Abstract
The yeast Yarrowia lipolytica has the ability to grow on substrates such as lipid or grease. The first step in the breakdown and metabolism of those hydrophobic substrates requires the actions of lipolytic enzymes. Lipases from Y. lipolytica, and particularly the lip2 enzyme, possess amazing properties in both aqueous and nonaqueous media. Those catalytic properties have enabled the development of many applications. Oil mill wastewater represents a serious risk of pollution due to its high contents of organic compounds, mainly fatty acids and triglycerides. Besides the physicochemical processes developed to treat those wastewaters, the Lip2p lipase was found as an attractive and low-cost biological alternative. In that field, they were also used successfully to degrade sludge for grease trap from the food industries. Applications were also developed in fine chemistry due to the abilities of Lip2p as an enantioselective catalyzer in organic medium for the synthesis of pharmaceutical drug used as a single enantiomer. Besides this, Lip2p was also found effective in the polymerization and modification processes of different fatty compounds of industrial interest. Microorganisms and their produced enzymes are also known for their role in traditional food making. The role of the lipase enzyme from Y. lipolytica in flavor development in cheese and fermented sausage is detailed in this review. Their involvement in the production of organic acids such as citric acid from hydrophobic compounds is also presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chemical Oxygen Demand
- Biochemical Oxygen Demand
- Lipase Production
- Olive Mill Wastewater
- Citric Acid Production
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The yeast Yarrowia lipolytica is often associated with proteinaceous or hydrophobic substrates such as lipid or grease. To assimilate these hydrophobic substrates, Y. lipolytica has developed an adaptative strategy in which lipolytic enzymes play a crucial role. Besides their physiological functions, the amazing catalytic properties of lipases have enabled the development of many industrial and/or environmental processes. The main applications developed with Y. lipolytica lipase will be discussed in this review. Of these, the first corresponds to the treatment of waste and sludge from the agro-food industry. The second leads to fine chemical production and enantioseparation of pharmaceutical compounds by lipase in aqueous or aqueous-free medium. The third area of application refers to traditional food manufacturing, while the last one is linked to the production of metabolites such as citric acid from hydrophobic substrates.
2 Waste Treatment
Many food industries generate in their process large amount of by-products with a high content of organic compounds that must be treated before their release. Nowadays, more ecological solutions are developed to substitute physicochemical process to degrade those by-products. Alternative applications involving the lipase from Y. lipolytica for the treatment of those wastes are presented in this section.
2.1 Treatment of Oil Mill Effluents
Wastewaters from the olive oil processing industry may cause severe pollution especially in the Mediterranean countries which account for about 95 % of the world olive production (Al-Malah et al. 2000). Olive mill wastewater (OMW) represents an annual volume of 3 × 107 m3 which is produced in a short period of time (from November to late February) (D’Annibale et al. 1998, 2004). The quality and the quantity of the different constituents of OMW are dependent on several factors: the type of olives, the cultivation system, or the production process (Lopes et al. 2009). OMW forms a dark acidic stable emulsion emitting a strong oily smell. It is composed of olive “vegetation waters,” waters from processing, olive pulp, and oil. OMW contains, in addition to fat and triglycerides, sugars, phosphate, polyphenols, polyalcohols, pectins, and metals. Another negative property of OMW is its extremely high organic content. Generally, OMW has a biochemical oxygen demand (BOD) ranging between 12,000 and 63,000 mg/l and a chemical oxygen demand (COD) value between 80,000 and 200,000 mg/l (Al-Malah et al. 2000). These values are around 200 to 400 times higher than typical municipal sewage (Cossu et al. 1993). Hence, OMW represents a serious source of water pollution if released into local rivers without any treatment. Several physicochemical processes, including simple evaporation, reverse osmosis, or ultrafiltration, have been proposed to reduce the polluting effect of OMW (Mameri et al. 2000; Al-Malah et al. 2000). Besides these, biological degradation is considered as a safe, effective, and low-cost process of removing harmful pollutants. For OMW, the conventional anaerobic systems of waste treatment have been experimented with. Although it yields to a methane production of 57 l/l of OMW, anaerobic cultures require an adaptation period of 15–25 days, which increase consequently the costs of storage (Andreoni et al. 1993). Therefore, several researches aimed to select microorganisms that are able to grow more rapidly using OMW as primary carbon source. In the early 1960s, Ros de Ursinos (1961) selected a strain of Torulopsis utilis that is able to grow on OMW that produced proteins and vitamins. Pilot experiments with this yeast yielded a 50 % in pollution. More recently, Scioli and De Felice (1993) tested the ability of different yeast species to grow on oil mill wastewaters and selected Y. lipolytica as the most adapted organism to grow in these conditions (Table 1). This yeast is particularly well suited to these applications since it has a GRAS status, limited nutritional requirements, and a capacity of adaption to stringent environmental conditions. In addition, Y. lipolytica is known to produce several different lipases with specific catalytic properties, indispensable for lipid and fat metabolization. The Y. lipolytica strain ATCC20255 isolated by Scioli and De Felice (1993) was found capable of reducing the initial COD value (146 g/l) by 80 % within 24 h in a 3.5-l tank fermenter with an increase of its biomass to 23 g/l despite the presence of large amount of phenols (200 mg/l). The production of lipase (770 U/l after 24 h), free and cell bound, could be detected and correlated to the fat degradation. At the same time, Scioli and Vollaro (1997), who obtained similar results with strain ATCC20255, also reported that after processing, the waters had a pleasant smell and did not exhibit the initial oily aspect and intense smell. With the aim to be more exhaustive, Lanciotti et al. (2005) tested the ability of 62 different Y. lipolytica isolates to grow in non-diluted and non-supplemented OMW and to reduce its COD level (Table 1). PO1 strain was found the most effective to significantly reduce both the COD (43 %) and the polyphenol (18 %) content. Other strains were found to produce citric acid or lipase at a high level in these conditions. From this work, it appears that among the different strains tested, a great variability in lipase activity occurred both in the yield of production and in the enzyme specificity. Besides this, Y. lipolytica ACA-DC-50109 cultivated on OMW-based media enriched with commercial-industrial glucose was found to present a citric acid production yield of 28.9 g/l (Papanikolaou et al. 2008) (Table 1). All of these suggest that the lipid content of OMW could be metabolized, upon the action of lipase, to synthesize valuable metabolites. Lopes et al. (2009) investigated OMW-based medium supplementation with either nitrogen (ammonium) or surfactant (Tween 80) on cell growth, COD, and phenol reduction as well as lipase synthesis. They showed that the lipase productivity was improved in the presence of ammonium sulfate (6 g/l), whereas an addition of Tween 80 had a negative effect on lipase activity and a significant positive effect on both cell growth and COD reduction. With the same Y. lipolytica strain (W29), Goncalves et al. (2009) reported a lipase production of 3,500 U/l in the same supplemented medium, showing that this particular strain could be used for the scale up of lipase production from OMW (Table 1).
All those applications for the treatment of OMW were first developed exclusively with free cells. However, Wu et al. (2009) recently demonstrated that the immobilization of Y. lipolytica cells by calcium alginate could present several advantages in the treatment of wastewater from the oil industry (Table 1). These authors reported a COD degradation rate significantly higher for immobilized cells than for free cells. They hypothesized to explain this phenomenon, that for a certain period, the alginate matrix acted as oil and COD adsorbent as well as immobilized carrier. The immobilization of cells also permitted a wider temperature range for COD reduction. Indeed, the suitable temperature for oil degradation by free and immobilized cells range from 25 to 30 °C and 25 to 35 °C, respectively, which is close to the optimal catalytic temperature of the main extracellular lipase Lip2p from Y. lipolytica CBS6303 (Destain et al. 1997). From a technological viewpoint, reuse of the immobilized cells is of great advantage. Indeed, this practice can decrease waste of cells, save time, and cut down cultivation costs. For an artificial oil wastewater containing 20 % of oil, immobilized cells could be reused for a maximum of 12 cycles for a total oil degradation of 2,351 mg. Even after the 12 utilizations, a 77 % degradation of the oil content could be obtained. However, for the COD reduction, the immobilized cells could only be reused for six cycles with a total COD degradation of 1,745 mg without any loss of the degradation capacity (Wu and Wan 2008). These authors also investigated the storage stability of oil degradation activity. They observed that the oil degradation was not significantly altered upon storage of both immobilized and free cells in distilled water at 4 °C.
Palm oil mill effluent (POME) is another possible source of inland water pollution. POME composition is somewhat different to OMW. It contains mainly lignocellulosic wastes with a mixture of carbohydrates and oil. POME also presents very high BOD and COD values of 246,000 and 11,000 mg/l, respectively (Oswal et al. 2002). In addition, incomplete extraction of palm oil from the palm nut could also lead to a substantial increase of COD values. POME treatment using the Y. lipolytica marine strain NCIM 3589 was investigated without any addition of nutriment or dilution (Table 1). This strain, known for its efficient lipase production, yielded to a 97 % and 80 % reduction of the COD and BOD, respectively, within 48 h. These results are comparable to those obtained previously with a pond treatment system (Chin et al. 1996). Oswal et al. (2002) reported that the initial acidic pH of POME became alkaline after treatment with Y. lipolytica probably due to the utilization of fatty acids present in the raw POME by this yeast.
2.2 Treatment of Sewage Sludge from the Food Industry
The food industries, especially those involved in ready-to-eat meal manufacturing, generate sewage sludge with high lipid contents and high COD values. Similarly to oil mill effluent, this waste must be treated before its release into nature. This is usually carried out through a stepwise process. Of these, one is constituted by a grease trap where lipids and grease could accumulate based on their relative low density (Ansenne et al. 1992). Sludge from this grease trap is acidic (pH 4.5–5.5) and contains a dry weight content ranging from 25 to 75 % and a COD value close to 4,000 mg/l. The dry matter is composed of up to 90 % of lipid and grease, mainly triglyceride, free fatty acid, and sterol ester (Thonart et al. 1997). Such effluents were used to grow different yeast and bacteria strains selected for their ability to grow on hydrophobic substrates and to secrete large amount of hydrolases, including lipases. From those experiments, a Y. lipolytica strain producing a large amount of the extracellular Lip2p lipase was selected. This strain was tested for its ability to reduce the lipid contents in a 6-m3 grease trap fed continuously with fresh sewage at a flow rate of 6 m3 every 24 h. This led to a significant reduction of the lipid content and the maintaining of the COD at a value of 3,000 mg/l during 33 weeks of treatments (Thonart et al. 1997).
In Asia and especially in Japan, where seafood is consumed at a high level per capita, the amount of the fish waste was estimated to be more than two million tons per year, and half is discarded as industrial waste (Hirai 2001). Fish waste mainly consists of viscera, heads, and fish bones as well as fish that are too small to be processed. Some of these wastes have been utilized for the production of condiments and valuable materials (Saito 2004). However, such kinds of processing often produce new waste in addition to the product. Fishmeal is widely used as a supplementary protein source for livestock and culturing fish. Processing of fish waste to fishmeal appeared as the best way to utilize this waste. However, as fish waste contains viscera, it has a relatively high lipid and a low protein content that confers on it a poor nutritive quality. Thus, the reduction of this lipid content could permit the utilization of fish waste for the production of fishmeal. In that context, different microorganisms were screened for their ability to metabolize lipids in fish samples without any additional materials. Yano et al. (2008) isolated the Y. lipolytica strain NRBC-10073 that was able to reduce the lipid content of minced anchovy samples by 30 % without any modification of the proteic composition. The lipid reduction by this strain was especially affected by the ratio of the surface area to the weight in the fermented minced samples and by the water content, suggesting the importance of the oxygen supply in the process. Hence, this work demonstrates that the fermentation by Y. lipolytica can improve the quality of fishmeal from fish waste.
Besides this, Dominguez et al. (2005) reported the utilization of waste cooking oil for lipase production by a Y. lipolytica strain. After 3 days of treatment, a biodegradation degree of 80 % was obtained, as indicated by the COD value, with an important lipase production. The high hydrolytic activity towards medium-chain-length ester hinted at the occurrence of both lipases and esterases in this process.
3 Fine Chemistry
The demand for compounds synthesized as a single enantiomer has increased during the last years. In that context, the utilization of enzymes, which enable stereoselective reaction, as catalyzers was found as an attractive alternative to the classical chiral chemistry. Applications involving the lipase from Y. lipolytica were developed recently for the resolution of racemic mixture and in the polymerization and modification of fat.
3.1 Resolution of Racemic Mixture
In recent years, the synthesis or resolution of optically active drugs and their intermediates has been intensively investigated due to the medical benefit of using single enantiomer. There are numerous examples in which the desired biological activity solely resides in one enantiomer of a chiral drug, with the other isomer being less potent, inactive, or even acting with cross-purpose effects (Tsai et al. 1997). Hence, the need for enantiomerically pure molecules, especially in the pharmaceutical industry, has grown since the legislation required investigations into the pharmacological effect of both enantiomers. The mark of drugs sold as single enantiomer has more than a 10 % growth per year and represented around $225 billion worldwide in 2005 (Bordes et al. 2009). Since classical ways to obtain pure enantiomer, i.e., chemical asymmetric synthesis, stereoselective crystallization, or chiral chromatography, are often expensive, the use of enzymes as catalyzers becomes very attractive. In that context, the extracellular lipase Lip2p from Y. lipolytica was found to be very effective for the resolution of 2-halogeno-carboxylic acids which are important intermediates in the synthesis pathways of a number of drugs (analgesics, prostaglandin, prostacyclin, semisynthetic penicillin) (Fig. 1). The resolution of 2-bromo-p-tolyacetic acid ethyl ester catalyzed by Y. lipolytica lipase Lip2p showed an (S)-enantiopreference of 28, which is similar to the best results obtained with the lipase from Burkholderia cepacia (E = 30). Moreover, Y. lipolytica lipase presents a higher catalytic activity and an (S)-enantiopreference, while B. cepacia lipase is (R)-enantiomer selective (Guieysse et al. 2004). For the 2-bromo-o-tolylacetic acid, a precursor of analgesics and non-peptide angiotensin II receptor antagonists, none of the commercial lipase was able to resolve the racemic mixture (Guieysse et al. 2003a). Only the Y. lipolytica lipase Lip2p was able to perform this resolution (Guieysse et al. 2003b). Despite enantioselectivity values obtained with Lip2p being promising, they might not be sufficient for pharmaceutical applications that require a high purity level. However, Lip2p is a good candidate to develop enantioselective catalysts through site-directed mutagenesis or directed evolution. Bordes et al. (2009) reported the improvement by site-directed mutagenesis of the enantioselectivity of the Y. lipase Lip2p for the resolution of 2-bromo-arylacetic ester. On the basis of a Lip2p structural model obtained by modeling techniques, five amino acid residues (T88, V94, D97, V232, V285) that form the hydrophobic substrate-binding site of the lipase were selected for site-directed mutagenesis. Position 232 was identified as crucial for the discrimination between enantiomers. Variant V232A displayed an enhanced enantioselectivity by one order of magnitude, whereas variant V232L exhibited a selectivity inversion. To further explore the diversity, position 232 was mutated by 19 other amino acids. Analysis of the obtained mutants led to the selection of the V232S variant, which has a tremendously increased E value compared to the wild-type enzyme for the resolution of 2-bromo-phenylacetic acid ethyl ester (58-fold) and 2-bromo-o-tolylacetic acid ester (16-fold). In addition to the gain in enantioselectivity, an eightfold increased velocity was observed for both substances (Bordes et al. 2009).
Ibuprofen is an arylpropionic acid related to the class of nonsteroidal, anti-inflammatory drugs. It is well known that the anti-inflammatory activity of this class of compounds is mainly due to the active (S)-enantiomer (Hutt and Caldwell 1984), and (S)-ibuprofen is 160-fold more active than its antipode in the in vitro synthesis of prostaglandin (Adams et al. 1976). In addition, (R)-ibuprofen displays toxicity due to its storage in fatty tissue as a hybrid glycerol ester, whose long-term effects are not known. In that context, Liu et al. (2009) reported the cyclic resolution of ibuprofen using coupled acid-base and lipase catalysis. They showed that the Lip2p lipase from Y. lipolytica had a higher affinity for the (S)-enantiomer. In their process, the unreacted (R)-enantiomer is extracted, racemized in a basic solvent–water mixture before being re-resolved. The (S)-ester was separated and hydrolyzed to (S)-ibuprofen in acidic dimethyl sulfoxide–water mixture. The high purity (S)-ibuprofen (ee = 0.98) was obtained using Lip2p lipase at pH = 8.
Optically pure amines can be used in the fine chemical industry as resolving agents, chiral auxiliaries, and chiral synthetic building blocks for pharmaceuticals as well as agrochemical compounds (Breuer et al. 2004; O'Donnell 2001). A variety of methodologies have been developed for the production of enantiopure amines. However, the latter are time-consuming, expensive, and still present a high risk of racemization. However, hydrolytic enzyme and especially lipase have been widely exploited to solve these problems. (±)α-Phenylethyl amine is widely used as a powerful intermediate in industrial asymmetric synthesis or chiral adjuvant (Juaristi et al. 1999). Wen et al. (2008) studied the enantioselective aminolysis of immobilized extracellular lipase from Y. lipolytica by catalyzing enantioselective acylation of (±) α-phenylethyl amine with acetic ester in a cosolvent medium. In their optimized condition, i.e., in hexane containing 3 % DMSO at 45 °C, the enantiomeric excess of the product markedly increased from 0.35 to 0.96 after 6 days of reaction with an E value close to 190. They also showed that the immobilized lipase could be reused in at least five consecutive batches with a high E value.
3.2 Polymerization Reaction and Modification of Fat
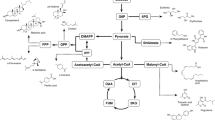
Polymerization reactions catalyzed by enzymes proceed generally through quimioselective, regiospecific, and stereoselective pathways. However, lipase-catalyzed reactions are quimioselective and proceed via the formation of an acyl-enzyme intermediate. For polyether synthesis by the so-called ring-opening polymerization (ROP), the key step is the reaction of the lactone ring with the lipase to provoke the ring opening and the formation of an acyl-enzyme intermediate (Fig. 2). The initiation step involves a nucleophilic attack of a water molecule onto the acyl carbon of the intermediate to produce a ω−hydroxycarboxylic acid (n = 1), the shortest propagating entities. In the propagation stage, the intermediate is nucleophilically attacked by the terminal hydroxyl group of a propagating polymer to produce a one-unit-more elongated polymer chain. The kinetics of the polymerization showed that the rate-determining step of the overall polymerization is the formation of the enzyme activated monomer. Therefore, the polymerization probably proceeds via an “activated monomer mechanism.” Based on that scheme, Barrera-Rivera et al. (2008) investigated the ring-opening polymerization reaction of the ε-caprolactone in the presence of n-heptane by the extracellular lipase from Y. lipolytica. After 360 h of reaction at 50 °C in the presence of 3 mmol of ε-caprolactone and 100 mg of enzyme, a 100 % conversion rate was obtained and a polyester molecule presented an average mass of 970 Da. The final polymers were found to correspond to an asymmetric α-hydroxy-ω-carboxylic acid poly (ε-caprolactones).
Scheme for the polyether synthesis by the so-called ring-opening polymerization (ROP) (inspired from Barrera-Rivera et al. 2008)
Besides these polymerization reactions, there has been considerable interest in the recent years in lipase-catalyzed reaction for the production of fatty acids, modification of oils and fats, and synthesis of various esters. The numerous substances obtained have many applications in the food, chemical, pharmaceutical, and medical sectors. Despite the fact that lipases are generally regarded as ester hydrolase, they can also catalyze the synthesis and transfer of ester with a regio- and/or stereospecificity. Ester transfer reaction (i.e., alcoholysis) does not directly involve water; they are usually carried out at a low water activity in order to hinder ester hydrolysis. Briand et al. (1994) investigated the alcoholysis of rapeseed oil by methanol using the extracellular lipase from Y. lipolytica. The transesterification reaction leads to an equilibrium state after 8 h with a reaction yield (methyl esters formed/total fatty acid initially present in the acylglycerols) of 73, 45, and 65 % for linolenic, linoleic, and oleic acid, respectively. The presence of methanol was found to favor transesterification reaction to the detriment of ester hydrolysis due to methanol inhibition of hydrolysis. This work demonstrated the ability of methyl-ester synthesis using the lipase from Y. lipolytica.
Monoacylglycerols (MAGs) are nonionic surfactant emulsifiers with their hydrophilic and hydrophobic parts. They are widely used in the food industry, with applications in dairy products, margarines, and bakery products. In addition, they present lubricant and plasticizing properties, and they are used in textile processing, production of plastics, and formulation of oil for different machineries (Esmelindro et al. 2008). Currently, MAG production is performed by chemical glycerolysis of fats and oils at high temperature in the presence of inorganic alkaline catalysts. Because this process is energy consuming, provided low reaction yield (30–40 %), and required product post-purification by molecular distillation, new techniques for MAG production were developed. These are mainly based on lipase-catalyzed reaction and proceed in organic medium, in solvent-free medium, in ionic liquids, or using compressed fluids as reaction media. In that context, Esmelindro et al. (2008) reported the production of MAG from olive oil in pressurized propane as solvent medium using different type of lipase, including the extracellular lipase from Y. lipolytica. Their results showed that lipase-catalyzed glycerolysis in compressed propane might be a potential alternative to conventional methods, as a high content of reaction products was obtained at mild temperatures (30 °C) and pressure conditions (30 bars) with a low solvent to substrate mass ratio (4:1) and in a short reaction time (3 h).
4 Applications in Traditional Food Making
It is well established that Y. lipolytica is naturally present in different kinds of food, underlining the importance of this yeast in the agro-food industry. The appearance of different yeasts, including Y. lipolytica, and their role in the production, ripening, or spoilage of traditional dairy products and meat products such as sausage have been studied for many years.
4.1 Cheese Ripening and Maturation
The production of mold-ripened cheeses, such as the Camembert and blue-veined cheese varieties, involves a maturation stage that is characterized by the growth of a complex ecology of yeast, bacteria, and filamentous fungi. The microbiological interactions and associated biochemical activities that occur during this stage determine product acceptability and value through their impact on sensory quality, shelf life, and safety (Addis et al. 1998). The role of yeast depends on the type of cheese: in some varieties they are responsible for spoilage, gassiness, slime formation, and discoloration, while in others they are involved in the ripening process and contribute to microbial interactions, texture changes, and biosynthesis of flavor compounds (Suzzi et al. 2001). Due to features such as proteolytic and lipolytic activities, yeast species including Y. lipolytica play an important role in the production of aroma precursors, especially amino acids, fatty acids, and ester. In particular, the dynamics of the free fatty acids (FFAs) release, which largely depends on the milk fat composition and microbial lipase selectivity and activity, determines the flavor of many dairy products (Suzzi et al. 2001). In order to better characterize this phenomenon, Addis et al. (1998) monitored the growth of yeast and bacteria during maturation of retail Camembert and blue-veined cheeses. Yeasts were found predominantly throughout the maturation process with Debaryomyces hansenii and Y. lipolytica, however, to a lesser extent, being the main constituent of the microbial flora. Among the 25 Y. lipolytica strains isolated from different Camembert and blue-veined cheese samples, 23 of them were found to present a lipolytic activity (varying from weak to strong) on tributyrin and butterfat agar. Suzzi et al. (2001), who performed a similar experiment, observed two different behaviors in terms of lipolytic activity. Some Y. lipolytica strains showed very high lipolytic activities over the first 3 days of maturation, producing the highest amounts of total FFAs. However, extended incubation of these strains resulted in a significant decrease of total FFA concentration. For other strains, characterized by a lower lipolytic activity after those 3 days, the total FFA content increased until the end of the maturation period. However, the authors reported that the specificity of the individual fatty acids released, and presumably their subsequent metabolisms, does not necessarily rely on the level of lipase production during the three first days of maturation. When the released FFAs are analyzed in more detail, it appears that short-chain FFAs (C4–C10) were produced by all the strains at low levels (1–2 % of the total FFAs) during the first days of maturation. After 6 days, longer-chain FFAs, such as palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), and linoleic (C18:2), decrease when strains producing high level of lipase are present. The linolenic acid (C18:3) released during the first 3 days tended to disappear whatever the level of lipase production. In regard to lipase specificity, all the isolated strains hydrolyzed both saturated and unsaturated fatty acids from milk fat with the liberation of high proportion of even-numbered carbon FFAs. The latter are then subsequently transformed by microbial enzymes such as lipoxygenases, epoxydases, and hydratases in the corresponding hydroxy acids which are then oxidized in shorter molecules including lactones, by means of α-, β-, and δ-oxidations (Fickers et al. 2005). The diminution of total FFAs occurring in the presence of certain Y. lipolytica strains suggests that these molecules are transformed into the corresponding alcohols, ketones, and lactones. It was also highlighted that the levels of the observed lipolytic activities did not correlate with genetic variations observed in the different producing strains (Suzzi et al. 2001).
By contrast to Camembert and blue-veined cheese, raclette is a semihard cheese. In this type of cheese, short-chain FFAs are important in the flavor development during ripening. Y. lipolytica was found responsible for the increase of n-butyric acid as a result of milk fat hydrolysis (Wyder et al. 1999). The lack of modification in n-butyric and n-caproic ratios during the maturation process suggests a lipase action rather than an esterase activity. Surprisingly, the authors found that Y. lipolytica did not seem to contribute to the breakdown of proteins and peptides despite its strong extracellular proteolytic capacity. Since practically no yeast cells were detected in the mature cheese, the action of yeasts in the maturation process could be attributed to enzymes released after cell lysis.
4.2 Fermented Sausage
In Europe, dry fermented sausages have a long tradition originating from Mediterranean countries since Roman times. Many types of fermented sausages have been developed together with processing conditions, additives, and ingredient formulation. Among the flavor products identified in dry sausages, the oxidation products of lipids, released upon meat fat hydrolysis by lipase, account for about 60 % of the total compounds which influence the flavor (Berdague et al. 1993). Lactic acid bacteria Micrococci and coagulase-negative Staphylococci have the most relevant role in the fermentative process and ripening. However, yeast and mold were also found associated with this process notably in the development of specific organoleptic characteristics (Samelis et al. 1993). Gardini et al. (2001) followed the yeast population during manufacturing and ripening of “salsiccia sotto sugna,” a typical salami of the Lucania region (Italy). Four different batches, from four different farms in Luciana, were studied. Although each batch showed a specific yeast population, Y. lipolytica strains were isolated in all of them and they were found to hydrolyze pork fat at different levels. Some of them produced high amounts of free fatty acids (FFAs) after 6 days of growth, while others produced a relatively low amount of FFAs, without significant differences in relation to the incubation time. A third group was responsible for the slight decrease of total FFAs in the medium after 6 days of incubation probably due to their metabolization or oxidization into flavor compounds. The major products of the lipolytic activity of Y. lipolytica strains from the third group were, in decreasing order, oleic, palmitic, stearic, linoleic, and myristic acid. This clearly demonstrates a specificity of the lipolytic enzymes for the positions sn1 and sn3 of the triglycerides. In fact, these are the positions of triglycerides most frequently occupied by unsaturated fatty acids in pork fat. Gardini et al. (2001) also reported that saturated and unsaturated fatty acids were present at similar concentrations at pH 5.5, while for other pH values, the saturated fatty acids were at lower concentrations. This highlights that Y. lipolytica lipase could favor at pH 5.5 the liberation of saturated FFAs rather than unsaturated. This tendency could have a positive effect by reducing the phenomenon of rancidity in which polyunsaturated FFAs are involved.
5 Production of Citric Acid
Citric acid (CA) is an intermediate of the tricarboxylic acid cycle (TCA) that holds a key position in the central metabolic fluxes in cells. Due to its acidulant, flavoring agent, and antioxidant properties, CA is used mainly in the food and beverage industry. In recent years, the consumption of citric acid and its salt, trisodium citrate, has reached worldwide 800,000 tons with an increase of 5 % per year (Kamzolova et al. 2005). Under certain conditions of fermentation, fungi, bacteria, and yeasts can produce CA in large amounts. Traditionally, the yeast strains, mostly belonging to Aspergillus niger, have been used for commercial production of CA from molasses, sucrose, or glucose (Kristiansen and Sinclair 1979). However, the production of CA with the use of fungi is associated with the accumulation of significant amounts of solid and liquid waste. As an alternative, there is a great interest in the possibility of CA production by yeast. Y. lipolytica is known to produce a wide range of organic acid, including TCA cycle intermediates, such as CA or isocitric acid (ICA). Many studies have been dedicated to this production using different sources of carbon (n-alkane, raw glycerol, ethanol). Y. lipolytica strains used in those processes are characterized by a greater resistance to high substrates and metal ion concentrations, thus allowing the use of less refined substrate (Rane and Sims 1993). In that context, Kamzolova et al. (2005, 2007) investigated the production of citric acid in a 3-l bioreactor using different Y. lipolytica strains grown in the presence of beef fat or olive oil. For Y. lipolytica strain 704, lipase production could be observed for the two hydrophobic substrate sources with a very high value of lipase activity (2,760 U/ml) when olive oil was used as carbon source. In the course of cell cultivation, glycerol and free fatty acid concentrations remain constant in the culture medium, suggesting that they were consumed simultaneously upon hydrolysis of triglycerides by lipase enzymes. FFA composition of the culture medium indicated that oleic and linoleic acids were the most representative hydrophobic substrates. However, the strain producing the higher amount of lipase was not the one that produced the larger quantity of citric acid. This clearly demonstrates that the two phenomena are not directly connected. A maximum of 135 g/l of citric acid was obtained at the end of the culture for strain Y. lipolytica 187/1. This value was significantly higher than those reported in the literature for other species (Klasson et al. 1989; Wojtatowicz et al. 1991). In addition, the undesired ICA was produced only in low amount (7.8 g/l). With Y. lipolytica strain Y-2373, the concentrations of ICA and CA obtained in similar conditions were 34 and 40 g/l, respectively, which correspond to an ICA/CA ratio of 0.85. However, Kamzolova et al. (2007) noticed that the composition of citric acids depends considerably on the pH value. At pH 6, the concentrations of ICA and CA were 55.4 and 21.7 g/l, respectively, with an ICA/CA ratio of 2.55:1, while at pH 4.5, the ratio of ICA to CA was 1:1.18. It is known that CA transport across the membrane is favored by low pH value (Peltsmane et al. 1988), whereas ICA transport is pH independent. This may explain why at pH 4.5, the ratio of ICA to CA changes in favor of CA. All of these suggest that plant oils appear as a promising substrate for citric acid production by Y. lipolytica.
6 Conclusions
The ability of Y. lipolytica to grow on raw substrate and to produce enzymes and metabolites in large amounts has received all the attention from industrials and academics for more than three decades. However, the availability nowadays of “omic” techniques and sophisticated molecular tools should permit rapid advances in the field.
References
Adams S, Bresloff P, Mason C (1976) Pharmacological difference between the optical isomers of ibuprofen: evidence for metabolic inversion of the (-)isomer. J Pharm Pharmacol 28:256–257
Addis E, Fleet G, Cox JM (1998) Evaluation of methods for the isolation and enumeration of yeast in mould-ripened cheese. In: Jakobsen M, Narvhus J, Viljoen BC (eds) Yeast in the dairy industry: positive and negative aspects. International Dairy Federation, Brussels, pp 109–116
Al-Malah K, Azzam M, Abu-lail N (2000) Olive mills effluent (OME) wastewater post treatment using activated clay. Sep Purif Technol 20:225–234
Andreoni V, Bonfanti P, Daffonchio D, Sorlini C, Villa M (1993) Anaerobic-digestion of olive mill-effluent: microbiological and processing aspects. J Environ Health Part A 28:2041–2059
Ansenne A, Destain J, Godefroid J, Thonart P (1992) La problématique des séparateur de graisse. Trib eau 558:33–40
Barrera-Rivera K, Flores-Carreon A, Martinez-Richa A (2008) Enzymatic ring opening polymerization of ε-caprolactone by a new lipase from Yarrowia lipolytica. J Appl Pol Sci 109:708–719
Berdague JL, Monteil P, Montel M, Talon R (1993) Effects of starter cultures on the formation of flavour compounds in dry sausage. Meat Sci 35:275–287
Bordes F, Cambon E, Dossat-Letisse V, Andre I, Croux C, Nicaud JM, Marty A (2009) Improvement of Yarrowia lipolytica lipase enantioselectivity by using mutagenesis targeted to the substrate binding site. Chembiochem 10:1705–1713
Breuer M, Ditrich K, Habicher T, Hauer B, Kesseler M, Stuurmer R, Zelinski T (2004) Industrial methods for the production of optically active intermediates. Angew Chem Int Ed 43:788–824
Briand D, Dubreucq E, Galzy P (1994) Enzymatic fatty esters synthesis in aqueous medium with lipase from Candida parapsilosis (Ashford) langeron and talice. Biotechnol Lett 16:813–818
Chin K, Lee S, Mohammad H (1996) A study of palm oil mill effluent treatment using a pond system. Water Sci Technol 34:119–123
Cossu R, Blakey N, Cannas P (1993) Influence of codisposal of municipal solid-waste and oil vegetation water on the anaerobic digestion of a sanitary-landfill. Water Sci Technol 27:261–271
D’Annibale A, Crestini C, Vinciguerra V, Sermanni GG (1998) The biodegradation of recalcitrant effluents from an olive mill by a white-rot fungus. J Biotechnol 61:209–218
D’Annibale A, Ricci M, Quaratino D, Federici F, Fenice M (2004) Panus tigrinus efficiently removes phenols, color and organic load from olive-mill wastewater. Res Microbiol 155:596–603
Destain J, Roblain D, Thonart P (1997) Improvement of lipase production from Yarrowia lipolytica. Biotechnol Lett 19:105–107
De Felice B, Pontecorvo G, Carfagna M (1997) Degradation of waste waters from olive oil mills by Yarrowia lipolytica ATCC 20255 and Pseudomonas putida. Acta Biotechnol 17:231–239
Dominguez A, Longo M, Sanroman M (2005) Recycling of waste cooking oil for lipase production by Yarrowia lipolytica. J Biotechnol 118S1:S170
Esmelindro A, Fiametti K, Ceni G, Corazza M, Treichel H, de Oliveira D, Oliveira J (2008) Lipase-catalysed production of monoglycerides in compressed propane and AOT surfactant. J Suercrit Fluids 47:64–69
Fickers P, Benetti PH, Wache Y, Marty A, Mauersberger S, Smit MS, Nicaud JM (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543
Gardini F, Suzzi G, Lombardi A, Galgano F, Crudele MA, Andrighetto C, Schirone M, Tofalo R (2001) A survey of yeasts in traditional sausages of southern Italy. FEMS Yeast Res 1:161–167
Goncalves C, Lopes M, Ferreira J, Belo I (2009) Biological treatment of olive mill wastewater by non-conventional yeast. Bioresour Technol 100:3759–3763
Guieysse D, Salagnad C, Monsan P, Remaud-Simeon M (2003a) Lipase-catalyzed enantioselective transesterification toward esters of 2-bromo-tolylacetic acids. Tetrahedron Asymmetry 14:317–323
Guieysse D, Salagnad C, Monsan P, Remaud-Simeon M (2003b) Towards a novel explanation of Pseudomonas cepacia lipase enantioselectivity via molecular modelling of the enantiomer trajectory into the active site. Tetrahedron Asymmetry 14:1807–1817
Guieysse D, Sandoval G, Faure L, Nicaud J, Monsan P, Marty A (2004) New efficient lipase from Yarrowia lipolytica for the resolution of 2-bromo-arylacetic acid esters. Tetrahedron Asymmetry 15:3539–3543
Hirai M (2001) New advanced technology for fish waste. Food Dev 35:11–13
Hutt A, Caldwell J (1984) The importance of stereochemistry in the clinical pharmacokinetics of the 2-arylpropionic acid non-steroidal anti-inflammatory drug. Clin Pharmacokinet 9:371–373
Juaristi E, Leon-Romo J, Reyes A, Escalante J (1999) Recent applications of alpha-phenylalanine in the preparation of enantiopure compounds. Tetrahedron Asymmetry 10:2441–2495
Kamzolova S, Morgunov I, Aurich A, Perevoznikova O, Shishkanova N, Stottmeister U, Finogenova T (2005) Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol Biotechnol 43:113–122
Kamzolova S, Finogenova T, Lunina O, Perevoznikova O, Minachova L, Morgunov I (2007) Characteristics of the growth on rapeseed oil and synthesis of citric and isocitric acids by Yarrowia lipolytica yeasts. Microbiology 76:20–24
Klasson T, Clausen J, Gaddy J (1989) Continuous fermentation for the production of citric acid from glucose. Appl Biochem Biotechnol 23:491–509
Kristiansen B, Sinclair C (1979) Production of citric acid in continuous culture. Biotechnol Bioeng 21:297–315
Lanciotti R, Gianotti A, Baldi D, Angrisani R, Suzzi G, Mastrocola D, Guerzoni M (2005) Use of Yarrowia lipolytica strains for the treatment of olive mill wastewater. Bioresour Technol 96:317–322
Liu Y, Wanf F, Tan T (2009) Cyclic resolution of racemic ibuprofen via coupled efficient lipase and acid-base catalysis. Chirality 21:349–353
Lopes M, Araujo C, Aguedo M, Gomes N, Goncalves C, Teixeira J, Belo I (2009) The use of olive mill wastewater by wild-type Yarrowia lipolytica strains: medium supplementation and surfactant presence effect by increased air pressure. J Chem Technol Biotechnol 84:533–537
Mameri N, Halet F, Drouiche M, Grib H, Lounici H, Pauss A, Piron D, Belhocine D (2000) Treatment of olive mill washing water by ultrafiltration. Can J Chem Eng 78:590–595
O’Donnell M (2001) The preparation of optically active alpha-amino acids from the benzophenone imines of glycine derivatives. Aldrichim Acta 34:3–15
Oswal N, Sarma PM, Zinjarde S, Pant A (2002) Palm oil mill effluent treatment by tropical marine yeast. Bioresour Technol 85:35–37
Papanikolaou S, Galiotou-Panayotou M, Fakas S, Komaitis M, Aggelis G (2008) Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour Technol 99:2419–2428
Peltsmane I, Raminya L, Finogenova T, Karklin R (1988) Biosynthesis and isolation of isocitric acide from n-alkane solution. Izv An Latv 487:93–95
Rane K, Sims K (1993) Production of citric acid by Candida lipolytica Y1905: effect of glucose concentration on the yield and productivity. Enzyme Microb Technol 15:646–651
Ros de Ursinos F (1961) Estudio del alpechin para su aprovechamiento industrial. VI. Aminoacidos presentes en la levardura Torulopsis utilis. Gras Aceit 12:161–165
Saito H (2004) Lipid and FA composition of the pearl oyster Pinctada fucata martensii: influence of season and maturation. Lipids 39:997–1005
Samelis J, Aggelis G, Metaxopoulos J (1993) Lipolytic and microbial changes during the natural fermentation and ripening of Greek dry sausages. Meat Sci 35:371–385
Scioli C, De Felice B (1993) Impiega de ceppi di lievito nella depurazione dei feflui dell’industria olearia. Ann Microbiol Enzymol 43:61–69
Scioli C, Vollaro L (1997) The use of Yarrowia lipolytica to reduce pollution in olive mill wastewaters. Water Res 31:2520–2524
Suzzi G, Larnote M, Galgano F, Andrighetto C, Lombardi A, Lanciotti R, Guerzoni M (2001) Proteolytic, lipolytic and molecular characterisation of Yarrowia lipolytica isolated from cheese. Int J Food Microbiol 69:69–77
Thonart P, Destain J, Zgoulli S, Antoine P, Godefroid J, Evrard P (1997) Les bacs à graisse: une solution aux problèmes des matières grasses pour les PME. Trib eau 586:41–46
Tsai S, Lin J, Chang C, Chen J (1997) Enzymatic synthesis of (S)-ibuprofen ester prodrug from racemic ibuprofen by lipase in organic solvents. Biotechnol Prog 13:82–87
Wen S, Tan T, Yu M (2008) Immobilized lipase YlLip2-catalysed resolution of alpha-phenylethyl amine in a medium with organic cosolvent. Process Biochem 43:1259–1264
Wojtatowicz M, Rymowicz W, Kautola H (1991) Comparison of different strains of the yeast Yarrowia lipolytica for citric acid production from glucose hydrol. Appl Biochem Biotechnol 31:165–174
Wu L, Wan J (2008) Investigation on the capability of disposing the grease wastewater with immobilized Yarrowia lipolytica. Chin J Environ Eng 4:482–486
Wu L, Ge G, Wan J (2009) Biodegradation of oil wastewater by free and immobilized Yarrowia lipolytica W29. J Environ Sci 21:237–242
Wyder M, Bachmann HP, Puhan Z (1999) Role of selected yeasts in cheese ripening: an evaluation in foil wrapped raclette cheese. Lebensm Wiss Technol 32:333–343
Yano Y, Oikawa H, Satomi M (2008) Reduction of lipids in fish meal prepared from fish waste by a yeast Yarrowia lipolytica. Int J Food Microbiol 121:302–307
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Fickers, P., Nicaud, JM. (2013). Biotechnological Applications of Yarrowia lipolytica Lipases: An Overview. In: Barth, G. (eds) Yarrowia lipolytica. Microbiology Monographs, vol 25. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38583-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-38583-4_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38582-7
Online ISBN: 978-3-642-38583-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)