Abstract
Azo dyes are known as industrially synthesized organic compounds, and these azo dyes are identified by their azo bonds (N=N). Mixtures of these synthetic dyes which are unbound to the fiber get released into the environment and that will ultimately lead to bioaccumulation. Bioaccumulation of these dyes constitutes a serious environmental hazard. Several physicochemical methods have been applied to the treatment of textile wastewater, but these methods have many limitations due to high cost, low efficiency, and secondary pollution problems. As an alternative to physicochemical methods, biological methods comprise bacteria, fungi, yeast, algae, and plants and their enzymes which received increasing interest due to their cost-effectiveness and eco-friendly nature.
Decolorization of toxic azo dyes by biological processes may take place either by biodegradation or biosorption. A variety of oxidative and reductive microbial enzymes may also be involved in the degradation of dyes. Azoreductase, peroxidase, laccase, and other important enzymes synthesized by these microbes have shown 80–90% efficacy in decolorizing the textile dyes. Green synthesis of nanoparticles and their mediated azo dye degradation are the latest and effective methods used for treatment of hazards effluent samples. Toxicity evaluation of pure dyes and degraded dye product using phytotoxicity and biotoxicity study is given a clear chart of the most effective methods This review provides an overview of decolorization and degradation of azo dyes by biological processes and establishes the fact that these Microbes and enzymes are significantly effective biological weapons against the toxic azo dyes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Azo dyes
- Biodegradation
- Azoreductase
- Peroxidases
- Laccase
- Nanoparticles
- Photocatalytic activity
- Toxicity study
5.1 An Introduction on History and Discovery of Dyes
The history of dye begins in 2600 BC, according to the earliest written record, with the use of dye stuffs in China. During that time these dyes were originally obtained from animal and vegetable sources. Also, Egyptian mummies were found to be wrapped with red-dyed clothes made of madder plants. The Egyptians commonly dye clothes using plant dyes and natural earth dyes. They also had very good knowledge of attaching the dye to the fabrics. Anthraquinone dye requires a metallic salt to impart color to the fabrics, and it is believed that to accomplish this, Egyptians used the salt alum (Nicholson and Shaw 2000). The majority of dyes that are used in the present world are chemically synthesized, and origin of these dyes can be traced back to organic chemistry. W.H. Perkin (1856) who is known as the “father of dye industry” accidentally discovered mauveine dye while trying to synthesize quinine, an antimalarial drug (Tyagi and Yadav 2001). Synthesis of dye is a very complex process. Distillate aromatic molecules should undergo reduction, oxidation, condensation, and nitration. Bismarck brown dye was the first commercial azo dye obtained by diazotization, and most of the dye used today is obtained through the same procedure. Based on the different chemical structures, dyes are divided into different classes. Azo dyes are the largest class of dye compounds since among the 100,000 existing dyes, more than 2000 dyes belong to azo dye group (Stolz 2001; Vijaykumar et al. 2007). Azo dyes are the most commercially important and extensively studied ones; few of those are shown in Figs. 5.1 and 5.2. This is because of the superior properties that are found in these dye classes when compared to other dyes. The chemical structure that is found in azo dyes and the bond that is responsible for the nondegradable property of these dyes are R-N=N-R. Azo dyes can be synthesized easily and can attach well to the fabrics and will not fade easily (Jeong 2008). Based on the number of N=N, azo dyes are classified as mono azo dyes, diazo dyes, triazo dyes, and polyazo dyes. Degradation of azo dyes is a very difficult process due to the presence of N=N. A total number of azo bonds, functional groups, and their arrangements greatly influence its degradation capacity (Rani et al. 2009; Grekova et al. 2012).
Structure of toxic azo dyes Methyl Red, Disperse Yellow, Reactive Orange II, Congo Red, and Methyl Orange. (Sudha et al. 2014)
5.1.1 Impact of Azo Dyes
Increasing urbanization, globalization, and industrialization have caused different types of environmental pollution. Among various industries, the textile industries discharge large volume of wastewater after dyeing process. As azo dyes have poor exhaustion properties, the remaining unbounded dye particles to the fiber get released into the environment and lead to bioaccumulation (Zolinger 1987). Among all available synthetic dyes , azo dyes are the largest class of dyes with the wide range of colors and structures, and it represents a major portion of the total dyes used in textile industries (Lang et al. 2013). In every textile industry, to dye 1 kg of fabric, 40–60 g of dyestuff is required, and after the dyeing process, approximately 15–20% of dye remains in the effluent (Baban et al. 2003; Babu et al. 2007). This effluent then becomes a highly toxic solution with toxic chemicals like reactive dyes and azo dyes (N=N). Toxic effluents after discharge into the environment cause adverse effects on the fertility of soil, plants, animals, aquatic organisms, and human beings (Mester and Tien 2000; Puvaneswari et al. 2006; Solıs et al. 2012; Saratale et al. 2013). Phytoplanktons present in the environment show abnormal coloration and reduction in the photosynthesis ability due to the absorbance of light by these dyes that enters the water ecosystem (Duran and Esposito 2000; Mester and Tien 2000). This also affects the pH, biochemical oxygen demand, and chemical oxygen demands and provided intense coloration in water and hence decreases the quality of water. The presence of these toxic and unnatural colors in water is aesthetically unpleasant and shows the presence of contamination in water. These dyes and their contamination will remain in the environment for longer period of time if not treated adequately (Olukanni et al. 2006). So far many physicochemical and biological methods were adapted for the removal of these toxic dyes. Some of those methods are found to be effective but also showed many negative side effects, and few are very expensive. Chemical methods used for the degradation of azo dye are found to increase the toxicity in the environment since most of the organic compounds are normally toxic. The advantages and disadvantages of the methods used for the removal of dyes from the textile effluents are summarized in Table 5.1 (Andrea et al. 2005). Azo dyes are mainly used in textile industries, but its applications are also seen in food, pharmaceutical, paper, cosmetics, and leather industries (Saratale et al. 2011). The more we use these toxic dyes, the more our environment will get polluted. Industrial effluent samples consisting of these azo dyes lead to bioaccumulation that causes severe toxic effects on the environment since these N=N make azo dyes highly toxic. Most of the dyes are soluble in water and can be absorbed by skin contact and also inhalation which can lead to allergy, risk of cancer, and skin and eye irritation and cause high toxicity if inhaled or consumed (Nikulina et al. 1995). Para-phenylenediamine is an aromatic amine which is present in almost all dyes and causes skin irritation, chemosis, permanent blindness, and lacrimation. Entry of para-phenylenediamine inside the body causes edema on the neck, tongue, and face and also respiratory distress. These dyes also cause disease like acute tubular necrosis, vomiting gastritis, hypertension, vertigo, urinary bladder cancer, splenic sarcomas, nuclear anomalies, and chromosomal aberrations (Table 5.2). Degradation of azo dyes is a bioremediation process which will remove toxicity from the environment . Therefore there is an urgent need for their removal and to reduce its toxicity before discharge of the waste effluent into the environment (Ayed et al. 2011). Research has been initiated in the field of biodegradation of azo dyes, i.e., azo dye degradation using microorganisms . Microbial degradation of azo dyes will also depend on the microbes such as bacteria, fungi, actinobacteria, bacterial consortium, and yeast and also on the culture condition provided. Biodegradation of azo dye is an easy, effective, and eco-friendly approach for the degradation and removal of toxic azo compounds from the environment. This review summarizes the recent achievements and methods that are used for the degradation of toxic azo dyes and also discusses the toxicity of degraded compounds and future perspective on the degradation of textile azo dyes.
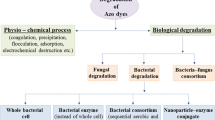
5.2 Biodegradation of Azo Dye
Physical and chemical methods available for the removal of azo dyes include coagulation, precipitation, adsorption, flotation, flocculation, mineralization, and electrochemical destruction (Gogate and Pandit 2004). Mentioned techniques have many disadvantages such as high cost, release of the residue, time, and also inability to reduce the toxicity of degraded compounds (Copper 1993; Maier et al. 2004). Moreover these techniques will only minimize the toxicity level and not be able to completely remove the toxicity of the dyes (Copper 1993; Maier et al. 2004). To replace these techniques, microbial degradation methods can be used which show complete degradation of azo dyes and also detoxify the toxic compounds (Pandey et al. 2007). Biological treatment of textile effluents is an eco-friendly approach, and it is also gaining much importance in today’s scenario. Microorganisms are very active in reducing azo dyes by secreting different enzymes like azoreductase, laccases , peroxidase , and hydrogenase. These reduced compounds are then broken down into smaller compounds which are then utilized as their energy source (Stozl 2001). The location of these reactions may be either intracellular or extracellular sites (Fig. 5.3). According to the available literature, microbes are more active under combined effect of aerobic and anaerobic conditions (Waleed and Muhammad 2014). Almost all microorganisms are capable of degrading azo dyes including bacteria, yeast, actinomycetes, fungi, algae, and consortium of these microbes (Table 5.3). All these microorganisms have developed special enzyme systems for the discoloration and degradation of azo dyes under certain environmental conditions (Anjali et al. 2007).
5.2.1 Degradation Using Bacteria
Degradation of azo dyes using bacteria is normally nonspecific and faster (Sudha et al. 2014). Many aerobic and anaerobic bacteria such as Staphylococcus sp., Enterococcus sp., Bacillus subtilis, Rhabdobacter sp., Xenophilus sp., Clostridium sp., Klebsiella sp., Acinetobacter sp., and Pseudomonas sp. have been reported in many studies for the degradation of toxic azo dyes (Olukanni et al. 2006; Vijaykumar et al. 2007; Lin and Leu 2008). Most of the bacteria produces azoreductase enzyme for the degradation of N=N, and few bacteria show their activity in the presence of specific carbon and nitrogen sources (Caughlin et al. 2002). Bacterial degradation, when done individually under the aerobic or anaerobic condition, will show only the degradation, but there will not be any mineralization. Many researchers have experimentally proved that the combined effect or aerobic and anaerobic treatment methods can be an effective method (Feigel and Knackmuss 1993; Chen et al. 2003). Many reports are available on degradation of mixture of azo dyes using bacteria (Table 5.4). Kolekar and Kisan (2013) reported degradation of mixture of textile dyes using Shewanella sp. strain KMK6 isolated from soil sample contaminated with dyes. This study using bacteria showed a decrease in the color of the mixture of dye and chemical oxygen demand. Also the toxic mixture got converted into nontoxic degraded product. In another study, 87% of degradation in the mixture of dyes was observed using novel bacterial strain Lysinibacillus sp. RGS with a reduction within 48 h (Saratale et al. 2013). In a recent report, two bacterial isolates Bacillus sp. and Aeromonas hydrophila isolated from textile mill effluent showed more than 90% of Reactive Green and provisional pink dye within 5 days with a dye concentration of 50 mg/L (Parimala and Suruthi 2016). Few other strains of bacteria like Pseudomonas fluorescens and Shewanella have also been reported for degradation of azo dyes (Liu et al. 2013; Godlewska et al. 2014). In another study plant and bacterial synergistic systems were used for treatment of textile effluents, and their consortium was used for the degradation of mixture of dyes. This treatment method showed 100% degradation for the mixture of dyes (Kabra et al. 2013). Anoxic culture of Aeromonas hydrophila was isolated and selected as dye degrading bacteria at a pH of 5.5–10 and at an optimum temperature of 20–30 °C (Naik and Singh 2012). Degradation parameter of textile effluent showed color and chemical oxygen demand removal when treated with culture of Bacillus subtilis (Jayan et al. 2011). Mixture of seven different dyes with different chemical structures showed 87% of degradation using B. laterosporus within 24 h when provided an optimum temperature of 40 °C. This study also came up with a very less toxic end product (Kurade et al. 2011). Biodegradation of selected dyes Reactive Black 5, Reactive Orange 16, Disperse Red 78, and Direct Red 81 was reported using bacterial isolates Providencia rettgeri and Pseudomonas sp. In this study, both isolates showed 97–99% degradation of all dyes within 30 h at a concentration of 100 mg/L (Harshad et al. 2014). It has been reported that mixed culture of bacteria can give better results when compared with the results shown by individual isolates.
5.2.1.1 Degradation Using Bacterial Consortium
Many bacterial isolates showed good azo dye degradation when applied together as a consortium rather than individually. Many reports are available on the dye degrading assays using bacterial consortium. Nigam et al. (1996) reported for the first time the combined ability of M. luteus, Micrococcus sp., and P. polymyxa in the degradation of azo dyes but individually showed no degradation. Similar work was carried by Moosi et al. (2007) using the same three isolates isolated from contaminated sites. A consortium of four bacterial isolates such as P. putida, P. fluorescens, B. cereus, and S. acidaminiphila showed degradation of Acid Red 88 within 24 h, but when inoculated individually, each isolate took more than 72 h for degradation (Khehra et al. 2005). In an another study, the effect of isolates Klebsiella sp., Bacillus sp., and Clostridium sp. showed good degradation ability under aerobic condition, while the same consortium showed no change under anaerobic condition (Cui et al. 2012). The fungal and bacterial consortium also plays a very good role and shows the high effect in azo dye degradation. Aspergillus sp. and Pseudomonas sp. together detoxified Rubine dye within 30 h (Lade et al. 2012). The same consortium showed very promising results by degrading 98% of textile effluents which consist of reactive dyes, disperse azo dyes, and sulfate within 35 h (Lade et al. 2012).
5.2.2 Degradation Using Fungi
A wide variety of fungal organisms are capable of decolorizing a wide range of textile azo dyes. Many of these fungi are employed either in living or inactive forms. Degradation of azo dyes using fungi has an advantage as it is cost-effective and production of sludge is very less and environmentally friendly. Fungi possess a strong ability to degrade complex organic molecules by producing extracellular enzymes such as laccase and lignin peroxidase ; hence researchers are paying more attention toward fungi-mediated dye degradation (Sudip et al. 2016). The mechanism of fungal degradation involves adsorption and enzymatic degradation or combination of both. Recently Wang et al. (2017) reported decolorization and degradation of Congo Red using Ceriporia lacerata a newly isolated white rot fungus isolated from decayed mulberry branches. This study showed 90% degradation of Congo Red dye with 48 h when 3 g of mycelia was inoculated in 20 mL of 0.1 mg/mL concentration of Congo Red solution. In another study two endophytic fungi, Phlebia sp. and Paecilomyces formosus, showed decolorization of Reactive Blue 19 and Reactive Black 5. Both isolates showed degradation activity with 0.1 g/ml of dye solution after 30 days (Ligia et al. 2017). Anand et al. (2017) reported biodegradation of Malachite Green using Aspergillus flavus. Aspergillus flavus showed complete degradation of 150 mg/L of dye solution within 8 days in Kirk’s medium under static condition in the presence of sucrose and sodium nitrate as effective carbon and nitrogen sources, respectively. Table 5.5 depicts some of the different dye mixtures decolorized by fungal degradation.
5.2.3 Degradation Using Yeast
The growth rate of filamentous fungi is normally slow when compared with yeast; hence yeasts have an advantage over fungi from a biotechnological view for degradation of azo dyes. Yeast is a resilient microbe and is able to resist different environmental conditions like pH, organic wastewater, and high salt concentration. According to our literature survey, the first study presenting degradation of azo dyes by breaking N=N was published by Mecke and Schmahl (1957). However, this subject was actually brought into action after several years (Olteanu et al. 2008). Many reports available on yeast-mediated degradation are using Candida curvata and Geotrichum candidum with 90% and above degradation effect. Kluyveromyces marxianus showed the removal of diazo dye Remazol Black with 89% of degradation (Ertugrul et al. 2009). Similarly Candida catenulata and Candida kefyr degraded 90% of amaranth dye using biosorption techniques (Zeroual et al. 2007). S. cerevisiae and C. tropicalis are very active yeast isolates with the capacity of degrading more than one azo dye including Remazol Blue, Reactive Black, and Reactive Red. The action of these strains changes according to the dye concentration and exposure time (Aksu 2013; Donmez 2012). In a recent study, 12 out of the 44 isolated yeast colonies showed degradation; Reactive brilliant red K2 and those isolates were identified as S. cerevisiae, Torulopsis candida, and Saccharomycopsis lipolytica. Hence this feasible and metabolically versatile yeast should be considered for bioremediation process since a majority of yeast species have never been studied for azo dye degradation process.
5.3 Enzyme Involved in the Degradation of Azo Dyes
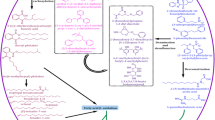
A number of microorganisms have been reported for the degradation of reactive azo dyes which include bacteria, yeast, fungi, and consortium of microorganisms and plants (Wesenberg et al. 2003; Olukanni et al. 2006). All these microbes have developed special enzyme systems for the degradation and discoloration of toxic azo dyes under suitable environmental conditions (Anjali et al. 2006). Although azo dyes have highly complex structural variations, they are degraded by a selected number of enzymes. Dye degrading enzymes are redox-active molecules which require a specific substrate for their action (Duran and Esposito 2000; Mester and Tien 2000). Microbes can either excrete the active enzymes into the used medium or the dye molecules move inside the microbial cell. Active enzymes are also potential in reducing or removing the toxicity from the dyes and effluents. The degrading capacity of microbes gets decreased by an increase in the concentration of dyes due to the microbial growth inhibition caused by the target molecules. To overcome this problem, we can extract the dye degrading enzymes from active microbes in bulk, and those enzymes can be used directly (Joshin and Chacko 2011). There are many reports on biologically synthesized dye degrading enzymes. Peroxidase , azoreductase, and laccases are the major and most promising enzymes involved in azo dye degradation (Abadulla et al. 2000). Azoreductase is a major and the most important group of enzyme synthesized from bacteria and fungi. The mechanism of these enzymes is reductive cleavage of azo bonds and converting them into colorless aromatic amines (Pandey et al. 2007). Figure 5.4 shows the proposed mechanism for the degradation of azo dyes using azoreductase under anaerobic condition. In intracellular and extracellular sites of the bacterial cell wall, the reducing molecules such as NADH, NADPH, and FADH2 help in the breaking of N=N (Zimmermann et al. 1982 and Zimmermann et al. 1984), while azoreductase plays a major role in the degradation process of bacteria, viz., Escherichia coli, Staphylococcus aureus, Rhodobacter sphaeroides, Enterococcus faecalis, and Bacillus sp. (Blumel and Stolz 2003, Yan et al. 2004 and Chen et al. 2005). Azoreductase enzyme extracted from E. faecalis YZ66 was able to degrade sulfonated azo dye Direct Red 18 and also detoxified their toxic effect (Sahasrabudhe et al. 2014). On the other hand, degradation of azo dyes using fungi generates two types of enzymes that are peroxidases and phenol oxidase (Ramya et al. 2010). Peroxidase enzymes are catalyzed in the presence of hydrogen peroxide (Fig. 5.5). These heme peroxidases are divided into different groups based on the organisms produced, substrate, and primary structure (Gumiero et al. 2010). The oxidative process of H2O2 which is catalyzed by chloroperoxidase was used for the degradation of azo dyes such as Orange G and S Yellow (Zhang et al. 2012). Lignin peroxidase enzyme isolated from Tagetes patula for the degradation of Reactive Blue 160 was reported by Patil and Jadha (2013). Another major enzyme that helps in the degradation of azo dyes is laccase enzyme (Fig. 5.6). These enzymes are also known as multicopper oxidase enzymes (MCO) as it belongs to the family of copper-containing polyphenol oxidases (Birhanli and Yesilada 2006; Arora and Sharma 2010; Giardina et al. 2010). Bertrand (1985) discovered laccase from the sap of a tree, Rhus vernicifera. Husain (2006) reported for the first time the importance of laccase enzyme in the degradation of textile color effluent. The major property of this enzyme that makes it a best azo dye degrading agent is its nonspecific oxidation capacity, a non-requirement of cofactors, and they do not require oxygen as an electron acceptor (Kalyani et al. 2012). In a recent study, laccase enzyme synthesized from white rot fungus, Ganoderma lucidum BCR 36123, showed 90% and above degradation azo dye Acid Orange AO7 (Chin et al. 2017). Purified laccases from mushroom Hypsizygus ulmarius showed degradation of azo dye Methyl Orange without using any redox mediator (Ravikumar et al. 2013). Enzymatic degradation of azo dyes also has significant potential to solve this problem due to their eco-friendly, inexpensive nature and also due to less production of sludges. Enzymatic processes are very promising for the degradation of toxic azo dyes; hence these enzymes can be considered as a molecular weapon for bioremediation of these dyes.
Proposed mechanism for the degradation of azo dyes by azoreductase by converting toxic chromophore group N=N into nontoxic NH2. (Courtesy: Keck et al. 1997)
Proposed mechanism for alternative asymmetrical and symmetrical cleavage of sulfonated azo dye by peroxidase enzymes generated from fungi. (Courtesy: McMullan et al. 2001)
Proposed mechanism of degradation using laccase enzyme, another major enzyme that helps in the degradation of azo dyes. (Courtesy: Andrea et al. 2005)
5.4 Nanoparticle-Mediated Photocatalytic Degradation
Photocatalytic degradation involves acceleration of a photoreaction in the presence of a catalyst. Light energy is absorbed by a provided semiconducting material which helps in the degradation of dyes. The concept of photocatalytic degradation using TiO2 as a substrate for water decomposition was brought by Akira and Honda (1972), which is known as Honda-Fujishima effect. One of the advanced methods that are used for degradation of azo dyes is photocatalytic degradation (Zhao and Zhang 2008). The concept of nanoparticle-mediated degradation is very simple: the semiconducting materials absorb light of equal or more energy that will lead to the generation of electrons. These electrons can then further generate free radicals for the oxidation of substrates (organic matters). In many previous studies, this method has been broadly and highly explored (Yizhong 2000; Loannis and Triantafyllos 2004). Andrea et al. (2014) reported UV-induced degradation of Methyl Red and Methyl Orange azo dyes in the presence of TiO2 nanoparticles which was immobilized at the bottom of the effluent passing channel. The concept of azo dye degradation using nanoparticles and photocatalysis is related to each other. Thermally active zinc oxide was used in a study for photocatalytic degradation of Congo Red, where the system showed 96% of degradation (Tapas and Naba 2014). In a recent report, ferric oxide Gallic nanostructures were used for the degradation of azo dyes. The nanostructure synthesized by two different ways was subjected to photocatalysis, and degradation percentage was compared (Minoo and Ali 2016). All these studies used chemically synthesized nanoparticles for azo dye degradation. Although these methods reduce the toxicity by a certain extent by breaking the azo bond, they can sustain moderate toxicity which may be due to the presence of nanoparticles. However, the toxic effect is less when compared with the chemical methods for effluent treatment . To overcome the abovementioned problem, researchers started green synthesis of nanoparticles and used them in azo dye degradation. Priyaragini et al. (2014) showed 84% and 85% degradation of Acid Red 79 and Acid Red 80, respectively, using marine actinobacterial-mediated TiO2 nanoparticles. Photocatalytic degradation of Rhodamine Blue in the presence of actinobacterial-mediated TiO2 nanoparticles showed 95% and above degradation effect (Veena et al. 2016). Similarly in another study, UV and solar photocatalytic degradation of azo dyes and dye effluents in different time intervals was tested using the crude extract of coconut-mediated silver nanoparticles (Mariselvam et al. 2016). Photocatalytic degradation of azo dye can be considered as an easy approach, however it is time consuming and effective method when compared to all other techniques. Photocatalytic activity was used first for self-cleaning property by Akira Fujishima (1972), and now it is used in the process of cleaning our environment and protects it from becoming toxic for the coming generations. When we talk about nanoparticle-mediated azo dye degradation, we should focus on biologically synthesized nanoparticles rather than going with chemically synthesized nanoparticles, since our purpose is to remove toxicity completely without leaving a single trace of toxic compounds. Also, the green synthesis of nanoparticles will make azo dye degrading product cheaper when compared with chemically synthesized nanoparticles. Hence, it can be concluded that nanoparticle-mediated photocatalytic degradation of azo dye is an easy, economical, fast, and eco-friendly technique.
5.5 Degraded Compounds and Their Toxicity
Degradation and decolorization of azo dyes only are not enough; emphasis should also be given to verify the detoxification of azo dyes as well. The degraded dye should break down into nontoxic compounds. After degradation, analysis researchers should focus whether the highly toxic azo dyes got converted to nontoxic compounds or not, and also the reduction in the toxicity levels can be checked. In this field the first attempt was done by removing the toxicity and mutagenicity of direct red in the presence of B. velezensis strain (Bafana et al. 2008). Toxic Remazol Black B was converted into nontoxic derivatives by using Zinnia angustifolia and Exiguobacterium aestuarii, a plant and bacterial remediation, respectively (Khandare et al. 2012). In another study, toxicity evaluation was done using Daphnia magna under microaerophilic process (Harshad et al. 2015). Here complete detoxification of all the selected textile azo dyes was observed. Few reports are available on toxicity removal by a combined effect of ozonation and biofilm reactor. Toxicity of azo dye solution decreased within 2 min when subjected to ozonation, but toxicity increased when kept for longer time. Along with the removal of toxicity, evaluation of toxicity is also a necessary process. Biotoxicity and phytotoxicity assays are two majorly used assays to evaluate the toxicity of degraded compounds. In many reported works, biotoxicity assay was done using brine shrimp eggs, and the test is known as brine shrimp hatchability test. This test is used widely since it uses convenient organisms for the evaluation of toxicity and is a simple and inexpensive method. In a study less toxic nature of degraded dye was evaluated by observing the survival of 50% of brine shrimp eggs at a much higher concentration than that of the azo dyes (Arun and Bhaskara Rao 2012). In a recent report, toxicity evaluation of degraded azo dye Direct Yellow 4 was reported using phytotoxicity assay. Here phytotoxicity assay showed a considerable decrease in the toxicity of degraded dyes when compared with the pure dye (Shazia et al. 2017). Mutagenicity, cytotoxicity, and phytotoxicity of biodegraded textile effluent using fungal ligninolytic enzyme have been evaluated in a recent report (Muhammad et al. 2016). The cytotoxicity (Allium cepa, Daphnia magna, and brine shrimp), phytotoxicity (Triticum aestivum), and mutagenicity study using Ames test revealed that biodegradation of textile effluent using fungal-mediated enzymes detoxifies the toxic compounds present.
5.6 Future Perspective
Wastewater discharge by textile industries has become a great environmental concern for scientists because of the prevailing hazards in our ecosystem. Accumulation of industrial dyestuffs and dye wastewater not only creates environmental pollution, but it can also lead to medical problems and problems in the exquisiteness of our environment. There should be technically possible and cost-effective treatment methods for the removal of these toxic dyestuffs from the environment since in the present world environment regulations are becoming even stricter. Dye degradation using microbes bears a significant potential in solving these problems since microbes and their products are eco-friendly, inexpensive, and easily available. This review clearly stated the importance of microbial dye degradation, nanoparticle -based degradation, photocatalytic degradation, enzymatic degradation, and toxicity of degraded compounds. As an emerging technique, using microbes and their mediated nanoparticles is an eco-friendly, less expensive, and easy way to degrade toxic dyes and to remove toxicity from our environment . Lab-scale work will be entirely different when it reaches to the industrial level. Microbial degradation of azo dyes should be focused using small-scale effluent treatment fermenter designing which later can be applied to different textile industries to treat these toxic dye-filled effluents. Similarly, all techniques should be studied with a design so that they can be applied at the industrial level. Industries should get involved with universities or research institutes to carry out these lab works to the next level. The enzyme responsible for degradation of these toxic dyes should be produced in a large amount with the help of industries and should be brought in action as soon as possible at least for small-scale textile industries or dyeing units. Also, there’s a need to formulate the effective product that can be delivered to remote places. These dyes get concentrated at the end of the food chain and lead to severe medical problems such as tumor, cancer, asthma, nervous disorder, and even death. So to avoid these entire problems and to protect our environment, textile effluents have to be free from toxic azo dyes and its toxicity before it reaches the environment.
5.7 Conclusion
Azo dyes constitute the largest and most versatile class of synthetic dyes used in a variety of industries including textile, pharmaceutical, food and cosmetics industries and represent major components in wastewater from these industrial dyeing processes. The presence of dyes imparts an intense color to effluents which leads to environmental as well as aesthetic problems. Many researchers are working on degradation of azo dyes; however, there is still a need to generate relative performance data on industrial effluents. Hence this review concludes that azo dye degradation is an extremely serious topic to be focused on, and it can be done using microbes, nanoparticles, and photocatalytic methods. As we are aware of the effect of water scarcity in our country, wastewater treatment is an issue that should be taken into consideration. Also, it’s our duty to keep our environment clean and to protect our natural resources from all toxic compounds. Azo dye also comes in the list of toxic compounds or environmental pollution-causing products; hence removal of these toxic dyes using all motioned techniques will help in the process of keeping our environment clean and healthy.
References
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco Paulo A, Gubitz GM (2000) Decolourization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol 66:3357–3362. https://doi.org/10.1128/AEM.66.8.3357-3362.2000
Akira F, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38. https://doi.org/10.1038/238037a0
Aksu Z (2013) Reactive dye bioaccumulation by Saccharomyces cerevisiae. Process Biochem 38:1437–1444. https://doi.org/10.1016/S0032-9592(03)00034-7
Amaral PFF, Fernandes DLA, Tavares APM, Xavier ABMR (2004) Decolorization of dyes from textile wastewater by Trametes versicolor. Environ Technol 25:1313–1320. https://doi.org/10.1080/09593332508618376
Ambrosio ST, Takaki GMC (2004) Decolorization of reactive azo dyes by Cunninghamella elegans UCP 542 undergo-metabolic conditions. Bioresour Technol 91:69–75. https://doi.org/10.1016/S0960-8524(03)00153-6
Ambrosio ST, Jose C, Junior V, Carlos A, Alves da S, Kaoru O, Aline E, Nascimento RLL, Galba MCT (2012) A biosorption isotherm model for the removal of reactive Azo dyes by inactivated mycelia of Cunninghamella elegans UCP542. Molecules 17:452–462. https://doi.org/10.3390/molecules17010452
Anand B, Keshaw RA, Harit J (2017) Biodegradation of malachite green by the ligninolytic fungus Aspergillus flavus. Clean Soil Air Water 45(4):1600045. https://doi.org/10.1002/clen.201600045
Andrea Z, Barbara G, Astrid R, Artur CP (2005) Degradation of Azo dyes by Trametes villosa Laccases over long periods of oxidative conditions. Appl Environ Microbiol 2:6711–6718. https://doi.org/10.1021/ie403506s
Andrea P, Giancarlo B, Mario P, Piero M, Valentina P, Domenico P (2014) Photocatalytic degradation of Azo dyes. Pilot plant investigation. Ind Eng Chem Res 53:2566–2257. https://doi.org/10.1021/ie403506s
Anjali P, Singh P, Iyengar L (2006) Review on bacterial decolourization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84. https://doi.org/10.1016/j.jtice.2010.06.006
Anjali P, Poonam S, Leela I (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84. https://doi.org/10.1016/j.ibiod.2006.08.006
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160:1760–1788. https://doi.org/10.1007/s12010-009-8676-y
Arun PAS, Bhaskara Rao KV (2012) Aerobic biodegradation of Azo dye by Bacillus cohnii MTCC 3616; an obligately alkaliphilic bacterium and toxicity evaluation of metabolites by different bioassay systems. Appl Microbiol Biotechnol 35:235–241. https://doi.org/10.1007/s00253-012-4492-3
Ayed L, Mahdhi A, Cheref A, Bakhrouf A (2011) Decolorization and degradation of azo dye methyl red by an isolated Sphingomonas paucimobilis: biotoxicity and metabolites characterization. Desalination 274:272–277. https://doi.org/10.1016/j.desal.2011.02.024
Baban A, Yediler A, Lienert D, Kemerdere N, Kettrup A (2003) Ozonation of high strength segregated effluents from a woollen textile dyeing and finishing plant. Dyes Pigments 58:93–98. https://doi.org/10.1016/S0143-7208(03)00047-0
Babu BR, Parande AK, Raghu S, Kumar TP (2007) Cotton textile processing: waste generation and effluent treatment. J Cotton Sci 11:141–153. http://journal.cotton.org
Bafana A, Chakrabarti T, Devi SS (2008) Azoreductase and dye detoxification activities of Bacillus velezensis strain AB. Appl Microbiol Biotechnol 77:1139–1144. https://doi.org/10.1007/s00253-007-1212-5
Ben MH, Ayed AY, Mosrati R, Corroler D, Ghedira K, Barillier D, Chekir GL (2010) Acid Violet 7 and its biodegradation products induce chromosome aberrations, lipid peroxidation, and cholinesterase inhibition in mouse bone marrow. Environ Sci Pollut Res Int 177:1371–1378. https://doi.org/10.1007/s11356-010-0323-1
Bertrand G (1985) Sur la laccase et sur le pouvoir oxydant de cette diastase. Comp Rendus L’Acad Sci 120:266–269. journal.cotton.org
Birhanli E, Yesilada O (2006) Increased production of laccase by pellets of Funalia trogii ATCC 200800 and Trametes versicolor ATCC 200801 in repeated-batch mode. Enzym Microb Technol 39:1286–1293. https://doi.org/10.1016/j.enzmictec.2006.03.015
Blumel S, Stolz A (2003) Cloning and characterization of the gene coding for the aerobic azoreductase from Pigmentiphaga kullae K24. Appl Microbiol Biotechnol 62:186–190. https://doi.org/10.1007/s00253-003-1316-5
Cerniglia CE, Zhuo Z, Manning BW, Federle TW, Heflich RH (1982) Mutagenic activation of the benzidine based dye direct black 38 by human intestinal microflora. Mutat Res 175:11–16. https://doi.org/10.1016/0165-7992(86)90138-7
Chen K, Wu J, Liou D, Hwang SJ (2003) Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol 101:57–68. https://doi.org/10.1016/S0168-1656(02)00303-6
Chen H, Hopper SL, Cerniglia CE (2005) Biochemical and molecular characterization of an azoreductase from Staphylococcus aureus a tetrameric NADPH dependent flavoprotein. Microbios 151:1433–1441. https://doi.org/10.1099/mic.0.27805-0
Chequer FMD, Angeli JPF, Ferraz ERA, Tsuboy MS, Marcarini JC, Mantovani MS, Oliveira DP (2009) The Azo dyes disperse red 1 and disperse orange 1 increase the micronuclei frequencies in human lymphocytes and in HepG2 cells. Mutat Res 676:83–86. https://doi.org/10.1016/j.mrgentox.2009.04.004
Chin YL, Chinh HW, Chui TM, Chi W (2017) Decolorization of azo dye and generation of electricity by microbial fuel cell with laccase-producing white-rot fungus on cathode. Appl Energy 188:392–198. https://doi.org/10.1016/j.apenergy.2016.12.044
Cooper P (1993) Removing color from dye house wastewaters a critical review of technology available. J Soc Dyers Col 109:97–101. https://doi.org/10.1111/j.1478-4408.1993.tb01536
Coughlin MF, Kinkle BK, Bishop PL (2002) Degradation of acid orange 7 in an aerobic biofilm. Chemosphere 46:11–19. https://doi.org/10.1016/S0045-6535(01)00096-0
Cui D, Li G, Zhao D, Gu X, Wang C, Zhao M (2012) Microbial community structures in mixed bacterial consortia for azo dye treatment under aerobic and anaerobic conditions. J Hazard Mater 222:185–192. https://doi.org/10.1016/j.jhazmat.2012.04.032
Dhaneshvar N, Ayazloo M, Khatae AR, Pourhassan M (2007) Biological decolorization of dye solution containing malachite green by microalgae Cosmarium sp. Bioresour Technol 29:1–7. https://doi.org/10.1016/j.biortech.2006.05.025
Donmez G (2012) Bioaccumulation of the reactive textile dyes by Candida tropicalis growing in molasses medium. Enzym Microb Technol 30:363–366. https://doi.org/10.1016/S0141-0229(01)00511-7
Duran N, Esposito E (2000) Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review. Appl Catal B Environ 28:83–99. https://doi.org/10.1016/S0926-3373(00)00168-5
Ertugrul S, San NO, Donmez G (2009) Treatment of dye (Remazol Blue) and heavy metals using yeast cells with the purpose of managing polluted textile wastewaters. Ecol Eng 35:128–134. https://doi.org/10.1016/j.ecoleng.2008.09.015
Feigel BJ, Knackmuss HJ (1993) Syntrophic interactions during degradation of 4-aminobenzenesulfonic acid by a two species bacterial culture. Arch Microbiol 159:124–132. https://doi.org/10.1007/BF00250271
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385. https://doi.org/10.1007/s00018-009-0169-1
Godlewska EZ, Przystas W, Sota EG (2014) Decolorisation of different dyes by two pseudomonas strains under various growth conditions. Water Air Soil Pollut 225:1–13. https://doi.org/10.1007/s11270-013-1846-0
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8:553–597. https://doi.org/10.1016/S1093-0191(03)00031-5
Grekova VM, Popov I, Vassilev D, Topalova Y (2012) Isolation and characterisation of microbial strain AZO29 capable of azo dye decolourization. Biotechnol Biotechnol Eq, XI anniversary scientific conference special edition on-line, pp 318–322. https://doi.org/10.1080/13102818.2009.10818428
Gumiero A, Murphy EJ, Metcalfe CL, Moody PCE, Raven EL (2010) An analysis of substrate binding interactions in the heme peroxidase enzymes: a structural perspective. Arch Biochem Biophys 500:13–20. https://doi.org/10.1016/j.abb.2010.02.015
Gurulakshmi M, Sudarmani DNP, Venba R (2008) Biodegradation of leather acid dye by Bacillus subtilis. Adv Biotech 7:12–18. https://doi.org/10.1016/j.abb.2010.02.015
Harazono K, Nakamura K (2005) Decolorization of mixtures of different reactive textile dyes by the white rot basidiomycete Phanerochaete sordida and inhibitory effect of polyvinyl alcohol. Chemosphere 59:63–68. https://doi.org/10.1016/j.chemosphere.2004.09.104
Harshad L, Avinash K, Diby P, Govindwa SP (2014) Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J 14:158–174. https://doi.org/10.17179/excli2014-642
Harshad L, Sanjay G, Diby P (2015) Low-cost biodegradation and detoxification of textile azo dye C.I. reactive blue 172 by Providencia rettgeri strain HSL1. J Chem. https://doi.org/10.1155/2015/894109
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26:201–221. https://doi.org/10.1080/07388550600969936
Idris A, Suhaimi MS, Zain NAM, Rashid R, Othman N (2014) Discoloration of aqueous textile dyes solution by Phanerochaete chrysosporium immobilized in modified PVA matrix. Desalin Water Treat 52:6694–6702. https://doi.org/10.1080/19443994.2013.819148
Jadhav JP, Govindwar SP (2007) Biotransformation of malachite green by Saccharomyces cerevisiae MTCC 463. Yeast 23:316–323. https://doi.org/10.1002/yea.1356
Jayan MA, Maragatham NR, Saravanan J (2011) Decolorization and physicochemical analysis of textile azo dye by Bacillus. Int J Appl Bioeng 5:35–39. https://doi.org/10.18000/ijabeg.10076
Jeong E (2008) Synthesis, mutagenicity and metabolism of substituted 4, 4′ aminoalkoxyazobenzene dyes. Pro Quest LLC:15–14. https://doi.org/10.1016/j.dyepig.2010.03.002
Jirasripongpun K, Nasanit R, Niruntasook J, Chotikasatian B (2007) Decolorization and degradation of C. I. reactive red by Enterobacter sp. Thammasat Int J Sci Tech 12:6–11. doi:123456789/13751/1/IJEB
Joshi SM, Inamdar SA, Telke AA, Tamboli DP, Govindwar SP (2010) Exploring the potential of natural bacterial consortium to degrade mixture of dyes and textile effluent. Int Biodeterior Biodegrad 64:622–628. https://doi.org/10.1016/j.ibiod.2010.07.001
Joshin T, Chacko KS (2011) Enzymatic degradation of azo dyes – a review. Int J Environ Sci 1:1250–1260. https://doi.org/10.1016/j.ibiod.2015.04.027
Kabra AN, Khandare RV, Govindwar SP (2013) Development of a bioreactor for remediation of textile effluent and dye mixture: a plant–bacterial synergistic strategy. Water Res 47:1035–1048. https://doi.org/10.1016/j.watres.2012.11.007
Kalyani DC, Patil PS, Jadhav JP, Govindwar SP (2007) Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp SUK1. Bioresour Technol 99:4635–4641. https://doi.org/10.1016/j.biortech.2007.06.058
Kalyani D, Dhiman SS, Kim H, Jeya M, Kim IW, Lee JK (2012) Characterization of a novel laccase from the isolated Coltricia perennis and its application to detoxification of biomass. Process Biochem 47:671–678. https://doi.org/10.1016/j.procbio.2012.01.013
Keck A, Klein J, Kudlich M, Stolz A, Knackmuss HJ, Mattes R (1997) Reduction of azo dyes by redox mediators originating in the naphthalene sulfonic acid degradation pathway of Sphingomonas sp strain BN6. Appl Environ Microbiol 63:3684–3690. PMCID: PMC168674
Keharia H, Madamwar D (2003) Bioremediation concepts for treatment of dye containing water: a review. Indian J Exp Biol 41:1061–1068. 123456789/17164
Khandar R, Rane NR, Waghmode TR, Govindwar SP (2012) Bacterial assisted phytoremediation for enhanced degradation of highly sulfonated diazo reactive dye. Environ Sci Pollut Res 19:1709–1718. https://doi.org/10.1007/s11356-011-0679-x
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2005) Decolorization of various azo dyes by bacterial consortium. Dyes Pigments 67:55–61. https://doi.org/10.1016/j.dyepig.2004.10.008
Kolekar YM, Kisan MK (2013) Decolorization of textile dye by Alishewanella sp. KMK6. Appl Microbiol Biotechnol 95:521–529. https://doi.org/10.1007/s00253-011-3698-0
Kolekar YM, Powar SP, Gawai KR, Lokhande PD, Shouche YS, Kodam KM (2008) Decolorization and degradation of disperse blue 79 and acid orange 10, by Bacillus fusiformis KMK5 isolated from the textile dye contaminated soil. Bioresour Technol 99:8899–9003. https://doi.org/10.1016/j.biortech.2008.04.073
Kolekar YM, Konde PD, Markad VL, Kulkarni SV, Chaudhari AU (2013) Effective bioremoval and detoxification of textile dye mixture by Alishewanella sp. KMK6. Appl Microbiol Biotechnol 97:881–889. https://doi.org/10.1007/s00253-012-3983-6
Kuhad RC, Sood N, Tripathi KK, Singh A, Ward OP (2004) Developments in microbial methods for the treatment of dye effluents. Adv Appl Microbiol 56:185–213. https://doi.org/10.1016/S0065-2164(04)56006-9
Kurade MB, Waghmode TR, Govindwar SP (2011) Preferential biodegradation of structurally dissimilar dyes from a mixture by Brevibacillus laterosporus. J Hazard Mater 192:1746–1755. https://doi.org/10.1016/j.jhazmat.2011.07.004
Lade HS, Waghmode TR, Kadam AA, Govindwar SJ (2012) Enhanced biodegradation and detoxification of disperse azo dye Rubine GFL and textile industry effluent by defined fungal bacterial consortium. Int Biodeterior Biodegrad 72:94–107. https://doi.org/10.1016/j.ibiod.2012.06.001
Lang, W, Sirisansaneeyakul S, Ngiwsara L, Mendes S, Martins LO, Okuyama M, Kimura A (2013) Characterization of a new oxygen-insensitive azo reductase from Brevibacillus laterosporus TISTR1911: toward dye decolorization using a packed-bed metal affinity reactor. Bioresour Technol 67:150, 298. https://doi.org/10.1016/j.biortech.2013.09.124
Li WY, Chen FF, Wang SL (2010) Binding of reactive brilliant red to human serum albumin: insights into the molecular toxicity of sulfonic azo dyes. Protein Pept Lett 175:621–629. https://doi.org/10.2174/092986610791112756
Ligia MCB, Julio CP, Ana LB, Vanessa K, Joao LA, Joao AP (2017) Activity of the endophytic fungi Phlebia sp. and Paecilomyces formosus in decolourisation and the reduction of reactive dyes’ cytotoxicity in fish erythrocytes. Environ Monit Assess 189:88–95. https://doi.org/10.1007/s10661-017-5790-0
Lin YH, Leu JY (2008) Kinetics of reactive azo-dye decolorization by Pseudomonas luteola in a biological activated carbon process. Biochem Eng J 39:457–467. https://doi.org/10.1016/j.bej.2007.10.015
Liu G, Zhou J, Meng X, Fu SQ, Wang J, Jin R, Lv H (2013) Decolorization of azo dyes by marine Shewanella strains under saline conditions. Appl Microbiol Biotechnol 97:4187–4197. https://doi.org/10.1016/j.bej.2007.10.015
Loannis K, Triantafyllos A (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations. J Appl Catal B 49:1–14. https://doi.org/10.1016/j.apcatb.2003.11.010
Machado KM, Compart LC, Morais RO, Rosa LH, Santos MH (2006) Biodegradation of reactive textile dyes by basidiomycetes fungi from Brazilian ecosystems. Braz J Microbiol 37:481–487. https://doi.org/10.1590/S1517-83822006000400015
Maier J, Kandelbauer A, Erlacher A, Cavaco PA, Gubits GM (2004) A new alkali – thermostable azoreductase from bacillus sp. strain SF. Appl Environ Microbiol 70:837–844. https://doi.org/10.1128/AEM.70.2.837-844.2004
Mariselvam R, Ranjitsingh AJA, Mosae SP, Abdullah AA, Murugan AM (2016) Spectral studies of UV and solar photocatalytic degradation of azo dye and textile dye effluents using green synthesized silver nanoparticles. Bioinorg Chem Appl 6:1–8. https://doi.org/10.1155/2016/8629178
McMullan G, Meehan C, Conneely A, Kirby N, Robinson T, Nigam P, Banat IM, Merchant R, Smyth WF (2001) Microbial decolorization and degradation of textile dyes. Appl Microbiol Biotechnol 56:81–87. https://doi.org/10.1007/s002530000587
Mecke R, Schmahl D (1957) Die spaltbarkeit der azo-bru¨cke durch hefe (Cleavage of azo bridge links by yeast). Arzneim-Forsch 7:335–340. https://doi.org/10.1007/698_2009_50
Meehan G, Banat IM, Mcmullan G, Nigum P, Smyth F, Marchant R (2000) Decolorization of Remazol black-B using a thermotolerant yeast, Kluyveromyces marxianus IMB3. Environ Int 26:75–79. https://doi.org/10.1016/S0160-4120(00)00084-2
Mester T, Tien M (2000) Oxidative mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants. Int Biodeterior Biodegrad 46:51–59. https://doi.org/10.1016/S0964-8305(00)00071-8
Minoo B, Ali RM (2016) Template assisted fast photocatalytic degradation of azo dye using ferric oxide–gallia nanostructures. RSC Adv 6:87555–87563. https://doi.org/10.1039/C6RA16317C
Molina GJM, Perez J, Manoz DJ, Guillen F, Moya R, Hernandez M, Arias ME (2009) Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int Microbiol 12:13–21 19440979
Moosvi S, Kher X, Madamwar X (2007) Isolation, characterization and decolorization of textile dyes by a mixed bacterial consortium JW-2. Dyes Pigments 74:723–729. https://doi.org/10.1016/j.dyepig.2006.05.005
Muhammad B, Munawar I, Hongbo H, Xuehong Z (2016) Mutagenicity, cytotoxicity and phytotoxicity evaluation of biodegraded textile effluent by fungal ligninolytic enzymes. Water Sci Technol 73:2332–2344. https://doi.org/10.2166/wst.2016.082
Naik C, Singh CR (2012) Isolation screening and development of Bacillus sp with decolorization and degradation capabilities towards reactive dyes and textile effluents. Recent Res Sci Technol 4:1–5. https://doi.org/10.1155/2015/628195
Nicholson PT, Shaw I (2000) Ancient Egyptian materials and technology. Cambridge University Press, Cambridge
Nigam P, Banat IM, Singh D, Marchant R (1996) Microbial process for the decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochem 31:435–442. https://doi.org/10.1016/0032-9592(95)00085-2
Nikulina GL, Deveikis DN, Pyshnov G (1995) Toxicity dynamics of anionic dyes in air of a work place and long term effects after absorption through skin. Med Tr Prom Ekol 6:25–28. https://doi.org/10.3923/jest.2016.188.197
Nordstrom F, Terrazas E, Welander U (2008) Decolorization of a mixture of textile dyes using Bjerkandera sp. Bol 13 Environ Tech 29:921–929. https://doi.org/10.1080/09593330802131628
Olteanu Z, Rosu CM, Mihasan M 2008 Preliminary consideration upon oxide-reductive system involved in aerobic biodegradation of some textile dyes. Analele Ştiinţifice ale Universităţii, Alexandru Ioan Cuza, Secţiunea Genetică şi Biologie Moleculară, TOM IX, pp 41–46 44
Olukanni OD, Osuntoki AA, Gbenle GO (2006) Textile effluent biodegradation potentials of textile effluent-adapted and non-adapted bacteria. Afr J Biotechnol 5:1980–1984. https://doi.org/10.12691/jaem-2-5-6
Ozer A, Gonul A, Meral T (2006) The removal of acid red 274 from wastewater: combined biosorption and biocoagulation with Spirogyra rhizopus. Dyes Pigments 71:83–89. https://doi.org/10.1016/j.dyepig.2005.06.004
Pakshi RK, Singh S (2010) Decolorization of synthetic wastewater containing azo dyes in a batch operated rotating biological contactor reactor with the immobilized fungus phanerochaete chrysosporium. Ind Eng Chem Res 49:7484–7487. https://doi.org/10.1021/ie1007079
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84. https://doi.org/10.1016/j.ibiod.2006.08.006
Parimala MC, Suruthi S (2016) Textile dye degradation using bacterial strains isolated from textile mill effluent. Int J Appl Res 2:337–341
Parshetti G, Kalme S, Saratale G, Govindwar S (2006) Biodegradation of malachite green by Kocuria rosea MTCC 1532. Acta Chim Slov 53:492–498. https://www.researchgate.net/profile/Ganesh_Saratale/publication/279903607
Patil AV, Jadha JP (2013) Evaluation of phytoremediation potential of Tagetes patula L. for the degradation of textile dye reactive blue 160 and assessment of the toxicity of degraded metabolites by cytogenotoxicity. Chemosphere 92:225–232. https://doi.org/10.1016/j.chemosphere.2013.01.089
Priyaragni S, Veena S, Swetha D, Karthik L, Kumar G, Bhaskara RKV (2014) Evaluating the effectiveness of marine actinobacterial extract and its mediated titanium dioxide nanoparticles in the degradation of azo dyes. J Environ Sci 26:1–8. https://doi.org/10.1016/S1001-0742(13)60470-2
Przystas W, Godlewska EZ, Grabinska ES (2013) Effectiveness of dyes removal by mixed fungal cultures and toxicity of their metabolites. Water Air Soil Pollut 224:1–9. https://doi.org/10.1007/s11270-013-1534-0
Puvaneswari N, Muthukrishnan J, Gunasekaran P (2006) Toxicity assessment and microbial degradation of azo dyes. Indian J Exp Biol 44:618–626. PMID: 16924831
Ramya M, Iyappan S, Manju A, Jiffe JS (2010) Biodegradation and decolorization of acid red by Acinetobacter radioresistens. Appl Environ Microbiol 70:837–844. https://doi.org/10.4172/2155-6199.1000105
Rani C, Jana AK, Bansal A (2009) Studies on the biodegradation of azo dyes by white rot fungi Phlebia radiata. In: Proceedings of international conference on energy and environment, pp 203–207
Ravikumar G, Kalaiselvi M, Gomathi D, Vidhya B, Devaki K, Uma C (2013) Effect of laccase from Hypsizygus ulmarius in decolorization of different dyes. J Pharm Sci 3:150–152. https://doi.org/10.7324/JAPS.2013.30128
Reid TM, Morton KC, Wang CY, King CM (1984) Mutagenicity of azo dyes following metabolism by different reductive/oxidative systems. Environ Mutagen 65:705–717. https://doi.org/10.1002/em.2860060508
Sahasrabudhe MM, Saratale RG, Saratale GD, Pathade GR (2014) Decolorization and detoxification of sulfonated toxic diazo dye C.I. direct red 81 by Enterococcus faecalis YZ66. J Environ Health Sci Eng 12:151–163. https://doi.org/10.1186/s40201-014-0151-1
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157. https://doi.org/10.1016/j.jtice.2010.06.006
Saratale RG, Gandhi SS, Purankar MV, Kurade MB (2013) Decolorization and detoxification of sulfonated azo dye CI Remazol red and textile effluent by isolated Lysinibacillus sp. RGS. J Biosci Bioeng 115:658–667. https://doi.org/10.1016/j.jbiosc.2012.12.009
Sharma P, Lakhvinder S, Dilboghi N (2009) Biodegradation of orange II dye by Phanerochaete chrysosporium in simulated wastewater. J Sci Ind Res 68:157–161. 123456789/2914
Shazia N, Haq NB, Munawar I, Ismat B, Shagufta K, Sana S, Misbah S, Abida K, Yusra S (2017) By-product identification and phytotoxicity of biodegraded direct yellow 4 dye. Chemosphere 169:474–484. https://doi.org/10.1016/j.chemosphere.2016.11.080
Solıs M, Solıs A, Perez HI, Manjarrez N, Flores M (2012) Microbial decolouration of azo dyes: a review. Process Biochem 47:1723–1748. https://doi.org/10.1016/j.procbio.2012.08.014
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56:69–80. https://doi.org/10.1007/s002530100686
Subramani AK, Byrappa KS, Ananda KM, Rai L, Ranganathaiah C, Yoshimura M (2007) Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon. Mater Sci 30:37–41. https://doi.org/10.1007/s12034-007-0007
Sudha M, Saranya A, Selvakumar G, Sivakumar N (2014) Microbial degradation of azo dyes: a review. Int J Cur Microbiol Appl Sci 3:670–690. https://doi.org/10.1007/s11274-012-1198-8
Sudip KS, Sumita R, Partha B, Sangeeta R (2016) Fungal decolouration and degradation of azo dyes: a review. Fungal Biol Rev 30:112–133. https://doi.org/10.1016/j.fbr.2016.06.003
Taha M, Adetutu EM, Shahsavari E, Smith AT, Ball AS (2014) Azo and anthraquinone dye mixture decolourization at elevated temperature and concentration by a newly isolated thermophilic fungus, Thermomucorindicae seudaticae. J Environ Chem Eng 2:415–423. https://doi.org/10.1016/j.jece.2014.01.015
Tamboli DP, Kurade MB, Waghmode TR, Joshi SM, Govindwar SP (2010) Exploring the ability of Sphingobacterium sp. ATM to degrade textile dye direct blue GLL, mixture of dyes and textile effluent and production of polyhydroxy hexadecanoic acid using waste biomass generated after dye degradation. J Hazard Mater 182:169–176. https://doi.org/10.1155/2014/410704
Tapas KR, Naba KM (2014) Photocatalytic degradation of Congo red dye on thermally activated zinc oxide. Int J Sci Res Environ Sci 2:457–469. https://doi.org/10.12983/ijsres-2014-p0457-0469
Topac FO, Dindar E, Ucaroglu S, Baskaya HS (2009) Effect of a sulfonated azo dye and sulfanilic acid on nitrogen transformation processes in soil. J Hazard Mater 1702:1006–1013. https://doi.org/10.1016/j.jhazmat.2009.05.080
Tsuboy MS, Angeli JPF, Mantovani MS, Knasmuller S, Umbuzeiro GA, Ribeiro LR (2007) Genotoxic, mutagenic and cytotoxic effects of the commercial dye CI disperse blue 291 in the human hepatic cell line HepG2. Toxicol in Vitro 21:1650–1655. https://doi.org/10.1016/j.tiv.2007.06.020
Tyagi OD, Yadav M (2001) A textbook of synthetic dye. Anmol Publications PVT Ltd, New Delhi
Vasconcelos DDLC, Ordaz NR, Mayer JG, Varaldo HP (2012) Aerobic biodegradation of a mixture of sulfonated azo dyes by a bacterial consortium immobilized in a two stage sparged packed bed biofilm reactor. Eng Life Sci 12:39–48. https://doi.org/10.1002/elsc.201000227
Veena S, Swetha D, Karhik L, Gaurav K, Bhaskara Rao KV (2016) Antibiofouling activity of marine actinobacterial mediated titanium dioxide nanoparticles. Indian J Geo Mar Sci 45:583–590. http://nopr.niscair.res.in/handle/123456789/35095
Vijaykumar MH, Vaishampayan PA, Shouche YS, Karegouda TB (2007) Decolourization of naphthalene-containing sulfonated azo dyes by Kerstersia sp. strain VKY1. Enzym Microb Technol 40:204–211. https://doi.org/10.1016/j.enzmictec.2006.04.001
Waghmode TR, Kurade MB, Govindwar SP (2011) Time dependent degradation of mixture of structurally different azo and non azo dyes by using Galactomyces geotrichum MTCC 1360. Int Biodeterior Biodegrad 65:479–486. https://doi.org/10.1016/j.ibiod.2011.01.010
Waleed M, Mohamed B (2014) Biodegradation of azo dye a review. Int Environ Sci 67:765–890. 262564975
Wang N, Yanliang C, Fuan W, Zhilin Z, Xiangyu X (2017) Decolorization and degradation of Congo red by a newly isolated white rot fungus, Ceriporia lacerata from decayed mulberry branches. Int Biodeterior Biodegrad 117:236–244. https://doi.org/10.1016/j.ibiod.2016.12.015
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187. https://doi.org/10.1016/j.biotechadv.2003.08.011
Yan B, Zhou J, Wang J, Du C, Hou H, Song Z, Bao Y (2004) Expression and characteristics of the gene encoding azoreductase from Rhodobacter sphaeroides AS1.1737. FEMS Microbiol Lett 236:129–136. https://doi.org/10.1016/j.femsle.2004.05.034
Yizhong W (2000) Solar photocatalytic degradation of eight commercial dyes in TiO2 suspension. J Water Res 34:990–994. https://doi.org/10.1016/S0043-1354(99)00210-9
Zeroual Y, Kim BS, Yang MW (2007) Decolorization of some azo dyes by immobilized Geotrichum sp. biomass in fluidized bed bioreactor. Appl Biochem Biotechnol 142:307–316. https://doi.org/10.1007/s12010-007-0037-0
Zhang J, Feng M, Jiang Y, Hu M, Li S, Zhai Q (2012) Efficient decolorization/ degradation of aqueous azo dyes using buffered H2O2 oxidation catalyzed by a dosage below ppm level of chloroperoxidase. Chem Eng J 191:236–242. https://doi.org/10.1016/j.cej.2012.03.009
Zhao M, Zhang J (2008) Wastewater treatment by photocatalytic oxidation of nano-ZnO. J Glob Environ Policy Japan 12:19. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.483.6772&rep=rep1&type=pdf
Zimmermann T, Kulla HG, Leisinger T (1982) Properties of purified Orange II azoreductase, the enzyme initiating azo dye degradation by pseudomonas KF46. Eur J Biochem 129:197–200. https://doi.org/10.1111/j.1432-1033.1982.tb07040
Zimmermann T, Gasser F, Kulla HG, Leisinger T (1984) Comparison of two bacterial azoreductases acquired during adaptation to growth on azo dyes. Arch Microbiol 138:37–43. https://doi.org/10.1007/BF00425404
Zolgharnein J, Asanjrani N, Bagtash M, Azimi G (2014) Multi-response optimization using Taguchi design and principle component analysis for removing binary mixture of alizarin red and alizarin yellow from aqueous solution by nano a-alumina. Spectrochim Acta A Mol Biomol Spectrosc 126:291–300. https://doi.org/10.1016/j.saa.2014.01.100
Zollinger H (1987) Synthesis, properties and application of organic dye and pigments. Color Chemistry, VCH, New York, pp 92–102. https://doi.org/10.1002/anie.200385122
Acknowledgment
Authors are very thankful to the management of VIT University and CSIR Delhi, India, for supporting this study.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sreedharan, V., Bhaskara Rao, K.V. (2019). Biodegradation of Textile Azo Dyes. In: Gothandam, K., Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Nanoscience and Biotechnology for Environmental Applications. Environmental Chemistry for a Sustainable World, vol 22. Springer, Cham. https://doi.org/10.1007/978-3-319-97922-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-97922-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-97921-2
Online ISBN: 978-3-319-97922-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)