0.014). Trimester of exposure and duration of exposure are clearly associated, with the early-exposure group having the shortest duration. Thus, the effects of timing of exposure cannot be separately estimated. We conducted a secondary analysis in which the PDI and BRS scores were regressed on timing of exposure group by trimester treated as a factor variable and then compared the results to the regression results for duration of exposure. Exposure group was also significant for both PDI and BRS (p = 0.049 and p = 0.049) but yielded a slightly less good fit than the linear duration model as measured by their respective log-likelihoods (PDI −219.1 vs. −218.8; BRS −246.6 vs. −245.9). The lowest PDI and BRS scale scores were seen in the children with the longest, continuous, exposure to SSRIs.
Neither Apgar total scores nor Apgar muscle tone scores at 1 or 5 min significantly predicted PDI or BRS scores. PDI scores were positively correlated with BRS total scores (p = 0.001, r = 0.45), motor quality factor scores (p < 0.001, r = 0.56), and orientation factor scores (p < 0.001, r = 0.52), but not with the emotional regulation factor scores (p = 0.41, r = 0.12).
We further considered whether age at examination might have affected the results. Age, itself, was not a significant predictor of outcome. Age-adjusted linear regression results for PDI and total BRS scores on exposure duration were qualitatively identical to previous results when age was included as a covariate. When these analyses were restricted to 28 infants between 12 and 24 months of age, the association for PDI and BRS total score remained significant (p = 0.03 and 0.01, respectively).
Discussion
Our data indicate that length of gestational exposure to SSRI antidepressants may have effects on neonatal adjustment and psychomotor development in infancy. Children born to mothers who used SSRI medication for a period of over 5 months were at greater risk for lower Apgar scores at delivery and delayed onset of spontaneous movements at birth. More frequent NICU admissions appear to be related to late gestational exposure, since we observed no association with duration of exposure.
Most previous investigations that have evaluated outcomes following late pregnancy exposure to SSRIs have not considered duration of exposure as a contributing factor, even though in many of these studies exposure occurred throughout gestation. There is one recent report from a Health Database analysis by Oberlander et al. (2008) that found an increased risk for reduced gestational age, for lower birth weight for gestational age, and for signs of respiratory distress with longer duration of prenatal SSRI exposure. However, it is unclear whether the logistic regression analysis of the effect of duration fully eliminated the effect of timing. An analysis of a matched subsample adjusted for maternal treatment characteristics using propensity score matching designed to correct for the effects of timing found a lower birth weight, only. In the present study, the duration model provided a better fit to the data than the timing of exposure (trimester) model, although the study cannot statistically exclude an effect of time of exposure. Another point worth considering with regard to timing is that serotonergic neurons and their projection networks evolve and change during brain development (Jacobs and Azmitia 1992). Therefore, the effects of the SSRIs on the developing brain may differ depending on the time of exposure, while a longer duration of exposure increases the chance that exposure occurs during critical periods of development.
Women in our study had good prenatal care and were essentially free of substance use, yet an increase in preterm births was noted in newborns exposed to SSRIs during late gestation compared to newborns exposed briefly during the first trimester confirming previous findings (Simon et al. 2002; Suri et al. 2007; Toh et al. 2009; Wisner et al. 2009).
Length of exposure to SSRIs was negatively associated with Apgar scores at 1 and at 5 minutes. The higher rate of newborn admissions to the NICU was not associated with duration of exposure, supporting the conclusions by Moses-Kolko et al. (2005) that late in utero exposure to SSRIs increases the risk for neonatal adaptation problems. Both lower Apgar scores and more frequent NICU admissions have been reported in clinical and registry studies that compared women with gestational SSRI use to medication free healthy controls or unmedicated depressed women (Casper et al. 2003; Chambers et al. 1996; Lund et al. 2009; Oberlander et al. 2002; Simon et al. 2002). Our data suggest that the risk for an Apgar of <8 and for a delayed onset of spontaneous movements at birth at both 1 and 5 min increased with the duration of gestational exposure to SSRIs. Still, it is important to note that the Apgar scores, including the activity subscale scores, improved at 5 min, suggesting a quick recovery over time. We observed no relationship between Apgar scores and developmental outcome assessed in infancy which would agree with the overall good prognosis for moderately reduced Apgar scores in normal weighed term infants described in the literature (Ehrenstein et al. 2009).
Consistent with previous research, we found no association between length of gestational SSRI exposure and mental development. However, longer prenatal exposure to SSRIs was associated with lower scores on the Bayley PDI and with reduced scores on the motor quality as well as the orientation/engagement and emotional regulation factors on the BRS.
The literature contains few longitudinal studies on the neurodevelopment of infants with gestational exposure to SSRIs. Two groups (Nulman et al. 1997, 2002; Oberlander et al. 2005) have reported normal motor developmental outcomes and one study noted advanced motor development (DiPietro et al. 2006) in children with gestational exposure to SSRIs compared to children of healthy controls. Psychomotor delay after in utero SSRI exposure has been recorded before by Simon et al. (2002) and has been reported by Casper et al. (2003). Of interest is a report by Pedersen et al. (2010) based on an analysis of data collected from the Danish National Birth Cohort. The investigators found a small developmental delay in gross motor function and a reduced ability for engagement in SSRI exposed children. Children with second or third trimester exposure to SSRI antidepressants showed a delay in sitting without support at 6 months and fewer of SSRI exposed children were able to occupy themselves for more than 15 min compared to not exposed children. Pedersen et al. (2010) found the delay in sitting without support to occur within the normal time range of development.
Similarly, the lowered psychomotor and motor quality ratings in our study can probably be best understood as a developmental delay, since clinically discernable functional motor symptoms were absent when the children were examined by the pediatric neurologist. A link between in utero exposure to SSRIs and motor control would not be entirely unexpected considering that muscle tone is under serotonergic control (Jacobs and Fornal 1995) and that alterations in muscle tone control can be side effects of the SSRI drugs (Laine et al. 2003; Loubinoux et al. 2002).
The reduced scores in the orientation and emotional regulation factor suggest less persistence—a behavior also observed in the study by Pedersen et al. (2010)—and a less easily engaged and flexible disposition in the children during the testing session. In contrast, Misri et al. (2006) and Oberlander et al. (2007) reported that levels of internalizing behaviors (withdrawal, anxiety, depression) and externalizing behaviors (attention difficulties, activity levels, problem solving) did not differ significantly in 4- to 5-year-old children with prenatal psychotropic medication exposure and those not exposed; mothers who were depressed reported more anxious/avoidant behavior and increased externalizing behaviors in their children than asymptomatic mothers. Oberlander et al. (2007) found elevated cord SSRI plasma levels to be associated with increasing parental reports of externalizing behaviors, but the effect was reduced after controlling for maternal depressed mood.
In rodents, developmental SSRI exposure causes a reduction in serotonin transporter (5-HTT) expression in adults (Hansen and Mikkelsen 1998). More specifically, Ansorge et al. (2004) have shown that chronic fluoxetine treatment early in development resulted in reduced exploratory and increased anxiety-related behavior, resembling the 5-HTT−/− phenotype in mice. Since these symptoms are the opposite of those induced by adult SSRI exposure, more research is required to answer the question to what extent conclusions about the effects of SSRI exposure in humans can be drawn from rodent studies (Homberg et al. 2009).
We cannot answer the question whether differences in depression severity during pregnancy may have contributed to the observed associations because our study lacked frequent maternal mood assessments. The data suggest overall comparable levels of illness severity in the groups given that the women in our sample had similar numbers of pre-pregnancy major depressive disorder episodes, similar mild to moderate levels of depressive symptoms during pregnancy and that the majority took antidepressant medications at the time of the follow-up evaluation. Two recent well-designed prospective studies did control for depressed mood and reported conflicting results. Suri et al. (2007) found the presence of depressive symptoms not to be associated with an increased risk of preterm birth, while Wisner et al. (2009) documented an effect of continued untreated depression on preterm birth rates.
Another limitation derives from the fact that the obstetricians who delivered the children were most likely or ought to have been aware of the mother’s diagnosis and treatment. This problem is unavoidable and is shared by other outcome studies, since keeping physicians blind to treatment status would raise ethical concerns. For the same reasons, random assignment was not possible. The sample size limits the statistical power for assessing some variables.
In conclusion, while the increased risks for greater difficulties in neonatal adjustment with longer prenatal exposure to SSRIs are consistent with findings from published studies, a further follow-up will be needed the clarify the nature of the motor and behavioral findings in infancy.
References
Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM (2007) Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 356:2684–2692
Ansorge M, Zhou M, Lira A, Hen R, Gingrich J (2004) Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306(5697):879–881
Bakker MK, Kolling P, van den Berg PB, de Walle HE, de Jong van den Berg LT (2008) Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol 65:600–606
Bayley N (1993) Bayley scales of infant development, 2nd edn. The Psychological Corporation, San Antonio
Beck A (1972) Depression: causes and treatment. University of Pennsylvania Press, Philadelphia
Beck A, Ward C, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Berard A, Ramos E, Rey E, Blais L, St-Andre M, Oraichi D (2007) First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 80:18–27
Casper R, Fleisher B, Lee-Ancajas J, Gilles A, Gaylor E, DeBattista A, Hoyme H (2003) Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 142:402–408
Chambers C, Johnson K, Dick L, Felix R, Jones K (1996) Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 335:1010–1015
Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA (2006) Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354:579–587
Diav-Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, Di Gianantonio E, Clementi M, Weber-Schoendorfer C, Schaefer C, Ornoy A (2008) Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Br J Clin Pharmacol 66:695–705
DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP (2006) Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev 77:573–587
DSM I (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Association, Washington, D.C
Ehrenstein V, Pedersen L, Grijota M, Nielsen GL, Rothman KJ, Sorensen HT (2009) Association of Apgar score at five minutes with long-term neurologic disability and cognitive function in a prevalence study of Danish conscripts. BMC Pregnancy Childbirth 9:14
First M, Spitzer RL, Gibbon M, Williams JBW (1997) Structured clinical interview for DSM IV axis I disorders. APA, Washington, D.C
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hansen H, Mikkelsen J (1998) Long term effects on serotonin transporter mRNA expression of chronic neonatal exposure to a serotonin reuptake inhibitor. Eur J Pharmacol 352:307–315
Hendrick V, Stowe Z, Altshuler L, Hwang S, Lee E, Haynes D (2003) Placental passage of antidepressant medications. Am J Psychiatry 160:993–996
Homberg JR, Schubert D, Gaspar P (2009) New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci 31:60–65
Jacobs B, Azmitia E (1992) Structure and function of the brain serotonin system. Physiol Rev 72:165–215
Jacobs B, Fornal C (1995) Serotonin and behavior, a general hypothesis. In: Bloom F, Kupfer D (eds) Psychopharmacology, the fourth generation of progress. Raven, New York, pp 461–469
Laine K, Heikkinen T, Ekblad U, Kero P (2003) Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry 60:720–726
Loubinoux I, Pariente J, Boulanouar K, Carel C, Manelfe C, Rascol O, Celsis P, Chollet F (2002) A single dose of the serotonin neurotransmission agonist paroxetine enhances motor output: double-blind, placebo-controlled, fMRI study in healthy subjects. Neuroimage 15:26–36
Loughhead AM, Fisher AD, Newport DJ, Ritchie JC, Owens MJ, DeVane CL, Stowe ZN (2006) Antidepressants in amniotic fluid: another route of fetal exposure. Am J Psychiatry 163:145–147
Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA (2007) First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 356:2675–2683
Lund N, Pedersen LH, Henriksen TB (2009) Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med 163:949–954
Mattson SN, Calarco K, Kao KK, Jones KL, Chambers CD (2004) Neurodevelopmental outcome of infants and toddlers exposed prenatally to selective serotonin reuptake inhibitors. Clinical and Molecular Teratology 70:261
Merlob P, Birk E, Sirota L, Linder N, Berant M, Stahl B, Klinger G (2009) Are selective serotonin reuptake inhibitors cardiac teratogens? Echocardiographic screening of newborns with persistent heart murmur. Birth Defects Res A Clin Mol Teratol 85:837–841
Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE, Oberlander TF (2006) Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 163:1026–1032
Mortensen JT, Olsen J, Larsen H, Bendsen J, Obel C, Sorensen HT (2003) Psychomotor development in children exposed in utero to benzodiazepines, antidepressants, neuroleptics, and anti-epileptics. Eur J Epidemiol 18:769–771
Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL (2005) Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 293:2372–2383
Nulman I, Rovet J, Stewart D, Wolpin J, Gardner H, Theis J, Kulin N, Koren G (1997) Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 336:258–262
Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G (2002) Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 159:1889–1895
Oberlander T, Eckstein Grunau R, Fitzgerald C, Ellwood A, Misri S, Rurak D, Riggs K (2002) Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatr Res 51:443–453
Oberlander T, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W (2005) Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics 115:411–425
Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE (2007) Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 161:22–29
Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C (2008) Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. Br J Psychiatry 192:338–343
Pastuszak A, Schick-Boschetto B, Zuber C (1993) Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). J Am Med Assoc 269:2246–2248
Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH (2009) Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. Bmj 339:b3569
Pedersen LH, Henriksen TB, Olsen J (2010) Fetal exposure to antidepressants and normal milestone development at 6 and 19 months of age. Pediatrics 125:e600–e608
Radloff L (1977) The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Simon G, Cunningham M, Davis R (2002) Outcomes following prenatal antidepressant exposure. Am J Psychiatry 159:2055–2061
Stowe Z, Hostetter A, Owens M, Ritchie J, Sternberg K, Cohen L, Nemeroff C (2003) The pharmacokinetics of sertraline excretion into human breast milk: determinants of infant serum concentrations. J Clin Psychiatry 64:73–80
Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J (2007) Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 164:1206–1213
Thompson B, Wasserman J, Matula K (1996) The factor structure of the behavior rating scale of the Bayley scales of infant development—II: cross-sample, cross-sectional, and cross-method investigations of construct validity. Educ Psychol Meas 56:460–474
Toh S, Mitchell AA, Louik C, Werler MM, Chambers CD, Hernandez-Diaz S (2009) Antidepressant use during pregnancy and the risk of preterm delivery and fetal growth restriction. J Clin Psychopharmacol 29:555–560
Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, Perel JM, Jones-Ivy S, Bodnar LM, Singer LT (2009) Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 166:557–566
Wogelius P, Norgaard M, Gislum M, Pedersen L, Munk E, Mortensen PB, Lipworth L, Sorensen HT (2006) Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology 17:701–704
Zeskind PS, Stephens LE (2004) Maternal selective serotonin reuptake inhibitors use during pregnancy and newborn neurobehavior. Pediatrics 113:368–375
Acknowledgments
We wish to thank the women and the children who participated in this study, and we appreciate the support of the Edwards and Matcovich Family Trust Fund.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study received support from the Edwards and Matcovich Family Trust Fund. The authors have no financial relationship with the Edwards and Matcovich Family Trust Fund. The Edwards and Matcovich Family Trust Fund had no input in any aspect or part of the study.
Rights and permissions
About this article
Cite this article
Casper, R.C., Gilles, A.A., Fleisher, B.E. et al. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology 217, 211–219 (2011). https://doi.org/10.1007/s00213-011-2270-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2270-z