Abstract
Meniscal tears are the most common knee injuries, and for years meniscectomy has been considered the gold-standard treatment. As it is now well-known that even partial deficiency of the meniscus could be destructive for the knee joint in the long-term, meniscal substitution has gained popularity. The collagen meniscus implant (CMI) is a resorbable collagen template that supports ingrowth of new tissue and finally regeneration of lost meniscus tissue. The surgical technique is arthroscopic and the post-operative care following CMI resembles that of meniscal suture, with restricted weight-bearing and cautious joint mobilization. Often, complex cases involve a combined procedure such as anterior cruciate ligament (ACL) reconstruction, corrective osteotomy or cartilage treatment, as demonstrated by the high rate of concurrent procedures reported in the literature. The results for both lateral and medial meniscal substitution are generally good in most patients, with evidence of scaffold resorption and in-growth of meniscus-like tissue. The chondroprotective effect of the CMI has not been completely demonstrated, despite encouraging evidence suggesting a reduction of the degenerative changes in the articular cartilage.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Introduction
Meniscal tears are the most common knee injuries, with a reported annual incidence of 61 per 100,000 people (Baker et al. 1985). For years, meniscectomy has been considered the gold-standard treatment for meniscal lesions, due to a lack of knowledge regarding the role of the meniscus and the long-term effects of its deficiency. In fact, it is now well-known that even partial deficiency of the meniscus can be destructive for the knee joint in the long-term. It has been reported that meniscectomy increases the risk of developing knee osteoarthritis after 10 years in approximately 20 % of patients for the medial meniscus and 40 % for the lateral meniscus (Chatain et al. 2003). This is due to the important and irreplaceable functions of the meniscus: increasing congruity of the joint, reducing contact stresses, shock absorption, stabilization, proprioception, and cartilage lubrification and nutrition (McBride and Reid 1988; McDevitt and Webber 1990). For these reasons, the management of meniscal tears has changed dramatically over the years, from aggressive management towards more conservative strategies. Therefore, meniscal substitution using allografts and, more recently, scaffolds has been proposed in cases involving irreparable lesions.
Rationale and Development
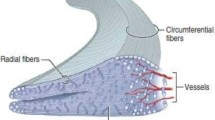
The collagen meniscus implant (CMI) is a resorbable collagen template that supports ingrowth of new tissue and, finally, regeneration of lost meniscus tissue (Stone et al. 1992; Fig. 1). It is a porous collagen–glycosaminoglycan (GAG) matrix of defined geometry, density, thermal stability, and mechanical strength (Li et al. 2002), composed of approximately 97 % purified type I collagen. The remaining portion of the CMI consists of GAGs, including chondroitin sulfate and hyaluronic acid. The type I collagen is isolated and purified from bovine Achilles tendon, then the collagen–GAG complex is chemically cross-linked to improve in vivo stability, handling, and implantation.
The suture pull-out strength of the fully hydrated CMI at 3 mm from the edge is greater than 20 N, permitting the implant to be properly positioned in the joint and fixed with sutures to the host meniscus remnant (Li et al. 2002).
In vitro studies have supported cellular ingrowth. The CMI has been tested on animal models, initially showing no evidence of cartilage wear or damage, and then signs of tissue regeneration (Stone et al. 1990, 1992). Later, animal studies confirmed these findings, showing healing of the implant-regenerated tissue to the host meniscus rim, and increasing amounts of tissue invasion and resorption of the CMI over time. This suggested a complete CMI resorption and replacement at 6 months in a canine model. Furthermore, magnetic resonance imaging (MRI) provided excellent correlation with the gross and histological observations, supporting the findings of continued tissue ingrowth and maturation over time.
These findings confirmed the safety of the device and showed the growth of meniscal-like tissue substituting the collagen CMI structure, allowing the possibility to implant CMI in humans.
Patient Selection and Pre-Operative Evaluation
Surgery for the meniscus-deficient knee should be considered only after a period of non-surgical treatment. When such treatment fails to provide relief of symptoms or to prevent joint space narrowing, CMI may be considered.
Accurate selection of patients and both clinical and radiological evaluation are mandatory in order to obtain a good result and prevent early failure.
Indication
CMI can be suggested in a patient who meets all of the following criteria:
-
Prior loss of meniscus tissue
-
Irreparable meniscus tears requiring partial meniscectomy
-
Either traumatic or chronic post-traumatic meniscus tear
-
Meniscus damage requiring more than 25 % removal
-
Intact anterior and posterior attachments of the meniscus
-
Intact rim over the entire circumference (except for the area of popliteal hiatus for lateral meniscus) of the involved meniscus
-
Anterior cruciate ligament (ACL) deficiencies corrected within 12 weeks of CMI surgery
-
Patients willing to follow a post-operative rehabilitation program
-
Patients should be capable of understanding and following the doctor’s instructions.
Treatment of an acute meniscal lesion with CMI is still a debated topic, as a multicentric study by Rodkey et al. (2008) reported no difference between CMI and partial meniscectomy for acute meniscal lesions at mid-term follow-up.
Contraindications
The main contraindications for CMI surgery are as follows:
-
Concomitant posterior cruciate ligament insufficiency of the involved knee
-
Grade IV degenerative cartilage disease in the affected joint
-
Uncorrected malalignment of the involved knee
-
Allergy to collagen of animal origin
-
Allergy to chondroitin sulfate of animal origin
-
Systemic or local infection
-
History of anaphylactoid reaction
-
Systemic administration of any type of corticosteriod, antineoplastic, immunostimulating or immunosuppressive agent within 30 days of surgery
-
Evidence of osteonecrosis in the involved knee
-
Medical history that is positive for, but not restricted to, the following diseases:
-
Rheumatoid arthritis
-
Severe degenerative osteoarthrosis.
-
Generally, the most common contraindication to CMI is advanced chondral degeneration, characterized by cartilage wear and radiographic evidence of osteophytes and femoral condyle flattening. Localized chondral defects may be addressed concomitantly with chondrocyte transplantation, osteochondral grafting, or synthetic scaffolds.
Malalignment is also reported to cause abnormal pressure on the affected compartment; therefore, a corrective osteotomy should be considered in the case of malalignment.
The lack of symptoms remains a controversial issue, as prophylactic CMI is not routinely recommended. In fact, the rehabilitation program after CMI is substantially different from that following partial meniscectomy. Furthermore, there is little evidence in favor of long-term prevention of arthrosis (Zaffagnini et al. 2011a).
Clinical Evaluation
An accurate history regarding knee trauma, injuries, and surgical procedures should be obtained. Knee pain, swelling, and mechanical symptoms exacerbated by physical activity are often present.
A physical examination should be performed and height, weight, and body mass index measured. With the patient standing, lower limb alignment is also evaluated. Range of motion (ROM) and ligament laxity are then assessed both for the affected and contralateral knee. Pain and tenderness should be reported for the affected compartment and ipsilateral quadriceps strength and circumference should be documented.
Radiological Evaluation
Accurate radiological planning is mandatory to correctly prepare for the surgery. Weight-bearing anteroposterior radiographs of both knees in full extension and a non-weight-bearing 45° flexion lateral radiograph are required. Rosenberg’s view (45° flexion weight-bearing posteroanterior radiograph) can be helpful to detect subtle joint space narrowing, while mechanical axis radiography is necessary in case of low limb malalignment. MRI should be performed whenever possible, to evaluate the meniscal defect, ligament lesions, subchondral bone pathology, and cartilage status.
Surgical Technique
CMI is a surgical procedure that is mainly performed arthroscopically; therefore, good surgical skills are required in order to achieve correct placement and fixation of the device and positive outcomes. Although medial and lateral CMI are different in shape and size, and lateral and medial compartments present different anatomical features, the surgical technique is similar.
Patient Position
The patient is positioned supine, with the knee flexed to 90°. A leg holder is placed 5 cm proximal to the superior pole of the patella in order to allow valgus stress to open the medial compartment, while opening of lateral compartment is achieved by flexing the leg over the contralateral knee in the “figure-of-four” position.
Arthroscopic Setting
A medial suprapatellar portal is usually performed for water inflow, while routine anteromedial (AM) and anterolateral (AL) portals are made for the scope and instruments. The AL portal is placed distally to the patella in the soft spot, about 1–2 cm lateral to the patellar tendon; the AM portal is placed at the same level, about 1–2 cm medial to the patellar tendon. When performing a lateral CMI, this portal is usually performed slightly above the joint line. Accessory portals may be required for a better view or access.
Once portals are established, a thorough arthroscopic examination is mandatory (Fig. 2a). Furthermore, ACL and cartilage status are controlled in order to correctly plan additional procedures.
Preparation of the Implant Bed
Any degenerate or unstable meniscal tissue is removed in order to obtain a full-thickness defect with a stable meniscal rim over the entire length using basket forceps and a motorized shaver (Fig. 2b). The meniscal rim should maintain a uniform width and extend to the red–red zone. When the defect reaches the white–red zone, bleeding is obtained by making puncture holes in the rim with a microfracture awl. The anterior and posterior attachments should be trimmed square (radially) to better match the CMI and improve fixation stability.
If the medial compartment is too tight for proper visualization, medial release with outside-in needle punctures and varus stress can be performed without the risk of any residual laxity. In contrast, tightness of the lateral compartment and not being able to place the CMI into the defect represent an intraoperative contraindication to a CMI, as secondary complications, poor healing, and lateral laxity are issues when lateral collateral ligament release is performed.
Preparation of the Collagen Meniscus Implant (CMI)
Once the implant bed is prepared, the defect is measured using a specially designed measuring device through the ipsilateral portal, starting from the posterior aspect of the lesion (Fig. 3a). The obtained measurement should be oversized by approximately 10 % when sizing the CMI in order to obtain a good press-fit and better stability. In case of disruption of the meniscus tissue at the popliteal hiatus, the measure should be oversized by 15–20 %, since the CMI may recess into the hiatus during fixation.
Once the correct size has been established, the dry CMI is removed from the sterile package and trimmed using a fresh scalpel (Fig. 3b). Care should be taken to match the shape of the CMI perfectly with the angles of the meniscal defect.
CMI Fixation
Once the device is adequately prepared, it is mounted on a curved atraumatic vascular clamp and directly inserted into the joint through the corresponding portal, which should be enlarged enough to accommodate the tip of the fifth finger (Fig. 4a). The device is then released in the proper position and the clamp retrieved without damaging cartilage surfaces (Fig. 4b). A blunt probe can also be useful to manipulate the implant into the correct position.
When the device is in the correct position, suturing to the capsule is performed. The new-generation meniscal repair takes the advantages of both the all-inside technique and the biomechanical proprieties of sutures. Sutures are placed vertically using a standard technique every 10–15 mm along the periphery of the device (Fig. 5), while the anterior and posterior borders are fixed using two horizontal sutures. This all-inside method is particularly indicated for the posterior third of the meniscus. When required, an inside-out suture placed every 5 mm could be used alone or in combination with the all-inside technique. This method, although more versatile, requires the execution of posterolateral or posteromedial approaches to retrieve the sutures. When performing lateral CMI, care should be taken while suturing the area of the popliteal hiatus, to avoid placing sutures directly through the popliteal tendon, because the physiological micromotion of this tendon might damage the still immature scaffold. If a 1–2 mm gap has been developed between anterior and posterior horns and the sutured CMI, a microfracture owl is used to scarify the synovium to stimulate a proliferative response at the gap interface. If the gap is more than 2 mm, the implant should be replaced.
The tip of the suturing device is inserted through the CMI (a) and the first “anchor” is released. Then the capsule is pinched just above the first passage of the stitch. After the release of the second “anchor” of the device, the stitch is pulled and locked (b). Finally the remaining suture is cut, obtaining a vertical stitch (c)
Once the CMI is sutured, its stability is checked with a probe, the tourniquet is released, and a drain (if used) is positioned with no suction.
Concurrent Procedures
Anterior Cruciate Ligament Reconstruction
ACL reconstruction is the most frequent procedure associated with CMI. Concomitant ACL reconstruction has been reported to create a more favorable environment for healing of the meniscus (Koski et al. 2000), and therefore combined ACL reconstruction and CMI are recommended. In such cases, CMI insertion and fixation should be performed before definitive fixation of the graft, in order to allow better medial or lateral joint opening during stress maneuvers. For the same reason, if the procedures are staged, CMI should be performed first. However, ACL reconstruction can be delayed no more than 12 weeks after CMI, as knee instability could compromise the healing of the scaffold.
Osteotomy
As lower limb malalignment is a contraindication to a CMI procedure, any axial deformity should be corrected before or concurrently with CMI. Closing wedge lateral high tibial osteotomy (HTO) (Marcacci et al. 2007) to correct varus deformity, which has been demonstrated to reduce tibial slope, reducing stress on native or reconstructed ACL (Ducat et al. 2012; Zaffagnini et al. 2013; Feucht et al. 2013), and closing wedge medial distal femoral osteotomy (DFO) to correct valgus deformity are recommended. When concurrent CMI and HTO or DFO are performed, the arthroscopic step and device implant should be performed first.
Cartilage Treatment
Advanced cartilage damage represents a contraindication to CMI, and therefore no clear recommendations regarding cartilage treatment and CMI are available. Generally, cartilage treatment, such as microfractures, osteochondral transplantation, or autologous chondrocyte implant (ACI), should be performed before CMI, in order to try to preserve the CMI device.
Rehabilitation
The main issue related to rehabilitation after a CMI is caution during the physiotherapy program, which is fundamental in order to allow CMI healing and promote good outcomes. The program covers a period of 6 months and offers a balanced combination of strengthening and motion exercises, providing protection to the newly formed tissue throughout the delicate process of regeneration. Although guidelines suggest return to full unrestricted sporting activity, the rehabilitation program should be tailored to the patient’s specific needs, expectations, and concurrent procedures.
Day 1–Day 30:
-
Brace: full extension, remove only for motion exercises
-
Motion: passive motion exercises using a continuous passive motion (CPM) machine (0–60°)
-
Weight-bearing: no weight-bearing (week 1), 30 % partial weight-bearing (week 2), 50 % partial weight-bearing (weeks 3–4)
-
Exercises: leg raises 30 × 2 (daily)
Week 5–Week 6:
-
Brace: full extension, remove only for motion exercises
-
Motion: passive motion exercises on CPM machine (0–90°)
-
Weight-bearing: 90 % partial weight-bearing
-
Exercises: leg raises 30 × 2 (daily)
Week 7–Week 8:
-
Brace: 0–90°, remove only for motion exercises
-
Motion: active motion exercises (increase ROM as tolerated)
-
Weight-bearing: full weight-bearing, abandon crutches
-
Exercises: leg raises 30 × 2 (daily), cycling without resistance (to a maximum 45 min)
Week 9–Week 16:
-
Brace: none
-
Motion: unrestricted full ROM
-
Weight-bearing: unrestricted full weight-bearing
-
Exercises: shallow knee bends from 0° to 90° 20 × 2 (daily), cycling with increased resistance (to a maximum 45 min), water exercises
Month 5–Month 6:
-
Brace: none
-
Motion: unrestricted full ROM
-
Weight-bearing: unrestricted full weight-bearing.
-
Exercises: lateral agility exercises 20–50 × 2 (daily)
Return to Sport:
-
Generally, return to sport is achieved after 6 months, although patient-specific issues or concurrent surgery such as cartilage treatment can lead to a prolonged rehabilitation period. In order to optimize the outcome, strict adherence to the program is mandatory, even when the patient feels able to return to his/her activities sooner than expected.
Risks and Complications
The risks related to a CMI are mainly produced by a less than perfect surgical technique. Nerve injuries have been reported after medial CMI, as a possible consequence of suture placement; also, laxity of the knee has been described, which could be produced by excessive medial or lateral release being performed to allow the opening of the affected compartment (Rodkey et al. 2008). Furthermore, when dealing with lateral meniscal substitution, improper scaffold fixation could entrap the popliteus tendon. Other complications such as pain, swelling, wound infection, and deep vein thrombosis have been reported in the literature, with incidences ranging from 0 % to 32 %.
These complications can produce failure of the implant, requiring reoperation. The failure rate ranges between 0 % and 12.5 %, and in the largest series of 160 medial CMIs (Rodkey et al. 2008) it was almost 10 %. The main causes of reoperation are persistent pain, swelling, infection, or mechanical failure of the scaffold.
Results
Clinical Results
A recent systematic review reported that satisfactory outcomes are achieved in approximately 70 % of patients treated with CMI (Harston et al. 2011), while 30 % did not receive any benefit; this is in contrast with studies that reported good/excellent results in more than 90 % of patients (Zaffagnini et al. 2012). The cornerstone study of CMI surgery is the randomized controlled trial performed by Rodkey et al. (2008), which compared medial CMI to medial meniscectomy at 5 years’ follow-up. This study, involving about 300 patients, showed that good results and a low reoperation rate were obtained in patients with chronic meniscal deficiency treated with CMI. Regarding acute meniscal lesion, no significant difference was found compared with the control group, thus limiting the indication of CMI to patients with previous meniscectomy. As the lateral CMI was developed more than 10 years after the medial CMI, there are few reports in the current literature on the results of the lateral procedures (Zaffagnini et al. 2011b, 2012; Hirschmann et al. 2013). Outcomes similar to those of the medial CMI have been reported (Zaffagnini et al. 2007), even if no randomized controlled trials or long-term follow-up studies are yet available.
The potential chondroprotective effect of the scaffold in the long-term has been studied by Zaffagnini et al. (2011) in a controlled clinical trial. They compared medial CMI with medial meniscectomy at 10-year follow-up, showing lower signs of knee osteoarthritis and better clinical results, particularly pain and knee function, using the CMI.
Second Look and Histologic Findings
Besides clinical results, several studies have focused their attention on the intra-articular behavior of the implanted CMI (Fig. 6). Stone et al. (1997) reported the presence of regenerated tissue similar to the fibrous composition of meniscal cartilage after 6 months. These findings were confirmed by Rodkey et al. (1999) at 24 months’ follow-up, when reduction of osteoarthritic degeneration was also noted. As suggested by these previous studies, Bulgheroni et al. (2010) showed a progressive resorption of the scaffold, with implant remnants visible at 3 years’ follow-up and complete absence of the implant at 5 years. Analogously, Rodkey et al. (2008) reported that only 10–25 % of the original implant was still present at the 1-year follow-up. These findings, similar to those reported by animal studies, suggest a progressive resorption of the scaffold, which is colonized and substituted by a fibrocartilaginous tissue similar to the meniscus.
Magnetic Resonance Imaging Findings
The behavior of the CMI has also been widely studied with MRI (Figs. 7 and 8). Genovese et al. (2007) developed an MRI score in order to assess the signal intensity and size of the scaffold. Various reports have shown that the scaffold tends to reduce its size over time, with a consistent reduction after 1 year that slowly progresses until 10 years. Regarding the signal intensity, the MRI findings showed a reduction of the implant signal that slowly tended to become isointense to the signal of native meniscus. However, this process, which suggests implant maturation, appears to be almost complete in only a small percentage of implants. In fact, most devices assume a signal that is slightly hyperintense compared to the native meniscus, and which is more similar to the newly implanted scaffold (Bulgheroni et al. 2010; Monllau et al. 2011; Zaffagnini et al. 2011, 2012).
Results of Associated Procedures
CMI is often combined with other surgical procedures; in fact, the rate of concomitant surgery ranges from 11 % to 79 %. The main procedures performed are to address knee stability, malalignment, or cartilage pathology. As a result, it is not easy to evaluate the real effect of the CMI when additional procedures are performed, as the results could be biased by their combination.
Hirschmann et al. (2013) reported a case series of 67 patients, 53 of whom were treated with medial or lateral CMI combined with ACL reconstruction. Those patients showed more bone marrow edema and fewer good clinical results, measured by the Lysholm score, than with isolated CMI at 1 year of follow-up.
Linke et al. (2006) performed a controlled study involving 60 patients with varus morphotype and medial meniscus loss. The control group underwent high tibial valgus osteotomy (HTVO) while the treatment group underwent HTVO combined with medial CMI. At 2 years’ follow-up, no significant differences were found between the groups in terms of knee function and pain. However, the improvement from baseline in the patients treated with CMI and HTVO was similar to that in other studies.
Due to the highly specialized and demanding procedures, reports of CMI and cartilage treatment are very few. Microfracture surgery is the most performed procedure, involving approximately 11–20 % of patients (Stone et al. 1997; Zaffagnini et al. 2011). Combined CMI and microfracture surgery does not appear to dramatically change the general outcomes, despite patients with cartilage injury requiring more careful management. Ronga et al. (2006) presented a report of a patient treated with combined medial CMI and ACL reconstruction, followed by medial femoral condyle MACI (matrix-applied characterized autologous cultured chondrocytes) 7 months later, due to a 5 cm2 chondral lesion. The authors reported good clinical results, with reduction of pain and scaffold invasion by blood vessels and cells, and production of new collagen fibrils without any signs of inflammatory reaction after 2 years.
Conclusions
CMI represents an attractive surgical option for irreparable meniscal lesions. Although a wide range of conditions can potentially be treated with CMI, good results are dependent on correct indications. Good/excellent results have been reported in 70–90 % of cases. The most interesting finding regarding the CMI is evidence of resorption of the scaffold and its substitution with a meniscus-like tissue, which represents a basis for theories relating to the potential effect of chondroprotection. However, the protective properties of the CMI are yet to be proven, as only a few controlled trials have been conducted. While the clinical benefits of CMI in the mid-term have been demonstrated, further studies are needed to confirm the long-term outcomes and its protective effects on articular cartilage.
References
Baker BE, Peckham AC, Pupparo F et al (1985) Review of meniscal injury and associated sports. Am J Sports Med 13(1):1–4
Bulgheroni P, Murena L, Ratti C et al (2010) Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee 17(3):224–229
Chatain F, Adeleine P, Chambat P et al (2003) A comparative study of medial versus lateral arthroscopic partial meniscectomy on stable knees: 10-year minimum follow-up. Arthroscopy 19(8):842–849
Ducat A, Sariali E, Lebel B et al (2012) Posterior tibial slope changes after opening- and closing-wedge high tibial osteotomy: a comparative prospective multicenter study. Orthop Traumatol Surg Res 98(1):68–74
Feucht MJ, Mauro CS, Brucker PU et al (2013) The role of the tibial slope in sustaining and treating anterior cruciate ligament injuries. Knee Surg Sports Traumatol Arthrosc 21(1):134–145
Genovese E, Angeretti MG, Ronga M et al (2007) Follow-up of collagen meniscus implants by MRI. Radiol Med 112(7):1036–1048
Harston A, Nyland J, Brand E et al (2011) Collagen meniscus implantation: a systematic review including rehabilitation and return to sports activity. Knee Surg Sports Traumatol Arthrosc 20(1):135–146
Hirschmann MT, Keller L, Hirschmann A et al (2013) One-year clinical and MR imaging outcome after partial meniscal replacement in stabilized knees using a collagen meniscus implant. Knee Surg Sports Traumatol Arthrosc 21(3):740–747
Koski JA, Ibarra C, Rodeo SA et al (2000) Meniscal injury and repair: clinical status. Orthop Clin North Am 31(3):419–436
Li S-T, Rodkey WG, Yuen D et al (2002) Type I collagen-based template for meniscus regeneration. In: Lewandrowski K-U, Wise DL, Trantolo DJ, Gresser JD, Yaszemski MJ, Altobelli DE (eds) Tissue engineering and biodegradable equivalents. Scientific and clinical applications. Marcel Dekker, New York, pp 237–266
Linke RD, Ulmer M, Imhoff AB (2006) Replacement of the meniscus with a collagen implant (CMI)]. Oper Orthop Traumatol 18(5–6):453–62
Marcacci M, Zaffagnini S, Giordano G et al (2007) High tibial osteotomy: the Italian experience. Op Tech Orthop 17(1):1–86
McBride ID, Reid JG (1988) Biomechanical considerations of the menisci of the knee. Can J Sport Sci 13(4):175–187
McDevitt CA, Webber RJ (1990) The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res 1990(252):8–18
Monllau JC, Gelber PE, Abat F et al (2011) Outcome after partial medial meniscus substitution with the collagen meniscal implant at a minimum of 10 years’ follow-up. Arthroscopy 27(7):933–943
Rodkey WG, Steadman JR, Li ST (1999) A clinical study of collagen meniscus implants to restore the injured meniscus. Clin Orthop Relat Res (367 Suppl):S281–S292
Rodkey WG, DeHaven KE, Montgomery WH 3rd et al (2008) Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am 90(7):1413–1426
Ronga M, Grassi FA, Manelli A et al (2006) Tissue engineering techniques for the treatment of a complex knee injury. Arthroscopy 22(5):576.e1–576.e3
Stone KR, Rodkey WG, Webber RJ et al (1990) Future directions. Collagen-based prostheses for meniscal regeneration. Clin Orthop Relat Res 1990(252):129–135
Stone KR, Rodkey WG, Webber R et al (1992) Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med 20(2):104–111
Stone KR, Steadman JR, Rodkey WG et al (1997) Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am 79(12):1770–1777
Zaffagnini S, Giordano G, Vascellari A et al (2007) Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surg Sports Traumatol Arthrosc 15(2):175–183
Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N et al (2011a) Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med 39(5):977–985
Zaffagnini S, Marcheggiani Muccioli GM, Grassi A et al (2011b) Arthroscopic lateral collagen meniscus implant in a professional soccer player. Knee Surg Sports Traumatol Arthrosc 19(10):1740–1743
Zaffagnini S, Marcheggiani Muccioli GM, Bulgheroni P et al (2012) Arthroscopic collagen meniscus implantation for partial lateral meniscal defects: a 2-year minimum follow-up study. Am J Sports Med 40(10):2281–2288
Zaffagnini S, Bonanzinga T, Grassi A et al (2013) Combined ACL reconstruction and closing-wedge HTO for varus angulated ACL-deficient knees. Knee Surg Sports Traumatol Arthrosc 21(4):934–941
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Marcacci, M., Grassi, A., Muccioli, G.M.M., Nitri, M., Zaffagnini, S. (2015). Meniscus Reconstruction Using a New Collagen Meniscus Implant. In: Doral, M.N., Karlsson, J. (eds) Sports Injuries. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36569-0_76
Download citation
DOI: https://doi.org/10.1007/978-3-642-36569-0_76
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36568-3
Online ISBN: 978-3-642-36569-0
eBook Packages: MedicineReference Module Medicine