Abstract
The hominoid cranium represents a tightly constrained, functionally and developmentally integrated structure subject to multiple selective influences. Modern apes are the remnant of a much more diverse radiation, raising issues about their suitability as models for earlier hominoids. Among gibbons the folivorous siamang is cranially distinctive. The markedly airorynchous Pongo is cranially highly variable and lacks the anterior digastric muscle, thereby contrasting with other hominoids (except Khoratpithecus). African apes share a common cranial pattern differentiated by varying growth rates, not duration. Airorhynchy is common among fossil hominoids and differentiates hominoids from non-hominoids, suggesting that African ape klinorhynchy is derived. Bonobos are cranially smaller, lighter, and less dimorphic than chimpanzees. These are comparatively uniform, with extensive overlap between subspecies, whereas gorillas display considerable contrasts, especially between east and west populations. Early Miocene hominoids are already cranially diverse, with most species probably soft- or hard-fruit feeders. Middle and Late Miocene forms from Africa, Europe, and Western Asia are thicker enameled with more strongly constructed crania suggesting harder diets, although Dryopithecus (soft frugivory) and Oreopithecus (folivory) are exceptions. South and East Asian fossil hominoid diets ranged from soft fruits through harder items to bulky, fibrous vegetation. All extant ape crania are relatively lightly constructed compared with fossil forms, again prompting questions about their suitability as adaptive models of earlier hominoids.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The hominoid fossil record has expanded markedly over the last two decades, sufficiently to indicate marked morphological diversity. This in turn reflects a major radiation or, more likely, series of radiations. The great bulk of the material is from Miocene contexts – apart from hominins there is still comparatively little from Plio-Pleistocene deposits – so that fossil and living hominoids are largely detached from one another. Whatever the details of this array, it is clear that the extant nonhuman apes represent but the surviving fragment of a significantly more numerous, geographically more extensive, and ecologically more diverse group of catarrhine primates. The extremely restricted modern comparative base and its (at best) tenuous links with the earlier material pose real challenges for adaptive and phylogenetic interpretations of the fossil hominoid record.
An outcome of this is that detailed phylogenies often differ appreciably from author to author, depending on the significance accorded to particular apomorphies and on the extent to which other similarities are deemed homoplasies. The upshot is a whole series of individual phylogenies and widespread disagreement about the status of particular groups which usually translate through into the taxonomies preferred by individual researchers. Since the thrust of this chapter is primarily adaptational, we do not concern ourselves with taxonomic or phylogenetic details; in what follows suprageneric categories are used informally and generally follow majority consensus usage. For those requiring more detailed information on phylogenetic issues concerning the Miocene hominoid record, the chapters “Fossil Record of Miocene Hominoids” and “Postcranial and Locomotor Adaptations of Hominoids,” Vol. 2, and the papers by Harrison (2002), and contributors in Hartwig (2002) are excellent recent surveys.
Cranial form is influenced by multiple factors. Functionally, the head houses the visual, olfactory, and auditory organs and those of vocalization, taste, and balance; it contains the openings for the respiratory and alimentary tracts; and it houses and protects the brain. It incorporates structures for food acquisition and processing, while postural and respiratory factors influence basicranial morphology. Its superficial tissues may be patterned and convey information to conspecifics about sex and ontogenetic status. The interplay of these features, and especially the size and configuration of those concerned with food processing relative to neurocranial proportions, may lead to the development of external structures such as crests and tori on the skull. There are clearly intense selection pressures determining effective developmental and functional integration of these varied aspects of cranial function throughout the individual life cycle.
Fleagle et al. (2010) undertook a 3D geometric morphometric study (Procrustes and Principal Components Analysis) of primate crania to quantify broad aspects of cranial diversity. Their first PC differentiated on cranial flexion, orbit size and orientation, and relative brain size, while PC 2 reflected differences in cranial height and snout length. Eulemur, Mandrillus, Pongo, and Homo represent the limits in cranial shape. Overall, hominoids display the greatest diversity in cranial shape among extant primate clades, although much of this is driven by the atypical and highly distinctive cranium of H. sapiens.
Adult African apes including humans, known as hominids , share a broadly common pattern of covariation in cranial traits, with the oral and zygomatic regions primary integrative influences and with a lesser contribution from the nasal region, i.e., those craniofacial components primarily associated with mastication (Ackermann 2002, 2005). This differs from the pattern in both Old and New World monkeys, in which the oral region is the exclusive primary contributor to facial integration. Ackermann suggests that this contrast may reflect innovatory functional or developmental shifts after the differentiation of hominoids from other Anthropoidea or be an allometric consequence of increased body size. Orangutans and gibbons were not represented in the analyses, but the extent to which they share the primary oral/zygomatic integrative pattern should help decide between these possibilities and assist in determining whether the pattern is a hominoid or hominid synapomorphy.
There are similar allometric patterns in the midface and common opposite relationships between lower and upper face in the adults. Whereas visual inspection and morphological distance place adult Pan troglodytes and Gorilla gorilla close together and Homo sapiens distant, craniofacial covariation patterns accord with molecular data in indicating closer affinity between P. troglodytes and H. sapiens, with G. gorilla distant (Ackermann 2002). Such concordance, however, does not hold throughout ontogeny, with differing patterns of affinity between juveniles and subadults of the above taxa on the one hand and infants and “adolescents” on the other (Ackermann 2005).
Nonetheless, some general patterns emerge: in particular, across the species earlier and later (subadult and adult) integration appears to reflect different drivers. Oral integration is especially influential in the earlier stages, as well as thereafter, but there are specific differences in the onset of zygomatic integration. In P. paniscus and P. troglodytes, it appears during the juvenile/adolescent periods, whereas in Gorilla it occurs from infancy, perhaps a correlate of its rapid growth. In all species, zygomatic integration intensifies in later ontogeny. Where evident, nasal integration occurs in mid-/late ontogeny, its intensity varying inversely with oral integration, suggesting that separate developmental modularities underlie these regions. While the most highly integrated species as adults, humans are more developmentally labile than the other African apes prior to maturity. While differing in detail, however, all species show a common pattern of intensified integration throughout development, with a particular shift toward more constrained variation around sexual maturity or just after. The extent to which these similarities reflect shared, genetically determined, developmental pathways, or common selection pressures associated with vital functional requirements – the need for effective food processing mechanisms, for instance – remains to be determined. In the latter case, some proportion of the resemblance could be homoplastic.
A recent study by Singh et al. (2012) based on covariation in 56 morphometric landmarks representing the functional modules of the face, vault, and basicranium (Moss and Young 1960; Moss 1973) extends the analysis of cranial integration to include Pongo as well as the African apes. The results point to complex integrated shape changes, but despite marked contrasts in adult cranial morphology, all species display close similarities in covariation patterns between the face, basicranium , and vault. The implication is that the pattern of hominoid cranial integration has been conserved at least since the separation of the Asian ape and hominid clades, presumably due to strong stabilizing selection constraining developmental processes.
While some cranial features are relatively invariant in catarrhines (e.g., positioning of orbits; structure of the auditory region), others (e.g., orbital size and shape) are highly variable within genera, species, and even subspecies (Seiffert and Kappelman 2001). Some features seem to be determined less by their “primary” function than by influences reflecting the interactions of other functional systems; e.g., the size and proportions of the orbits appear to be determined more by the growth trajectories of the mid- and upper face and by requirements to resist the biomechanical forces generated by food processing as they affect those regions than by the dimensions of the visual organs housed within them (Schultz 1940). Other traits (e.g., the structure of the nasal floor and premaxilla/palatal relationships; Ward and Kimbel 1983; Ward and Pilbeam 1983) exhibit contrasts, the functional basis of which is poorly understood, but which serve as useful phylogenetic indicators (see below).
The compilation of long lists of character states as the raw data for computer-based cladistic analyses has been criticized by some (Rak 1983; Suwa et al. 1997; Asfaw et al. 1999, 2002) as resulting in the fragmentation or “atomization” of morphology as multiple discrete traits, rather than an integrated whole. It is therefore worth noting here the recent accounts that stress the importance of broader functional and developmental perspectives in analyzing morphology and its evolutionary/phylogenetic and adaptive contexts (Lovejoy et al. 1999, 2003; Lieberman et al. 2000a, b; McCollum and Sharpe 2001; Rae and Koppe 2000; Ackermann 2002, 2005; Singh et al. 2012). These build upon earlier studies such as those of Moss and Young (1960), Moss (1973), Enlow (1968, 1990), and Cheverud (1982, 1996); and biomechanical analyses such as that of Endo (1966); see also Rak (1983).
An example of this approach is McCollum’s analysis of Paranthropus cranial morphology (McCollum 1997, 1999; McCollum and Sharpe 2001), which concludes that limited changes in the relative growth rates of jaws and teeth on the one hand and of the orbit and upper face on the other would be sufficient to produce in mature individuals the distinctive set of features that characterize the robust australopithecine cranium/face. Such growth rate changes are doubtless under simple, limited genetic control and, as such, are readily elicited in appropriate selective contexts. It is not difficult to envisage comparable pressures operating on Miocene hominoids, and so a variety of cranial forms thereby rapidly resulting from relatively limited genetic changes. So, for example, the contrasting morphologies of Proconsul and Afropithecus might both be derived relatively simply from an Oligocene precursor such as Aegyptopithecus, and purely phenetic measures of affinity between these forms could be seriously awry as indicators of phylogenetic relationship.
One outcome of cladistic studies has been the general recognition of the pervasiveness of homoplasy in the fossil record. From an adaptive perspective, instances of homoplasy can provide important clues as to the contexts of, and likely selective forces impacting on, hominoid communities. In such cases, the influence of phylogenetic constraint and contingency may be considerable. Minor initial differences between spatially distributed populations of a single species (or of closely related species), when further influenced by bottlenecking or other stochastic factors – easily occurring in small, localized arboreal groups, where gaps in tree cover impede gene flow – may result in significantly different morphological outcomes as responses to common selection pressures associated with similar niches. The evolution from the nasal/palatal structure seen in Proconsul and other Early Miocene forms of distinct anatomical configurations for that region in Middle/Late Miocene Afro-European and Asian hominoid s may be an example of such a process and its outcomes.
A fundamental division of extant hominoids is that between gibbons (hylobatids) and large-bodied apes – the Asian orangutan (Pongo) and the African chimpanzee and bonobo (Pan ), gorilla (Gorilla), and human (Homo), although the last taxon will be discussed elsewhere. Pongo and Pan are both largely frugivorous, with common dental adaptations (large anterior teeth and relatively small check teeth with enamel wrinkling) but differing in cranial features, whereas the more herbivorous Gorilla closely resembles Pan cranially despite its contrasting dietary niche (see below). These differing patterns of affinity illustrate the importance of developmental constraints and phylogenetic inertia in determining morphology and thus the lack of any necessary one-to-one correspondence between morphology and adaptation (for further discussion of this, see below).
It is possible in principle to extend the limited insights provided by the few extant great apes into the earlier radiation by supplementing them with modeling based on early hominins, which can be thought of as phenetically and adaptively “apes” in some respects. Apart from the dangers of circular reasoning (using modern ape data as inputs into constructing early hominin models that are then used to “extend” the ape comparator base) and the appropriateness of such models (what form and degree of terrestrial orthogrady, if any, is compatible with using hominins as analogues for non-hominins?), however, there are major issues of contextual relevance.
All extant apes (here and throughout meaning non-hominin hominoids) and early hominins are essentially from tropical contexts (forest, woodland, and savannah) with none present in higher latitudes, reflecting a comparatively narrow environmental range compared with earlier ape habitats. Even incorporating early hominins within the comparator base provides a time depth of little more than 4+ Myr, characterized by broadly modern faunas that include groups rare or absent in the earlier record. In contrast, Miocene hominoids are components of markedly distinct and diverse faunas, often including entire mammalian families now extinct. So community relationships within earlier faunas will have differed from contemporary ecological webs, and the place(s) of earlier hominoids in their ecological communities are unlikely to correspond closely to those of modern ape analogues. An obvious primate example of this is the expansion and radiation of cercopithecoids over the last 10 Myr or thereabouts, so forming a major dimension of the community ecology of all recent hominoids, unlike that of earlier taxa. Floral communities also fluctuated as climatic conditions changed, with notable contrasts between Early to Middle Miocene habitats and those of the Late Miocene and Pliocene.
Against these differentiating features are some factors that make for modeling continuity: the range of potential (plant) food items is limited, and their physical properties even more so, limiting the nature and magnitude of the masticatory forces influencing hominoid cranial morphology. Metabolic and biomechanical constraints on body size and on locomotor form and activity, allometric influences on growth, and the functional and developmental interdependences of cranial form noted above all allow for a more comparative approach to hominid cranial variation. Below we review the probable ancestral condition for Hominoidea, then examine some aspects of cranial form in extant nonhuman hominoids before summarizing craniodental information on the more complete fossil forms.
Ancestral Hominoid Cranial Morphology
The combination of outgroup analysis of extant forms and the morphology of stem catarrhines provides an indication of the ancestral hominoid cranial morphotype. The Fayum fossil primates represent an early diversification of basal catarrhines, presumably reflecting dietary specialization. For example, the small and dentally and gnathically primitive Catopithecus (35.5–36 Ma) combines the characteristic 2.1.2.3 dental formula, postorbital closure (primarily formed from the zygomatic), fused frontals, and C1/P3 honing facet with triangular upper molars with only limited hypocone development and lower molars with high trigonids and sharp crests. Catopithecus had a deep and projecting face, with an especially broad premaxilla; small, widely separated orbits; and a small neurocranium with anteriorly prominent temporal lines merging to form a sagittal crest along the rear half of the vault and well-developed nuchal crests. The tympanic region is like that of platyrrhines, not catarrhines, and the mandibular symphysis is unfused. The anterior dentition displays broad, spatulate incisors and projecting, dimorphic canines, suggesting a predominantly frugivorous niche.
Many of these features, including the contribution of the premaxilla to facial proportions, small neurocranium with marked muscle attachments and pronounced ectocranial cresting, and ceboid-like tympanic region, are also seen in the younger (33.1–33.4 Mya) and dentally more derived propliopithecid Aegyptopithecus. The zygomatic is again deep and the face in general strongly constructed, with a characteristic angled profile, and the mandibular symphysis is fused. The gonial region is strongly constructed and the ramus broad and high. The interorbital distance is again broad, with bony septa separating the high, narrow orbits and the interorbital region projects anteriorly from the medial orbital margins. Semicircular supraorbital tori extend over each orbit and, meeting medially, anteriorly bound a diamond-shaped frontal planum, whose posterior limits are defined by the anterior temporal lines. The anterior teeth are small compared to the postcanine dentition, making a narrow anterior palate. The molars are inflated and highly bunodont, especially the second, and the elongated lower third molar has a centrally placed hypoconulid; the trigonid is reduced in occlusal area and height and lacks the paraconid, while the talonid is expanded with a large distal fovea. The upper molars are quadritubercular, with a well-developed hypocone. There is marked canine dimorphism, with upper canine honing capabilities increased by a lengthening of the anterior surface of P3. Overall morphology points to the generation of greater occlusal pressures than in Catopithecus and a craniofacial form better able to withstand the resulting forces.
When the details of stem catarrhine facial morphology are considered with the evidence from extant outgroups of the Catarrhini (e.g., Platyrrhini), it is possible to infer the major changes that underlie the ancestral hominoid craniofacial skeleton. Unlike stem catarrhines or platyrrhines, hominoids are characterized by a palate that is wide at the level of the canines, nasals that are nonprojecting and lie near the medial orbital margin in transverse section, and a premaxillomaxillary suture that contacts the nasals inferiorly near the nasal aperture (Rae 1999). Unlike previous interpretations, it is also evident that the overall shape of the ancestral hominoid morphotype is more cercopithecine-like (Benefit and McCrossin 1991), with tall zygoma and a deep face. This suggests that the shared craniofacial configuration of gibbons and colobine monkeys (short face, sloping zygoma) is convergent.
Extant Hominoids
Hylobates
Gibbons represent a radiation of small-bodied, brachiating suspensory hominoid species with attendant postcranial specializations, distinguished from each other primarily by pelage color and patterning and by vocalization. Four main groups are usually recognized, sometimes accorded subgeneric or generic rank, depending on the author. Three groups – Hylobates hoolock, H. concolor, and H. syndactylus – are comparatively well defined; the H. lar group is more problematic. Valuable reviews of extant gibbon characteristics and diversity include Groves (1972), Marshall and Sugardjito (1986), and Groves (2001); see also Geissmann (2002) and Mootnick and Groves (2005) for recent findings on gibbon diversity that support generic distinction, although the traditional use of the single genus Hylobates is maintained here.
Gibbons are craniodentally primitive in some characteristics (see above), compared with other extant hominoids, whether by plesiomorphy (McNulty 2004) or reversal (Rae 2004); appreciation of this led to the realization that similarities with Miocene taxa, such as Limnopithecus and Pliopithecus, previously taken as grounds for regarding these as likely gibbon ancestors, do not betoken any especially close phylogenetic relationship. The upshot is that, in the absence of a fossil record other than dental remains from Quaternary deposits of China and Indonesia referable to the modern genus, the early evolutionary history of gibbons is wholly obscure.
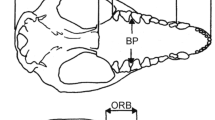
Overall, the Hylobates skull is rather lightly constructed (Fig. 1). The neurocranium is thin walled and the vault low and ovoid in profile, with a capacity of about 80–125 cm3. The frontal extends rearward between the parietals, and in most individuals the sphenoid sutures with the parietal on the vault wall. The orbits are rectangular and relatively large, with strongly developed lateral margins; a torus also develops laterally above the orbits but is not continuous, fading out medially. The lacrimal fossa extends beyond the orbital rim onto the maxilla, and the interorbital breadth is large; the short, broad nasals are usually fused above the ovoid nasal aperture. Overall the face is short, broad, and fairly projecting. Within the nasal cavity, the premaxilla and maxillary palatine process are separated by broad palatine fenestra linking the nasal and oral cavities (Fig. 2 upper left); the vomer extends only as far as the fenestra, and the bony nasal septum is continued anteriorly by the premaxillary prevomer, which fuses to the vomer and forms a small bony crest in the incisive region in all gibbon species except the smallest, H. klossi (McCollum and Ward 1997). The palate and mandible are long; both corpus and symphysis are comparatively lightly built, although external thickening of the latter may be evident in some individuals, as well as the usual internal reinforcement by a superior transverse torus. The ramus is short, broad, and vertical, with some expansion of the gonial region.
Subnasal morphology of hominoids seen in sagittal section. Upper left: Morotopithecus, showing no overlap of the premaxilla on the maxilla (the primitive condition seen in extant Hylobates and most fossil hominoids). Lower left: Pongo, showing the smooth overlapped subnasal condition also seen in Sivapithecus. Right: Pan (upper) and Gorilla (lower) showing the stepped overlapped condition usual in extant African apes (Modified after Ward and Kimbel (1983))
Reflecting gibbons’ predominantly frugivorous niche, the anterior dental arcade is relatively broad compared with the rear. The upper incisors are markedly heterodont – I1 broad and spatulate and I2 narrow and pointed – the lowers more similar, vertically implanted, and subequal in size. The canines are long, curving, transversely slightly narrowed, and sharply pointed, with minimal sexual dimorphism. There is well-developed honing of the upper canine against the long, highly compressed anterior face of the sectorial P3, which is orientated in line with the molars. Cheek teeth exhibit considerable metric and morphological variation, but the rear molars are usually reduced compared with the first and especially the second molars except in H. ( Symphalangus) syndactylus (see below).
The basicranium is long, with the foramen magnum and occipital condyles well behind the auditory meatus; there is no distinct mastoid process. The nuchal area is quite extensive, rising well up the occipital, with a distinct crest laterally that usually fades medially, although it may be continuous in some individuals. A sagittal crest is usually absent but may occur in small-brained individuals.
Detailed accounts of intra- and interspecific variation in Hylobates are given in Groves (1972, 2001) and Marshall and Sugardjito (1986) as above. Albrecht and Miller (1993) summarize their reanalysis, with caveats, of Creel and Preuschoft’s (1976) craniometric data: canonical variate analysis (CVA) reveals H. hoolock, H. concolor, and H. syndactylus as cranially distinct from each other and from the H. lar group. This consists of a primary cluster including H. lar, H. agilis, H. moloch, and H. muelleri subspecies, with H. pileatus as an outlier and H. l. vestitus and H. klossi grouped together as a second, distinct, outlier. A subsequent analysis (Creel and Preuschoft 1984) produced patterns of resemblance that generally accord with geographical distribution but not always with the usually recognized species limits. A recent study by Leslie (2010) extends analysis to the relative orientation of internal cranial features and their variation across the recognized hylobatid groupings; the findings generally accord with those of the earlier studies based on external cranial features.
The only distinctive form noted here is the siamang H. (S.) syndactylus (Fig. 1, lower). This large, heavily built gibbon is more folivorous than other taxa and has a larger cranial capacity, a long, broad palate, and an inflatable air sac in the throat to aid calling. Postorbital constriction is more marked, and, despite the larger cranial capacity, sagittal cresting is both more frequent and larger than in other gibbons, an allometric correlate of greater body size (see below).
In the dentition , the canines are less lingually curved than in other gibbons, the protocone on P3 and P4 larger; and on the upper molars, crowns are elongated, the hypocone variable in size, and lingual cingula almost always absent. Third molar reduction occurs in only a minority of cases, and some individuals possess supernumerary molars. Again consistent with its more folivorous niche, relative shearing-crest development is greater than in other gibbon species (Kay and Ungar 1997, 2000). H. (S.) syndactylus has a larger, more airorhynchous (i.e., more dorsally flexed) face than other gibbons (Shea 1988) – see below.
Pongo
The Asian great ape, the orangutan, exhibits a distinctive overall cranial form (Fig. 3). In profile the large face is markedly prognathic subnasally, with a projecting, convex alveolar clivus. The comparatively small neurocranium is set above the facial skeleton, so that both frontal and occipital contours are relatively vertical. The orbits are elliptical, with their major axis vertical, and are surmounted by separate semicircular supraorbital costae rather than a continuous torus. The interorbital distance is very small, the ethmoid correspondingly constricted and set at a lower level than in the African apes (Shea 1988). There is no frontoethmoid sinus (Fig. 4), and the floor of the anterior cranial fossa forms a large part of the orbital roof (Winkler et al. 1988). In the fossa, the two wings of the frontal bone fail to meet behind the ethmoid, which retains contact with the sphenoid. The nasal bones are small, typically fused at an early age, and continue beyond the frontomaxillary suture, extending as a narrow wedge into the glabellar region of the frontal. On the medial orbital wall the lacrimal sutures with the ethmoid.
The midface region is short, the zygomatics are wide, deep, and flared, and there is usually a pronounced notch on the zygomatic process of the maxilla. The nasal cavity is tall and broad, the maxillary sinuses invade the interorbital pillar (sometimes as far superior as the frontal), and the lateral maxillary walls are obliquely inclined. The convex nasoalveolar clivus passes smoothly into the nasal cavity, extensively overlapping the anteriorly thin maxillary palatine process without a stepped incisive fossa; the fossa and canal are narrow, the latter long and orientated almost horizontally (Fig. 2 lower left). The vomer usually extends to the rear of the incisive canal but occasionally does not, in which case a small prevomer may be present (Ward and Kimbel 1983; Ward and Pilbeam 1983; McCollum and Ward 1997). Overall the palate is orientated anterosuperiorly.
The mandible is massive, the symphysis reinforced by a robust superior transverse torus and an especially pronounced inferior transverse torus extending back as far as P4 or M1 (Brown 1997). The corpus is deep and comparatively short. As in the African apes, there is a strongly developed platysma muscle extending laterally over much of the facial musculature and strongly attached to the swollen base of the mandibular corpus from the symphysis to the area of masseter insertion. Brown and Ward (1988) speculate that the massive platysma is associated with the orangutan’s extensive laryngeal air sac system – greater than in other apes – aiding the regulation of air pressure and volume within the sac during vocalization. A distinctive feature of Pongo is the absence of the anterior digastric muscle (and so of the digastric fossae on the base of the symphysis) and associated separation of the posterior digastric from the hyoid and stylohyoid muscle (Dean 1984; Brown and Ward 1988). Instead the large posterior digastric, originating on the cranial base adjacent to rectus capitis lateralis, inserts onto the gonial region between the medial pterygoid and masseter muscles, acting to depress the mandible. The orangutan’s mylohyoid muscle is especially well developed, as are the geniohyoids. Rectus capitis lateralis, originating from a narrow area on the front of the atlas and inserting on the basioccipital anterior to the foramen magnum, is a more fan-shaped muscle than its homologue in the chimpanzee .
The cranial base is wider than in the African apes (Dean and Wood 1981, 1984), but the eustachian process is much smaller, providing the origin for only tensor palati, with levator palati originating from the apex of the petrous temporal (Dean 1985). The mastoid processes are poorly developed. In the articular region, there is a long preglenoid plane, an indistinct articular eminence, and a prominent postglenoid tubercle. The roof of the glenoid fossa is coronally oblique, slightly sloping inferomedially, so that the entoglenoid is less prominent than in the African apes. The temporomandibular ligament is well developed laterally but lacking the deeper horizontal band, suggesting closer approximation of the rear of the working condyle and the postglenoid tubercle during chewing (Aiello and Dean 1990).
The foramen magnum and occipital condyles are set well back on the skull base. A nuchal crest is present in all mature individuals, and a prominent sagittal crest develops posteriorly in most males, uniting with the nuchal crest but, reflecting the orangutan’s greater airorhynchy, typically not extending as far beyond the rear of the vault proper as in Gorilla (see below). Anteriorly the temporal muscles diverge as lines or simple crests bounding a triangular area of the frontal. As in the African apes, the bulk of the temporalis muscle is orientated obliquely, with an emphasis on the posterior fibers.
The dentition reflects the orangutan’s predominantly frugivorous niche. The upper incisors are the most heteromorphic of any extant hominoid: I1 is very broad and spatulate, but I2 is smaller, more pointed, and more convex in curvature. Well-developed median and marginal ridges reinforce the incisor crowns in biting. Lower incisors, high crowned and narrower than the uppers, are also reinforced by lingual ridging. Canines are conical, markedly dimorphic, and especially robust in males; females display more pronounced lingual cingula. Upper premolars are bicuspid; P3 is sectorial with a narrow, elongate protoconid as the honing face; P4 is bicuspid. Upper molars are more oval in occlusal outline than in other apes (Swarts 1988; Swindler and Olshan 1988; Uchida 1998b). Cheek teeth are relatively large compared to body size, low crowned, and with extensive, deep secondary wrinkling that further increases occlusal area. Molar shearing crests are rather well developed considering the emphasis on fruit (although significant quantities of bark and leaves are also ingested), exceeding those of chimpanzee species but considerably less than gorillas (Kay and Ungar 1997). They perhaps provide an instance of phylogenetic inertia, suggesting a more folivorous ancestor.
Orangutans are remarkably variable in cranial morphology (Wood Jones 1929; Röhrer-Ertl 1988a, b; Winkler 1988). Röhrer-Ertl (1988b) has shown that the most stable region is the midface, other cranial areas varying according to age, sex, dental eruption and masticatory development, hormonal status, dietary composition, and tooth use. Both the neurocranium and face exhibit greater growth in breadth than in length or height, a differential that is more marked in males than in females. While there is much individual and intrapopulational diversity, at least some variation reflects geographic factors: Groves (1971, 1986, 2001) and Röhrer-Ertl (1988a, b) review cranial patterning and Brown (1997) mandibular form, while Uchida (1998b) summarizes dental differences. Within a context of admittedly high variability, Sumatran orangutans are characterized by an oblique but straight (not concave) facial profile with highly protuberant anterior teeth, a convex cheek region lacking a suborbital fossa, relatively short nasals, a shorter neurocranium but with a longer nuchal region, and a longer foramen magnum. The mandibular symphysis tends to be long and narrow, with an extensive inferior transverse torus. Dentally they exhibit relatively small paracones on P3 and M1 compared with their Bornean counterparts, M1 larger than M2 rather than subequal, and a broader M3.
Bornean orangutans have a generally more prognathous and concave facial profile , display a distinct suborbital fossa on the cheek, and have more labially positioned incisors, a “trumpet-shaped” nasal aperture that becomes triangular in cross section at the level of the nasal tubercle (Röhrer-Ertl 1988a), and a more prominent interorbital pillar (Groves 2001). Their mandibles are deeper and broader anteriorly, and the symphysis is usually larger, thicker, and more bulbous than that of Sumatran orangutans. Taylor (2006) explored the relationships between feeding behavior, diet, and mandible morphology, specifically the greater exploitation of bark and relatively tough vegetation during low fruit periods by some Bornean orangutan populations compared with Sumatran ones. She found that the Bornean mandibles display a relatively deeper corpus, deeper and wider symphysis, and relatively greater condylar area, arguing that these features enable greater load resistance to masticatory and incisal forces, reflecting ingestion of harder food items. There is a gradient within Borneo, with populations in NE Kalimantan and Sabah (P. p. morio) displaying fullest expression of these traits, those in SW Kalimantan (P. p. wurmbii) rather less, and with those in NW Kalimantan (P. p. pygmaeus) generally intermediate between P. p. morio and the Sumatran mandibles, thus implying a spectrum of hard food exploitation in Bornean orangutans.
There is other craniodental differentiation within Borneo between populations from Sabah, NW and SW Kalimantan separated by the Kapuas River (Groves 1986, 2001; Courtney et al. 1988; Groves et al. 1992), often of comparable magnitude to that between Bornean and Sumatran orangutans. For example, Taylor and Schaik (2007) document variability in absolute and relative brain size in orangutan populations, finding significantly smaller brains among the north east Kalimantan/Sabah group (P. p. morio) compared with those from elsewhere in Borneo and from Sumatra. They relate these findings to differences in resource quality and life history: P. p. morio has the least productive habitat, lowest energy intake during extended periods of scarcity, and the shortest interbirth intervals, arguing that brain size and prolonged food scarcity may be inversely correlated. Uchida (1998b) was unable to identify any consistent pattern of dental differences between Pongo populations from W Borneo, SW Borneo, and Sumatra, with the Bornean groups often as distinct from each other as either was from the Sumatran sample. Bornean orangutans were significantly different from each other (but not from Sumatra) in P4 and M1 shape, but virtually identical in their narrow M3 shape, with Sumatran orangutans having broader rear molars. Differences in molar cusp proportions showed similarly inconsistent patterning between the three groups. There were no obvious links to dietary differences, and Uchida concluded that on dental evidence, river and mountain systems within Borneo were as significant biogeographic barriers and so promoters of differentiation, as flooding of the Sunda shelf.
Bornean and Sumatran orangutans have generally been accorded subspecific status as Pongo pygmaeus pygmaeus and P. p. abelii, respectively (Schwartz 1988). In his latest revision, however, Groves (2001) distinguishes them as separate species (P. pygmaeus and P. abelii) on the basis of the more comprehensive morphological information now available and molecular differences well above levels usually associated with subspecies, which indicate a long period (c. 1.5 Ma) of isolation between the two forms. He also formalizes the intra-Bornean diversity noted above as subspecies of P. pygmaeus. This taxonomic framework, which is also followed, for example, by Taylor (2006), is reinforced by a study of multiple genetic loci which extends Sumatran and Bornean orangutan divergence back to 2.7–5.0 Mya, with isolation thereafter (Steiper 2006). The data also point to contrasting population histories, with Bornean orangutans having undergone recent population expansion beginning 39–64 Kya, while Sumatran populations remained stable.
The Sumatran and Bornean orangutans also exhibit developmental contrasts. Uniquely, male Sumatran orangutans may delay for many years full expression of secondary sexual characters, including their characteristic cheek flanges, whereas such long delays are much less common among Bornean males. Pradhan et al. (2012) relate such flexible developmental arrest to sociobiological factors and in particular to the potential for high-ranking males (flanged or unflanged) to monopolize sexual access to females. When the potential is low, no developmental arrest is the prevailing pattern, whereas at high monopolization potential the flexible, arrested development pattern is the stable one. Their model accords with field data indicating different monopolization potentials between Bornean and Sumatran flanged males and a lower proportion of these in the Sumatran orangutan population. Harrison and Chivers (2007) relate the evolution of developmental arrest to the onset of longer, more severe periods of low food availability reflecting climate change 3–5 Mya, with females dispersing more widely in search of food and adult flanged males less able to effectively guard a female harem, so providing an opening for the unflanged male as a quiet, quick, opportunistic “sexual predator.”
Hominoids exhibit more dorsal flexing of the face relative to the cranial base (airorhynchy) than non-hominoids; their orbital axes and palates are both shifted more dorsally relative to their degree of basicranial flexion than those of other primates (Ross and Ravosa 1993; Ross and Henneberg 1995). While the functional basis for this is disputed (Ross and Ravosa 1993) and may well have multiple causes, within this context many of the orangutan’s distinctive features can be plausibly related to its extreme airorhynchy (Delattre and Fenart 1956, 1960; Biegert 1964; Shea 1985, 1988; Brown and Ward 1988). Biegert (1964) argued that the hypertrophied laryngeal sac in Pongo is a prime determinant of its skull form, comparing it with the enlarged hyoid and associated throat organs of Alouatta. Shea (1985, 1988) and Brown and Ward (1988) have criticized this interpretation. Shea considers laryngeal specialization as just one potential determinant of airorhynchy, interacting with other factors, largely unknown. Brown and Ward consider the Pongo-Alouatta analogy invalid in view of contrasts in the submandibular anatomy of these two genera, and it has also been rejected by Hershkovitz (1970) and Zingeser (1973).
Shea argues that pronounced dorsal flexion of the face links Sivapithecus and Pongo and that a degree of airorhynchy (although not to the extent seen in these two genera) is primitive for catarrhines and hominoids generally. On this view, the more ventral positioning of the face relative to the neurocranium seen in African apes and hominids is synapomorphous and, as such, a significant phylogenetic indicator (see also Ross and Ravosa 1993; Ross and Henneberg 1995; and below). The distinctiveness of Pongo is emphasized by its pattern of ectocranial suture closure (Cray et al. 2010). Vault suture synostosis is similar to Gorilla (but contrasts with that of Pan and Homo – see below), but the lateral-anterior pattern of fusion, with its strong superior to inferior gradient, is unique to Pongo, reflecting its relative phylogenetic isolation among hominoids.
The African Apes
As is well known, the African apes (hereinafter meaning gorillas, chimpanzees, and bonobos, i.e., non-hominin hominines) share a basic similarity of cranial form and in many respects are scaled variants of a common bauplan (Figs. 5 and 6). Many of the craniodental differences between them have been related, with varying degrees of success, to differences in dietary niche (see Chaps. “The Paleoclimatic Record and Plio-Pleistocene Paleoenvironments,” Vol. 1, “Geological Background of Early Hominid Sites in Africa,” Vol. 1, “Paleosols,” Vol. 1, “Quaternary Geology and Paleoenvironments,” Vol. 1, “Zoogeography: Primate and Early Hominin Distribution and Migration Patterns,” Vol. 1, and Modeling the Past: Archaeology, Vol. 1). Taylor (2002) also provides a useful recent summary of African ape diets. Within a highly variable context of local preferences and seasonal fluctuations and with considerable overlap in the fruits exploited, gorillas are, broadly speaking, more folivorous than chimpanzees . Gorillas consume less fruit than chimpanzees and exploit leaves, pith, bark, bamboo, and terrestrial herbaceous items. The eastern mountain gorilla (G. g. beringei) is the most exclusively folivorous form; the western lowland gorilla (G. g. gorilla) exploits the most varied diet, with a significant fruit component. In contrast, chimpanzee diets are dominated by fruits, although it is unclear whether the bonobo (P. paniscus) exploits more terrestrial herbaceous vegetation than the common chimpanzee (P. troglodytes) (Taylor 2002).
Top (Upper) Frontal and profile views of Gorilla gorilla gorilla skull (Photograph courtesy of C.P. Groves). (Lower) Frontal and profile views of G. g. diehli skull (Photograph courtesy of E. Sarmiento). Bottom (Upper) Frontal and profile views of G. g. graueri skull (Photograph courtesy of C.P. Groves). (Lower) Frontal and profile views of G. g. beringei skull (Photograph courtesy of C.P. Groves)
Compared with the orangutan, African apes exhibit longer, lower, narrower neurocrania set at a lower level relative to the facial skeleton (klinorhynchy). The frontal contour is low and retreating, the parietal region flat, and the occipital more curved than in the large-bodied Asian ape. There is a prominent supraorbital torus that is usually continuous across the glabellar region as well as above each orbit, although in some P. troglodytes individuals it may be divided by a slight depression. A supratoral sulcus, its lateral limits defined by the anterior temporal lines, delimits the torus from the frontal squama. The orbits are subrectangular, usually broader than high, and interorbital breadth is greater than in the orangutan, reflecting the broader ethmoid of African apes. On the medial orbital wall, the ethmoid’s orbital plate is reduced, and the ethmolacrimal suture is usually much less extensive than in the orangutan and in some individuals may be replaced by contact of the interposed frontal and maxilla. There is an extensive frontoethmoid sinus (Fig. 4). On the floor of the anterior cranial fossa, the frontal may separate the ethmoid from the sphenoid, more commonly in Gorilla (>50 %) than Pan (15 %). Frontotemporal contact predominates on the lateral cranial wall of the chimpanzee and gorilla , but sphenoparietal contact is common in the bonobo .
The root of the maxillary zygomatic process arises relatively close to the occlusal plane, above M1 or M2. In the chimpanzee , the zygoma’s facial (malar) aspect is limited in height and breadth; in the gorilla, it is deeper and extends further laterally. In both apes, it is remarkably thin in sagittal cross section when compared with most early hominins but is strengthened by the sagittal angulation of its upper and lower portions. Rak (1983) has emphasized the structural importance of the zygomatic region as a transverse buttress, linking the lateral and medial components of the face and resisting masticatory forces. In both gorilla and chimpanzee, the zygoma’s temporal process is sharply angled from its malar surface, with the zygomatic arches orientated parasagittally/posteriorly slightly divergent (Pan) and parasagittaly/posteriorly slightly convergent (Gorilla), reflecting differing ratios of mid-facial and bitemporal breadths in the two genera. The greater facial breadth in Gorilla means that the masseters, especially their anterior fibers, have a greater lateral component to their contraction than in Pan .
The zygomatic arch is thin in cross section but vertically deeper, its inferior border marked anteriorly for the superficial masseter fibers, and in Gorilla posteriorly scalloped for the origin of the muscle’s deeper portion. A part of this, sometimes differentiated as the zygomaticomandibularis muscle, fuses with anterior temporalis fibers, to attach to the temporalis tendon, the coronoid process, and anterior ramus edge (Raven 1950; Sakka 1984; Aiello and Dean 1990). In mature male gorillas, the arch is reinforced sagittally in its mid-region by a “step” with convex upper border which increases its vertical depth compared with immediately adjacent areas and strengthened transversely toward its rear by the broad, flat base of the temporal’s zygomatic process. Additional support against the masseter’s pull is provided by the temporalis fascia, inserting on the upper border of the zygomatic arch; again, it is particularly extensive in male gorillas .
Anteriorly the face is braced against masseteric force by the zygomatic buttress (see above) and by the beam of the supraorbital torus, which links with the zygoma via its frontal process (Rak 1983). The greater facial breadth of Gorilla combined with its more marked postorbital constriction and so deeper infratemporal fossa means that the lateral component of the torus is unsupported behind by the anterior neurocranial wall and so is massively thickened vertically and sagittally, while the postorbital bar is broadened compared with Pan. These structures, the canine roots, and nasal septum also reinforce the palate and face against bending (sagittal), torsional (coronal), and shearing forces generated during biting by the anterior teeth. Such forces are highest rostrally and of greatest magnitude in large-jawed forms such as Gorilla (Preuschoft et al. 1986).
Within the nasal cavity, the incisive canal is wide and the fossae are broad and bowl shaped. In Pan, the extent by which the premaxilla overlaps the palate, and so the length of the incisive canal, is comparable to that in the orangutan, although the canal is angled more steeply than in the latter because of the African ape’s less convex premaxilla (Fig. 2 upper right). In Gorilla, the overlap is much less and the incisive canal shorter, and there is always a distinct step in the nasal floor between premaxilla and palate (Fig. 2 lower right). In Pan , the step is much less marked and may be absent altogether in about one third of individuals, who evince a smooth floor comparable to that of the orangutan (McCollum and Ward 1997). In Gorilla, a long prevomer is interposed between the vomer and the premaxilla, with the inferior parts of both the former bones descending into the incisive canal, dividing its posterior wall and eventually partitioning it into two channels. A septal groove along the nasal sill is seen only in younger individuals; in adults, it is confined to the rear of the sill. In Pan, the prevomer is much smaller, and, while it descends into the incisive canal to divide the posterior wall, together with the vomer, complete partitioning into two channels is much less frequent than in Gorilla. Unlike the latter, a septal groove is present on the nasal sill in adults as well as younger individuals.
Fusion of the facial aspect of the premaxillomaxillary suture in chimpanzees begins prenatally and is usually completed before the permanent dentition is fully erupted, with the nasal aspect being completely fused around the eruption of M2. Facial growth in Gorilla continues for longer, with both facial and nasal aspects of the premaxillomaxillary suture and the prevomeral-vomeral sutures remaining open until well into maturity (McCollum and Ward 1997). Accessory premaxillary sutures are also quite common (>20 %) in Gorilla, indicating separate ossification centers for the palate and facial components of the premaxilla (Schultz 1950).
The palate is long in both Pan and Gorilla ; externally it is shallow anteriorly with no clear alveolar border but deeper along the postcanine row. Internally, the maxillary palatine process of Pan is distinctive in thickening anteriorly and containing the palatine recess, a medial extension of the maxillary sinus. Laterally the maxillary alveolar process is thin, with the contours of the tooth roots evident; medially the process is thicker. Rak (1983) argues that the maxillary zygomatic process acts as a mid-palatal buttress, reinforcing the hard palate against shearing stresses generated between the chewing and balancing sides of the dental arcade, primarily from the latter’s medial pterygoid muscle. Both medial and lateral pterygoids are particularly well developed in Gorilla.
As in the orangutan, the preglenoid plane of African apes is long, the articular eminence only slightly developed so the glenoid fossa is sagittally shallow, and the postglenoid tubercle is well developed. The roof of the glenoid fossa is coronally more horizontal than in the orangutan and the entoglenoid more distinctly differentiated from it, especially in Gorilla, where it is very large, extending beyond the level of the articular eminence and preventing any medial shift of the condyle prior to moving onto the preglenoid plane (Du Bruhl 1977). In some of these features and in temporal bone shape overall, Pan is more derived than Gorilla (Lockwood et al. 2004). Terhune (2012) notes that joint surfaces in the mandibular fossa are sagittally extended in chimpanzees, whereas in gorillas the surfaces are sagittally contracted and in orangutans intermediate, and that much variation is associated with morphologies that promote gape rather than bite force. A prominent temporomandibular ligament is present in Gorilla and is apparently variably developed in Pan (Aiello and Dean 1990).
In Pan species, the dentitions are basically similar, although P. paniscus teeth are smaller and less sexually dimorphic than those of P. troglodytes. A comparative study of root length development (Dean and Vesey 2008) revealed that in P. troglodytes, anterior tooth root growth rose quickly to higher rates and then plateaued, with the highest rates in canines, followed by incisors (the reverse of the H. sapiens pattern). In both modern humans and apes, molar tooth roots grew in a nonlinear pattern, with peak rates reducing from M1 to M3. A recent study (Boughner et al. 2012) showed no significant differences in the relative timing of permanent tooth crown and root formation in bonobos and chimpanzees. Similarly, dental topographic analyses that reflect contrasts in occlusal form related to diet among primate species identified differences between wear stages within subspecies in surface slope, relief, and angularity, but failed to differentiate between Pan subspecies (Klukkert et al. 2012). Discriminant analysis of size transformed and untransformed molar traits (Pilbrow 2006), however, yielded more effective separation (see below).
Smith et al. (2010) present data on crown and root formation in Taï Forest chimpanzees to evaluate claims that wild chimpanzees display delayed dental development compared with captive ones. They conclude that crown formation onset and development markedly overlap captive chimpanzees, whereas root development may be accelerated in captive specimens, and wild individuals fall near the middle or latter half of captive eruption ranges. Overall the authors conclude that while minor developmental differences are evident in some comparisons, the results do not show a consistent pattern of slower tooth formation in wild individuals. A later paper (Smith and Boesch 2011) extends the analysis to estimate that delayed tooth emergence in wild individuals is more moderate than previously recorded, averaging about 1 SD of the captive distribution, rising to 1.3 SD if age estimate criteria are relaxed; M1 emergence is estimated at 3.66–3.75 years in wild chimpanzees. The authors point out that “wild” data are usually skewed, often deriving from diseased, debilitated, or otherwise pathologically affected corpses of immatures, who cannot be considered fully representative of a healthy population.
The maxillary incisors are curved mesiodistally, with I1 larger than I2, although the difference is smaller in P. paniscus than in P. troglodytes. In the mandibular incisors, these proportions are usually reversed. The upper canine is larger in males than females of both species; its mesial surface is more convex in P. troglodytes and, with the lingual surface, displays grooving absent in a small sample of P. paniscus (Swindler 1976). In the upper jaw, M1 and M2 are subequal in size, M3 reduced, with the hypocone the smallest cusp and reducing progressively along the molar row. Reduction is more pronounced in P. paniscus, and the cusp may even be completely absent from M1 and M2 in some individuals, whereas it is always present on those teeth in P. troglodytes. The hypocone may be entirely absent on some M3s of both species but is more weakly developed in bonobos (fully developed in 21 % of P. troglodytes teeth, compared with only 9 % of P. paniscus). The preprotocrista (anterior transverse crest between paracone and protocone) is more angled and transversely orientated in P. paniscus, running from closer to the protocone to mesial of the paracone rather than to its tip, as in P. troglodytes. The distoconule, an accessory cusp between hypocone and metacone, is absent in bonobos but present in all chimpanzee subspecies, generally at low frequency but up to 40 % of M3 in one collection of P. t. troglodytes (Kinzey 1984). A lingual cingulum is often present, most frequently on M1 but larger on M3 and better developed (longer distally) in bonobos than chimpanzees.
M2 is usually the largest mandibular molar, M3 the smallest; a Y-5 cusp pattern is almost universal on M1 but only occurs in <50 % of cases on M3. The talonid is extensive, and a buccal cingulum is rarely (5–10 %) present (Swindler 1976). In P. paniscus, the metaconid is usually opposite the protoconid rather than distal to it as in P. troglodytes, resulting in a greater relative distance and a deeper groove, between the metaconid and entoconid in the former species (Kinzey 1984). Nonetheless, the two cusps are closely adjacent compared with Gorilla. The hypoconulid is usually slightly buccally positioned in chimpanzees and more centrally (lingually) placed in bonobos, while a tuberculum sextum is often present between hypoconulid and entoconid in the former species but more rarely in the latter, which Kinzey (1984) suggests may be associated with the more lingually positioned hypoconulid. Pan molars are often wrinkled but not to the extent seen in Pongo. Skinner et al. (2009) demonstrated that shape contrasts in the enamel-dentine junction of M1 and M2, especially in the relative height and position of the dentine horns, dentine crown height, and the shape of the base, serve to differentiate Pan species and subspecies, so extending the utility of teeth with worn occlusal surfaces for systematic identification.
Central to lateral incisor proportions in Gorilla are comparable to those of Pan, although compared to the postcanine teeth, the incisors are much smaller. Canines are large and markedly dimorphic, in the female projecting less beyond the other teeth. Contrary to the sequence in Pan, but like the orangutan, P4 erupts before P3, which is sectorial but with a vestigial metaconid, a large distal fossa for the P3 protocone, and a well-developed lingual cingulum. On the upper molars, the hypocone is larger relative to the other cusps than in other apes; the mesial fossa is narrow, the distal one wide, and a lingual cingulum is usually present. On the lower molars, the metaconid and entoconid are widely separated, and there is an extensive talonid basin to receive the large protocone of the upper molar. A tuberculum intermedium is often present between metaconid and entoconid on M1 and is almost invariably so on M2 and M3; a tuberculum sextum may also occur between the entoconid and the buccally positioned hypoconulid. A buccal cingulum is usually present on M1, on about 50 % of M2, and on a minority of M3; overall, it is both more common and better developed in Gorilla than in other extant apes. In the upper jaw, M2 is usually the largest tooth; in the lower jaw, M1 is the smallest, with M2 and M3 subequal (Swindler 1976). Dimorphism in dental dimensions is extensive in Gorilla, with most teeth differing significantly in size between the sexes. Tooth enamel is smooth, without the wrinkling displayed by Pongo and Pan. Supernumerary molars may occur, more often in the upper jaw than the mandible.
McCollum (2007) investigated the relationships of diet, incisor wear, and incisor crown breadth in western lowland gorillas and chimpanzees, confirming that incisor dimensions are broadly similar in the two apes. She found that incisor wear was greater in the more folivorous gorilla than in the frugivorous chimpanzee, questioning Hylander’s suggestion that larger incisors and enhanced resistance to wear are associated with frugivory and the need for greater incisal processing of large fruits. Using a more extensive database, however, Deane (2009) has demonstrated that increased mesiodistal incisor length and greater incisor crown curvature are correlated with greater frugivory, so reaffirming Hylander’s proposed link. Hard-object frugivores show greater curvature than soft-object frugivores, while mixed folivores/frugivores display intermediate degrees of curvature compared with frugivores and folivores . Frugivores also have mesiodistally wider I1, I2, and I2 crowns relative to their labiolingual lengths, while folivores have labiolingually broader crowns than mixed folivore/frugivores, and those of hard-object frugivores are broader than those of soft-object frugivores. McCollum and Deane’s conflicting findings may result from their differing databases – two species with overlapping diets in McCollum’s study compared with a larger number of taxa and wider dietary spectrum in Dean’s case – and in their scaling to adjust for body size differences.
Cray et al. (2008) have shown that cranial vault suture closure mirrors consensus phylogeny, with H. sapiens, P. troglodytes, and G. gorilla sharing a similar lateral-anterior closure pattern, while G. gorilla displays a distinct vault pattern that follows a strong posterior to anterior gradient. P. troglodytes is thus more like H. sapiens in suture synostosis, in accord with these two species sharing a common ancestor after the Gorilla clade split off. P. paniscus was not included in the analysis.
Temporal muscles are well marked on the cranial walls in the chimpanzee, often forming raised ridges which in mature males may occasionally meet to form a sagittal crest. In male gorillas, a pronounced sagittal crest is present, thickened at the top where the two temporal laminae abut, and highest toward the rear of the vault where it unites with the nuchal crest, forming a beak-like posterior projection at the rear of the skull. The crest, besides enlarging the area for temporalis attachment, improves the power of the cheek teeth by increasing the relative length of the muscle insertion axis compared with the load and also serves to increase the effective height of the neurocranium, thereby enhancing its resistance to the vertical forces generated during mastication (Davis 1964).
A compound T/N crest (Robinson 1958) forms laterally in chimpanzees from the juxtaposition of the temporal and nuchal muscles, but these diverge medially, and there the perimeters of the temporal and nuchal muscles are marked by lines, slightly raised ridges, or a simple nuchal crest. In male gorillas, the nuchal muscles develop medially as well as laterally beyond the neurocranium proper, producing a compound T/N crest uniting with the sagittal crest as above and resulting in an extensive, triangular-shaped nuchal area.
Temporalis fibers originate from the lower part of the nuchal crest’s anterior surface but do not attain its rim, which provides attachment for the occipitofrontalis scalp muscle (Sakka 1984; Aiello and Dean 1990). Medially trapezius and laterally sternocleidomastoideus insert on the posterior rim of the nuchal crest, with below these the rhomboids (medially), and the fleshy, laterally extensive splenius capitis muscles. Deep to these is the heavy semispinalis capitis, which may be divisible into medial, thick biventer, and more lateral, straplike, complexus portions (Aiello and Dean 1990), although this separation is said to be uncommon in Pan (Swindler and Wood 1973) and is not indicated in Raven’s (1950) account of Gorilla anatomy.
On the cranial base, rectus capitis lateralis lies immediately lateral to the mid-rear portion of the occipital condyle in Gorilla and Pongo and to the front mid-portion of the condyle in Pan (Dean 1984; Raven 1950). It is unclear whether the rather more anterior insertion of the muscle in the chimpanzee reflects individual variation or a specific trait. Just lateral and slightly posterior to this muscle is the digastric; see above for its distinctive form in the orangutan. Just in front of the foramen magnum and close to the midline are the closely adjacent rectus capitis anterior muscles, and ventral to these the longus capitis muscles. The basilar suture fuses early in the African apes and the orangutan, severely limiting its utility for determining individual age (Poe 2011).
Nishimura et al. (2006) have documented vocal tract growth and development in three chimpanzees. In early infancy, they show rapid laryngeal descent with consequent changes in vocal tract proportions as a result of descent of the laryngeal skeleton relative to the hyoid. Subsequently, the hyoid also descends relative to the palate, maintaining rapid laryngeal descent, as in humans. They conclude that descent of the larynx evolved before the Pan-hominin split for a function unrelated to speech and that human speech capabilities resulted from facial flattening rather than laryngeal descent.
Individual Species Morphology and Intraspecific Diversity
Pan
Pan troglodyte s
The commonly recognized subspecies may be distinguished cranially as below, based primarily on Groves (2001).
P. t. troglodytes possesses a very broad head combined with a comparatively narrow muzzle, a continuous, straight, medially thickened supraorbital torus, more concave facial profile, and more gradually sloping occipital than other subspecies. On the medial orbital wall, ethmo-lacrimal contact is very common, while supernumerary bones on the lambdoid suture are rare, as are multiple infraorbital foramina.
P. t. verus also has a broad, rather flat-topped cranium but a broader muzzle, a less sharply concave facial profile, and a more steeply curved occipital. The supraorbital torus is arched over each orbit and is laterally well developed. Ethmo-lacrimal contact is very rare, while additional bones at lambda and along the lambdoid suture are very common. The frequency of a single infraorbital foramen bilaterally is higher than in other subspecies.
P. t. schweinfurthii has a more rounded skull than other subspecies, with an elongated, gently sloping occipital. The facial profile tends to be straight or only slightly concave, and the muzzle narrow, although interorbital breadth is high. The straight, continuous supraorbital torus is thinner than in other subspecies, especially laterally, but is prominent at glabella. Multiple infraorbital foramina are very common, and frontotemporal contact at pterion virtually universal. In cranial nonmetric traits, generally it resembles P. t. troglodytes but is rather smaller and less sexually dimorphic than that subspecies. Despite this, Angst (quoted in Groves et al. 1992) has reported a higher average cranial capacity for P. t. schweinfurthii – 420 cm3 – compared with virtually identical capacities for P. t. troglodytes and P. t. verus (401 and 404 cm3, respectively). Highly variable in size and cranial proportions, P. t. schweinfurthii may incorporate more than one subspecies.
P. t. vellerosus is a recently recognized subspecies from Nigeria to Cameroon (Gonder et al. 1997), identified on mtDNA sequencing that showed it to be a sister taxon of P. t. verus. Cranially it is unlike P. t. verus but similar to P. t. troglodytes and P. t. schweinfurthii in its high frequency of ethmo-lacrimal contact and low frequency of Wormian bones at lambda and along the lambdoid suture (Groves 2001).
A study of chimpanzee molar development (Smith et al. 2007) indicated marked within cusp, between cusp, and between tooth variation in enamel formation times and in cuspal initiation and completion sequences, pointing to the need to take account of significant variation when interpreting hominoid and hominin developmental data. In contrast, discriminant analysis of upper and lower molar morphometrics (Pilbrow 2006) to assess the efficacy of dental evidence in distinguishing chimpanzee populations differentiated on geographical criteria (river boundaries) provided more consistent findings. The results showed clear distinction of P. paniscus (see below) and P. troglodytes at all molar positions, while within the latter P. t. verus was distinct from other P. troglodytes populations, P. t. vellerosus was also clearly differentiated, and P. t. troglodytes and P. t. schweinfurthii were dentally similar.
Pan paniscus
Bonobos are characterized by relatively smaller heads and teeth than common chimpanzees, but by comparably sized upper limbs, rather lighter, more slender trunks, and heavier hind limbs (Susman 1984; Zihlman 1984). The bonobo skull is smaller, smoother, and more lightly built than that of the chimpanzee, the mandible appreciably shorter, and the face considerably less prognathic and reduced in height (Fig. 5). Reflecting the less projecting face and jaws, the cranial base is more tightly flexed, with a mean angle of 140° compared with 145° in the chimpanzee (Cramer 1977). This flexion results from a basicranial growth pattern to adulthood in P. paniscus that resembles that of P. troglodytes curtailed at the subadult (M2 eruption) stage (Laitman and Heimbuch 1984; see also below). The bonobo supraorbital torus is thinner and the supratoral sulcus weaker, while the frontal squama rises (and the occipital descends) more steeply than is usual in P. troglodytes. It is more common (57 %) for the sphenoid and parietal to suture at pterion (contrast P. troglodytes above), while on the orbital wall frontomaxillary contact is more frequent than in chimpanzees (24 % and 9 %, respectively, Cramer 1977). Following CT scanning, Balzeau et al. (2009a, b) provide further information on the type specimen of P. paniscus, including details of its internal cranial anatomy.
While bonobos exhibit some canine dimorphism, there are only very limited differences between sexes in the size of the incisors and cheek teeth (see above). Similarly, mean endocranial capacity is virtually identical in males and females at c. 350 cm3 compared with 404 and 375 cm3, respectively, in P. troglodytes (Cramer 1977). The nuchal area may be bounded by a low ridge or line, but a true crest with sharply defined rim is absent, as is any sign of sagittal cresting. Consistent with its more neotenous form, P. paniscus shows earlier closure of the facial component of the premaxillary/maxillary suture than P. troglodytes and much higher frequency of a completely open palatal component (>93 % cf. 19 %, respectively, of individuals with M1 erupted; Braga 1998). This early synostosis results in a vertically and horizontally shorter face and reduced dental arch, consistent with the bonobo’s significantly smaller incisors, compared with P. troglodytes. Kinzey (1984) notes the greater degree of incisor wear in P. paniscus than P. troglodytes, which he suggests may be related to a greater incidence of pith and leaf petioles in the diet; he also speculates that the combination of a more transversely orientated and angled preprotocrista, with a more mesially sited metaconid and deeper groove between protoconid and hypoconid into which the crest occludes (see above), produces a more efficient shearing mechanism that again may reflect a more folivorous dietary component in bonobos.
Comparison of small samples of immature captive and wild female P. paniscus with P. troglodytes showed similar patterns of skeletal fusion in the two captive groups with the pattern of tooth eruption to bone fusion also generally consistent between species save for minor variations in late juveniles and subadults. While displaying similar patterns, direct age comparisons showed skeletal growth in the captive bonobo group to be accelerated compared with both captive and wild P. troglodytes samples (Bolter and Zihlman 2012).
Morphometric studies illustrate the relative homogeneity of chimpanzee cranial form compared with other great apes. While usually distinguishing P. paniscus from P. troglodytes, differentiation within the latter is, not unexpectedly, less secure, with extensive overlap between subspecies; see, for example, Shea and Coolidge (1988). These authors found that discrimination just about reached the subspecies threshold and that separation was considerably less than in orangutans or gorillas (see below). They considered that this comparative uniformity might reflect a more recent differentiation of P. troglodytes subspecies, more frequent or extensive contact – and so gene flow – between them, marked ecological flexibility for the species overall so precluding close matching of subspecific features to habitat, or any combination of these. A subsequent study (Groves et al. 1992), with specimens sorted by location rather than subspecies, produced neither meaningful geographic patterning nor subspecific grouping among males. Female crania, however, exhibited better separation, with P. paniscus distinct, P. t. schweinfurthii grading geographically toward P. t. troglodytes, and with evidence for east–west differentiation within P. t. schweinfurthii based on facial proportions.
Shea et al. (1993) compare the results of both raw and size-adjusted analyses. For the former, there is 100 % correct classification for P. paniscus females and about 75 % correct classification for P. troglodytes, of which P. t. verus and P. t. schweinfurthii are furthest apart, according with their geographic separation. Confining the analysis to P. troglodytes, however, removes this geographic gradient, with maximal separation now between P. t. troglodytes and schweinfurthii. As expected, size adjustment reduces separation of P. paniscus from P. troglodytes, so that the distance between P. t. verus and P. t. schweinfurthii, now the most widely divergent subspecies, approaches that between the latter and P. paniscus. Principal Components Analysis shows P. paniscus clustering with immature P. troglodytes crania along PC 1, indicating their common growth trajectories and emphasizing that shape contrasts between bonobo and chimpanzee reflect the smaller size and truncated growth of the former relative to the latter, within which the major differences between P. t. troglodytes and P. t. schweinfurthii are also due to size and associated allometric factors (see below).
Separate analysis of mandibular variation in Pan accords generally, but not completely, with the above (Taylor and Groves 2003). Mandibular separation within P. troglodytes is less than that within Gorilla, but contrasts between P. paniscus and P. troglodytes are greater than Gorilla, and there is clear separation of bonobos and chimpanzees. There is extensive overlap of P. troglodytes subspecies, maximally between P. t. schweinfurthii and P. t. troglodytes, and greatest distinction between the latter and P. t. verus (contrast to Shea et al.’s cranial finding of greatest overlap between P. t. troglodytes and P. t. verus). Size adjustment again reduces separation, so that bonobos, while remaining the most distinctive, now partly overlap with chimpanzees; and P. t. verus, while still the most isolated of chimpanzee subspecies, is now furthest from P. t. schweinfurthii (as on the cranial data). P. t. verus’s; distinctiveness on mandibular traits, while relatively slight (Taylor and Groves 2003), nonetheless accords with Braga’s finding (1998) that premaxillomaxillary suture closure differs significantly between P. t. verus and other subspecies, with P. t. verus displaying later complete closure of the suture’s facial component and earlier closure of its palatal component compared with P. t. troglodytes and P. t. schweinfurthii (Braga 1998). This points to a longer, deeper lower face in P. t. verus than other subspecies.
A recent morphometric study of mandibular form (Robinson 2012) broadly accords with Taylor and Groves’ findings: size-adjusted corpus shapes in P. paniscus and P. troglodytes could be assigned with 93 % accuracy, with much of the shape differences size related, but subspecies could only be correctly identified <75 % of the time. Robinson’s findings indicate symphyseal shape to be especially informative in distinguishing Pan species, with potential implications for hominin systematics.
Zihlman et al. (2008) present cranial and postcranial data on 25 P. t. verus individuals of known age and sex from Taï National Park, Cote d’Ivoire, and compare them with a P. t. schweinfurthii sample from Gombe National Park, Tanzania, with P. paniscus as an additional comparator. Taï males and females differ in cranial capacity and, as do the Gombe sexes, in facial dimensions. The Tai sample has a smaller cranial capacity, longer palate and mandible, and greater trunk dimensions and limb lengths; most variation is in females, with males differing only in humeral and femoral lengths. A further study by Neubauer et al. (2012) of endocranial volumes (EV) in an ontogenetic series of Taï forest chimpanzees showed brain size to increase rapidly during early ontogeny and for sexual dimorphism in EV, with males larger than females, to be evident before adult EV was attained. The mean adult EV in this Taï Forest sample was just under 380 cm3.
Gorilla
Most accounts of Gorilla cranial diversity are based on Groves’ highly influential morphometric analysis of variation in 45 traits from >700 gorilla skulls, grouped by origin into 19 and 10 geographic localities for crania and mandibles, respectively (Groves 1967, 1970). D2 values were calculated for each of the ten cranial and six mandibular representative variables, allowing the localities to be grouped into eight larger regions which could be further combined on the basis of intra- and intergroup differences into three clusters: a relatively homogeneous western cluster (four regions, of which the Cross River sample was rather more distant from the other three), a distinctive eastern group from the Virunga volcano region, and a further eastern group (three regions). These correspond to the western lowland gorilla (G. g. gorilla), the eastern highland gorilla (G. g. beringei), and the eastern lowland gorilla (G. g. graueri) (Fig. 6).
G. g. gorilla is the smallest subspecies, with fairly broad face, small jaws and teeth, a short palate, a single mental foramen under P3 or P4 (more usually under the latter), and a jaw condyle without a cleft. G. g. graueri is the largest subspecies, with a high, narrow face; larger jaws and teeth; and a longer palate. The mental foramen is often multiple and set under P3, while the jaw condyle is often cleft. The mountain gorilla, G. g. beringei, is distinguished by a low, broad face; very large jaws and teeth; a very long tooth row and palate; anteriorly sited (under C or P3) multiple mental foramina; and a jaw condyle that is usually cleft.
Stumpf et al. (1998), adjusting for size, demonstrated that the Cross River sample was more distinctive than Groves’ original analyses indicated, so providing support to the growing movement advocating its recognition as a further subspecies, G. g. diehli (see Sarmiento and Oates 1999, 2000; Groves 2001, 2003). G. g. diehli is distinguished by its shorter skull, shorter molar row, narrower palate, shorter cranial base, and more steeply angled nuchal plane than other western gorillas, which Sarmiento and Oates speculate may be associated with a diet of smaller, drier, and harder food items than that of other western gorillas.
A further reanalysis of Groves’ data (Stumpf et al. 2003) confirmed a primary east–west separation on the latter’s smaller values for palatal and tooth row lengths, nasal aperture and nasal bone breadths, lateral facial height, and supraorbital torus thickness. They also demonstrated the distinctiveness of the Cross River and Virunga populations from other west and eastern groups on the basis of their narrower interorbital breadths, narrower palates, and reduced lateral facial height. Analyses restricted to the western populations further indicate the distinctiveness of G. g. diehli on overall and neurocranial lengths, bicanine and bimolar breadths, interorbital and neurocranial widths, palatal length, and medial and lateral facial heights. Stumpf et al., however, emphasize that the fundamental distinction is between east and west Gorilla populations, with the corollary that G. g. graueri is more closely related to G. g. beringei than it is to western lowland gorillas. The implications of this, together with recent data from molecular and other studies, have led Groves (2001, 2003) to revise his earlier taxonomy and to differentiate western and eastern gorillas at the species level as G. gorilla (G. g. gorilla and G. g. diehli) and G. beringei (G. b. beringei and G. b. graueri), respectively. This also accords with the zoogeographical evidence, but for consistency with other sources referred to herein, we retain the traditional single species classification.
Leigh et al. (2003), however, apply Wright’s FST (an indicator of microdifferentiation, measuring the extent to which subdivision within species – i.e., between subspecies – departs from random mating) to Groves’ craniometric data and to discrete trait variation and reach different conclusions. Their approach requires assumptions about population sizes and the heritability of craniometric traits but is considered to be robust, especially, when subspecies sizes differ markedly, as they do in Gorilla. FST calculated from the craniometric data yields unexpectedly low levels of between group variation, only c. 20 % between subspecies compared with 80 % within subspecies assuming equal population sizes. Adjusting for different population estimates between the subspecies results in even lower values of FST, with correspondingly more variation within subspecies. FST derived from discrete trait analysis gives rather higher, but still modest, levels of divergence. Leigh et al. argue that much gorilla variation reflects ontogenetic changes and sexual dimorphism, and as such is intra-subspecific, and that their results offer no support for differentiating eastern and western gorillas at the specific level. See also Albrecht et al. (2003) for a detailed analysis of Gorilla cranial diversity at locality, deme, subspecies and species levels, and its potential evolutionary and sociobiological implications. Interestingly, genetic data indicate much deeper levels of differentiation among African ape species than the morphological evidence does (Gagneux et al. 1999; Lockwood et al. 2004).
Caillaud et al. (2008) explore the possible sociobiological basis for sexual dimorphism – specifically male body size and head crest development – in G. g. gorilla, through photogrammetry and field observations. Their findings show the number of females belonging to a mature male correlates with head crest size, body length, and musculature and that female numbers at male-male encounters, where larger individuals could be expected to be at an advantage, strongly affect the number of male agonistic displays. Exaggerated male traits therefore convey a mating advantage, whether through male-male fighting or female mate choice. Similar drivers may well be influencing the evolution of dimorphism in other, larger-bodied gorilla taxa.
Breuer et al. (2012) also explored the relationships of body length, crest size, and gluteal muscle size with long-term male reproductive success in western gorillas. They found that all three traits correlated with the average number of mates per male, but that only crest size and gluteal development significantly correlated with offspring survival and the annual rate of siring offspring who survive to weaning.
Dental variation in Gorilla is considerable: molar shapes and cusp proportions, relatively invariant within subspecies, differ between subspecies, as do tooth dimensions (Uchida 1998a). Male (but not female) G. g. beringei canines are larger than those of G. g. gorilla and G. g. graueri. In the postcanine dentition, G. g. graueri is, surprisingly, significantly larger than G. g. beringei, which is larger than G. g. gorilla. While upper molars of beringei show B-L enlargement, those of graueri are expanded in both length and breadth. G. g. gorilla has wider incisors relative to molar length than the eastern subspecies, while G. g. beringei displays higher crowned cheek teeth with sharper cusps and ridges than G. g. gorilla. G. g. graueri has a relatively smaller talonid on P4 and, together with G. g. beringei, has larger distal cusps on the upper and lower molars than G. g. gorilla. Patterns of dental sexual dimorphism differ among gorilla subspecies: G. g. beringei displays greatest dimorphism in canine and lower molar size, graueri greatest dimorphism in upper molars, with G. g. gorilla least dimorphic both in canines and molars. This reflects a larger canine relative to molar size in females of this subspecies, which Uchida considers to reflect heightened female-female competition, possibly related to greater frugivory. She stresses the importance of local dietary adaptation influencing tooth form and proportions, with considerable variation but with more extensive frugivory in G. g. gorilla and lowland G. g. graueri than in highland populations of that subspecies and G. g beringei.
Similarly, Pilbrow (2010) has analyzed molar morphometrics to assess gorilla geographical diversity. Her results support species distinction between G. gorilla and G. beringei, with subspecies G. g. diehli, G. g. gorilla, G. b. graueri, G. b. beringei, and a possible further subspecies, G. b. rex-pygmaeorum; dental metrics thus accord with other evidence for gorilla population diversity. Dental separation increased with altitude differences but not geographical distance, so that altitudinal segregation better explains gorilla population divergence better than isolation by distance. Pilbrow argues that the historic center of gorilla distribution was West Africa and that Plio-Pleistocene climatic oscillations combined with mountain building promoted drift and population differentiation. Further analyses of the gorilla masticatory system are discussed below.
Allometric and Biomechanical Studies
The greater size of the gorilla relative to the chimpanzee is an instance of peramorphosis (Shea 1983c). Length of maturation is comparable in all three African ape species, but gorillas grow much more rapidly and to greater sizes than chimpanzees, while bonobos grow somewhat more slowly than chimpanzees (rate hypermorphosis ) to rather lesser sizes (although there is considerable overlap); within each species, males grow for longer than females (time hypermorphosis). The pattern indicates that the interspecies differences reflect selection for greater body size, perhaps associated with increasing terrestrial folivory rather than selection for delayed maturation (Shea 1983c).
Significant differences between Pan and Gorilla growth in body weight only become apparent after about 2 years, with gorillas pulling away increasingly strongly from the chimpanzee growth curve thereafter (Shea 1983d; Fig. 6). Since neural growth is predominantly prenatal/immediately postnatal, there is no corresponding increase in Gorilla brain size in later ontogeny; the natural consequence is that, while having absolutely larger brains than chimpanzees, gorillas have lower brain–body ratios and lower encephalization quotients, with male value particularly depressed compared with females. “In the case of the African pongid species … the developmental pathway utilized to increase body size ensures that relative brain size decreases as a consequence” (Shea 1983d, p 58). It follows that attempted explanations of behavioral and/or ecological contrasts between the species based on differences in relative brain size should be regarded with skepticism.
Shea (1983a, 1984) also summarizes evidence that the differences in body form between adult P. troglodytes and P. paniscus result from ontogenetic scaling. The extension of common growth allometries to different end sizes holds within the skull, trunk, and limbs but not between them, so that adult bonobos do not match any single stage in chimpanzee ontogeny. Relative to the latter, the P. paniscus skull is most strongly reduced in size, forelimbs and trunk less reduced, and hind limbs not reduced at all, so that for a given body size, P. paniscus has a smaller skull than P. troglodytes. This is turn results in a more paedomorphic cranial shape compared with the chimpanzee through the decoupling of growth rates for the head and body, with the former slowed relative to the latter – an instance of neoteny. The selective factors underlying this process are obscure, although Shea speculates that the reduced sexual dimorphism and different social organization of P. paniscus compared with P. troglodytes may be important drivers in the evolution of its distinctive cranial proportions.
A more recent morphometric study by Lieberman et al. (2007) also concluded that the bonobo skull is largely paedomorphic relative to the chimpanzee , but that not all shape differences between the species, particularly in the face, could be explained in such terms, and that other developmental differences were also responsible for the contrasts in form. Durrleman et al. (2012) document ontogenetic changes in endocranial size and shape in sizeable samples of P. paniscus (n = 60) and P. troglodytes (n = 59) aged on dental criteria. They identify in bonobos an early, strong anisotropic endocranial expansion and bending due to localized expansion of the frontal pole, occipital lobe, and superior parietal lobe, which contrasts with developmentally later endocranial expansion in the chimpanzee. Patterns of expansion also differ in magnitude between the species, with a phase of rapid increase in endocranial volume occurring later in chimpanzees than bonobos.
Earlier suggestions (Ackermann and Krovitz 2002) of a common cranial postnatal ontogenetic shape trajectory or of separate but parallel shape trajectories that merely accentuate differences established in early (prenatal) ontogeny have been refuted by Cobb and O’Higgins (2004), who show hominin postnatal shape trajectories to be divergent with differing shape changes between species, even in early postnatal ontogeny (Vidarsdottir and Cobb 2004). The directions of scaling trajectories between Pan species, however, are not significant (so changing postnatal facial shape in a similar manner from different starting points), whereas those between Pan and Gorilla are directionally distinct.
Cobb and O’Higgins (2007) explore the role of ontogenetic scaling in determining sexual dimorphism in the facial skeleton of African apes (G. g. gorilla, P. paniscus and P. troglodytes). Using geometric morphometric analysis, they found that on average males and females shared a common ontogenetic shape trajectory and a common ontogenetic scaling trajectory until around M2 eruption. Thereafter, males and females diverged from each other and from the common juvenile trajectories within each species, indicating ontogenetic scaling as a mechanism until around “puberty” and the development of secondary sexual characters, but that subsequent sexual dimorphism occurs through divergent trajectories and not via ontogenetic scaling.
In general, the degree of adult cranial sexual dimorphism is greater in the larger apes (gorilla and orangutan) than in the chimpanzee and bonobo. Gorilla males display more size and shape variability than females, and a similar difference appears to be present in Pongo, but not in Pan (O’Higgins and Dryden 1993). Most of the differences reflect greater male facial prognathism, in turn a consequence of canine dimorphism. Adult cranial dimorphism appears to result from distinct mechanisms in the African and Asian apes. Whereas in Pan and Gorilla cranial dimorphism follows from extending the growth period of males for most cranial proportions (Shea 1983c), in Pongo only about half the growth allometries exhibit this process, with the other half displaying accelerated growth in males compared with females (Leutnegger and Masterson 1989a, b).
Male and female chimpanzees display significant cranial size differences but no shape differences, perhaps because the period of extended growth is a short one and/or the scaling coefficients are minor, so resulting in insignificant shape differences given the comparatively modest size of chimpanzees. As with the bonobo-chimpanzee comparison above, these differing patterns of cranial dimorphism in the great ape genera have been linked to socioecological contrasts between them (O’Higgins and Dryden 1993); see also Caillaud et al. (2008) and above.
Shea has also explored allometric influences on African ape craniofacial and dental form and their relationships to diet using bivariate and multivariate techniques. Many facial proportions in the bonobo, chimpanzee, and gorilla exhibit ontogenetic scaling, i.e., a common pattern of size/shape change. There are also instances, however, where this does not obtain: for example, chimpanzees have shallower zygomatic roots, narrower bizygomatic breadths, smaller infratemporal fossae, and narrower anterior cranial bases than bonobos with the same basicranial lengths. In other words, these features are reduced in chimpanzees compared with the values expected in bonobos ontogenetically scaled to their sizes (Shea 1984). Similarly, in those features in which chimpanzees are reduced relative to bonobos, gorillas tend to be reduced relative to chimpanzees (Shea 1984). As Shea points out, such allometrically adjusted analyses point to the opposite conclusion from that usually drawn from the study of absolute skull sizes – cranially; bonobos are relatively the most robust, and gorillas relatively the most gracile of the African apes .
Additionally, gorillas have significantly longer and higher cranial vaults, higher orbits, and longer foramen magnums than chimpanzee crania of equivalent basicranial lengths. They also exhibit longer, more projecting nasal regions that are sited lower on the face, than comparably sized chimpanzees. As overall skull size increases in the sequence bonobo-chimpanzee-gorilla, the three species also exhibit relatively narrower faces and neurocrania, reflecting in the latter case, the fact that increased brain size results primarily from growth in length, not width, during the prenatal and early postnatal phases.
During late postnatal growth, occipital length and breadth in gorillas increase appreciably compared with chimpanzees, reflecting the development of sagittal and nuchal crests as a functional response to the enlarged temporal and nuchal muscles “outgrowing” their areas of attachment on the exterior cranial wall. This, in turn, is a consequence of the respectively positive and negative allometric relationships between splanchnocranial and neurocranial proportions and body size. Dental metrics indicate that gorillas have relatively smaller incisors and relatively larger cheek teeth than comparably sized chimpanzees, while, surprisingly, temporal fossa area (and so temporal muscle size) becomes relatively smaller across the three species as size increases. Despite these differences, the predominant pattern among the African apes is essentially one of similarity in craniofacial growth. Multivariate analysis yields a common allometry vector incorporating >93 % of total variance confirming this general picture, with a second vector (3.4 %) distinguishing chimpanzees and gorillas.
Shea considers that the differences in midface proportions between Pan and Gorilla may reflect differences in soft-tissue function or dietary contrasts, although the influence of the latter is by no means clear. In fact, he notes that while dental contrasts between the two apes can fairly clearly be linked to diet, no significant reorganization of the face occurs, with its form being primarily determined by the endpoints of common allometric trajectories. This suggests that the face and masticatory apparatus may be less strongly coupled to diet than is the dentition and/or that chimpanzee and gorilla diets, while differing in their constituent items, may not differ appreciably in their physical properties, in particular the force required to process them. For Shea, the African ape masticatory complex provides an example of an integrated functional system preadapted to extension into new size ranges and dietary shifts.
Molar crown area scales positively in hominoids, so that larger forms have relatively as well as absolutely larger crown areas, associated with their generally increased folivory, primarily achieved through increased tooth lengths rather than breadths (Demes et al. 1986). There are associated increases in palatal and mandibular lengths and relative narrowing of upper and lower dental arcades in larger taxa. Allometrically determined snout elongation produces greater bite force in larger animals, by lengthening the horizontal distance between the mandibular joint and the molar row, which Demes et al. have shown scales with a mean value of c. +1.6 in hominoids. Bite force is maintained by lengthening the masticatory muscles’ power arms, by increasing their cross-sectional area, or by a combination of these. The temporal muscle’s power arm scales from +1.16 (male great apes) to +1.62 (female gibbons) and that of the masseters and medial pterygoids, which is strongly influenced by facial height, between +1.54 and +1.64. A rough estimate of temporalis cross-sectional area scales from c. +1.4 to +1.9, indicating that the greater load of the allometrically lengthened lever arm is more than matched in larger species by the positive allometry of the power arm and of muscle cross-sectional area and so muscle force.
Larger species produce more bite force for their size than smaller ones due to the allometric changes in masticatory biomechanics following from increased body size, a point neatly illustrated by Demes et al., who demonstrate that skulls of H. klossi and P. paniscus enlarged isometrically to the size of H. (S.) symphylangus and G. gorilla, respectively, produce lesser bite forces than the “real” latter two forms do. Bite pressure (bite force/crown area) is maintained if bite force increases at the same rate as crown area; broadly similar relationships for these variables hold within hylobatids and great apes, indicating that in hominoids, crown area and bite force increase at about the same rate, at least over the size range of extant taxa. Estimated bite pressure is generally greater in great apes than hylobatids, although the orangutan is an exception here. Bite pressure shows no obvious relationship to between-species differences in size or diet; interestingly, P. troglodytes males produce the greatest scaled bite pressure, exceeding even G. gorilla males. Within species, males generally produce greater pressures than females, although whether this is selected for (implying differences in food processing or paramasticatory activity between the sexes) or is a by-product of selection for larger body size is a moot point. Demes et al. make the important point that similar allometric relationships obtaining within hylobatids and great apes provide strong evidence that biomechanical constraints associated with increased body size elicit similar functional responses across the Hominoidea. This is strong presumptive evidence that they should also be applicable to fossil forms.
Ravosa (2000) undertook such a combined allometric analysis of mandible size and form in fossil and extant apes, comparing them with cercopithecoids. Deeper corpora counter parasagittal bending while the more robust cross sections of larger species, especially the fossils, counter axial torsion. The positive allometry of corpus and symphysis cross sections suggests increased masticatory stresses due to greater balancing side muscle activity during powerful mastication, probably reflecting a tougher, harder diet. In addition, the allometry of jaw length and breadth point to greater wishboning stresses at the symphysis at the end of the masticatory powerstroke, countered by a thicker symphysis and increased anterior jaw breadth. After allometric scaling, the most robust mandibles include Proconsul africanus and P. nyanzae, Rangwapithecus, Turkanapithecus, Afropithecus, Ankarapithecus, Lufengpithecus, and Ouranopithecus; the more slender include hylobatids, Simiolus, Hispanopithecus laietanus, Pan paniscus, and P. troglodytes (see also below).
In the broader context of fossil (as well as extant) hominoids, even G. gorilla has a comparatively low and slender corpus, only average symphyseal height and a slightly broader than expected symphysis for its mandible length. Pongo has a higher but rather thinner corpus than expected and a higher symphysis of expected width; P. paniscus has a corpus and symphysis of expected height but rather thinner than expected, while P. troglodytes has a shallower, narrower corpus and symphysis, although the latter is closer to the values expected on jaw length than the former. These findings have implications for the dietary reconstruction of fossil forms: many Middle/Later Miocene hominoids are notably more robust than modern apes and so are plausibly reconstructed as exploiting harder, more resistant food items requiring substantial force in processing, while the categorization of proconsulids as “frugivorous ” may also well underplay the variety and toughness of their dietary items. Such reconstruction, however, is difficult to reconcile with the evidence provided by dental morphology and wear patterns in Proconsul and by some other aspects of jaw form (see below).
In some respects, hominoid and cercopithecoid mandibular cross-sectional scaling patterns are similar; smaller apes are notably gracile, resembling cercopithecine proportions, but larger ones have both deeper and relatively wider corpora more reminiscent of colobines to resist greater axial torsion during chewing – perhaps reflecting larger, more laterally placed masseters that contribute relatively more to unilateral mastication, together with the medial pterygoids. This is especially so in the largest apes which have absolutely and relatively very thick corpora exceeding colobine proportions, suggesting diets with at least comparable, and very possibly greater, physical properties of hardness and/or toughness.
Smaller apes also display symphyseal curvature comparable to cercopithecines, whereas, with increasing body size, curvature reduces to a shallower, colobine-like, arc, eventually falling below even that in the largest apes. This has traditionally been interpreted in dietary terms (frugivores requiring large incisors and so a wide anterior dental arcade), but the bulk of the fossil evidence points to diets other than frugivory. Ravosa (2000) therefore interprets the broader anterior dental arcade combined with a relatively thick symphysis as hominoid adaptations to resist concentrated wishboning forces at elevated levels resulting from a hard-object diet. The exception to this is Afropithecus, which has a notably narrow, tightly curved anterior mandible with, presumably, correspondingly concentrated wishboning forces, raising interesting issues about dietary composition and food processing activities in that genus. Again, findings from the dental evidence do not easily accord with those based on mandibular proportions.
More recently, Taylor (2002, 2003) has used morphometric methods to investigate masticatory variation in African apes as a function of dietary differences. She compared allometrically adjusted mandibular, cheek, and facial dimensions, quantifying masticatory parameters associated with bite force and load resistance in ontogenetic series of bonobo, chimpanzee, and gorilla, to test the hypothesis that more folivorous forms would show greater development of these features.
The results are complex. Unsurprisingly, all species show allometric increases during growth in traits indicating improved muscle and bite force and the capacity to resist greater loads. Masticatory muscle sizes are especially strongly allometric. After adjusting for allometry, however, only a few traits differ consistently across African ape species as predicted by dietary preferences. A more resistant diet is generally correlated with a thicker mandibular corpus, although the thicker corpus of chimpanzees compared with bonobos is not matched by evidence of corresponding dietary differences. Compared with Pan, Gorilla has a relatively wider mandibular corpus to resist axial torsion, a wider symphysis so resisting “wishboning,” a higher temporomandibular joint which contributes to improved mechanical advantage of the jaw lever and distributes forces more evenly along the cheek teeth, and a higher mandibular ramus, increasing the moment arm of the temporal and masseter muscles and providing a larger attachment area for the latter and the medial pterygoids. Moreover, within Gorilla, eastern gorillas exhibit greater values than western ones and also have larger masseter muscle than the latter, in accord with their more resistant diet.
Other analyses, however, do not conform to the pattern predicted from diet; for example, gorillas do not have the relatively deeper corpora expected to resist parasagittal bending (Hylander 1979a, b), and there was no regular association of the deeper symphysis providing increased resistance to bending and shearing forces with greater folivory (see also Ravosa’s findings summarized above, although Demes et al. (1984) and Wolff (1984) considered the torsional resistance of the gorilla mandible “remarkable,” and maximal at the symphysis). Overall, gorillas do not have the shorter deeper faces and more anteriorly positioned masticatory muscles predicted to improve the mandible’s power arm ratio and to reduce bending moments in the face, and there was no consistent differentiation of bonobos and chimpanzees. Taylor concludes that while some of the distinctive craniofacial features of the African apes can plausibly be considered as dietary adaptations, the link is not especially strong. Dental development and allometric and other ontogenetic constraints are doubtless important influences, while more information is needed on the composition, variability, and especially the physical properties of ape diets. The equivocal nature of these results accords with those from some other studies; for example, despite their dietary contrasts, Rak’s indices quantifying relationships between the palate, masseter origin, and their positions relative to the calvaria fail to discriminate between Pan and Gorilla (Rak 1983: table 3, p 25).
Further analysis of the Gorilla masticatory system with larger samples, and including G. g. graueri in the analysis, confirms and extends the earlier findings (Taylor 2003). G. g. beringei has a significantly larger face than the other two subspecies and differs from G. g. graueri in the same features that distinguish it from G. g. gorilla and which differentiate Gorilla and Pan. Despite being more folivorous, however, G. g. graueri does not differ from G. g. gorilla in those features, and G. g. beringei fails to express the full set of masticatory traits predicted by its diet. In this last respect, it may well be the case that an investigative model assuming optimization of each and every variable is simply inappropriate; rather than a spectrum or continuum of values for every trait, some may be more appropriately considered in terms of thresholds. For example, if food availability is not a constraint and provided the face is structurally sufficiently strong to resist masticatory forces, there seems little reason to suppose that selection will necessarily promote further shortening and deepening of the face in hominoid folivores to achieve “optimal” values, particularly if to do so will disrupt pervasive, well-established allometric trajectories. Covariation within a tightly integrated functional system, such as the head, is likely to impose multiple constraints on the variation of any given character or character complex.
Taylor et al. (2008) report a detailed analysis of the relationships between jaw form, diet, and food properties in orangutans, chimpanzees, and gorillas, using area moments of inertia and condylar area ratios to estimate moments imposed on the mandible to assess relative ability to counter mandibular loads. They took data on elastic modulus and fracture toughness of food types to derive food material properties and generated bending and twisting moments on the mandible to estimate minimally required bite forces. Based on food properties, they hypothesized improved resistance to mandibular loads in Pongo p. wurmbeii compared to African apes and in Gorilla b. beringei compared to Pan t. schweinfurthii. The predictions were, in fact, only applicable when bite forces were estimated from maximum fracture toughness of non-fruit, non-leaf vegetation; for all other tissues (fruit, leaves) and material properties, results were contrary to predictions. As food material properties changed, moments imposed on the mandible changed, so altering ratios of relative load resistance to moment, so that species appear over- or under-designed for the moments imposed on the mandible. Reliable estimates of average and maximum bite forces from food material properties accordingly require information about the physical properties of the full range of dietary items.
A recent study (Taylor and Vinyard 2013) incorporates data on masticatory muscle physiologic cross-sectional area (PCSA) and muscle fiber length and broadly confirms an allometric influence on masticatory power while underlining the need for caution when inferring chewing forces from skull form. Hominoid muscle fiber architecture reflects both absolute size and allometric influences; PCSA is close to isometry relative to jaw length in anthropoids but trends to positive allometry in hominoids. Extant large-bodied apes therefore probably generate absolutely and relatively greater muscle forces compared with hylobatids and monkeys, possibly reflecting changes in food composition, ingestive behavior, and/or increased emphasis on mastication as opposed to ingestion with greater body size. The study also revealed that craniometric estimates of masseter and temporalis PCSA may be seriously awry, underestimating the actual figures by >50 % in gorillas and overestimating masseter PCSA by up to 30 % in humans.
Given the lack of concordance in masticatory morphology and diet in living hominoids, and the discongruities between craniofacial morphology and dental evidence among fossil forms, detailed dietary reconstruction based on craniofacial form in fossil hominoids appears questionable. There seems no secure basis on which to go beyond the most general of statements about dietary properties. Shea’s conclusions about the relative decoupling of masticatory morphology and diet in the African apes may be applicable to hominoids generally (see also Daegling and Hylander (1998, 2000) and Daegling (2004)). Dental evidence (crown proportions and morphology, crest development and structure, chemical composition, and wear patterns) may well prove a better guide to hominoid diets than the analysis of craniofacial form, no matter how elaborate the biomechanical models employed (Ungar 1998; Teaford and Ungar 2000). These provisos should be kept in mind in relation to the following summary of craniofacial form in fossil hominoids, which for its framework draws especially on the chapters by Harrison, Begun, Kelley, and Ward and Duren in Hartwig (2002).
Fossil Hominoids
Africa
Oligocene/Early Miocene African Hominoids
The splitting event between the two crown groups of the Catarrhini, apes and Old World monkeys, was an African phenomenon, as the earliest members of both lineages are found in sub-Saharan Africa. The earliest evidence for the divergence is known from late Oligocene (25.2 Ma) deposits in the Rukwa Rift Basin in southwestern Tanzania; Nsungwepithecus gunnelli shows evidence of bilophodont, a cercopithecoid synapomorphy, while Rukwapithecus fleaglei, from the same deposits, shares two dental traits with crown hominoids – M2 hypoconulid positioned buccally and a hypoconid positioned opposite the lingual notch between the metaconid and the entoconid (Stevens et al. 2013). The dental similarities of the latter taxon with Rangwapithecus from the early Miocene of Kenya may suggest a similar dietary adaptation, namely, folivory (see below).
Kamoyapithecus
The other known late Oligocene catarrhine that has been considered a possible hominoid is Kamoyapithecus, from Lothidok, Kenya (Leakey et al. 1995). No explicit shared derived traits link the form with later hominoids. Although very little can be said about the maxillary bone preserved in the type specimen, the dentition is consistent with a high degree of anterior tooth use, albeit with thinner dental enamel than that seen in later, similarly sized forms, such as Afropithecus.
More extensive evidence from Early Miocene sites in East Africa indicates an array of forms, variously regarded as hominoid or non-hominoid (see chapter “Fossil Record of Miocene Hominoids,” Vol. 2). Evidence from the oldest (19–20 Mya) sites of Songhor and Koru (Kenya) points to predominantly tropical forest habitats, with later (16–17 Mya) sites on Rusinga Island (Kenya) ranging from flood plain to riverine contexts. The picture here is of drier, more seasonal environments than Songhor or Koru, but with persistent wooded conditions, varying from forest to deciduous woodland according to rainfall (Andrews 1996; Andrews et al. 1997). Similar habitats are indicated at the rather later Middle Miocene sites of Maboko Island (15–16 Mya) and Fort Ternan (14.5 Mya; see below).
Micropithecus
The small (ca. 3.5 kg) Micropithecus contrasts the bulk of the Early Miocene non-cercopithecoid fauna dentally in having relatively larger anterior teeth compared to the cheek teeth, broad incisors, and narrow premolars and molars, with only weakly developed occlusal ridges. It differs cranially from the similarly sized Dendropithecus in its shallow, broader palate and nasal aperture, short face and clivus, and moderately high and lightly built mandible corpus, with only modest symphyseal tori. Phylogenetic analyses (Rae 1993, 1997; Stevens et al. 2013) suggest that this taxon may be a stem hominoid, as it lacks some of the characteristic hominoid cranial synapomorphies. The frequency of dental pitting and pit shape in Micropithecus clarki (19–20 Mya) points to folivory (Ungar et al. 2004), while M. leakeyorum (15–16 Mya) shows similarities to the rather earlier Simiolus enjessi (16.5–18 Mya) that probably also reflect folivorous adaptations (Harrison 1989; Benefit 1991).
Proconsul
At least two broad cranial morphologies are represented among the Lower Miocene fossils. The genus Proconsul is particularly well known, with species differing in size and dental and gnathic details, but linked by fundamental similarities in craniodental morphology (Fig. 7). Knowledge of Proconsul is primarily based on the material recovered from sites on Rusinga Island, Kenya, including much of a skull in 1948 (Le Gros Clark and Leakey 1951) and a partial skeleton from 1951 and subsequently (Napier and Davis 1959; Walker and Teaford 1989; Walker et al. 1993) supplemented by other material. Initially assigned to the type species P. africanus, the 1948 and 1951 specimens were later transferred to P. heseloni (Walker et al. 1993; Walker 1997) on the basis of differences in dental size and morphology and in mandibular proportions and symphyseal reinforcement from the type of P. africanus from Koru.
Compared with later hominoids, the P. heseloni cranium is lightly constructed: the globular neurocranium largely lacks pronounced tori or crests, although the medial portion of the nuchal crest is evident above the steeply angled nuchal area, and the external occipital protuberance is located high on the skull rear. The frontal is short but broad, reflecting the limited postorbital constriction, while the superior temporal lines are prominent anteriorly and converge toward the vault rear but do not meet to form a crest. On the face, the premaxilla rises on either side of the nasal aperture, contacting the nasal bones above their tip, so excluding the maxilla from the rim of the nasal aperture, which narrows inferiorly between the central incisor roots above a short nasoalveolar clivus. Above it, the nasal bones are nonprojecting, long, and narrow, extending upward beyond the frontomaxillary suture and expanding in breadth in the glabellar region. There is a prominent jugum above the upper canine root and a shallow canine fossa. The lightly built zygomatic arch originates low down, curving backward and upward, and has a well-developed malar tuberosity. The subrectangular orbits are widely separated, surmounted by weak supraorbital ridges and with a slightly swollen glabella over a large frontal sinus (which may represent a frontoethmoid sinus; see Rossie 2005) between, but there is no distinct supraorbital torus.
The palate is long, rectangular, and shallow. A large, transversely broad incisive fossa joins directly with the nasal cavity, so there is no true incisive canal, and the hard palate is retracted from the subnasal alveolar process (Ward and Pilbeam 1983; McCollum and Ward 1997). There is a pronounced tuberosity on the alveolar process behind M3. The maxillary sinus extends anteriorly to the premolars and laterally into the root of the zygomatic arch. The articular eminence and postglenoid process are well developed, and the auditory region has a tubular ectotympanic as in modern catarrhines, while the prominent, well-pneumatized mastoid process is coronally narrow and rather bladelike.
The mandibular symphysis exhibits a moderately to well-developed superior transverse torus but a much weaker and variable inferior torus, absent altogether in some individuals. The corpus displays limited lateral buttressing below the cheek teeth and shallows posteriorly; the relatively high ramus is lightly constructed, and the gonial region only slightly marked by muscle attachments. The slightly earlier, similarly sized and evidently closely related P. africanus differs from P. heseloni in numerous dental details, mandibular proportions, and symphyseal reinforcement: the P. africanus corpus is deeper anteriorly and posteriorly shallows more strongly, while the symphysis lacks an inferior transverse torus but bears a pronounced superior torus (Walker et al. 1993). An inferior transverse torus is also absent in the larger P. nyanzae.
Views differ on Proconsul’s brain size and encephalization: Walker et al. estimated the 1948 cranium’s capacity as 167 cm3 and inferred that P. heseloni was more encephalized than modern cercopithecoids of similar size. Manser and Harrison (1999), however, using foramen magnum area as a size surrogate, estimated brain size as the markedly lower 130 cm3 and close to the mean encephalization value for anthropoids. The endocast’s relatively small frontal lobe and cortical sulcal pattern were considered primitive and cercopithecoid by Le Gros Clark and Leakey (1951) and Le Gros Clark (1962) but definitely not so by Radinsky (1974) who judged it hominoid and most like gibbons. More recently, Falk (1983) has argued that the sulcal pattern resembles that of extant New World monkeys, such as Ateles, rather than any group of catarrhines and approximates the inferred common ancestral sulcal pattern for Anthropoidea.
Relative shearing-crest lengths in those Proconsul species studied (P. heseloni, P. nyanzae, and P. major, the last sometimes referred to the genus Ugandapithecus; see below) are less than those of any extant ape and considered to indicate frugivory (Kay and Ungar 1997), while the proportion of pits to scratches on molar wear facets (37–39 %) also indicates soft-fruit eating (Ungar 1998; Ungar et al. 2004). Despite the findings of Ravosa (2000), see above, mandibular form and proportions are also compatible with this interpretation. At the symphysis, the prominent superior transverse torus and minimal or absent inferior torus produce a broadly triangular cross section, especially in larger individuals, resistant to torsional or bending stresses produced by medial (jaw opening) or lateral (masticatory power stroke) bending (Hylander 1984; Brown 1997). Below the cheek teeth, the corpus is relatively deep vertically and narrow coronally, and, while it may be reinforced by the rear of the superior transverse torus, the mylohyoid ridge, and the lateral eminence, there is relatively little change in cross-sectional shape along the corpus compared with other fossil apes (Brown 1997). This suggests chewing activity generating comparatively high vertical forces but only limited transverse or torsional forces during food processing.
It is also possible to infer aspects of the locomotor pattern of fossil taxa with reference to their cranial morphology via the semicircular canals of the middle ear; there is a general correlation between the radius of curvature of the organ of balance and the relative agility of primate taxa (Ryan et al. 2012). The ancestral catarrhine adaptation, judged from stem forms such as Aegyptopithecus, is that of a medium-slow arboreal quadruped. P. heseloni, however, is characterized by larger semicircular canals, which suggests more agile, medium-speed locomotion, much like that seen in extant macaques.
One species previously assigned to Proconsul is now considered by some to be distinct at the generic level (Senut et al. 2000); Ugandapithecus is held to contain as many as four species of varying sizes and chronological ages (Pickford et al. 2009b), including the only-just-named Proconsul meswae (Harrison and Andrews 2009). The largest (and least contentious) of the species, U. major from Songhor in Kenya and Napak in Uganda, has little in the way of cranial material preserved, although all of the taxa assigned to Ugandapithecus are said to share a distinctive mandibular corpus that becomes shallower posteriorly. It is worth noting, however, that the generic distinction is not recognized universally (MacLatchy and Rossie 2005).
Afropithecus
The contemporary Afropithecus turkanensis contrasts markedly with Proconsul in its cranial form (Leakey and Walker 1997) (Fig. 8). The large face is dominated by the long domed muzzle and deep, flaring zygomatic processes. The projecting premaxilla forms a deep nasoalveolar clivus and extends up on both sides of the broad, oval nasal aperture to contact the narrow, medially elevated nasal bones. There is an extensive maxillary sinus; the shallow palate displays large paired openings for the incisive foramen. The canine roots form prominent juga; the root of the zygomatic process is deep, anteriorly inferiorly sloping, and originates low down on the face. The cordiform orbits are broader than high, inferolaterally sloping and widely separated. The glabellar region is prominent, the slender supraorbital torus curving above each orbit and delimiting a frontal trigon with its lateral limits marked by well-defined anterior temporal lines which merge to form a distinct sagittal crest. Postorbital constriction is marked, and the temporalis muscles are well developed. The mandibular corpus is very deep with a distinct fossa, while the ascending ramus is set obliquely to the corpus. The symphysis bears moderate superior and inferior transverse tori, a long and strongly sloping subincisive planum, and a low-genial pit, implying a deep, narrow tongue.
Overall facial proportions of Afropithecus are reminiscent of A. zeuxis, but absolutely much larger; finite element scaling analysis reveals marked size contrasts but minimal shape differences in the snout and some shape differences (but reduced size contrasts) in the zygomatic and maxillary tuberosity regions (Leakey et al. 1991). These authors conclude that the similarities in Aegyptopithecus and Afropithecus facial form indicate the persistence into the Early Miocene of a functionally integrated mosaic of features that characterized the primitive hominoid face. Benefit and McCrossin (1991) draw attention to craniofacial similarities between Aegyptopithecus, Afropithecus, and the Miocene cercopithecoid Victoriapithecus, indicating that many of these facial traits are primitive catarrhine characters rather than basal hominoid synapomorphies.
With its large, procumbent, and mesially inclined upper central incisors; stout, low-crowned canines; and cheek teeth covered with very thick enamel and complex wrinkling, Afropithecus has been compared, especially in its anterior dentition, to pithecines exploiting seeds and hard fruits, where the incisors crop food items and the large canines apply considerable force to puncture hard fruits, as in Chiropotes (Leakey and Walker 1997; Kinzey 1992). Cusp morphology, the high incidence of pitting in the single Afropithecus individual sampled – at 43 %, the highest of the early African forms studied (Ungar et al. 2004) – and a lack of prominent shearing crests on the cheek teeth are also consistent, like the anterior dentition , with frugivory.
Overall, Leakey and Walker (1997) conclude that Afropithecus was a sclerocarp forager. Other aspects of mandible reinforcement (e.g., strong basal buttressing, a pronounced lateral tubercle where the oblique line meets the corpus, a hollowed buccal surface above the mental foramen with a marked canine jugum anteriorly) that support this interpretation are also seen in Sivapithecus (see below) and probably reflect comparable biomechanical responses to reliance on food items with similar physical properties rather than any especially close phylogenetic link. The somewhat smaller but otherwise similar Heliopithecus from the early Middle Miocene of Saudi Arabia may be no more than specifically distinct from Afropithecus (Andrews et al. 1978; Andrews and Martin 1987).
Morotopithecus
The large (chimpanzee-sized) hominoid Morotopithecus bishopi (Gebo et al. 1997), based on the palate from Moroto initially assigned to P. major (Pilbeam 1969), resembles Afropithecus in many respects and may be congeneric with it. It is of Early (20–21 Mya) or Middle (15–17 Mya) Miocene age, depending on 40Ar39/Ar dating (Gebo et al. 1997) or faunal correlation (Pickford et al. 1999). It combines an anteriorly broad palate with comparatively narrow, procumbent incisors offset by a pronounced diastema from the large, stout canines, whose massive roots form pronounced juga. The molars resemble those of P. major in their bunodont cusps, wrinkled enamel, and beaded lingual cingulum, but contrast in their relative sizes, while the anterior dentition is much larger and the interobital breadth narrower than in P. major. Overall the face is relatively long and narrow, with a broad nasal aperture, a short clivus, and an extensive maxillary sinus. The undoubted resemblances in face and dentition to Afropithecus may reflect dietary convergence in exploiting hard-cased fruits rather than phylogenetic propinquity. Postcrania referred to Morotopithecus resemble those of Proconsul in some respects but are also markedly more derived in the direction of modern hominoids in the lumbar, shoulder, hip, femur, and knee regions and point to forelimb suspension and slow brachiation, as well as climbing and quadrupedal activity in an arboreal habitat (MacLatchy 2004).
Nyanzapithecus
Nyanzapithecus is much less well known, but premaxillary and maxillary fragments of two species, N. vancouveringorum from the Early Miocene (17–18.5 Mya) and N. pickfordi from the Middle Miocene (15–16 Mya), indicate contrasts with Afropithecus in their smaller size; shorter faces; low, broad nasal apertures; and robust premaxillary regions (Harrison 2002). These species and the rather smaller Middle Miocene N. harrisoni (13–15 Mya) display broad, strongly built upper and lower incisors, while the cheek teeth are long and narrow; the molars bear low, expanded cusps and rounded occlusal crests. Mabokopithecus, represented by two isolated rear lower molars and an almost complete mandible, is dentally very similar to N. pickfordi and may well be congeneric with Nyanzapithecus, in which case the former genus has priority.
Rangwapithecus
The Early Miocene (19–20 Ma) Rangwapithecus gordoni, similar in size and probable locomotor pattern to the smaller Proconsul species, contrasts dentally with them in numerous respects, including molars with low cusps and well-developed crests, enamel wrinkling, and a pronounced wear differential. Cranial material indicates a comparatively short premaxilla and long, narrow palate widening toward the rear. The maxillary sinus is deep and the zygomatic root set low down the face above M1–M2; the mandible is deep and the symphysis reinforced with a pronounced superior transverse torus. Kay and Ungar (1997) and Ungar et al. (2004) have argued that, on the basis of its molar crest development (greater than that of any other Early Miocene form) and the low incidence and long, narrow form of dental pitting, Rangwapithecus is likely to have been folivorous. Dental proportions and macrowear, facial morphology, palatal proportions, and mandible structure are all compatible with this interpretation.
Turkanapithecus
The somewhat younger (16.6–17.7 Mya) Turkanapithecus kalakolensis is another medium-sized form, rather smaller than P. heseloni, and represented by a partial cranium preserving the upper dentition save for the incisors and a mandible with left M2 and right M3 (Fig. 8). The skull exhibits a relatively short face with broad, domed snout; a wide, oval nasal aperture flanked by prominent canine pillars, with expanded nasal bones; and a broad, flat interorbital region above. The palate is narrow, with posteriorly convergent tooth rows, and there is an extensive maxillary sinus. The zygomatic process originates low down on the face, and the arch is relatively deep and flaring. This, combined with pronounced postorbital constriction, makes for a deep infratemporal fossa and, presumably, well-developed temporalis muscle – a view also supported by the strongly marked and convergent temporal lines, pointing to a sagittal crest. The rear of the saddle-shaped glenoid cavity is bounded by a well-developed postglenoid process, but there is no distinct articular eminence. The nuchal area is comparatively short and the crest strongly developed, reflecting both the rugged facial architecture and comparatively small neurocranium, estimated at c. 85 cm3 – absolutely and relatively smaller than P. heseloni (Manser and Harrison 1999).
Given this cranial morphology, it is rather surprising that the mandibular symphysis displays neither strongly developed superior nor inferior transverse tori. The corpus is shallow and relatively slender, with constant depth below the molars, while the ramus is broad, low, and sloping, with an expanded gonial region, so according with zygomatic architecture indicating well-developed masseter muscles – and a knoblike condyle. Upper first premolars are large, and while both upper and lower molar teeth increase in size posteriorly, the gradient is much less than in comparably sized Proconsul species. Overall, craniodental features suggest a resistant diet, possibly consisting of hard-cased fruits or leaves.
Dendropithecus
There is also a cluster of small- to medium-sized forms (perhaps 3–9 kg), usually grouped together as dendropithecoids. The siamang-sized Dendropithecus macinnesi, based on material originally assigned to Limnopithecus, displays narrow, high-crowned incisors; strongly dimorphic canines; broad premolars; and molars with high cusps, sharp occlusal crests, and well-defined foveae. The palate is narrow, as is the nasal aperture; the maxillary sinus is extensive and the mandible corpus low and robust, with the symphysis reinforced by fairly prominent superior and inferior transverse tori. Among Early Miocene hominoids, Dendropithecus has the least well-developed molar shearing crests of those studied other than Proconsul and a fairly high incidence of pitting, pointing to a predominantly soft-fruited dietary niche (Kay and Ungar 1997; Ungar et al. 2004).
Limnopithecus
Other small Early Miocene forms, known principally from isolated teeth and part jaws, include Limnopithecus legetet and L. evansi. These have a short lower face, with anteriorly positioned orbits; narrow, elliptical nasal aperture; and a shallow clivus, an inflated maxillary sinus, and a shallow, lightly built mandible reinforced symphyseally by a strongly developed superior transverse torus but with an inferior torus that is weak (L. evansi) or absent (L. legetet). L. legetet combines broad, low-crowned incisors and small canines with an ovoid P3 that suggests only part development of the C–P3 honing complex, and cheek teeth with high, sharp cusps and occlusal crests. L. evansi has narrower, higher crowned incisors, larger canines, and a better developed sectorial face on P3, cheek teeth with lower, rounded cusps, and less sharply developed occlusal crests. Relative shearing-crest development suggests a fairly folivorous niche (Kay and Ungar 1997).
Kalepithecus
Less well known than these forms is the broadly contemporary Kalepithecus songhorensis, similar in dental size to L. legetet, but differing in most other respects. The anterior teeth are relatively large, with I1 broader and more spatulate, the upper premolars relatively narrow but the molars relatively broad; P3 is moderately sectorial, the short, broad lower molars with low, rounded, and expanded cusps, and poorly developed occlusal crests. Kalepithecus contrasts with other Early Miocene forms in its inferiorly broad nasal aperture and deep clivus, while dental morphology and proportions suggest a frugivorous diet.
Middle and Late Miocene African Hominoids
Fossil hominins apart, evidence of African hominoids from the Middle Miocene onward is limited, especially, when contrasted with the comparatively abundant Early Miocene material. Nonetheless, recent discoveries have both significantly increased the number of fossils (Ward and Duren 2002) and led to major reappraisals of earlier finds, notably of the material assigned to “Kenyapithecus” africanus (Leakey 1967), which has been reallocated to distinct taxa, with consequent systematic and phyletic implications.
Nacholapithecus
One such taxon is the large, markedly dimorphic Nacholapithecus kerioi (Ishida et al. 1999, 2004), based on material from Middle Miocene (15 Mya) sites in Samburu District, Kenya. Cranial remains display overlap of the posterior premaxilla and hard palate, forming a short incisive canal (Kunimatsu et al. 2004), which may indicate an intermediate condition between the nonoverlapping subnasal configuration of gibbons and fossil stem hominoids and the longer incisive canal that is a diagnostic trait of the clade that includes the extant large-bodied apes (Nakatsukasa and Kunimatsu 2009), or Hominidae. The incisive foramen is small, while the face bears strong canine pillars and deep fossae, and the zygomatic process originates low down on the maxilla. The mandible corpus is tall but thin, with a near vertical symphysis, a moderate inferior transverse torus, and a lateral fossa below the premolars. I1 is high crowned and robust, while both lower incisors are tall and narrow. Canines are low crowned, upper premolars display marked cusp heteromorphy, and molars are thick enameled.
Equatorius
Other material previously assigned to “K.” africanus has been incorporated, along with new discoveries (including a part skeleton (KNM-TH 28860) from Kipsaramon, Tugen Hills, and multiple finds at Maboko Island and adjacent localities, Kenya), in another, broadly contemporary (14–15.5 Mya) large-bodied, dimorphic species Equatorius africanus (Ward et al. 1999). While initially criticized – see, for example, Begun (2000) and Benefit and McCrossin (2000) and response by Kelley et al. (2000) – the current consensus is that E. africanus is a valid taxon. The species is characterized by broad I1s with marginal ridges, markedly asymmetrical lateral incisors, with a spiral lingual cingulum, and relatively large upper premolars with reduced cusp heteromorphy (contra Nacholapithecus). The procumbent, narrow lower incisors are tall, whereas the mandibular canines are low crowned with convergent roots. The thick-enameled, bunodont lower molars increase markedly in size along the tooth row.
The maxilla exhibits a very low, broad root for the zygomatic process and an extensive sinus extending into the premolar region, while the mandible displays a long, inclined sublingual planum, a prominent inferior transverse torus, and a robust corpus. The partial skeleton and other postcranial fossils indicate some resemblances to earlier forms such as Proconsul and Afropithecus, but with forelimb and hind limb contrasts that point to significant terrestriality (Sherwood et al. 2002; Patel et al. 2009). It remains to be determined whether ground vegetation formed an appreciable component of Equatorius’ diet, but dental similarities to Afropithecus (and “Heliopithecus”), especially in canine form and premolar proportions, suggest resistant foods, such as seeds and/or hard-cased fruits, as major dietary items.
Kenyapithecus
The genus Kenyapithecus is retained for the rather later (14 Mya) species K. wickeri, known from partial jaws and isolated teeth from Ft. Ternan, Kenya. The maxilla exhibits marked canine fossae, a relatively low and anteriorly positioned origin for the zygomatic process above M1, little extension of the maxillary sinus into the inflated alveolar process (in contrast to Equatorius), and a relatively highly arched palate. The upper incisors are markedly heteromorphic, with I2 much smaller than I1, which is reinforced by strong lingual marginal ridges extending across the base of the crown surface. The upper canines exhibit marked dimorphism: robust, tall, and externally rotated in presumed males, more conical in females. P4 is relatively broad with subequal cusps; M1 is quadritubercular and lacks a lingual cingulum, while M2 is similar but larger. The postcanine teeth are closely packed, with low cusp relief and appreciable wear.
The mandible , considered female, displays a shallow symphysis, sharply retreating at 30–40° to the alveolar margin, with pronounced inferior transverse torus extending to below the mesial root of M1, rather weak superior torus and long sublingual planum, a short incisor row, and a robust, comparatively shallow but thick corpus. As reconstructed, the mid-lower face overall was broad and flat, with wide cheeks and a short snout (Andrews 1971; Walker and Andrews 1973). The relatively tall-crowned lower canine bears only a slight lingual cingulum, while P3 is obliquely set and sectorial, with a distinct honing facet for the upper canine, and P4 bears prominent mesial and smaller, lower distal cusps; the poorly known lower molars apparently lack buccal cingula.
The narrow anterior dental arcade, procumbent incisors with curved roots, anteriorly positioned zygomatic origin, restricted maxillary sinus and mandible with markedly sloping symphysis, pronounced inferior transverse torus, and shallow, robust corpus differentiate K. wickeri from most other African hominoids. Andrews (1971) and Walker and Andrews (1973) interpret these traits as a functional set adapted for powerful chewing activity with a strong lateral grinding component and pronounced incisal action. There are similarities, especially in the anterior dentition, with Afropithecus, and, as with that genus, pithecines have been proposed as the most plausible dietary analogues (Leakey and Walker 1997; McCrossin and Benefit 1997). This model of K. wickeri as a sclerocarp feeder, exploiting hard-cased/hard-stoned fruits, seeds, and nuts, is compatible with reconstruction of the Ft. Ternan environment as drier and more seasonal than many earlier African sites, predominantly closed-canopy woodland with both open country and forested conditions nearby (Andrews 1996; Andrews et al. 1997). A second species of this genus, K. kizili (Kelley et al. 2008), has been named for material found outside of Africa and will be treated below.
Otavipithecus
Broadly contemporary at 13 + 1 Mya, the more southerly Otavipithecus namibiensis is known from a part mandible and frontal bone from Berg Aukas, Namibia (Conroy 1997; Conroy et al. 1992). The incisor region of the mandible is narrow and the symphysis reinforced by a short inferior transverse torus. The corpus is robust, of constant depth, and relatively long, with the ramus originating behind M3 and with a distinct retromolar space. Premolar and molar cusps are inflated and bunodont, mesial, and distal foveae small and enamel thin. The frontal bears superciliary ridges rather than a transverse torus, with marked temporal ridges adjacent to glabella, relatively wide interorbital dimensions and an extensive frontal sinus. The last of these traits has been used to link Otavipithecus with extant hominines (Pickford et al. 1997), but the current lack of clarity as to the polarity of frontal sinus evolution in catarrhines renders this interpretation premature at best. The narrow incisor region does not support a niche of specialized frugivory, while the thin enamel and minimal wear differential on the molar teeth point to a nonabrasive diet; there are no obvious dental adaptations to folivory. Conroy argues that O. namibiensis probably subsisted on a range of plant foods that required little preparation by the anterior teeth prior to chewing.
Samburupithecus
A later Miocene (9.5 Mya) large-bodied species from the Samburu Hills of north central Kenya named Samburupithecus kiptalami is known only from one left maxilla with P3–M3 crowns and the canine alveolus (Ishida and Pickford 1997). The palate displays a marked arch, a shallow postcanine fossa, a low origin for the zygomatic root, and invasion of the zygomatic process by the extensive maxillary sinus. The nasal floor has a sharp margin, and the tooth row is straight from the canine alveolus to M3. The three-rooted premolars have elongated crowns with coequal main cusps, while the molars display inflated, bunodont cusps and thick enamel. S. kiptalami’s affinities are unknown; some workers consider it to show some similarities with the gorilla, although there are also undoubted differences, e.g., the size of lingual cingulae. This and other traits make it most likely that Samburupithecus is a late-surviving part of the stem hominoid radiation that included Proconsul (Olejniczak et al. 2009).
Two newly discovered taxa have increased our knowledge of the later Miocene of Africa substantially. Nakalipithecus nakayamai is a large ape (>50 kg) from the early Late Miocene (9.9–9.8 Mya) site of Nakali, Kenya (Kunimatsu et al. 2007). Although known from only a few dentognathic remains, its striking resemblance to the slightly younger Ouranopithecus from Greece suggests that the popular paleobiogeographic scenario of large-bodied apes originating in Eurasia before reinvading Africa (Stewart and Disotell 1998) may need to be reexamined. The mandible of the holotype has a well-developed simian shelf (inferior transverse torus) and the thick dental enamel, reduced molar cingulae and reduced upper premolar cusp heteromorphy seen in Eurasian forms that have been considered more closely related to living hominids than African forms, although it retains the primitive non-compressed lower mesial premolar shape. A second large-bodied form, Chororapithecus is known from dental remains found in 10–10.5 Mya deposits in the Afar region of Ethiopia (Suwa et al. 2007). Aside from their similar size, the Chororapithecus molars are also similar to those of Gorilla in their relative development of molar shearing crests associated with folivory. Although not linked directly with the extant taxon, partly due to its thicker enamel caps, the resemblance suggests to the describers that it may belong to same clade. Two other possible new taxa, Kogolepithecus from the ca. 17 Mya site of Moroto II in Uganda (Pickford et al. 2003), and a single mandibular fragment from between 11 and 5 Mya in Niger (Pickford et al. 2009a) are too fragmentary to allow any convincing analysis.
Other, later taxa, closer to the Mio-Pliocene boundary (Sahelanthropus, Orrorin), are as yet only incompletely described but are claimed as basal hominins (see Senut, chapter “The Miocene Hominoids and the Earliest Putative Hominids,” Vol. 3). The known time span of Pan has recently been extended by the recovery of four fossil teeth (r and l I1, l M1, r M3) from Middle Pleistocene deposits of the Kapthurin Formation of the Tugen Hills, Kenya, within the eastern Rift (McBrearty and Jablonski 2005). The broad, spatulate incisors bear deep mesial and distal foveae separated by a prominent lingual tubercle and the molars are low crowned, while all teeth exhibit thin enamel. The large hypocone on M1 suggests P. troglodytes rather than P. paniscus, although McBrearty and Jablonksi are cautious in attributing specific identity, preferring assignment to Pan sp. indet. The finds date from around 0.5+ Mya, and the site lies some 600 km east of the present chimpanzee range. The teeth were discovered close to localities yielding part mandibles of Homo (H. erectus or H. heidelbergensis/H. rhodesiensis) pointing to sympatry and suggesting that adaptive scenarios which reconstruct differentiation of chimpanzee and hominin populations through the Rift Valley acting as an isolating barrier are unlikely to be correct. Fossil evidence for bonobos and gorillas is entirely lacking.
Europe and Asia Minor
While there is limited evidence dating from 15 to 17 Mya, the bulk of the European and west Asian hominoid material is from later Miocene sites between 6 and 12 Mya (Kay and Simons 1983; Pilbeam 2002). Many of these suggest subtropical seasonal forest or woodland as the dominant habitat; there is evidence of swamp conditions at some sites, while possibly harsher, more open environments are indicated at sites in Greece and Turkey yielding Ouranopithecus and Ankarapithecus, respectively.
Griphopithecus
The earliest evidence (13.5–17 Mya) consists of several low-crowned, large-cusped, and thick-enameled molars from Germany, Austria, and Slovakia assigned to Griphopithecus, a genus better known from Turkey, where a mandible with cheek teeth from Çandır and maxillary teeth from Paşalar dating from c. 15 Mya are referred to G. alpani (Alpagut et al. 1990). Like their European counterparts, the lower molars have bunodont, thick-enameled cusps, and well-developed buccal cingula. The upper central incisors are relatively narrow with a distinct median lingual pillar, and the male canines, especially in the upper jaw, are robust and comparatively low crowned. The mandible is strongly constructed, with both a prominent superior and well-developed inferior transverse torus at the symphysis and a long, shallowly inclined planum alveolare.
Heizmann and Begun (2001) suggest that Griphopithecus evolved from an Afropithecus/Heliopithecus-like thick-enameled ancestor and that this feature, together with the associated trait of low-dentine penetrance, was crucial to the expansion and success of dentally modern hominoids in the more seasonal Middle and Late Miocene habitats of Eurasia. However, at least one successful European form – Dryopithecus – had comparatively thin enamel, pointing to this as either a secondarily derived trait evolved from a thicker-enameled European ancestor or that the genus represents a second hominoid radiation into Europe.
Also at Paşalar, the species Kenyapithecus kizili (Kelley et al. 2008) is found. It has been linked to the African genus primarily due to the lack of lingual pillars in the upper central incisors, by which it differs from the other Paşalar hominoid Griphopithecus, although there are also some maxillary similarities. Unusually, the entire hypodigm of the taxon is thought to be from a single birth cohort, as all are the same developmental age and show identical enamel hypoplasias (Kelley 2008). As the molar teeth are extremely similar to those of Griphopithecus, it is assumed that their adaptation was similar, as well.
Dryopithecus
The thin molar enamel of Dryopithecus results in frequent dentine exposure, especially on the cusps, which lie close to the crown margins around a broad, shallow fovea. Below the narrow, low P3 crown enamel extends onto the anterior root, pointing to at least partial honing against the upper canine (Begun 2002), while the upper central incisors are narrow and high crowned. Larger (presumed male) mandibles are generally more robust than in the Early Miocene east African forms but are not as strongly built as more thickly enameled taxa (Begun 2002). All species in which evidence is available display a relatively high root for the maxillary zygomatic process. Compared with Proconsul and other Early Miocene forms, Dryopithecus has reduced cingula, relatively short lower molars (Szalay and Delson 1979), expanded occlusal surfaces, and reduced molar crown flare and a mandibular symphysis reinforced by a prominent inferior transverse torus.
Until recently, the type species, D. fontani (11–12 Mya), was among the less well-known fossil hominoids cranially. New material from Catalonia, NE Spain (Moyà-Solà et al. 2009a, b), however, has dramatically improved our understanding of this chimpanzee-sized ape. A partial cranium dated to 11.8 Mya preserves several hominoid and hominid synapomorphies: a large nasal aperture with its widest portion located inferiorly and its margin constructed primarily of the maxilla, over a hard palate that is wide anteriorly. Unlike other large-bodied apes, however, the zygomatic slopes posteriorly from the orbital margin to the root, although there is mid-facial prognathism as well. The authors describe the steep face and vertical orientation of the nasal aperture as resembling extant Gorilla, in contrast to earlier stem hominoids with more sloping anterior maxillae. The subnasal morphology is stepped, as in extant hominines, but the premaxilla does not overlap the maxilla at the midline, which suggests a more primitive condition than that seen in Nacholapithecus. It has rather broader canines than other species, frequent cingula on the lower molars, while in larger mandibles the corpus shallows markedly from the symphysis toward the rear, unlike other species.
Rudapithecus
Although formerly placed in Dryopithecus, the slightly younger (9.5–10 Mya) Rudapithecus hungaricus (Kivell and Begun 2009) is also well known cranially (Fig. 9). Comparable in tooth size to D. fontani, it differs in its labio-lingually thicker incisors, narrower canines, reduced molar cingula, and more tapered M3. The mandibles have weak symphyseal tori, but the corpus is reinforced below M1–M2 by a lateral eminence. Cranial morphology is comparatively well known from several incomplete specimens from Rudabanya, Hungary (Begun and Kordos 1997; Kordos and Begun 2001). The braincase is elongated, with a flat frontal displaying moderate postorbital constriction and strong anterior temporal ridges; supraorbital reinforcement is weak, but there is a fair-sized frontal sinus.
At the skull, rear inion is relatively highly positioned, while the mandibular fossa is transversely deep, with marked entoglenoid and postglenoid processes. The face is moderately projecting and deflected downward. The maxillary sinuses are larger than those of the East African fossils, and the nasal aperture has a broad base with subvertical sides. Again, in contrast to the early East African specimens where preserved, the subnasal floor is stepped, with the rear of the subnasal alveolar process extending over the palatal process of the maxilla, and an incisive canal is present. The long and projecting premaxilla is sagittally and transversely convex. The preserved semicircular canals suggest that Rudapithecus practiced relatively slow locomotion (Ryan et al. 2012).
Hispanopithecus
A smaller contemporary of Rudapithecus and also previously considered a species of Dryopithecus (Cameron 1997), Hispanopithecus laietanus is known from several sites in NE Spain (Begun 2002). Two partial skeletons have been discovered: a presumed male (39 kg) from Can Llobateres (Moyà-Solà and Köhler 1996) and a female (22–25 kg) from Can Feu (Alba et al. 2012). Mandibular teeth resemble those of Rudapithecus, but the premolars are relatively smaller, the molar cusps more rounded and expanded around the occlusal margins, and M3 less tapered.
CLI-18800 preserves the upper dentition: the incisors are like those of Rudapithecus, the canines have strongly curved roots and narrow crowns, and the molar teeth increase in size posteriorly. In its known craniofacial structures, Hispanopithecus shows many similarities with Rudapithecus, including periorbital and maxillary morphology, the supraorbital region, frontal sinuses, and a locomotor pattern reconstructed as low, as determined via semicircular canal size. There are also contrasts, however, with CLI-18800 displaying a very high root for the zygomatic process and a relatively deep and flatter anterior aspect of the zygoma. The upper incisor row is more strongly curved, and the premaxilla is strongly biconvex. Overall the facial profile of Hispanopithecus is more concave.
The slightly earlier (c. 10.5 Mya) H. crusafonti, also from NE Spain, is known only from a mandible and some isolated teeth. Dentally slightly larger than H. laietanus, it is distinguished by comparatively broad upper canines, relatively longer upper premolars than in Rudapithecus, and relatively broader upper molars than H. laietanus. The mandible combines an exceptionally robust corpus with comparatively small tooth crowns; the symphysis is reinforced by a strong inferior transverse torus and the corpus bulges laterally below M1–M3. Hispanopithecus differs from the geographically close Anoiapithecus in that the molar crowns are relatively narrower, as are the buccal cuspulids, the hypoconulid is less centrally placed, and cingulids are lacking (Alba et al. 2012).
Overall, dental features of all three genera, such as relative shearing-crest development (Ungar 1996; Kay and Ungar 1997), pitting incidence of >35–<40 % (Ungar et al. 2004), thin enamel and dentine penetrance, and molar flare (Singleton 2003) accord with reconstructions of these forms as a frugivores, probably primarily soft-fruit feeders, in mildly seasonal subtropical forests (Andrews 1996). This is also consistent with mandibular corpus cross section which in many cases is like that of similar-sized Proconsul, although larger specimens resemble Sivapithecus in their shallow, more robust, and almost triangular section below M3 – proportions particularly effective in resisting torsion (Brown 1997) and raising the possibility of a tougher, more fibrous component in the diet of larger individuals to sustain their greater body bulk.
Anoiapithecus
Another new European Miocene form with a well-preserved cranium is A. brevirostris, known from the Middle Miocene (11.9 Mya) locality of Abocador de Can Mata in the Vallès-Penedès Basin of Spain (Moyà-Solà et al. 2009a). This unique short-faced taxon possesses the classic hominid features of a wide anterior palate, a nasal aperture widest at the base, but the upper premolars are moderately heteromorphic. There is also a frontal sinus, in which it differs from the condition seen in Pierolapithecus; the maxillary sinus is situated above the roots of the molars. The latter trait is considered to indicate a restricted sinus, but is often seen in extant hominoids that do not demonstrate reduced sinus volume. The molars themselves have thick enamel, cusps set toward the center of the tooth, small, compressed upper canines, and a robust mandible with a weak superior transverse torus. The extreme orthognathism and dentognathic traits could indicate a functional regime optimized for strong vertical crushing.
Pierolapithecus
The recently discovered Middle Miocene (12.5–13 Mya) partial skeleton of Pierolapithecus catalaunicus from Barranc de Can Vila 1, Els Hostalets de Pierola, Barcelona, Spain, provides extensive new evidence of European hominoids (Moyà-Solà et al. 2004). The specimen (IPS 21350) includes a virtually complete face and lower frontal, the upper dentition, and much postcranial material. The Pierolapithecus face is exceptionally prognathous compared with other Middle and Late Miocene Eurasian hominoids and, as such, is reminiscent of Afropithecus. It is low, with slender superciliary arches merging into a moderately swollen glabella region, below which the upper face is transversely flat. The orbits are broader than high, and the interorbital distance wide. In profile, the nasal bones are concave and salient over the wide piriform aperture, the inferior margin of which is well anterior to the tips of the nasal bones. The nasoalveolar clivus is high, convex, and markedly projecting. The deep, laterally expanded and strongly constructed zygomatics slope anteroinferiorly, with their root originating high above the alveolar margin of M1. Internally, the paranasal sinus configuration of this taxon shows two specific similarities to extant Pongo: the maxillary sinus is limited in its anterior extent, but extends posteriorly toward the ethmoid, and the frontal sinus is absent (Pérez de los Ríosa et al. 2012). This, combined with the fact that the subnasal architecture does not include an overlapping premaxilla, suggests that Pierolapithecus bears no special relationship with extant African large-bodied apes, but may instead be part of the pongine clade.
The palate is short, wide, and deep, with the anterior dentition arcuate. I1 is low crowned and procumbent, the large C low crowned and compressed. P3 and P4 are of similar size with reduced cusp heteromorphy, while M1 and M2 crowns are long and relatively narrow, with M3 reduced. The lingual cusps are situated toward the crown edge and all the cheek teeth lack cingula.
This combination of dental and facial features distinguishes Pierolapithecus from all other Miocene hominoids; contrasts with Dryopithecus include the lower, more prognathous face; more anteriorly positioned zygomatic roots; shorter wider palate; larger, low-crowned anterior teeth; and relative molar crown size. In the view of Moyà-Solà et al. (2004), shared derived features of the two genera that link them to extant great apes include upper facial flatness, nasal bone projection and aperture form, a high zygomatic root, high nasoalveolar clivus, and deep palate. The postcranium also reveals shared derived traits with extant hominoids: a broad, shallow thorax and stiff lumbar region to the trunk, a dorsally positioned scapula, and apelike carpal bones with ulnar retreat from the wrist joint and evidence for the fibrous capsule of a semilunar meniscus, providing enhanced wrist abduction and supination. These traits, however, are combined with metacarpal and phalangeal features indicating the hand was palmigrade during locomotion and the fingers are short, as in monkeys, pointing to a dissociation in ape phylogeny between orthograde posture and climbing/below-branch suspension. The latter appears to have evolved later than the former and may well have arisen independently in several distinct lineages.
Similarities in craniofacial form and dental proportions with Afropithecus suggest a sclerocarp feeder, consistent with reconstructions of the Pierolapithecus habitat, based on the associated fauna, as wooded and relatively humid. Several Late Miocene hominoids (Ouranopithecus, Ankarapithecus, Graecopithecus) exhibit notably more ruggedly constructed crania and derive from drier, more fluctuating, and possibly more open environments.
Ouranopithecus
Ouranopithecus macedoniensis, represented by several jaws, teeth, and a fairly complete face from the c. 9-Myr-old northern Greek sites of Ravin de la Pluie, Xirochori, and Nikiti 1 (Fig. 10), is the largest European hominoid known, with males estimated to have been about female gorilla size. The face is strongly reinforced in its mid- and upper portions, pointing to powerful masticatory forces; the rectangular orbits are separated by a broad, stout interorbital area and laterally bounded by massive orbital pillars, while the nasal aperture is flanked by thick nasomaxillary (canine) pillars with broad, deep canine fossae beyond. The zygomatic region is very deep with, unusually for hominoids, a low origin for the zygomatic root on the maxilla. The premaxilla is strongly built, comparatively long, and markedly convex sagittally and transversely, with clivus, subnasal fossa, and incisive canal resembling Dryopithecus. The supraorbital torus bulges moderately above the orbits but less so medially; the strongly marked temporal lines indicate powerful anterior temporalis fibers.
The palate is deep and anteriorly broad; the incisors are set in an arc, offset from the other teeth by a pronounced diastema. The upper incisors are markedly heterodont, with the central teeth thick and broad and the laterals narrow and peg shaped; lower incisors are tall and slightly flared. Larger (male) canines are tall and laterally compressed but small when compared with molar size, while smaller (female) canines are very low crowned and almost premolariform.
Brown (1997) considers such low canines compatible with a greater degree of lateral mandibular movement than in modern apes. Both upper and (and especially) lower anterior premolars are triangular, the latter with a prominent mesial beak and lacking an anterior vertical honing facet from the upper canine. Instead wear occurs on the tip and along the protocristid, a feature judged reminiscent of Australopithecus afarensis (de Bonis and Koufos 2001). P4 resembles P3 in shape – another claimed Australopithecus – like trait and unlike other fossil and extant apes, while P4 is molarized, being elongate and with a high talonid. The molars are relatively large, with M2 and M3 the largest teeth; individual cusps are expanded, and both upper and lower rear molars possess accessory cusps. There is a marked wear gradient along the molar row: enamel is very thick and the cusps are worn almost flat before dentine appears.
Mandibles are robust, with thicker corpora than in extant apes, and larger (male) specimens are deep as well as thick. The symphysis is strongly reinforced by a broad, long planum and prominent inferior transverse torus. The lateral eminence originates opposite M3, and the ramus ascends between M2 and M3. The gonial region is extensive and displays strongly marked ridging for the superficial masseter and medial pterygoid muscles. The condyle is relatively large and sagittally strongly convex.
Facial structure, muscle impressions, dental structure and proportions, occlusal morphology, and attritional wear gradients in Ouranopithecus all contrast with Dryopithecus and suggest extremely powerful masticatory activity, especially of the cheek teeth. This is further supported by microwear patterns which reveal a very high ratio of pits compared with striations on phase 2 facets of the cheek teeth (at >58 %, the highest of any fossil ape studied) and distinct wear on the incisors (Ungar 1996; Ungar et al. 2004), while the Ouranopithecus shearing quotient (and so relative shearing-crest development) is lower than that of any modern hominoid or other European fossil ape studied (Ungar and Kay 1995; Kay and Ungar 1997). In all these features, Ouranopithecus resembles extant hard-object feeders, pointing to exploitation of a similar niche – perhaps seeds, nuts, roots, and tubers, and other terrestrial vegetation. There are obvious resemblances here to some reconstructions of early (Plio-Pleistocene) hominine dietary niche(s), although these are unlikely to mirror any specially close phylogenetic link. De Bonis and Koufos (1997) argue that this model of Ouranopithecus’ diet is consistent with reconstruction of its open habitat (De Bonis et al. 1992), although Andrews (1996) and Andrews et al. (1997) urge caution, considering the overall fauna to be undiagnostic other than indicating a strongly seasonal, possibly harsh, environment.
The poorly known Graecopithecus freybergi (von Koenigswald 1972), based on a single mandible from Tour la Reine, Pyrgos, Greece, and dated around 6.5–8 Mya, is often regarded as congeneric or even conspecific with O. macedoniensis (Martin and Andrews 1984; Andrews 1996), although Begun (2002) makes a strong case for its retention as a separate taxon based on molar size and mandible proportions.
Ankarapithecus
Ankarapithecus meteai (Ozansoy 1965), from sites in the Sinap Formation of Anatolia, Turkey, dated at c. 10 Mya, is a strongly built form known from cranial material including the type mandible and a partial face (Fig. 10), together with undescribed postcrania (Alpagut et al. 1996; Begun and Gulec 1998). The face is tall and markedly prognathic in both midface (unlike Sivapithecus and Pongo) and premaxillary regions. The clivus is biconvex, with large, low-crowned, and labiolingually thick central incisors and smaller lateral incisors. Male upper canines are relatively low crowned, and their roots form strong juga converging on the broad nasal aperture, with relatively shallow canine fossae beyond. The palate is deep, with the root of the zygomatic process set comparatively high above M1 and into which the large maxillary sinuses extend. The vertically orientated, laterally flaring zygomatic process imparts strong anteriorly and laterally directed components to masseteric action, while the deep temporal fossa allows for a powerful temporalis muscle. The subnasal fossa is stepped and the incisive fossa large. The orbits are square, with a narrow interorbital space, very long nasal bones, broad, rounded orbital pillars, and prominent anterior temporal lines. Rather surprisingly in view of the rugged mid- and lower face, the superciliary arches above the orbits do not form a true torus.
The massive mandible is strongly buttressed, with a very deep, narrow, and vertical symphysis and the inferior traverse torus extending to the level of M1. The rear of the corpus is very thick and the ramus broad. The lower incisors are labiolingually thick, narrow, and tall crowned, set almost vertically in a straight line between the low-crowned canines, which in males are more massive than in Dryopithecus or Ouranopithecus in basal section, while female canines are more premolariform, as in the latter genus. P3 is large, oval, and elongated, with a large mesial beak comparable to Dryopithecus but smaller than Ouranopithecus, and P4 is large and relatively broad, while M1 is small relative to M2. Upper and lower molars are broader relative to their lengths than in other later Miocene forms; their occlusal surfaces have broad, flat cusps and shallow basins and lack cingula.
Andrews and Alpagut (2001) provide a valuable functional analysis of this taxon as a hard-object feeder in dry seasonal subtropical forest (Andrews 1996), illuminating aspects of A. meteai morphology and also that of other Miocene hominoids. In many features, Ankarapithecus resembles Ouranopithecus: dentally in the large, low-crowned and worn incisors; large cheek teeth; thick enamel; flat occlusal wear; poor shearing-crest development; and also in aspects of facial architecture and mandibular reinforcement. However, there are also differences: the supraorbital region differs, the Ankarapithecus interorbital region is narrower as are the lateral orbital margins, the midface is more prognathic, and the zygomatic root originates higher on the maxilla, while the mandibular corpus is massively thickened under the rear molars, so that it is actually broader than deep, whereas that of Ouranopithecus is deeper and narrower in cross section.
Oreopithecus
Oreopithecus bambolii, a comparatively large-bodied form dating from 6 to 7 Mya, is represented by multiple specimens from northern Italy, including a largely complete skeleton, making it the best-known European fossil primate. This has not prevented protracted debate about its affinities, although in recent years there has been a growing consensus that Oreopithecus is a primitive hominoid. Whatever its phyletic status, it is clear that Oreopithecus differs markedly in adaptive features and inferred niche from other Late Miocene European and West Asian hominoids.
The Oreopithecus skull combines a relatively small, low, but globular neurocranium with a deep, broad, and moderately projecting face that reflects anterior placement and projection of the midface and nasal region, for the premaxilla and clivus are short and comparatively vertical. The supraorbital torus is well developed, the interorbital region broad, and the nasal bones short and salient. Strong canine pillars are bounded by shallow canine fossae, while the zygomatic process is comparatively deep with a low, anteriorly placed root originating above P4/M1, and the zygomatic arch is long, flaring, and upwardly curved posteriorly. The alveolar region has deep but restricted sinuses. The saddle-shaped articular eminence is broad and long, with a large entoglenoid process. The articular and tympanic portions of the temporal are not fused, but the temporal petrous is hominid-like in its shallow, indistinct subarcuate fossa. The mastoid is broad and continuous with the extensive, strongly marked nuchal area. Zygomatic flare and mandibular proportions point to powerful, fleshy temporal muscles, as do the deep nuchal and sagittal crests, meeting high on the skull rear; the sagittal crest continues well forward before dividing into two prominent anterior temporal lines.
The strongly built mandible is large, with corpus height decreasing slightly along the cheek teeth row, and with strong reinforcement provided by the pronounced lateral eminence below the molar region. The ramus is broad and high with an expanded gonial region. There are pronounced markings for the masseters and medial pterygoids, while the condylar processes, below the broad and convex condyles, display strong markings for the lateral pterygoids.
Many of these cranial features can be considered representative of the primitive catarrhine morphotype (Harrison 1986); the exceptions are those features of the maxilla, zygomatic region, and mandible summarized above that can be related to masticatory power (see below). The dentition is highly derived: the incisor teeth are small overall and vertically implanted; those in the mandible are labiolingually compressed, while the uppers are heteromorphic with I1 exhibiting a distinctive projecting lingual cusp. Canines are basally stout but not very tall and only loosely interlock, with a diastema small or absent. Larger (male) upper canines are strongly compressed and with a sharp rear edge, the lowers are more rounded in section; smaller (female) upper canines are rather incisiform. Much canine wear is from the tips; there was some C1/C1 honing, and some larger upper canines show evidence of slight honing against P3, but smaller ones lack this, and the anterior face of C1 did not hone against I2. The lower premolars are bicuspid, with P3 oval in outline and P4 more rectangular; upper premolars are oval with subequal cusps. Upper and lower molars are elongated and bear tall, spiky cusps with deep notches between. Besides the four main upper molar cusps, a metaconule is positioned centrally on the crista obliqua, often linked to the hypocone by a crest. On the lower molars, the protoconid and hypoconid (buccally) and meta- and entoconid (lingually) are joined by sharp crests to a well-developed centroconid on the cristid obliqua, so mirroring the upper molars in their distinctive occlusal pattern. The hypoconulid is frequently split into several smaller cusps. M1 usually bears a small paraconid, which is rarely present on M2 and never on M3. M1 and M2 are subequal in size, and M3 is the largest tooth. The inner ear shows adaptations to medium-slow agility, somewhat more active than in Rudapithecus/Hispanopithecus, but marginally less so than Proconsul.
Although it displays some primitive features, the postcranial skeleton is apelike, indicating a degree of orthogrady, forelimb suspension, and strong grasping capabilities in the feet. The thorax is broad, the lumbar region short, and the iliac blades are short and broad, with a prominent anterior inferior iliac spine. The forelimbs are much longer than the hind limbs and display multiple adaptations to stability and hyperextension at the elbow joint and rotation in the forearm. There were a wide range of movements at the wrist, with short palms and long, curved fingers. The femora show weight-bearing adaptations at hip and knee, with flexible, wide-ranging movements at the ankle, and a short midfoot with long, strongly muscled digits including a powerful, opposed hallux.
Oreopithecus’ reduced anterior dentition, expanded cheek teeth with complex occlusal morphology, relatively deep and orthognathic face, small neurocranium combined with large ectocranial crests and powerful chewing muscles, and robust mandible all point to a specialized folivorous diet of bulky, relatively low-grade food items which, judging by postcranial morphology and proportions, it exploited largely via an underbranch milieu. This conclusion is reinforced by study of relative shearing-crest development (Ungar and Kay 1995; Kay and Ungar 1997), which shows it to have the highest shearing quotient of any catarrhine studied, substantially in excess of any extant or other fossil hominoid. Further support is provided by dental microwear patterns, with a very low proportion (17 %) of pitting on phase II facets (Ungar 1996; King 2001; Ungar et al. 2004), consistent with extreme folivory. This wholly accords with reconstructions of Oreopithecus’ paleoenvironment, which indicates lowland mixed broad-leaved and coniferous forest, with bushes, ferns, and sedges accumulated under swampy conditions (Andrews et al. 1997; Harrison and Rook 1997). However, see Alba et al. (2001) for an alternative and, to our minds, less convincing interpretation of the Oreopithecus skull based on biomechanical constraints associated with orthograde posture and bipedalism. These authors derive Oreopithecus cranial morphology from a Dryopithecus-like ancestor by a process of neoteny.
South and East Asia
Sivapithecus
The best-known Asian fossil ape genus is Sivapithecus, from Late Miocene (8.5–12.7 Mya) deposits in the Siwalik Hills of India and Pakistan and including material assigned to Ramapithecus prior to the 1980s (Pilbeam 2002). Sivapithecus is rare throughout the Siwalik record, comprising only c. 1 % of the mammalian community (Ward 1997). Despite this, aspects of its cranial morphology are relatively well known through discoveries over the last three decades; in particular, a partial skull (GSP 15000) from Potwar, Pakistan, provides much information on facial and gnathic morphology (Fig. 11). It consists of the left side of the face with zygomatic arch, palate, mandible, and complete adult dentition (Pilbeam 1982).
The specimen indicates many similarities with Pongo in its overall facial proportions (but see below): the orbits are taller than broad and high-set, ovoid in outline, and the zygomatic foramina are large. The interorbital distance is very narrow, while the lateral orbital pillars are slender, especially sagittally, and there are distinct supraorbital ridges but no continuous torus.
Postorbital constriction is marked, with the anterior temporal lines strongly convergent, implying a well-developed sagittal crest in larger individuals. The frontal rises more steeply above the orbits than in extant nonhuman African apes and is orangutan-like in its contour and the absence of a frontoethmoid sinus.
The nasoalveolar clivus is long and strongly curved, intersecting the alveolar plane at a shallow angle. As in the orangutan but in contrast to Dryopithecus, Ouranopithecus, and Ankarapithecus, the nasal floor is smooth, with the premaxilla curving into the nasal cavity and joining the palatal process without a step; the incisive fossa and incisive foramen are both tiny, linked by a very narrow incisive canal. The long, medially convergent canine roots are externally rotated, while the deep zygomatic process is thin and Pongo-like in its flare. These features result in exceptionally prominent canine pillars reinforcing the robust anterior midface and well-marked canine fossae lateral to them (Ward and Pilbeam 1983). The GSP 15,000 mandible is deep and strongly built, the symphysis exhibits pronounced buttressing, the corpus is of fairly constant depth along the tooth row, while the ramus is high and broadest at the level of the occlusal plane, tapering slightly superiorly. The facial contour is Pongo-like in its marked concavity, nasoalveolar clivus projection, superoposterior slope to the zygomatic process and lateral orbital pillar, and the upward inclination of the zygomatic arch itself.
Nonetheless, there are contrasts with the orangutan: the Sivapithecus midface is much longer, the nasal bones especially so, and the maxillary sinus is more restricted (Ward 1997). The mandible in particular contrasts with Pongo in most features other than its high ramus; while highly variable (Brown 1997), all Sivapithecus specimens display markings for the anterior digastric muscles which are absent in orangutans (see above), and the inferior transverse torus does not extend as posteriorly as in Pongo (Brown 1997). There are also contrasts in corpus cross section: Sivapithecus specimens have robust, broad corpora, ovoid below the premolars, more triangular below M3, with smaller specimens relatively shallow, larger ones deeper. They show marked relief, with an intertoral sulcus near the lingual base below the cheek teeth, and a pronounced lateral eminence which continues to the base, whereas in orangutans the lateral eminence is usually a much less prominent swelling restricted to the upper part of the corpus. Sivapithecus mandible proportions have been interpreted as resisting sagittal bending loads on the balancing side and pronounced torsional and shearing loads on the working side, associated with powerful molar action and, as a possible secondary factor, incisal biting (Kelley and Pilbeam 1986; Brown 1997).
Dental features accord with this interpretation: the upper incisors are strongly heteromorphic, and the central teeth are very wide and spatulate with a heavily crenulated, extended lingual tubercle; the laterals are much narrower. The canines are moderately tall, compressed, and outwardly rotated and display only limited dimorphism. P3 is larger than P4, while the mandibular premolars are broad, with P3 expanded mesiobuccally and displaying only limited evidence of upper canine honing. The thickly enameled molars lack cingula and display expanded, bunodont cusps and so limited occlusal foveae. In the upper jaw, M2 is the largest tooth, while M3 is the largest of the relatively short and broad lower molars. The cheek teeth are closely packed, with clear interproximal wear facets and, despite the thick enamel, often display a pronounced wear gradient with, in older individuals such as GSP 15,000, destruction of crown relief and extensive dentine exposure.
Overall, evidence points to Sivapithecus as a frugivore/hard-object feeder, possibly nuts, seeds, bark, or hard-pitted fruits, requiring powerful mastication by the postcanine teeth. Earlier scenarios of the genus as an open habitat form, exploiting terrestrial vegetation, have been replaced by reconstructions of its environment as predominantly seasonal tropical or subtropical closed-canopy forest or woodland, albeit with patchiness and expanding areas of more open grassland in the later phase of its presence in the Siwalik record (Andrews et al. 1997; Ward 1997). While this shift could reflect broader climatic changes that, through the contraction and break up of its forested habitat, eventually resulted in the extinction of Sivapithecus, it also might be the case that the taxon was more adapted to open habitats than previously thought. Analysis of some Siwalik carpals suggests that Sivapithecus may have been a knuckle-walker (Begun and Kivell 2011).
Three Sivapithecus species are recognized, differentiated primarily on dental proportions: S. sivalenis from Siwalik sites dating between 8.5 and 9.5 Mya is the type species. S. indicus, represented by GSP 15,000 and other material, is earlier (10.5–12.5 Mya) and with absolutely rather smaller teeth than S. sivalensis, but with a proportionately larger M3 compared with M2, and with a rather shorter premaxillary region. A humerus with a retroflexed and mediolaterally strongly curved shaft and a prominent deltopectoral crest is assigned to this species. S. parvada is a recently recognized, appreciably larger form, dating around 10 Mya. Its I1 is particularly wide relative to its breadth, the premolars, especially the lower ones, are expanded relative to molar size, and M3 is again much larger than M2. The mandible’s symphysis and anterior corpus region are exceptionally deep, while a humerus referred to S. parvada broadly resembles that of S. indicus but is much bigger, implying larger body size overall.
Gigantopithecus
Many workers regard the Asian genus Gigantopithecus (von Koenigswald 1952) – known from massive mandibles and individual teeth from the Late Miocene/Pleistocene of southern China, Vietnam, and the Siwaliks of India and Pakistan – as closely related to Sivapithecus. While extremely large, it is characterized by a reduced anterior dentition, with relatively small lower incisors and low-crowned but basally large canines without honing facets, strongly worn down from the tip and functionally incorporated in the premolar/molar rows. The expanded premolars are strongly molarized: P3 is bicuspid with a large talonid and is larger than P4, which is almost square with a large trigonid taller than the talonid. The upper molars are almost square, the lowers elongated; all have very thick enamel, high crowns, and low cusps. The symphysis is reinforced by a moderate superior transverse torus and a much more extensive inferior torus that may extend as far back as M1.
Gigantopithecus giganteus is known from specimens found from the 1960s onward from Haritalyangar and other Siwalik sites, especially a mandible CYP359/68 (Pilbeam et al. 1977), usually considered late in the Siwalik sequence at <7 Mya (Johnson et al. 1983). However, Pillans et al. (2005) argue for a date of 8.6 Mya for the mandible and report a newly discovered M2 from the earlier (8.85 Mya) HD site at Haritalyangar which also yielded an incisor assigned to Sivapithecus, demonstrating sympatry of the two genera. Fossils of the more recent G. blacki are even larger than those of G. giganteus, with more pronounced molarization of the premolars, so that P3 is more distinctly bicuspid and P4 longer, while the molars are higher crowned, with low, expanded cusps, and multiple accessory cusplets. The mandibular symphysis is long and powerfully buttressed; the corpus is strongly reinforced by a thick lateral torus originating below M1 and corpus depth increases posteriorly (Weidenreich 1945). Given these and other contrasts between the South and East Asian species, Cameron has recently reassigned the Haritalyangar mandible to Indopithecus (Cameron 2001, 2003; Pillans et al. 2005).
The size of Gigantopithecus fossils almost certainly precludes arboreality, and most reconstructions are of a ground-dwelling pongine exploiting a low-grade, bulky diet – perhaps bamboo (present at both Siwalik and Chinese localities) – although more varied diets, including fruits, have been suggested (Ciochon et al. 1990). Whatever the details, dental and gnathic features clearly indicate an extremely powerful masticatory apparatus with a premium on occlusal crushing and grinding by the cheek teeth, together with an anterior dentition capable of generating considerable bite forces for cropping food items. The short, premolariform canines, together with the mandible’s symphyseal and corporal proportions, point to the generation of powerful torsional and shearing forces during food processing. The same environmental changes that reduced forest and woodland cover, leading to the extinction of Sivapithecus, may well have favored the evolution of Gigantopithecus as a form better adapted to more open conditions and the exploitation of terrestrial vegetation (Ward 1997; Pillans et al. 2005).
Lufengpithecus
Lufengpithecus is another large-bodied Asian pongin, represented by extensive material, including several distorted crania and numerous teeth, from the Late Miocene (c. 8 Mya) of Lufeng, Yunnan province, southern China. Dental metrics indicate a degree of sexual dimorphism rather greater than that of any extant ape (Kelley and Qinghua 1991; Kelley 1993; Schwartz 1997). Cranial morphology contrasts with Sivapithecus in the relatively shorter midface, square orbits, broad interorbital and glabellar regions, and more prominent supraorbital torus above the medial superior orbital margins, resembling more the Dryopithecus pattern. The nasoalveolar clivus is relatively short and the mandibular symphysis strengthened by a moderate superior and strongly developed inferior transverse torus. The corpus is narrow and columnar with little sculpting or buttressing (Brown 1997), presumably resistant to vertical occlusal forces, but less well adapted to torsional or shearing forces generated by the cheek teeth.
The upper central incisors are tall and narrow but relatively thick and buttressed by a median pillar on the lingual surface, while the lower incisors are relatively narrow and moderately procumbent. Presumed male canines, especially the lowers, are tall, sharply tapering, and relatively slender. Molar enamel is moderately thick, and the crowns are narrow with the cusps situated toward the rim so that the foveae are extensive, and the occlusal surface bears a complex pattern of crenulations. Overall molar occlusal morphology is remarkably like that of the orangutan (Ward 1997), suggesting a frugivorous niche, as do the similarities with Dryopithecus. Paleoenvironmental indicators point to swampy moist tropical forest conditions, with ferns and epiphytes in which – again unlike Sivapithecus – Lufengpithecus was common, representing >33 % of the mammalian fossils (Andrews et al. 1997).
Kelley (2002) recognizes three species of Lufengpithecus: L. lufengensis, the type species, is the best known; L. keiyuanensis and L. hudienensis have smaller postcanine teeth and rather greater molar cingulum development than L. lufengensis, with L. keiyuanensis possibly also having thinner enamel.
Khoratpithecus
The recent discovery of teeth and a part mandible from Middle and Late Miocene deposits at sites in northern Thailand, and so within the geographical range of Pleistocene Pongo, sheds further light on orangutan ancestry (Chaimanee et al. 2003, 2004). The finds have been assigned to the new genus Khoratpithecus and display numerous dental and gnathic similarities with Pongo, as well as with Lufengpithecus and, to a lesser extent, Sivapithecus. Khoratpithecus piriyai (9–7 Mya) is a large form (estimated 70–80 kg body weight) from a locality in Khorat, NE Thailand, and represented by a mandible body with the left canine – right I2 roots – and with the right canine and all cheek teeth crowns preserved on both sides (Chaimanee et al. 2004). The symphysis is strongly sloping, thicker in overall cross section than usual in Pongo, with a weaker superior traverse torus, shallow genial fossa, and strongly developed inferior torus that, while wide and extending to below the anterior part of the M1 crown, is less posteriorly extensive than in the orangutan. While the geniohyoid muscle facets are distinct, Khoratpithecus, like Pongo, lacks any impression for the anterior digastric muscle. The corpus is uniformly deep, with a marked depression on the lateral surface below the C/P3 region and thickening posteriorly, accentuated by a pronounced lateral eminence below M3.
Judging by anterior jaw proportions and alveoli, the procumbent incisors were larger than in Lufengpithecus but smaller than Pongo and arranged in a slightly convex arc. Enamel wrinkling, while present, is less complex than in the orangutan, the P4 is shorter, and the molar cusps are more centrally located than in the modern ape. The site indicates a riverine setting with palms and dipterocarps, together with proboscids, anthracotheres, pigs, rhinos, bovids, and rare Hipparion, corresponding to the Upper Nagri/Lower Dhok Pathan Formation faunas in the Siwalik sequence.
An earlier species, Khoratpithecus chiangmuanensis (13.5–10 Mya), is based on upper and lower teeth of a single individual from Ban Sa in the Chiang Muan basin (Chaimanee et al. 2003). Enamel wrinkling, markedly heterodont upper incisors, P3 crown form, lack of molar cingula and comparable degrees of relative enamel thickness, and dentine penetrance again align it with Pongo, but it differs from the latter in its smaller central incisors, in the weaker median lingual pillar of the upper and lower incisors, in the greater buccal flare of the lower molar crowns, and in its less intensive enamel wrinkling.
Contextual evidence associated with K. chiangmuanensis indicates a mosaic of tropical freshwater swamps and lowland forest that contrasts with the temperate flora from Lufeng, instead resembling modern African habitats such as those in the southern Sudan around the source of the White Nile. Chaimanee et al. take this to indicate a Middle Miocene floral and faunal dispersal corridor linking South East Asia and Africa that may have been critical in hominoid dispersion. Overall, Khoratpithecus closely resembles Lufengpithecus in its dentition, but its similarities with Pongo, especially the absence of digastric fossae, point to closer affinity with the modern genus than any other fossil ape.
Conclusions
Early Miocene hominoids already show considerable craniodental diversity, probably associated with dietary niche differentiation. Better known genera (Afropithecus, Proconsul) have contrasting morphologies but span a range of frugivory: Proconsul sp. and Dendropithecus were probably soft-fruit feeders, Afropithecus exploited hard-cased fruits. Among the less well-known genera, some (e.g., Morotopithecus, Turkanapithecus) bear cranial and/or dental similarities to Afropithecus, suggesting a hard-fruit niche, and that the most familiar cranial morphology – that of Proconsul – is not necessarily characteristic of many Early Miocene hominoids. Limnopithecus and Micropithecus appear to have been folivores on the basis of their dentition .
Middle and Late Miocene forms from the region (Nacholapithecus, Equatorius, Kenyapithecus, Samburupithecus) are thick enameled and probably hard-cased fruits and seed feeders, while environmental evidence suggests more open, rather drier, and more seasonal habitats. The more southerly Otavipithecus is an exception, with thin enamel and minimal dental wear, pointing to a soft-fruit diet.
By the Middle Miocene, hominoids are known from Europe and Western Asia. The earliest (Griphopithecus) are thick enameled, as are many later genera which are also generally more robust cranially than Proconsul, some especially so. At least one successful genus (Dryopithecus), however, has thin enamel and an only moderately strongly constructed cranium, although occlusal area, inferior symphyseal reinforcement, and, in larger individuals, mandibular cross section are expanded compared with Proconsul. Dryopithecus also contrasts with early East African fossils in its stepped nasal floor with the alveolar process overriding the palate and an incisive canal present. Overall, evidence suggests Dryopithecus primarily exploited soft fruits. The recently described, markedly prognathous Pierolapithecus is much more reminiscent of Afropithecus in its morphology and, as such, probably a sclerocarp feeder.
Broadly contemporary with the younger Dryopithecus species at 9–10 Mya, Ouranopithecus and Ankarapithecus are more strongly built forms whose cranial reinforcement, muscle markings, gnathic proportions, and dental features all point to impressive masticatory power and hard-object feeding, characteristics shared with the less well-known and rather younger Graecopithecus. Of about the same age (7–8 Mya) is the (masticatory power apart) generally contrasting Oreopithecus. This genus retains many primitive cranial traits together with features making for enhanced chewing capability and a distinctive dentition adapted to specialized folivory.
A similar trend to more robust morphologies is seen in South Asian hominoids, although details differ. The Late Miocene Sivapithecus (8.5–<13 Ma) is broadly Pongo-like in many aspects of cranial morphology, including periorbital proportions, mid-/lower facial prognathism and cheek orientation, nasal floor structure, and the presence of a narrow incisive canal, and remains among the most convincing instances of a comparatively close phylogenetic link between fossil and extant hominoid taxa, although Sivapithecus is unlikely to be directly ancestral to the orangutan. Cranial and dental features of Sivapithecus point to a frugivorous/hard-object feeding niche in increasingly patchy, fragmented habitats. These same environmental shifts probably underlie the appearance of Gigantopithecus [Indopithecus] giganteus in the Siwalik record, the oldest example of which is sympatric with Sivapithecus. Gigantopithecus mandibles are massive and powerfully reinforced, while occlusal area is expanded through increased molar size, premolar molarization, and incorporation of the low-crowned, worn flat canines into the cheek teeth rows. These features are evident in G. giganteus but even more pronounced in the later, East Asian, G. blacki (2–<1 Mya). Given its size, Gigantopithecus probably exploited bulky, low-grade terrestrial vegetation, with bamboo as the most likely predominant food source.
Also from East Asia and roughly contemporary with latest Sivapithecus and earliest Gigantopithecus is Lufengpithecus. In some respects, this is cranially rather reminiscent of Dryopithecus, contrasting with Sivapithecus in its broad upper face, supraorbital development and orbital proportions, thinner enamel, in its mandibular section which is less resistant to torsion and shear forces, and in its molar occlusal pattern which is more Pongo-like than that of Sivapithecus, all of which suggest a frugivorous diet .
The immediate ancestry of the extant great apes is obscure, while the entire early evolution of hylobatids is unknown. Both the distribution and diversity of contemporary Hylobates and Pongo populations indicate that climatic changes and associated sea-level fluctuations have been major determinants of their evolution over the last 2+ Ma. Pongo in particular is cranially highly variable: there are appreciable differences between Bornean groups separated by major river barriers, as well as marked contrasts between these and Sumatran orangutans, leading to recent proposals for species-level distinction between the two island populations. All orangutans lack the anterior digastric muscle, thereby contrasting with virtually all other hominoids including Sivapithecus, which does, however, share with Pongo a distinctively airorhynchous cranial form. The recently discovered Middle/Late Miocene Khoratpithecus from Thailand displays jaw and dental affinities with Sivapithecus and especially Lufengpithecus and Pongo, sharing with the last a lack of any indication of the anterior digastric muscle.
The notably klinorhynchous African apes contrast in this respect and exhibit multiple similarities that indicate a common cranial pattern differentiated by varying growth periods. Since a degree of airorhynchy seems common among fossil hominoids and differentiates hominoids from non-hominoids, the African ape condition appears derived. Despite the comparatively full Miocene fossil record, there are no especially convincing candidates for modern African ape ancestry, although Chororapithecus is a potential link. The sequence and timing of splitting of the gorilla, chimpanzee, and hominin clades is uncertain, although some evidence (dental, gnathic, temporal) suggests that Gorilla is more primitive and Pan more derived. Differentiation of bonobos and chimpanzees and of east and west gorilla populations perhaps occurred in the Late Pliocene/Pleistocene. Recently recovered fossil teeth provide evidence of Pan (probably P. troglodytes) from c. 0.5 Mya in the East African Rift.
While there are broad associations between African ape diet and cranial form, more detailed analyses fail to show an exact correspondence, in part because of dietary variability and also because of cranial variation. Evidence suggests that cranial features are less closely determined by diet than are characteristics of the dentition .
Bonobos show reduced levels of sexual dimorphism in craniodental features compared with chimpanzees, perhaps reflecting differences in sexual behavior and social organization. Surprisingly, bonobos are relatively the most robust of the African apes in some cranial traits, gorillas relatively the most gracile. When viewed in the broader context of the hominoid fossil record, all the living great ape crania are comparatively lightly constructed, raising issues about their representative nature or otherwise for the functional and adaptive modeling of earlier hominoids.
Cross-References
Dental Adaptations of African Apes
Estimation of Basic Life History Data of Fossil Hominoids
Evolution of the Primate Brain
Evolutionary Biology of Ape and Monkey Feeding and Nutrition
Fossil Record of Miocene Hominoids
General Principles of Evolutionary Morphology
Geological Background of Early Hominid Sites in Africa
Modeling the Past: Archaeology
Paleoecology: An Adequate Window on the Past?
Postcranial and Locomotor Adaptations of Hominoids
Potential Hominoid Ancestors for Hominidae
Quaternary Geology and Paleoenvironments
The Biotic Environments of the Late Miocene Hominoids
The Ontogeny-Phylogeny Nexus in a Nutshell: Implications for Primatology and Paleoanthropology
The Paleoclimatic Record and Plio-Pleistocene Paleoenvironments
Zoogeography: Primate and Early Hominin Distribution and Migration Patterns
References
Ackermann RR (2002) Patterns of covariation in the hominoid craniofacial skeleton: implications for paleoanthropological models. J Hum Evol 42:167–187
Ackermann RR (2005) Ontogenetic integration of the hominoid face. J Hum Evol 48:175–197
Ackermann RR, Krovitz GE (2002) Common pattern of facial ontogeny in the hominid lineage. Anat Rec 219:142–147
Aiello L, Dean C (1990) An introduction to human evolutionary anatomy. Academic, London
Alba DM, Moyà-Solà S, Köhler M, Rook L (2001) Heterochrony and the cranial anatomy of Oreopithecus: some cladistic fallacies and the significance of developmental constraints in phylogenetic analysis. In: de Bonis L, Koufos GD, Andrews P (eds) Hominoid evolution and climatic change in Europe 2: phylogeny of the Neogene hominoid primates of Eurasia. Cambridge University Press, Cambridge, pp 284–315
Alba DM, Almécija S, Casanovas-Vilar I, Méndez JM, Moyà-Solà S (2012) A partial skeleton of the fossil great ape Hispanopithecus laietanus from Can Feu and the mosaic evolution of crown-hominoid positional behaviors. PLoS ONE 7(6):e39617
Albrecht GH, Miller JMA (1993) Geographic variation in primates. A review with implications for interpreting fossils. In: Kimbel WH, Martin LB (eds) Species, species concepts and primate evolution. Plenum Press, New York, pp 123–161
Albrecht GH, Gelvin BR, Miller JMA (2003) The hierarchy of intraspecific craniometric variation in gorillas: a population-thinking approach with implications for fossil species recognition studies. In: Taylor AB, Goldsmith ML (eds) Gorilla biology: a multidisciplinary perspective. Cambridge University Press, Cambridge, pp 62–103
Alpagut B, Andrews P, Martin L (1990) New Miocene hominoid specimens from the Middle Miocene site at Paşalar, Turkey. J Hum Evol 19:397–422
Alpagut B, Andrews P, Fortelius M, Kappelman J, Temiszoy L, Celebi H, Lindsay W (1996) A new specimen of Ankarapithecus meteai from the Sinap Formation of central Anatolia. Nature 382:349–351
Andrews P (1971) Ramapithecus wickeri mandible from Fort Ternan, Kenya. Nature 231:192–194
Andrews P (1996) Palaeoecology and hominoid palaeoenvironments. Biol Rev 71:257–300
Andrews P, Alpagut B (2001) Functional morphology of Ankarapithecus meteai. In: de Bonis L, Koufos GD, Andrews P (eds) Hominoid evolution and climate change in Europe: vol 2 phylogeny of the Neogene hominoid primates of Eurasia. Cambridge University Press, Cambridge, pp 213–230
Andrews P, Martin L (1987) The phyletic position of the Ad Dabtiyah hominoid. Bull Br Mus (Nat Hist) Geol Ser 41:383–393
Andrews P, Hamilton WR, Whybrow PJ (1978) Dryopithecines from the Miocene of Saudi Arabia. Nature 274:249–250
Andrews P, Begun DR, Zylstra M (1997) Interrelationships between functional morphology and paleoenvironments in Miocene hominoids. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 29–58
Asfaw B, White T, Lovejoy O, Latimer B, Simpson S, Suwa G (1999) Australopithecus garhi: a new species of early hominid from Ethiopia. Science 284:629–634
Asfaw B, Gilbert WH, Beyene Y, Hart WK, Renne PR, Wolde Gabriel G, Vrba ES, White TD (2002) Remains of Homo erectus from Bouri, Middle Awash, Ethiopia. Nature 416:317–320
Balzeau A, Gilison E, Wendelen W, Coudyzer W (2009a) Internal cranial anatomy of the type specimen of Pan paniscus and available data for study. J Hum Evol 56:205–208
Balzeau A, Gilison E, Wendelen W, Coudyzer W (2009b) Erratum to ‘Internal cranial anatomy of the type specimen of Pan paniscus and available data for study’ [J Hum Evol 56 (2009) 205–208]. J Hum Evol 57:91–93
Begun D (2000) Middle Miocene hominoid origins. Science 287:2375a
Begun D (2002) European hominoids. In: Hartwig WC (ed) The primate fossil record. Cambridge University Press, Cambridge, pp 339–368
Begun DR, Gulec E (1998) Restoration of the type and palate of Ankarapithecus meteai: taxonomic and phylogenetic implications. Am J Phys Anthropol 105:279–314
Begun DR, Kivell TL (2011) Knuckle-walking in Sivapithecus? The combined effects of homology and homoplasy with possible implications for pongine dispersals. J Hum Evol 60(2):158–170
Begun DR, Kordos L (1997) Phyletic affinities and functional convergence in Dryopithecus and other Miocene and living hominids. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 291–316
Benefit BR (1991) The taxonomic status of Maboko small apes. Am J Phys Anthropol 85(Supp 12):50–51
Benefit BR, McCrossin ML (1991) Ancestral facial morphology of Old World higher primates. Proc Natl Acad Sci U S A 88:5267–5271
Benefit BR, McCrossin ML (2000) Middle Miocene hominoid origins. Science 287:2375
Biegert J (1964) The evaluation of characteristics of the skull, hands and feet for primate taxonomy. In: Washburn SL (ed) Classification and human evolution. Methuen, London, pp 116–145
Bolter DL, Zihlman AL (2012) Skeletal development in Pan paniscus with comparisons to Pan troglodytes. Am J Phys Anthropol 147:629–636
Boughner JC, Dean MC, Wilgenbusch CS (2012) Permanent tooth mineralisation in bonobos (Pan paniscus) and Chimpanzees (P. troglodytes). Am J Phys Anthropol 149:560–571
Braga J (1998) Chimpanzee variation facilitates the interpretation of the incisive suture closure in South African Plio-Pleistocene hominids. Am J Phys Anthropol 105:121–135
Breuer T, Robbins AM, Boesch C, Robbins MM (2012) Phenotypic correlates of male reproductive success in western gorillas. J Hum Evol 62:466–472
Brown B (1997) Miocene hominoid mandibles: functional and phylogenetic perspectives. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 153–171
Brown B, Ward SC (1988) Basicranial and facial topography in Pongo and Sivapithecus. In: Schwartz JH (ed) Orang-utan biology. Oxford University Press, New York, pp 247–260
Caillaud D, Levrero F, Gatti S, Menard N, Raymond M (2008) Influence of male morphology on male mating status and behavior during interunit encounters in western lowland gorillas. Am J Phys Anthropol 135:379–388
Cameron DW (1997) A revised systematic scheme for the Eurasian Miocene fossil Hominidae. J Hum Evol 33(4):449–477
Cameron DW (2001) The taxonomic status of the Siwalik late Miocene hominid Indopithecus (=‘Gigantopithecus’). Himal Geol 22:29–34
Cameron DW (2003) A functional and phylogenetic interpretation of the late Miocene Siwalik hominid Indopithecus and the Chinese Pleistocene hominid Gigantopithecus. Himal Geol 24:19–28
Chaimanee Y, Jolly D, Benammi M, Tafforeau P, Duzer D, Mousssa I, Jaeger J-J (2003) A Middle Miocene hominoid from Thailand and orangutan origins. Nature 422:61–65
Chaimanee Y, Suteethorn V, Jintasakul P, Vidthyanon C, Marandat B, Jaeger J-J (2004) A new orang-utan relative from the Late Miocene of Thailand. Nature 427:439–441
Cheverud JM (1982) Phenotypic, genetic and environmental morphological integration in the cranium. Evolution 36:499–516
Cheverud JM (1996) Developmental integration and the evolution of pleiotropy. Am Zool 36:44–50
Ciochon RE, Piperno DL, Thompson RG (1990) Opal Phytoliths found on the teeth of the extinct ape Gigantopithecus blackii: implications for paleodietary studies. Proc Natl Acad Sci U S A 87:8120–8124
Cobb SN, O’Higgins P (2004) Hominins do not share a common postnatal ontogenetic shape trajectory. J Exp Zool (Mol Dev Evol) 302B:302–321
Cobb SN, O’Higgins P (2007) The ontogeny of sexual dimorphism in the facial skeleton of the African apes. J Hum Evol 53:176–190
Conroy GC (1997) Reconstructing human origins: a modern synthesis. Norton, New York, 557 pp
Conroy GC, Pickford M, Senut B, Van Couvering J, Mein P (1992) Otavipithecus namibiensis, first Miocene hominoid from southern Africa. Nature 356:144–148
Courtney J, Groves CP, Andrews P (1988) Inter- or intra-island variation? An assessment of the differences between Bornean and Sumatran Orang-utans. In: Schwartz JH (ed) Orang-utan biology. Oxford University Press, New York, pp 19–31
Cramer DL (1977) Craniofacial morphology of Pan paniscus. Contrib Primatol 10:1–64
Cray J, Meindl RS, Sherwood CC, Lovejoy CO (2008) Ectocranial suture closure in Pan troglodytes and Gorilla gorilla: pattern and phylogeny. Am J Phys Anthropol 136:394–399
Cray J, Cooper GM, Mooney MP, Seigel MI (2010) Brief communication: ectocranial suture closure in Pongo: pattern and phylogeny. Am J Phys Anthropol 143:473–477
Creel N, Preuschoft H (1976) Cranial morphology of the lesser apes. In: Rumbaugh DM (ed) CrabGibbon and siamang, vol 4. Karger, Basel, pp 219–303
Creel N, Preuschoft H (1984) Systematics of the lesser apes: a quantitative taxonomic analysis of craniometric and other variables. In: Chivers DJ, Preuschoft H, Brockelman WY, Creel N (eds) The lesser apes. Edinburgh University Press, Edinburgh, pp 562–613
Daegling DJ (2004) Relationship of strain magnitude to morphological variation in the primate skull. Am J Phys Anthropol 124:346–352
Daegling DJ, Hylander WJ (1998) Biomechanics of torsion in the human mandible. Am J Phys Anthropol 105:73–87
Daegling DJ, Hylander WL (2000) Experimental observation, theoretical models, and biomechanical inference in the study of mandibular form. Am J Phys Anthropol 112:541–551
Davis DD (1964) The giant panda: a morphological study of evolutionary mechanisms. Fieldiana: Zool Mem 3:1–339
De Bonis L, Bouvrain G, Geraads D, Koufos GD (1992) Diversity and paleoecology of Greek late Miocene mammalian faunas. Palaeogeogr Paleoclimatol Paleoecol 91:99–121
De Bonis L, Koufos GD (1997) The phylogenetic and functional implications of Ouranopithecus macedoniensis. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 317–326
De Bonis L, Koufos GD (2001) Phylogenetic relationships of Ouranopithecus macedoniensis (Mammalia, primates, Hominoidea, Hominidae) of the late Miocene deposits of Central Macedonia (Greece). In: de Bonis L, Koufos GD, Andrews P (eds) Hominoid evolution and climatic change in Europe 2: phylogeny of the Neogene hominoid primates of Eurasia. Cambridge University Press, Cambridge, pp 254–268
Dean MC (1984) Comparative myology of the hominoid cranial base. I. The muscular relationships and bony attachments of the digastric muscle. Folia Primatol 43:234–248
Dean MC (1985) Comparative myology of the hominoid cranial base. II: The muscles of the prevertebral and upper pharyngeal region. Folia Primatol 44:40–51
Dean MC, Vesey P (2008) Preliminary observations on increasing root length during the eruptive phase of tooth development in modern humans and great apes. J Hum Evol 54:258–271
Dean MC, Wood B (1981) Metrical analysis of the basicranium of extant hominoids and Australopithecus. Am J Phys Anthropol 54:63–71
Dean MC, Wood BA (1984) Phylogeny, neoteny and growth of the cranial base in hominoids. Folia Primatol 43:157–180
Deane A (2009) First contact: understanding the relationship between hominoid incisor curvature and diet. J Hum Evol 56:263–274
Delattre A, Fenart R (1956) Analyse morphologique du splanchnocrane chez les primates et ses raports avec le prognathisme. Mammalia 20:169–323
Delattre A, Fenart R (1960) L’Hominisation du Crane. CNRS, Paris
Demes B, Preuschoft H, Wolf JEA (1984) Stress-strength relationships in the mandibles of hominoids. In: Chivers DJ, Wood BA, Bilsborough A (eds) Food acquisition and processing in primates. Plenum Press, New York, pp 369–390
Demes B, Creel N, Preuschoft H (1986) Functional significance of allometric trends in the hominoid masticatory apparatus. In: Else J, Lee P (eds) Primate evolution. Cambridge University Press, Cambridge, pp 229–237
Du Bruhl EL (1977) Early hominid feeding mechanisms. Am J Phys Anthropol 47:305–320
Durrleman S, Pennec X, Trouve A, Ayache N, Braga J (2012) Comparison of the endocranial ontogenies between chimpanzees and bonobos via temporal regression and spatiotemporal registration. J Hum Evol 62:74–88
Endo B (1966) Experimental studies on the mechanical significance of the form of the human facial skeleton. J Fac Sci Univ Tokyo (Sec 5) 3:1–106
Enlow DH (1968) The human face: an account of the postnatal growth and development of the craniofacial skeleton. Harper & Row, New York
Enlow DH (1990) Handbook of facial growth. Saunders, Philadelphia
Falk D (1983) A reconsideration of the endocast of Proconsul africanus: implications for brain evolution. In: Ciochon RL, Corruccini RS (eds) New interpretations of ape and human ancestry. Plenum Press, New York, pp 239–248
Fleagle JG, Gilbert CC, Baden AL (2010) Primate cranial diversity. Am J Phys Anthropol 142:565–578
Gagneux P, Wills C, Gerloff U, Tautz D, Morin PA, Boesch C, Fruth B, Hohlmann G, Ryder OA, Woodruff DS (1999) Mitochondrial sequences show diverse evolutionary history of African hominoids. Proc Natl Acad Sci U S A 96:5077–5082
Gebo DL, Mac Latchy L, Kityo R, Deino A, Kingston J, Pilbeam D (1997) A hominoid genus from the Early Miocene of Uganda. Science 276:401–404
Geissmann T (2002) Taxonomy and evolution of gibbons. Evol Anthropol Suppl l1:28–31
Gonder MK, Oates JF, Disotell TR, Forstner MR, Morales JC, Melnick DJ (1997) A new west African chimpanzee subspecies? Nature 388:337
Groves CP (1967) Ecology and taxonomy of the gorilla. Nature 213:890–893
Groves CP (1970) Population systematics of the gorilla. J Zool (Lond) 161:287–300
Groves CP (1971) Pongo pygmaeus. Mamm Species 4:1–6
Groves CP (1972) Systematics and phylogeny of gibbons. In: Rumbaugh D (ed) Gibbon and siamang, vol 1. Karger, Basel, pp 1–89
Groves CP (1986) Systematics of the great apes. In: Swindler DR, Erwin J (eds) Comparative primate biology, vol 1. Liss, New York, pp 187–217
Groves CP (2001) Primate taxonomy. Smithsonian Inst, Washington
Groves CP (2003) A history of gorilla taxonomy. In: Taylor AB, Goldsmith ML (eds) Gorilla biology: a multidisciplinary perspective. Cambridge University Press, Cambridge, pp 15–34
Groves CP, Westwood C, Shea BT (1992) Unfinished business: Mahalanobis and a clockwork orang. J Hum Evol 22:327–340
Harrison T (1986) A reassessment of the phylogenetic relationships of Oreopithecus bambolii Gervais. J Hum Evol 15:541–583
Harrison T (1989) A new species of Micropithecus from the middle Miocene of Kenya. J Hum Evol 18:537–557
Harrison T (2002) Late Oligocene to middle Miocene catarrhines from Afro-Arabia. In: Hartwig WC (ed) The primate fossil record. Cambridge University Press, Cambridge, pp 311–338
Harrison T, Andrews P (2009) The anatomy and systematic position of the early Miocene proconsulid from Meswa Bridge, Kenya. J Hum Evol 56(5):479–496
Harrison ME, Chivers DJ (2007) The orang-utan mating system and the unflanged male: a product of increased food stress during the late Miocene and Pliocene? J Hum Evol 52:275–293
Harrison T, Rook L (1997) Enigmatic anthropoid or misunderstood ape? The phylogenetic status of Oreopithecus bambolii reconsidered. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 327–362
Hartwig WC (2002) The primate fossil record. Cambridge University Press, Cambridge, 544 pp
Heizmann EPJ, Begun DR (2001) The oldest Eurasian hominoid. J Hum Evol 41:463–481
Hershkovitz P (1970) Cerebral fissure patterns in platyrrhine monkeys. Folia Primatol 13:213–240
Hylander W (1979a) The functional significance of primate mandibular form. J Morphol 160:223–240
Hylander W (1979b) Mandibular function in Galago crassicaudatus and Macaca fascicularis: an in vivo approach to stress analysis of the mandible. J Morphol 159:253–296
Hylander W (1984) Stress and strain in the mandibular symphysis of primates: a test of competing hypotheses. Am J Phys Anthropol 64:1–46
Ishida H, Pickford M (1997) A new late Miocene hominoid from Kenya: Samburupithecus kiptalami gen et sp nov. C R Acad Sci Paris 325:823–829
Ishida H, Kunimatsu Y, Nakatsukasa M, Nakano Y (1999) New hominoid genus from the Middle Miocene of Nachola, Kenya. Anthropol Sci 107:189–191
Ishida H, Kunimatsu Y, Takano T, Nakano Y, Nakatsukasa M (2004) Nacholapithecus skeleton from the Middle Miocene of Kenya. J Hum Evol 46:69–103
Johnson GD, Opdyke ND, Tandon SK, Nanda AC (1983) The magnetic polarity stratigraphy of the Siwalik Group at Haritalyangar (India) and a new last appearance datum for Ramapithecus and Sivapithecus. Palaeogeog Palaeoclim Palaeoecol 44:223–249
Kay RF, Simons EL (1983) A reassessment of the relationship between later Miocene and subsequent Hominoidea. In: Ciochon RL, Corruccini RS (eds) New interpretations of ape and human ancestry. Plenum Press, New York, pp 577–624
Kay RF, Ungar PS (1997) Dental evidence for diet in some Miocene Catarrhines with comments on the effects of phylogeny on the interpretation of adaptation. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 131–151
Kelley J (1993) Taxonomic implications of sexual dimorphism in Lufengpithecus. In: Kimbel WH, Martin LB (eds) Species, species concepts and primate evolution. Plenum Press, New York, pp 429–458
Kelley J (2002) The hominoid radiation in Asia. In: Hartwig WC (ed) The primate fossil record. Cambridge University Press, Cambridge, pp 369–384
Kelley J (2008) Identification of a single birth cohort in Kenyapithecus kizili and the nature of sympatry between K. kizili and Griphopithecus alpani at Paşalar. J Hum Evol 54(4):530–537
Kelley J, Pilbeam DR (1986) The dryopithecines: taxonomy, comparative anatomy and phylogeny of Miocene large hominoids. In: Swindler DR, Irwin J (eds) Comparative primate biology 1: systematics, evolution and anatomy. Liss, New York, pp 361–411
Kelley J, Qinghua X (1991) Extreme sexual dimorphism in a Miocene hominoid. Nature 352:151–153
Kelley J, Ward S, Brown B, Hill A, Downs W (2000) Middle Miocene hominid origins. Science 287:2375a
Kelley J, Andrews P, Alpagut B (2008) A new hominoid species from the Middle Miocene site of Paşalar, Turkey. J Hum Evol 54(4):455–479
King T (2001) Dental microwear and diet in Eurasian Miocene catarrhines. In: de Bonis L, Koufos GD, Andrews P (eds) Hominoid evolution and climate change in Europe. vol 2: phylogeny of the Neogene hominoid primates of Eurasia. Cambridge University Press, Cambridge, pp 102–117
Kinzey WG (1984) The dentition of the pygmy chimpanzee, Pan paniscus. In: Susman RL (ed) The pygmy chimpanzee. Evolutionary biology and behavior. Plenum Press, New York, pp 65–88
Kinzey WG (1992) Dietary and dental adaptations in the Pitheciinae. Am J Phys Anthropol 88:499–514
Kivell TL, Begun DR (2009) New primate carpal bones from Rudabánya (late Miocene, Hungary): taxonomic and functional implications. J Hum Evol 57(6):697–709
Klukkert ZS, Teaford MF, Ungar PS (2012) A dental topographic analysis of chimpanzees. Am J Phys Anthropol 148:276–284
Kordos L, Begun DR (2001) A new cranium of Dryopithecus from Rudabanya. Hungary J Hum Evol 41:689–700
Kunimatsu Y, Ishida H, Nakatsukasa M, Nakanoc Y, Sawadad Y, Nakayama K (2004) Maxillae and associated gnathodental specimens of Nacholapithecus kerioi, a large-bodied hominoid from Nachola, northern Kenya. J Hum Evol 46:365–400
Kunimatsu Y, Nakatsukasa M, Sawada Y, Sakai T, Hyodo M, Hyodo H, Itaya T, Nakaya H, Saegusa H, Mazurier A, Saneyoshi M, Tsujikawa H, Yamamoto A, Mbua E (2007) A new Late Miocene great ape from Kenya and its implications for the origins of African great apes and humans. Proc Natl Acad Sci U S A 104:19220–19225
Laitman JH, Heimbuch RC (1984) A measure of basicranial flexion in Pan paniscus, the pygmy chimpanzee. In: Susman RL (ed) The pygmy chimpanzee evolutionary biology and behavior. Plenum Press, New York, pp 49–63
Le Gros Clark WE (1962) The antecedents of man: an introduction to the evolution of the primates. Edinburgh University Press, Edinburgh
Le Gros Clark WE, Leakey LSB (1951) The Miocene Hominoidea of East Africa. Fossil mammals of Africa. Br Mus Nat Hist 1:1–117
Leakey LSB (1967) An early Miocene member of Hominidae. Nature 213:155–163
Leakey M, Walker A (1997) Afropithecus function and phylogeny. In: Begun DR, Ward CV, Rose MD (eds) Function, phylogeny, and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 225–239
Leakey MG, Leakey REF, Richtsmeier JT, Simons EL, Walker AC (1991) Similarities in Aegyptopithecus and Afropithecus facial morphology. Folia Primatol 56:65–85
Leakey MG, Ungar P, Walker A (1995) A new genus of large primate from the late Oligocene of Lothidok, Turkana District, Kenya. J Hum Evol 28:519–531
Leigh SR, Relethford JH, Park PB, Konigsberg LW (2003) Morphological differentiation of Gorilla subspecies. In: Taylor AB, Goldsmith ML (eds) Gorilla biology: a multidisciplinary perspective. Cambridge University Press, Cambridge, pp 104–131
Leslie ER (2010) A comparative analysis of internal cranial anatomy of the hylobatidae. Am J Phys Anthropol 143:250–265
Leutnegger W, Masterson TJ (1989a) The ontogeny of sexual dimorphism in the cranium of Bornean orang-utans (Pongo pygmaeus pygmaeus): I. Univariate analyses. Z Morph Anthropol 78:1–14
Leutnegger W, Masterson TJ (1989b) The ontogeny of sexual dimorphism in the cranium of Bornean orang-utans (Pongo pygmaeus pygmaeus): II. Allometry and heterochrony. Z Morph Anthropol 78:15–24
Lieberman DE, Pearson OM, Mowbray KM (2000a) Basicranial influence on overall cranial shape. J Hum Evol 38:291–315
Lieberman DE, Ross CF, Ravosa MJ (2000b) The primate cranial base: ontogeny, function, and integration. Yrbk of Phys Anthropol 43:117–169
Lieberman DE, Carlo J, Ponce de León M, Zollikofer CPE (2007) Geometric morphometric analysis of heterochrony in the cranium of chimpanzees and bonobos. J Hum Evol 52:647–662
Lockwood CA, Kimbel WH, Lynch JM (2004) Morphometrics and hominoid phylogeny: support for a chimpanzee-human clade and differentiation among great ape subspecies. Proc Natl Acad Sci U S A 101:4356–4360
Lovejoy CO, Cohn MJ, White TD (1999) Morphological analysis of the mammalian postcranium: a developmental perspective. Proc Natl Acad Sci U S A 96:13247–13252
Lovejoy CO, McCollum MA, Reno PL, Rosenman BA (2003) Developmental biology and human evolution. Ann Rev Anthropol 32:85–109
MacLatchy L (2004) The oldest ape. Evol Anthropol 13:90–104
MacLatchy L, Rossie J (2005) The Napak hominoid: still Proconsul major. In: Liebermann DE, Smith RJ, Kelley J (eds) Interpreting the past. Brill Academic Publishers, Boston, pp 15–28
Manser J, Harrison T (1999) Estimates of cranial capacity and encephalization in Proconsul and Turkanapithecus. Am J Phys Anthropol 108(Suppl 28):189
Marshall J, Sugardjito J (1986) Gibbon systematics. In: Swindler DR, Erwin J (eds) Comparative primate biology 1: systematics, evolution and anatomy. Liss, New York, pp 137–185
Martin L, Andrews P (1984) The phyletic position of Graecopithecus freybergi Koenigswald. Cour Forschung Inst Senckenberg 69:25–40
McBrearty S, Jablonski NG (2005) First fossil chimpanzee. Nature 437:105–108
McCollum MA (1997) Palatal thickening and facial form in Paranthropus: examination of alternative developmental models. Am J Phys Anthropol 103:375–392
McCollum MA (1999) The robust australopithecine face: a morphogenetic perspective. Science 284:301–305
McCollum MA (2007) Rethinking incisor size and diet in anthropoids: diet, incisor wear and incisor breadth in the African apes. Am J Phys Anthropol 133:986–993
McCollum MA, Sharpe PT (2001) Developmental genetics and early hominid craniodental evolution. Bioessays 23:481–493
McCollum MA, Ward SC (1997) Subnasoalveolar anatomy and hominoid phylogeny: evidence from comparative ontogeny. Am J Phys Anthropol 102:377–405
McCrossin ML, Benefit BR (1997) On the relationships and adaptations of Kenyapithecus a large-bodied hominoid from the Middle Miocene of eastern Africa. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 241–267
McNulty KP (2004) A geometric morphometric assessment of hominoid crania: conservative African apes and their liberal implications. Ann Anat-Anat Anz 186:429–433
Mootnick A, Groves C (2005) A new generic name for the Hoolock gibbon (Hylobatidae). Int J Primatol 26(4):971–976
Moss ML (1973) A functional cranial analysis of primate craniofacial growth. Symp IV Int Cong Primatol 3:191–208
Moss ML, Young RW (1960) A functional approach to craniology. Am J Phys Anthropol 18:281–292
Moyà-Solà S, Köhler M, Alba DM, Casanovas-Vilar I, Galindo J (2004) Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science 306:1339–1344
Moyà-Solà S, Köhler M (1996) A Dryopithecus skeleton and the origins of great-ape locomotion. Nature 379:156–159
Moyà-Solà S, Alba DM, Almécija S, Casanovas-Vilar I, Köhler M, de Esteban-Trivigno S, Robles JM, Galindo J, Fortuny J (2009a) A unique Middle Miocene European hominoid and the origins of the great ape and human clade. Proc Natl Acad Sci U S A 106:9601–9606
Moyà-Solà S, Köhler M, Alba DM, Casanovas-Vilar I, Galindo J, Robles JM, Cabrera L, Garces M, Almecija S, Beamud E (2009b) First partial face and upper dentition of the Middle Miocene hominoid Dryopithecus fontani from Abocador de Can Mata (Valles-Penedes Basin, Catalonia, NE Spain): taxonomic and phylogenetic implications. Am J Phys Anthropol 139(2):126–145
Nakatsukasa M, Kunimatsu Y (2009) Nacholapithecus and its importance for understanding hominoid evolution. Evol Anthropol 18:103–119
Napier JR, Davis PR (1959) The forelimb and associated remains of Proconsul africanus. Fossil Mamm Afr (Br Mus Nat Hist, London) 16:1–69
Neubauer S, Gunz P, Schwarz U, Hublin J-J, Boesch C (2012) Brief communication: endocranial volumes in an ontogenetic sample of chimpanzees from the Taï Forest National Park, Ivory Coast. Am J Phys Anthropol 147:319–325
Nishimura T, Mikami A, Suzuki J, Matsuzawa T (2006) Descent of the hyoid in chimpanzees: evolution of face flattening and speech. J Hum Evol 51:244–254
O’Higgins P, Dryden IL (1993) Sexual dimorphism in hominoids: further studies of craniofacial shape differences in Pan, Gorilla and Pongo. J Hum Evol 24:189–205
Olejniczak AJ, Begun DR, Mbua E, Hublin J-J (2009) Phyletic affinities of Samburupithecus kiptalami: a late Miocene proconsulid. Am J Phys Anthropol Sup 48:202–202
Ozansoy F (1965) Etude des gisements continentaux et des mammiferes du Cenozoique de Turquie. Mem de la Soc geol de France (ns) 44:1–92
Patel BA, Susman RL, Rossie JB, Hill A (2009) Terrestrial adaptations in the hands of Equatorius africanus revisited. J Hum Evol 57(6):763–772
Pérez de los Ríosa M, Moyà-Solà S, Alba DM (2012) The nasal and paranasal architecture of the Middle Miocene ape Pierolapithecus catalaunicus (Primates: Hominidae): phylogenetic implications. J Hum Evol 63(3):497–506
Pickford M, Moyà-Solà S, Köhler M (1997) Phylogenetic implications of the first African Middle Miocene hominoid frontal bone from Otavi, Namibia. C R Acad Sci Paris Earth & Planet Sci 325:459–466
Pickford M, Senut B, Gommery D (1999) Sexual dimorphism in Morotopithecus bishopi, and early Middle Miocene hominoid from Uganda, and a reassessment of its geological and biological contexts. In: Andrews P, Banham P (eds) Late Cenozoic environments and hominid evolution: a tribute to Bill Bishop. Geological Society, London, pp 27–38
Pickford M, Coppens Y, Senut B, Morales J, Braga J (2009a) Late Miocene hominoid from Niger. C R Palevol 8:413–425
Pickford M, Senut B, Gommery D, Musiime E (2009b) Distinctiveness of Ugandapithecus from Proconsul. Estud Geol 65(2):183–241
Pilbeam DR (1969) Tertiary Pongidae of East Africa: evolutionary relationships and taxonomy. Bull Peabody Mus Nat Hist (Yale Univ) 31:1–185
Pilbeam DR (1982) New hominoid skull material from the Miocene of Pakistan. Nature 295:232–234
Pilbeam DR (2002) Perspectives on the Miocene Hominoidea. In: Hartwig WC (ed) The primate fossil record. Cambridge University Press, Cambridge, pp 303–310
Pilbeam DR, Meyer GE, Badgley C, Rose MD, Pickford MHL, Behrensmeyer AK, Shah SMI (1977) New hominoid primates from the Siwaliks of Pakistan and their bearing on hominid evolution. Nature 270:689–695
Pilbrow V (2006) Population systematics of chimpanzees using molar morphometrics. J Hum Evol 51:646–662
Pilbrow V (2010) Dental and phylogeographic patterns of variation in gorillas. J Hum Evol 59:16–34
Pillans B, Williams M, Cameron D, Patnaik R, Hogarth J, Sahni A, Sharma JC, Williams F, Bernor RL (2005) Revised correlation of the Haritalyangar magneto stratigraphy, Indian Siwaliks: implications for the age of Miocene hominids Indopithecus and Sivapithecus, with a note on a new hominid tooth. J Hum Evol 48:507–515
Poe DJ (2011) Brief communication: testing the usefulness of the basilar suture as a means to determine age in great ape skeletons. Am J Phys Anthropol 144:162–165
Pradhan GR, van Noordwijk MA, van Schaik C (2012) A model for the evolution of developmental arrest in male orangutans. Am J Phys Anthropol 149:18–25
Preuschoft H, Demes B, Meyer M, Bar HF (1986) The biomechanical principles realised in the upper jaw of long-snouted primates. In: Else JC, Lee PC (eds) Primate evolution. vol 1 proc tenth congress Int Primatol Society. Cambridge Univ Press, Cambridge, pp 249–264
Radinsky LB (1974) The fossil evidence of anthropoid brain evolution. Am J Phys Anthropol 41:15–28
Rae T (1993) Phylogenetic analysis of proconsulid facial morphology. Ph.D. Dissertation, S.U.N.Y. at Stony Brook
Rae T (1997) The early evolution of the hominoid face. In: Begun D, Ward C, Rose M (eds) Function, phylogeny, and fossils: Miocene hominoid evolution and adaptations. Plenum, New York, pp 59–77
Rae T (1999) Mosaic evolution in the origin of the Hominoidea. Folia Primatol 70:125–135
Rae TC (2004) Miocene hominoid craniofacial morphology and the emergence of great apes. Ann Anat-Anat Anz 186:417–422
Rae T, Koppe T (2000) Isometric scaling of maxillary sinus volume in hominoids. J Hum Evol 38:411–423
Rak Y (1983) The australopithecine face. Academic, New York, 169 pp
Raven HC (1950) Regional anatomy of the gorilla. In: Gregory WK (ed) The anatomy of the gorilla. Columbia, New York, pp 15–188
Ravosa MJ (2000) Size and scaling in the mandible of living and extinct apes. Folia Primatol 71:305–322
Robinson JT (1958) Cranial cresting patterns and their significance in the Hominoidea. Am J Phys Anthropol 16:397–428
Robinson C (2012) Geometric morphometric analysis of mandibular shape diversity in Pan. J Hum Evol 63:191–204
Röhrer-Ertl O (1988a) Research history, nomenclature, and taxonomy of the orang-utan. In: Schwartz JH (ed) Orang-utan biology. Oxford, New York, pp 7–18
Röhrer-Ertl O (1988b) Cranial growth. In: Schwartz JH (ed) Orang-utan biology. Oxford, New York, pp 201–224
Ross C, Henneberg MJ (1995) Basicranial flexion, relative brain size and facial kyphosis in Homo sapiens and some fossil hominids. Am J Phys Anthropol 98:575–593
Ross C, Ravosa MJ (1993) Basicranial flexion, relative brain size and facial kyphosis in non-human primates. Am J Phys Anthropol 91:305–324
Rossie JB (2005) Anatomy of the nasal cavity and paranasal sinuses in Aegyptopithecus and early Miocene African catarrhines. Am J Phys Anthropol 126:250–267
Ryan TM, Silcox MT, Walker A, Mao XY, Begun DR, Benefit BR, Gingerich PD, Köhler M, Kordos L, McCrossin ML, Moyà-Solà S, Sanders WJ, Seiffert ER, Simons E, Zalmout IS, Spoor F (2012) Evolution of locomotion in Anthropoidea: the semicircular canal evidence. Proc R Soc B 279(1742):3467–3475
Sakka M (1984) Cranial morphology and masticatory adaptations. In: Chivers DJ, Wood BA, Bilsborough A (eds) Food acquisition and processing in primates. Plenum, New York, pp 415–427
Sarmiento EE, Oates JF (1999) Cross river gorillas: a neglected subspecies. Gorilla J 19:1–2
Sarmiento EE, Oates JF (2000) The Cross River gorillas: a distinct subspecies, Gorilla gorilla diehli Matschie 1904. Am Mus Novit 3304:1–55
Schultz AH (1940) The size of the orbit and of the eye in primates. Am J Phys Anthropol 26:389–408
Schultz AH (1950) Morphological observations on gorillas. In: Gregory WK (ed) The anatomy of the gorilla. Columbia, New York, pp 227–254
Schwartz JH (1988) History, morphology, paleontology, and evolution. In: Schwartz JH (ed) Orang-utan biology. Oxford, New York, pp 69–85
Schwartz JH (1997) Lufengpithecus and hominoid phylogeny. Problems in delineating and evaluating phylogenetically relevant characters. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum, New York, pp 363–388
Seiffert ER, Kappelman J (2001) Morphometric variation in the hominoid orbital aperture: a case study with implications for the use of variable characters in Miocene catarrhine systematics. J Hum Evol 40:301–318
Senut B, Pickford M, Gommery D, Kunimatsu Y (2000) A new genus of Early Miocene hominoid from East Africa: Ugandapithecus major (Le Gros Clark & Leakey, 1950). C R Acad Sci Paris 331:227–233
Shea BT (1983a) Paedomorphosis and neoteny in the pygmy chimpanzee. Science 222:521–522
Shea BT (1983b) Size and diet in the evolution of African ape cranio-dental form. Folia Primatol 40:32–68
Shea BT (1983c) Allometry and heterochrony in the African apes. Am J Phys Anthropol 62:275–289
Shea BT (1983d) Phyletic size change and brain/body allometry: a consideration based on the African pongids and other primates. Int J Primatol 4:34–62, *should be 1983a
Shea BT (1984) An allometric perspective on the morphological and evolutionary relationships between Pygmy (Pan paniscus) and Common (Pan troglodytes) Chimpanzees. In: Susman RL (ed) The pygmy chimpanzee: evolutionary biology and behavior. Plenum, New York, pp 89–130
Shea BT (1985) On aspects of skull form in African apes and orangutans, with implications for hominoid evolution. Am J Phys Anthropol 68:329–342
Shea BT (1988) Phylogeny & skull form in the hominoid primates. In: Schwartz JH (ed) Orang-utan biology. Oxford Univ Press, New York, pp 233–246
Shea BT, Coolidge HJ (1988) Craniometric differentiation and systematics in the genus Pan. J Hum Evol 17:671–685
Shea BT, Leigh SR, Groves CP (1993) Multivariate craniometric variation in chimpanzees. Implications for species identification in paleoanthropology. In: Kimbel WH, Martin LB (eds) Species, species concepts and primate evolution. Plenum, New York, pp 265–296
Sherwood RJ, Ward S, Hill A, Duren DL, Brown B, Downs W (2002) Preliminary description of the Equatorius africanus partial skeleton (KNM-TH 28860) from Kipsaramon, Tugen Hills, Baringo District, Kenya. J Hum Evol 42:63–73
Singh N, Harvati K, Hublin J-J, Klingenberg CP (2012) Morphological evolution through integration: a quantitative study of cranial integration in Homo, Pan, Gorilla and Pongo. J Hum Evol 62:155–164
Singleton M (2003) Functional and phylogenetic implications of molar flare variation in Miocene hominoids. J Hum Evol 45:57–79
Skinner MM, Gunz P, Wood BA, Boesch C, Hublin J-J (2009) Discrimination of extant Pan species and subspecies using the enamel-dentine junction morphology of lower molars. Am J Phys Anthropol 140:234–243
Smith BH, Boesch C (2011) Mortality and the magnitude of the ‘wild effect’ in chimpanzee tooth emergence. J Hum Evol 60:34–46
Smith TM, Reid DJ, Dean MC, Olejniczak AJ, Martin LB (2007) Molar development in common chimpanzees (Pan troglodytes). J Hum Evol 52:201–216
Smith TM, Smith BH, Reid DJ, Siedel L, Vigilant L, Hublin J-J, Boesch C (2010) Dental development of the Tai Forest chimpanzees revisited. J Hum Evol 58:363–373
Steiper ME (2006) Population history, biogeography, and taxonomy of orangutans (Genus: Pongo) based on a population genetic meta-analysis of multiple loci. J Hum Evol 50:509–522
Stevens NJ, Seiffert ER, O’Connor PM, Roberts EM, Schmitz MD, Krause C, Gorscak E, Ngasala S, Hieronymus TL, Temu J (2013) Palaeontological evidence for an Oligocene divergence between Old World monkeys and apes. Nature 497(7451):611–614
Stewart C-B, Disotell TR (1998) Primate evolution-in and out of Africa. Curr Biol 8:582–588
Stumpf RM, Fleagle JG, Jungers WL, Oates JF, Groves CP (1998) Morphological distinctiveness of Nigerian gorilla crania. Am J Phys Anthropol Suppl 26:213
Stumpf RM, Polk JD, Oates JF, Jungers WL, Heesy CP, Groves CP, Fleagle JG (2003) Patterns of diversity in gorilla cranial morphology. In: Taylor AB, Goldsmith ML (eds) Gorilla biology: a multidisciplinary perspective. Cambridge University Press, Cambridge, pp 35–61
Susman RL (1984) Pan paniscus, the pygmy chimpanzee. In: Susman RL (ed) The pygmy chimpanzee evolutionary biology and behavior. Plenum Press, New York, pp 49–63
Suwa G, Asfaw B, Beyene Y, White TD, Katoh S, Nagaoka S, Nakaya H, Uzawa K, Renne P, Wolde Gabriel G (1997) The first skull of Australopithecus boisei. Nature 389:489–492
Suwa G, Kono RT, Katoh S, Asfaw B, Beyene Y (2007) A new species of great ape from the late Miocene epoch in Ethiopia. Nature 448:921–924
Swarts JD (1988) Deciduous dentition: implications for hominoid phylogeny. In: Schwartz JH (ed) Orang-utan biology. Oxford University Press, New York, pp 263–270
Swindler DR (1976) Dentition of living primates. Academic, London, 308 pp
Swindler DR, Olshan AF (1988) Comparative and evolutionary aspects of permanent dentition. In: Schwartz JH (ed) Orang-utan biology. Oxford University Press, New York, pp 271–282
Swindler DR, Wood CD (1973) An atlas of primate gross anatomy. Baboon, chimpanzee, and man. University of Washington Press, Seattle
Szalay F, Delson E (1979) Evolutionary history of the primates. Academic, New York
Taylor AB (2002) Masticatory form and function in the African apes. Am J Phys Anthropol 117:133–156
Taylor AB (2003) Ontogeny and function of the masticatory complex in Gorilla: functional, evolutionary, and taxonomic implications. In: Taylor AB, Goldsmith ML (eds) Gorilla biology: a multidisciplinary perspective. Cambridge University Press, Cambridge, pp 132–193
Taylor AB (2006) Feeding behavior, diet and the functional consequences of jaw form in orangutans, with implications for the evolution of Pongo. J Hum Evol 50:377–393
Taylor AB, Groves CP (2003) Patterns of mandibular variation in Pan and Gorilla and implications for African ape taxonomy. J Hum Evol 44:529–561
Taylor AB, Schaik ABv (2007) Variation in brain size and ecology in Pongo. J Hum Evol 52:59–71
Taylor AB, Vinyard CJ (2013) The relationships among jaw-muscle fiber architecture, jaw morphology, and feeding behavior in extant apes and modern humans. Am J Phys Anthropol 151:120–134
Taylor AB, Vogel ER, Dominy NJ (2008) Food material properties and mandibular load resistance abilities in large-bodied hominoids. J Hum Evol 55:604–616
Teaford MF, Ungar PS (2000) Diet and the evolution of the earliest human ancestors. Proc Natl Acad Sci U S A 97:13506–13511
Terhune CE (2012) Dietary correlates of temporomandibular morphology in the great apes. Am J Phys Anthropol 150:260–276
Uchida A (1998a) Variation in tooth morphology of Gorilla gorilla. J Hum Evol 34:55–70
Uchida A (1998b) Variation in tooth morphology of Pongo pygmaeus. J Hum Evol 34:71–79
Ungar PS (1996) Dental microwear of European Miocene catarrhines: evidence for diets and tool use. J Hum Evol 30:335–366
Ungar PS (1998) Dental allometry, morphology and wear as evidence for diet in fossil primates. Evol Anthropol 6:205–217
Ungar PS, Kay RF (1995) The dietary adaptations of European Miocene catarrhines. Proc Natl Acad Sci U S A 92:5479–5481
Ungar PS, Teaford MF, Kay RF (2004) Molar microwear and shearing crest development in Miocene catarrhines. Anthropologie 42(1):21–35
Vidarsdottir US, Cobb S (2004) Inter- and intra-specific variation in the ontogeny of the hominoid facial skeleton: testing assumptions of ontogenetic variability. Ann Anat 186:423–428
von Koenigswald GHR (1952) Gigantopithecus blacki von Koenigswald, a giant fossil hominoid from the Pleistocene of southern China. Anthropol Pap Am Mus Nat Hist 43:292–425
von Koenigswald GHR (1972) Ein Unterkiefer eines fossilen Hominoiden aus dem Unterpliozän Griechenlands. Proc K Ned Akad Wet Amst B 75:385–394
Walker A (1997) Proconsul function and phylogeny. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 209–224
Walker A, Andrews P (1973) Reconstruction of the dental arcades of Ramapithecus wickeri. Nature 244:313–314
Walker A, Teaford MF (1989) The hunt for Proconsul. Sci Am 260:76–82
Walker A, Teaford MF, Martin L, Andrews P (1993) A new species of Proconsul from the early Miocene of Rusinga/Mfangano Islands, Kenya. J Hum Evol 25:43–56
Ward S (1997) The taxonomy and phylogenetic relationships of Sivapithecus revisited. In: Begun D, Ward CV, Rose MD (eds) Function, phylogeny and fossils: Miocene hominoid evolution and adaptations. Plenum Press, New York, pp 269–290
Ward S, Duren DL (2002) Middle and late Miocene African hominoids. In: Hartwig WC (ed) The primate fossil record. Cambridge University Press, Cambridge, pp 385–387
Ward S, Kimbel W (1983) Subnasal alveolar morphology and the systematic position of Sivapithecus. Am J Phys Anthropol 61:157–171
Ward S, Pilbeam D (1983) Maxillofacial morphology of Miocene hominoids from Africa and Indo-Pakistan. In: Ciochon R, Corruccini R (eds) New interpretations of ape and human ancestry. Plenum Press, New York, pp 211–238
Ward S, Brown B, Hill A, Kelley J, Downs W (1999) Equatorius: a new hominoid genus form the Middle Miocene of Kenya. Science 285:1382
Weidenreich F (1945) Giant early man from Java and South China. Anthropol Pap Am Mus Nat Hist 40:1–134
Winkler LA (1988) Variation in the suboccipital anatomy. In: Schwartz JH (ed) Orang-utan biology. Oxford University Press, New York, pp 191–200
Winkler LA, Conroy GC, Vannier MW (1988) Sexual dimorphism in exocranial and endocranial dimensions. In: Schwartz JH (ed) Orang-utan biology. Oxford University Press, New York, pp 225–232
Wolff JEA (1984) A theoretical approach to solve the chin problem. In: Chivers DJ, Wood BA, Bilsborough A (eds) Food acquisition and processing in primates. Plenum Press, New York, pp 391–406
Wood Jones F (1929) Man’s place among the mammals. Arnold, London
Zihlman A (1984) Body build and tissue composition in Pan paniscus and Pan troglodytes, with comparisons to other hominoids. In: Susman RL (ed) The pygmy chimpanzee evolutionary biology and behavior. Plenum Press, New York, pp 49–63
Zihlman AL, Stahl D, Boesch C (2008) Morphological variation in adult chimpanzees (Pan troglodytes verus) of the Tai National Park, Cote d’Ivoire. Am J Phys Anthropol 135:34–41
Zingeser MR (1973) Dentition of Brachyteles arachnoides with reference to alouattine and ateline affinities. Folia Primatol 20:351–390
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Bilsborough, A., Rae, T.C. (2015). Hominoid Cranial Diversity and Adaptation. In: Henke, W., Tattersall, I. (eds) Handbook of Paleoanthropology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39979-4_35
Download citation
DOI: https://doi.org/10.1007/978-3-642-39979-4_35
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39978-7
Online ISBN: 978-3-642-39979-4
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences