Abstract
The amount of fly ash disposed as industrial wastes are increasing with increasing coal demands all over the world. Although fly ash is generally disposed by landfill, the demand of effective utilization of them is increasing because of the limitation of the disposal site; 80% of coal ash is utilized such as a material for cements though less of them are disposed by landfill. Considering the preparation of landfill area and environmental issues, it is very meaningful to discuss the utilization of fly ash except a cement usage. Most of coal is mined by open-pit mining method in Indonesia. A broad post-mining area is built after the mining operation. The broad area has to be revegetated in terms of environmental conservation. However, soil acidification caused by mixing acid sulfate rocks or soils mined in the operation influences the revegetation. As the plant growth is inhibited under the acidic conditions, the utilization of fly ash which has higher neutralizing capacity due to its alkalinity is expected in order to improve the conditions of seedbed in the revegetation area. In this paper, the utilization of fly ash for preparation of seedbed in disturbed land in Indonesian open-cut coal mine is discussed by means of laboratory pot trials by using simulated acidic soil with a mixture of pyrite, fly ash which has higher alkalinity, and Acacia mangium which is a typical species planted for fast growing tree in Indonesia. The results suggested that the appropriate mixture of fly ash to neutralize the acidic soil can improve the plant growth under the acidic condition.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Rehabilitation of post-mine land is necessary for sustainable development of open-cut mining. Minerals are mined with the creation of the large disturbed land in open-cut mining. Thus, rehabilitation of post-mine land is required at the end of the development for environmental conservation. In open-cut mining, topsoil, which is formed from the surface down to approximately 1.0 m depth, is stored during the excavation stage, followed by the placement in surface layer in post-mine land during rehabilitation since they contain much nutrition that is useful for plant growth (Sheoran et al. 2010). However, the shortage of topsoil attributing to the loss while hauling soil results in the difficulty of rehabilitation in some cases. Furthermore, the loss of topsoil caused by soil erosion or landslide causes the inhibition of plant growth in the post-mined land, leading to the failure of rehabilitation (Sheoran et al. 2010; Takeuchi and Shimano 2009). Therefore, it is important to secure enough amount of topsoil for rehabilitation in the disturbed land of open-cut mining.
In many mines, acid sulfate soils and/or rocks are mined as waste materials with excavation of minerals (Ueno 2004). These soils and/or rocks consist of several kinds of minerals such as silicate and sulfide minerals (e.g. pyrite: FeS2). This sulfide mineral leads to Acid Mine Drainage (AMD) when they are exposed to oxygen and water during excavation process as follows (Nugraha et al. 2009; Nordstrom et al. 2000; Nordstrom and Alpers 1999):
The waste materials are classified as Potentially Acid Forming (PAF) by means of several geochemical tests: PAF is the source of AMD. PAF is, generally, backfilled under the ground to prevent the occurrence of AMD. However, they are mixed in topsoil and backfilled in surface layer in the post-mining land in some cases (Matsumoto et al. 2015). In this situation, the mixing of such waste materials consisting of PAF in topsoil causes soil acidification, and this affects revegetation process in mining operation. In fact, the failure of revegetation by soil acidification attributed to metal dissolutions with PAF was reported in Indonesian open-cut coal mines (Ogata 2016). However, another researcher reported vegetation growth can be promoted if the soil quality is good for plant growth, although the soil contains high metal contents (Anawar et al. 2013).
As for the vast amount of fly ash produced as the industrial wastes in coal-fired power plants, the effective utilization ways are expected in some applications as alternatives for natural materials. Some of fly ash is utilized such as a material for cements though the others are disposed in ash dams. Considering the preparation and maintenance costs of landfill area and environmental issues, it is very meaningful to discuss the utilization of fly ash except a cement usage. Besides, the potential to utilize fly ash for biomass production has been suggested (Pandey et al. 2009). Several studies have shown that the fly ash application on topsoil can promote the plant growth because it can increase pH of the acid soil (Capp and Adams 1971; Srivastava and Chhonkar 2000; Brown et al. 1997), improve the soil texture (Fail and Wochok 1977), and supply the trace element which is necessary for plant growth (Khan and Khan 1996). On the other hand, careful consideration is needed when the amendment of topsoil using fly ash is discussed because the excess addition of fly ash causes the decrease in plant growth due to the salinity of the soils and impact from toxic elements (Singh and Siddiqui 2003; Matsi and Keramidas 1999). Given these facts, several researchers suggested the allowable range of fly ash addition to the soil based on pot experiment (Matsi and Keramidas 1999; Mishra and Shukla 2009). However, the use of fly ash for soil amendment is a complicated topic because the properties of fly ash and acidic soils are site specific.
The present work is focused on addition of fly ash which has high alkaline to two acidic soils which have different metal leachate potential. Therefore, the utilization of fly ash for preparation of seedbed in disturbed land in open-cut mine is discussed by means of laboratory pot trials using simulated acidic soil with a mixture of pyrite, fly ash which has higher alkalinity, and Acacia mangium which is a typical species planted for fast growing tree in the beginning stage of revegetation.
2 Materials and Methods
2.1 Preparation of Simulated Soil for Pot Trials
Simulated acid soil was prepared by mixing decomposed granite soil and pyrite. In this study, two types of acidic soils were prepared to simulate the different potential of metal leachate from the soil. Besides, fly ash (FA) was taken in the coal-fired power plant in Japan. Table 1 shows fundamental properties of each sample. Paste pH and EC tests were carried out by the dissolution process with sample and deionized water. The mixing ratio of sample and deionized water was 1:2.5 in the paste pH (Kabala et al. 2016) and 1:5 in the paste EC (Slavich and Petterson 1993), respectively, to compare the standard (Research Committee of Japanese Institute of Landscape Architecture 2000). From the Table 1, the acidic soil 2 shows the lower pH and higher EC compared to acidic soil 1, meaning that the potential of metal leachate is higher. Fly ash has the higher alkalinity, and the dominant particle size is silt.
Paste pH and EC, dissolved metal ion analysis, and permeability test based on ASTM D5084-10 standard were conducted for ten types of samples which were prepared by changing the mixture rate of simulated acidic soil and fly ash (Tables 2 and 3). Paste pH rises with an increase in mixing ratio of fly ash in both acidic soils 1 and 2. Above all, excess addition of fly ash causes soil alkalization in acidic soil 1 because the paste pH shows alkaline when the mixture ratio of fly ash is more than 20%. Paste EC declines under the neutral pH condition when the fly ash mixture ratio is 5 and 10% for acidic soil 1 and more than 10% for acidic soil 2, respectively. This fact indicates that metal dissolution can be minimized by controlling the soil pH. However, the remediation of paste EC in acidic soil 2 is not enough due to the high potential of metal dissolution from acidic soil based on the standard (Research Committee of Japanese Institute of Landscape Architecture 2000). The hydraulic conductivity is slightly decreased with an addition of fly ash. Table 3 shows the result of metal ion analysis by using the water from paste EC test. The major metal contents eluted in AMD, such as Al, Fe, and Mn, were measured in this test. The dissolution of Al causes the inhibition of plants’ growth due to the elongation of roots of plants (Kochian et al. 2005; Kikui et al. 2005). Additionally, Fe derived from the pyrite is a dominant metal ion eluted under the acidic conditions, and its concentration is higher in acidic soil 2.
2.2 Laboratory Pot Trials
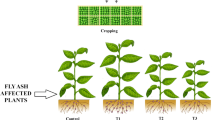
In order to discuss the applicability of fly ash on acidic conditions to promote the plant growth, A. mangium was planted on the prepared soils mixed with acidic soil and fly ash described in previous section. The plants were placed in the phytotron glass room G-9 in Biotron Application Center, Kyushu University, under the following conditions: at 30 °C room temperature and 70% relative humidity assuming the tropical climate. In this test, five plants were planted in each soil, and the height and the basal diameter were measured every a week. At the end of the test, the dry weight of A. mangium was measured. The pot trials continued for 11 weeks until a clear distinction was observed. All pots were supplied with 500 mL water every 3~4 day, while liquid fertilizer HYPONeX formula 6-10-5 diluted to 1000 ppm with water was additionally added weekly. The data obtained from the laboratory pot trials are processed by using Tukey-kramer method to indicate the significant differences among each result.
3 Results and Discussion
3.1 Preparation of Simulated Acidic Soil
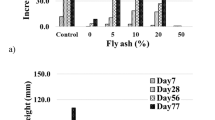
Figure 1a, b shows the photograph of A. mangium at the end of the test. We also prepared the pots of decomposed granite soil without pyrite and fly ash as a control pot. It can be clearly observed that the growth of A. mangium is promoted with mixing of fly ash in acidic soil 1, whereas most of the plants do not grow in acidic soil 2. Figures 2a, b and 3a, b show the increment of plant height and basal diameter. These increments are found in acidic soil 1 with 5, 10, and 20% of fly ash with elapsed time though the other soil shows the slight increase or no improvement due to the death of plant. The height increase in the plants is improved in acidic soil 1. The acidic soil 1 with 10% of fly ash addition showed the highest height (47.3 mm) followed by 5% of fly ash addition (40.6 mm) at the end of the test, while the height increase in the control pots is 110.5 mm. The basal diameter increase showed similar pattern with that of height. Maximum basal diameter was found in acidic soil 1 with 10% of fly ash (0.90 mm) followed by 5% of fly ash addition (0.86 mm) while the basal diameter of the control pots is 1.80 mm. A slight increment or no improvements of both of plant height and basal diameter are found in acidic soil 2.
Dry weight of A. mangium is a proper indicator of the impact of soil conditions on the plant growth in terms of biomass. Multiple comparison analysis of dry weight revealed significant differences between the pots in acidic soil 1 (Fig. 4). The dry weight is significantly increased in acidic soil 1 with 5% (0.88 g) and 10% (0.71 g) of fly ash followed by 20% of fly ash (0.39 g) while the weight of the control pots is 1.14 g. The 5% addition of fly ash seems to be appropriate to promote the plant growth because the results did not show statistical difference with a control. The pots of acidic soil 1 show the addition of 0 and 50% does not promote plant growth. Considering that the inhibition of the growth of plants by aluminum is a major problem in acidic conditions, the content of Al dissolved under the acidic condition may affect the growth of A. mangium in the 0% of fly ash. The dry weight is declined with an increase in fly ash addition under the alkaline conditions. The decline of the dry weight is probably due to salinity caused by sulfate, chloride, carbonate, bicarbonate from fly ash. Additionally, the reduction in absorbing nutrient under the alkaline condition is also a factor to inhibit the plant growth. On the other hand, the results of acidic soil 2 do not show statistically significant difference between the pots. The significant inhibitions of plant growth in acidic soil 2 are due to the osmotic stress caused by high salinity and the specific effects of individual ions. Previous research indicated water uptake by the root is reduced if there is osmotic stress (Bernstein 1975). The electric conductivity of acidic soil 2 shows quite higher compared to the standard value (Table 2), meaning that the water uptake by roots is inhibited due to the effects of osmotic stress. Besides, the specific ions should influence the plant growth because excessive metal ions dissolved. Therefore, the metal accumulation in the plant body will be investigated as the future task.
4 Conclusions
Vegetation process is necessary after mining operation in terms of environmental conservation. However, acid sulfate soils attributed to the process of backfill of waste rocks in post-mining area inhibit plant establishment in the revegetation stage in some cases. Using fly ash which has alkaline properties seems to be an effective way to neutralize acidic soils. In this research, laboratory pot trials were performed with A. mangium for 11 weeks in order to understand the effects of fly ash addition on plant growth in acid-topsoil. According to the results, it is possible to improve the plant growth under the acidic soil by remediating soil conditions with fly ash addition though excess of fly ash inhibits the plant growth due to the salinity and alkalization of soils. Moreover, it is difficult to improve the plant growth by using fly ash if the acidic soil has the high potential of metal dissolution. In summary, effective revegetation by using fly ash is possible to be performed by evaluating the effect of metal dissolution from acidic soil and fly ash on plant growth.
References
Anawar, H.M., Canha, N., Santa-Regina, I., Freitas, M.C.: Adaptation, tolerance, and evolution of plant species in a pyrite mine in response to contamination level and properties of mine tailings: sustainable rehabilitation. J. Soils Sed. 13(4), 730–741 (2013)
Bernstein, L.: Effects of salinity and sodicity on plant-growth. Ann. Rev. Phytopathol. 13, 295–312 (1975)
Brown, T.H., Bland, A.E., Wheeldon, J.M.: Pressurized fluidized bed combustion ash. 2. Soil and mine spoil amendment use options. Fuel 76(8), 741–748 (1997)
Capp, J.P., Adams, L.M.: Reclamation of coal mine wastes and strip spoil with fly ash. In: Proceedings of Economic use of surface-mined land and mine refuse symposium, pp. 26–37 (1971)
Fail, J.L., Wochok, Z.S.: Soybean growth on fly ash-amended strip mine spoils. Plant Soil 48(2), 473–484 (1977)
Kabala, C., Musztyfaga, E., Galka, B., Labunska, D., Manczynska, P.: Conversion of soil pH 1:2.5 KCl and 1:2.5 H2O to 1:5 H2O: conclusions for soil management, environmental monitoring, and international soil databases. Pol. J. Environ. Stud. 25(2), 647–653 (2016)
Khan, M.R., Khan, M.W.: The effect of fly ash on plant growth and yield of tomato. Environ. Pollut. 92(2), 105–111 (1996)
Kikui, S., Sasaki, T., Maekawa, M., Miyao, A., Hirochika, H., Matsumoto, H., Yamamoto, Y.: Physiological and genetic analyses of aluminium tolerance in rice, focusing on root growth during germination. J. Inorg. Biochem. 99(9), 1837–1844 (2005)
Kochian, L.V., Pineros, M.A., Hoekenga, O.A.: The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274(1–2), 175–195 (2005)
Matsi, T., Keramidas, V.Z.: Fly ash application on two acid soils and its effect on soil salinity, pH, B, P and on ryegrass growth and composition. Environ. Pollut. 104(1), 107–112 (1999)
Matsumoto, S., Ogata, S., Dwiki, S., Hideki, S., Sasaoka, T.: Fundamental research on evaluation of acid-topsoil for effective revegetation at post-mining land in indonesian open cast coal mine. Proc. Int. Sym. Earth Sci. Technol. 2015, 65–69 (2015)
Mishra, L.C., Shukla, K.N.: Effects of fly-ash deposition on growth, metabolism and dry-matter production of maize and soybean. Environ. Pollut. Ser. Ecol. Biol. 42(1), 1–13 (2009)
Nordstrom, D.K., Alpers, C.N.: Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the iron mountain superfund site, California. Proc. Natl. Acad. Sci. USA 96(7), 3455–3462 (1999)
Nordstrom, D.K., Alpers, C.N., Ptacek, C.J., Blowes, D.W.: Negative pH and extremely acidic mine waters from Iron mountain, California. Environ. Sci. Technol. 34(2), 254–258 (2000)
Nugraha, C., Shimada, H., Sasaoka, T., Ichinose, M., Matsui, K., Manege, I.: Geochemistry of waste rock at dumping area. Int. J. Min. Reclam. Environ. 23(2), 132–143 (2009)
Ogata, S., Matsumoto, S., Hideki, S., Sasaoka, T.: Study on effective revegetation at post-mining land in Indonesian open-cut coal mine. Proc. Int. Sym. Earth Sci. Technol., 352–356 (2016)
Pandey, V.C., Abhilash, P.C., Singh, N.: The Indian perspective of utilizing fly ash in phytoremediation, phytomanagement and biomass production. J. Environ. Manage. 90(10), 2943–2958 (2009)
Research Committee of Japanese Institute of Landscape Architecture: Ground maintenance manual in landscape planting. J. Jpn. Inst. Landscape Archit. 63(3), 224–241 (2000). (in Japanese)
Sheoran, V., Sheoran, A.S., Poonia, P.: Soil reclamation of abandoned mine land by revegetation: a review. Int. J. Soil Sed. Water 3(2), 1–21 (2010)
Singh, L.P., Siddiqui, Z.A.: Effects of fly ash and Helminthosporium oryzae on growth and yield of three cultivars of rice. Bioresour. Technol. 86(1), 73–78 (2003)
Slavich, P.G., Petterson, G.H.: Estimating the electrical-conductivity of saturated paste extracts from 1-5 soil, water suspensions and texture. Aust. J. Soil Res. 31(1), 73–81 (1993)
Srivastava, A., Chhonkar, P.K.: Amelioration of coal mine spoils through fly ash application as liming material. J. Sci. Ind. Res. 59(4), 309–313 (2000)
Takeuchi, K., Shimano, K.: Vegetation succession at the abandoned Ogushi sulfur mine, central Japan. Landscape Ecol. Eng. 5(1), 33–44 (2009)
Ueno, K.: A mechanism of soil acidification in acid sulfate soils. Ann. Rep. Res. Inst. Biol. Funct. 4, 25–33 (2004). (in Japanese)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Hamanaka, A., Yamasaki, H., Sasaoka, T., Shimada, H., Matsumoto, S. (2019). Application of Fly Ash to Acidic Soil to Improve Plant Growth in Disturbed Land of Open-Cut Mining. In: Widzyk-Capehart, E., Hekmat, A., Singhal, R. (eds) Proceedings of the 18th Symposium on Environmental Issues and Waste Management in Energy and Mineral Production. SWEMP 2018. Springer, Cham. https://doi.org/10.1007/978-3-319-99903-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-99903-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99902-9
Online ISBN: 978-3-319-99903-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)