Abstract
Gasification is a multiphase process that converts the solid fuels into useful synthetic gas. Despite the potential of biomass gasification as a clean technique to produce a high caloric value syngas, it is not as well looked upon as its advantages suggest. In the present study, an ASPEN plus model of biomass gasification was developed with considering the kinetics of the reactions and tar cracking mechanism. Firstly, the model was validated against the experimental data in the literature. Next, a parametric study was done to investigate and evaluate the performance and produced gas composition of air–steam gasification of biomass. The effect of various operational parameters, such as the reaction temperature, equivalence ratio (ER) and steam/biomass ratio (S/B) on the gas product composition, products yield and the gasifier performance was investigated. The results showed that the increase of reaction temperature not only can enhance the H2 content in the gas stream, but inhibit the formation of tar. The achieved optimal conditions for production of maximum H2 content (16.18 vol %) were as follows: reaction temperature of 800 °C, S/B of 0.8 and ER of 0.211.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil fuels are the world's most important source of energy, and if demand for energy increases at the same pace, their share of total energy supply is projected to reach 80% by 2040 [1]. This scenario could lead to irreparable damage to the environment because of global warming associated with fossil fuels. Biomass, a mixture of organic constituents, is known as a renewable energy source and has great potential to replace fossil fuels because of its availability, flexibility and calorific value [2]. Unlike other renewable energies, biomass can be used both to generate electricity and to produce basic chemicals such as methanol, ethanol and dimethyl ether (DME) [3].
There are two main strategies for biomass conversion: (1) biochemical routes such as fermentation and anaerobic digestion, and (2) thermochemical routes like devolatilization, gasification and combustion [4]. Among a wide variety of biomass conversion pathways, gasification has attracted more attention due to its greater environmental compatibility and economic advantages. Biomass gasification is a complicated process to convert biomass into fuel gases or other valuable products in a partial oxidation atmosphere. The produced fuel gas (so-called syngas) mainly consists of H2, CO, CO2, CH4, H2O, N2 and some impurities like tars, NH3 and H2S [5]. Tar is a complex mixture of polycyclic aromatic hydrocarbons (PAHs), which can lead to difficulties in downstream engines but these problems could be overcome via catalytic and/or thermal tracking of tars. The quality of producer syngas is strongly affected by feed properties and operational conditions such as reaction temperature and gasification agent. Among all available gasification agents (air, oxygen, steam or mixture of these agents), air is more common due to its extensive abundance at no cost [6]. However, biomass gasification with air often produces a fuel gas rich in N2 (50–65%) and consequently low in heating value (4–6 MJ Nm−3) [7]. A mixture of air and steam may be used in cases in which the dilution of the gaseous fuel with N2 may lead to serious problems in further applications [8].
The main technical barrier for syngas production is tar formation because it not only reduces the caloric value of the produced syngas but may also cause serious technical problems to the system [9]. The produced syngas from biomass gasification is not currently feasible for industrial applications because it has a high level of tar content, which ranges from 0.1 to 150 g Nm−3 [10]. However, in order to use the produced syngas in most applications, the tar content must be less than 0.05 g Nm−3 [11]. Among all available options for tar reduction, catalytic conversion and thermal cracking are the most used techniques. Thermal cracking is more common for tar cracking because it economically degrades the complex condensable hydrocarbons into simple fuel gases (e.g., H2, CH4).
Process simulation on the basis of experimental parameters can save considerable time and cost. However, simulation of biomass gasification is a complex process because it involves a series of thermochemical processes and complicated phenomena which occur mostly simultaneously. In general, there are three main models for simulation of biomass gasification, which are as follows: equilibrium models, kinetic models, and artificial neural network (ANN) models. A number of studies have been performed on simulation of biomass gasification. Yu et al. [12] used two validated models based on minimizing Gibbs free energy and reaction kinetics to compare the accuracy of the models. The authors concluded that the kinetic models are more accurate than equilibrium models. Moreover, the researchers analyzed the produced gas compositions under various operating conditions (equivalence ratio, temperature, moisture content and biomass composition). The results showed that the biomass composition significantly affects the quality of the syngas. Hejazi al. [13] developed a kinetic model for simulation of steam gasification of biomass in a bubbling fluidized bed. The main focus of their study was on the prediction of the product gas composition at different temperatures and steam/biomass ratios. Using a coarse-grained CFD-DEM approach, Ostermeier et al. [14] studied the overall behavior of a bubbling fluidized bed reactor during steam gasification of wood by using a simulation tool (ANSYS Fluent). To support a reliable model, heat and mass transfer and solids collisional behavior were considered in their model.
However, there still is a lack of knowledge concerning the tar formation, which impedes proper designing and upscaling of fluidized bed reactors. The main purpose of this work is to develop and validate an ASPEN plus model for predicting the gas composition with considering tar concentration. The model firstly validated against two sets of previously published experimental data. Next, a parametric study was performed to investigate the effects of equivalence ratio (ER), gasifier temperature and steam/biomass ratio (S/B) on the gas compositions, tar concentration and gasifier performance indicators.
Model description
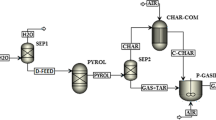
The main purpose of this study is to develop an ASPEN plus model of biomass gasification with air and steam as gasification agent. Figure 1 shows a flowsheet diagram of the gasification system. The following assumptions were used in the model: (1) ash is not considered; (2) char is considered as 100% carbon; (3) heat losses are not considered in the calculations; (4) biomass devolatilization is considered as an instantaneous process. Biomass is considered as a non-conventional component. Enthalpy and density of feedstock was calculated by using HCOALGEN and DCOALIGT, respectively, based on the proximate and ultimate analyses (Table 1). Firstly, biomass was dried by using a RYIELD reactor (block ID: DR) by converting a portion of feedstock into water. The moisture content in biomass was specified by using a calculator block which takes into consideration the chemical composition of biomass. The dried feed was then transferred to a second RYIELD reactor (block ID: DECOM) to decompose the feedstock into its constituent elements by specifying the yield distribution according to the biomass characteristics presented in Table 1. To separate the condensable materials (tars) from solid material (char) and volatiles, a separator (block ID: SP1) was used. A RGIBBS reactor (block ID: TAR COMB) was used to simulate the tar combustion which uses a Gibbs free energy minimization approach for equilibrium calculations. The combustion products, solid char and volatiles were transferred to a RPLUG reactor (block ID: CHF) to simulate char gasification using kinetic data available in the literature. The kinetic parameters used for the simulation of gasification section can be found elsewhere [15, 16].
Data analysis
The char conversion efficiency (CCE) and cold gas efficiency (CGE), which are two important indicators of the gasification process, were calculated as follows [9]:
where YG, LHVsyngas, LHVfeed and C are the dry gas yield (Nm3 kg−1), the lower heating value of the produced syngas (MJ Nm−3), the lower heating value of feedstock (MJ kg−1) and carbon content in the feedstock according to the ultimate analysis. LHVsyngas is calculated as follows [17]:
Here, [H2], [CO] and [CH4] are the volume fraction of the H2, CO and CH4 in the dry producer gas, respectively.
Results and discussion
Model validation
In order to validate the model, experimental results from pine sawdust gasification in a fluidized bed gasifier [18] were selected. The produced gas compositions at different conditions were compared with experimental data. The comparison of model predictions and experimental results reported by Ma et al. [18] are shown in Fig. 2. From Fig. 2, it can be observed that the model predictions show good agreement with the experimental results; however, the predicted H2 concentrations show a slight deviation from experimental data, attributed to the relevant kinetic expressions used in the present model and lack of sufficient kinetic data for simulation of tar conversion.
Comparison between the simulated results and the reported data by Ma et al. [18] at different ERs
Parametric study
The reaction temperature is an important parameter in biomass gasification. The reaction temperature was varied from 700 to 850 °C, while ER is kept constant. Figure 3 shows the variation in dry gas composition with change in reaction temperature from 700 to 850 °C. The syngas (H2 and CO) concentration increased with increasing temperature, while CO2 content showed a decreasing trend. The major reactions involved in biomass gasification are as follows: water–gas shift reaction (CO + H2O → CO2 + H2, ∆H = -41 kJ/mol), water–gas reaction (C + H2O → CO + H2, ∆H = 131 kJ/mol), Boudouard reaction (C + CO2 → 2CO, ∆H = 172 kJ/mol), methane–steam reforming reaction (CH4 + H2O → CO + 3H2, ∆H = 206 kJ/mol), and tar cracking reaction (CnHm + nH2O → (n + m/2) H2 + nCO, ∆H > 0). The increase of syngas concentration with increasing temperature can be attributed to the water–gas reaction prevailing at higher temperature as reported by Hejazi et al. [19]. In general, higher temperature favors the endothermic reactions like water–gas reaction and Boudouard reaction, increasing syngas concentration and decreasing CO2 content. The CH4 content also decreased with increasing temperature from 700 to 850 °C, which is consistent with the result reported by Hejazi et al. [19]. From the above analysis, it can be seen that increasing temperature would be helpful in enhancing the syngas quality and quantity.
Variations in product gas yield, H2 yield and tar yield with increasing temperature from 700 to 850 °C are shown in Fig. 4. As can be observed in Fig. 4, tar yield decreased from 5.85 g Nm−3 to 2.74 g Nm−3 as the reaction temperature increased from 700 to 850 °C, which can be attributed to the acceleration of tar cracking reaction. According to literature reports [20], higher temperature is more favorable for tar elimination, and subsequently high quality syngas production. The gas yield increased obviously as the reaction temperature increased, peaking at 1.52 Nm3 kg−1 at 850 °C, which can be attributed to the higher rate of endothermic reactions such as water–gas reaction and Boudouard reaction at higher temperatures. From Fig. 4, it can be seen that the H2 yield mostly increased with increasing temperature and reached a maximum value of 18.82 g/kg at 850 °C which is probably due to the higher rate of water–gas reaction as well as tar cracking reaction.
Figure 5 shows the variation in CCE and CGE with change in reaction temperature from 700 to 850 °C. From Fig. 5, it can be observed that CCE increased by 5.58% as the reaction temperature increased from 700 to 850 °C. Since the water–gas reaction and Boudouard are endothermic, elevating the temperature promotes these reactions toward more char consumption and syngas production. CGE also increased from 46.49 to 50.15% with increasing temperature from 700 to 850 °C. As gasification is mainly an endothermic process, product flow rate enhances with increasing reaction temperature. These trends are in agreement with experimental data reported in the literature [21].
Figure 6 shows the variation in the produced gas composition with change in ER in the range of 0.169–0.255. ER is defined as the ratio of the actual air/fuel to the stoichiometric air/fuel. From Fig. 6, it can be observed that CO and CH4 contents decreased as ER increased from 0.169 to 0.255, while CO2 showed an increasing trend. Increasing ER means increasing the air added to the gasifier. Increasing the air causes the process to shift toward the char combustion zone, and thus the concentrations of CO and CH4 decrease and the content of CO2 increases. As can be seen in Fig. 6, H2 content slightly increased with increasing ER in the range of 0.169–0.255, which might be attributed to the increase of tar cracking reaction.
Figure 7 shows the variation in product gas yield, H2 yield and tar yield with change in ER from 0.169 to 0.255. It can be observed from Fig. 7 that, as the ER increased, the gas yield increased slowly which is probably due to the higher rate of char conversion via endothermic water–gas and Boudouard reactions. Increase in the gas yield can also be due to the dilution effect of N2 in the air. This increase in the gas yield with increasing ER is in agreement with the literature [22]. The yield of tar exhibited a trend that slightly decreased when the ER increased. This can be explained by more tar conversion taking place because of increased endothermic nature of the process. A slight reduction in the tar yield with the increase of ER can be due to keeping the temperature constant. As expected, H2 yield increased from 10.25 g/kg to 19.52 g/kg with increasing ER from 0.169 to 0.255, which can be attributed to the higher rate of tar conversion with the increase of ER.
The effects of ER on CCE and CGE are shown in Fig. 8. As shown in Fig. 8, with the increase in ER, the CCE sharply increased from 51.63 to 87.41%, probably because the increased ER (air) promoted the char combustion, causing the CO2 content to rise. However, it should be noted that excessive air will enhance the combustion reactions and gradually decrease the quality of syngas. As ER increased in the range of 0.169–0.255, CGE slightly decreased from 36.6 to 56.17%. The char in the biomass reacts with the oxygen content in the air via the oxidation reactions, thereby causing the increase of CO2 content and the decrease of energetic species.
Variations in product gas composition with increasing S/B are shown in Fig. 9. The reaction temperature (800 °C) and ER (0.211) were kept constant, while S/B changed in the range 0.0–0.48. From Fig. 9, it can be seen that the H2 content increased by 20.9% as S/B increased from 0.0 to 0.48. The increase of steam addition can enhances tar cracking and water–gas shift reactions. These two reactions utilize heavier hydrocarbons and water vapor to produce hydrogen. The decrease of CO content with increasing S/B was due to CO reacting with water vapor in water–gas shift reaction, which leads to an increase in the contents of H2 and CO2. The production rate of CH4 also decreased with higher S/B due to CH4 reacting with water vapor in the methane–steam reforming reaction, increasing H2 production. The decrease of CO production with the increase of S/B is due to the fact that the water–gas shift reaction is the dominant reaction over the methane-reforming reaction for the S/B range studied [5].
Figure 10 shows the effect of S/B on the product gas yield, H2 yield and tar yield at 800 °C and ER of 0.211. When S/B ranged from 0.0 to 0.48, the gas yield increased with the increase in S/B, which may be attributed to the increase of char conversion, promoting the production of carbonaceous gas species. When S/B increased from 0.0 to 0.48, the tar yield slightly decreased from 5.84 g Nm−3 to 5.28 g Nm−3. This may be due to the increase in the endothermic nature of the tar cracking reaction with the increase in the S/B. With the increase of S/B from 0.0 to 0.48, the H2 yield reached its highest level. This trend may be due to the increase in the steam content in the gasifier with the increase in the S/B; the tar cracking and water–gas shift reactions would have been enhanced, causing the H2 yield to rise.
The variations in CCE and CGE with change in S/B are shown in Fig. 11. As can be seen in Fig. 11, CCE slightly increased with increasing S/B in the range of 0.0–0.48. In fact, with the increase in S/B, the tar cracking reaction is more dominant than the water–gas reaction, causing the char conversion to improve. When S/B increased in the range of 0.0–0.48, CGE increased by 6.7% which may be attributed to the fact that the rise in S/B promotes the tar conversion and gas production, thereby increasing CGE.
Conclusions
In the present study, a comprehensive ASPEN plus model was developed to simulate biomass gasification at various conditions. In the model, the produced syngas compositions, products yield and gasifier performances were predicted using a series of chemical reactions with kinetics. This study showed the following:
-
The syngas (H2 and CO) concentration increased with increasing temperature, while CO2 content showed a decreasing trend.
-
Higher temperatures, higher equivalence ratios (ERs) and higher steam/biomass ratios (S/Bs) were found to increase the H2 content in the gas stream and tar cracking.
-
The gas yield slightly increased with the increase of the reaction temperature, ER and S/B.
-
Both the char conversion efficiency (CCE) and cold gas efficiency (CGE) slightly increased with the increase of reaction temperature, ER and S/B.
References
Tauqir W, Zubair M, Nazir S. Parametric analysis of a steady state equilibrium-based biomass gasification model for syngas and biochar production and heat generation. Energy Convers Manag. 2019;199:111954.
Hiloidhari M, Baruah DC, Kumari M, Kumari S, Thakur IS. Prospect and potential of biomass power to mitigate climate change: a case study in India. J Clean Prod. 2019;220:931–44.
Nowicki L, Antecka A, Bedyk T, Stolarek P, Ledakowicz S. The kinetics of gasification of char derived from sewage sludge. J Therm Anal Calorim. 2011;104:693–700.
Strezov V, Moghtaderi B, Lucas JA. Thermal study of decomposition of selected biomass samples. J Therm Anal Calorim. 2003;72:1041–8.
Puig-Gamero M, Argudo-Santamaria J, Valverde JL, Sánchez P, Sanchez-Silva L. Three integrated process simulation using aspen plus®: pine gasification, syngas cleaning and methanol synthesis. Energy Convers Manag. 2018;177:416–27.
Jeong YS, Kim JW, Seo MW, Mun TY, Kim JS. Characteristics of two-stage air gasification of polystyrene with active carbon as a tar removal agent. Energy. 2021;219:119681.
Campoy M, Gómez-Barea A, Villanueva AL, Ollero P. Air–steam gasification of biomass in a fluidized bed under simulated autothermal and adiabatic conditions. Ind Eng Chem Res. 2008;47:5957–65.
Upadhyay DS, Panchal KR, Sakhiya AKV, Patel RN. Air-steam gasification of lignite in a fixed bed gasifier: influence of steam to lignite ratio on performance of downdraft gasifier. Energy. 2020;211:118187.
Yang H, Wang C, Xu S, Liu R. Biomass gasification over hematite in a decoupled dual loop gasifier. Fuel Process Technol. 2019;192:140–6.
Hasler P, Nussbaumer T. Gas cleaning for IC engine applications from fixed bed biomass gasification. Biomass Bioenergy. 1999;16:385–95.
Zhang LH, Xu CB, Champagne P. Overview of recent advances in thermochemical conversion of biomass. Energy Convers Manag. 2010;51:969–82.
Yu J, Smith JD, Golpour H, Alembath A, Al-Rubaye H, Gao X. Validation and application of a kinetic model for downdraft biomass gasification simulation. Chem Eng Technol. 2019. https://doi.org/10.1002/ceat.201900304.
Hejazi B, Grace JR, Bi X, Mahecha-Botero A. Kinetic model of steam gasification of biomass in a bubbling fluidized bed reactor. Energy Fuels. 2017;31:1702–11.
Ostermeier P, Fischer F, Fendt DS, Spliethoff H. Coarse-grained 784 CFD-DEM simulation of biomass gasification in a fluidized bed reactor. Fuel. 2019;55(785):115790.
Miao Q, Zhu J, Barghi S, Wu C, Yin X, Zhou Z. Modeling biomass gasification in circulating fluidized beds. Renew Energy. 2013;50:655–61.
Corella J, Sanz A. Modeling circulating fluidized bed biomass gasifiers: a pseudo–rigorous model for stationary state. Fuel Process Technol. 2005;86:1021–53.
Kallis KX, Pellegrini Susini GA, Oakey JE. A comparison between miscanthus and bioethanol waste pellets and their performance in a downdraft gasifier. Appl Energy. 2013;101:333–40.
Ma Z, Ye J, Zhao C, Zhang Q. Gasification of rice husk in a downdraft gasifier: the effect of equivalence ratio on the gasification performance, properties, and utilization analysis of byproducts of char and tar. Bioresour. 2015;10:2888–902.
Hejazi B, Grace JR, Bi XT, Mahecha-Botero A. Kinetic model of steam gasification of biomass in a dual fluidized bed reactor: comparison with pilot plant experimental results. Energy Fuels. 2017;31:12141–55.
Zhai M, Wang X, Zhang Y, Dong P, Qi G, Huang Y. Characteristics of rice husk tar secondary thermal cracking. Energy. 2015;93:1321–7.
Liu L, Huang Y, Cao J, Liu C, Dong L, Xu L, Zha J. Experimental study of biomass gasification with oxygen-enriched air in fluidized bed gasifier. Sci Total Environ. 2018;626:423–33.
Das B, Bhattacharya A, Datta A. Kinetic modeling of biomass gasification and tar formation in afluidized bed gasifier using equivalent reactor network (ERN). Fuel. 2020;280:118582.
Acknowledgements
This paper is supported by President Fund of Xi'an Technological University (Grand: XAGDXJJ15004) and Shaanxi Key Research and Development Plan (2020GY-147).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, L., Cao, Y. & Bai, Y. Development of a comprehensive simulation model for H2-rich syngas production by air–steam gasification of biomass. J Therm Anal Calorim 147, 8069–8075 (2022). https://doi.org/10.1007/s10973-021-11041-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11041-5