Abstract
Multiple intraluminal impedance in combination with pH measurement on a single catheter in the esophagus allows the detection of bolus movements, even of very low volume. With this technology, which has become the new diagnostic standard for gastroesophageal reflux, it is possible to analyze bolus direction, velocity, height, and clearance. With simultaneous recording of the symptom under investigation, a temporal symptom-reflux association can be established.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastroesophageal reflux
- Disease
- Assessment
- pH monitoring
- Bioelectrical impedance
- Treatment
- Complications
- Surgical treatment
Electrical impedance is defined as the relation of voltage (U) to current (I). It is measured in Ohm (Ω) and is, similar to resistance, inversely proportional to electrical conductivity. The multiple intraluminal impedance (MII) technology is based on the measurement of impedance in an organ lumen and the change of impedance during the passage of a bolus through this lumen. To measure impedance, cylindrical electrodes are placed on a hollow catheter. For signal transduction and registration, these electrodes are connected via thin wires inside the catheter with impedance-voltage converters and a display/recording unit outside the body. Impedance is measured bipolarly between two electrodes on a catheter. These two electrodes form an impedance channel, representing a defined area of the luminal organ. The total length of all channels is defined by the number of and distance between the electrodes [6].
Impedance changes characteristically depend on the content of the bolus. The electrical conductivity of air is close to zero, whereas the electrical conductivity of a liquid bolus is high, even compared to the conductivity of the muscular organ wall. All components (air, bolus, organ wall, body) together form a volume conductor around the catheter and the impedance electrodes (Fig. 78.1).
Multiple intraluminal (electric) impedance procedure. Cross section through a luminal organ inside the body, organ wall, intraluminal impedance catheter, air, and bolus. Arrow: direction of bolus movement. Impedance (Z) is measured bipolarly between adjacent electrodes (= impedance channel, Z n ). Defined electrode distance (x) and number of channels (n)
By high-frequency registration (50–100 Hz registration rate) in the esophagus, it is possible to distinguish between the resting phase, the bolus passage, and the muscular contraction in every single impedance channel. During the resting phase, due to the close proximity of the relaxed muscles (low conductivity) to the measurement electrodes, the impedance baseline is recorded. The increase of impedance just before the bolus passage, e.g., during a swallow, represents air that is propelled in front of the bolus. This is followed by a decrease of impedance after entry of the bolus into the measuring segment. The consecutive increase of impedance represents bolus exit and the contracted muscle wall after bolus passage through the measuring segment. Finally, muscles relax and impedance values return to baseline [3].
By placing multiple impedance channels consecutively on a single catheter inside a luminal organ, e.g., the esophagus, it is possible to determine the direction, height, and velocity of the bolus movement [6].
All impedance channels are recorded digitally and displayed simultaneously. Stationary or portable recording systems are available. Impedance measurements are analyzed using dedicated software and manually-visually to detect the typical changes during the passage of a bolus. If these changes appear in the most proximal impedance channels first and then in the more distal impedance channels, this is interpreted as antegrade bolus movement, e.g., a swallow. If these changes appear in the most distal impedance channels first and then in the more proximal impedance channels, this is interpreted as retrograde bolus movement, e.g., a gastroesophageal reflux (GER) (Fig. 78.2). As the software allows the use of different time scales on the screen, longer episodes can be displayed completely, but also a more detailed analysis can be performed using a magnified view.
Typical impedance changes during bolus passage through multiple measuring segments. Cross section through a luminal organ. Thick arrow: direction of bolus movement. Impedance (Z) over time (t). Impedance values of channel 2 (Z 2), 6 (Z 6), and 10 (Z 10) at the time of bolus passage. Typical sequential impedance changes (thin arrow) during bolus passage, first appearing in Z 10, then in Z 6, and ultimately in Z 2. t 1: bolus passage through Z 10, bolus entry in Z 6, and air entry in Z 2. t 2: bolus exit from Z 10, bolus passage through Z 6, and bolus entry in Z 2. t 3: return to baseline in Z 10, contracted muscular wall in Z 6, and bolus exit from Z 2
In a pilot study, the conductivity and impedance of various tissues, liquids, and foodstuff were measured in vitro using an eight-channel impedance catheter. A measuring current of <6 μA was used, as this is well below the stimulation threshold of human nerves and muscles and therefore does not interfere with the impedance registration of gastrointestinal motility. The theoretical principle of the technology was confirmed with these experiments, and the typical phases of a bolus passage were verified [6].
In vivo studies in the esophagus and duodenum of healthy volunteers confirmed the bolus phases found in vitro and the general potential of the method. Typical impedance patterns during swallows and muscular contractions were recorded and analyzed [3].
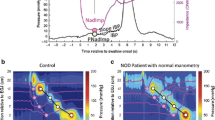
In studies on healthy adults, fluoroscopy, manometry, and MII of the esophagus were performed simultaneously. These studies delivered information regarding normal motility during the antegrade passage (swallow) of solid meals and liquids. The potential of recording GER and its clearance was demonstrated, the temporal relation of pressure changes in the esophagus, and the different impedance phases were analyzed (Fig. 78.3). The passage of air and a bolus results in relevant changes of impedance, but only little pressure increase. Maximum pressure is only experienced when the bolus exits the measuring segment.
Typical impedance and pressure changes during bolus passage through a measuring segment. Cross section through a luminal organ. Arrow: direction of bolus movement. Impedance (Z) and pressure (P) over time (t). Increase of impedance and slight increase of pressure in phase 2. Maximum pressure increase (phase 4) during segmental muscular contraction. (A) Entry of bolus head and return of impedance to baseline; (B) maximum bolus conductivity and lowest impedance; (C) maximum luminal occlusion
From the defined electrode distance and the time difference between appearances of the typical impedance pattern in the neighboring impedance channels, the bolus velocity and length could be calculated for every organ segment. With this technology, it is possible to analyze changes of bolus velocity and length during the passage through the esophagus. A difference between the bolus velocity and the contraction wave velocity became apparent.
In a first clinical trial, adults with reflux esophagitis and healthy controls were examined with MII and manometry. MII patterns of the upper and lower esophagus were compared after test meals. A significant reduction of bolus velocity in the aboral direction was recorded in all study individuals. Velocity was hereby inversely proportional to the viscosity of the test meal. Patients with reflux esophagitis showed a significant delay of bolus passage in the distal esophagus as compared to healthy controls; impedance values stayed well above baseline and only slowly returned to baseline after the second swallow. As no corresponding changes were documented with manometry, these were most likely non-occluding contractions.
In the next trials, MII was combined with pH-metry to study adults with reflux esophagitis. Impedance data was analyzed for the typical pattern of retrograde bolus movement; additionally, the pH of the GER episode could be defined. Shortly after the development of impedance and pH registration on a single catheter, the technology was applied in infants [7]. Impedance channels covered the lumen from the hypopharynx to the most distal esophagus [11].
The most proximal impedance channel displaying a drop of impedance resembling bolus presence was defined as height reached by the reflux. Most GER in infants reached the most proximal channel and occurred early postprandially.
The beginning of a bolus reflux was defined as a drop of impedance of at least 50 % of the impedance baseline prior to the reflux episode. It was shown that even very small bolus volumes were detectable by intraluminal impedance [4].
Volume clearance was defined as the time interval from bolus entry until bolus exit, i.e., the return of impedance values to at least 50 % of the impedance baseline prior to the GER. This was derived from in vitro studies and known to correspond to a clearance of more than 90 % of the bolus from the measuring segment [6]. Acid clearance was defined as the time interval from the drop of pH below threshold (usually pH 4) until its return to values above pH 4. Due to the chemical definitions, GER with pH <4 was defined as being acid GER, with pH 4–7 as weakly acidic GER, and with pH >7 as being nonacid or alkaline GER. In almost all GER, volume clearance was shorter than acid clearance and achieved after a single swallow.
All studies in infants clearly demonstrated that the major amount of GER episodes were weakly acidic, with pH monitoring alone showing a very low sensitivity for GER detection in all pH ranges.
This was found to be the new definition of gastroesophageal reflux in children [1, 5]. Color-coding of impedance changes improved visual analysis of the tracings significantly (Fig. 78.4). To date, multiple combined impedance-pH measurements have been performed in clinical routine in children of all age groups worldwide [9, 10]. International standards have been created for the procedure. The establishment of normal values and the validation of the automated analysis software in pediatric patients are in the focus of current collaborative efforts. The most recently published international guideline on gastroesophageal reflux in children describes combined multiple esophageal impedance-pH recording as being superior to pH monitoring alone for evaluation of GER-related symptom association [2, 8].
References
Dalby K, Nielsen RG, Markoew S, Kruse-Andersen S, Husby S. Reproducibility of 24-hour combined multiple intraluminal impedance (MII) and pH measurements in infants and children. Evaluation of a diagnostic procedure for gastroesophageal reflux disease. Dig Dis Sci. 2007;52:2159–65.
Loots CM, Benninga MA, Davidson GP, et al. Addition of pH-impedance monitoring to standard pH monitoring increases the yield of symptom association analysis in infants and children with gastroesophageal reflux. J Pediatr. 2009;154:248–52.
Nguyen HN, Silny J, Matern S. Multiple intraluminal electrical impedancometry for recording of upper gastrointestinal motility: current results and further implications. Am J Gastroenterol. 1999;94:306–17.
Peter CS, Wiechers C, Bohnhorst B, et al. Detection of small bolus volumes using multiple intraluminal impedance in preterm infants. J Pediatr Gastroenterol Nutr. 2003;36:381–4.
Rosen R, Lord C, Nurko S. The sensitivity of multichannel intraluminal impedance and the pH probe in the evaluation of gastroesophageal reflux in children. Clin Gastroenterol Hepatol. 2006;4:167–72.
Silny J. Intraluminal multiple electric impedance procedure for measurement of gastrointestinal motility. J Gastrointest Mot. 1991;3:151–62.
Skopnik H, Silny J, Heiber O, et al. Gastroesophageal reflux in infants: evaluation of a new intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 1996;23:591–8.
Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guideline: joint recommendations of NASPGHAN and ESPGHAN. J Pediatr Gastroenterol Nutr. 2009;49:498–547.
van Wijk M, Benninga M, Omari T. Role of multichannel intraluminal impedance technique in infants and children. J Pediatr Gastroenterol Nutr. 2009;48:2–12.
Wenzl T. Investigating esophageal reflux with the intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 2002;34:261–8.
Wenzl TG, Moroder C, Trachterna M, et al. Esophageal pH monitoring and impedance measurement: a comparison of two diagnostic tests for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2002;34:519–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Wenzl, T.G. (2017). Esophageal Intraluminal Impedance. In: Till, H., Thomson, M., Foker, J., Holcomb III, G., Khan, K. (eds) Esophageal and Gastric Disorders in Infancy and Childhood. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-11202-7_78
Download citation
DOI: https://doi.org/10.1007/978-3-642-11202-7_78
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-11201-0
Online ISBN: 978-3-642-11202-7
eBook Packages: MedicineMedicine (R0)