Abstract
The development of functional imaging techniques applicable to neuroscience and covering a wide range of spatial and temporal scales has greatly facilitated the exploration of the relationships between cognition, behaviour and electrical brain activity. For mammals, the neocortex plays a particularly profound role in generating sensory perception, controlling voluntary movement, higher cognitive functions and planning goal-directed behaviours. Since these remarkable functions of the neocortex cannot be explored in simple model preparations or in anesthetised animals, the neural basis of behaviour must be explored in awake behaving subjects. Because neocortical function is highly distributed across many rapidly interacting regions, it is essential to measure spatiotemporal dynamics of cortical activity in real-time. Extensive work in anesthetised mammals has shown that in vivo Voltage-Sensitive Dye Imaging (VSDI) reveals the neocortical population membrane potential dynamics at millisecond temporal resolution and subcolumnar spatial resolution. Here, we describe recent advances indicating that VSDI is also already well-developed for exploring cortical function in behaving monkeys and mice. The first animal model, the non-human primate, is well-suited for fundamental exploration of higher-level cognitive function and behavior. The second animal model, the mouse, benefits from a rich arsenal of molecular and genetic technologies. In the monkey, imaging from the same patch of cortex, repeatedly, is feasible for a long period of time, up to a year. In the rodent, VSDI is applicable to freely moving and awake head-restrained mice. Interactions between different cortical areas and different cortical columns can therefore now be dynamically mapped through VSDI and related to the corresponding behaviour. Thus by applying VSDI to mice and monkeys one can begin to explore how behaviour emerges from neuronal activity in neuronal networks residing in different cortical areas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cat
- Cortical response field
- Evoked response
- Horizontal connections
- Lateral spread
- Monkey
- Mouse
- Orientation map
- Orientation selectivity
- Sensorimotor cortex
- Spatiotemporal response
- Spikes
- Spontaneous activity
- Subthreshold response
- V1
- V2
- Visual cortex
- Whisker
1 Introduction
The activity of highly distributed neural networks is thought to underlie sensory processing, motor coordination and higher brain functions. These intricate networks are composed of large numbers of individual neurons, which interact through synaptic connections in complex dynamically regulated spatiotemporal patterns. To understand network properties and functions, it is helpful to begin by studying the ensemble activity of neuronal populations. Functionally related networks of neurons are often spatially organised and imaging techniques are therefore ideally suited to monitoring neural network activity. Having defined the spatiotemporal dynamics of the ensemble population network activity, it may then be possible to begin to understand the relationships and contributions of single neurons and their synaptic connections to the larger networks that are essential for generating percepts and controlling behaviour.
The high performance of the mammalian brain is thought to result, in part, from computations taking place in the neocortex. The neocortex is organized into cortical columns (Mountcastle 1957; Hubel and Wiesel 1962), which have lateral dimensions of a few hundred microns. Interactions within and between cortical columns occur on the time scale of milliseconds. To follow neuronal computations at the fundamental level of cortical columns in real-time therefore requires a spatial resolution of ~100 μm and a temporal resolution of ~1 ms. In vivo Voltage-Sensitive Dye Imaging (VSDI) (Grinvald et al. 1984; Orbach et al. 1985) fulfils these technical requirements and should help resolve many outstanding fundamental questions. Electrical communication in cortical networks comprises two basic signals: subthreshold potentials (reflecting dendritic synaptic integration) and suprathreshold action potentials (forming the neuronal output). VSDI appears to relate primarily to spatiotemporal patterns of the subthreshold synaptically-driven membrane potentials. VSDI in behaving animals therefore allows a correlation of subthreshold synaptic processing with perception and other higher brain functions.
Sensory perception is an active process, in which information is often acquired by self-generated movements of sensors. Multiple brain regions concerning motor control and different sensory modalities must cooperate to generate the mental construct of the world around us. Obvious examples that we are familiar with from our own experiences are vision and touch. In vision we actively move our eyes to examine visual targets of interest. For touch, we move our fingers into contact with objects to obtain tactile information about shape and texture. In order to understand sensory perception it is therefore of critical importance to investigate the neural activity of highly distributed sensorimotor networks in awake behaving animals. Here we describe how in vivo VSDI (Grinvald et al. 1984; Shoham et al. 1999; Grinvald and Hildesheim 2004) can be applied to provide such information on cortical spatiotemporal dynamics during behaviour. We describe technical advances and first results from VSDI of behaving animals and how VSDI can be combined with intracortical microstimulation, single-unit recording and local-field-potential recording from awake animals. We conclude by discussing further technical developments to overcome current limitations and additional questions that are likely to be explored.
2 Voltage-Sensitive Dyes
To explore the neuronal basis of behavior it is important to be able to image cortical dynamics without signal averaging. This is now possible through the development of new voltage-sensitive dyes (Shoham et al. 1999) with excitation and emission spectra in the far red. These new dyes reduce pulsation artefacts and haemodynamic noise, since they operate beyond the major absorption band of haemoglobin. This advance has enabled the functional architecture and dynamics of cortical information processing to be revealed at subcolumnar spatial and millisecond temporal resolution in behaving animals.
Intracellular recordings in vivo under anesthesia combined with simultaneous VSDI, both in the cat visual cortex (Grinvald et al. 1999) and in the rodent somatosensory cortex (Petersen et al. 2003a, b; Ferezou et al. 2006; Berger et al. 2007) indicate that the VSD signal correlates closely with changes in subthreshold membrane potential (red and blue traces in Fig. 11.1). In vitro measurements show microsecond VSD response times and, in vivo, VSD signals correlate on the millisecond timescale with membrane potential recorded in individual neurons. However, it should be noted that these simultaneous recordings have so far only been carried out under anesthesia and in the future it will be important to repeat these experiments combining VSDI and intracellular electrophysiology in awake behaving animals.
Voltage-sensitive dye imaging of behaving monkeys. Setup for VSD imaging of cortical dynamics in behaving monkeys. The exposed monkey cortex is covered with a sealed cranial window (bottom left inset), which acts as if the monkey had a transparent skull. After the cortex is stained with a suitable voltage-sensitive dye (blue/orange molecules in top right inset), it is illuminated with excitation light of the appropriate wavelength (630 nm; 30 nm bandwidth, in this case). A sequence of images of the fluorescing cortex is taken with a fast camera (100–10,000 Hz) using a macroscope offering a numerical aperture of ~0.4. During image acquisition the monkey fixates on a visual stimulus or the eyes are covered with shutters in order to monitor ongoing activity. The acquired images are digitized and transferred to the computer controlling the experiment. Functional maps or movies of the cortical activity are analysed and displayed on a colour monitor. Various types of electrical recording, microstimulation or tracer injection are often carried out simultaneously with the imaging. The similarity between the two traces above the electrode, comparing intracellularly recorded electrical activity (blue) from one cell and VSD population activity (red), indicate that, in vivo, VSD measures mostly subthreshold synaptic membrane potential changes. Spikes recorded in the intracellular recording are truncated to increase vertical scale, but spikes are indicated by blue lines at the top. Spikes were not detected in the optical signal
The spatial resolution of VSDI in awake animals is primarily limited by light scattering, movement artefacts and signal-to-noise ratio. Each camera pixel will receive fluorescence originating from dendrites, axons and somata belonging to a population of neurons. Since the VSD stains membranes, dendrites and axons will dominate the total observed fluorescence. Dendrites and axons can extend for considerable lateral distances, which could therefore strongly affect spatial resolution of VSDI. In practice, however, VSDI has been shown to provide high resolution maps clearly differentiating activity in nearby cortical columns (Petersen et al. 2003a; Ferezou et al. 2006) and offering a spatial resolution better than 50 μm (Shoham et al. 1999; Grinvald et al. 1999). One possible explanation may be that synaptic processing on different dendritic branches is spatially mapped with respect to cortical columns.
3 Imaging from Awake Behaving Animals
A major technical impediment to imaging in awake animals is movement of the brain, as is the case for all forms of in vivo brain imaging. Movements are of course particularly large during wakefulness and overt movements are often an integral part of behavioural paradigms in animal experiments. A critical aspect of imaging brain function during behaviour is therefore to obtain a stable view of the brain.
The most obvious paradigm is to fix the head relative to the imaging apparatus. Head-fixation is routinely applied in neurophysiological animal experiments and has proven to be extremely useful both in constraining behavioural variability and in facilitating measurements. Head-fixation bars can be attached to the skull of monkeys (Fig. 11.1) and mice (Fig. 11.2a). The animals rapidly learn to accept head-restraint during a period of behavioural habituation. As for imaging in anesthetised animals, a sealed recording chamber should be used to minimise brain movements relative to the skull. Furthermore, to facilitate staining in monkeys, the resected dura matter is substituted with artificial dura mater (Fig. 11.3). A careful cleaning procedure allowed repeated VSDI sessions in monkeys 2–3 times each week over a period longer than a year (Arieli et al. 2002).
Voltage-sensitive dye imaging of awake and freely moving mice. (a) Setup for voltage-sensitive dye imaging of the sensorimotor cortex in an awake head-restrained mouse. An implanted metal head-fixation post is attached to a fixed mechanical arm. The tandem lens optics are turned to include as large an area of sensorimotor cortex as possible in the focal depth of the epifluorescence tandem lens optics. With the cortex stained with RH1691, the excitation light (630 nm) is reflected by a dichroic mirror (650 nm) and focused on the brain through an objective. Emitted fluorescence is collected by the same lens and imaged on to a fast camera with large well-depth. Simultaneous filming of behaviour enables correlation of neocortical activity with sensorimotor behavioural events. (b) For imaging freely moving mice, the experimental setup is almost identical, except that a flexible fiber optic image bundle relays light from the focal plane of the tandem lens imaging system to the mouse brain. The fibers are arranged in a orderly fashion at each end of the fiber (right). Each individual fiber measures 10 μm in diameter and they are assembled in a multistep process first involving 6 × 6 multifibers that are then wound to form a 300 × 300 array of fibers extending approximately 3 × 3 mm
The silicone dural substitute for long-term chronic recordings. (a) Scalp, dura and cortex prior to the surgery. (b) When the dural substitute was positioned in its proper position, the cut edge of the natural dura was enfolded and touched the silicon ring in the middle of the sheet, in order to stabilize it and prevent the natural dura from growing back into the central area used for imaging. (c) Within a few weeks after the operation the dura had grown around the ring, thus creating a good seal of the cortex. (d) View from above 2 weeks after the artificial dural transplantation. (e, f) Cortical reaction to dural implant. The cortical vascularisation 12 months (e) and 3 months (f) after implantation of artificial dura. Note the new growth of blood vessels originating from the Lunate sulcus and other sites (big arrows). This neovascularization is different from the blood vessels over the cortical tissue since it has the shape of ‘hair like’ growth. Note that cortical blood vessels also react: the veins show new ‘growth cones’ which were directed towards the new microvascularization (small arrows)
A tandem-lens macroscope (Ratzlaff and Grinvald 1991) can then be mounted in a stable location relative to the fixed head position for epifluorescent voltage-sensitive dye imaging with suitable illumination and camera equipment (similar to that used for imaging anesthetised animals, except for additional equipment necessary for controlling and monitoring behaviour). Voltage-sensitive dye imaging of awake head-restrained mice (Petersen et al. 2003b; Ferezou et al. 2007) and awake head-restrained monkeys (Slovin et al. 2002; Seidemann et al. 2002; Omer and Grinvald 2008; Fekete et al. 2009) has begun to reveal cortical correlates of sensorimotor processing.
An alternative approach, which has been successfully tested in mice (Ferezou et al. 2006), is to image voltage-sensitive dye signals via flexible fiber optics (Fig. 11.2b). Fiber optic bundles containing well-ordered arrays of individual fibers are able to efficiently transmit images from one end of the fiber to the other. At each end, the fibers are held rigidly in place, but in between the ends, the fibers can run separately from each other allowing high flexibility and a considerable range of rotation. Under these conditions the animal is therefore relatively free to move around within the tether limits of the light-weight fiber optics. Excitation light can be passed in one direction through the same fibers that also relay emitted fluorescence back from the animal. One end of the fiber bundle can be placed in the location normally imaged by the tandem lens epifluorescence macroscope. The other end of the fiber optic image bundle can be placed in direct contact with the brain surface and firmly anchored on the skull. Movement artefacts are surprisingly small in this imaging configuration (Ferezou et al. 2006; Flusberg et al. 2008), perhaps because of correlated movement of the fiber bundle and the brain.
4 Integrating VSDI with Electrode Techniques
VSDI can easily be combined with standard electrode-based techniques. Whereas optimal mechanical stability for imaging is provided by sealed cranial windows, these do not allow electrodes to be introduced. One approach is to cover the cortical surface with agarose and stabilise it by a cover slip. A small gap between the edge of the cover slip and the wall of the recording chamber allows oblique entry of electrodes into the rodent cortex (Petersen et al. 2003a, b; Berger et al. 2007; Ferezou et al. 2006, 2007). For cats and monkeys a ‘sliding-top cranial window’ was developed with a removable microdrive-positioned electrode (Arieli and Grinvald 2002). VSDI can then be combined with microstimulation, extracellular recording (single- and multiple-unit recording and local field potential), or intracellular recording, and the targeted injection of tracers. Recordings can either be made simultaneously and/or can be targeted to specific regions based on the functional imaging data.
5 Imaging the Monkey Visual Cortex During Behaviour
5.1 Dynamics of the Spread of Retinotopically Evoked Activity in Macaque Visual Cortex
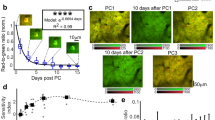
Using VSDI in awake behaving monkeys, Slovin et al. (2002) imaged cortical dynamics with high spatial and temporal resolution from the same patch of cortex for up to 1 year (Fig. 11.4). In the original study, the visual cortices of trained macaques were stained 1–3 times a week. Immediately after each staining session, the monkey started to carry out a simple behavioural task, while the visual cortex was imaged using VSDI. The first important task of this study was to show that such repeated use of VSDI does not interfere with normal cortical function. The functional maps obtained by VSDI were confirmed by imaging based on intrinsic signals (Grinvald et al. 1986) over a period of up to a year (Fig. 11.4b). Orientation preference maps can be used as a sensitive assay to assess any potential cortical damage since orientation preference is formed in the cortex itself rather than lower brain areas. The imaging results, as well as the behavioural performance, indicated that pharmacological side-effects or photodynamic damage were negligible. Having established this point, Slovin et al. (2002) studied the spatiotemporal representation of a point stimulus in primary and secondary visual cortex (Fig. 11.4c), quantifying the latency and time course of the cortical response (Fig. 11.4d). The individual traces indicate that evoked activity can be detected without signal averaging in the awake monkey, offering VSDI as an important tool to explore real-time cortical dynamics. This is beneficial because averaging may mask important variability among the instantaneous responses. In an example behavioural experiment, the cortical dynamics as the monkey makes a saccade are depicted in Fig. 11.5. Evidently despite the high speed of the eye movements, an evoked response was detected also along the cortical representation of the fast eye movement.
Normal cortical architecture and function is preserved throughout long-term chronic VSDI for up to a year. (a) A large area of exposed cortex in excellent condition after almost a year of repeated VSDI, intrinsic imaging and electrical recording in this monkey. (b) The functional VSDI maps of ocular dominance in two sites (left and right) and orientation (middle), which were recorded from the same patch of cortex in recording sessions separated by up to a year, are virtually identical (compare top and bottom rows of images). (c) Dynamics of retinotopic activation. Top: retinotopic activation of V1 and V2 (width of cortical area about 14 mm). Bottom: the spread of cortical dynamics along horizontal (upper) and vertical (lower) axes as a function of time. Time is marked by different colours. (d) Single-trial evoked responses in the awake monkey. As in the anaesthetized animal, the response is variable. Scale bar: 1 mm
Fast saccadic eye movement generates a cortical response along its track. (a) Schematic representation of the visual stimulus. (b) Time series of the average optical signal triggered by the onset of a saccade to the new visual stimulus averaged over 17 trials. The first few frames show the fully developed evoked response to the small (0.5°) single isoluminant drifting grating, which was turned on 500–800 ms earlier. After a saccadic eye movement to the stimulus (t = 0), the activity on the cortex is shifted to a more foveal location (lateral direction). The thin black line in the first frame denotes approximately the V1/V2 border. Scale bar 3 mm. (c) Black-and-white image of the blood vessel pattern from the imaged cortical areas, the V1/V2 border was detected by obtaining an ocular-dominance map with intrinsic imaging. A anterior, P posterior, L lateral, M medial
5.2 Visualization of Neuronal Assemblies
With the improvements in the signal-to-noise ratio of in vivo VSDI offered by the blue dyes, evoked activity can be detected without signal averaging in the awake monkey (Fig. 11.4d; Slovin et al. 2002), as previously found in anaesthetized cats (Arieli et al. 1995, 1996; Tsodyks et al. 1999; Kenet et al. 2003). This finding indicates that cortical spatiotemporal dynamics per se can now be explored in awake behaving monkeys. Hebb (1949) suggested that neocortical neurons operate in assemblies, defined as networks of neurons, local or widespread, that communicate coherently to carry out the computations that are required for various behavioral tasks. Neurons located within the same pixel of a VSDI movie might belong to the same or different neuronal assemblies. It is therefore of great interest to monitor the spiking activity of a single neuron in the context of larger network dynamics, to reveal the co-active nodes in the assembly during a given computation. This can be achieved by combining single unit (or multiple-unit recording) with real-time optical VSD imaging of the dynamics of coherent neuronal assemblies. First, a single unit with the desired tuning properties is selected. Second, VSDI and unit recordings are conducted for a long time without any signal averaging. During off-line analysis, which involves performing spike-triggered averaging on the optical data, the firing of a single neuron serves as a time reference to selectively visualize only the population activity that was synchronized with it. With a sufficient number of spikes, any neuronal activity not time-locked to these spikes will be averaged out and the net, clean spatiotemporal pattern of coherent activity will be thus obtained.
Such spike-triggered averaging of VSD signals can be performed on both evoked and spontaneous activity. Even in primary sensory areas, there is a large amount of spontaneous on-going electrical activity, primarily subthreshold activity, in the absence of any sensory input. Some of this activity is coherent over large cortical areas. Is this spontaneous activity just network noise? How large is it relative to evoked activity? Does it affect evoked activity? And does it have an important functional role? VSDI is ideally suited to exploring such questions at the level of neuronal assemblies. To compare the amplitude of coherent, ongoing activity with that of evoked activity (both representing large subthreshold activity, to which VSDI is sensitive), spike-triggered averaging was used during both spontaneous- and evoked-activity imaging sessions with anaesthetized cats (Grinvald et al. 1991; Arieli et al. 1995, 1996; Tsodyks et al. 1999; Kenet et al. 2003). Surprisingly, the amplitude of coherent, spontaneous, ongoing activity in neuronal assemblies was nearly as large as that of evoked activity. Large, coherent, ongoing activity was also found in the somatosensory cortex (Petersen et al. 2003b; Ferezou et al. 2006, 2007). The large fluctuations of ongoing activity affect how far cortical neurons are from their firing threshold. Therefore, the amplitude of ongoing activity indicates that it might have an important role in shaping spatiotemporal patterns evoked by sensory input. The idea that network activity can converge to cortical states is a central concept in theoretical brain research. What are the dynamics of intrinsic cortical states? It is now possible to visualize certain aspects of cortical networks and their states in action, at a high spatiotemporal resolution. Such studies can provide insights into the dynamic interplay between activated internal cortical representations and incoming sensory input. The previous studies on anesthetized cats are reviewed in the previous chapter. In those VSDI studies we reported that spontaneous ongoing cortical activity in areas 17 and 18 represented dynamic spatial patterns, and about 20 % of these patterns resembled the functional architecture of orientation domains. These patterns covered large cortical areas—up to the entire imaged areas of up to 6 × 6 mm (Grinvald et al. 1989; Arieli et al. 1995, 1996; Tsodyks et al. 1999; Kenet et al. 2003).
To find whether these results are relevant to the awake behaving primate, Omer and Grinvald (2008) performed VSDI of ongoing cortical activity in the visual cortices of awake monkeys simultaneously with measurements of single unit activity and the local-field potential. They found coherent activity also in the awake monkey (Fig. 11.6). This figure shows the tuning properties of the reference cell (Fig. 11.6a), the border between the two imaged areas V1 and V2 (Fig. 11.6b), time course of the coherent activity in area V1 (red) and V2 (blue; Fig. 11.6c) and the instantaneous spatial pattern of the assembly activity just before the action potential of the reference neuron (Fig. 11.6d). Overall, the dynamics were very different from that found in anesthetized cats and in a single epoch it was not possible to recognize any pattern that looked like the known functional architecture. Nevertheless, it was found that such patterns do appear: the pairwise correlation among pixels of known functional architecture as a function of the orientation difference between these pixels or their ocularity index had the same stereotypic shape for evoked activity and for spontaneous epochs. Similar results were obtained in anesthetized monkeys. However, in the anesthetized monkeys, Omer and Grinvald found that spontaneous cortical activity resembled the functional architecture just like in the anesthetized cat (unpublished). These results underscore the importance of carrying out experiments on awake behaving animals since the spatiotemporal patterns of activity are so drastically different.
Visualization of coherent neuronal assemblies across visual areas. (a) The tuning properties of a single cell in V1 of primary visual cortex of the awake monkey, which was used for the spike triggered averaging. (b) Cartoon of the imaged area showing the V1/V2 border that was identified by mapping the ocular dominance columns in V1. (c) The time course of spike triggered average (STA) of the optical signal from V2 (blue curve) as well as the optical signal from V1 (red curve) on the time of the firing of the V1 reference neuron. Evidently, whenever this cell fired, there was activity over a large area in V1 as well as V2. In fact, in this case, whenever this cell in V1 fired there was larger coherent activity in V2 than in V1. (d) The average spatial pattern 60 ms before the V1 action potential occurred is shown. Courtesy of David Omer and Amiram Grinvald; unpublished results
5.3 VSDI Reveals Complex Distributed Activity Evoked by Intracortical Microstimulation
Microstimulation in various brain regions has been used extensively to affect neuronal and behavioural responses. However, many questions remain about the spatiotemporal patterns of activity that are evoked by microstimulation and their characteristics as a function of various parameters such as the current strength and the electrode shape (Tehovnik et al. 2006).
Intracortical stimulation has been used as a tool to discover the pattern of muscle and/or movement representation in motor areas of the cortex (Slovin et al. 2003). The direct and synaptic spread of activation induced by stimulation has been the subject of considerable uncertainty and controversy. To help resolve some of these issues we used voltage sensitive-dye (VSD) imaging to examine the patterns of activation evoked by different stimulation parameters in the primary motor cortex (M1) of an awake monkey. Single cathodal pulses in M1 (15–30 μA, 0.2 ms duration) evoked short latency VSD responses that continued for 40–50 ms after the onset of stimulation. These responses spread in an asymmetric manner from the stimulation site in M1; the width of the activated area was about 1.5–3 mm. Single pulses larger than 15 μA also evoked activity at spatially separate sites within M1. Short trains of pulses in M1 (2–20 pulses, 330–500 Hz, 30–100 μA) produced activation that spread from the electrode site to adjacent premotor areas and to the contralateral hemisphere. A distinct hyperpolarization followed the activation evoked by a high current (>60 μA), short stimulus train (2–20 pulses). Finally, long stimulus trains (70 μA, 200 pulses, 330 Hz) evoked activation that spread over large portions of M1 and the adjacent premotor areas. These responses outlasted the stimulus train by several hundred milliseconds. Our results emphasize the importance of considering the spread of activation when evaluating the results of M1 stimulation.
VSDI studies on the effect of microstimulation in the frontal and motor cortices of awake monkeys have also recently shed light on the evoked spatiotemporal dynamics. The frontal eye field and neighbouring area ‘8Ar’ of the primate cortex are involved in the programming and execution of fast eye movements. Electrical microstimulation in these regions elicits short-latency contralateral saccades. A combination of VSDI and microstimulation has been used to determine how the spatiotemporal dynamics of microstimulation-evoked activity are converted into saccade plans. Microstimulation was shown to elicit neural activity with complex spatiotemporal dynamics, both inhibitory and excitatory; these dynamics depend on the stimulated area, and have important behavioural correlates. The observed spread was large and depended on the amplitude of the microstimulation (Seidemann et al. 2002). These results emphasize the importance of further characterization of microstimulation-evoked activity for the interpretation of its behavioural effects and what it has revealed about the neural basis of cognition (Newsome et al. 1989; Cohen and Newsome 2004). VSDI seems to be one of the most appropriate tools to nail down these issues, which also have important implications for neural prosthesis and brain machine interfaces.
5.4 Imaging the Correlates of Go/No-Go Delayed Response Task in the Motor Cortex
Electrical recording studies during Go/No-Go tasks (Strick et al. 2003) have found that M1 neurons display changes in activity, which begin primarily after a Move cue, whereas PMd neurons display changes in activity, which can begin during Instructed Delay periods. We used both intrinsic imaging and VSD imaging in a trained monkey to examine the spatiotemporal patterns of subthreshold population activity, which underlies these neuronal responses.
One monkey was trained to perform a Go/No-Go task. The monkey began a trial by placing his hand over a small photodiode placed in front of him. This caused a small white square to appear on a computer screen. After a variable precue period (3,000–4,000 ms), the white square was changed to red (Go mode) or green (No-Go mode). Then, after a variable Instructed Delay period (300–1,500 ms), a luminance change of the square told the monkey to follow the prior instruction: uncover (Go) or continue to cover the photodiode (No-Go).
Intrinsic imaging showed patches of activation in PMd and in M1 for the Go mode. In the No-Go mode, small amplitude responses were present in the PMd, but not in M1. Changes in VSD signals were present in both PMd and M1 during the Go mode. However, changes in VSD signals were much smaller in M1 during the No-Go mode. In the PMd, the VSD signal changes began shortly after the onset of the Go or No-Go Instructions (i.e., at the start of the Instructed Delay periods). In M1, the signal changes appeared to be locked to movement onset as confirmed by single unit recordings. These observations suggest that the preparation to perform or withhold a motor response is associated mainly with marked subthreshold activity in the PMd.
6 Imaging the Mouse Sensorimotor Cortex During Behaviour
Mice are nocturnal animals living in tunnels. For much of their lives they therefore receive impoverished visual input, and instead they rely heavily upon olfactory and somatosensory information. The prominent mystacial whiskers surrounding the snout form a sensitive array of tactile detectors, allowing texture discrimination (von Heimendahl et al. 2007) and object location (Knutsen et al. 2006; Mehta et al. 2007). Sensory information is signalled to the primary somatosensory cortex via a first synapse in the brainstem and a second synapse in the thalamus. The thalamus in turn directly signals to the neocortex. Whisker-responsive thalamic neurons mainly project to the primary somatosensory cortex. Each whisker is represented in the primary somatosensory cortex by an anatomically identifiable structure, termed a barrel. These barrels are somatotopically arranged in a stereotypical manner matching the layout of the whiskers on the snout (Woolsey and van der Loos 1970). As one might expect from such an anatomical map, the functional cortical sensory processing evoked by a single whisker deflection indeed begins within the related barrel column, i.e. if the C2 whisker is deflected, then the neurons in the C2 barrel column are the first to respond (reviewed by Petersen 2007). Many of the pyramidal neurons located in a given barrel column project dense axonal arborisations into neighbouring cortical columns (Petersen et al. 2003a) and indeed also to other cortical regions such as secondary somatosensory cortex and motor cortex (Chakrabarti and Alloway 2006; Ferezou et al. 2007). Consistent with such anatomical data, sensory signals can propagate extensively across the sensorimotor cortex, which can be directly visualised by voltage-sensitive dye imaging (Orbach et al. 1985; Kleinfeld and Delaney 1996; Derdikman et al. 2003; Petersen et al. 2003a; Ferezou et al. 2006, 2007; Berger et al. 2007). Here, we will focus on how different behavioural states in awake mice affect both sensory processing and spontaneous cortical activity.
6.1 State-Dependent Processing in Mouse Sensorimotor Cortex
In order to deliver a well-controlled whisker deflection in awake mice, we attach a small metal particle to the C2 whisker and evoke movement of this whisker by driving a brief (1–2 ms) current pulse through an electromagnetic coil to generate a magnetic field which exerts a force on the metal particle attached to the whisker. Such a stimulus evokes a small, brief and reproducible whisker deflection (Ferezou et al. 2006). The evoked sensory response was imaged in head-fixed mice, whose sensorimotor cortex had been stained with voltage-sensitive dye RH1691 through a large craniotomy (Ferezou et al. 2007). When the mouse was not spontaneously moving, defining a period of quiet wakefulness, deflection of the C2 whisker evoked a sensory response, which at first was localised to the C2 barrel column (Fig. 11.7a, upper). In the following milliseconds the depolarisation spread across the barrel field, presumably through local excitatory synaptic connections between neighbouring cortical columns. In addition, a second localised spot of activity was evoked in the whisker motor cortex, after a delay of ~8 ms following the first signals in S1. This initially localised secondary sensory response in motor cortex also spread over the following milliseconds and within 50 ms of the stimulus a surprisingly large cortical area had depolarised in response to a single whisker deflection. Interestingly, the mouse often began to actively whisk following such a sensorimotor response. The propagating sensory response may serve as a wake-up call, bringing the mouse from an initially quiet state into one optimised for actively processing subsequent sensory stimuli. Rapid signalling of sensory information to motor cortex may be important in general for optimising motor control. For example when we touch an object with our hands, we change our movements appropriately as informed by sensory feedback. Rodents also often change their pattern of whisker movements following whisker-object contact, perhaps as a way to improve the quality of sensory information.
State-dependent processing of sensory information in the sensorimotor cortex of the awake mouse. (a) A large fraction of the sensorimotor cortex was stained with voltage-sensitive dye including primary somatosensory barrel cortex (S1) and the whisker motor cortex (M1). A small piece of metal was attached to the whisker allowing brief magnetic pulses to evoke a whisker deflection and subsequent sensory processing. The whisker-related behaviour was filmed simultaneously with voltage-sensitive dye imaging allowing direct correlation of cortical activity with behaviour. The image of the mouse nose and whisker is a superposition of many frames. The upper image extends over a quiet prestimulus period. The lower image extends over a period of spontaneous whisking in the prestimulus period. Deflection of the whisker during these two different behavioural periods evokes dramatically different sensory responses. During quiet wakefulness, the response is initially localised but rapidly spreads across S1 and a secondary sensory response is observed in motor cortex. The same stimulus applied during a period of spontaneous whisking, evokes a small amplitude sensory response that remains localised to S1. (b) Voltage-sensitive dye imaging of barrel cortex in freely moving mice through fiber optics provides further evidence for state-dependent sensory processing of tactile information. During a quiet period the deflection of a whisker evoked a large-amplitude propagating sensory response. During active whisking, the same stimulus barely evoked a measurable response. (c) State-dependent processing is also a prominent feature of whole-cell recordings of awake head-restrained mice. Whisker deflection during quiet wakefulness evoked a large-amplitude depolarisation, whereas during active whisking periods the same stimulus evoked only a very small and brief sensory response. There is therefore good agreement between voltage-sensitive dye imaging data and whole-cell recording data
During exploratory behaviour, mice move their whiskers back and forth at high frequencies (typically around 10 Hz) scanning the environment surrounding their snout. Mice also spontaneously whisk during head-restraint and by chance some of our magnetic whisker deflections occurred during such active whisking bouts. Interestingly, the sensory-evoked responses were dramatically different during active states. The sensory responses were again initially localised to the C2 barrel column, but instead of spreading across large cortical areas, the response remained localised and had only small amplitude and a brief duration (Fig. 11.7a, lower). The ongoing behaviour of the mouse therefore makes a dramatic difference to sensory processing. Here in this example, it is again clear that neocortical spatiotemporal patterns of activity must be directly explored during behaviour, since brain states and behavioural states play such profound roles.
In order to investigate if a similar state-dependence of sensory processing occurs in freely moving mice, we used fiber optic image bundles to visualise the membrane potential dynamics of the primary somatosensory barrel cortex (Ferezou et al. 2006). The fiber array we used only extended across somatosensory barrel cortex and did not include motor cortex. Similar to the observations made in head-restrained mice, we found that a brief whisker stimulus delivered during quiet wakefulness evoked a large, spreading sensory response (Fig. 11.7b, upper panel) whereas the stimulus delivered during active whisking was strongly suppressed (Fig. 11.7b, lower panel). The propagating sensory responses evoked by a single whisker stimulation observed during anaesthesia (Orbach et al. 1985; Kleinfeld and Delaney 1996; Derdikman et al. 2003; Petersen et al. 2003a) are therefore not artefacts of anaesthesia, per se, but appear prominently during some behavioural states of wakefulness (Ferezou et al. 2006, 2007). That a large part of the cortex can be informed about a single whisker deflection might allow integration of sensory information from different whiskers and even different modalities, which might be crucial for associative learning and sensory perception.
In separate experiments, we examined whether we could find single cell correlates of the voltage-sensitive dye imaging data. In head-fixed mice, we obtained whole-cell recordings from excitatory neurons and again stimulated the C2 whisker by magnetic pulses (Crochet and Petersen 2006). In good agreement with the voltage-sensitive dye imaging experiments, we found that brief, small-amplitude sensory responses were evoked during active whisking periods whereas larger amplitude sensory responses were evoked during quiet wakefulness (Fig. 11.7c). Similar to observations from anesthetised animals (Petersen et al. 2003a, b; Ferezou et al. 2006; Berger et al. 2007), in awake mice there is therefore also good agreement between membrane potential measurements made at the single cell level through whole-cell recordings and membrane potential measurements made at the ensemble level with voltage-sensitive dye.
6.2 State-Dependence of Spontaneous Dynamics in Mouse Sensorimotor Cortex
Having identified a profound role of the prestimulus behaviour in governing sensory processing, we were obviously interested to examine what changes in brain states accompany the changes in behavioural states. During quiet wakefulness in head-fixed mice, using voltage-sensitive dyes, we imaged propagating waves of depolarisation spreading across the sensorimotor cortex in complex patterns (Fig. 11.8a). Quantified over time at a given location on the cortical surface, these appear as slow oscillations in the local ensemble membrane potential. Although a detailed analysis of these spontaneous activity patterns has not yet been carried out, in some cases we found correlated depolarisations in primary somatosensory cortex and motor cortex (Fig. 11.8a), which was not very different from the sensory-evoked response. Spontaneous activity patterns in sensorimotor cortex may, in part, reflect major signalling pathways between different cortical areas, as already discussed for the occurrence of spontaneous orientation maps in the visual cortex (Kenet et al. 2003). Analysis of these spontaneous patterns of activity might therefore give insight into the functional connectivity between cortical areas, something that has also already been studied for spontaneous dynamics recorded through functional magnetic resonance imaging (Vincent et al. 2007).
State-dependent spontaneous activity in the sensorimotor cortex of the awake mouse. (a) Spontaneous activity was imaged across the sensorimotor cortex in awake head-restrained mice. During quiet wakefulness (when the whisker was not moving), propagating waves of depolarisation can be observed in complex spatiotemporal patterns. Quantified across regions of interest located in S1 (red) and M1 (blue), the membrane potential changes appear as slow oscillations (grey shading indicates the period shown in the above sequence of images). (b) In a freely moving mouse, spontaneous waves of activity can also be imaged during quiet wakefulness through fiber optics. The signal changes were quantified across a region of interest in the C2 barrel column and period shown in the image sequence (left) is shaded in grey. (c) Dual whole-cell recordings from nearby neurons in the barrel cortex indicate that correlated, slow, large-amplitude membrane potential changes dominate during quiet wakefulness. During active whisking periods, the membrane potential fluctuations show lower variance and are less correlated. Both of these factors will decrease the amplitude of spontaneous voltage-sensitive dye signals during whisking. During quiet wakefulness, it is likely that the strong correlations of membrane potential changes in nearby neurons forms the basis of the travelling waves of spontaneous activity imaged with voltage-sensitive dye
In freely moving mice, we also observed waves of activity propagating across the primary somatosensory barrel cortex during quiet wakefulness (Fig. 11.8b). The waves of activity during rest are therefore not imposed by head-restraint, but appear to be a normal physiological pattern of cortical activity during rest. However, during active whisking we did not observe such large-amplitude propagating waves of spontaneous activity.
In order to understand why spontaneous activity was not resolved during active periods, we obtained dual whole-cell recordings from pyramidal neurons in the barrel cortex of awake head-fixed mice (Poulet and Petersen 2008). During quiet wakefulness, we observed slow, highly-correlated, large-amplitude subthreshold membrane potential changes, which qualitatively resemble those observed under anaesthesia (Lampl et al. 1999; Petersen et al. 2003b). Action potential activity occurred independently in each neuron across our dual recording data set, despite remarkably high correlations in subthreshold membrane potential changes. These highly correlated subthreshold membrane potential changes are likely to provide the basis for the large-amplitude propagating waves of depolarisation imaged with voltage-sensitive dye. Each depolarised oscillation recorded in an individual neuron is therefore likely to reflect a propagating wave of activity travelling across the location of the recorded neuron.
Interestingly, the spontaneous membrane potential fluctuations differ dramatically comparing periods of quiet wakefulness to periods of active whisking. The slow large-amplitude membrane potentials are suppressed during active periods (Crochet and Petersen 2006; Poulet and Petersen 2008). The remaining higher frequency membrane potential changes have lower amplitude giving an overall lower variance to the membrane potential fluctuations. This reduction in the amplitude of spontaneous membrane potential fluctuations therefore provides one reason that spontaneous activity is less obvious during active periods in the voltage-sensitive dye images. A second and equally important factor is that the remaining membrane potential fluctuations are more independent in nearby neurons during active periods. The correlation coefficients of membrane potentials recorded in nearby neurons indicates that synchrony is approximately halved during active periods (Poulet and Petersen 2008). Since our method of voltage-sensitive dye imaging measures ensemble membrane potential, we need correlated changes in membrane potentials of nearby neurons in order to measure a signal. Both the decorrelation and the reduction in amplitude of the membrane potential fluctuations during active periods will reduce voltage-sensitive dye signals relating to spontaneous activity. Nonetheless, even during active behaviours and active brain states, it is likely that useful smaller amplitude signals will be uncovered in the future by more detailed analysis of spontaneous activity in mice imaged with voltage-sensitive dye.
7 Conclusions and Future Perspectives
Voltage-sensitive dye imaging of awake animals is beginning to provide a unique insight into the spatiotemporal dynamics of cortical processing during behaviour. VSDI provides information on cortical dynamics with millisecond temporal resolution and subcolumnar spatial resolution. Correlations of spatiotemporal dynamics of VSDI with behaviour indicate sequential activation of different cortical areas. Combining VSDI with extracellular recordings has begun to shed light on the larger neuronal network dynamics that through concerted interactions drive spiking in individual neurons. Combining VSDI with intracortical microstimulation reveals the spatiotemporal extent of microstimulation, which is important for the interpretation of motor mapping experiments. The ease, with which VSDI can be combined with other techniques, makes it broadly applicable to a wide range of neurophysiological experiments. There are of course also many limitations to what has so far been accomplished with VSDI and there is a great need for further technical development.
-
1.
Perhaps most importantly, in vivo VSDI is currently limited to the exposed areas of the cortical surface. In the future, use of fiber optic microendoscopes may provide a way to image from deep structures in the brain (Flusberg et al. 2005). In this context it is interesting to note that the results of VSDI and functional MRI in behaving monkeys (Logothetis et al. 2001) may complement each other in a very helpful manner. VSDI can provide temporal dynamics and higher spatial resolution to the static images obtained by fMRI. On the other hand, fMRI can contribute details about activity in the entire brain including subcortical areas.
-
2.
The application of VSD to the cortical surface ensures even staining of the underlying neuronal networks, but the approach inherently lacks cell-type specificity. High resolution in vivo imaging using two photon microscopy (Denk et al. 1990) of sparsely labeled individual neurons would allow investigation of membrane potential dynamics in single cells, without however providing information on the population activity. Genetically encoded fluorescent functional reporter proteins (Miyawaki et al. 1997) would provide a possible alternative to synthesised dyes allowing the targeting to specific cell types. There has been progress in developing voltage-sensitive fluorescent proteins (Siegel and Isacoff 1997; Sakai et al. 2001; Ataka and Pieribone 2002; Dimitrov et al. 2007; Lundby et al. 2008; Akemann et al., 2010; Kralj et al., 2011; Jin et al., 2012; St-Pierre et al., 2014) giving hope for future prospects of cell-type specific VSDI. Transgenic animals expressing voltage-sensitive fluorescent proteins now begin to allow simple repetitive imaging of cortical activity in preparations with sealed cranial windows (Trachtenberg et al. 2002; Madisen et al. 2015). This is particularly important since, so far, repetitive staining and imaging of the same cortical area with VSDs has not yet been accomplished in mice (although as described above this works well in monkeys).
-
3.
VSDI appears to be dominated by subthreshold membrane potential dynamics. On the other hand calcium signals from neuronal networks are dominated by suprathreshold action potential activity (Stosiek et al. 2003). It would therefore be of great interest to combine VSDI with calcium imaging in order to simultaneously image sub- and supra-threshold activity (Berger et al. 2007). In future experiments, such combined imaging techniques should be applied to awake behaving animals.
-
4.
So far, only very simple behaviours have been imaged with VSD. It will be of great interest to image more complex behaviours in order to study the spatiotemporal dynamics of learned behaviours.
References
Akemann W, Mutoh H, Perron A, Rossier J, Knpfel T (2010) Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat Methods 7:643–649
Arieli A, Grinvald A (2002) Combined optical imaging and targeted electrophysiological manipulations in anesthetized and behaving animals. J Neurosci Methods 116:15–28
Arieli A, Shoham D, Hildesheim R, Grinvald A (1995) Coherent spatio-temporal pattern of on-going activity revealed by real time optical imaging coupled with single unit recording in the cat visual cortex. J Neurophysiol 73:2072–2093
Arieli A, Sterkin A, Grinvald A, Aertsen A (1996) Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273:1868–1871
Arieli A, Grinvald A, Slovin H (2002) Dural substitute for longterm imaging of cortical activity in behaving monkeys and its clinical implications. J Neurosci Methods 114:119–133
Ataka K, Pieribone VA (2002) A genetically targetable fluorescent probe of channel gating with rapid kinetics. Biophys J 82:509–516
Berger T, Borgdorff AJ et al (2007) Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J Neurophysiol 97:3751–3762
Chakrabarti S, Alloway KD (2006) Differential origin of projections from SI barrel cortex to the whisker representations in SII and MI. J Comp Neurol 498:624–636
Cohen MR, Newsome WT (2004) What electrical microstimulation has revealed about the neural basis of cognition. Curr Opin Neurobiol 14:169–177
Crochet S, Petersen CCH (2006) Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci 9:608–610
Denk W, Strickler JH, Webb WW (1990) Two-photon laser scanning fluorescence microscopy. Science 248:73–76
Derdikman D, Hildesheim R, Ahissar E, Arieli A, Grinvald A (2003) Imaging spatiotemporal dynamics of surround inhibition in the barrels somatosensory cortex. J Neurosci 23:3100–3105
Dimitrov D, He Y et al (2007) Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS One 2, e440
Fekete T, Pitowsky I, Grinvald A, Omer DB (2009) The representational capacity of cortical tissue. Comput Neurosci 178:31–39
Ferezou I, Bolea S, Petersen CCH (2006) Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron 50:617–629
Ferezou I, Haiss F et al (2007) Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56:907–923
Flusberg BA, Cocker ED et al (2005) Fiber-optic fluorescence imaging. Nat Methods 2:941–950
Flusberg BA, Nimmerjahn A et al (2008) High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat Methods 5:935–938
Grinvald A, Hildesheim R (2004) VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci 5:874–885
Grinvald A, Anglister L, Freeman JA, Hildesheim R, Manker A (1984) Real-time optical imaging of naturally evoked electrical activity in intact frog brain. Nature 308:848–850
Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN (1986) Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324:361–364
Grinvald A, Frostig RD et al (1989) Optical imaging of activity in the visual cortex. In: Lam D, Glibert CD (eds). MIT Press, Cambridge, MA
Grinvald A, Bonhoeffer T et al (1991) Optical imaging of architecture and function in the living brain. In: Squire L (ed) Memory, organization and locus of change. Oxford University Press, Oxford
Grinvald A, Shoham D et al (1999) In-vivo optical imaging of cortical architecture and dynamics. In: Windhorst U, Johansson H (eds) Modern techniques in neuroscience research. Springer, Berlin
Hebb D (1949) The organization of behavior. Wiley, New York, NY
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interactions and functional architecture in the cat’s visual cortex. J Physiol 160:106–154
Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA (2012) Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 75:779–785
Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A (2003) Spontaneously occurring cortical representations of visual attributes. Nature 425:954–956
Kleinfeld D, Delaney KR (1996) Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage-sensitive dyes. J Comp Neurol 375:89–108
Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE (2011) Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods 9:90–95
Knutsen PM, Pietr M, Ahissar E (2006) Haptic object localization in the vibrissal system: behavior and performance. J Neurosci 26:8451–8464
Lampl I, Reichova I, Ferster D (1999) Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron 22:361–374
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157
Lundby A, Mutoh H, Dimitrov D, Akemann W, Knöpfel T (2008) Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One 3, e2514
Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, Gu H, Mills M, Cheng A, Tasic B, Nguyen TN, Sunkin SM, Benucci A, Nagy A, Miyawaki A, Helmchen F, Empson RM, Knpfel T, Boyden ES, Reid RC, Carandini M, Zeng H (2015) Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85:942–958
Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D (2007) Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol 5, e15
Miyawaki A, Llopis J et al (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388:834–835
Mountcastle VB (1957) Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol 20:408–434
Newsome WT, Britten KH, Movshon JA (1989) Neuronal correlates of a perceptual decision. Nature 341:52–54
Omer DB, Grinvald A (2008) The dynamics of evoked and ongoing activity in the behaving monkey. Rev Neurosci 19:S50
Orbach HS, Cohen LB, Grinvald A (1985) Optical mapping of electrical activity in rat somatosensory and visual cortex. J Neurosci 5:1886–1895
Petersen CCH (2007) The functional organization of the barrel cortex. Neuron 56:339–355
Petersen CCH, Grinvald A, Sakmann B (2003a) Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 23:1298–1309
Petersen CCH, Hahn TTG, Mehta M, Grinvald A, Sakmann B (2003b) Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A 100:13638–13643
Poulet JFA, Petersen CCH (2008) Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454:881–885
Ratzlaff EH, Grinvald A (1991) A tandem-lens epifluorescence macroscope: hundred-fold brightness advantage for wide-field imaging. J Neurosci Methods 36:127–137
Sakai R, Repunte-Canonigo V, Raj CD, Knöpfel T (2001) Design and characterization of a DNA-encoded, voltage-sensitive fluorescent protein. Eur J Neurosci 13:2314–2318
Seidemann E, Arieli A, Grinvald A, Slovin H (2002) Dynamics of depolarization and hyperpolarization in the frontal cortex and saccade goal. Science 295:862–865
Shoham D, Glaser DE et al (1999) Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron 24:791–802
Siegel MS, Isacoff EY (1997) A genetically encoded optical probe of membrane voltage. Neuron 19:735–741
Slovin H, Arieli A, Hildesheim R, Grinvald A (2002) Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys. J Neurophysiol 88:3421–3438
Slovin H, Strick PL, Hildesheim R, Grinvald A (2003) Voltage sensitive dye imaging in the motor cortex I. Intra- and intercortical connectivity revealed by microstimulation in the awake monkey. Soc Neurosci Abstr 554(8)
Stosiek C, Garaschuk O, Holthoff K, Konnerth A (2003) In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A 100:7319–7324
Strick P, Grinvald A, Hildesheim R, Slovin H (2003) Voltage sensitive dye imaging in the motor cortex II. Cortical correlates of Go/No-Go delayed response task. Soc Neurosci Abstr 918(8)
St-Pierre F, Marshall JD, Yang Y, Gong Y, Schnitzer MJ, Lin MZ (2014) High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci 17:884-889
Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK (2006) Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol 96:512–521
Trachtenberg JT, Chen BE et al (2002) Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420:788–794
Tsodyks M, Kenet T, Grinvald A, Arieli A (1999) The spontaneous activity of single cortical neurons depends on the underlying global functional architecture. Science 286:1943–1946
Vincent JL, Patel GH et al (2007) Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86
von Heimendahl M, Itskov PM, Arabzadeh E, Diamond ME (2007) Neuronal activity in rat barrel cortex underlying texture discrimination. PLoS Biol 5, e305
Woolsey TA, Van der Loos H (1970) The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17:205–242
Acknowledgements
AG’s research was funded by the Weizmann Institute of Science, The Grodetsky Center, the Goldsmith, Glasberg, Heineman and Korber foundations, BMBF, ISF grants, Ms. Enoch and the EU daisy grants. CCHP’s research was funded by grants from the Swiss National Science Foundation and SystemsX.ch.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Grinvald, A., Petersen, C.C.H. (2015). Imaging the Dynamics of Neocortical Population Activity in Behaving and Freely Moving Mammals. In: Canepari, M., Zecevic, D., Bernus, O. (eds) Membrane Potential Imaging in the Nervous System and Heart. Advances in Experimental Medicine and Biology, vol 859. Springer, Cham. https://doi.org/10.1007/978-3-319-17641-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-17641-3_11
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17640-6
Online ISBN: 978-3-319-17641-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)