Abstract
Bio-syngas production from biomass gasification via pressurized interconnected fluidized beds was described. The interconnected fluidized beds technology separates the gasification and combustion processes of biomass, and the heat is transferred from combustor to gasifier by bed materials, while extra heat needed in gasification process is provided by additional biomass burning in the combustor. The simulation of the whole process was carried out with Aspen Plus software. The effects of gasification temperature (T g), gasification pressure (p g) and steam to biomass ratio (S/B) on bio-syngas production were studied. The results showed that gasification temperature, gasification pressure, and S/B had great influences on the bio-syngas composition and to achieve high carbon conversion and yield of high-quality bio-syngas, the suitable gasification temperature is around 750 °C, and the gasification pressure and S/B could not too high.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Since biomass is one kind of renewable, clean energy, and can achieve the goal of CO2 zero-emission and reduce the greenhouse effect during its industrial utilization, its development and employment has obtained great attentions all over the world [1]. Biomass gasification is considered as a key technology in reaching targets for renewable energy and CO2 emissions reduction [2]. After proper purification and conditioning, bio-syngas from biomass gasification composed mainly of CO and H2 can be synthesized to methane, methanol, gasoline, and diesel oil [3–6]. So many researchers have done a lot of work about the bio-syngas production from biomass gasification, including gasification agents, reactors, catalysts, and so on. For instance, the oxygen-rich air was used as the gasification agents to avoid the dilution of bio-syngas with the inert nitrogen of air, while some researchers used a mixture of air and steam as gasification agents to produce bio-syngas with relatively higher H2/CO ratio to meet the requirements of the further synthetic reactions [7–9].
This article intends to study the laws of bio-syngas production with biomass gasification from a new perspective of simulation. The process simulation with Aspen Plus software was carried out to demonstrate the effects of operating parameters, like gasification temperature, gasification pressure and the ratio of steam to biomass fed into the gasifier (S/B), on the bio-syngas composition, yield, and carbon conversion of biomass. These results provided reference data for the further study of biomass gasification.
2 Materials and Methods
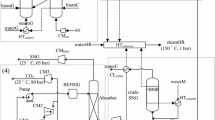
Gasifier is the core equipment of the biomass gasification process, which can be classified as fixed bed gasifiers and fluidized bed ones. The interconnected fluidized beds gasifiers are evolved from the conventional fluidized bed gasifiers [10]. The scheme of biomass gasification in interconnected fluidized beds is illustrated in Fig. 122.1 [11]. It is in a loop with end-to-end configuration composed of a circulating fluidized bed as a combustor, and a bubbling fluidized bed as a gasifier. The circulating fluidized bed is designed for combustion fed with air as gasification agent and the bubbling fluidized bed for biomass gasification fed with steam as gasification agent. The gasification-required heat is achieved by means of the circulation of bed particles (sand, ash, etc.), which serve as the heat carrier and circulate in the system. In this way, the gasification and combustion processes are separated from each other [12, 13].
The whole biomass fed into the interconnected fluidized beds is divided into two parts, which are BiomC and BiomG, respectively. One part (BiomG) is fed into the bubbling fluidized bed (gasifier), where the biomass mixes with the steam and hot bed particles, and the intense exchange of heat and mass occurs. Then the volatile compounds in the biomass evaporate, followed by the pyrolysis of the biomass. The reactions between gaseous product after pyrolysis combined with solid residual and steam occur, where gases such as CO and H2 are generated [12]. The reactions occurring in the gasifier include [14, 15]:
The other part (BiomC) is burnt in a circulating fluidized bed (combustor) where the bed particles carry a great deal of heat. The flue gas carrying hot bed particles from the combustor passes through a separator, where the hot particles are separated from the flue gas and pass into the gasifier, providing the heat needed for the biomass gasification. The unseparated particles are expelled from the system in the form of flowing ash with the flue gas. The char mixed with the cold bed particles in the gasifier is back-passed into the combustor to combust.
3 Process Simulation
Based on the application of Aspen Plus software, the following assumptions were made [12, 16]:
-
(1)
The combustor and the gasifier were operated under a steady state, and the reactions reached the chemical equilibrium.
-
(2)
Pressure losses in the combustor and the gasifier were not considered.
-
(3)
The product gas of biomass gasification included H2, CO, CO2, CH4, H2O, N2, H2S, NH3, COS, and SO2 and the solid products were ash and unburnt carbon. Tar was not taken into account.
-
(4)
Ash in biomass and bed particles (sand) were inert and would not participate in the chemical reactions.
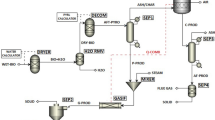
The simulation flowchart of bio-syngas production from biomass gasification in pressurized interconnected fluidized beds was shown in Fig. 122.2. The whole model mainly consisted of two basic modules, a gasification module and a combustion module. The gasification module was composed of a pyrolyzer and a gasifier, and the combustion module included a decomposer and a combustor. The pyrolyzer block corresponded to Ryield block of Aspen Plus, whose function was to decompose biomass into simple components. Unreacted char from the gasifier was separated by a cyclone, and sent to the combustor of the combustion module. The bed materials from the combustor (sand) were circulating, whereby the heat was carried from the gasification module to the combustion module. Water was heated in the steam generator with the flue gas from the combustor to provide the gasifier with steam as the gasification agent.
The whole model of gasification module and combustion module was based on the principle of minimization of Gibbs free energy, which originated from the Rgibbs block of Aspen Plus. The Gibbs free energy is minimal when the chemical equilibrium for the process is achieved [17]. Based on mass balance, chemical equilibrium, and energy balance between the gasifier and the combustor, the mathematical model for the gasification process was set up.
4 Operating Conditions and Primary Parameters
The biomass sample was the pine sawdust from Jiangsu Province, China. The proximate analysis and the ultimate analysis of biomass were illustrated in Table 122.1.
The operating conditions and primary parameters in the simulation were shown in Table 122.2.
5 Results and Discussion
5.1 Influences on Product Gas Composition
The product gas from biomass gasifier was mainly composed of bio-syngas (H2 + CO), CO2 and CH4. Figures 122.3, 122.4, 122.5 showed the influences on its composition.
The product gas composition was shown as a function of gasifier temperature in Fig. 122.3. In the case where the gasifier pressure remained 0.4 MPa and the S/B was 0.6, the bio-syngas content was varied around 60–90 mol % at the gasifier temperature range of 650–950 °C and with the rise of gasifier temperature, it increased and kept nearly constant at the temperature of about 800 °C, whilst the CO2 and CH4 content decreased correspondingly. The product gas composition in the biomass gasifier is the result of the combination of a series of complex and competing reactions, as given in reactions (122.1)–(122.5). Reactions (122.1), (122.3), and (122.5) occurring in the gasifier are intensive endothermic processes, while the reactions (122.2) and (122.4) are exothermic ones. As a result, higher temperatures favor the endothermic reactions and the production of bio-syngas.
The effect of gasification pressure on product gas composition was shown in Fig. 122.4, which indicated that as the gasification temperature was 750 °C and S/B was 0.6, with the increase of gasification pressure, the bio-syngas content decreased, while the CO2 and CH4 content increased correspondingly. This was because the increase of pressure would promote the gasification reactions toward the direction that led to volume reduction, which favored the reaction (122.4), while led the reactions (122.3) and (122.5) to the opposite direction.
Figure 122.5 indicated the influence of S/B on product gas composition. When the gasification temperature was 750 °C and the gasification pressure remained 0.4 MPa, as the S/B increased, the bio-syngas content was not affected so evidently, while CH4 content decreased and the CO2 content increased. More S/B meant more steam participated in the reactions, which would promote reactions (122.1), (122.2), and (122.5) toward the right direction.
5.2 Influences on Carbon Conversion of Biomass
One parameter of carbon conversion of biomass can be used to investigate the effect of different operating parameters in the gasifier on the gasification process. The carbon conversion of biomass was defined in Eq. (122.6).
The effect of gasification temperature on carbon conversion at different gasification pressure was indicated in Fig. 122.6. When S/B was kept 0.6, with the rise of gasification temperature, the carbon conversion of biomass decreased correspondingly at the same gasification pressure. This was because higher gasification temperature meant more biomass was fed into the combustor and less biomass into the gasifier, which resulted in a decrease of carbon conversion.
Figure 122.6 also showed that at the same gasification temperature, with the increase of gasification pressure, the carbon conversion of biomass increased, too, which indicated that higher gasification pressure favored the carbon conversion.
S/B also had great influence on the carbon conversion of biomass. As shown in Fig. 122.7, in the case where gasification temperature was 750 °C and the gasification pressure was 0.4 MPa, with the increase of S/B, the carbon conversion increased and then decreased, and reached maximum for the S/B of about 0.4.
As the S/B was lower than 0.4, there was not enough steam to react with the biomass, and reactions (122.1), (122.2), and (122.5) may not reach a state of completion. With the increase of S/B, more steam took part in the above reactions and made the carbon conversion increase. However, larger S/B meant excessive water was fed into the system, which resulted in more carbon that was needed in the combustor to provide heat to make the water evaporate and overheat. Thus, less biomass went into the gasifier, which led to a decrease of the carbon conversion correspondingly.
5.3 Influences on Bio-Syngas Yield
Bio-syngas yield is another important parameter to describe the gasification process of biomass, which can be defined as
Figure 122.8 showed the influence of gasification temperature on bio-syngas yield at different gasification pressure. In the case where S/B was 0.6, bio-syngas yields all increased with the rise of gasification temperature and almost kept a high value after the temperature reached 750 °C and above. The reason was that higher temperature favored the bio-syngas production, while the gasification pressure had a weak effect on it at high temperature.
S/B also had great influence on the bio-syngas yield, as shown in Fig. 122.9. In the case where the gasification temperature maintained 750 °C and the gasification pressure was 0.4 MPa, bio-syngas yield increased at first, reached the maximum value when S/B was around 0.6, and then decreased. The reason was somewhat the same as the explanation of the variant of carbon conversion of biomass. With the increase of S/B, more steam participated in the biomass gasification process and promoted the bio-syngas production, while the lager S/B value meant more water was needed in the system and resulted in the reduction of biomass fed in the gasifier. As a result, the bio-syngas yield decreased as S/B was larger than 0.6.
6 Conclusions
With the Aspen Plus software, simulation of bio-syngas production from biomass gasification via pressurized interconnected fluidized beds was carried out. Some valuable results were obtained as follows.
-
(1)
Bio-syngas content in the product gas was influenced by the gasification temperature, pressure, and S/B. It increased with the gasification temperature rising, while the gasification pressure and S/B had a weak effect on it.
-
(2)
When S/B remained 0.6, the carbon conversion of biomass decreased with the increase of gasification temperature at the same gasification pressure, while it increased with the rise of gasification pressure at the same gasification temperature. In the case where gasification temperature was 750 °C and the gasification pressure 0.4 MPa, with the increase of S/B, the carbon conversion increased and then decreased, and reached maximum as the S/B was about 0.4.
-
(3)
When S/B was kept 0.6, bio-syngas yield almost increased with the increase of gasification temperature at different gasification pressure. It kept a high value as the gasification temperature was higher than 750 °C.
-
(4)
To achieve a high content of bio-syngas and high carbon conversion and bio-syngas yield, the gasification temperature could be higher than 750 °C, and the gasification pressure and S/B could not be too high.
References
Fatih M, Mustafa B, Havva B (2009) Potential contribution of biomass to the sustainable energy development. Energy Convers Manag 50:1746–1760
Turn S, Kinoshita C, Zhang Z et al (1998) Experimental investigation of hydrogen production from biomass gasification. Int J Hydrogen Energy 23:641–648
Pengmei L, Zhenhong Y, Chuangzhi W et al (2007) Bio-syngas production from biomass catalytic gasification. Energy Convers Manag 48:1132–1139
Jan K, Tilman JS, Serge MAB (2010) Production of synthetic natural gas (SNG) from coal and dry biomass-technology review from 1950 to 2009. Fuel 89:1763–1783
Zhang XT, Chang J, Wang TJ et al (2006) Investigation into methanol synthesis from biomass derived syngas over Cu-Zn-Al-Li catalysts. Chi J Pro Eng 6:104–107 (in Chinese)
Lu YX, Wang TJ, Li YP et al (2008) Direct synthesis of liquefied petroleum gas from biomass synthesis gas. J Fuel Chem Tech 36:246–249 (in Chinese)
Dennis YCL, Yin XL, Wu CZ (2004) Review on the development and commercialization of biomass gasification technologies in China. Renew Sust Energy Rev 8:565–580
Su DR, Zhou ZQ, Xie JJ et al (2011) Biomass oxygen enriched-steam gasification in an atmospheric fluidized bed for singes production. Trans Chin Soc Agric Mach 42:100–104 (in Chinese)
Song FE, Tan YS, Xie HJ et al (2009) Study on preparation of dimethyl ether from biomass-derived syngas. Mod Chem Ind 29:306–308 (in Chinese)
Jose′ C, Jose′ M, Gregorio M (2007) Review on dual fluidized-bed biomass gasifiers. Ind Eng Chem Res 46:6831–6839
Christoph P, Reinhard R, Hermann H (2004) In-bed catalytic tar reduction in a dual fluidized bed biomass steam gasifier. Ind Eng Chem Res 43:1634–1640
Yanan Z, Jun X, Laihong S (2009) Simulation of methanol production from biomass gasification in interconnected fluidized beds. Ind Eng Chem Res 48:5351–5359
Bischi A, Langørgenb øy, Saanum I et al (2011) Design study of a 150kWth double loop circulating fluidized bed reactor system. Int J Greenh Gas Con 5:467–474
Ajay K, David D, Milford AH (2009) Thermochemical biomass gasification: a review of the current status of the technology. Energies 2:556–581
Hugo D, Enrique S, Jahirul M et al (2011) Catalytic steam gasification of biomass: catalysts, thermodynamics and kinetics. Chem Rev 111:5404–5433
Gao Y, Xiao J, Shen L (2008) Hydrogen production from biomass gasification in interconnected fluidized beds. Acta Energiae Solaris Sinica 29:894–899 (in Chinese)
Qu YX (2011) Numerical simulation and software for chemical processes. Chem Ind Press, Beijing (in Chinese)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (2010CB732206).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Feng, F., Song, G., Shen, L., Xiao, J. (2014). Simulation of Bio-syngas Production from Biomass Gasification via Pressurized Interconnected Fluidized Beds. In: Zhang, TC., Ouyang, P., Kaplan, S., Skarnes, B. (eds) Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012). Lecture Notes in Electrical Engineering, vol 250. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37922-2_122
Download citation
DOI: https://doi.org/10.1007/978-3-642-37922-2_122
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37921-5
Online ISBN: 978-3-642-37922-2
eBook Packages: EngineeringEngineering (R0)