Abstract
A deeper understanding of the brain is likely to require detailed, quantitative descriptions at several levels, ranging from the molecular to the behavioral, as well as an understanding of the relations among these levels. Taking the single neuron as the basic building block, I will here outline recent progress in linking different levels of description, including anatomical and molecular properties on the one hand (“structure”) and electrochemical activity on the other (“function”), whereby these properties are always considered to be interdependent on the activity of other neurons in the network and the behavior of the organism as a whole.
One key methodological advance has been the ability to both record activity from single neurons and observe their structural properties, in intact animals during specific brain states and/or behaviors. In this chapter, I will describe such methods in some detail, and illustrate with some key examples how observations on single-cell physiological and anatomical properties (membrane potential fluctuations and associated currents, morphology, molecular expression profile), in combination with network and behavioral properties (specifically focusing on navigation and the representation of space), can provide unique insights into hippocampal function.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Place cells
- Grid cells
- Head-direction cells
- Interneurons
- Oscillations
- Juxtacellular
- Patch-clamp
- Head-fixed
- Anesthetized
- Freely moving
Overview
Although “understanding the brain” is a stated ultimate goal for many neuroscientists, it remains unclear what such an understanding would entail for a structure whose defining characteristic is perhaps its complexity. In this chapter, I assume it would require, for a start, a detailed description of the brain at several levels, ranging from the molecular to the behavioral (Fig. 1). The challenge will be to not only describe each of these levels in greater detail but to reveal how they are connected. Following the classic “neuron doctrine” and taking the single neuron as the basic building block (ignoring for the moment the role of non-neuronal cells), an immediate task is therefore to describe neurons in terms of their anatomical and molecular properties on the one hand (“structure”) and their electrochemical activity on the other (“function”). As Fig. 1 illustrates, neuronal activity not only depends on the underlying structure of the neuron but also on the activity of other neurons in the network and, eventually, the behavior of the organism (taken here to mean any interaction of the organism with its environment). Importantly, these connections can all be bidirectional. For example, even the firing of a single neuron can, by influencing a population of neurons, change the behavior of an animal (Brecht et al. 2004). Equally, the firing of a single neuron can change its anatomical shape, as well as its molecular expression profile.
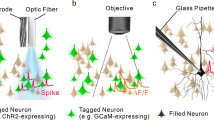
Multilevel description of neural circuits underlying behavior. Left panel: Structure and function interact (blue arrows), in the sense that structure underlies function, and function shapes structure in the brain (plasticity).In a similar manner, each of the displayed levels of description is linked to other levels of description (blue arrows; note that additional arrows have been left out for clarity). Middle panel: For each level of description in the left panel, different methods are presented. Juxta- or intracellular recording (highlighted), which forms the focus of this chapter, is merely one part of an array of methods that can ideally be deployed in concert to allow observations at several levels of description simultaneously. Right panel: For each method in the middle panel, the interface with the physical world is depicted – the use of a glass pipette (shown in blue) allows juxta- or intracellular recording (black arrow); the pipette also enables filling of a single cell (green; gray triangles represent other neurons), which can then be further analyzed by the methods indicated by arrows (dashed arrow for mRNA sequencing indicates a potential application only; this method has not been used in vivo for hippocampal recordings, nor with drug-free mice). Note that although both imaging and extracellular electrophysiology methods typically are used as readouts of neuronal population activity, they do of course also enable observation of single neuron activity, albeit with lower temporal (imaging) or spatial (extracellular) resolution than juxta- or intracellular recordings. The latter methods are most easily combined with methods to describe structure, allowing one to span all included levels of description in a single experiment, including behavior (depicted on bottom right by a camera (black) and a rat running along an oval track (red stippled arrow))
This chapter will outline progress that has been made toward recording the activity of single neurons in intact animals. I will particularly focus on relatively new methods and methodologies that allow us to combine observations on single-cell physiological and anatomical properties (membrane potential fluctuations and associated currents, morphology, molecular expression profile) with synaptic properties (connectivity and plasticity), network properties (oscillatory patterns at different spatial and temporal scales), and behavioral properties (specifically focusing on navigation and the representation of space).
Clearly, this chapter can only give a glimpse of recent advances, but I hope to convey some of the excitement of the field based on the huge possibilities provided by novel technology. On a practical level, these possibilities will also provide a major challenge in the coming years as the accompanying “big data” thinking and technology is incorporated into neuroscience (Sejnowski et al. 2014). The deeper insights we will gain will enable us to intervene ever more effectively, making it possible to ultimately develop more effective treatments for a wide range of devastating neurological and psychiatric disorders. However, the brain is not like other organs, in the sense that it is deeply tied up to what we are as humans. This is what makes our field so fascinating, but it also reminds us of our responsibility to consider potential ethical and societal implications in the face of growing possibilities.
I will not dwell on these aspects but will provide an overview of the techniques presently available, focusing on methods to record “identified” cells in living rodents. In the first section of this chapter, I will provide some examples of data gathered with these techniques, linking anatomical and functional parameters. I will touch upon work related to place cells, grid cells, head-direction cells, and interneurons (categories which need not be mutually exclusive). In the second section, I will describe the techniques in more detail. Finally, I will present a brief outlook on future developments.
Although work done in other organisms ranging from invertebrates to primates (including humans) has also provided great insights, this chapter will focus on research performed in the rodent hippocampal region, including parahippocampal areas such as the pre- and parasubiculum and the entorhinal cortex.
Introduction
There are three main factors that make recording from identified cells difficult. The first is the thorny issue of what exactly constitutes “identification.” Simply put, it means being able to identify a particular cell as belonging to a certain class. The difficulty is that there are many different levels at which a class can be defined and many different parameters that can be used. Such parameters can include molecular data, such as a cell’s protein or mRNA expression profiles (Fig. 2a–b), or anatomical data related to its overall shape or location (Fig. 2c). Parameters based on electrophysiological characterization can include responses to current injection (Fig. 2d) or a combination of spike shape and firing rate/pattern. In the latter case, firing patterns can, for example, be described in terms of their phase locking to local field potential (LFP) oscillations (Fig. 2f) and their relationship to behavioral parameters (Fig. 2g) such as the location of the animal or the orientation of its head (defining place cells and head-direction cells, respectively). Finally, both physiological and anatomical methods have recently been developed to study connectivity (Fig. 2e). Decades of research have shown that many of these parameters are often correlated. Although single parameters might appear as a graded continuum, and there is still much debate regarding exactly what constitutes a “cell type” (Bota and Swanson 2007; Battaglia et al. 2013; Seung and Sümbül 2014; Armañanzas and Ascoli 2015), the fact that clusters can be identified in this high-dimensional parameter space gives some justification to the idea that discrete “cell types” exist. Even if the number of cell types in any one area may be quite large (in the range of tens, see, e.g., Klausberger and Somogyi 2008), the identification of such discrete “building blocks” is extremely helpful in the quest to understand the microcircuitry of the brain.
Cell types in the hippocampal formation. (a). Expression of 20 common biomarkers for a subset of cell types in the hippocampal area CA1 based on literature mining (green is positive expression, blue is negative, orange is mixed expression based on different experimental protocols, red is unresolved mixed expression). (b)ThemRNA-based molecular profile of hippocampal area CA1 pyramidal cells shows distinct clusters (black rectangles) of gene expression (colors represent expression mRNA level for each gene (y-axis) for each analyzed cell (x-axis)). (c) Morphology of cell types defined in the superficial layers of the MEC. Black, dendrites and somata; red, axons. Ln layer n, Ld lamina dissecans. (d) Responses to current injection in awake mice for two pyramidal cell types in the subiculum, with either a bursty (top) or regular (bottom) firing pattern. (e) The two cell types shown in D (same color scheme) have different local connectivity profiles relative to each other and to PV-expressing interneurons (yellow), based on recording and stimulation in vitro from up to eight cells in parallel. (f) Firing patterns of several cell types recorded in area CA1 in anesthetized rats, in relation to different locally recorded cortical oscillations. (g) Spatial firing patterns of three common functionally defined cell types in the hippocampal formation: place cells (top row), HD cells (middle row), and grid cells (bottom row). Rate maps (left top, bottom) show color-coded firing rate as a function of location; a polar plot (left middle)shows HD cell firing rate as a function of the direction in which the animal’s head is facing (letters denote four cardinal directions). Plots on the right show AP firing locations (green dots) and paths traveled by the animal (black lines). (Figures taken and adapted with permission: (a) from Wheeler et al. 2015; (b) from Zeisel et al. 2015, (c) from Canto et al. 2008, (d–e) from Böhm et al. 2015, (f) from Somogyi et al. 2014; (g) from Hartley et al. 2014)
Assuming we can come up with a plan on how exactly to identify a cell type, the second issue to consider is how to collect the required parameters for such an identification (Fig. 1). One way is to record a single cell in the intact brain as an animal performs a behavioral task and then use the recording pipette to selectively “tag” the recorded cell by filling it with a dye (usually biocytin or neurobiotin). After the brain is removed from the skull and processed, the recorded cell can then be recovered and further analyzed. Contact with a target cell is usually established “blindly,” typically based on monitoring the resistance at the pipette tip (Zhu and Connors 1999; Margrie et al. 2002). More recently, single-cell recordings have also been combined with imaging, allowing a neuron to be targeted under visual control, either via a genetically defined fluorescent marker (Margrie et al. 2003) or based on the absence of fluorescence relative to a locally injected dye background (so-called shadow-patching (Kitamura et al. 2008)). The latter method was even used to record from dendrites in vivo under visual control. Although the limited penetration of light makes such imaging-dependent methods difficult to apply to deeper-lying structures such as the hippocampus, it has been done (Grienberger et al. 2014) by removal of the overlying cortical areas (Mizrahi et al. 2004; Dombeck et al. 2010).
Another possibility is to “tag” not just a single cell but rather a specific population of cells, by using genetics to induce expression of a protein based on a particular promoter. Besides using promoters for known cell types, an “ensemble” of active cells can be labeled by making use of the so-called immediate early genes such as c-Fos, whose expression can be rapidly induced by neuronal activity (Guzowski et al. 2005; Reijmers et al. 2007; Tonegawa et al. 2015). Finally, expression can be limited to a subpopulation of cells in a particular area or with particular connections by using viral stereotactic injections. All of these methods can be used to drive expression of fluorescent proteins or, perhaps more interestingly, calcium- or voltage indicators which allow imaging of the activity of the transfected neurons (Looger and Griesbeck 2012). It is even possible to extract and further process the imaged volume, enabling, e.g., post hoc immunohistochemistry or even full reconstruction of optically recorded cells with electron microscopy (Bock et al. 2011; Briggman et al. 2011; Langer and Helmchen 2012). Alternatively, the expression of light-dependent channels such as channelrhodopsin can be used to enable light-induced activity in transfected neurons. Such activity can then be used to identify transfected cells in extracellular recordings (Lima et al. 2009) and can even enable the generation of “false memories” (Ramirez et al. 2013). However, both extracellular and optical recordings have limitations in terms of describing neuronal activity (see “Readouts of Neuronal Function” below), and detailed anatomical analysis of imaged cells is still relatively difficult and limited to small volumes. Therefore, single-cell approaches, as described in this chapter, arguably provide the greatest amount of combined anatomical and functional information per recorded cell.
One way to exponentially increase the amount of anatomical information one can derive from a particular cell, albeit at the cost of destroying the cell’s morphology, is to use a glass pipette not to fill the recorded cell but rather to “harvest” material from it and isolate mRNA for further analysis (Lambolez et al. 1992; Martina et al. 1998). Progress in this field means that it is now possible in principle to obtain the “full” transcriptome from a single cell (at least from the soma), as recently published for mouse hippocampal and neocortical neurons (Zeisel et al. 2015; Tasic et al. 2016); this can even be combined with patch-clamp recordings in anesthetized animals (Cadwell et al. 2016). However, the level of technical and biological noise in the acquired data precludes the detection of low-abundance transcripts, making this method still prone to false negatives, depending on the amount of transcripts that can be collected (Okaty et al. 2011). Because cleanly harvesting mRNA material from single recorded cells in intact awake animals is not possible so far, post hoc immunohistochemistry and in situ hybridization (ISH) are still the most commonly used methods to determine molecular expression profiles. Sensitivity and specificity can be an issue for these methods, depending on the available probe/antibody. Since these methods depend on the discriminability of different markers, the spectral overlap of various fluorescent markers typically limits analysis to four different molecules per tissue sample. Usually, the brain is cut into thin sections such that a single labeled cell typically extends over many sections, making the overall number of testable molecules still relatively large, particularly for markers present on the cell’s extensive axonal or dendritic trees (see, e.g., Lasztóczi et al. 2011; Viney et al. 2013). However, it is clear that any such analysis can only ever reveal a snapshot of a cell’s full genetic expression profile and is limited by prior knowledge of which markers to test for.
The final challenge related to in vivo recording of identified cells is achieving recordings that are stable over sufficiently long time scales to achieve electrophysiological or behaviorally related characterization of the recorded cell. Recording stability always appears to involve some kind of trade-off. For instance, one can achieve stable long-term recordings from freely moving animals, even over many days, using extracellular methods based on tetrodes or silicone probes (Buzsáki et al. 2015) or recently developed imaging methods (Helmchen et al. 2013; Ziv et al. 2013). Such methods can offer access to relatively large populations of cells over long time scales. The trade-off is that the identity of the recorded cells remains largely unknown. Although genetically encoded calcium sensors or light-sensitive channels can give some information on the identity of the recorded cells, and have thus provided a major boost to our understanding of neural circuits, such methods still typically rely on a single promoter or a single injection site (in the case of virally induced expression), thus severely limiting the amount of available anatomical information. Unique genetic markers identifying single-cell types are likely to be rare. For imaging methods, post hoc identification of the imaged area can provide detailed anatomical information, as mentioned above, but these methods are still quite cost- and labor-intensive and limited to small volumes, which can be problematic particularly in the case of axonal trees which often extend over large distances.
In this chapter, I will focus on whole-cell patch-clamp and juxtacellular recordings of single cells in vivo. These methods allow high temporal resolution recordings (including, for patch-clamp recordings, subthreshold membrane potential fluctuations) from single cells, together with post hoc analysis of both the morphology and molecular expression profile of the recorded cell. The trade-off is that the number of cells one can record with such methods tends to be very small (often just one cell per animal), and recording duration is limited to the timescale of minutes (or hours, in exceptional cases). To achieve stable recordings, researchers have either recorded from anesthetized animals (Fig. 3a; Kitai et al. 1976; Pinault 1996; Margrie et al. 2002; Klausberger et al. 2003), performed head fixation to record from drug-free animals (Fig. 3b; Harvey et al. 2009; Domnisoru et al. 2013; Schmidt-Hieber and Häusser 2013), or used other methods to record single identified cells from freely moving animals (Fig. 3c; Lee et al. 2006, 2014a; Long et al. 2010; Herfst et al. 2012; Tang et al. 2014a). I will address these three different preparations and the abovementioned techniques for recording single neurons in more detail in the two Experimental Techniques sections below, but first I will summarize some recent results obtained with these methods.
Single-cell recordings in vivo. (a) Anesthetized rat in stereotaxic frame. (b) Methods to record from awake head-fixed rodents. Left: A mouse running on a treadmill. Two pipettes are shown for simultaneous intracellular (whole-cell patch-clamp) membrane potential (Vm) recording and extracellular recording of the LFP. Middle: A mouse running on an airlifted ball whose movements drive a virtual reality (VR) stimulation, which is projected onto a toroidal screen largely surrounding the mouse (inset shows top view). Note the presence of a water tube to deliver water rewards for motivation. AAM angular amplification mirror, RM reflecting mirror. Right: Mouse running on an air-lifted platform (orange), providing 3D stimulation. (c) Method to record from freely moving rodents by patching a single cell under anesthesia and then injecting antagonists to quickly wake the animal. (d) Similar method, except that recording is performed in awake mice after habituation to head fixation. (e) A miniaturized drive (micromanipulator) with pipette holder implanted onto the head of a rat, allowing the search for and recording of cells to take place in freely moving animals. Note this method has mainly been applied for juxtacellular recordings. (Figures taken and adapted with permission: (a) from Moore et al. 2014; (b) left panel from Bittner et al. 2015, middle panel from Harvey et al. 2009, right panel from Kislin et al. 2014; (c-–d) from Lee et al. 2014a; (e) from Tang et al. 2014a)
Cell Types: Linking Anatomical and Functional/Behavioral Classification
I will here focus on the link, provided by in vivo single-cell recording studies, between “anatomical” and “functional” cell types. The former, extensively classified in vitro, includes, for instance, stellate and pyramidal cells, but also many types of interneurons, whereas the latter category, identified in often classic chronic extracellular recordings, includes place cells (O’Keefe and Dostrovsky 1971; O’Keefe 1976), grid cells (Hafting et al. 2005), and head-direction cells (Taube et al. 1990), among others (Cacucci et al. 2004; Solstad et al. 2008; Krupic et al. 2012; Kropff et al. 2015). We are now finally starting to bring together these two major classification systems, although clearly there are many open questions remaining.
Place Cells
Place cells, firing selectively when an animal is at a particular position (the cell’s place field), have long been considered to be pyramidal cells based on electrophysiological parameters. However, it is becoming increasingly clear that pyramidal cells are not a homogeneous population. Recent reports suggest there are two main classes of pyramidal cells in hippocampal area CA1, mostly based on anatomical position within the stratum pyramidale, but also related to the innervation by PV cells and expression of calbindin (Slomianka et al. 2011; Lee et al. 2014b). Both classes can be place cells, but the sublayer position of recorded cell somata correlates to several functional parameters, including the likelihood of place cell firing, both in freely moving and head-fixed animals (Mizuseki et al. 2011; Danielson et al. 2016). Others have described differences between pyramidal cells based on morphology, intrinsic firing patterns (bursting versus more regular firing), and the expression of metabotropic glutamate expression (Graves et al. 2012). The relation of the latter groups with the abovementioned functional parameters remains unclear, because the functional parameters were thus far only measured in vivo with calcium imaging and extracellular recordings, methods that make further anatomical analysis of the recorded cells in those studies either difficult or impossible.
Beyond the question of anatomical identity, whole-cell recordings in freely moving and head-fixed animals have revealed a great deal about the mechanisms underlying place-specific firing (Fig. 4). As the animal approaches a cell’s place field, a ramp-like membrane depolarization and an increase in the amplitude of intracellular theta oscillations were recorded from CA1 pyramidal cells in head-fixed mice (Fig. 4a–b; Harvey et al. 2009). Whole-cell recordings from freely moving animals showed a similar ramp-like depolarization in place cells (Fig. 4c), in contrast to the conspicuously flat membrane potentials of silent cells; furthermore, place cells were shown to have a lower spike threshold than non-place-modulated silent cells (see Table 1) and were intrinsically more “bursty,” even when these same cells were recorded under anesthesia prior to any exploration (Epsztein et al. 2011; see Experimental Techniques, section “Freely Moving Animals”). Interestingly, despite these differences, many silent cells can be induced to display place-specific firing by injecting a small constant depolarizing current (Fig. 4d–e; Lee et al. 2012). This suggests that all CA1 pyramidal cells may be receiving spatially modulated inputs at their dendrites. The presence of spikelets was also modulated by the animal’s location (Epsztein et al. 2010), suggesting possible axonal interaction among pyramidal cells encoding similar locations. Finally, a recently published seminal paper (Bittner et al. 2015) used head-fixed mice running on a linear track treadmill to show that dendritic plateau potentials drive the previously described somatic ramp-like membrane depolarization and complex burst firing of place cells (Fig. 4f). These plateau potentials were shown to depend on coincident input from entorhinal cortex and CA3. Furthermore, using intracellular induction of plateau potentials, the authors were able to rapidly induce place-selective firing at the location the animal was at when the induction took place (Fig. 4g). This place-selective firing is likely due to plateau potential-mediated enhancement of the amplitude of spatially modulated EPSPs (Fig. 4h). Thus, it appears that not only does each pyramidal cell in CA1 receive spatially tuned input, but it receives spatially tuned input for all potential locations, and the convergence of input from the entorhinal cortex and CA3 determines which particular cell codes for which location (Table 2).
Place cell mechanisms revealed by intracellular recordings from CA1 pyramidal cells. (a) Intracellular recording from head-fixed mouse in VR, showing a depolarizing ramp in the membrane potential of a place cell (top trace), and plateau potentials underlying burst firing (bottom trace) when a mouse crosses a place field (outlined in gray). (b) Depolarizing ramps (top) and increased theta power were observed as mice crossed virtual place fields. (c) In recordings from freely moving mice, a similar firing pattern was found when animals crossed the place field (traces as in a). (d) Injecting depolarizing current (83 pA in this example, left panel) can cause spatial firing to appear in previously silent cells (0 pA, right panel). (e) Spatially selective depolarizing ramp (red) induced by depolarizing current. (f) Recordings from head-fixed mice running on a treadmill also found that place-field firing (top right) was associated with a depolarizing ramp and plateau potentials (bottom right). (g) Inducing a plateau potential at any particular position could induce long-term place-selective firing at this location. (h) Input amplitude potentiation was suggested by an increase in Vm residuals (Vm – mean Vm) after place field (PF) induction. (Figures taken and adapted with permission: (a) from Long and Lee 2012, adapted from Harvey et al. 2009; (b) from Harvey et al. 2009; (c) top panel from Lee et al. 2014a, middle and lower panels from Long and Lee 2012, adapted from Epsztein et al. 2011; (d–e) from Lee et al. 2012; (f–h) from Bittner et al. 2015)
It should be noted that place cells have been recorded not only in CA1 but in all hippocampal subfields including the dentate gyrus and subiculum. It is beyond the scope of this chapter to compare the properties of place cells across subfields, but notable differences do exist both at the single cell and ensemble level (Lee etal. 2004; Mizuseki et al. 2012), consistent with anatomical differences in terms of inputs and local microcircuits. For instance, granule cells tend to have several place fields (Jung and McNaughton 1993), whereas CA1 and CA3 place cells are classically thought to have one place field, although this depends on the size of the environment (Fenton et al. 2008; Rich et al. 2014).
Grid Cells
Grid cells fire when the animal is at specific locations spaced in a periodic manner such that they form a regular lattice extending over the entire environment (Hafting et al. 2005). The anatomical substrate of grid cells, particularly in MEC layer 2 (L2) where most “pure” grid cells have been reported based on tetrode recordings in rat (Sargolini et al. 2006), remains an unresolved issue. MEC L2 has classically been described as containing two main types of principal cells: pyramidal cells, expressing calbindin and Wfs1 and projecting to the contralateral MEC (Varga et al. 2010) and CA1 stratum lacunosum moleculare (Kitamura et al. 2014), and stellate cells, selectively projecting to the dentate gyrus and CA3 (Varga et al. 2010). In fact, a recent in vitro study challenged this ontology, instead finding four distinct types based on electrophysiological and morphological parameters; importantly, these types also showed distinct (local) connectivity patterns (Fuchs et al. 2016; but see also Winterer et al. 2017). The possible misclassification of intermediate cell types in older reports may be one reason why there have been quite different results regarding the question of whether grid cells in MEC L2 are stellate or pyramidal cells.
Whole-cell patch-clamp recordings show that grid cells can be recorded in head-fixed mice navigating a VR environment (Domnisoru et al. 2013; Schmidt-Hieber and Häusser 2013), but due to the technical difficulties of these experiments, the number of recovered cells was low in these studies. Only one of the reports included a characterization of the spatial firing properties of pyramidal cells, finding that most (6/9) recovered grid cells in L2 were stellate cells (Domnisoru et al. 2013). Other work based on juxtacellular recordings suggested that grid cells in layer 2 include a disproportionate number of calbindin-positive pyramidal cells, which are also significantly more theta-rhythmic than reelin-positive stellate cells (Ray et al. 2014; Tang et al. 2014b). Finally, a third report based on calcium imaging and the marker Wfs1 to delineate pyramidal cells suggests there is an equal proportion of grid cells among pyramidal and stellate cells (Sun et al. 2015). This brief overview highlights the complexities of even answering such a basic question as the anatomical identity of grid cells. This may be due to differences between rats and mice, as well as methodological differences: VR versus freely moving conditions, long versus short recordings, chronic versus acute conditions (and potential differences in behavior), the precision of anatomical localization, and the probability of isolating the activity of single cells (particularly difficult in extracellular recordings where neighboring cells display coincident firing, as may be the case in MEC L2 (Heys et al. 2014)).
Head-Direction Cells
Head-direction (HD) cells fire only when an animal’s head is facing a particular direction relative to the environment (Taube et al. 1990). Based on juxtacellular recordings from freely moving rats, it was recently found that all recovered HD cells in the presubiculum (PrS), a major input area of the MEC, were pyramidal neurons (Tukker et al. 2015), with spiny dendrites extending across all layers, mostly including apical tufts in layer 1 that suggest these cells are likely to receive input from the thalamus, another area known to contain a large proportion of HD cells (for review, see Taube 2007). Interestingly, very weak HD tuning was also found in both putative and identified fast-spiking interneurons. Another recent study was able to record HD cells in head-fixed rats by placing them on a rotating platform, thus taking advantage of the improved success and recovery rates associated with head fixation while at the same time generating the vestibular input necessary for HD cell firing (Preston-Ferrer et al. 2016). In this report, juxtacellular recording and labeling were used to show that long-range axonal projections of PrS HD cells targeted layer 3 of the MEC. These two studies together showed the presence of HD-tuned, non-theta-rhythmic pyramidal cells in PrS providing inputs to MEC layer 3, supporting the idea that grid cells in this layer are likely to receive excitatory HD-tuned inputs.
Virtually all models of grid cells require some directionally selective input, although the anatomical origin of such input is not usually explicitly mentioned. Although the aforementioned studies suggest the PrS input may supply this input, it remains to be shown that indeed grid cells are among the targets of the HD PrS inputs. Interestingly, other studies have also shown that there may be “masked” HD tuning in grid cells (Brandon et al. 2011; Bonnevie et al. 2013), as well as a large proportion of HD cells and conjunctive grid x HD cells (Sargolini et al. 2006) specifically in layer 3 of the MEC. In apparent contrast, a report based on juxtacellularly filled cells as well as tetrode recordings (Tang et al. 2015) recently reported rather weak HD tuning in this layer. This could be partially due to differences in the recording and/or training methods, but it seems likely that other factors such as the precise definition of HD cells and the anatomical localization of recordings may also explain the divergent results. The latter point is related to the fact that there is a gradient of HD tuning in layer 3, with many of the strong HD cells located in the most dorsal part of MEC (Giocomo et al. 2014). Based on recent genetic and anatomical work, this dorsal region may correspond to some extent to the parasubiculum (Ramsden et al. 2015), which contains a much higher proportion of HD cells (Tang et al. 2016). This example emphasizes the importance of precise anatomical localization, which is generally more limited in extracellular recordings, but also reminds us that macroanatomical definitions of brain areas may need to be adapted as we acquire new insights on the basis of molecular markers or connectivity (Boccara et al. 2015; Ramsden et al. 2015; Ishihara and Fukuda 2016).
HD cells, like most functionally defined cell types, are typically defined based on a somewhat arbitrary cutoff within a wider distribution of tuning strengths. In fact, a quantification of the extent to which particular functional parameters could explain the variance in unit firing rate, based on extracellular recordings in several hippocampal areas, found that many cells tend to code for several parameters, to different extents (Sharp 1996; see also Sargolini et al. 2006; Hardcastle et al. 2017). This contrasts to some extent with the often clearer categorization of anatomical or neurochemical cell types, e.g., based on the presence or absence of a particular marker. It may therefore not be feasible to find an anatomical substrate for each functional cell type, and instead we may find certain functional tuning parameters correlating to a greater or lesser extent with particular anatomical parameters. Certainly it is important to keep in mind that functional cell type classifications are often a shorthand for a more complex reality. Furthermore, the precise definitions of functional cell types often vary between studies, and these differences matter.
Interneurons
Historically, the question of the anatomical identity of functional cell types has mostly been limited to principal cells; this is partly because many extracellular electrophysiology studies, where functional cell types have been discovered, have excluded “fast-spiking” units and partly because principal cells are, as the name implies, the majority. In principle, there is no reason any particular functional cell type could not include interneurons, and in fact it is likely that many GABAergic interneurons display some form of functional tuning (Kepecs and Fishell 2014). Thus, depending on how “inclusive” the criteria are, interneurons can either be included as, e.g., HD cells, or excluded. However, in terms of understanding a cortical circuit, it seems clear that GABAergic HD cells can have quite different functions than glutamatergic HD cells. Therefore, it makes sense to treat interneurons as a separate category and ask the complementary question, i.e., what is the function of anatomically identified interneuron cell types?
This question was briefly touched upon in the paragraph on HD cells; for the PrS, so far little is known beyond the fact that fast-spiking interneurons, including at least some PV neurons, show weak but significant HD tuning (Tukker et al. 2015). In general, there is a large body of work showing a similar trend: relatively weak tuning in interneurons has been found in visual cortex, hippocampus, and many other brain areas (Kubie et al. 1990; Maurer et al. 2006; Ego-Stengel and Wilson 2007; Kerlin et al. 2010). In the MEC, a study combining extracellular recordings with optogenetics recently showed that PV cells, although not displaying any HD or grid-like spatial coding, do encode some spatial information (Buetfering et al. 2014). This study is also one of the first to tackle the question of how functionally and anatomically defined cell types are connected with each other, a crucial issue that mostly still remains in the realm of modeling. They showed that PV cells receive inputs from many nonaligned grids, thus explaining their lack of “gridness.”
Another recent study from the same laboratory used visually guided intracellular patch-clamp recordings in ketamine-/xylazine-anesthetized mice to show odor-evoked firing in four out of four GAD67 neurons in the lateral entorhinal cortex (LEC; Leitner et al. 2016); although all these cells had dense axonal arborization in the superficial layers, their electrophysiological properties were very heterogeneous. Of course, more cells need to be recorded, preferably in drug-free conditions, and additional knowledge of the molecular expression profile of these cells would also be very informative, but even this small sample suggests that several types of GABAergic interneuron respond to odor in the LEC.
Unfortunately, there are many different classes of PV cells in the MEC, and even more classes of GAD67 cells in the LEC, which could not be discerned in these studies and could explain some of the reported variability. Indeed, most reports of interneuron functional tuning reported thus far are either based on spike shape and firing pattern, as in the case of the classic “theta cells” in the hippocampus (Fox and Ranck 1975; Kubie et al. 1990) or, more recently, based on the expression of one of a small set of genetic markers (e.g., Royer et al. 2012). Although such studies are insightful, GABAergic interneurons form an incredibly heterogeneous population (Canto et al. 2008; Klausberger and Somogyi 2008; Somogyi et al. 2014; Nassar et al. 2015; Ferrante et al. 2017), and no electrophysiological or single genetic marker can suffice to discern the various types that have been described thus far. For instance, PV cells in the hippocampus include preferentially soma-targeting basket cells, axon initial segment-targeting axo-axonic cells (also known as chandelier cells), proximal dendrite-targeting bistratified cells, and distal dendrite-targeting oriens-lacunosum moleculare (O-LM) cells (Klausberger et al. 2003, 2004). Each of these cell types can have its own connectivity, plasticity, and expression patterns (including neuropeptides, receptors, channels, etc.). Considering the recent use of genetic methods, it should also be mentioned that protein expression patterns are regulated developmentally, and thus the expression of, e.g., Cre recombinase under the control of a particular promoter may not necessarily reflect protein expression in the adult. This was recently shown for neurons in the hippocampus expressing both Cre and Flp recombinases under the control of parvalbumin and somatostatin promoters, respectively; surprisingly, a majority of these neurons were found to be immunonegative for PV (Fenno et al. 2014). Based on their morphologies, these cells were identified as mostly O-LM interneurons, a cell type originally described as PV-expressing in rat (Klausberger et al. 2003) but recently reported to be PV-immunonegative in mice (Varga et al. 2012).
Interestingly, although O-LM cells and bistratified cells both express PV and SOM, a recent study showed that they play very different roles in the circuit during fear learning (Lovett-Barron et al. 2014). Since O-LM cells target almost exclusively the stratum lacunosum moleculare, whereas bistratified cells target the neighboring stratum radiatum, calcium imaging of SOM axons restricted to these layers could be used to selectively image putative O-LM and bistratified cells. A contextual fear conditioning task in head-fixed mice running on a treadmill was used to reveal that O-LM but not bistratified or other PV cells responded to aversive stimuli. This was mediated by a cholinergic input signal from the medial septum, which the O-LM cells can respond to because they express cholinergic receptors. The response of SOM expressing putative O-LM cells to aversive air puff stimuli was also shown via visually guided juxtacellular recordings in head-fixed mice (Schmid et al. 2016). This same paper used calcium imaging and pharmacology to reveal that this response was acetylcholine-dependent and impaired in a mouse model of Alzheimer’s disease (AD). The deficit in the AD mouse was linked to a reduced number of presynaptic cholinergic cells in the medial septum and an acetylcholine-dependent fear conditioning deficit in these animals. Thus, O-LM cells may be a potential target for cholinergic drugs that could compensate the well-known degeneration of cholinergic neurons in AD: by targeting cholinergic receptors on O-LM cells, future therapeutics could potentially reverse learning deficits by repairing the O-LM cell-mediated modulation of the entorhinal input onto CA1 pyramidal cells. This example serves to illustrate the importance of knowing, e.g., which other cell types also express acetylcholine receptors, and what their synaptic targets may be. For instance, SOM cells in the hippocampus have also been shown to include long-range interneurons projecting to the MEC (Melzer et al. 2012), which presumably have very different functions from O-LM cells.
A full description of hippocampal interneuronal heterogeneity is beyond the scope of this chapter (see also chapter “Fast and Slow GABAergic Transmission in Hippocampal Circuits”). It is clear, however, that the large diversity of these cells makes the precise identification of cells both important and difficult, particularly in combination with behavior. Earlier intracellular and juxtacellular recordings from anesthetized rats have shown that specific subtypes of GABAergic interneuron play specific roles in the generation of various oscillations of the local field potential (LFP) related to particular behavioral states (Ylinen et al. 1995b; Klausberger et al. 2003, 2004, 2005; Jinno et al. 2007; Tukker et al. 2007; Fuentealba et al. 2008; Lasztóczi et al. 2011). Many, but not all, of these findings were confirmed in juxtacellular recordings in freely moving rats (Lapray et al. 2012; Viney et al. 2013; Katona et al. 2014) and head-fixed mice (Varga et al. 2012, 2014). For the future, it will be important to investigate to what extent the functional tuning of different interneuron classes differs and to relate this to connectivity either directly (e.g., using viral tracing methods in combination with whole-cell recordings in head-fixed mice (Rancz et al. 2011; Vélez-Fort et al. 2014; Wertz et al. 2015)) or based on in vitro results (Couey et al. 2013; Böhm et al. 2015; Fuchs et al. 2016), particularly if those in vitro results can include more extensive cell type classifications, e.g., based on morphology (Jiang et al. 2013, 2015).
What Have We Learned? An Example
It is very difficult, at this stage, to already draw major conclusions based on the previously described work. We are only just beginning to have some overview of the roles of different cells in behavior and in driving network phenomena like cortical oscillations. A major challenge will be to bring together the results of in vivo single-cell studies as described here with in vitro work on the one hand and extracellular, imaging, and behavioral studies on the other, to form a unified picture of hippocampal microcircuits.
Our most advanced understanding is perhaps related to hippocampal place cells (Fig. 4), whose place-specific firing was recently reported to rely on dendritic plateau potentials and convergent inputs from CA3 and layer 3 of the EC, as described above (Bittner et al. 2015). However, there are still many open questions even for this most studied “cell type,” including the nature of the specific input provided by layer 3 of the EC (Sargolini et al. 2006; Suh et al. 2011; Tang et al. 2015) and the role of specific types of interneurons in CA1. As one specific example, consider the role of bistratified cells in the hippocampus (Fig. 5; for a more detailed review, see Müller and Remy 2014). Paired recordings in hippocampal area CA1 in vitro, combined with LM reconstructions and EM analysis (Buhl et al. 1994), first showed these cells, which express PV, neuropeptide Y (NPY), and SOM (Klausberger et al. 2004), to selectively innervate pyramidal cell dendrites co-aligned with Schaffer collateral input from CA3 in strata radiatum and oriens. Bistratified cells receive direct inputs from PV basket cells (Cobb et al. 1997), such that somatic and dendritic inhibition can be coordinated in a complementary manner (Lovett-Barron et al. 2012). The latter in vitro study also showed that direct inputs from CA3 pyramidal cells onto bistratified cells may help these cells to regulate the impact of inputs from CA3 onto CA1 pyramidal cells. Such a role is consistent with recordings from CA1 in urethane-anesthetized rats suggesting that these cells are among the most strongly phase-locked to gamma oscillations (Tukker et al. 2007), which are likely generated in CA3 (Csicsvari et al. 2003). Thus, bistratified cells may ensure that the dendrites of CA1 pyramidal cells can effectively process the gamma-rhythmic inputs from CA3 cell assemblies; alternatively, they may also block transfer of information during certain brain states, for instance, by releasing NPY or SOM in response to high-frequency firing, which bistratified cells display both during movement and sleep (Katona et al. 2014). The latter authors speculated that this slow peptide release may be one mechanism for the termination of SWRs, during which identified bistratified cells have been shown to strongly increase their firing rates in anesthetized (Klausberger et al. 2004), head-fixed (Varga et al. 2014), and freely moving (Katona et al. 2014) rodents. Interestingly, the firing rate increase in bistratified cells appeared to be different depending on the extent of its dendritic tree in stratum radiatum (Varga et al. 2014), raising the question whether bistratified cells should be further subdivided into two separate cell types or not. Like gamma oscillations, SWRs are also generated in CA3; in fact, gamma oscillation power and synchrony across CA3 and CA1 were recently shown to increase during SWRs, in a manner that was predictive of the quality of “replay” of past experiences (Carr et al. 2012).
Hippocampal bistratified interneurons characterized via in vivo juxtacellular recordings in different rodent preparations. (a) LFP recording (top) and simultaneously recorded APs from a single cell recorded in urethane plus ketamine-xylazine anesthesia. Scale bars: horizontal 0.1 s; vertical 0.2 mV; (b) firing probability of seven different bistratified cells (gray is average) at different phases of simultaneously recorded theta oscillations, showing that bistratified cells preferably fire at the trough of the theta cycle. (c) Bistratified cells were filled with neurobiotin (blue) and shown to be immunopositive for parvalbumin (green, bottom left), somatostatin (red), and neuropeptide Y (green, bottom right). Scale bar, 20 μm. (d) LFP recording (top) and simultaneously recorded APs from a bistratified cell recorded in a head-fixed mouse running on an air-lifted ball; bottom trace shows theta-filtered (5–10 Hz) LFP. (e) Theta modulation strength for eight recorded bistratified cells (green), showing that these cell preferentially fired at the trough of theta. Note that other recorded cell types (PV basket cells, red, and axo-axonic cells, blue) were more strongly modulated and fired at earlier theta phases. (f) Example filled bistratified cell (axon blue, dendrites red) which was filled with neurobiotin (inset, red) and immunopositive for somatostatin (inset, green). (g) LFP recording (top) and simultaneously recorded APs (bottom) from a bistratified cell recorded from a freely moving mouse; inset shows autocorrelogram illustrating theta-rhythmic firing of the recorded cell during periods when the animal was moving. (h) Polar plot showing average firing rate as a function of theta phase for five recorded bistratified cells (red), with preferred phase for each cell individually shown as red circles. O-LM cells are shown in green. (i) Cell recorded in g (axon black, dendrites red) filled with neurobiotin (inset, red), immunopositive for PV both in axon (yellow arrow) and dendrite (yellow arrowhead). (Figures taken and adapted with permission: (a) from Somogyi et al. 2014; (b–c) from Klausberger et al. 2004; (d–f) from Varga et al. 2014; (g–i) from Katona et al. 2014)
In general, it seems plausible that bistratified cells are involved in gating the transfer of information from CA3 to CA1 during sharp-wave ripple events as well as gamma oscillations (Buzsaki 2006). One possible way in which this gating might be regulated is via bistratified cell-mediated inhibition of dendritically generated plateau potentials (Lovett-Barron et al. 2012), which were recently shown, as mentioned above (Fig. 4f–g), to be important for the generation of burst firing in pyramidal cells underlying place selectivity (Bittner et al. 2015). In contrast, bistratified cells did not appear to be involved in fear conditioning (Lovett-Barron et al. 2014). Like many other interneuron types in the hippocampus, bistratified cells also show theta-modulated firing (Fig. 5; Klausberger et al. 2004; Katona et al. 2014; Varga et al. 2014), at a similar phase as O-LM cells but different from other interneuron types. Although both theta and gamma oscillations have been linked to the coding of an animal’s movement speed, there is unfortunately no report on the speed dependence of bistratified cell firing. Furthermore, recent reports have shown two or even three types of gamma oscillations in CA1, with different underlying mechanisms, which could all be relevant for place cell firing and spatial navigation (Lasztóczi and Klausberger 2014, 2016; Colgin 2015). The relation of these oscillations with bistratified cells, or indeed any other specific interneuron type recorded in awake animals, remains unknown (except PV basket cells; Lasztóczi and Klausberger 2014).
The work presented here on bistratified cells serves as an example to illustrate the insights that can be gained from recording identified cell types in the hippocampus (based on anatomical and molecular characterization). There are still very few studies directly relating the activity of bistratified cells to navigational function, which may be partly due to the fact that simply very few studies have been done in freely moving or head-fixed animals engaged in spatial tasks, and most of those studies have focused on first understanding place-specific activity of CA1 pyramidal cells (Harvey et al. 2009; Epsztein et al. 2010, 2011; Lee et al. 2012; Bittner et al. 2015). It is indeed still challenging to achieve long and mechanically stable recordings in moving animals, even if they are head-fixed, and to recover recorded cells such that one can perform the necessary anatomical and molecular characterization to robustly identify neuron types. For interneurons specifically, the presence of many relatively small populations of specific cell types poses further challenges. Bistratified cells, for instance, comprise just 6% of all interneurons (Bezaire and Soltesz 2013), which themselves are estimated to comprise just ∼10% of all hippocampal neurons. However,even such relatively small populations have been shown to perform important roles within the hippocampal microcircuit. Comparing the activity of bistratified cells with other interneuron types, including other SOM- or PV-expressing cells, supports the idea that different cell types have specific roles within the neural circuitry underlying hippocampal function (Somogyi et al. 2014).
Experimental Techniques: Rodent Preparations
Anesthetized Animals
Urethane has been a very commonly used (non-recovery) anesthetic for several decades. It has a broad spectrum of actions, including potentiation of GABA-A, nicotinic acetylcholine, and glycine receptors, while inhibiting NMDA- and AMPA-type glutamate receptors (Hara and Harris 2002); furthermore, it has been shown to inhibit glutamate release (Moroni et al. 1981), and LFPs recorded in the hippocampus of urethane-anesthetized animals are similar to those observed during entorhinal lesion (Ylinen et al. 1995b). Although it has pronounced effects on LFP and unit firing (Buzsáki et al. 1983, 1986), the overall network appears relatively intact, and in fact urethane can induce a regularly cycling series or brain states reminiscent of sleep, both in rats (Clement et al. 2008) and mice (Pagliardini et al. 2013). As with all anesthetics, the dosage is a crucial determinant of the effects.
Because of the irreversible nature of its effects, and the fact that urethane is highly carcinogenic, in many situations other anesthetics are preferred. Ketamine, an NMDA antagonist, is often used as an alternative. Its action is relatively short-lasting and on its own it generally provides insufficient anesthetic effect. Therefore it is typically combined with an adrenergic receptor type α2 agonist such as xylazine or medetomidine. This way, a surgical depth of anesthesia can be reached, and the recovery can be aided by an antagonist (e.g., atipamezole). Often, ketamine-xylazine is combined with a relatively low concentration of urethane; by giving top-ups at specific intervals, the experimenter can then have finer control over the depth of anesthesia (Klausberger et al. 2003). At lighter planes of anesthesia, brain state can be additionally influenced by external stimuli such as a foot- or tail-pinch, which can be used to elicit theta oscillations in the hippocampus or entorhinal area (Dickson et al. 1994; Klausberger et al. 2003). Importantly, anesthetized animals can still respond to sensory stimuli, as evidenced by neuronal responses in sensory cortices (Stosiek et al. 2003; Ohki et al. 2005).
In situations where fast recovery is essential, such as when a whole-cell patch-clamp recording established during anesthesia needs to be maintained as the animal wakes up (Lee et al. 2006), a cocktail of drugs including medetomidine, midazolam, and fentanyl has been used. These drugs were selected because administration of atipamezole, flumazenil, and naloxone could be used to end the anesthesia such that behavior could recover within 1–5 min.
For many applications, isoflurane has proven a convenient anesthetic. It is an inhalant that works, as least in part, by reducing transmitter release (Hemmings et al. 2005), particularly at glutamatergic synapses (Westphalen and Hemmings 2006). A recent paper showed that different brain states could be elicited, depending on the concentration: at low doses, exploratory or REM-like brain waves could be detected in the hippocampus, whereas at higher doses, isoflurane elicited slower oscillations more akin to quiet resting or slow-wave sleep (Lustig et al. 2016). One advantage of this method is that one can directly adapt the concentration in response to behavioral signs, resulting in a more reliable stable level of anesthesia. Injections, particularly intraperitoneal injections, can sometimes be misdirected depending on the experience and skill of the experimenter; this can result in unstable anesthesia or even death. Particularly for drugs that often require repeated injections (e.g., ketamine-xylazine), the instability and loss of animals can be serious issues, especially when working with mice.
The exact effects and mechanisms of anesthesia are not always well understood, and certainly a full discussion (including other commonly used anesthetics) is beyond the scope of this chapter. However, it is important to emphasize that anesthetics can and typically do have substantial effects on neuronal activity. For instance, dendritic calcium spikes (measured in vitro) can be differentially affected by urethane versus ketamine-xylazine (Potez and Larkum 2008). Firing rates, spike bursting, and neuronal synchrony have all been shown to be affected by (a relatively high dose of) urethane anesthesia (Greenberg et al. 2008). As a final example, a relatively brief exposure to isoflurane was recently found to affect the phosphorylation state of a wide range of proteins (Kohtala et al. 2016), which may in turn affect neuronal activity. However, as stated above, the overall network often seems relatively intact, and thus indeed many findings from anesthetized animals have been confirmed in awake animals, and the anesthetized animal remains a convenient preparation for in vivo investigations. In particular, the increased mechanical stability achievable in anesthetized animals allows much longer recording times compared to awake animals. The overall time the animal can be used in an experiment also tends to be much longer under anesthesia, allowing more recordings per animal compared to awake conditions, where session times are more limited. Furthermore, these recordings do not require training or habituation of the animals. Thus, it is time efficient and the model of choice for the first implementary steps of a new technique. Finally, anesthetized preparations allow a broad spectrum of profound surgical intervention which might be problematic in the light of animal welfare in awake in vivo preparations.
Head-Fixed Awake Animals
Clearly, the goal of many studies is to establish a link between neuronal activity and behavior. For single-cell recordings, head-fixed animals in many cases provide the best compromise between recording stability on the one hand and a relatively rich behavioral repertoire on the other (for recent reviews, see Minderer et al. 2016; Thurley and Ayaz 2017).
Although head fixation does limit the behavioral repertoire, animals can nevertheless be trained, for example, to press a lever (Guo et al. 2014), lick (Houweling and Brecht 2008), or whisk (Gao et al. 2003) in response to a stimulus. In fact, head fixation can be an advantage since stimuli can be presented in a very controlled manner (O’Connor et al. 2009). By adding the possibility for animals to move all their limbs on an air-cushioned Styrofoam ball (Dombeck et al. 2007; Fuhrmann et al. 2015) or cylinder (Domnisoru et al. 2013), one can increase the behavioral possibilities of the animal. These options are also the most relevant for the study of navigation and spatial memory. Running is often accompanied by brain movement, also in head-fixed animals, and this can be an issue: one study reported motion limited to 5 μm in the head-fixed mouse, being greatest in the rostrocaudal axis (Dombeck et al. 2007); another study reported cranial movement up to 40 μm in the head-fixed rat along the axis of the pipette (Fee 2000). The latter study was able to move the pipette with a piezo device to compensate for measured motion, thus enabling longer intracellular recordings even in moving animals. Of course, brain and/or residual cranial motion may be variable depending on the area recorded from, as well as the precise methods used for head fixation, but overall it seems that in terms of stability mice may provide an advantage over larger, stronger rats.
In terms of stimuli, allowing the animals to run provides additional proprioceptive input, which can be crucial for certain types of navigational processes such as path integration. Furthermore, linear treadmills (Royer et al. 2012) and movable platforms (Kislin et al. 2014; Nashaat et al. 2016) can offer a relatively wide range of somatosensory and visual stimulation. Spherical treadmills have also been combined with moveable walls to provide a “tactile virtual reality” system (Sofroniew et al. 2014); this same study also showed that mouse behavior on the ball resembled natural behavior in terms of running speed and stride and whisking frequencies. However, most studies combine the spherical treadmill with a visually presented virtual reality (VR), either via an array of screens or a toroidal projection system (Harvey et al. 2009). Rats can navigate in VR as long as the visual stimuli extend over a sufficient angle (Hölscher et al. 2005) and can even be trained to lick to indicate recognition of a previously learned location (Cushman et al. 2013). Mice can also perform spatial memory tasks, as shown in a one-dimensional VR environment using just a single widescreen LCD monitor (Youngstrom and Strowbridge 2012), and have been successfully trained to perform a VR T-maze decision task (Harvey et al. 2012). Thus, head fixation, particularly in combination with VR, allows one to employ a relatively wide range of stimuli and tasks relevant for studying hippocampal microcircuitry, ranging from simple running along a one-dimensional corridor to complex tasks in two dimensions.
However, some caveats are in order. First of all, head fixation does limit the animal’s motion; for instance, rearing (Lever et al. 2006) is obviously not possible nor are orienting head movements (Monaco et al. 2014). Secondly, the sensory input tends to be limited: visual cues are all relatively distal, and local somatosensory cues tend to be non-informative. These issues can be resolved to some extent by using a treadmill (Royer et al. 2012) or moveable platform (Kislin et al. 2014; Nashaat et al. 2016) with physical objects on it. However, for any head-fixed system, the lack of vestibular inputs is unavoidable and should be carefully considered particularly in light of its potential importance for certain aspects of navigational function. For instance, place cells could not be detected when rats navigated a virtual 2D environment (Aghajan et al. 2015), suggesting that the spatial maps generated in the more commonly used one-dimensional VR environments may be more related to internal mechanisms keeping track of self-motion than external cues, although there is likely to be heterogeneity among place cells (Chen et al. 2013). The importance of vestibular input was underlined by another recent study which found that in body-tethered rats, which were free to move their head and thus generate intact vestibular inputs, grid, place, and border cells could all be recorded in a 2D VR environment (Aronov and Tank 2014).
A reported lack of correlation between theta oscillations and speed in VR, which clearly differs from real-world results (Ravassard et al. 2013), is also consistent with a role for vestibular inputs in the mechanisms underlying theta oscillations (Russell et al. 2006; Jacob et al. 2014). Interestingly, the absence of speed-dependent theta oscillations did not abolish theta phase precession (Ravassard et al. 2013), a form of temporal coding whereby place cells fire at progressively earlier phases of the theta cycle as an animal crosses a place field (O’Keefe and Recce 1993). The fact that theta phase precession was also intact in a 2D environment (Aghajan et al. 2015), where no place cells could be detected, suggests that theta phase precession may be independent of both speed-dependent theta oscillations and representations of the current location, in support of a recent model positing that theta phase precession represents “mind travel” related to imagined movement rather than actual movement (Sanders et al. 2015).
More generally, the limitations of the virtual reality system in terms of ecological behavior are offset by greater control over different modalities and over the relationship between, e.g., animal motion and the generated visual flow. This has been used successfully to disentangle the relevance of particular inputs to the hippocampal representation of space (Chen et al. 2013; Ravassard et al. 2013; Acharya et al. 2016).
A final point to consider is that time and effort are required to get animals to habituate to head fixation and perform tasks (Schwarz et al. 2010; Guo et al. 2014). Training typically relies on positive feedback (usually water), whereby the animals undergo food or water restriction. The severity of the restriction regime depends on the difficulty of the task to be performed; for relatively simple tasks, such as running on a ball, it may even be sufficient to provide sugar-water to unrestricted animals (Schmidt-Hieber and Häusser 2013), although this is atypical. With appropriate training, rats will even initiate head fixation voluntarily and remain head-fixed for relatively short durations (Scott et al. 2013), potentially enabling high-throughput automated approaches.
Freely Moving Animals
Extracellular recordings have been possible from freely moving animals for many decades (see also chapter “Spatial, Temporal, and Behavioral Correlates of Hippocampal Neuronal Activity: A Primer for Computational Analysis”), and recently the advent of optogenetics has allowed the use of optrodes in freely moving animals to record from genetically identified populations of cells or populations of cells with specific anatomical targets (Buzsáki et al. 2015; Grosenick et al. 2015; Wu et al. 2015). In addition, various miniaturized microscopes have been developed (Helmchen et al. 2001; Ferezou et al. 2006; Flusberg et al. 2008; Sawinski et al. 2009), which can also be used to image genetically and/or anatomically identified populations of cells in freely moving animals, even in deeper-lying brain regions such as the hippocampus (Ziv et al. 2013). Via targeted light stimulation, one can even selectively manipulate cells (Packer et al. 2015). These are important developments that are, however, outside the scope of this chapter. Electrophysiological tools still have the advantage of being able to directly record and manipulate membrane potentials and spiking activity at high temporal resolution. In head-fixed and particularly anesthetized applications, single-cell electrophysiology is also relatively cheap and easy to implement and enables relatively straightforward labeling and recovery of recorded cells. In freely moving animals, however, it is still a challenging and relatively rarely used technique. The technique has been made possible in part by the development of lightweight, miniature recording equipment that can be implanted on the head, including miniature headstages but also microdrives and holders for glass pipettes (Lee et al. 2006; Long et al. 2010; Herfst et al. 2012; Tang et al. 2014a).
One approach has been to search for cells (using current pulses and monitoring resistance at the pipette tip) under anesthesia, when the brain is relatively stable, and then waking up the animal after a successful recording has been initiated and the pipette has been “anchored” in place (Lee et al. 2006; Tang et al. 2014a). This method has been used with some success to perform whole-cell patch-clamp recordings of CA1 hippocampal pyramidal cells (Lee et al. 2009, 2012; Epsztein et al. 2010, 2011) and has also been applied to perform juxtacellular recordings in the medial entorhinal cortex and hippocampal area CA1 (Burgalossi et al. 2011; Herfst et al. 2012). Refinements of this “anchoring” method have recently enabled whole-cell patch-clamp recordings from drug-free animals (Lee et al. 2014a).
By using a microdrive implanted on the head, it is also possible to search for cells and perform single-cell recordings in fully drug-free animals (Long et al. 2010; Tang et al. 2014a). This method was used to perform intracellular recordings with sharp electrodes from CA1 pyramidal cells in mice (English et al. 2014) and has also been successfully used to perform juxtacellular recordings in the hippocampus (Lapray et al. 2012; Viney et al. 2013; Katona et al. 2014; Diamantaki et al. 2016a, 2016b), medial entorhinal cortex (Ray et al. 2014; Tang et al. 2014b), presubiculum (Tukker et al. 2015), and parasubiculum (Tang et al. 2016) of freely moving rats.
Experimental Techniques: Readouts of Neuronal Function
Intracellular Recordings
For decades, intracellular recordings with sharp electrodes have been performed in vivo (see Long and Lee 2012, for review), but more recently the whole-cell patch-clamp approach has also been adapted for intracellular recording in vivo (Zhu and Connors 1999; Margrie et al. 2002). The key advantage of the latter approach is that patch-clamp pipette tips are considerably larger, providing lower resistance and thus better electrical access to the inside of the cell. In vivo, these recordings are usually performed blindly, particularly in deep tissues such as the hippocampus. The probability of recording from a particular cell type or layer may therefore be limited. Overall, success rates depend on a thoroughly prepared brain surface and possibly on a slow approach of the pipette through the tissue. Also taking the welfare of the animal into account, which sets practical limits to the recording time in awake animals (particularly when head-fixed), it can be challenging to achieve a successful recording. Newer techniques allow visual guidance (Kitamura et al. 2008) or, for deeper-lying brain structures, the use of optogenetic tools to pre-identify target neurons for intracellular recording and labeling (Muñoz et al. 2014). Even for a deep-lying structure such as the hippocampus, whole-cell recordings have been combined with calcium imaging (Grienberger et al. 2014), revealing the nature of burst firing in CA1 pyramidal cells in head-fixed mice under light isoflurane anesthesia.
Whole-cell patch-clamp recordings offer a number of benefits compared to extracellular recordings and/or calcium-imaging approaches. First of all, subthreshold activity can be measured at high temporal resolution. Although in vivo access resistance is often relatively high, limiting access to compartments further from the soma, it is possible to differentiate inhibitory and excitatory inputs to some extent either by clamping the membrane potential or by extracting putative excitatory and inhibitory synaptic potentials from the recorded voltage traces (Tao et al. 2015). Another possibility is to use pharmacological manipulation to isolate particular synaptic inputs. One limitation of the aforementioned methods is that it can be difficult or impossible to simultaneously observe temporally overlapping inhibition and excitation.
A series of papers has used intracellular recordings to elucidate the roles of excitation and inhibition in the generation of so-called sharp-wave-associated ripples (SWRs; Fig. 6). SWRs are fast oscillations (∼100–200 Hz) thought to play a role in the replay of previously experienced events, linked to memory consolidation, as well as the possible planning of future events (Buzsáki 2015). Early intracellular recordings from urethane- and ketamine-anesthetized mice showed the presence of ripple-frequency membrane oscillations in vivo and suggested a main role for perisomatic inhibition (Ylinen et al. 1995a). A later study combining in vitro experiments with recordings from awake head-fixed mice first showed that SWRs were also coupled to phasic excitation, which preceded hyperpolarization (Maier et al. 2011; see also Hulse et al. 2016; Fig. 6a). More recently, sharp recordings from freely moving mice showed that action potential firing of CA1 pyramidal cells was often suppressed during simultaneously recorded SWRs despite a large (threshold-exceeding) membrane depolarization, indicating the presence of shunting inhibition (English et al. 2014; Fig. 6b–c). Finally, there is also a reported variability among principal cells in terms of the role of inhibitory versus excitatory drive during SWRs. In CA1, intracellular recordings from urethane-anesthetized rats, as well as juxtacellular recordings from freely moving rats, showed that deep-lying pyramidal cells were more inhibited, while more superficial cells were more excited during SWRs, a finding that correlated to calbindin immunoreactivity (Valero et al. 2015; Fig. 6d–f). In the subiculum, both intracellular and juxtacellular recordings in awake head-fixed mice showed that burst-firing cells were more depolarized, whereas regular firing cells were more hyperpolarized during SWRs (Böhm et al. 2015); interestingly, these cells also had different connectivity within the network as shown in vitro by simultaneous intracellular recordings of up to eight cells (Fig. 2e). Both of these reports suggest the presence of different principal cell types playing different roles in the generation of SWRs, whereby recorded firing rates and membrane potential were correlated to either a molecular marker and precise anatomical location (Valero et al. 2015) or to intrinsic electrophysiological properties and connectivity (Böhm et al. 2015).
Mechanisms underlying SWR generation in CA1. (a) Whole-cell patch-clamp recordings of CA1 pyramidal cells in awake head-fixed mice reveal phasic excitatory currents (bottom trace) coincident with SWRs detected in the LFP recorded with a separate electrode(top trace). (b) Intracellular recordings of a CA1 pyramidal cell (left inset) from a freely moving mouse also showed a membrane potential depolarization (bottom trace, Vm) during SWRs (top trace, LFP) followed by a relatively long-lasting after hyperpolarization (AHP). (c) These recordings also revealed that during SWRs (green) action potential firing was reduced despite cells being depolarized above threshold. This was not the case during pre-ripple control intervals (gray). (d) Intracellular recordings from anesthetized rats revealed that during SWRs, CA1 pyramidal cells could be either predominantly depolarized (green) or hyperpolarized (red). Simultaneously recorded LFPs are shown in black. (e) These firing patterns were found in more superficial calbindin-immunopositive (CB+) and deeper-lying calbindin-immunonegative (CB-) cells, respectively. (f). Scheme showing hippocampal connectivity related to firing patterns of different cell types during SWRs. (Figures taken and adapted with permission: (a) from Maier et al. 2011; (b–c) from English et al. 2014; (d–f) from Valero et al. 2015)
In general, the ability to record subthreshold oscillations or ramps and their voltage dependence is an important advantage of intracellular recordings, particularly if they can be related to the behavior of the animal (e.g., its location or speed; see below). It is even possible to record signatures of dendritic spiking (Kamondi et al. 1998; Harvey et al. 2009; Epsztein et al. 2011; Grienberger et al. 2014; Bittner et al. 2015) and of axonal events (Epsztein et al. 2010; Chorev and Brecht 2012; Apostolides et al. 2016). Importantly, this recording method also allows cells to be labeled and recovered, enabling further classification to be performed post hoc based on precise anatomical localization as well as morphological, immunohistochemical, and/or ISH characterization. Even complete filling of axonal arbors is possible, which can enable tracking of long-range projections over several millimeters (Oberlaender et al. 2012). Besides filling cells based on the injection of a dye, cells can also be labeled by infusing a plasmid that then drives the expression of a fluorescent protein; this method has been used to transfect single intracellularly recorded cells in the visual cortex not only with a fluorescent protein but also with a receptor allowing selective infection by a rabies mutant (injected 2 days later) which retrogradely labels monosynaptically connected presynaptic cells (Rancz et al. 2011).
One caveat is that the washout of cytosol can be an issue for certain markers, e.g., calbindin (Müller et al. 2005), particularly for longer whole-cell patch-clamp recordings (less so for sharp electrodes). It should also be noted that labeling is typically worse for recordings from freely moving animals, particularly for longer axons. The reduced recovery rate is likely due to the fact that cells recorded from freely moving animals are often lost due to mechanical disturbance, rather than being actively terminated via slow withdrawal of the pipette, as typically done in anesthetized animals (Rancz et al. 2011).
In contrast to extracellular recording methods, which rely on unit isolation algorithms that are biased for cells with high firing rates (Pedreira et al. 2012) and are likely to miss many cells in any particular brain volume (Henze et al. 2000; Shoham et al. 2006; Wolfe et al. 2010), the single-cell recording approach has no bias for cells firing at high rates and can also be used to record silent cells (Margrie et al. 2002; Epsztein et al. 2011). This is not to say this approach cannot have a bias: there may be unknown factors that make some cells more “patchable” than others.
Another advantage of this method is that one can manipulate single cells’ membrane potential and spiking activity with high precision. Surprisingly, eliciting action potentials during whole-cell patch-clamp recordings of a single pyramidal cell in vibrissae motor cortex was able to induce whisker movement, both in ketamine-/xylazine-anesthetized and awake rats (Brecht et al. 2004). In the hippocampus, silent cells stimulated can be suddenly, and reversibly, turned into place cells (Lee et al. 2012; Bittner et al. 2015).
There are also a few clear disadvantages that should be considered. First, one can only record a small number of cells per animal. This is due partly to the difficulty in achieving and maintaining a high-quality stable recording and partly to the fact that one must avoid confusing the identity of possibly labeled cells. In theory, this confusion could be avoided by labeling different cells with different colors, but in practice this turns out to be difficult, and labeling still mostly depends on neurobiotin or biocytin. Second, recording times are short. This places limits on, e.g., the extent of space that can be covered by an exploring rodent during a recording. This is important, for example, when recording grid cells, where a grid-like firing pattern only gradually becomes apparent as the animal moves throughout the arena. Third, it is still very difficult to do paired intracellular recordings in vivo, so that it remains difficult to make statements about functional connectivity or synchrony between identified cells. However, it has been achieved in some brain areas, using either sharp electrode (Lampl et al. 1999; Crochet et al. 2005) or patch-clamp recordings (Poulet and Petersen 2008; Jiang et al. 2013; Jouhanneau et al. 2015). Perhaps in the future, automated intracellular recordings will make it feasible to record from larger number of cells in parallel; good automated performance on single cells was recently demonstrated in both in anesthetized (Kodandaramaiah et al. 2012) and awake head-fixed animals (Desai et al. 2015).
Finally, combining intracellular recordings with extracellular (Bruno and Sakmann 2006; Quilichini et al. 2010) or optogenetic approaches (Muñoz et al. 2014; Pala and Petersen 2015) is likely to give many more insights into connectivity in the behaving animal. Antidromic stimulation, applied either optogenetically (Ciocchi et al. 2015) or electrically (e.g., Long et al. 2010), can also be used to obtain information regarding the synaptic targets of a recorded cell.
Juxtacellular Recordings
Juxtacellular recordings, also known by the more precise term of juxtasomatic recordings, are essentially cell-attached recordings, which can be used to record spiking activity with a high signal-to-noise ratio and to inject (charged) dye into the recorded cell (Pinault 1996). This method shares many of the advantages and disadvantages of whole-cell patch-clamp recordings, with some important differences, the most important one being that one cannot access subthreshold activity with juxtacellular recordings, i.e., this method only provides a physiological readout of a cell’s outputs.
However, it is considerably simpler to perform than whole-cell recording. It is also less invasive, so that there is no washout of the cytosol, leading to a perhaps more physiological state of the cell. This method still retains many of the same advantages as described above for whole-cell recordings. There is no clear bias for cells with a higher firing rate, as silent cells can also be recorded and identified (Burgalossi et al. 2011; Herfst et al. 2012; Diamantaki et al. 2016a), although there could be an unknown bias regarding which cells are more easily labeled with this method. In fact, the pipettes used, e.g., for interneuron recordings in the Klausberger and Somogyi labs have a much smaller tip and higher impedance (Klausberger et al. 2003; Lapray et al. 2012), possibly because this configuration is more successful for recording and/or labeling interneurons. However, this has not been shown systematically, and also larger “patch” pipette tips have been used to successfully record and label interneurons both in the presubiculum and medial entorhinal cortex (Tukker et al. 2015), although indeed the proportion of successfully labeled cells may be lower.
In general, the recovery of recorded cells and anatomical characterization is a key advantage of this method. This has proven particularly useful for distinguishing differences among parvalbumin-positive (PV) interneurons in the hippocampus (Klausberger et al. 2003, 2004; Tukker et al. 2007, 2013; Lapray et al. 2012; Varga et al. 2012, 2014; Viney et al. 2013), although many other cell types have also been successfully characterized with this method. With long waiting times after the labeling procedure, even axons over many millimeters can be traced (Jinno et al. 2007; Viney et al. 2013; Arszovszki et al. 2014; Preston-Ferrer et al. 2016).
Finally, manipulation of cell firing is possible with a relatively high degree of control. This has been combined with a behavioral readout in head-fixed animals to show that, surprisingly, manipulation of activity in single cells can modulate behavior (Houweling and Brecht 2008; Doron et al. 2014). A more recent report demonstrated that in the dentate gyrus, juxtacellular stimulation of a silent granule cell in a freely moving rat can induce that “primed” cell to selectively fire again when the rat subsequently revisits the priming location (Diamantaki et al. 2016b).
The Future
Identifying cell types in the future will ideally be done in vivo, based on one or a very small number of easily identifiable “markers.” At the moment, we are still in the process of determining which cell types may exist in various parts of the brain, and this endeavor is likely to continue for some time. While methods to describe neurons at any single level (anatomical, molecular, physiological, functional) are increasingly “scaled up,” as will be outlined in this section, the relatively sparse data obtained by single-cell experiments, spanning multiple levels, offers the opportunity to directly study how these levels are related to each other. In the near future we are likely to see the combination of single-cell methods as described in this chapter with more extensive analysis methods at the anatomical and molecular level. In parallel, it is becoming increasingly feasible to combine extensive, high-resolution “structure” descriptions (anatomy and genetics) with quantitative descriptions of behavior and large-scale electrophysiological readouts, e.g., via calcium- or voltage-sensitive dyes (Bock et al. 2011; Begemann and Galic 2016). In all these approaches, one crucial step to dealing with the complexity of such large datasets will be a dimensionality reduction, which is offered by the concept of cell types. Although limited in scale, research so far indicates that indeed there exist tendencies of particular combinations of properties to co-occur, and identifying these cell types will be a major focus for the coming years.
New methods are being developed for more sophisticated, “data-driven” ways to define cell types (Armañanzas and Ascoli 2015), for example, based on fuzzy set theory (Battaglia et al. 2013) or nonparametric Bayesian inference techniques (Jonas and Kording 2015), or to at least improve the consistency of cell identification within the community (DeFelipe et al. 2013). In order to derive potential cell types from the wealth of previously collected data, a number of efforts are under way to collate data from published reports into more formalized database-like structures (Bota et al. 2003; Ascoli et al. 2007; Wheeler et al. 2015), in order to make this data more readily available (and searchable). These initiatives are also likely to help the adoption of a more standardized nomenclature for describing properties of cell types (Ascoli et al. 2008) and cell types themselves (Hamilton et al. 2016). More generally, neuroscientific data is becoming ever more digital and quantitative (Helmstaedter et al. 2013; Budd et al. 2015), enabling data sets to be shared and compared more easily (Ascoli 2015; Teeters et al. 2015). Although such sharing is becoming more common, its usefulness will depend to some extent on the adoption of some standard data formats including more formalized representations of “metadata” (Garcia et al. 2014; Zehl et al. 2016). The adoption of common standards may be helped by large-scale initiatives such as the European Human Brain Project, the American BRAIN initiative, as well as institutions such as the Allen Brain Institute, all of which have cell type classification as one of their primary aims.
Although many experiments are already using, e.g., genetic markers to identify cell types in vivo, at present such experiments still entail considerable compromises. By limiting “identity” to a single promoter (e.g., parvalbumin to delineate fast-spiking interneurons in the cortex), one risks confounding several different cell types with potentially very different functional properties and roles. One solution to this is via “intersectional” approaches, where genetics can be based on several promoters (Fenno et al. 2014; Madisen et al. 2015). However, such approaches still depend on the identification of a very small number of genes that uniquely identify a particular cell type. The development of improved mRNA sequencing methods will certainly help in the quest for unique markers (Zeisel et al. 2015; Cadwell et al. 2016; Fuzik et al. 2016; Tasic et al. 2016), but they may not necessarily exist for every cell type.
The anatomical analysis of labeled cells is also advancing, particularly through the use of new viral tracing (Nassi et al. 2015) and tissue-processing (Chung and Deisseroth 2013) methods in combination with modern imaging approaches to map out long-range circuits throughout the brain (Ragan et al. 2012; Osten and Margrie 2013; Niedworok et al. 2016). Novel ISH techniques in combination with such imaging approaches are also likely to advance our understanding greatly (Sylwestrak et al. 2016). Expanding ISH and immunohistochemical methods with improved fluorophores with sharp emission spectra (Resch-Genger et al. 2008) would greatly improve the resolution with which cell types can be studied, by increasing the amount of molecules that can be tested for any particular tissue sample. The sequencing of mRNA material from single cells is also likely to improve, together with the quantitative techniques for finding clusters in such datasets; it may soon become routine to extract the complete transcriptome for any single recorded cell and read out the cell type unambiguously.
Another advance related to anatomical analysis is likely to come from the increased availability of EM reconstructions of ever-larger volumes of brain tissue (Plaza et al. 2014; Seung and Sümbül 2014; Swanson and Lichtman 2016). As recently shown in a series of studies focusing on the retina (Helmstaedter et al. 2013; Kim et al. 2014; Sümbül et al. 2014), these methods have the potential to greatly improve the resolution of anatomical data both with regard to morphology and synaptic connectivity. It is in principle already possible to functionally characterize and label a single neuron in vivo (e.g., a grid cell), via methods illustrated in this chapter, and reconstruct a sizeable portion of tissue around this cell in EM. One could theoretically identify all synaptically connected cells in this volume by their reconstructed morphology. This assumes that cell types can indeed be recognized by morphology alone, as in the retina (Helmstaedter et al. 2013). Future work will have to show to what extent this is also the case in the cortex. At the moment, the scale of EM reconstructed volumes in the cortex is still rather limited (Berning et al. 2015; Kasthuri et al. 2015), and the process remains slow and expensive (Marblestone et al. 2014). Dealing with the immense data sets such an endeavor will produce, eventually including, e.g., an entire mouse brain (Mikula and Denk 2015) will also pose new challenges, which may in part be solved by machine learning approaches (Lichtman et al. 2014; Helmstaedter 2015).
Readouts of neuronal activity are also rapidly developing. On the one hand, extracellular recordings from freely moving animals can be made with ever-increasing numbers of channels at ever-higher densities, making it possible to sample large populations of neurons from one or several brain areas (Buzsáki et al. 2015) during a wide range of behaviors. Similarly, miniaturization and improvement of calcium- and voltage-sensitive imaging approaches are also enabling optical recordings from deep-lying brain structures in freely moving animals at increasing temporal and spatial resolution and scale (Ziv and Ghosh 2015; Kim et al. 2016; Lin and Schnitzer 2016). For both optical and electrophysiological methods, novel wireless technology and more advanced ways of stimulating cells are also likely to increase possibilities to manipulate and record neurons from freely moving animals engaging in natural behaviors such as social interaction (Hasegawa et al. 2015; Kale et al. 2015; Park et al. 2015).
Combining these population-scale methods with opto- or chemogenetics, based on novel viral and genetic methods, will allow future investigators to record from and manipulate large numbers of anatomically and/or genetically defined populations (Grosenick et al. 2015). The caveat here is again that the “cell-type resolution” of such methods is likely to remain limited, as long as our understanding of cell types in the brain remains incomplete. Thus, it is important that the development of single-cell patch-clamp and juxtacellular recordings from behaving animals is also continuing.
Although relatively few laboratories are using these methods in freely moving animals, recent advances have made this method ever more feasible (Lee et al. 2014a; Tang et al. 2014a; Wang et al. 2016). Clearly, for many complex behaviors where one does not know all variables driving neural activity, it is preferable to record from animals engaging in behaviors as close to “natural” as possible. However, for understanding many “simpler” tasks, VR is an increasingly popular option which makes both imaging and single-cell recordings much simpler in awake animals. To what extent navigation can be studied effectively in VR is still a debated issue (Minderer et al. 2016), but since practical considerations also constrain most spatial navigation studies in humans to VR paradigms, the use of VR in rodents may actually be beneficial for allowing a cross-species comparison of neural circuits underlying spatial navigation. Such translatability may be particularly important for studies on animal models of diseases such as Alzheimer’s disease, where difficulties in navigation are very common (Lithfous et al. 2013). The fact that VR enables strong experimental control over both the animal’s behavior and the stimuli it is exposed to may help to unravel the roles of vestibular, auditory, visual, somatosensory, motor, and higher-order (path-integration) systems in navigation and other tasks (Chen et al. 2013; Cushman et al. 2013; Ravassard et al. 2013; Aronov and Tank 2014; Acharya et al. 2016; Kautzky and Thurley 2016). The question of how the hippocampal system generates an abstract higher-level representation of space based on such inputs is very much unsolved, but the combination of VR with modern viral, genetic, and large-scale recording and imaging tools in head-fixed animals gives us a good chance of addressing this question at the necessary level of detail.
Finally, the complexity of behavior itself may remain the biggest challenge. In Drosophila, a pioneering paper has recently correlated automatically detected behavioral modules (“behaviotypes”) with neuronal function from over a thousand neuron lines (Vogelstein et al. 2014). The first steps have also been made toward automated, non-biased analyses of behavior in mice (Wiltschko et al. 2015). Clearly, there is huge potential in this field to look at more complex relationships between stimuli and behavior, and at how such relationships may change over time through plastic processes in the brain. It will be interesting to see how, in the future, the combination of advanced behavioral analysis with other approaches described above will shed new light on the fundamental functioning of neural circuits, both in health and disease.
References
Acharya L, Aghajan ZM, Vuong C, Moore JJ, Mehta MR (2016) Causal influence of visual cues on hippocampal directional selectivity. Cell 164:197–207
Aghajan ZM, Acharya L, Moore JJ, Cushman JD, Vuong C, Mehta MR (2015) Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nat Neurosci 18:121–128
Apostolides PF, Milstein AD, Grienberger C, Bittner KC, Magee JC (2016) Axonal filtering allows reliable output during dendritic plateau-driven complex spiking in CA1 neurons. Neuron 89:770–783
Armañanzas R, Ascoli GA (2015) Towards the automatic classification of neurons. Trends Neurosci 38:307–318
Aronov D, Tank DW (2014) Engagement of neural circuits underlying 2D spatial navigation in a rodent virtual reality system. Neuron 84:442–456
Arszovszki A, Borhegyi Z, Klausberger T (2014) Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Front Neuroanat 8:53
Ascoli GA et al (2008) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9:557–568
Ascoli GA (2015) Sharing neuron data: carrots, sticks, and digital records. PLoS Biol 13:e1002275
Ascoli GA, Donohue DE, Halavi M (2007) NeuroMorpho.Org: a central resource for neuronal morphologies. J Neurosci 27:9247–9251
Battaglia D, Karagiannis A, Gallopin T, Gutch HW, Cauli B (2013) Beyond the frontiers of neuronal types. Front Neural Circuits 7:13
Begemann I, Galic M (2016) Correlative light electron microscopy: connecting synaptic structure and function. Front Synap Neurosci 28
Berning M, Boergens KM, Helmstaedter M (2015) SegEM: efficient image analysis for high-resolution connectomics. Neuron 87:1193–1206
Bezaire MJ, Soltesz I (2013) Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 23:751–785
Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC (2015) Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci 18:1133–1142
Boccara CN, Kjonigsen LJ, Hammer IM, Bjaalie JG, Leergaard TB, Witter MP (2015) A three-plane architectonic atlas of the rat hippocampal region. Hippocampus 25:838–857
Bock DD, Lee W-CA, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC (2011) Network anatomy and in vivo physiology of visual cortical neurons. Nature 471:177–182
Böhm C, Peng Y, Maier N, Winterer J, Poulet JFA, Geiger JRP, Schmitz D (2015) Functional diversity of subicular principal cells during hippocampal ripples. J Neurosci 35:13608–13618
Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser M-B (2013) Grid cells require excitatory drive from the hippocampus. Nat Neurosci 16:309–317
Bota M, Dong H-W, Swanson LW (2003) From gene networks to brain networks. Nat Neurosci 6:795–799
Bota M, Swanson LW (2007) The neuron classification problem. Brain Res Rev 56:79–88
Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME (2011) Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332:595–599
Brecht M, Schneider M, Sakmann B, Margrie TW (2004) Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 427:704–710
Briggman KL, Helmstaedter M, Denk W (2011) Wiring specificity in the direction-selectivity circuit of the retina. Nature 471:183–188
Bruno RM, Sakmann B (2006) Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312:1622–1627
Budd JML, Cuntz H, Eglen SJ, Krieger P (2015) Editorial: quantitative analysis of neuroanatomy. Front Neuroanat 9:143
Buetfering C, Allen K, Monyer H (2014) Parvalbumin interneurons provide grid cell-driven recurrent inhibition in the medial entorhinal cortex. Nat Neurosci 17:710–718
Buhl EH, Halasy K, Somogyi P (1994) Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368:823–828
Burgalossi A, Herfst L, von Heimendahl M, Förste H, Haskic K, Schmidt M, Brecht M (2011) Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron 70:773–786
Buzsaki G (2006) Rhythms of the brain, 1st edn. Oxford University Press, New York
Buzsáki G (2015) Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25:1073–1188
Buzsáki G, Leung LW, Vanderwolf CH (1983) Cellular bases of hippocampal EEG in the behaving rat. Brain Res 287:139–171
Buzsáki G, Czopf J, Kondákor I, Kellényi L (1986) Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res 365:125–137
Buzsáki G, Stark E, Berényi A, Khodagholy D, Kipke DR, Yoon E, Wise KD (2015) Tools for probing local circuits: high-density silicon probes combined with optogenetics. Neuron 86:92–105
Cacucci F, Lever C, Wills TJ, Burgess N, O’Keefe J (2004) Theta-modulated place-by-direction cells in the hippocampal formation in the rat. J Neurosci 24:8265–8277
Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shen S, Bethge M, Tolias KF, Sandberg R, Tolias AS (2016) Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol 34:199–203
Canto CB, Wouterlood FG, Witter MP (2008) What does the anatomical organization of the entorhinal cortex tell us? Neural Plast 2008:381243
Carr MF, Karlsson MP, Frank LM (2012) Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75:700–713
Chen G, King JA, Burgess N, O’Keefe J (2013) How vision and movement combine in the hippocampal place code. Proc Natl Acad Sci U S A 110:378–383
Chorev E, Brecht M (2012) In vivo dual intra- and extracellular recordings suggest bidirectional coupling between CA1 pyramidal neurons. J Neurophysiol 108:1584–1593
Chung K, Deisseroth K (2013) CLARITY for mapping the nervous system. Nat Methods 10:508–513
Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T (2015) Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348:560–563
Clement EA, Richard A, Thwaites M, Ailon J, Peters S, Dickson CT (2008) Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS ONE 3:e2004
Cobb SR, Halasy K, Vida I, Nyiri G, Tamas G, Buhl EH, Somogyi P (1997) Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience 79:629–648
Colgin LL (2015) Do slow and fast gamma rhythms correspond to distinct functional states in the hippocampal network? Brain Res 1621:309–315
Couey JJ, Witoelar A, Zhang S-J, Zheng K, Ye J, Dunn B, Czajkowski R, Moser M-B, Moser EI, Roudi Y, Witter MP (2013) Recurrent inhibitory circuitry as a mechanism for grid formation. Nat Neurosci 16:318–324
Crochet S, Chauvette S, Boucetta S, Timofeev I (2005) Modulation of synaptic transmission in neocortex by network activities. Eur J Neurosci 21:1030–1044
Csicsvari J, Jamieson B, Wise KD, Buzsáki G (2003) Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37:311–322
Cushman JD, Aharoni DB, Willers B, Ravassard P, Kees A, Vuong C, Popeney B, Arisaka K, Mehta MR (2013) Multisensory control of multimodal behavior: do the legs know what the tongue is doing? PLoS ONE 8:e80465
Danielson NB, Zaremba JD, Kaifosh P, Bowler J, Ladow M, Losonczy A (2016) Sublayer-specific coding dynamics during spatial navigation and learning in hippocampal area CA1. Neuron 91:652–665
DeFelipe J et al (2013) New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14:202–216
Desai NS, Siegel JJ, Taylor W, Chitwood RA, Johnston D (2015) MATLAB-based automated patch-clamp system for awake behaving mice. J Neurophysiol 114:1331–1345
Diamantaki M, Frey M, Berens P, Preston-Ferrer P, Burgalossi A (2016a) Sparse activity of identified dentate granule cells during spatial exploration. Elife 5
Diamantaki M, Frey M, Preston-Ferrer P, Burgalossi A (2016b) Priming spatial activity by single-cell stimulation in the dentate gyrus of freely moving rats. Curr Biol 26:536–541
Dickson CT, Trepel C, Bland BH (1994) Extrinsic modulation of theta field activity in the entorhinal cortex of the anesthetized rat. Hippocampus 4:37–51
Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW (2007) Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56:43–57
Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW (2010) Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci 13:1433–1440
Domnisoru C, Kinkhabwala AA, Tank DW (2013) Membrane potential dynamics of grid cells. Nature 495:199–204
Doron G, von Heimendahl M, Schlattmann P, Houweling AR, Brecht M (2014) Spiking irregularity and frequency modulate the behavioral report of single-neuron stimulation. Neuron 81:653–663
Ego-Stengel V, Wilson MA (2007) Spatial selectivity and theta phase precession in CA1 interneurons. Hippocampus 17:161–174
English DF, Peyrache A, Stark E, Roux L, Vallentin D, Long MA, Buzsáki G (2014) Excitation and inhibition compete to control spiking during hippocampal ripples: intracellular study in behaving mice. J Neurosci 34:16509–16517
Epsztein J, Lee AK, Chorev E, Brecht M (2010) Impact of spikelets on hippocampal CA1 pyramidal cell activity during spatial exploration. Science 327:474–477
Epsztein J, Brecht M, Lee AK (2011) Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70:109–120
Fee MS (2000) Active stabilization of electrodes for intracellular recording in awake behaving animals. Neuron 27:461–468
Fenno LE et al (2014) Targeting cells with single vectors using multiple-feature boolean logic. Nat Methods 11:763–772
Fenton AA, Kao H-Y, Neymotin SA, Olypher A, Vayntrub Y, Lytton WW, Ludvig N (2008) Unmasking the CA1 ensemble place code by exposures to small and large environments: more place cells and multiple, irregularly arranged, and expanded place fields in the larger space. J Neurosci 28:11250–11262
Ferezou I, Bolea S, Petersen CCH (2006) Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron 50:617–629
Ferrante M, Tahvildari B, Duque A, Hadzipasic M, Salkoff D, Zagha EW, Hasselmo ME, McCormick DA (2017) Distinct functional groups emerge from the intrinsic properties of molecularly identified Entorhinal interneurons and principal cells. Cereb Cortex 27:3186–3207
Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RPJ, Ko TH, Burns LD, Jung JC, Schnitzer MJ (2008) High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat Methods 5:935–938
Fox SE, Ranck JB (1975) Localization and anatomical identification of theta and complex spike cells in dorsal hippocampal formation of rats. Exp Neurol 49:299–313
Fuchs EC, Neitz A, Pinna R, Melzer S, Caputi A, Monyer H (2016) Local and distant input controlling excitation in layer ii of the medial entorhinal cortex. Neuron 89:194–208
Fuentealba P, Begum R, Capogna M, Jinno S, Márton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T (2008) Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron 57:917–929
Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S (2015) Locomotion, Theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal Glutamatergic circuit. Neuron
Fuzik J, Zeisel A, Máté Z, Calvigioni D, Yanagawa Y, Szabó G, Linnarsson S, Harkany T (2016) Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nat Biotechnol 34:175–183
Gao P, Ploog BO, Zeigler HP (2003) Whisking as a “voluntary” response: operant control of whisking parameters and effects of whisker denervation. Somatosens Mot Res 20:179–189
Garcia S, Guarino D, Jaillet F, Jennings T, Pröpper R, Rautenberg PL, Rodgers CC, Sobolev A, Wachtler T, Yger P, Davison AP (2014) Neo: an object model for handling electrophysiology data in multiple formats. Front Neuroinform 8:10
Giocomo LM, Stensola T, Bonnevie T, Van Cauter T, Moser M-B, Moser EI (2014) Topography of head direction cells in medial entorhinal cortex. Curr Biol 24:252–262
Graves AR, Moore SJ, Bloss EB, Mensh BD, Kath WL, Spruston N (2012) Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 76:776–789
Greenberg DS, Houweling AR, Kerr JN (2008) Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci 11:749–751
Grienberger C, Chen X, Konnerth A (2014) NMDA receptor-dependent multidendrite Ca(2+) spikes required for hippocampal burst firing in vivo. Neuron 81:1274–1281
Grosenick L, Marshel JH, Deisseroth K (2015) Closed-loop and activity-guided optogenetic control. Neuron 86:106–139
Guo ZV, Hires SA, Li N, O’Connor DH, Komiyama T, Ophir E, Huber D, Bonardi C, Morandell K, Gutnisky D, Peron S, Xu N, Cox J, Svoboda K (2014) Procedures for behavioral experiments in head-fixed mice. PLoS ONE 9:e88678
Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA (2005) Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol 15:599–606
Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806
Hamilton DJ, Wheeler DW, White CM, Rees CL, Komendantov AO, Bergamino M, Ascoli GA (2016) Name-calling in the hippocampus (and beyond): coming to terms with neuron types and properties. Brain Inform
Hara K, Harris RA (2002) The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94:313–318
Hardcastle K, Maheswaranathan N, Ganguli S, Giocomo LM (2017) A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron 94:375–387.e7
Hartley T, Lever C, Burgess N, O’Keefe J (2014) Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci 369:20120510
Harvey CD, Collman F, Dombeck DA, Tank DW (2009) Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461:941–946
Harvey CD, Coen P, Tank DW (2012) Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484:62–68
Hasegawa T, Fujimoto H, Tashiro K, Nonomura M, Tsuchiya A, Watanabe D (2015) A wireless neural recording system with a precision motorized microdrive for freely behaving animals. Sci Rep 5:7853
Helmchen F, Fee MS, Tank DW, Denk W (2001) A miniature head-mounted two-photon microscope. High-resolution brain imaging in freely moving animals. Neuron 31:903–912
Helmchen F, Denk W, Kerr JND (2013) Miniaturization of two-photon microscopy for imaging in freely moving animals. Cold Spring Harb Protoc 2013:904–913
Helmstaedter M (2015) The mutual inspirations of machine learning and neuroscience. Neuron 86:25–28
Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W (2013) Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500:168–174
Hemmings HC, Yan W, Westphalen RI, Ryan TA (2005) The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol 67:1591–1599
Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G (2000) Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84:390–400
Herfst L, Burgalossi A, Haskic K, Tukker JJ, Schmidt M, Brecht M (2012) Friction-based stabilization of juxtacellular recordings in freely moving rats. J Neurophysiol 108:697–707
Heys JG, Rangarajan KV, Dombeck DA (2014) The functional micro-organization of grid cells revealed by cellular-resolution imaging. Neuron 84:1079–1090
Hölscher C, Schnee A, Dahmen H, Setia L, Mallot HA (2005) Rats are able to navigate in virtual environments. J Exp Biol 208:561–569
Houweling AR, Brecht M (2008) Behavioural report of single neuron stimulation in somatosensory cortex. Nature 451:65–68
Hulse BK, Moreaux LC, Lubenov EV, Siapas AG (2016) Membrane potential dynamics of CA1 pyramidal neurons during hippocampal ripples in awake mice. Neuron 89:800–813
Ishihara Y, Fukuda T (2016) Immunohistochemical investigation of the internal structure of the mouse subiculum. Neuroscience 337:242–266
Jacob P-Y, Poucet B, Liberge M, Save E, Sargolini F (2014) Vestibular control of entorhinal cortex activity in spatial navigation. Front Integr Neurosci 8:38
Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ (2013) The organization of two new cortical interneuronal circuits. Nat Neurosci 16:210–218
Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Patel S, Tolias AS (2015) Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350:aac9462
Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JDB, Fuentealba P, Bushong EA, Henze D, Buzsáki G, Somogyi P (2007) Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci 27:8790–8804
Jonas E, Kording K (2015) Automatic discovery of cell types and microcircuitry from neural connectomics. Elife 4:e04250
Jouhanneau J-S, Kremkow J, Dorrn AL, Poulet JFA (2015) In vivo monosynaptic excitatory transmission between layer 2 cortical pyramidal neurons. Cell Rep 13:2098–2106
Jung MW, McNaughton BL (1993) Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3:165–182
Kale RP, Kouzani AZ, Walder K, Berk M, Tye SJ (2015) Evolution of optogenetic microdevices. Neurophotonics 2:31206
Kamondi A, Acsády L, Buzsáki G (1998) Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J Neurosci 18:3919–3928
Kasthuri N et al (2015) Saturated reconstruction of a volume of neocortex. Cell 162:648–661
Katona L, Lapray D, Viney TJ, Oulhaj A, Borhegyi Z, Micklem BR, Klausberger T, Somogyi P (2014) Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron 82:872–886
Kautzky M, Thurley K (2016) Estimation of self-motion duration and distance in rodents. R Soc Open Sci 3:160118
Kepecs A, Fishell G (2014) Interneuron cell types are fit to function. Nature 505:318–326
Kerlin AM, Andermann ML, Berezovskii VK, Reid RC (2010) Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67:858–871
Kim EJ, Jacobs MW, Ito-Cole T, Callaway EM (2016) Improved monosynaptic neural circuit tracing using engineered rabies virus glycoproteins. Cell Rep 15(4):692–699
Kim JS, Greene MJ, Zlateski A, Lee K, Richardson M, Turaga SC, Purcaro M, Balkam M, Robinson A, Behabadi BF, Campos M, Denk W, Seung HS, EyeWirers (2014) Space-time wiring specificity supports direction selectivity in the retina. Nature 509:331–336
Kislin M, Mugantseva E, Molotkov D, Kulesskaya N, Khirug S, Kirilkin I, Pryazhnikov E, Kolikova J, Toptunov D, Yuryev M, Giniatullin R, Voikar V, Rivera C, Rauvala H, Khiroug L (2014) Flat-floored air-lifted platform: a new method for combining behavior with microscopy or electrophysiology on awake freely moving rodents. J Vis Exp:e51869
Kitai ST, Kocsis JD, Preston RJ, Sugimori M (1976) Monosynaptic inputs to caudate neurons identified by intracellular injection of horseradish peroxidase. Brain Res 109:601–606
Kitamura K, Judkewitz B, Kano M, Denk W, Häusser M (2008) Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat Methods 5:61–67
Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S (2014) Island cells control temporal association memory. Science 343:896–901
Klausberger T, Somogyi P (2008) Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321:53–57
Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P (2003) Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421:844–848
Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P (2004) Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci 7:41–47
Klausberger T, Marton LF, O’Neill J, Huck JHJ, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P (2005) Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci 25:9782–9793
Kodandaramaiah SB, Franzesi GT, Chow BY, Boyden ES, Forest CR (2012) Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat Methods 9:585–587
Kohtala S, Theilmann W, Suomi T, Wigren H-K, Porkka-Heiskanen T, Elo LL, Rokka A, Rantamäki T (2016) Brief Isoflurane anesthesia produces prominent Phosphoproteomic changes in the adult mouse Hippocampus. ACS Chem Neurosci
Kropff E, Carmichael JE, Moser M-B, Moser EI (2015) Speed cells in the medial entorhinal cortex. Nature 523:419–424
Krupic J, Burgess N, O’Keefe J (2012) Neural representations of location composed of spatially periodic bands. Science 337:853–857
Kubie JL, Muller RU, Bostock E (1990) Spatial firing properties of hippocampal theta cells. J Neurosci 10:1110–1123
Lambolez B, Audinat E, Bochet P, Crépel F, Rossier J (1992) AMPA receptor subunits expressed by single purkinje cells. Neuron 9:247–258
Lampl I, Reichova I, Ferster D (1999) Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron 22:361–374
Langer D, Helmchen F (2012) Post hoc immunostaining of GABAergic neuronal subtypes following in vivo two-photon calcium imaging in mouse neocortex. Pflugers Arch 463:339–354
Lapray D, Lasztoczi B, Lagler M, Viney TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T (2012) Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci 15:1265–1271
Lasztóczi B, Klausberger T (2014) Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron 81:1126–1139
Lasztóczi B, Klausberger T (2016) Hippocampal place cells couple to three different gamma oscillations during place field traversal. Neuron 91:34–40
Lasztóczi B, Tukker JJ, Somogyi P, Klausberger T (2011) Terminal field and firing selectivity of cholecystokinin-expressing interneurons in the hippocampal CA3 area. J Neurosci 31:18073–18,093
Lee I, Rao G, Knierim JJ (2004) A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron 42:803–815
Lee AK, Manns ID, Sakmann B, Brecht M (2006) Whole-cell recordings in freely moving rats. Neuron 51:399–407
Lee AK, Epsztein J, Brecht M (2009) Head-anchored whole-cell recordings in freely moving rats. Nat Protoc 4:385–392
Lee D, Lin B-J, Lee AK (2012) Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337:849–853
Lee D, Shtengel G, Osborne JE, Lee AK (2014a) Anesthetized- and awake-patched whole-cell recordings in freely moving rats using UV-cured collar-based electrode stabilization. Nat Protoc 9:2784–2795
Lee S-H, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I (2014b) Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82:1129–1144
Leitner FC, Melzer S, Lütcke H, Pinna R, Seeburg PH, Helmchen F, Monyer H (2016) Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex. Nat Neurosci 19:935–944
Lever C, Burton S, O’Keefe J (2006) Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci 17:111–133
Lichtman JW, Pfister H, Shavit N (2014) The big data challenges of connectomics. Nat Neurosci 17:1448–1454
Lima SQ, Hromádka T, Znamenskiy P, Zador AM (2009) PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE 4:e6099
Lin MZ, Schnitzer MJ (2016) Genetically encoded indicators of neuronal activity. Nat Neurosci 19:1142–1153
Lithfous S, Dufour A, Després O (2013) Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: insights from imaging and behavioral studies. Ageing Res Rev 12:201–213
Long MA, Lee AK (2012) Intracellular recording in behaving animals. Curr Opin Neurobiol 22:34–44
Long MA, Jin DZ, Fee MS (2010) Support for a synaptic chain model of neuronal sequence generation. Nature 468:394–399
Looger LL, Griesbeck O (2012) Genetically encoded neural activity indicators. Curr Opin Neurobiol 22:18–23
Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun X-H, Nicoud J-F, Zemelman BV, Sternson SM, Losonczy A (2012) Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci 15(423–430):S1–S3
Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A (2014) Dendritic inhibition in the hippocampus supports fear learning. Science 343:857–863
Lustig B, Wang Y, Pastalkova E (2016) Oscillatory patterns in hippocampus under light and deep isoflurane anesthesia closely mirror prominent brain states in awake animals. Hippocampus 26:102–109
Madisen L et al (2015) Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85:942–958
Maier N, Tejero-Cantero A, Dorrn AL, Winterer J, Beed PS, Morris G, Kempter R, Poulet JFA, Leibold C, Schmitz D (2011) Coherent phasic excitation during hippocampal ripples. Neuron 72:137–152
Marblestone AH, Daugharthy ER, Kalhor R, Peikon ID, Kebschull JM, Shipman SL, Mishchenko Y, Lee JH, Dalrymple DA, Zamft BM, Kording KP, Boyden ES, Zador AM, Church GM (2014) Conneconomics: the economics of dense, large-scale, high-resolution neural Connectomics. bioRxiv:1214
Margrie TW, Brecht M, Sakmann B (2002) In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch 444:491–498
Margrie TW, Meyer AH, Caputi A, Monyer H, Hasan MT, Schaefer AT, Denk W, Brecht M (2003) Targeted whole-cell recordings in the mammalian brain in vivo. Neuron 39:911–918
Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P (1998) Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci 18:8111–8125
Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL (2006) Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. J Neurosci 26:13485–13492
Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H (2012) Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science 335:1506–1510
Mikula S, Denk W (2015) High-resolution whole-brain staining for electron microscopic circuit reconstruction. Nat Methods 12:541–546
Minderer M, Harvey CD, Donato F, Moser EI (2016) Neuroscience: virtual reality explored. Nature 533:324–325
Mizrahi A, Crowley JC, Shtoyerman E, Katz LC (2004) High-resolution in vivo imaging of hippocampal dendrites and spines. J Neurosci 24:3147–3151
Mizuseki K, Diba K, Pastalkova E, Buzsáki G (2011) Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci Available at: http://www.ncbi.nlm.nih.gov/pubmed/21822270. Accessed 23 Aug 2011
Mizuseki K, Royer S, Diba K, Buzsáki G (2012) Activity dynamics and behavioral correlates of CA3 and CA1 hippocampal pyramidal neurons. Hippocampus 22:1659–1680
Monaco JD, Rao G, Roth ED, Knierim JJ (2014) Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat Neurosci 17:725–731
Moore JD, Deschênes M, Kurnikova A, Kleinfeld D (2014) Activation and measurement of free whisking in the lightly anesthetized rodent. Nat Protoc 9:1792–1802
Moroni F, Corradetti R, Casamenti F, Moneti G, Pepeu G (1981) The release of endogenous GABA and glutamate from the cerebral cortex in the rat. Naunyn Schmiedebergs Arch Pharmacol 316:235–239
Müller C, Remy S (2014) Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Front Synaptic Neurosci 6:23
Müller A, Kukley M, Stausberg P, Beck H, Müller W, Dietrich D (2005) Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. J Neurosci 25:558–565
Muñoz W, Tremblay R, Rudy B (2014) Channelrhodopsin-assisted patching: in vivo recording of genetically and morphologically identified neurons throughout the brain. Cell Rep 9:2304–2316
Nashaat MA, Oraby H, Sachdev RNS, Winter Y, Larkum ME (2016) Air-Track: a real-world floating environment for active sensing in head-fixed mice. J Neurophysiol 116:1542–1553
Nassar M, Simonnet J, Lofredi R, Cohen I, Savary E, Yanagawa Y, Miles R, Fricker D (2015) Diversity and overlap of parvalbumin and somatostatin expressing interneurons in mouse presubiculum. Front Neural Circuits 9:20
Nassi JJ, Cepko CL, Born RT, Beier KT (2015) Neuroanatomy goes viral! Front Neuroanat 9:80
Niedworok CJ, Brown APY, Jorge Cardoso M, Osten P, Ourselin S, Modat M, Margrie TW (2016) aMAP is a validated pipeline for registration and segmentation of high-resolution mouse brain data. Nat Commun 7:11879
O’Connor DH, Huber D, Svoboda K (2009) Reverse engineering the mouse brain. Nature 461:923–929
O’Keefe J (1976) Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109
O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171–175
O’Keefe J, Recce ML (1993) Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3:317–330
Oberlaender M, Ramirez A, Bruno RM (2012) Sensory experience restructures thalamocortical axons during adulthood. Neuron 74:648–655
Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC (2005) Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433:597–603
Okaty BW, Sugino K, Nelson SB (2011) Cell type-specific transcriptomics in the brain. J Neurosci 31:6939–6943
Osten P, Margrie TW (2013) Mapping brain circuitry with a light microscope. Nat Methods 10:515–523
Packer AM, Russell LE, Dalgleish HWP, Häusser M (2015) Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat Methods 12:140–146
Pagliardini S, Gosgnach S, Dickson CT (2013) Spontaneous sleep-like brain state alternations and breathing characteristics in urethane anesthetized mice. PLoS ONE 8:e70411
Pala A, Petersen CCH (2015) In vivo measurement of cell-type-specific synaptic connectivity and synaptic transmission in layer 2/3 mouse barrel cortex. Neuron 85:68–75
Park SI, Shin G, Banks A, McCall JG, Siuda ER, Schmidt MJ, Chung HU, Noh KN, Mun JG-H, Rhodes J, Bruchas MR, Rogers JA (2015) Ultraminiaturized photovoltaic and radio frequency powered optoelectronic systems for wireless optogenetics. J Neural Eng 12:56002
Pedreira C, Martinez J, Ison MJ, Quian Quiroga R (2012) How many neurons can we see with current spike sorting algorithms? J Neurosci Methods 211:58–65
Pinault D (1996) A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods 65:113–136
Plaza SM, Scheffer LK, Chklovskii DB (2014) Toward large-scale connectome reconstructions. Curr Opin Neurobiol 25:201–210
Potez S, Larkum ME (2008) Effect of common anesthetics on dendritic properties in layer 5 neocortical pyramidal neurons. J Neurophysiol 99:1394–1407
Poulet JFA, Petersen CCH (2008) Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454:881–885
Preston-Ferrer P, Coletta S, Frey M, Burgalossi A (2016) Anatomical organization of presubicular head-direction circuits. Elife 5
Quilichini P, Sirota A, Buzsáki G (2010) Intrinsic circuit organization and theta-gamma oscillation dynamics in the entorhinal cortex of the rat. J Neurosci 30:11128–11142
Ragan T, Kadiri LR, Venkataraju KU, Bahlmann K, Sutin J, Taranda J, Arganda-Carreras I, Kim Y, Seung HS, Osten P (2012) Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat Methods 9:255–258
Ramirez S, Liu X, Lin P-A, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S (2013) Creating a false memory in the hippocampus. Science 341:387–391
Ramsden HL, Sürmeli G, McDonagh SG, Nolan MF (2015) Laminar and dorsoventral molecular organization of the medial entorhinal cortex revealed by large-scale anatomical analysis of gene expression. PLoS Comput Biol 11:e1004032
Rancz EA, Franks KM, Schwarz MK, Pichler B, Schaefer AT, Margrie TW (2011) Transfection via whole-cell recording in vivo: bridging single-cell physiology, genetics and connectomics. Nat Neurosci 14:527–532
Ravassard P, Kees A, Willers B, Ho D, Aharoni D, Cushman J, Aghajan ZM, Mehta MR (2013) Multisensory control of hippocampal spatiotemporal selectivity. Science 340:1342–1346
Ray S, Naumann R, Burgalossi A, Tang Q, Schmidt H, Brecht M (2014) Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science 343:891–896
Reijmers LG, Perkins BL, Matsuo N, Mayford M (2007) Localization of a stable neural correlate of associative memory. Science 317:1230–1233
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5:763–775
Rich PD, Liaw H-P, Lee AK (2014) Place cells. Large environments reveal the statistical structure governing hippocampal representations. Science 345:814–817
Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G (2012) Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci 15:769–775
Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK (2006) Lesions of the vestibular system disrupt hippocampal theta rhythm in the rat. J Neurophysiol 96:4–14
Sanders H, Rennó-Costa C, Idiart M, Lisman J (2015) Grid cells and place cells: an integrated view of their navigational and memory function. Trends Neurosci 38:763–775
Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M-B, Moser EI (2006) Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312:758–762
Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JND (2009) Visually evoked activity in cortical cells imaged in freely moving animals. Proc Natl Acad Sci U S A 106:19557–19562
Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis H-R, Schwarz I, Schmidt B, Schwarz MK, Remy S, Fuhrmann M (2016) Dysfunction of somatostatin-positive interneurons associated with memory deficits in an Alzheimer’s disease model. Neuron 92:114–125
Schmidt-Hieber C, Häusser M (2013) Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat Neurosci 16:325–331
Schwarz C, Hentschke H, Butovas S, Haiss F, Stüttgen MC, Gerdjikov TV, Bergner CG, Waiblinger C (2010) The head-fixed behaving rat—procedures and pitfalls. Somatosens Mot Res 27:131–148
Scott BB, Brody CD, Tank DW (2013) Cellular resolution functional imaging in behaving rats using voluntary head restraint. Neuron 80:371–384
Sejnowski TJ, Churchland PS, Movshon JA (2014) Putting big data to good use in neuroscience. Nat Neurosci 17:1440–1441
Seung HS, Sümbül U (2014) Neuronal cell types and connectivity: lessons from the retina. Neuron 83:1262–1272
Sharp PE (1996) Multiple spatial/behavioral correlates for cells in the rat postsubiculum: multiple regression analysis and comparison to other hippocampal areas. Cereb Cortex 6:238–259
Shoham S, O’Connor DH, Segev R (2006) How silent is the brain: is there a “dark matter” problem in neuroscience? J Comp Physiol A 192:777–784
Slomianka L, Amrein I, Knuesel I, Sørensen JC, Wolfer DP (2011) Hippocampal pyramidal cells: the reemergence of cortical lamination. Brain Struct Funct 216:301–317
Sofroniew NJ, Cohen JD, Lee AK, Svoboda K (2014) Natural whisker-guided behavior by head-fixed mice in tactile virtual reality. J Neurosci 34:9537–9550
Solstad T, Boccara CN, Kropff E, Moser M-B, Moser EI (2008) Representation of geometric borders in the entorhinal cortex. Science 322:1865–1868
Somogyi P, Katona L, Klausberger T, Lasztóczi B, Viney TJ (2014) Temporal redistribution of inhibition over neuronal subcellular domains underlies state-dependent rhythmic change of excitability in the hippocampus. Philos Trans R Soc Lond B Biol Sci 369:20120518
Stosiek C, Garaschuk O, Holthoff K, Konnerth A (2003) In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A 100:7319–7324
Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S (2011) Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science 334:1415–1420
Sümbül U, Song S, McCulloch K, Becker M, Lin B, Sanes JR, Masland RH, Seung HS (2014) A genetic and computational approach to structurally classify neuronal types. Nat Commun 5:3512
Sun C, Kitamura T, Yamamoto J, Martin J, Pignatelli M, Kitch LJ, Schnitzer MJ, Tonegawa S (2015) Distinct speed dependence of entorhinal island and ocean cells, including respective grid cells. Proc Natl Acad Sci U S A 112:9466–9471
Swanson LW, Lichtman JW (2016) From Cajal to Connectome and beyond. Annu Rev Neurosci 39:197–216
Sylwestrak EL, Rajasethupathy P, Wright MA, Jaffe A, Deisseroth K (2016) Multiplexed intact-tissue transcriptional analysis at cellular resolution. Cell 164:792–804
Tang Q, Brecht M, Burgalossi A (2014a) Juxtacellular recording and morphological identification of single neurons in freely moving rats. Nat Protoc 9:2369–2381
Tang Q, Burgalossi A, Ebbesen CL, Ray S, Naumann R, Schmidt H, Spicher D, Brecht M (2014b) Pyramidal and stellate cell specificity of grid and border representations in layer 2 of medial entorhinal cortex. Neuron 84:1191–1197
Tang Q, Ebbesen CL, Sanguinetti-Scheck JI, Preston-Ferrer P, Gundlfinger A, Winterer J, Beed P, Ray S, Naumann R, Schmitz D, Brecht M, Burgalossi A (2015) Anatomical organization and spatiotemporal firing patterns of layer 3 neurons in the rat medial entorhinal cortex. J Neurosci 35:12346–12,354
Tang Q, Burgalossi A, Ebbesen CL, Sanguinetti-Scheck JI, Schmidt H, Tukker JJ, Naumann R, Ray S, Preston-Ferrer P, Schmitz D, Brecht M (2016) Functional architecture of the rat parasubiculum. J Neurosci 36:2289–2301
Tao C, Zhang G, Xiong Y, Zhou Y (2015) Functional dissection of synaptic circuits: in vivo patch-clamp recording in neuroscience. Front Neural Circuits 9:23
Tasic B et al (2016) Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19:335–346
Taube JS (2007) The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30
Taube JS, Muller RU, Ranck JBJ (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10:420–435
Teeters JL et al (2015) Neurodata without Borders: creating a common data format for neurophysiology. Neuron 88:629–634
Thurley K, Ayaz A (2017) Virtual reality systems for rodents. Curr Zool 63:109–119
Tonegawa S, Liu X, Ramirez S, Redondo R (2015) Memory engram cells have come of age. Neuron 87:918–931
Tsuno Y, Chapman GW, Hasselmo ME (2015) Rebound spiking properties of mouse medial entorhinal cortex neurons in vivo. Eur J Neurosci 42:2974–2984
Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T (2007) Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 27:8184–8189
Tukker JJ, Lasztóczi B, Katona L, Roberts JDB, Pissadaki EK, Dalezios Y, Márton L, Zhang L, Klausberger T, Somogyi P (2013) Distinct dendritic arborization and in vivo firing patterns of parvalbumin-expressing basket cells in the hippocampal area CA3. J Neurosci 33:6809–6825
Tukker JJ, Tang Q, Burgalossi A, Brecht M (2015) Head-directional tuning and theta modulation of anatomically identified neurons in the presubiculum. J Neurosci 35:15391–15395
Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, de la Prida LM (2015) Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci 18:1281–1290
Varga C, Lee SY, Soltesz I (2010) Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci 13:822–824
Varga C, Golshani P, Soltesz I (2012) Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. PNAS 109:E2726–E2734
Varga C, Oijala M, Lish J, Szabo GG, Bezaire M, Marchionni I, Golshani P, Soltesz I (2014) Functional fission of parvalbumin interneuron classes during fast network events. Elife 3
Vélez-Fort M, Rousseau CV, Niedworok CJ, Wickersham IR, Rancz EA, Brown APY, Strom M, Margrie TW (2014) The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron 83:1431–1443
Viney TJ, Lasztoczi B, Katona L, Crump MG, Tukker JJ, Klausberger T, Somogyi P (2013) Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat Neurosci 16:1802–1811
Vogelstein JT, Park Y, Ohyama T, Kerr RA, Truman JW, Priebe CE, Zlatic M (2014) Discovery of brainwide neural-behavioral maps via multiscale unsupervised structure learning. Science 344:386–392
Wang Y, Liu Y-Z, Wang S-Y, Wang Z (2016) In vivo whole-cell recording with high success rate in anaesthetized and awake mammalian brains. Mol Brain 9:86
Wertz A, Trenholm S, Yonehara K, Hillier D, Raics Z, Leinweber M, Szalay G, Ghanem A, Keller G, Rózsa B, Conzelmann K-K, Roska B (2015) Presynaptic networks. Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Science 349:70–74
Westphalen RI, Hemmings HC (2006) Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine-evoked release. J Pharmacol Exp Ther 316:216–223
Wheeler DW, White CM, Rees CL, Komendantov AO, Hamilton DJ, Ascoli GA (2015) Hippocampome.org: a knowledge base of neuron types in the rodent hippocampus. Elife 4
Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, Datta SR (2015) Mapping sub-second structure in mouse behavior. Neuron 88:1121–1135
Winterer J, Maier N, Wozny C, Beed P, Breustedt J, Evangelista R, Peng Y, D’Albis T, Kempter R, Schmitz D (2017) Excitatory microcircuits within superficial layers of the medial entorhinal cortex. Cell Rep 19:1110–1116
Wolfe J, Houweling AR, Brecht M (2010) Sparse and powerful cortical spikes. Curr Opin Neurobiol 20:306–312
Wu F, Stark E, Ku P-C, Wise KD, Buzsáki G, Yoon E (2015) Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88:1136–1148
Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G (1995a) Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15:30–46
Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsaki G (1995b) Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus 5:78–90
Youngstrom IA, Strowbridge BW (2012) Visual landmarks facilitate rodent spatial navigation in virtual reality environments. Learn Mem 19:84–90
Zehl L, Jaillet F, Stoewer A, Grewe J, Sobolev A, Wachtler T, Brochier TG, Riehle A, Denker M, Grün S (2016) Handling metadata in a neurophysiology laboratory. Front Neuroinform 10:26
Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S (2015) Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347:1138–1142
Zhu JJ, Connors BW (1999) Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol 81:1171–1183
Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ (2013) Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 16:264–266
Ziv Y, Ghosh KK (2015) Miniature microscopes for large-scale imaging of neuronal activity in freely behaving rodents. Curr Opin Neurobiol 32:141–147
Acknowledgments
The author wishes to thank Andrea Burgalossi, Constance Holman, Nikolaus Maier, and Peter Somogyi for helpful comments on earlier versions of this chapter and Linda Hahn-Tukker for help with the figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tukker, J.J. (2018). Recording Identified Neurons in Awake and Anesthetized Rodents. In: Cutsuridis, V., Graham, B., Cobb, S., Vida, I. (eds) Hippocampal Microcircuits. Springer Series in Computational Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-99103-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-99103-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99102-3
Online ISBN: 978-3-319-99103-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)