Abstract
Reprogramming of host metabolism is a common strategy for improving desired compounds in host cells and is essential to generate overproducing strains in biotechnology. As a promising feedstock converter, Yarrowia lipolytica has been engineered to extend its bioproduction ability related to the synthesis of new value-added molecules relevant to human food and disease treatment. New synthetic tools have been reported and new enzymes with biotechnological importance are recovered. Additionally, metabolic events occurring during substrate utilization and recombinant protein production have been elucidated. Its contributions as feed and in controlling disease in the food industry have also been provided. Likewise, the recent abilities of Yarrowia lipolytica in the bioconversion of food waste into single-cell protein have been reported. These aforementioned events made the novelty of this review compared to the existing ones on this oleaginous yeast.
Key points
• The production of biolipids by the heterotrophic yeast Yarrowia lipolytica is examined.
• A Summary of information concerning new value-added molecules has been highlighted.
• Special focus on the importance of Yarrowia lipolytica in regulating the immune system has been provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yeasts in the Saccharomycotina subphylum have proven to be useful platforms for the production of a diverse range of pharmaceutical, industrial, biotechnological, food, feed (e.g. food additive in diet), and biodiesel compounds. The oleaginous yeast Y. lipolytica is viewed as a more attractive tool to exploit renewable resources for microbial lipid production; a reliable source for biodiesel production. Y. lipolytica is also a well-established oleaginous dimorphic yeast extensively analysed to comprehend molecular events related to the synthesis and bioconversion of a wide range of molecules. A current report unveiled the predominant role of sugar signaling pathways, such as cAMP-PKA (cyclic AMP [cAMP]–dependent protein kinase [protein kinase A {PKA}]–dependent pathway, in controlling Y. lipolytica dimorphism. The same study reported an ovoid morphology in the conditions of residual glucose concentration below a threshold value around 0.35−0.37 mg/L while the elongated forms are stimulated at this threshold progressively accelerated with the increase in residual glucose levels (Lesage et al. 2021).
To get more insights into the reactions companying substrates utilization by this species, a novel experiment has been performed. Indeed, by applying the Genome-scale metabolic models GSMM (iYli21) coupled with transcriptomic data to Y. lipolytica type strain W29, to predict its nutrient utilization, commonly used biochemical reactions as well as specific reactions have been reported. For example, the commonly noticed reactions encompassed reactions from the tricarboxylic acid (TCA) cycle of central metabolism, oxidative phosphorylation, and purine metabolism for energy and material supply. As far as specific reactions are concerned, when glucose and glycerol are employed as sole carbon sources, only glycolytic reactions were observed while gluconeogenesis, as well as fatty acid oxidation reactions, was triggered when fatty acids (alkane and glycerolipid) are used as the sole carbon sources (Guo et al. 2022). Y. lipolytica Po1f genome has recently been demonstrated, using the CRISPR-Cas9 system, to contain a putative diamine oxidase (DAO-1) enzyme with promising potential for the decomposition of biogenic amines, such as tyramine, histamine, putrescine, and cadaverine, in food (Kettner et al. 2021). Y. lipolytica has been also engineered by introducing three modules for de novo production of the natural compound scutellarin; a molecule employed in drugs used to treat cerebrovascular and cardiovascular diseases. These modules are p-coumaric acid production module expressing phenylalanine ammonia-lyase, cinnamate-4-hydroxylase and 4-coumaroyl-CoA ligase, naringenin production module expressing chalcone synthase, chalcone isomerase and flavone synthase II, and scutellarin production module expressing flavone-6-hydroxylase, cytochrome P450 reductase, flavonoid-7-O-glucuronosyltransferase, and UDP-glucose dehydrogenase. The production of scutellarin by engineered strain deriving from Y. lipolytica W29 strain, carrying Cas9 on the KU70 locus, was estimated as 94.79 mg/L in flask condition and 346 mg/L in a fed-batch bioreactor (Wang et al. 2022). Scutellarin is a natural flavonoid compound known for its cardiovascular preservative role and, recently, its role in restraining the transendothelial migration of cells in the aggressive subtype of breast cancer; triple-negative breast cancer, has been elucidated (Mei et al. 2022). This brought to light the high biological platform potential of Y. lipolytica for cancer therapy.
The predisposition of the oleaginous yeast Y. lipolytica as a probiotic organism with detrimental effects on pathogens has also been confirmed. For example, when the marine yeasts Y. lipolytica Yl-N6 and Debaryomyces hansenii CBS8339 are simultaneously administered orally by feed and water to the white shrimp Penaeus vannamei post-larvae, they triggered upregulation of the expression of gene penaeidin and lectin in the shrimp. It increased this organism’s survival (shrimp) after infection by the pathogen bacterial Vibrio parahaemolyticus IPNGS16 (Licona-Jain et al. 2022). This elucidates the superior feature of this species as probiotics relevant for the control of infectious disease in the food industry.
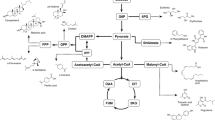
Beyond its importance in the biosynthesis of value-added molecules, Y. lipolytica can significantly contribute to plant resistance to pathogens. This species can stimulate a series of signals that activate several genes assuming a key role in plant (asparagus) resistance to Fusarium disease (Godana et al. 2022). Figure 1 highlights all the genes involved in the biocontrol steps. Other parameters regarding the biocontrol exerted by Y. lipolytica on asparagus disease causal agent Fusarium proliferatum encompassed the stimulation of respiratory-related enzymes phosphofructokinase (PFK), pyruvate kinase (PK), citrate synthase (CS), isocitrate dehydrogenase of the mitochondrion (ICDHm), α-ketoglutaricdehydrogenase (α-KGDH), succinodehydrogenase (SDH), 6-phosphate-dehydrogenase (G-6-PDH), 6-phosphogluconate dehydrogenase (6-PGDH), NAD kinase (NADK), and cytochrome oxidase (CCO). These relations allow safeguarding a high energy status, and a high reduction state (Hu et al. 2021).
Regulation of resistance genes in asparagus upon exposure to Y. lipolytica. This species can stimulate a series of signals (signal transduction pathways of salicylic acid and jasmonate, Ca2+signal transduction activated by CML19, reactive oxygen output expressed by RBOHE, RBOHF) that activate several genes assuming a key role in plant (asparagus) resistance to Fusarium disease (Godana et al. 2022). The genes spanned PR1 genes, PGIP2, PLP2, and FMO1, antioxidant genes (PER genes, PNC1, Sb03g046810) and glutathione S-transferase (GST) genes, genes related to secondary metabolites (CCR, CAD, DIR21, PAL, PER genes, CYP75B2, CYP73A13, UGT92A1, UGT73C6, F3H-1, CCoAMT5, ALDH2C4, and BGLU12), EMP-TCA pathway genes (FRK2, PFK3 and MSTRG.32630) and genes related to the cell wall and membrane SBH1, SBH2, LRX, and PERK (Godana et al. 2022)
A combinatorial gene overexpression method has also been applied to Y. lipolytica grown on waste cooking oil (WCO) to enhance limonene biosynthesis. The highest titer production under optimal fermentation conditions was 91.24 mg/L for D-limonene and 83.06 mg/L for L-limonene on WCO (Li et al. 2022a, c, d).
This review highlights general trends and updates the most recent developments in technologies to improve Yarrowia lipolytica competitiveness in biotechnology. We highlight for the first time the mechanism underlying the proneness of this species in controlling pathogens. We further discuss how Y. lipolytica as a cell factory contributes to human milk synthesis and converts diverse feedstocks into the product of interest at high rates and yields, depending on these different approaches.
Contribution of Yarrowia lipolytica in the food and feed industry
The GRAS (Generally Recognized As Safe) status of the non-conventional yeast Y. lipolytica has spurred interest in exploiting this host for food and feed bioproduction. The outstanding potential of this yeast for human food has been underpinned. In 2022, the European Food and Safety Authority (EFSA) authorised to use the biomass of Y. lipolytica, as a novel food supplement at maximum use levels of 3 g per day for children ranging from 3 to 9 years of age and 6 g per day for children from 10 years of age, adolescents and adults (EFSA, 2022). On the lignocellulosic agricultural waste subjected to hydrolysis, oat bran hydrolysate, Y. lipolytica displayed biomass total yield and total productivity values of 0.141 g/g and 0.078 g/h, respectively, and protein contents in yeast biomass ranging from 30.5 to 44.5% of dry weight. On other lignocellulosic agricultural waste (rye bran), Y. lipolytica exhibited high content of commercially desirable exogenous amino acid (leucine 3.38 g, lysine 2.93 g, threonine 2.31 g per 100 g of dry mass) and an array of unsaturated fatty acid composed majorly of oleic acid (59.28%; Drzymała et al. 2020). It has been provided evidence that an engineered Y. lipolytica gsy1Δ-LPAAT2 strain expressing lysophosphatidic acidacyltransferases with palmitoyl-Coenzyme A specificity and cultured under nitrogen-limited conditions, on glycerol or palm oil or a mixture of the two substrates, generated fatty acid (FA) composition that resembles human milk fat with ~19% palmitic acid (16:0), ~3% palmitoleic acid (16:1), ~13% stearic acid (18:0), ~50% oleic acid (18:1) and ~15% linoleic acid (18:2), with more than 60% of the total 16:0 in triacylglycerol at the sn-2 position and 16:0 representing nearly 40% of all the FA at the sn-2 position (Bhutada et al. 2022). Non-conjugated linoleic acid (C18:2) presence in milk fat is highly preferred in human metabolism due to the beneficial role of this fatty acid species in lowering blood lipids, restraining immune responses, and triggering lipid metabolism (Belury 2002).
Furthermore, Jach and Malm (2022) reported that the biomass of Y. lipolytica gathered protein, exogenous amino acids, bioavailable essential trace minerals, and unsaturated fatty acids. Again, the protein biomass of Y. lipolytica cultivated in industrial glycerol or biofuel waste accommodates 0.68–11.5 mg/100g of thiamine (vitamin B1), 1.1–6.9 mg/100g of riboflavin (vitamin B2), 2.53–6.50 mg/100g of pyridoxine (vitamin B6), 184–330 mg/100g of folic acid (vitamin B9), and 5.2–11.2 mg/100g cyanocobalamin or vitamin B12 (Jach and Malm, 2022). Vitamins are noticeable for their significant contribution to several biochemical processes in humans and their deficiency or depletion are associated with characteristic symptoms, e.g., Beri-Beri disease (Vit. B1). Therefore, the protein biomass of Y. lipolytica can be considered a valuable source of vitamin supplementation.
As its contribution to the food industry keeps increasing, the molecular regulatory mechanism that occurred during the transformation of (+)-valencene to (+)-nootkatone (grapefruit aroma employed as aromatics) in Y. lipolytica was examined in detail in the report of Li et al. (2022a, c, d). This report highlighted the up-regulation of the expression of genes related to the biosynthesis of secondary metabolites and most ATP-binding cassette (ABC) transporters and the down-regulation of genes implicated in energy metabolism. Furthermore, an induction of enzymes involved in (+)-valencene biotransformation prone to inhibition by cytochrome P450 inhibitors was described. And finally, several up-expressed genes linked to cytochrome P450 and dehydrogenase (gene2800, gene2911, gene3152) were identified that may be involved in the biotransformation.
The contribution of Y. lipolytica as quality feed in the diet has been illustrated through the report of zootechnical, hematological, and immune response parameters exhibited by the plasma and kidney of the fish Nile Tilapia (Oreochromis niloticus); the second largest group of farmed fish worldwide. On the plasma, it has been unveiled that fermented biomass of the yeast Y. lipolytica enhances zootechnical parameters in tilapia, augments the number of neutrophils, monocytes, and the levels of lysozyme, myeloperoxidase, as well as nitrite/nitrate content in the blood of the fish. Additionally, the fermented biomass of this oleaginous yeast does not compromise survival or affect the hematological parameters of the fish but rather enhances the levels of myeloperoxidase with no perturbation on the levels of lysozyme and nitrite/nitrate content of the fish kidney (Neuls et al. 2021). These data indicate the promising role that Y. lipolytica may exert as a nutritional component of the aquafeed industry.
Molecules deriving from the biotransformation of natural starting materials are viewed as natural, as stated by the United States and European Union regulations; consequently, interest in the biotechnological synthesis of natural flavor molecules has recently increased (Yu et al. 2014). Natural molecules include lactones identify as a group of additives containing ester bonds and exhibiting an intense, specific aroma that can affect food intake and preferences. The role of Y. lipolytica in aroma synthesis in the industry has been also investigated. Y. lipolytica NCIM 3590 in optimized conditions converted 5 g/L ricinoleic acids, under a continuously stirred tank reactor, into 200 mg/L/h of γ-decalactone. More specifically, 80% pure γ-decalactone with an overall recovery of 85% of the product was achieved after a three-step purification method (Kothari et al. 2022). Noteworthy, the compound γ-decalactone is a lactone with a low aroma threshold employed as an additive in the production of beverages or food (Schrader et al. 2004). Considering that the extraction and isolation of natural food additives can be sometimes expensive, a microbial route for their synthesis can be viewed as a viable option and a breakthrough in the food industry.
Enzymes synthesis features of Yarrowia lipolytica
Although a recent bioconversion of food waste to single-cell protein (SCP) has been reported for the oleaginous yeast Y. lipolytica, its ability in recombinant protein synthesis should also be considered. Indeed, 38.8 ± 0.2% w/w biomass dry weight (BDW) with a chemical oxygen demand removal rate of 85.5 ± 0.7% were reported during a two-stage fermentation process implicating a step of anaerobic fermentation of food waste to volatile fatty acids preceding conversion to SCP (Yang et al. 2022). Y. lipolytica represents, therefore, an effective measure to remove additional pollutants source in the environment, avoid long-term ecological problems, and improve the food industry.
Recombinant enzymes that can help to mitigate a huge number of environmental problems through the deconstruction of lignocellulose biomass, degradation of recalcitrant substrates from nature or that can be valorised for the synthesis of value-added compounds hold great promise for sustainable biotechnology. Protein engineering efforts focused on supplying variants of extended substrate scope or augmented operational and thermal stability. Catalytic units of the endogenous lipases Lip11 from Y. lipolytica are known to contain, serine, histidine, and aspartate residues. Lip11 has been engineered for improving its activity. It has been revealed that the insertion of the point mutation in the C-terminus of Lip11, from the yeast Y. lipolytica MSR80, impedes putative glycosylation residue and reduces Lip11 stability and catalytic activity while mutating the putative glycosylation residue (N17) located towards the N-terminus in the structure of Lip11 leads to a catalytically efficient variant (N1) with improved thermal and acid stability (Kashyap and Gupta, 2021). By creating N-truncations in the 58-residue extended terminus of the native or endogenous lipase of Y. lipolytica MSR80, Lip11, a lipase with abolished substrate inhibition, enhanced catalytic activity, stability, and efficiency has been obtained (Kashyap and Gupta 2022). To see how these lipases clustered with other lipases from fungi, a phylogenetic tree has been designed (Fig. 2). Clearly, the lipases of Y. lipolytica are separated from those of the other fungi. This may inform more targeted approaches for improving lipase’s role in this yeast.
Protein sequence-based phylogenetic tree obtained from multiple alignments of different protein sequences of fungi lipases. The accession numbers of the related lipase sequences given in the bracket. The position of lipase Lip 11 from Yarrowia lipolytica is highlighted in red. Multiple sequence alignment was performed using MUSCLE (Multiple Sequence Comparison by Log-expectation) in MEGA 11. A phylogenetic tree was obtained in MEGA 11 (Tamura et al. 2021) with a Neighbor-Joining tree model with 1000 replicates. Scale bar: 0.2. Yarrowia lipolytica isolate Lip7 (Lip7, GenBank: ADN93266.1), Yarrowia lipolytica Lip8 (Lip8, GenBank: RDW51668.1), Yarrowia lipolytica isolate lipase 11 (Lip11, GenBank: AFH77826.1), Yarrowia lipolytica isolate lipase 2 (Lip2, GenBank: ADL57415.1), Yarrowia lipolytica isolate lipase 12 (Lip12,GenBank: RDW52422.1), Yarrowia lipolytica isolate lipase 1 (Lip1,GenBank: RDW55890.1), Yarrowia lipolytica isolate lipase 3 (Lip3,GenBank: QNP97273.1), Yarrowia lipolytica isolate lipase 4 (Lip4,GenBank: QNQ00654.1), Yarrowia lipolytica isolate partial lipase 5 (Lip5,GenBank: ALM55103.1), Yarrowia lipolytica isolate lipase 9 (Lip9,GenBank: AHA84098.1), Yarrowia lipolytica isolate lipase 14 (Lip14,GenBank: RDW55471.1), compared to other lipases from fungi found in NCBI database Malassezia restricta strain CBS 7877 lipase (Lip4, GenBank: AYO44747.1), Malassezia restricta strain CBS7877 lipase (Lip1, GenBank: AYO44746.1), Malassezia restricta strain 7877 lipase (Lip2, GenBank: AYO44072.1), Rhodotorula toruloides lipase (Lip2, GenBank GEM07177.1), Candida albicans SC5314 lipase 8 (Lip8, GenBank: KHC79859.1), Candida albicans SC5314 lipase 3 (Lip3, GenBank: KHC89171.1), Trichophyton rubrum putative lipase 4 (Lip4, GenBank: DQ778062.1), Candida albicans SC5314 lipase 6 (Lip6, GenBank: KHC89143.1), Candida albicans P78042 lipase 9 (Lip9, GenBank: KHC67886.1), Candida albicans SC5314 lipase 5 (Lip5, GenBank: KHC79813.1), Candida albicans lipase 10 (Lip10, GenBank: KHC89142.1), Candida albicans SC5314 lipase 4 (Lip4, GenBank: KHC82116.1), Candida albicans SC5314 lipase 1 (Lip1, GenBank: KHC89141.1), Trichophyton rubrum putative lipase 3 (Lip3, GenBank: ABG67899.1), and Trichophyton rubrum putative lipase 4 (Lip4, GenBank: ABG67900.1)
The yeast strains Y. lipolytica YEAST-1 have been shown to promote the growth and enhance the salinity tolerance of the plant Triticum aestivum L. by secreting extracellular amino-cyclopropane-1-carboxylate deaminase (Hussein et al. 2022). Moreover, Y. lipolytica is shown to produce fatty acids on a seawater medium (Dobrowolski et al. 2019). The dimorphic Y. lipolytica RIY368 exhibited superior features over Pichia pastoris RIY311 towards synthesis and secretion of extracellular Candida Antarctica lipase B (CalB) in a bioreactor, with the maximal CalB production levels being reported in half the cultivation time necessary for maximal production by P. pastoris (Theron et al. 2020). The amount of recombinant enzymes (lipase CalB from Candida antarctica) can be improved in Y. lipolytica when the yeast is cultured on in situ fibrous bed bioreactor (isFBB) containing glycerol; designed by exploiting sugarcane bagasse as a cell immobilization support. The maximum lipase titer achieved using the isFBB culture mode was 38%, 33%, and 49% higher than those estimated using the batch, pulsed fed-batch (PFB), and continuous fed-batch (CFB) cultures, respectively (Mou et al. 2021). Relying on the engineering of Y. lipolytica for high-level production of highly enriched lipase B (CalB) is a promising strategy in the chemical industry knowing that CalB is notified as a robust enzyme, which keeps its activity in harsh industrial conditions, like in high solvent content (Carrea and Riva, 2000).
Heterologous protein secretion in this dimorphic species has been reported to be improved by the mean of thermal treatment conditions or genetic engineering. For example, it has been demonstrated that heterologous protein production of a recombinant Y. lipolytica strain, overproducing a heterologous raw starch digesting alpha-amylase (SoA), grown on a crude glycerol-based medium increases in response to decreased temperature; 20°C (Kubiak et al. 2021). Heterologous protein secretion can be improved in Y. lipolytica (Po1f strain) by co-cloning genes (RPL3, SSA5, and SSA8) encoding secretory helpers (SHs) with an easy-to-track reporter in the targeted strain at a decreased temperature (25°C; Korpys-Woźniak et al. 2021). A two-step strategy (co-transformation of haploid strains with different vectors and construction of diploid strains from various haploid transformants) for designing recombinant strains that facilitates the simple integration of several expression cassettes encoding heterologous proteins into Y. lipolytica genome has been successfully implemented. To this end, up to three expression vectors containing different heterologous cDNAs for P450scc system proteins and P45017α, have been integrated into Y. lipolytica for the purpose of constructing heterologous expression of multi-component enzyme systems in the yeast (Novikova et al. 2021). A well-established microbial protein production host may remove the cost and time-intensive bottleneck within the design of new bioprocesses. Other approaches for protein secretion in Y. lipolytica include the coupling of the TEF intron sequence with native secretion signal lip2pre-pro devoid of pro sequence (optimized construct) for enhancing protein secretion (e.g. T4 lysozyme). This combination led to an increase in the secretion of extracellular titer of T4 lysozyme by 17-fold. The secretion yield is further improved by combining the overexpression of enzymes in the endoplasmic reticulum (S. cerevisiae ERV29 encoding ER exit receptor) and Golgi body (S. cerevisiae STE13 encoding dipeptidyl aminopeptidase A), with the deletion of phosphatidic acid phosphatase gene (PAH1, important for diacylglycerol biosynthesis) in a strain containing lip2pre-intron signal. The resulting yield in Δpah1 strain with the lip2pre-intron signal is estimated as a 50-fold enhancement of T4-lysozyme secretion (Wang and Blenner 2022).
It has been disclosed that exposure to environmental stress factors can affect the overproduction of recombinant secretory proteins (rs-Prots) and the physiology of the oleaginous yeast Y. lipolytica. In the case of batch cultivations of Y. lipolytica metabolically burdened strains (GGY251 and GGY178 strains), it has been provided evidence that the notified decrease in rs-Prot production under adverse environmental conditions (pH 3/7) and oxygen availability (kLa 28/110 h−1) is predominantly due to emergence of a less-producing cell subpopulation rather than the decrease of the synthetic capacity of the whole cell population (Gorczyca et al. 2022). These authors further highlighted that the significantly burdened producer cells exhibited a higher demand for the carbon source, even if the growth of the cell is compromised. Of note, metabolic burden refers to the processes in which a decrease in the growth rate precedes an increase in recombinant protein content (Bentley et al. 2009).
Moreover, Y. lipolytica (strain GGY237) overproducing rs-Prot and submitted to hyperosmolarity (3 Osm kg−1) stress factors, in batch bioreactor cultures displayed an upregulated heat-shock proteins (HSPs) and aldo–keto reductases, a downregulated central carbon metabolism encompassing glycolysis, tricarboxylic acid cycle, and fatty acid synthesis, as well as a downregulated translation (elongation factors, several aa-tRNA synthetases), amino acid biosynthesis and ribosome biogenesis. Hyperosmolarity triggered the synthesis of polyols and drastically restricted citric acid synthesis and growth (Kubiak-Szymendera et al. 2022). Y. lipolytica strains subjected to high-level expression of different r(s)-Prot-encoding genes respond to perturbations imposed by the synthesis of proteins by exhibiting oxidative and unfolded protein stress (CTT1, PXMP2/4, HAC1), glycosylation (ALGs, KTRs, MNTs, MNNs), folding and translocation (SSAs, SSEs), non-conventional protein secretion (NCE102), transcriptional regulators (FLO11, MHY1, D01353g, RSFA, E23925g or MAF1), vacuolar proteolysis targets (ATGs, VPSs, HSE1, PRB1, PRC1, PEP4) or growth arrest (CLN1) upon rs-Prots overproduction (Korpys-Woźniak and Celińska, 2021).
The quality of Y. lipolytica as microbial cell factories (e.g. Y. lipolytica strain YLY) for simultaneous production of lipase and single-cell protein (SCP), employed as feed additives, has been reported during growth on the substrates (sugarcane molasses, waste cooking oil, and crude glycerol), with sugarcane molasses substrate being the cheapest feedstock favoring production of 16420 U/mL of lipase and 151.2 g/L of single-cell protein at 10-L fermentation scale (Yan et al. 2018). Of note, crude glycerol, as a major by-product deriving from the biodiesel industry, can be an environmental threat at a higher quantity and needs to be valorized via biotechnology processes transforming it into value-added compounds with significant economic benefits. Y. lipolytica Lip2 lipase showed promising features for the leather industry by efficiently degreasing sheepskins, with 6 mg of lipase/kg of raw skin sufficient for successful degreasing in only 15 min at pH 8 and 30°C (Moujehed et al. 2022).
Molecular events linked to lipid metabolism in Yarrowia lipolytica
Beyond food and enzyme synthesis, Y. lipolytica is also an important cell factory for lipids and citrate production. Y. lipolytica has been widely regarded as a good oleaginous yeast candidate with wide industrial application prospects based on its wide substrate spectrum, and excellent fatty acid composition for high-quality biodiesel. Other applications based on Y. lipolytica features are highlighted in Table 1. Generally identified as oleaginous yeast that cannot naturally metabolize C-5 substrates (e.g. pentose-specific transporters YALI0C04730p and YALI0B00396p need to be overexpressed for D-xylose assimilation; Ryu et al. 2018), a recent report highlighted the natural ability of some undomesticated strain of Y. lipolytica on xylose bioconversion. For example, in bioreactor culture conditions, it has been indicated that the undomesticated strain YB420 metabolized xylose to sustain cell growth as well as to keep high lipid levels and contained proteins linked to lipid metabolism (e.g. lipase, NADPH generation, lipid regulators, and β-oxidation) that are activated in a xylose-containing medium. The same authors found that the conventional strain CBS7504 (or W29) decomposed cell biomass and displayed a lipid degradation phenotype when xylose is the sole carbon source (Walker et al. 2021). Typical metabolic pathways for NADPH supply are depicted in Fig. 3.
Schematic diagram of lipogenesis with emphasis on NADPH synthesis in the oleaginous yeast Yarrowia lipolytica. The metabolic pathway is based on Wasylenko et al. (2015) and Wu et al. (2019) publications. Full arrows indicate direct reactions. Dashed arrow indicated multiple steps before the final reactions. PPP: pentose phosphate pathway, G-3-P: glycerol-3-phosphate acyltransferase, TCA: tricarboxylic acid
The lipid synthesis in Y. lipolytica is affected by the presence of a high carbon source content and low concentration of nitrogen in the environment (Coşgun et al. 2022). Knowledge of the lipid biosynthesis in Y. lipolytica indicated that, under carbon and nitrogen-limited chemostat cultures conditions, lipid accumulation in Y. lipolytica (e.g. strains derived from Po1g (Leu−)) is linked to the regulation of amino-acid biosynthesis, giving rise to the rewiring of carbon flux during nitrogen limitation from amino acids to lipids, rather than the transcriptional monitoring of lipid metabolism (Kerkhoven et al. 2016).
Additionally, the mechanism underlying the regulation of one of the key enzymes affecting lipogenesis in oleaginous yeasts has been uncovered in this species. Specifically, there are relationships between the two subunits of the ATP citrate lyase (ACL), an enzyme that is known for its role in causing the conversion of citrate to acetyl-CoA in an ATP-dependent manner. Indeed, it has been highlighted during lipogenesis that the subunit Acl1 encoded by the ACL1 gene augmented the protein levels of Acl2 encoded by the ACL2 gene (Anche and Fakas 2022). Of note, ACL known to convert citrate to acetyl-CoA for fatty acid biosynthesis is inactive in Y. lipolytica cultured in a nitrogen-rich medium but is significantly upregulated in Y. lipolytica cultured in a nitrogen-limited medium (Zhang et al. 2016).
Lipids biosynthesis in Y. lipolytica can typically follow two routes: de novo accumulation of cellular lipids in a medium containing non-lipid carbon sources (e.g. saccharides, glycerol) and ex novo microbial oil synthesis which involves fatty acids uptake from the environment. The de novo lipid accumulation led to acetyl-CoA formation triggered by the inactivation of the Krebs cycle in sugar-based media, while in the ex novo route of final products or intermediates of fatty acid β-oxidation are incorporated into triacylglycerol molecules in hydrophobic substrates (oils, alkane, etc., Ratledge and Wynn, 2002; Beopoulos et al. 2011).
It has been recently provided evidence that the de novo lipid synthesis pathway in Y. lipolytica KKP 379 wild-type strain is linked, at least in part, to the limitation of the nitrogen source in the medium while the ex novo pathway in the same yeast is activated in a lipid-rich medium (e.g. olive oil-rich media) for intracellular lipids production. Yet the expression of genes encoding the enzymes of the de novo pathway was not completely abrogated (e.g. ATP-citrate lyase; Fabiszewska et al. 2022).
A metabolic lever contributing to citrate overproduction has been recently elucidated in Y. lipolytica. It has been reported that the alternative oxidase (AOX) protein, linked to reactive oxygen species (ROS) synthesis, exerted a central role in redirecting carbon flux, derived from glucose, toward citric acid production or lipid accumulation, and more specifically in citrate/lipid flux balance in Y. lipolytica W29 wild-type strain. Indeed, adding a specific AOX inhibitor (n-Propyl Gallate or nPG) in batch cultures leads to two-fold overproduction of citrate (20.5 g/L) at stationary phase compared to 10.9 g/L obtain with no nPG addition condition (da Veiga et al. 2021). A report identified the mevalonate along with Methyl erythritol phosphate (MEP) pathways essential for isoprenoid biosynthesis in the oleaginous yeast Y. lipolytica. This report also demonstrated that the MEP pathway is activated in Y. lipolytica Po1d cultivated in nitrogen-limiting conditions (Dissook et al. 2021).
As Y. lipolytica strains are well-known for their higher potential for the production of a wide range of lipids, different approaches to optimize the recovery of the intracellular lipids from this oleaginous yeast and used them as feedstock toward an economically feasible biofuel and oleochemical production have been envisioned. Biofuels have some attractive environmental benefits compared to fossil fuel resources all over the world because of the absence of adverse effects on the environment. To this end, some nanoparticles coated with axially oriented graphene, with no inhibitory effects in the exponential growth of yeast and weak concentration-dependent inhibition at the entry of the stationary phase, have been employed to extract 50% of total intracellular lipids from Y. lipolytica cells, grown in nitrogen restricted medium (Pandit et al. 2021). This highlights a promising environmentally friendly and cost-effective way to extract lipids from oleaginous yeasts. It has been previously recognized that Y. lipolytica is an auxotroph for thiamine as it is unable to biosynthesize this vitamin that typically contains pyrimidine (4-amino-5-hydroxymethylpyrimidine) and a thiazole (4-methyl-5-β-hydroxyethylthiazole) moiety. The mechanism underlying thiamine auxotrophy in Y. lipolytica is now described in the work of Walker et al. (2020). They unveiled, in Y. lipolytica native genome, the absence of 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate synthase (THI13) gene responsible for the de novo thiamine biosynthesis and demonstrated that thiamine presence strongly affected lipid biosynthesis as its supplementation significantly improved growth, sugar assimilation and augmented the lipid production in the yeast strain YlSR001 (Walker et al. 2020). The knowledge acquired from lipid metabolism and substrate utilization may allow redesigning the existing biological systems to gain new functions.
Metabolic engineering approaches for lipid and synthesis of natural molecules
These methods encompassed the use of strategy to augment precursor supply, organelles targeting for integration of synthetic pathways, the overexpression of enzymes involved in lipid metabolism, and the deletion of enzymes from competing pathways.
Fatty acid metabolism is known to be vital for the biogenesis of cellular components. The unique molecular and chemical characteristics of very-long-chain fatty acids (FAs with 22 or more carbons) and their restricted natural abundance make them attractive targets for biosynthesis. The oleaginous yeast Y. lipolytica contains native diacylglycerol acyltransferases among which the enzyme YlDga1p was recently found to be crucial for the accumulation of very-long-chain fatty acids (VLCFA, e.g. behenic acid (C22:0), erucic acid (C22:1Δ13)). It has been shown that engineered Y. lipolytica strain, YL53 (Δmfe, pTEF-YlDGA1, 8UAS-pTEF-TaFAE1, overexpressing both YlDga1p (Y. lipolytica YlDga1) and 3-ketoacyl-CoA synthase (Thlaspi arvense TaFAE1), and in which fatty acid degradation pathway is disrupted by deletion of YlMFE1-mediated β-oxidation, generated 120 μg of VLCFAs per g of produced biomass, representing 34% of total fatty acids in biomass (Gajdoš et al. 2022). An efficient method for microbial lipid production from acetate-containing waste streams has been achieved using engineered strains of Y. lipolytica Po1f. Indeed, the first step of engineering has been performed through the overexpression of the key enzyme of acetyl-CoA synthetase. This led to an accumulation of 9.2% microbial lipids from acetate under shake flask fermentation conditions. The second step consisted in overexpressing a second key enzyme of acetyl-CoA carboxylase (ACC1) and fatty acid synthase (FAS) in the Y. lipolytica strain generated in the first step. This led to the bioconversion of acetate into lipid with a 25.7% increase in lipid content. Finally, the cultivation of co-substrate made with glycerol and acetate in fed-batch fermentation conditions generated a lipid content of 41.7% representing an increase of 68 (with glycerol) and 95% with acetate (Chen et al. 2021).
A method focused on replacing the native Δ9 fatty acid desaturase (Ole1p) with homologs from other species and changing the expression of both Ole1p and the Δ12 fatty acid desaturase (Fad2p) to interrupt the palmitoleic acid, minimized linoleic acid content in TAG, and significantly reduced oleic acid content to approximately 40 percent of the total fatty acids, permitted to approach triacyl-glycerol (TAG) storage lipids in Y. lipolytica (derived from the W29 background strain Y-63746) mimicking cocoa butter (Konzock et al. 2022). Concerning the well-known preference of Y. lipolytica for glycerol over glucose, a study recently confirmed that the rapid assimilation of glycerol is due to the presence of six active transporters encoding genes, YALI0C04730g (STL2), YALI0C16522g (STL3), YALI0B17138g (STL6), YALI2C00079g (STL8), YALI0E05665g (FPS1), or YALI0F00462g (FPS2); dedicated for glycerol import in Y. lipolytica DSM 3286 (Erian et al. 2022). It is worth noting that the preference of glycerol over glucose is suggested to rely on the diminished growth rate of glucose compared to glycerol, the presence of one hexose transporter along with at least three genes coding for proteins associated with glycerol transport, and the absence of homologues for genes described to be implicated in carbon catabolite repression (in the presence of glucose) in the genome of Y. lipolytica (Workman et al. 2013).
As cytosol is the main target for the production of high-value chemicals in microorganisms and this organelle is a crossroad for multiple metabolic pathways that may limit the efficient synthesis of the desired molecules (biosynthesis bottlenecks), a study was initiated to express the astaxanthin biosynthesis pathway in different sub-organelles (lipid body, endoplasmic reticulum or peroxisome) of the oleaginous yeast Y. lipolytica (Ma et al. 2021). The overall goal was to examine the impact of this strategy on the synthesis of astaxanthin. It has been found in the individual organelles expressing β-carotene ketolase and hydroxylase (enzymes converting β-carotene to astaxanthin), an accelerated conversion of β-carotene (C40H56) to astaxanthin accompanied by a drastic decreased in the accumulation ketocarotenoid intermediates. A further increase in astaxanthin synthesis estimated as 858 mg/L of astaxanthin in fed-batch fermentation occurred when the two enzymes were expressed in the three aforementioned organelles at the same time. In addition to the carotenoid astaxanthin, Y. lipolytica is used for the synthesis of carotenoid precursors Zeaxanthin (a C40 hydroxyl-carotenoid). β-Carotene-producing strain expressing genes, such as crtE, crtB, crtI, and carRP, were engineered using crtZ genes encoding β-carotene hydroxylase from different organisms. The use of crtZ from the bacterium Pantoea ananatis leads to 21.98 ± 1.80 mg/L of zeaxanthin in a medium rich in yeast extract peptone dextrose while 3.20 ± 0.11 mg/g has been estimated in a synthetic yeast nitrogen base medium to culture the cells. Additionally, a large amount of lycopene and β-carotene have been identified in zeaxanthin-producing strains (Xie et al. 2021). Likewise, to design a β-carotene-producing Y. lipolytica strain with higher β-carotene contents, Y. lipolytica was engineered in two steps. In the first step, multiple copies of 13 genes linked to the β-carotene biosynthesis pathway have been inserted in the genome of strain T1 leading to an engineered strain exhibiting an 11.7-fold increase in β-carotene content compared with the initial strain T1. In the second step, metabolic stress linked to the gradual change of cells from oval-shaped yeast to hyphae has been prevented by deleting CLA4 and MHY1 genes to maintain yeast form. This step allowed a further increase of the β-carotene production by 139% and reached 7.6 g/L and 159 mg/g DCW β-carotene in fed-batch fermentation (Liu et al. 2021a).
Two steps of metabolic engineering methods have been recently applied to generate 4.86 g/L retinol (vitamin A) in Y. lipolytica. A first step has given rise to β-carotene-producing strains exhibiting a high-lipid-production ability through the overexpression of heterologous β-carotene biosynthetic genes, endogenous geranylgeranyl pyrophosphate synthase, FAD1 encoding flavin adenine dinucleotide synthetase, and deletion of several genes with a central role in carotenoid production, followed by a second step where 11 copies of β-carotene 15,15′-dioxygenase (BCO) gene from marine bacterium 66A03 (Mb.Blh) integrated into the previously created β-carotene producer strain from the first step coupled with the addition of antioxidant butylated hydroxytoluene (BHT), allowed to recover the highest retinol producer Y. lipolytica strain CJ2104 producing 4.86 g/L bio-based retinol and 0.26 g/L retinal in fed-batch fermentation in a 5-L bioreactor (Park et al. 2022). Vitamin A (or retinol) is a micronutrient whose deficiency is connected to obesity (Viroonudomphol et al. 2003). The amounts in utero of the metabolites derived from dietary retinol, such as retinoic acid (RA), are controlled by the maternal intake of dietary vitamin A and are critical for monitoring the size of the lymphocyte pool and the resistance to infection in the offspring (van de Pavert et al. 2014). Therefore, a microbial source for retinol supplementation has a strong potential to address clinical cases related to retinol deficiency.
A genome-wide functional genetic screen platform specifically adapted to non-conventional microorganisms has been successfully designed for Y. lipolytica. To overcome the undesirable regulatory mechanisms from substrates that generally encourage the activity of some enzymes with inhibitory effects that are detrimental to the production of the molecules of interest in engineered microorganisms, two effective approaches to reduce the effect of enzyme inhibition have been applied to Y. lipolytica Po1f strain for the overproduction of the natural molecule carotenoid, especially β-carotene. The first strategy employed structure-guided protein engineering combined with phylogenetic datasets, to produce protein variants with mutated (substitutions) areas responsible for substrate inhibition. This method, informed by the strong substrate-inhibited effect of lycopene cyclase enzyme during the synthesis of carotenoids, completely impeded substrate inhibition without reducing enzyme activity and allowed to generate strain with a single mutation Y27R endowed with a noticeable increase of β-carotene production. The second strategy focused on reducing the formation rate of lycopene relative to its conversion rate and establishing a geranylgeranyl pyrophosphate synthase (GGPPS)-mediated metabolic flow restrictor that monitors the substrate lycopene formation rate, which has been implemented to maintain sub-inhibitory levels of lycopene and high lycopene conversion into β-carotene. The two strategies allowed the creation of a strain capable of producing 39.5 g/L β-carotene (98% selectivity) with a 0.165 g/L/h volumetric productivity in bioreactor fermentation and to reach lycopene titer of 17.6 g/L and productivities of 0.073 g/L/h (Ma et al. 2022).
Y. lipolytica is a good candidate for the synthesis of plant triterpenoids; a group of specialized metabolites exploited in pharmaceutical industries. For example, it has been used as a microbial cell factory to produce asiatic, madecassic, and arjunolic acids. The report showed a different amount of the aforementioned molecules resulting from the expression cytochrome P450 monoxygenases enzymes, CaCYP716C11p, CaCYP714E19p, and CaCYP716E41p, from the Centella asiatica in Y. lipolytica HiMas strain. Indeed, 8.9 mg/g DCW maslinic acid has been obtained by expressing CaCYP716C11p in oleanolic acid-producing HiMas strain, 4.4 mg/g DCW arjunolic acid was estimated after expressing codon-optimized CaCYP714E19p HiMas strain and an increase to 9.1 mg/g DCW was unveiled by swapping the N-terminal domain of CaCYP714E19p with the N-terminal domain from a Kalopanax septemlobus cytochrome P450 in HiMas strain (Arnesen et al. 2022).
The association of Y. lipolytica M53-S with Trichoderma reesei Rut C-30 during the co-fermentation of distillers grains, conducted under one-pot solid-state fermentation condition at initial moisture of 55%, pH of 5.0, NaCl addition of 0.02 g/gds and DGS mass of 200 g in 144 h, allowed a maximum erythritol production of 267.1 mg/gds (Liu et al. 2022a). This confirmed for the first time the co-fermentation potential of this oleaginous for erythritol production and the advantages of using polymicrobial culture systems for complex substrate co-utilization. Additionally, Y. lipolytica has been coupled to Trichosporon cutaneum for the bioconversion of acid-hydrolyzed spentwash-based dual-stage fermentation into lipid and volatile fatty acid (VFA). The experiment generated lipid yields ranging from 29.8 to 35%, lipid titer (0.89 g/L), and total VFA of 16 g/L have been notified with Trichosporon cutaneum, whereas only 5.5 g/L of total VFA is estimated with Y. lipolytica (Rachapudi et al. 2022). Another example of erythritol production has been provided. Heterologous overexpression of the sugar alcohol phosphatase as well as expression of native glycerol kinase (GK), and transketolase (TKL) in Y. lipolytica Po1f (MATa leu2-270 ura3-302 xpr2-322 axp1) grown on glycerol lead to the synthesis of 27.5 ± 0.7 g/L erythritol during batch growth and 58.8 ± 1.68 g/L erythritol during fed-batch growth in shake-flasks conditions. The production of erythritol gives rise to intracellular metabolites such as amino acids, sugar alcohols, and polyamines (Jagtap et al. 2021). Besides, concerning polyamines production, a yield of 4.5 g/L has been reported when the gene 4HPPD encoding 4-hydroxyphenylpyruvic acid dioxygenase enzyme implicated in homogentisic acid (direct precursor to pyomelanin) is overexpressed in aromatic amino acid (AAA)-overproducing chassis strain of Y. lipolytica (JMY8208). The aforementioned strain contains three copies of the 4HPPD overexpression cassette linked with the increase in pyomelanin yield (Larroude et al. 2021). Pyomelanin is a polymer of homogentisic acid (2,5-dihydroxyphenylacetic acid) with a multifaceted nature that can be exploited in numerous applications. For example, it may act as reporter genes and can be employed in cosmetics, dyes, colorings, and sunscreens (Weiner 1997). Therefore, Y. lipolytica is a promising biological platform to furnish acceptable quantities of a marketable pigment.
Y. lipolytica is used as a host system to synthesize high-value compounds; wax esters (WE), by expressing genes encoding fatty acyl-CoA reductases and wax ester synthase in the yeast genome. The overall production reached 7.6 g/L WE with a yield of 0.31 (g/g) from waste cooking oil (WCO) within 120h (Soong et al. 2021). The WE can be used to fill various industry niches. For example, Demski et al. (2022) entrapped moth sex pheromone precursors in WE to efficiently control the insect pests, moths (of the order Lepidoptera), known to significantly compromise the yield security of food and fiber crops.
It is worth noting that, a yeast-to-filament transition is also stimulated by neutral-alkaline pH. Indeed, it has been highlighted that the pH-responsive transcription factor Ylrim101 encoded by the gene YlRIM101, from Y. lipolytica Rim101, is the predominant regulator of alkaline-induced filamentation as its control of the expression of the majority of alkaline-regulated cell wall protein genes including cell surface glycosidase gene YlPHR1 assuming a vital role in growth, cell wall function, and filamentation at alkaline pH. This control is significantly impeded upon the deletion of YlRIM101 (Shu et al. 2021). The same authors revealed the filamentation regulatory role of the Msn2/Msn4-like transcription factor, Mhy1, that particularly monitored both alkaline- and glucose-triggered filamentation (Shu et al. 2021).
Synthesis of oleochemicals and organic acids
Recently, a metabolite-driven system has been emerged to influence numerous biological processes including oleochemicals production (fatty acid) and lipid biosynthesis. Y. lipolytica has been extensively used for producing metabolic intermediate and organic acids. Nicotinamide adenine dinucleotide phosphate (NADPH)-producing a flux of Y. lipolytica has been constructed. The integration of synthetic pathways, converting glycolytic NADH into the lipid biosynthetic precursors NADPH or acetyl-CoA, resulted in an engineered Y. lipolytica strain exhibiting productivity of 1.2 g/L/h and a process yield of 0.27 g fatty acid methyl esters/g-glucose, representing some 25% increase over previously engineered yeast strains (Qiao et al. 2017). When acetyl-CoA carboxylase (ACC) gene from Y. lipolytica ACC (YlACC1) is expressed in Saccharomyces cerevisiae CEN.PK2-1C, it highly increased the production and accumulation of malonyl-CoA in the concerned yeast (Pereira et al. 2022).The strain Y. lipolytica ARA9 grows on the crude glycerol-based medium (endorsing both substrate and osmotic agent roles) produces a five-carbon sugar alcohol compound; D-arabitol, in fed-batch fermentation conditions and in the presence of nitrogen source, NH3·H2O, acting as pH regulator. D-Arabitol production and productivity reported are 118.5 g/L and 1.10 g/L/h, respectively. When nitrogen source exceeds, the activities of cellular events such as gluconeogenesis and pentose phosphate pathways, implicated in D-arabitol synthesis, improve. These events are accompanied by an augmented expression of nucleotide and structural proteins encouraging cell growth and supporting D-arabitol biosynthesis. Under nitrogen-limited conditions, reactive oxygen species elimination, and heat shock protein response occurred within the stressed cells (Yang et al. 2021).
Y. lipolytica (Yl) W29 strain is employed for the synthesis of a high amount of organic acids, especially bio-based succinic acid (SA). The global strategy used to this end can be gathered in two steps. First, an engineered strain Y. lipolytica PGC62-SYF containing fumarate reductase encoding gene TbFrd from Trypanosoma brucei to improve the carbon flow of the reductive TCA bypass, an expressed library of the succinyl-CoA synthetase β subunit encoding gene YlScs2, endogenous isocitrate lyase YlIcl to augment the flux via the oxidative TCA pathway, and malate synthase YlMls along with mitochondrial citrate transporter YlYhm2 to improve glyoxylate bypass have been designed. This strain (Y. lipolytica PGC62-SYF) has been employed to generate Y. lipolytica PGC62-SYF-Mae, in which cell membrane transporter SpMae1 and mitochondrial transporter YlDic1 were expressed. The latter strain (Y. lipolytica PGC62-SYF-Mae) was produced in Fed-batch fermentation conditions with glucose as the sole carbon resource 101.4 g/L SA with a productivity of 0.70 g/L/h and a yield of 0.37 g/g glucose (Jiang et al. 2021). Additionally, the amount of bio-based SA obtained from the bioconversion of municipal organic biowaste by the engineered yeast strain Y. lipolytica PSA02004 has been estimated as 42.2 g/L succinic acids with 0.38 g/g yield and 0.84 g/L/h productivity in fed-batch conditions. This amount drastically increase upon gradual decrease of pH from 6 to 5.5 and reached 54.4 g/L succinic acids with 0.44 g/g yield and 0.82 g/L/h productivity and 43% lower NaOH consumption (Stylianou et al. 2021). This data also highlights the strong contribution of pH to organic acid production in Y. lipolytica. On the one hand, the essential intermediate for vitamin B12 biosynthesis; 5-Aminolevulinic acid (5-ALA), has been biosynthesized for the first time in Y. lipolytica by co-expressing genes, linked to C4 and C5 pathway, in succinate dehydrogenase (SDH)-deficient Y. lipolytica. The best-engineered strain, PGC62-IAL (MatA, xpr2–322, axp-2, leu2–270, ura3–302, ΔSdh5::loxP, ΔAch1::loxP, ScPck, ScHemI, StHemA, EcHemL), cultivated in shake flask fermentation containing glycerol substrate generate a titer of 5-ALA was 1050 mg/L. The titer was improved to 2216.8 mg/L in the fed-batch fermentation and estimated as 0.024 g/g glucose by excluding the amount of by-products; succinic acid, and porphyrin compounds (Cui et al. 2021a). Another report revealed the higher impact of the nitrogen-limited medium on SA-producing Y. lipolytica strains was initiated by the interruption of biomass growth and redirection of carbon flux toward succinic acid synthesis. For example cultivation of strains, PGC01003 and PGC202, engineered for succinic acid, in nitrogen-limited conditions in fed-batch mode with glycerol as carbon and energy source indicated production of 19 g/L SA with an overall yield of 0.23 g/g and overall productivity of 0.23 g/L/h for the strain PGC01003 while strain PGC202 produced 33 g/L succinic acid with an overall yield of 0.12 g/g and a productivity of 0.57 g/L/h (Billerach et al. 2021).
On the other hand, high-value organic acid has been produced in an engineered strain of Y. lipolytica Po1g ku70Δ. Indeed, by coupling the expression of cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus (in cytosol or peroxisome) to the overexpression of 10 genes implicated in the production pathway of acetyl-CoA (LIP2 encoding lipases, POX1-6, MFE1, POT1, and PEX10 encoding proteins essential for peroxisome assembly). with the deletion of peroxisome genes (isocitrate lyase and carnitine acetyltransferases encoding genes), an itaconic acid titer up to 54.55 g/L was reached in a 5 L bioreactor employing waste cooking oil as substrate (Rong et al. 2022).
Metabolic engineering tools and growing awareness of climate change have recently spurred efforts to design sustainable cell factories that employed eco-friendly bioprocesses and solely depended on renewable non-food bioresources as feedstocks.
Bioplastic degradation and bioremediation features of Yarrowia lipolytica
There has been a growing interest in eliminating plastic waste from the environment. Enzymes produced from Y. lipolytica can effectively treat a range of pollutants, but engineered strains showed superior characteristics as they could be more potent than natural strains and exhibited greater degradative capacities, as well as rapid adaptation to diverse pollutants used as substrates. Y. lipolytica was identified as a synthetic plastics degrader. An engineered Y. lipolytica (strain Po1f) was coupled with another microorganism to simultaneously decompose synthetic plastics (polyethylene terephthalate or PET) and produce polyhydroxybutyrate (PHB) enzyme in a one-step fermentation strategy. Indeed, Y. lipolytica Po1f expressing the PETase from Ideonalla sakaiensis with a signal peptide from lipase and contributing to the hydrolysis of bis(2-hydroxyethyl) terephthalate (BHET) and PET powder into the monomers terephthalate (TPA) and ethylene glycol (EG), was combined with TPA-degrading Pseudomonas stutzeri strain engineered by the means of a recombinant plasmid expressing enzymes for biosynthesis of PHB encoded by the phbCAB operon from Ralstonia eutropha. This co-cultivation strategy implicating the two engineered strains lead to a total production of 0.31g/L TPA from the hydrolyzation of PET in 228 h (Liu et al. 2021b). Kosiorowska et al. (2022a) improved the ability of Y. lipolytica in degrading PET by adding olive oil to the culture medium. It allowed releasing of up to 66 % of terephthalic acid into the medium. A modified Y. lipolytica A101, AJD2 pAD CUT_FS strain, expressing an extracellular cutinase from Fusarium solani, was demonstrated to decompose amorphous PET powder at 28°C to release terephthalic acid (TPA) and mono-(2-hydroxyethyl)-terephthalic acid (MHET), whose quantities were increasing daily, and estimated as 1.51g/L and 0.45g/L, respectively after 240h of bioreactor fermentation (Kosiorowska et al. 2022b). Furthermore, improving Y. lipolytica robustness against lignocellulosic-derived inhibitors has been claimed as a promising option for the transition to a bio-based economy.
Evidence of aliphatic polyester-degrading ability of Y. lipolityca has also been provided. The overexpressed native lipase Lip2 combined with the expressed cutinases from Fusarium solani f. sp. Pisi and Trichoderma reesei, in the strain A101 of Y. lipolytica rendered this strain capable of decomposing polyester at a pH ranging from 4.0 to 9.0; with the expression of the highest esterase activity being notified at pH 9.0. The engineered Y. lipolytica strain, co-expressing cutinase from F. solani and native lipase from Y. lipolytica, decomposed 0.5 g of polycaprolactone film within 144 h in shake flask conditions (Kosiorowska et al. 2021).
Its catalyst feature for bioremediation/biosensing of mixed pollutants was illustrated through the identification of Ylehd, an epoxide hydrolase with promiscuous haloalkane dehalogenase capable of catalysing structurally diverse epoxides and bromoorganics and whose expression was shown to be induced on 1,2-Epoxyoctane (EO) and 1-Bromodecane (BD) (Bendigiri et al. 2017). Furthermore, a co-expression of diacylglycerol acyltransferase (DGA1, YALI0E32769g) and transketolase (TKL1, YALI0E06479g) in Y. lipolytica cultured on both glycerol and glucose leads to higher SCO synthesis and increasing lipid content by 40% over the control strain overexpressing DGA1 (Dobrowolski and Mirończuk, 2020). Hamimed et al. (2022), revealed the bioremediation ability of Y. lipolytica strain (CLIB40) on saline wastewater. Indeed, they showed that this strain can remove 97.49% chemical oxygen demand (COD), 98.90 % phosphorus and 92.21% of salt from dephenolated olive mill wastewater (DOMW)/tuna wash processing wastewater (TWPW) mixtures. Other advantages linked to Y. lipolytica biology are highlighted in Fig. 4.
Numerous advantages linked to the oleaginous yeast Yarrowia lipolytica. The design is based on the work of Gul et al. 2019; Zhang et al. 2022; Parey et al. 2021; Wang et al. 2021; Xie et al. 2022; Elkins et al. 2022; Domenzain et al. 2022 and Zainuddin et al. 2022. Different colors are retained for more visibility
Biomass-degrading features of Yarrowia lipolytica
With the increasing environmental issues initiated by fossil fuels and their rapid depletion, the requirement for a renewable resource that can solve these environmental issues in a greener way has emerged. Biodiesel production from one of the richest sources of natural sugars, lignocellulosic biomass, has been reported for this oleaginous yeast. Y. lipolytica has been demonstrated to convert sugarcane bagasse hydrolysates, deriving from alkaline pre-treatment combined with ultrasonication, into 16.39 g/L yeast biomass, which is further submitted to in-situ transesterification with K2CO3 catalyst and ex-situ transesterification with KOH catalyst to yield 80% and 63% biodiesel respectively (Vasaki et al. 2022). Yet, it should be stressed that Y. lipolytica can exhibit a slow growth on lignocellulosic hydrolysates due to the presence of residual phenolic aldehydes; an inhibitor that also stimulates poor cell growth and metabolism in other oleaginous yeasts such as Rhodosporidium toruloides, Rhodotorula glutinis (Zhang et al. 2022).
Generally, the use of cellulosic biomass necessitates a pre-conversion to highly concentrated biomass hydrolysates that will thereafter be used as substrate and subject to bioconversion by microbes. Wei et al. (2021a) conferred cellulolytic activity (important for cellulosic biomass decomposition) to Y. lipolytica by knocking out the sucrose non-fermenting 1 (SNF1) gene-mediated lipid and protein biosynthesis processes inhibition and knocking in the cellulase cassette fused with the recyclable selection marker URA3 gene in a lipid-accumulating Y. lipolytica strain overexpressing both ATP citrate lyase (ACL) and diacylglycerol acyltransferase 1 (DGA1) genes. They reported improved cell growth and lipid accumulation upon SNF1 gene disruption, a drastic reduction of cellular saturated fatty acid level, as well as saturated to unsaturated fatty acid ratio, and reduced endoplasmic reticulum stress in the mutant YL163t compared to its parent strain Po1g ACL-DGA1 (Wei et al. 2021a). Enabling efficient cellulosic biomass valorization by the oleaginous yeast Y. lipolytica would greatly facilitate industrial cellulosic biorefineries knowing its versatility and robustness as an industrial production platform. Additional tools and applications available for Y. lipolytica are highlighted in Table 1. As a trade-off between growth and production may impact the overall production of engineered microbes, any approach to predict metabolic reactions that influence metabolite production can strongly improve growth and allow for the achievement of high production of value-added molecules in oleaginous yeast.
New computer-assisted and synthetic tools for Yarrowia lipolytica engineering
Machine learning methods coupled with metabolic pathways engineering have been attracting much attention to synthesize natural and non-natural compounds. A deep learning-based guide design algorithm tool, also known as DeepGuide, allows to accurately predict the activity of 20 nt Streptococcus pyogenes Cas9 single guide RNA (sgRNA) with an NGG PAM, as well as 25 nt Lachnospiraceae bacterium Cas12a sgRNA with a TTTV PAM, has been developed for Y. lipolytica. This algorithm is made of three interconnected neural networks spanning convolutional autoencoder (CAE) exploiting the k-mers from the genome of interest, a convolutional fully connected neural network (FCCN) trained via back-propagation from input pairs of sgRNA sequences and their corresponding cutting score (CS) values, and a small fully connected network that is employed to capture nucleosome occupancy data (Baisya et al. 2022). The dimorphic yeast Y. lipolytica, a representative hemiascomycota yeast, attracts much attention as it is highly amenable to genetic perturbations. Nevertheless, the low homologous recombination (HR) efficiency hinders its accurate genetic manipulation during microbial cell factory construction, and any method that can enhance HR activity and down-regulate the non-homologous end joining (NHEJ) is viewed as a suitable solution for the high amount of value-added biological chemicals. Considering that the NHEJ preference (major repair pathway for DNA double-strand breaks) of Y. lipolytica, can restrict lipid production, a genome-scale trackable mutagenesis library, allows for randomly inserting DNA across the chromosomes, more specifically in both nucleosome-occupancy regions and nucleosome-free regions, has been designed for Y. lipolytica. This mutagenesis approach focused on NHEJ-mediated integration and enhanced β-carotene biosynthesis and acetic acid tolerance rapidly (Liu et al. 2022b). It has been uncovered that metabolic engineering-designed Y. lipolytica strains, by the mean of Non-homologous end joining (NHEJ)-mediated integration targeting YALI0_A00913g (“A1 gene”) for deletion, displayed an enhanced protein synthesis process as well as fatty alcohol overproduction phenotype when transformed with fatty acyl-CoA reductase gene (FAR) and grown in batch conditions (Li et al. 2022b).
Furthermore, a Golden Gate modular cloning system (YALIcloneNHEJ) for robust DNA assembly and exploiting the Non-homologous end-joining (NHEJ)-mediated random integration system has been designed and used in Y. lipolytica for the overproduction of the sesquiterpene (-)-α-bisabolol. To this end, some constructs for the biosynthesis route and improvement of the flux in the mevalonate pathway have been employed. The engineered strain produced 4.4 g/L (-)-α-bisabolol (Li et al. 2022a, c, d).
This species has been used to develop artificial chromosomes (ylAC) enabling rapid and efficient assembly of multiple genes (genes for xylose utilization XYL1, XYL2, and XKS1), genes for cellobiose consumption (CBP1, CDT1, and scPGM2)) and chromosomal elements in a single step in vivo, in less than one week, into a complete, independent, and linear supplementary chromosome with a yield over 90%. The design ylAC can be genetically conserved over several generations either under selective conditions or, without selective pressure, exploiting an essential gene as the selection marker (Guo et al. 2020).
Conclusion
The review improved our understanding of molecular events occurring in Yarrowia lipolytica cells upon substrate utilization and protein overproduction. We notified that this yeast has unlimited abilities in the synthesis of desired molecules and even its morphological transition can be controlled for up-scaling the biosynthesis of targeted compounds in hyper-producer strains. This oleaginous yeast possessed a feature for designing some sustainable biocontrol strategies in the food industry. More Y. lipolytica strains with biocontrol characteristics against pathogens should be investigated. Its feature concerning the bioconversion of agro-industrial residues containing inhibitors should be extended to diversify the low-cost substrates it can use to generate cost-effective value-added products by exploiting the new available synthetic tools.
Data availability
Not applicable
References
Anche VC, Fakas S (2022) Regulation of ATP-Citrate lyase during lipogenesis in the oleaginous Yeast Yarrowia lipolytica. 36(S1). https://doi.org/10.1096/fasebj.2022.36.S1.R6042
Arnesen JA, Belmonte DAA, Jayachandran S, Dahlin J, Rago D, Andersen AJC, Borodina I (2022) Engineering of Yarrowia lipolytica for the production of plant triterpenoids: asiatic, madecassic, and arjunolic acids. Metab Eng Commun 14:e00197. https://doi.org/10.1016/j.mec.2022.e00197
Baisya D, Ramesh A, Schwartz C, Lonardi S, Wheeldon I (2022) Genome-wide functional screens enable the prediction of high activity CRISPR-Cas9 and -Cas12a guides in Yarrowia lipolytica. Nat Commun 13(1):922. https://doi.org/10.1038/s41467-022-28540-0
Belury MA (2002) DIETARY CONJUGATED LINOLEIC ACID IN HEALTH: Physiological effects and mechanisms of action. 22(1):505-531. https://doi.org/10.1146/annurev.nutr.22.021302.121842
Bendigiri C, Zinjarde S, RaviKumar A (2017) Ylehd, an epoxide hydrolase with promiscuous haloalkane dehalogenase activity from tropical marine yeast Yarrowia lipolytica is induced upon xenobiotic stress. Sci Rep 7(1):11887. https://doi.org/10.1038/s41598-017-12284-9
Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS, William E. Bentley Introduction by (2009) Plasmid-encoded protein: the principal factor in the “metabolic burden” associated with recombinant bacteria. 102(5):1283-1297. https://doi.org/10.1002/bit.22292
Beopoulos A, Nicaud JM, Gaillardin C (2011) An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol 90(4):1193–1206. https://doi.org/10.1007/s00253-011-3212-8
Bhutada G, Menard G, Bhunia RK, Hapeta PP, Ledesma-Amaro R, Eastmond PJ (2022) Production of human milk fat substitute by engineered strains of Yarrowia lipolytica. Metab Eng Commun 14:e00192. https://doi.org/10.1016/j.mec.2022.e00192
Billerach G, Preziosi-Belloy L, Lin CSK, Fulcrand H, Dubreucq E, Grousseau E (2021) Impact of nitrogen deficiency on succinic acid production by engineered strains of Yarrowia lipolytica. J Biotechnol 336:30–40. https://doi.org/10.1016/j.jbiotec.2021.06.001
Carrea G, Riva S (2000) Properties and Synthetic Applications of Enzymes in Organic Solvents. 39(13):2226-2254. https://doi.org/10.1002/1521-3773(20000703)39:13<2226::AID-ANIE2226>3.0.CO;2-L
Chen L, Yan W, Qian X, Chen M, Zhang X, Xin F, Zhang W, Jiang M, Ochsenreither K (2021) Increased lipid production in Yarrowia lipolytica from acetate through metabolic engineering and cosubstrate fermentation. ACS Synth Biol 10(11):3129–3138. https://doi.org/10.1021/acssynbio.1c00405
Coşgun A, Günay ME, Yıldırım R (2022) Analysis of lipid production from Yarrowia lipolytica for renewable fuel production by machine learning. Fuel 315:122817. https://doi.org/10.1016/j.fuel.2021.122817
Cui Z, Zhu Z, Zhang J, Jiang Z, Liu Y, Wang Q, Hou J, Qi Q (2021a) Efficient 5-aminolevulinic acid production through reconstructing the metabolic pathway in SDH-deficient Yarrowia lipolytica. Biochem Eng J 174:108125. https://doi.org/10.1016/j.bej.2021.108125
Cui Z, Zheng H, Zhang J, Jiang Z, Zhu Z, Liu X, Qi Q, Hou J, Kivisaar M (2021b) A CRISPR/Cas9-mediated, homology-independent tool developed for targeted genome integration in Yarrowia lipolytica. 87(6):e02666-20. https://doi.org/10.1128/AEM.02666-20
Demski K, Ding BJ, Wang HL, Tran TNT, Durrett TP, Lager I, Löfstedt C, Hofvander P (2022) Manufacturing specialized wax esters in plants. Metab Eng 72:391–402. https://doi.org/10.1016/j.ymben.2022.05.005
Dissook S, Kuzuyama T, Nishimoto Y, Kitani S, Putri S, Fukusaki E (2021) Stable isotope and chemical inhibition analyses suggested the existence of a non-mevalonate-like pathway in the yeast Yarrowia lipolytica. Sci Rep 11(1):5598. https://doi.org/10.1038/s41598-021-85170-0
Dobrowolski A, Mirończuk AM (2020) The influence of transketolase on lipid biosynthesis in the yeast Yarrowia lipolytica. Microb Cell Factories 19(1):138. https://doi.org/10.1186/s12934-020-01398-x
Dobrowolski A, Drzymała K, Rzechonek DA, Mituła P, Mirończuk AM (2019) Lipid Production From Waste Materials in Seawater-Based Medium by the Yeast Yarrowia lipolytica. Front Microbiol 10:547. https://doi.org/10.3389/fmicb.2019.00547
Domenzain I, Sánchez B, Anton M, Kerkhoven EJ, Millán-Oropeza A, Henry C, Siewers V, Morrissey JP, Sonnenschein N, Nielsen J (2022) Reconstruction of a catalogue of genome-scale metabolic models with enzymatic constraints using GECKO 2.0. Nat Commun 13(1):3766. https://doi.org/10.1038/s41467-022-31421-1
Dourou M, Economou CN, Aggeli L, Janák M, Valdés G, Elezi N, Kakavas D, Papageorgiou T, Lianou A, Vayenas DV, Certik M, Aggelis G (2021) Bioconversion of pomegranate residues into biofuels and bioactive lipids. J Clean Prod 323:129193. https://doi.org/10.1016/j.jclepro.2021.129193
Drzymała K, Mirończuk AM, Pietrzak W, Dobrowolski A (2020) Rye and oat agricultural wastes as substrate candidates for biomass production of the non-conventional yeast Yarrowia lipolytica 12(18):7704
EFSA. Safety of an extension of use of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022;20(7):7450. https://doi.org/10.2903/j.efsa.2022.7450
Elkins JG, Rodriguez M, Cannon ON, Connatser RM, Oguntimein GB, Kass MD, West B, Davison BH (2022) n-Butanol or isobutanol as a value-added fuel additive to inhibit microbial degradation of stored gasoline. JFUECO:100072. https://doi.org/10.1016/j.jfueco.2022.100072
Erian AM, Egermeier M, Marx H, Sauer M (2022) Insights into the glycerol transport of Yarrowia lipolytica. 39(5):323-336. https://doi.org/10.1002/yea.3702
Fabiszewska A, Paplińska-Goryca M, Misiukiewicz-Stępień P, Wołoszynowska M, Nowak D, Zieniuk B (2022) Expression profile of selected genes involved in storage lipid synthesis in a model oleaginous yeast species Yarrowia lipolytica. 23(3):1041
Gajdoš P, Urbaníková V, Vicenová M, Čertík M (2022) Enhancing very long chain fatty acids production in Yarrowia lipolytica. Microb Cell Factories 21(1):138. https://doi.org/10.1186/s12934-022-01866-6
Godana EA, Zhang X, Hu W, Zhao L, Gu X, Zhang H (2022) Transcriptome analysis of asparagus in response to postharvest treatment with Yarrowia lipolytica. Biol Control 169:104906. https://doi.org/10.1016/j.biocontrol.2022.104906
Gorczyca M, Kaźmierczak J, Fickers P, Celińska E (2022) Synthesis of secretory proteins in Yarrowia lipolytica: effect of combined stress factors and metabolic load. 23(7):3602
Gul JF, Hamayun M, Hussain A, Jan G, Iqbal A, Khan A, Lee IJ (2019) An endophytic isolate of the fungus Yarrowia lipolytica produces metabolites that ameliorate the negative impact of salt stress on the physiology of maize. BMC Microbiol 19(1):3. https://doi.org/10.1186/s12866-018-1374-6
Guo ZP, Borsenberger V, Croux C, Duquesne S, Truan G, Marty A, Bordes F (2020) An artificial chromosome ylAC enables efficient assembly of multiple genes in Yarrowia lipolytica for biomanufacturing. Commun Biol 3(1):199. https://doi.org/10.1038/s42003-020-0936-y
Guo Y, Su L, Liu Q, Zhu Y, Dai Z, Wang Q (2022) Dissecting carbon metabolism of Yarrowia lipolytica type strain W29 using genome-scale metabolic modelling. Comput Struct Biotechn 20:2503–2511. https://doi.org/10.1016/j.csbj.2022.05.018
Hamimed S, Gamraoui A, Landoulsi A, Chatti A (2022) Bio-nanocrystallization of NaCl using saline wastewaters through biological treatment by Yarrowia lipolytica. Environ Technol Innov 26:102338. https://doi.org/10.1016/j.eti.2022.102338
Hu W, Zhang X, Godana EA, Gu X, Zhao L, Zhang H (2021) Yarrowia lipolytica reduces the disease incidence of asparagus infected by Fusarium proliferatum by affecting respiratory metabolism and energy status. Biol Control 159:104625. https://doi.org/10.1016/j.biocontrol.2021.104625
Hussein KA, Tohamy TA, El-Maraghy SS (2022) Amino-cyclopropane-1-carboxylate deaminase (ACCD) producing yeasts improved salinity tolerance of Triticum aestivum L. Rhizosphere 23:100548. https://doi.org/10.1016/j.rhisph.2022.100548
Jach ME, Malm A (2022) Yarrowia lipolytica as an alternative and valuable source of nutritional and bioactive compounds for humans. Molecules (Basel, Switzerland) 27(7). https://doi.org/10.3390/molecules27072300
Jagtap SS, Bedekar AA, Singh V, Jin YS, Rao CV (2021) Metabolic engineering of the oleaginous yeast Yarrowia lipolytica PO1f for production of erythritol from glycerol. Biotechnol Biofuels 14(1):188. https://doi.org/10.1186/s13068-021-02039-0
Jiang Z, Cui Z, Zhu Z, Liu Y, Tang YJ, Hou J, Qi Q (2021) Engineering of Yarrowia lipolytica transporters for high-efficient production of biobased succinic acid from glucose. Biotechnol Biofuels 14(1):145. https://doi.org/10.1186/s13068-021-01996-w
John-White M, Gardiner J, Johanesen P, Lyras D, Dumsday G, Atomi H (2019) β-Aminopeptidases: Insight into enzymes without a known natural substrate. 85(15):e00318-19. https://doi.org/10.1128/AEM.00318-19
Kashyap A, Gupta R (2021) Disrupting putative N-glycosylation site N17 in lipase Lip11 of Yarrowia lipolytica yielded a catalytically efficient and thermostable variant accompanying conformational changes. Enzym Microb Technol 151:109922. https://doi.org/10.1016/j.enzmictec.2021.109922
Kashyap A, Gupta R (2022) N-truncation in lipase Lip11 from Yarrowia lipolytica alleviates substrate inhibition with improved stability and efficiency ensuing distinct structural modifications. Process Biochem 116:185–196. https://doi.org/10.1016/j.procbio.2022.03.013
Kerkhoven EJ, Pomraning KR, Baker SE, Nielsen J (2016) Regulation of amino-acid metabolism controls flux to lipid accumulation in Yarrowia lipolytica. NPJ Syst Biol Appl 2(1):16005. https://doi.org/10.1038/npjsba.2016.5
Kettner L, Braun C, Seitl I, Pross E, Fischer L (2021) Production and characterization of a new diamine oxidase from Yarrowia lipolytica. J Biotechnol 340:39–46. https://doi.org/10.1016/j.jbiotec.2021.08.015
Konzock O, Matsushita Y, Zaghen S, Sako A, Norbeck J (2022) Altering the fatty acid profile of Yarrowia lipolytica to mimic cocoa butter by genetic engineering of desaturases. Microb Cell Factories 21(1):25. https://doi.org/10.1186/s12934-022-01748-x
Korpys-Woźniak P, Celińska E (2021) Global transcriptome profiling reveals genes responding to overproduction of a small secretory, a high cysteine- and a high glycosylation-bearing protein in Yarrowia lipolytica. Biotechnol Rep 31:e00646. https://doi.org/10.1016/j.btre.2021.e00646
Korpys-Woźniak P, Kubiak P, Celińska E (2021) Secretory helpers for enhanced production of heterologous proteins in Yarrowia lipolytica. Biotechnol Rep 32:e00669. https://doi.org/10.1016/j.btre.2021.e00669
Kosiorowska KE, Połomska X, Wang G, Borodina I, Mirończuk AM (2021) Efficient biodegradation of aliphatic polyester by genetically engineered strains of the yeast Yarrowia lipolytica. Int Biodeterior Biodegradation 161:105232. https://doi.org/10.1016/j.ibiod.2021.105232
Kosiorowska KE, Moreno GAD, Iglesias R, Leluk K, Mirończuk AM (2022a) Production of PETase by engineered Yarrowia lipolytica for efficient poly(ethylene terephthalate) biodegradation. Sci Total Environ 157358. https://doi.org/10.1016/j.scitotenv.2022.157358
Kosiorowska KE, Biniarz P, Dobrowolski A, Leluk K, Mirończuk AM (2022b) Metabolic engineering of Yarrowia lipolytica for poly(ethylene terephthalate) degradation. Sci Total Environ 831:154841. https://doi.org/10.1016/j.scitotenv.2022.154841
Kothari SD, Vadgama RN, Bhat KH, Lali AM, Odaneth AA (2022) Process optimization for production and purification of γ-decalactone from ricinoleic acid using Yarrowia lipolytica NCIM 3590. Biocatal Agric Biotechnol 39:102285. https://doi.org/10.1016/j.bcab.2022.102285
Kubiak M, Białas W, Celińska E (2021) Thermal treatment improves a process of crude glycerol valorization for the production of a heterologous enzyme by Yarrowia lipolytica. Biotechnol Rep 31:e00648. https://doi.org/10.1016/j.btre.2021.e00648
Kubiak-Szymendera M, Skupien-Rabian B, Jankowska U, Celińska E (2022) Hyperosmolarity adversely impacts recombinant protein synthesis by Yarrowia lipolytica-molecular background revealed by quantitative proteomics. Appl Microbiol Biotechnol 106(1):349–367. https://doi.org/10.1007/s00253-021-11731-y
Larroude M, Onésime D, Rué O, Nicaud JM, Rossignol T (2021) A Yarrowia lipolytica Strain Engineered for Pyomelanin Production. Microorganisms 9(4). https://doi.org/10.3390/microorganisms9040838
Lesage J, Timoumi A, Cenard S, Lombard E, Lee HLT, Guillouet SE, Gorret N (2021) Accelerostat study in conventional and microfluidic bioreactors to assess the key role of residual glucose in the dimorphic transition of Yarrowia lipolytica in response to environmental stimuli. New Biotechnol 64:37–45. https://doi.org/10.1016/j.nbt.2021.05.004
Li M, Zhang J, Bai Q, Fang L, Song H, Cao Y (2022a) Non-homologous end joining-mediated insertional mutagenesis reveals a novel target for enhancing fatty alcohols production in Yarrowia lipolytica. 13. https://doi.org/10.3389/fmicb.2022.898884
Li S, Rong L, Wang S, Liu S, Lu Z, Miao L, Zhao B, Zhang C, Xiao D, Pushpanathan K, Wong A, Yu A (2022b) Enhanced limonene production by metabolically engineered Yarrowia lipolytica from cheap carbon sources. Chem Eng Sci 249:117342. https://doi.org/10.1016/j.ces.2021.117342
Li X, Ren JN, Fan G, He J, Zhang LL, Pan SY (2022c) Genomic and transcriptomic analysis screening key genes for (+)-valencene biotransformation to (+)-nootkatone in Yarrowia lipolytica. Microbiol Res 260:127042. https://doi.org/10.1016/j.micres.2022.127042
Li YW, Yang CL, Shen Q, Peng QQ, Guo Q, Nie ZK, Sun XM, Shi TQ, Ji XJ, Huang H (2022d) YALIcloneNHEJ: An efficient modular cloning toolkit for NHEJ integration of multigene pathway and terpenoid production in Yarrowia lipolytica. 9. https://doi.org/10.3389/fbioe.2021.816980
Licona-Jain A, Racotta I, Angulo C, Luna-González A, Escamilla-Montes R, Cortés-Jacinto E, Morelos-Castro RM, Campa-Córdova ÁI (2022) Combined administration routes of marine yeasts enhanced immune-related genes and protection of white shrimp (Penaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol 124:192–200. https://doi.org/10.1016/j.fsi.2022.04.004
Liu M, Zhang J, Ye J, Qi Q, Hou J (2021a) Morphological and metabolic engineering of Yarrowia lipolytica to increase β-carotene production. ACS Synth Biol 10(12):3551–3560. https://doi.org/10.1021/acssynbio.1c00480
Liu P, Zhang T, Zheng Y, Li Q, Su T, Qi Q (2021b) Potential one-step strategy for PET degradation and PHB biosynthesis through co-cultivation of two engineered microorganisms. Engineering Microbiology 1:100003. https://doi.org/10.1016/j.engmic.2021.100003
Liu L, Wang Z, Zheng Z, Li Z, Ji X, Cong H, Wang H (2021c) Secretory expression of an alkaline alginate lyase with heat recovery property in Yarrowia lipolytica. 12. https://doi.org/10.3389/fmicb.2021.710533
Liu X, Yu X, He A, Xia J, He J, Deng Y, Xu N, Qiu Z, Wang X, Zhao P (2022a) One-pot fermentation for erythritol production from distillers grains by the co-cultivation of Yarrowia lipolytica and Trichoderma reesei. Bioresour Technol 351:127053. https://doi.org/10.1016/j.biortech.2022.127053
Liu X, Liu M, Zhang J, Chang Y, Cui Z, Ji B, Nielsen J, Qi Q, Hou J (2022b) Mapping of nonhomologous end joining-mediated integration facilitates genome-scale trackable mutagenesis in Yarrowia lipolytica. ACS Synth Biol 11(1):216–227. https://doi.org/10.1021/acssynbio.1c00390
Ma Y, Li J, Huang S, Stephanopoulos G (2021) Targeting pathway expression to subcellular organelles improves astaxanthin synthesis in Yarrowia lipolytica. Metab Eng 68:152–161. https://doi.org/10.1016/j.ymben.2021.10.004
Ma Y, Liu N, Greisen P, Li J, Qiao K, Huang S, Stephanopoulos G (2022) Removal of lycopene substrate inhibition enables high carotenoid productivity in Yarrowia lipolytica. Nat Commun 13(1):572. https://doi.org/10.1038/s41467-022-28277-w
Mei XY, Zhang JN, Jia WY, Lu B, Wang MN, Zhang TY, Ji LL (2022) Scutellarin suppresses triple-negative breast cancer metastasis by inhibiting TNFα-induced vascular endothelial barrier breakdown. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-022-00873-y
Mihreteab M, Stubblefield BA, Gilbert ES (2021) Enhancing polypropylene bioconversion and lipogenesis by Yarrowia lipolytica using a chemical/biological hybrid process. J Biotechnol 332:94–102. https://doi.org/10.1016/j.jbiotec.2021.03.015
Mou JH, Tahar IB, Wang ZY, Ong KL, Li C, Qin ZH, Wang X, Lin CSK, Fickers P (2021) Enhancing the recombinant protein productivity of Yarrowia lipolytica using in situ fibrous bed bioreactor. Bioresour Technol 340:125672. https://doi.org/10.1016/j.biortech.2021.125672
Moujehed E, Zarai Z, Khemir H, Miled N, Bchir MS, Gablin C, Bessueille F, Bonhommé A, Leonard D, Carrière F, Aloulou A (2022) Cleaner degreasing of sheepskins by the Yarrowia lipolytica LIP2 lipase as a chemical-free alternative in the leather industry. Colloids Surf B: Biointerfaces Biointerfaces 211:112292. https://doi.org/10.1016/j.colsurfb.2021.112292
Neuls L, Souza VJ, Romão S, Bitencourt TB, Ramos CJR, Parra JEG, Cazarolli LH (2021) Immunomodulatory effects of Yarrowia lipolytica as a food additive in the diet of Nile tilapia. Fish Shellfish Immunol 119:272–279. https://doi.org/10.1016/j.fsi.2021.10.011
Novikova LA, Yovkova V, Luzikov VN, Barth G, Mauersberger S (2021) Recombinant Yarrowia lipolytica strains for the heterologous expression of multi-component enzyme systems: expression of mammalian steroidogenic proteins. J Biotechnol 339:42–52. https://doi.org/10.1016/j.jbiotec.2021.07.012
Pandit S, Konzock O, Leistner K, Mokkapati V, Merlo A, Sun J, Mijakovic I (2021) Graphene coated magnetic nanoparticles facilitate the release of biofuels and oleochemicals from yeast cell factories. Sci Rep 11(1):20612. https://doi.org/10.1038/s41598-021-00189-7
Parey K, Lasham J, Mills DJ, Djurabekova A, Haapanen O, Yoga EG, Xie H, Kühlbrandt W, Sharma V, Vonck J, Zickermann V (2021) High-resolution structure and dynamics of mitochondrial complex I-Insights into the proton pumping mechanism. 7(46):eabj3221. https://doi.org/10.1126/sciadv.abj3221
Park H, Lee D, Kim JE, Park S, Park JH, Ha CW, Baek M, Yoon SH, Park KH, Lee P, Hahn JS (2022) Efficient production of retinol in Yarrowia lipolytica by increasing stability using antioxidant and detergent extraction. Metab Eng 73:26–37. https://doi.org/10.1016/j.ymben.2022.06.001
van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labão-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O’Toole T, Mizee MR, Habani Y, Haak E, Santori FR et al (2014) Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508(7494):123–127. https://doi.org/10.1038/nature13158
Pereira H, Azevedo F, Domingues L, Johansson B (2022) Expression of Yarrowia lipolytica acetyl-CoA carboxylase in Saccharomyces cerevisiae and its effect on in-vivo accumulation of Malonyl-CoA. Comput Struct Biotechnol J 20:779–787. https://doi.org/10.1016/j.csbj.2022.01.020
Qiao K, Wasylenko TM, Zhou K, Xu P, Stephanopoulos G (2017) Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat Biotechnol 35(2):173–177. https://doi.org/10.1038/nbt.3763
Rachapudi VS, Sai TG, Venkata MS (2022) Dual-stage biorefinery to convert spentwash hydrolysate into oleochemicals using Trichosporon cutaneum and Yarrowia lipolytica. Bioresour Technol 354:127146. https://doi.org/10.1016/j.biortech.2022.127146
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51. https://doi.org/10.1016/s0065-2164(02)51000-5
Rong L, Miao L, Wang S, Wang Y, Liu S, Lu Z, Zhao B, Zhang C, Xiao D, Pushpanathan K, Wong A, Yu A (2022) Engineering Yarrowia lipolytica to produce itaconic acid from waste cooking oil. 10. https://doi.org/10.3389/fbioe.2022.888869
Rossi S, Parrotta L, Del DS, Rosa MD, Patrignani F, Schluter O, Lanciotti R (2021) Effect of Yarrowia lipolytica RO25 cricket-based hydrolysates on sourdough quality parameters. LWT 148:111760. https://doi.org/10.1016/j.lwt.2021.111760
Ryu S, Trinh CT, Elliot MA (2018) Understanding functional roles of native pentose-specific transporters for activating dormant pentose metabolism in Yarrowia lipolytica. 84(3):e02146-e02117. https://doi.org/10.1128/AEM.02146-17
Schrader J, Etschmann MM, Sell D, Hilmer JM, Rabenhorst J (2004) Applied biocatalysis for the synthesis of natural flavour compounds--current industrial processes and future prospects. Biotechnol Lett 26(6):463–472. https://doi.org/10.1023/b:bile.0000019576.80594.0e
Shu T, He XY, Chen JW, Mao YS, Gao XD, Mitchell AP (2021) The pH-Responsive transcription factors YlRim101 and Mhy1 regulate alkaline pH-induced filamentation in the dimorphic yeast Yarrowia lipolytica. 6(3):e00179-21. https://doi.org/10.1128/mSphere.00179-21
Soong YHV, Zhao L, Liu N, Yu P, Lopez C, Olson A, Wong HW, Shao Z, Xie D (2021) Microbial synthesis of wax esters. Metab Eng 67:428–442. https://doi.org/10.1016/j.ymben.2021.08.002
Sreeharsha RV, Sai TG, Venkata MS (2022) Dual-stage biorefinery to convert spentwash hydrolysate into oleochemicals using Trichosporon cutaneum and Yarrowia lipolytica. Bioresour Technol 354:127146. https://doi.org/10.1016/j.biortech.2022.127146
Stylianou E, Pateraki C, Ladakis D, Damala C, Vlysidis A, Latorre-Sánchez M, Coll C, Lin CSK, Koutinas A (2021) Bioprocess development using organic biowaste and sustainability assessment of succinic acid production with engineered Yarrowia lipolytica strain. Biochem Eng J 174:108099. https://doi.org/10.1016/j.bej.2021.108099
Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Theron CW, Vandermies M, Telek S, Steels S, Fickers P (2020) Comprehensive comparison of Yarrowia lipolytica and Pichia pastoris for production of Candida antarctica lipase B. Sci Rep 10(1):1741. https://doi.org/10.1038/s41598-020-58683-3
Vargas O, Gutiérrez MS, Caruffo M, Valderrama B, Medina DA, García K, Reyes-Jara A, Toro M, Feijóo CG, Navarrete P (2021) Probiotic yeasts and Vibrio anguillarum infection modify the microbiome of Zebrafish Larvae. 12. https://doi.org/10.3389/fmicb.2021.647977
Vasaki M, Sithan M, Ravindran G, Paramasivan B, Ekambaram G, Karri RR (2022) Biodiesel production from lignocellulosic biomass using Yarrowia lipolytica. Energy Convers Manag X 13:100167. https://doi.org/10.1016/j.ecmx.2021.100167
da Veiga MJ, Jolicoeur M, Schwartz L, Peres S (2021) Fine-tuning mitochondrial activity in Yarrowia lipolytica for citrate overproduction. Sci Rep 11(1):878. https://doi.org/10.1038/s41598-020-79577-4
Viroonudomphol D, Pongpaew P, Tungtrongchitr R, Changbumrung S, Tungtrongchitr A, Phonrat B, Vudhivai N, Schelp FP (2003) The relationships between anthropometric measurements, serum vitamin A and E concentrations and lipid profiles in overweight and obese subjects. Asia Pac J Clin Nutr 12(1):73–79
Walker C, Ryu S, Giannone RJ, Garcia S, Trinh CT, Atomi H (2020) Understanding and eliminating the detrimental effect of thiamine deficiency on the oleaginous yeast Yarrowia lipolytica. 86(3):e02299-e02219. https://doi.org/10.1128/AEM.02299-19
Walker C, Dien B, Giannone RJ, Slininger P, Thompson SR, Trinh CT, Tullman-Ercek D (2021) Exploring proteomes of robust Yarrowia lipolytica isolates cultivated in biomass hydrolysate reveals key processes impacting mixed sugar utilization, lipid accumulation, and degradation. 6(4):e00443-21. https://doi.org/10.1128/mSystems.00443-21
Wang W, Blenner MA (2022) Engineering heterologous enzyme secretion in Yarrowia lipolytica. Microb Cell Factories 21(1):134. https://doi.org/10.1186/s12934-022-01863-9
Wang G, Olofsson-Dolk M, Hansson FG, Donati S, Li X, Chang H, Cheng J, Dahlin J, Borodina I (2021) Engineering yeast Yarrowia lipolytica for methanol assimilation. ACS Synth Biol 10(12):3537–3550. https://doi.org/10.1021/acssynbio.1c00464
Wang Y, Liu X, Chen B, Liu W, Guo Z, Liu X, Zhu X, Liu J, Zhang J, Li J, Zhang L, Gao Y, Zhang G, Wang Y, Choudhary MI, Yang S, Jiang H (2022) Metabolic engineering of Yarrowia lipolytica for scutellarin production. Synth Syst Biotechnol 7(3):958–964. https://doi.org/10.1016/j.synbio.2022.05.009
Wasylenko TM, Ahn WS, Stephanopoulos G (2015) The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab Eng 30:27–39. https://doi.org/10.1016/j.ymben.2015.02.007
Wei H, Wang W, Knoshaug EP, Chen X, Van WS, Bomble YJ, Himmel ME, Zhang M (2021a) Disruption of the Snf1 gene enhances cell growth and reduces the metabolic burden in cellulase-expressing and lipid-accumulating Yarrowia lipolytica. 12. https://doi.org/10.3389/fmicb.2021.757741
Wei LJ, Cao X, Liu JJ, Kwak S, Jin YS, Wang W, Hua Q, Atomi H (2021b) Increased accumulation of squalene in engineered Yarrowia lipolytica through deletion of PEX10 and URE2. 87(17):e00481-21. https://doi.org/10.1128/AEM.00481-21
Weiner RM (1997) Biopolymers from marine prokaryotes. Trends Biotechnol 15(10):390–394. https://doi.org/10.1016/S0167-7799(97)01099-8
Workman M, Holt P, Thykaer J (2013) Comparing cellular performance of Yarrowia lipolytica during growth on glucose and glycerol in submerged cultivations. AMB Express 3(1):58. https://doi.org/10.1186/2191-0855-3-58
Wu Y, Xu S, Gao X, Li M, Li D, Lu W (2019) Enhanced protopanaxadiol production from xylose by engineered Yarrowia lipolytica. Microb Cell Factories 18(1):83. https://doi.org/10.1186/s12934-019-1136-7
Xie Y, Chen S, Xiong X (2021) Metabolic engineering of non-carotenoid-producing yeast Yarrowia lipolytica for the biosynthesis of zeaxanthin. 12. https://doi.org/10.3389/fmicb.2021.699235
Xie X, Yang K, Lu Y, Li Y, Yan J, Huang J, Xu L, Yang M, Yan Y (2022) Broad-spectrum and effective rare earth enriching via Lanmodulin-displayed Yarrowia lipolytica. J Hazard Mater 129561. https://doi.org/10.1016/j.jhazmat.2022.129561
Yan J, Han B, Gui X, Wang G, Xu L, Yan Y, Madzak C, Pan D, Wang Y, Zha G, Jiao L (2018) Engineering Yarrowia lipolytica to simultaneously produce lipase and single cell protein from agro-industrial wastes for feed. Sci Rep 8(1):758. https://doi.org/10.1038/s41598-018-19238-9
Yang L, Kong W, Yang W, Li D, Zhao S, Wu Y, Zheng S (2021) High D-arabitol production with osmotic pressure control fed-batch fermentation by Yarrowia lipolytica and proteomic analysis under nitrogen source perturbation. Enzym Microb Technol 152:109936. https://doi.org/10.1016/j.enzmictec.2021.109936
Yang R, Chen Z, Hu P, Zhang S, Luo G (2022) Two-stage fermentation enhanced single-cell protein production by Yarrowia lipolytica from food waste. Bioresour Technol 361:127677. https://doi.org/10.1016/j.biortech.2022.127677
Yu Y, Qian C, Li X (2014) Distributed and collaborative traffic monitoring in software defined networks. Paper presented at the Proceedings of the third workshop on Hot topics in software defined networking, Chicago, Illinois, USA,
Zainuddin MF, Kar FC, Mohamed MS, Abdul RNA, Halim M (2022) Production of single cell oil by Yarrowia lipolytica JCM 2320 using detoxified desiccated coconut residue hydrolysate. PeerJ 10:e12833. https://doi.org/10.7717/peerj.12833
Zhang Y, Bao J (2022) Tolerance of Trichosporon cutaneum to lignin derived phenolic aldehydes facilitate the cell growth and cellulosic lipid accumulation. J Biotechnol 343:32–37. https://doi.org/10.1016/j.jbiotec.2021.09.009
Zhang H, Wu C, Wu Q, Dai J, Song Y (2016) Metabolic Flux Analysis of Lipid Biosynthesis in the Yeast Yarrowia lipolytica Using 13C-Labled Glucose and Gas Chromatography-Mass Spectrometry. PLoS One 11(7):e0159187. https://doi.org/10.1371/journal.pone.0159187
Zhang X, Wang H, Li Q, Yin Z, Qi H, Yang J, Wang X, Xiao W, Zhang L (2022) Development of organogels for Live Yarrowia lipolytica encapsulation. J Am Chem Soc 144(23):10251–10258. https://doi.org/10.1021/jacs.2c00847
Zhu HZ, Jiang S, Wu JJ, Zhou XR, Liu PY, Huang FH, Wan X (2022) Production of high levels of 3S,3′S-astaxanthin in Yarrowia lipolytica via iterative metabolic engineering. J Agric Food Chem 70(8):2673–2683. https://doi.org/10.1021/acs.jafc.1c08072
Acknowledgments
The authors would like to thank all persons who provided the proofreading format of this publication.
Author’s information
Université Paris-Saclay, Institut Micalis, Diversité génomique et fonctionnelle des levures, domaine de Vilvert, 78350 Jouy-en-Josas, France, sanyadaniel86@yahoo.com.
Author information
Authors and Affiliations
Contributions
D.R.A.S. conceived, designed, wrote, and edited the review paper. DO has revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanya, D.R.A., Onésime, D. New roles for Yarrowia lipolytica in molecules synthesis and biocontrol. Appl Microbiol Biotechnol 106, 7397–7416 (2022). https://doi.org/10.1007/s00253-022-12227-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12227-z