Abstract
Tropical scleractinian corals are dependent to varying degrees on their photosymbiotic partners. Under normal levels of temperature and irradiance, they can provide most, but not all, of the host’s nutritional requirements. Heterotrophy is required to adequately supply critical nutrients, especially nitrogen and phosphorus. Scleractinian corals are known as mesozooplankton predators, and most employ tentacle capture. The ability to trap nano- and picoplankton has been demonstrated by several coral species and appears to fulfill a substantial proportion of their daily metabolic requirements. The mechanism of capture likely involves mucociliary activity or extracoelenteric digestion, but the relative contribution of these avenues have not been evaluated. Many corals employ mesenterial filaments to procure food in various forms, but the functional morphology and chemical activities of these structures have been poorly documented. Corals are capable of acquiring nutrition from particulate and dissolved organic matter, although the degree of reliance on these sources generally has not been established. Corals, including tropical, deep- and cold-water species, are known as a major source of carbon and other nutrients for benthic communities through the secretion of mucus, despite wide variation in chemical composition. Mucus is cycled through the planktonic microbial loop, the benthos, and the microbial community within the sediments. The consensus indicates that the dissolved organic fraction of mucus usually exceeds the insoluble portion, and both serve as sources for the growth of nano- and picoplankton. As many corals employ mucus to trap food, a portion is taken back during feeding. The net gain or loss has not been evaluated, although production is generally thought to exceed consumption. The same is true for the net uptake and loss of dissolved organic matter by mucus secretion. Octocorals are thought not to employ mucus capture or mesenterial filaments during feeding and generally rely on tentacular filtration of weakly swimming mesozooplankton, particulates, dissolved organic matter, and picoplankton. Nonsymbiotic species in the tropics favor phytoplankton and weakly swimming zooplankton. Azooxanthellate soft corals are opportunistic feeders and shift their diet according to the season from phyto- and nanoplankton in summer to primarily particulate organic matter (POM) in winter. Cold-water species favor POM, phytodetritus, microplankton, and larger zooplankton when available. Antipatharians apparently feed on mesozooplankton but also use mucus nets, possibly for capture of POM. Feeding modes in this group are poorly known.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Mesoplankton
- Microplankton
- Nanoplankton
- Picoplankton
- Microbial loop
- Dissolved and particulate organic carbon
- Nitrogen
- Phosphorus
- Mucus secretion
- Tentacle capture
- Mesenterial filaments
- Tropical
- Temperate and polar scleractinians
- Octocorals
- Antipatharians
1 Introduction
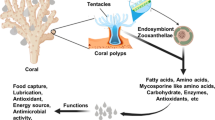
Corals are ecological engineers capable of modifying their physicochemical environment by virtue of their growth, form, and metabolism. Corals and the reefs they support are among the richest, most complex, and astonishingly diverse ecosystems on Earth, although they compose only a fractional percent of its surface. To add incongruity, they also live in nutritionally poor waters, a paradox first noted by Charles Darwin (1842). A partnership with endosymbiotic algae enables tropical reef corals to flourish in such environments through the conservation of nutrients, including nitrogen and phosphorus, and by retention and recycling within the reef ecosystem. These complex processes are easier to describe generally than they are to fully explain. The diverse sources and forms of nutrition that are available to corals and other members of the reef benthos are additionally dependent on complex associations with heterotrophic bacteria, archaea, and viruses whose residence in hospite are not simply influential, but are vital to their hosts (e.g., Rosenberg et al. 2007; Silveira et al. 2017). Corals may also house an assortment of fungi, filamentous skeletal algae, and cyanobacteria (Ainsworth et al. 2010) whose roles in many cases have yet to be clarified. The host and its suite of typical residents are often referred to collectively as the holobiont, whose composition is environmentally influenced through a tangle of feedback loops that are only now beginning to be unraveled.
Corals are threatened by climate change, and research emphases have become more focused on that issue, particularly as warmer or eutrophic waters appear to promote the sequestration of nutritional resources by symbiotic algae at the expense of their hosts (Wooldridge 2017; Baker et al. 2018). Ultimately, this shift from symbiosis to parasitism may presage the well-known expulsion of the coral’s symbiotic partners and a change in the nature of their microbial communities (e.g., Sokolow 2009; Bourne et al. 2009; Work and Meteyer 2014). This review focuses on the complexities presented by diverse sources of food, modes of feeding, secretory activities, and the nutritive sources available to corals in a variety of environmental situations, whether they act as hosts in symbiotic relationships or not. As used here, the term “corals” will refer to those that produce calcified skeletons (stony corals, order Scleractinia); however, reference will also be made to octocorals including those that produce flexible collagenous or composite calcite/protein skeletons (gorgonian octocorals), a scleritic or hydraulic skeleton (soft corals, order Alcyonacea), and skeletons composed of chitin and protein (black or wire corals, order Antipatharia).
2 Autotrophic Nutrition: A Brief Overview
Most shallow-water tropical corals regardless of taxonomic alliance associate with photosynthetic dinoflagellate symbionts of the genus Symbiodinium, a phototroph commonly referred to as zooxanthellae (Figs. 18.1 and 18.2). The relationship is intimate with the symbiont housed inside a host-cell vacuole, the symbiosome (Fig. 18.1), through which the two partners typically exchange nutritive and other molecules. Carbon is fixed during photosynthesis by zooxanthellae, and 50 to >90% of it is translocated to the host within a few hours (Muscatine et al. 1981; Dubinsky and Jokiel 1994; Kopp et al. 2013), although different rates have been reported under suboptimal conditions (e.g., Tremblay et al. 2012a; Seeman 2013). Glycerol, lipids, and glucose are thought to be especially abundant among the translocated forms of carbon, and appear to provide more than enough for the respiratory needs of the zooxanthellae and the host (Battey and Patton 1984; Burriesci et al. 2012 and contained references). Indeed, when expressed on a daily basis, the photosynthetic/respiration (P:R) ratio can be greater than 1:1 suggesting that the symbiosis is autotrophic, i.e., that all daily metabolic requirements including respiration and growth of the skeleton and tissue are met through photosynthesis and translocation of its products. However, photosynthetic or translocation capacity may differ within the same coral colony during the day due to self-shading, light fluctuation, depth, turbidity, and the genetic capacity of the Symbiodinium clade or density, among other factors (Warner et al. 2006; Ulstrup et al. 2007; Cooper et al. 2011).
Carbon translocation may also fail to account for periodic energy expenses including growth, gamete, or mucus production by the host (Muscatine et al. 1981; Davies 1984; Sorokin 1995) and may not be energetically balanced even when such requirements are taken into account (Falkowski et al. 1984; Edmunds and Davies 1989). While models suggest that 60% or more of the nitrogen available to the host can be recycled through autotrophy, it is clear that internal recycling cannot go on indefinitely without the influx of new sources to support growth and replace secretory losses (Tremblay et al. 2012a; Gustafsson et al. 2013). In addition, there may be differences in the use of products that are acquired through photosynthesis by the host and those obtained by heterotrophy. It has been suggested, for example, that simple autotrophic molecules are used for short-term energy requirements such as respiration, whereas more complex organic molecules are used for storage (e.g., Bachar et al. 2007; Tremblay et al. 2015). Moreover, translocated nutrients alone are deficient in phosphorous and nitrogen and thus provide “junk food,” calories without nutritional quality (Falkowski et al. 1984; Davies 1984). Thus, while shallow-water tropical species may be autotrophic to some degree, they must rely on prey items or on dissolved sources dissolved or particulate matter to replenish inorganic nitrogen, phosphorus, and other nutrients that cannot be adequately supplied by symbiotic algae (Muscatine and Porter 1977; Sorokin 1995).
2.1 A Need to Feed
Exogenous nutrient availability may be reflected by the density of zooxanthellae in hospite (Muscatine et al. 1989), by an increase in the size of symbiont cells, by the number of starch granules, and by the increased accumulation of intracellular lipids (Rosset et al. 2015). Lipogenesis is primarily a function of the chloroplasts (Crossland et al. 1980), and the assembly of these biomolecules may vary among zooxanthellae clades, the depth ranges of the host, or levels of environmental stress (e.g., Tchernov et al. 2004; Cooper et al. 2011). Synthesis begins with fatty acids and continues as storage lipids (i.e., triacylglycerol and wax esters), which are translocated to the host (Patton et al. 1977; Yamashiro et al. 1999; Grottoli et al. 2004). Lipids are an important indicator of coral vitality, and shallow-water coral tissue accumulates them as “fat bodies,” which may constitute 30–40% of the dry tissue weight (Stimson 1987). Such stores are important as food reserves that may compensate for a reduction in autotrophic output. Fatty acids can be synthesized endogenously by zooxanthellae but are most probably derived from heterotrophic feeding (Treignier et al. 2008; Imbs et al. 2010). However, there may be considerable differences among coral species in the amount of stored lipid reserves and in the ability of the host to replenish them under stress (Stimson 1987; Ferrier-Pagès et al. 2011a; Imbs and Yakovleva 2012).
The relative ratio of heterotrophy to autotrophy varies greatly. In some cases, heterotrophy meets as little as 15% of the coral’s daily metabolic requirement (Houlbrèque and Ferrier-Pagès 2009). In general, it appears that the importance of exogenous food for symbiotic corals becomes especially critical at low irradiance levels. Corals that have been assessed are able to maintain or increase levels of fatty acids, sterols, and protein concentrations as long as they are fed (Al-Moghrabi et al. 1995; Treignier et al. 2008). In Stylophora pistillata, heterotrophy combined with high irradiance levels results in high translocation rates of carbon to the host compared with fed colonies at lower irradiance levels. This “overproduction” in turn results in the release of excess organic compounds described below (Tremblay et al. 2014). Likewise, fed colonies of S. pistillata better resist irradiance stress and oxidative damage compared with unfed colonies (Levy et al. 2016). More specifically, other species have demonstrated the ability to resist temperature stress and its repercussions through the provision of a lipid-enriched diet (Tagliafico et al. 2017a).
Autotrophy may also differ seasonally among symbiotic coral species living in temperate and seasonally marginal environments. Colonies of the symbiotic Cladocora caespitosa form reefs in the Mediterranean Sea. This species reaches compensation irradiance in low-light conditions and is adapted to relatively high levels of turbidity. As a result, it can occur to a depth of 40 m. Summer conditions favor both skeletal and tissue growth, much of it from autotrophy. If colonies are experimentally fed under these conditions, they release excess carbon as mucus similar to tropical species (Hoogenboom et al. 2010; Tremblay et al. 2011, 2012b). However, zooplankton supplies are greater in cooler weather than in summer, and supplementation from heterotrophy is evident from the shifts in carbon and nitrogen isotope ratios during winter months. Thus, external sources of food compensate for photosynthetic limitations (Piniak 2002; Rodolfo-Metalpa et al. 2008; Ferrier-Pagès et al. 2011b). A similar compensation mechanism may be found among corals living on tropical mesophotic reefs (Schlichter et al. 1986; Eyal et al. 2015). Here at depths of up to 150 m, symbiotic species are likely to be light limited and more dependent on heterotrophy compared with their shallow-water relatives (e.g., Gattuso et al. 1993; Einbinder et al. 2009; Lesser et al. 2010). By contrast, symbiosis in the NW Atlantic species Oculina arbuscula is facultative. Colonies that retain their photosymbionts year-round obtain most of their nutrition from them, but naturally occurring aposymbiotic colonies rely on a diet of phyto- and zooplankton 12 μm or smaller, in addition to organic matter in the sediment judging from the isotopic signatures (Leal et al. 2014a).
The symbiotic relationship is fraught with delicacy in the face of environmental change. Beyond their narrow range of temperature or irradiance, zooxanthellae become photosynthetically impaired and begin to undergo a process of expulsion and degrees of bleaching. Hosts in those conditions vary in their capacity to obtain heterotrophic nutrition (Palardy et al. 2008; Grottoli et al. 2006; Rodrigues and Grottoli 2007; Ferrier-Pagès et al. 2011a), and if bleaching is severe, heterotrophy must provide 100% of coral metabolic requirements. While some species are able to make up for autotrophic impairment by heterotrophy, others cannot, even in the presence of food (e.g., Grottoli et al. 2006; Hughes and Grotolli 2013). Similarly, periodic episodes of turbid water may cause a shift from autotrophy to other forms of nutrition including the capture of particulate matter, even if it is a poor-quality food source. Under prolonged light reduction and fouling of the coral feeding surfaces, some species may be unable to obtain appropriate amounts or adequate forms of nutrition from these sources or may be overwhelmed by respiratory requirements imposed by nonnutritive particle removal (e.g., Anthony 1999; Anthony and Fabricius 2000; Browne et al. 2014).
3 Heterotrophic Nutrition in Scleractinians
3.1 From the Water Column
All scleractinian corals are heterotrophs that obtain at least part of their nutrition by suspension feeding. Corals are polytrophic feeders, obtaining food by several mechanisms. Tentacle capture and/or mucociliary entrapment of zooplankton and nanoplankton plays a prominent role. Most scleractinians exhibit characteristic responses to the presence of finely ground animal food (e.g., minced crab or clam tissue) including muscular activity of the tentacles, contraction of the oral disk, wide dilation of the oral region, secretion of mucus, and, in some, eversion of the mesenterial filaments (e.g., Carpenter 1910; Boschma 1925; Muscatine 1973) described below. Similar reactions occur when corals are exposed to millimolar (or lower) concentrations of amino acids (Mariscal and Lenhoff 1968; Goreau et al. 1971).
3.1.1 Tentacle Capture and the Use of Cnidae
Suspension-feeding invertebrates consume plankton and other sources of organic material by straining, sieving, or otherwise trapping them from the water column. Active suspension feeders are those animals that employ a pumping mechanism to drive currents to trap food (e.g., bivalve molluscs, ascidians, and some annelid worms, among many other groups). Passive suspension feeders, by contrast, depend exclusively on ambient water movements to drive water past their food-gathering surfaces. With some exceptions (e.g., xeniid octocorals, decribed below), corals of all affinities fall into this category and employ tentacle capture, with assistance to varying degrees by mucus entrapment. Tentacles act as sieves that rely on their spacing, number, and shape to trap particles larger than their pass-through elements (LaBarbara 1984). Scleractinian coral tentacles are relatively simple conical structures, but they can be quite numerous (e.g., Fig. 18.2). Thus, the simple act of tentacle projection produces a canopy that slows the flow of water, thereby facilitating food capture (Johnson and Sebens 1993). However, relatively low flow rates are often crucial because higher flows can induce mechanical deformation of the exposed feeding surfaces and may cause detachment of prey (Wijgerde et al. 2012).
While some scleractinians exhibit a diminished number of cnidae and/or reduced tentacles (Schlichter and Brendelberger 1998; Goldberg 2002a, 2004), nematocysts and spirocysts are typically densely populated in the tentacles and are used to subdue and increase retention of prey items that might otherwise escape. Most scleractinian corals develop tentacles that are studded with wartlike “batteries” of nematocysts that may be helpful in taking relatively large planktonic prey (Fig. 18.2; Sebens et al. 1996). However, while venoms and neurotoxins are known to be associated with nematocysts (see below), those from corals have not been assessed, nor have adhesive spirocysts, the most abundant type of cnida in tentacles of several coral groups (Watson and Mariscal 1983; Goldberg and Taylor 1996; Strömberg and Östman 2017). Spirocysts characteristically develop internal tubules that appear to form a weblike mesh once they have been externalized by discharge, and this is thought to entangle prey (Mariscal et al. 1977). These are glutinant or adhesive cnidae that contrast with penetrant nematocysts (Mariscal 1984), but spirocysts have not been observed in the act of taking prey or particulate food. Interestingly, the cnidome of tropical octocorals may be diminished in number or in diversity compared with their stony relatives (Mariscal and Bigger 1977; Schmidt and Moraw 1982; Sammarco and Coll 1992). However, this generality may fail as more species are examined, and it may not apply to nonsymbiotic species (e.g., Piraino et al. 1993; Yoffe et al. 2012).
Satiation mechanisms have been examined in anemones, but not in corals, and appear to be controlled in part by the inhibition of discharge by certain types of nematocysts rather than by prey ingestion. However, there is also a marked decline in feeding efficiency due to the failure of discharged nematocysts to maintain adherence of food items to the tentacle in sated anemones, and this results in prey being dropped out of reach. Adhesive forces in turn, measured in micronewtons, are the sum of contributions arising from the stickiness of the tentacle mucus to the prey and the number of cnidae (nematocysts and spirocysts) discharged, multiplied by the mean force needed to pull individual cnidae out of the tentacle (Thorington and Hessinger 1996). Although numerous spirocysts discharge on stimulation, the adherence forces imparted by them are extremely low compared with nematocysts (Thorington and Hessinger 1998), leaving the question of spirocyst function in the dark. Furthermore, cnidae must be released for prey to be ingested, and this entails the wholesale disengagement of the nematocyst and its supporting cells from the tentacle as prey is consumed. This complex action of nematocyst release occurs in response to chemosensitization of prey-derived molecules (Thorington and Hessinger 1998). Both nematocyst discharge inhibition and adhesive failure are restored when no food has been available for 48 h or more (Thorington et al. 2010).
Although the venom of medically significant cnidarians has been examined (e.g., Hessinger 1988; Tezcan 2016), their composition is complex and is often not known in detail. They appear to contain about 250 types of peptides, proteins, enzymes, and proteinase inhibitors, as well as non-proteinaceous substances including purines, quaternary ammonium compounds, biogenic amines, and betaines (Frazão et al. 2012). Few of these have been characterized. Nematocyst venom from sea anemone cnidae contains phospholipases and cytolysins including membrane disruptors such as perforins and hemolysins (reviewed by Frazão et al. 2012; Jouiaei et al. 2015). This suggests a potential key role in extracellular digestion, but that aspect has yet to be examined. The contamination of nematocyst preparations with toxins of different origin is an additional confounding issue. For example, when cnidae are isolated en masse from tentacles for analysis, as they typically are, they may be contaminated with mucus discharge, and the mucus from certain anemones appears to contain toxins (Moran et al. 2012). Mucus secretion, whether toxic by itself or not, is an important aspect of coral feeding as described below.
3.1.2 Feeding and Digestion by Mesenterial Filaments
The mouths of each polyp are primed to open widely in the presence of food, and these openings lead to a muscular and ciliated pharynx before entering the coelenteric cavities. Each of these digestive regions is subdivided by mesenteries, muscular folds of the gastrodermis, and mesoglea whose edges become developed into longitudinal ribbonlike extensions. These “complete mesenteries” are arranged like spokes on a wheel in cross section, and among scleractinians, there are typically 8–12 of them arranged in pairs. In most species, the mesenteries extend half way or farther down the entire length of the coelenteron but rarely extend to its entire length as in anemones (Duerden 1902). There are also shorter partitions of the body wall (incomplete mesenteries) that do not reach the coelenteron. However, all mesenteries, whether complete or not, form thin, threadlike filaments that extend from the aboral ends of the mesenteries and coil toward the base of the coelenteron. A pair of mesenteries corresponds to the number of tentacles; thus, a polyp with 24 or 48 tentacles will have twice that number of mesenterial filaments, many of which have been illustrated in detail by Duerden (1902). Transverse sections typically reveal a bulbous tip, heavily invested with nematocysts and mucocytes, although the predominance of one cell type over the other changes along the filament length.
Most coral species are capable of extracoelenteric digestion of food. Mesenterial filaments accomplish this by extrusion through the mouth, temporary openings on the colonial surface, or the column walls (Duerden 1902; Matthai 1918). Each filament exhibits a stalk 2–10 cm long and some exhibit a U-shaped groove containing ciliated cells (Muscatine 1973; Goldberg 2002b). The stalk described by the latter author is unequally bilobed with the larger side exhibiting a well-developed muscular band. The opposite lobe is densely packed with cnidae, mucocytes, collar cells, and zymogen cells. The filament tip is spatulate (Fig. 18.3) and is packed with characteristic zymogen, mucus cells and nematocysts similar to the bulbous filament tips reported from sea anemones (Van-Praët 1985; Shick 1991), although the predominance of one cell type over the other changes along the length of the filament. Zymogen granules are typical of digestive cells and include proenzymes, inactive forms of trypsin, pepsin, and other proteases. Boschma (1925) noted that after food is brought into the coelenteron, vacuoles in the mesenterial filaments become acidic and then alkaline after about 2 days, judging from pH-sensitive dyes, suggesting a pepsin-trypsin-like digestive sequence. Yonge (1930a) established that corals digest mucus-coated food extracellularly by secretion of proteases from the mesenterial filaments. Particles were then phagocytosed or completely solubilized and transported within the filaments by either diffusion or by amoebocytes to complete the intracellular phase of digestion. Raz-Bahat et al. (2017) used histochemistry and RNA transcripts to determine that chymotrypsinogen occurs in the mesenterial filaments of Stylophora pistillata. They also report short and long filaments with different cellular arrangements emanating from incomplete and complete mesenteries, respectively, assigning a digestive role to the long ones. However, the fine structure of filaments implies that they have other functions. The occurrence of a ciliated groove along the filament length indicates a role in small particle transport, while the collar cells suggest a phagocytic capacity (Goldberg 2002b). Roles of absorption and egestion (e.g., of zooxanthellae) appear to be associated with the regions proximal to the filament tip as shown by Yonge (1930a). Similar work that extends what is known of coral digestion from the early twentieth century has returned little additional illumination.
Left: Toluidine blue-stained transverse sections of a mesenterial filament from the scleractinian Mycetophyllia reesi. Numerous, darkly staining mucocytes (mu) occur at the tip; a single large nematocyst is shown (arrow), but many others are hidden below the surface. The muscular lobe and mucus cells are shown on the stalk. Scanning microscopy of the tip (inset, upper right) shows the grooved nature of the stalk, otherwise invisible in section (after Goldberg 2002b). Right: sections taken from varying depths along a filament from Meandrina sp. shows the changing nature of the cellular constituents, especially the proportion of nematocysts and mucocytes. The dark center extending through the filament represents mesoglea (after Duerden 1902)
The relatively shallow depth of information available on the structure-function relationships of mesenterial filaments has implications for properly assessing the nature and extent of heterotrophy. Filament extrusion can be an impressive event involving much of the colony (Fig. 18.4). Corals can use these structures for aggressive and competitive behavior by extending them onto nearby corals and digesting the tissue down to the skeleton within 12 h. A dominant hierarchy has been established showing that certain species are always winners compared with less aggressive species (Lang 1973; reviewed by Lang and Chornesky 1990). However, the cellular mechanics and event sequences of digestion, as well as the nutritive value to the aggressor, are unknown. Likewise, many scleractinians are unspecialized detritus feeders that use their mesenterial filaments to sweep the surrounding sediment for food (Goreau et al. 1971) or to clear the substratum for colony expansion (Roff et al. 2009). In addition, the role of the filaments in absorption of dissolved organic and particulate matter remains to be assessed, as does the entire potential scope of extracoelenteric feeding. The study by Wijgerde et al. (2011), for example, found that >96% of Artemia nauplii fed to Galaxea fascicularis were not ingested, but instead were captured by mesenterial filaments and digested externally. Their study counterintuitively indicates that zooplankton digested extracoelenterically by G. fascicularis colonies provides them with far more carbon, nitrogen, and phosphorus than does coelenteric digestion. The role of zooplankton nutrition from the perspective of coelenteric digestion is reviewed below.
3.1.3 Capture of Mesozooplankton
A few corals are able to consume phytoplankton (e.g., Leal et al. 2014a), but most are thought to be active zooplankton predators (Porter 1974; Muscatine et al. 1981; Sebens et al. 1996). Such exogenous food supplies play a large role in the nutritive requirements of growth, skeleton formation, and respiration (Ferrier-Pagès et al. 2003; Houlbrèque et al. 2004a; reviewed by Houlbrèque and Ferrier-Pagès 2009). Yet the degree to which corals are able to sustain themselves by zooplankton feeding is controversial, and some authors have found this food source inadequate for metabolic needs (Clayton and Lasker 1982; Yamamuro et al. 1995; Sammarco et al. 1999). Indeed a tentative ceiling for zooplanktonic heterotrophy as a carbon source for tropical scleractinians is thought to be about 60–66% (Grottoli et al. 2006; Palardy et al. 2008; Houlbrèque and Ferrier-Pagès 2009) although the contribution by nano- and picoplankton will be important as a means of reconciling the true scope of such calculations (see below). Corals may also face challenges of nutrition limitation for other reasons.
Mesozooplankton (Table 18.1) is the typical size range of food that is easily observed during coral feeding. Within this group, corals appear to select poor swimmers, and these plankters are taken disproportionately irrespective of colony morphology or polyp size (Palardy et al. 2005). The natural supply of plankton may vary considerably on a seasonal basis and during the lunar cycle. Thus, selective feeding on favored types of plankton may be cyclically limited (Palardy et al. 2006). While natural plankton is often experimentally supplied, Artemia nauplii or cysts often serve as surrogates. Freshly hatched nauplii range from 428 to 517 μm, and hydrated cysts vary from 220 to 285 μm in diameter depending on the strain (Vanhaecke and Sorgeloos 1980; Léger et al. 1987), and these fall within the size range of mesozooplanktonic prey.
Observations of coral feeding suggest that they are relatively inefficient mesoplanktonic predators, as significant numbers of them are often captured and then released, or escape (Hii et al. 2009; Wijgerde et al. 2011). Some types of zooplankton are more likely to contact tentacles at low flow speeds, either due to chance abetted by erratic swimming behavior or to settlement by gravity onto feeding surfaces (e.g., Johnson and Sebens 1993). Conversely, certain copepods that are strong swimmers may be able to avoid tentacles at low to moderate flow speeds, but may be unable to elude interception with stronger flow or more turbulent conditions (Sebens et al. 1996, 1998; Wijgerde et al. 2012). In addition, colony morphology modifies flow and feeding success. Branched corals appear to be more successful in relatively high flows compared with mound-shaped or flat coral colonies, due to drag and turbulence imposed by branching (Sebens and Johnson 1991).
Laboratory experiments have found that capture is proportional to prey density, increasing with availability until satiation (e.g., Palardy et al. 2006; Tagliafico et al. 2017b). Corals that recently consumed prey fed less frequently than those that had not eaten for a few days (Ferrier-Pagès et al. 2003). There are species-specific differences in prey capture, perhaps due to feeding mechanisms, colonial morphology, number and arrangement of tentacles, polyp density, or surface to volume ratio (Lewis and Price 1975; Johnson and Sebens 1993; Sebens et al. 1996; Hii et al. 2009). In addition, the capture of zooplankton is also dependent on polyp expansion and feeding times. Depending on the species and the environment, coral polyps may be expanded both day and night, only during the day, or only at night when zooplankton concentrations are highest (Lewis and Price 1975). This may be due to the emergence of demersal plankton at dusk, migration of plankton from the open ocean, or release of gametes or larvae (e.g., Heidelberg et al. 2004; Yahel et al. 2005). However, expansion itself is inducible and can be modulated by the interaction of light, current flow, and the presence of food (Levy et al. 2000, 2003).
3.1.4 Capture of Micro-, Nano-, and Picoplankton
Several studies have examined the contribution of small plankton groups to the scleractinian coral diet. Nanoplankton includes a diverse group of photosynthetic and heterotrophic flagellates, including dinoflagellates, as well as coccolithophorids, ciliates, and diatoms that range in size from 2 to 20 μm. Picoplankton is the fraction of the plankton community that is an order of magnitude smaller and includes bacterial as well as eukaryotic heterotrophs and phototrophs. One particularly important group of algal picoplankton is a group of coccoid cyanobacteria less than a micron in diameter, which is responsible for 80–90% of the total carbon production annually or daily in oligotrophic tropical marine waters (Longhurst and Pauly 1987; Stocker 1988; Partensky et al. 1999).
The small planktonic groups (micro-, nano-, picoplankton) contribute >50% of the chlorophyll biomass in reef waters whether from Japan (Tada et al. 2003), the Great Barrier Reef (Furnas and Mitchell 1986), French Polynesia (Legendre et al. 1988), or Fiji’s Great Astrolabe Reef (Charpy and Blanchot 1999). Picoplankton in these environments may contain up to 106 bacterial cells ml−1, 104–105 cyanobacteria ml−1, and as many as 104 flagellates ml−1 (Sorokin 1991; Ferrier-Pagès and Gattuso 1998). Micro-, nano-, and picoplankton may thus constitute an important food source for corals, but verifying their consumption has been indirect by necessity. Field studies have examined the depletion of these organisms as incoming flows pass over reef communities (Patten et al. 2011), but more specific experimental evidence is hampered not only by their small size, but also by their delicate and readily digested architecture. Thus, assessing the fate of these organisms requires radiotracers, chlorophyll autofluorescence, and DAPI stains to demonstrate their incorporation into coral tissue (Sorokin 1973; Ferrier-Pagès et al. 1998a; Houlbrèque et al. 2004b; Tremblay et al. 2012a). In most cases, feeding on picoplankton is deduced from the clearance rate of flow tanks after comparing those with and without corals. Ferrier-Pagès et al. (1998b) incubated Stylophora pistillata with 3H-labeled bacteria and ciliates (<30 μm) and found that the amount of radioactivity in coral tissue is proportional to the amount disappearing from the medium. Bak et al. (1998) observed that fluorescently labeled bacteria are taken up and captured by mesenterial cells of the Caribbean coral Madracis mirabilis. Further studies of three different coral species revealed that autotrophic and heterotrophic nanoflagellates represent 84–94% of the total ingested carbon and 52–85% of the total ingested nitrogen (Houlbrèque et al. 2004b, 2006; reviewed by Houlbrèque and Ferrier-Pagès 2009). Although a source of dietary nitrogen, cyanobacterial picoplankton is not clearly selected as a component of the scleractinian diet. However, this does not appear to be the case for sponges (e.g., Hanson et al. 2009; McMurray et al. 2016), ascidians (Ribes et al. 2005; Jacobi et al. 2017), or certain octocorals described below.
3.1.5 The Microbial Loop, POM, and DOM in the Water Column: A Brief Overview
Water column activities in the plankton include multiple feedback loops beginning with carbon fixation by phytoplankton, a group including photosynthetic diatoms, dinoflagellates, and others shown in Table 18.1. Particulate organic matter (POM) is derived from primary producers and zooplanktonic grazing food webs among other sources, despite early studies concluding that such contributions were minimal. However, the importance of this material has been demonstrated by examining reef nutrient budgets (Genin et al. 2009; Sorokin and Sorokin 2010; Wyatt et al. 2013). In part, the reason for the underestimate was the focus on larger particles, often sampled using plankton nets, when in fact most particulates in oligotrophic waters are <5 μm (Ferrier-Pagès and Gattuso 1998; Wyatt et al. 2013). Conversely, the relative contribution of allochthonous particulates and other nutrients on reefs compared with those originating in situ may be dependent on local factors including riverine input, upwelling, runoff, offshore plankton blooms, and other factors. Thus, in some environments, the import of allochthonous particulates may be substantial (Wyatt et al. 2010, 2013; Briand et al. 2015), but others suggest that phytoplankton-derived organic carbon and import from oceanic currents, for example, contribute to only a minor fraction, perhaps up to 13% of the total reef primary production (e.g., Alldredge et al. 2013). Regardless of the amount, phytoplankton and grazing zooplankton are important sources of POM as well as dissolved organic matter (DOM).
Up to half of the carbon fixed by phototrophs in the water column is released as DOM (Roshan and DeVries 2017), which includes simple sugars, amino acids, lipids, vitamins, chlorophyll, humic acids, and larger organic molecules up to 100,000 Da. This material is the largest exchangeable reservoir of organic material on Earth and is defined operationally as the fraction of organic matter that is not retained by filtration. This is often a glass fiber filter with a pore size of 0.7 μm, but there is no universally agreed upon filter or pore diameter (Carlson and Hansell 2015).
The autotrophs supply plumes of nutrients in the form of POM and DOM through leakage of photosynthates, cell lysis and death, or the production of cellular exudate (e.g., Passow 2002; Stocker 2012). The latter material is a sticky, nearly invisible mucus produced by some species, especially diatoms, that causes an increase in size through flocculation, and the sinking of the resulting particulates. These materials and processes initiate the microbial loop (Azam et al. 1983) as illustrated in Fig. 18.5. Larger zooplankton (mesozooplankton) and microplankton feed by grazing on phytoplankton cells as well as on microplanktonic ciliates and detritus (Calbet and Saiz 2005; Nakajima et al. 2017a). They contribute to POM and DOM by death, as well as by ammonium and phosphate leakage, ‘sloppy grazing’, and fecal matter (e.g., Møller et al. 2003). Small fecal pellets from 3 to 150 μm in diameter are ubiquitous in the upper reaches of the ocean and exceed larger fecal particulates by three orders of magnitude in the upper 2 km in some areas. These “minipellets” contain intact bacteria and picoplankton and originate from microzooplanktonic grazers including ciliates, protozoans, heterotrophic dinoflagellates, radiolarians, and foraminiferans (Turner 2002).
Dissolved organic matter is taken up by phytoplankton but more so by heterotrophic bacteria and archaea due to their absorptive capacity and high surface to volume ratios. In addition, the secretion of hydrolases solubilizes and reduces the size of POM, thereby releasing more DOM (reviewed by Azam and Malfatti 2007). It is estimated that heterotrophic flagellates and ciliates consume roughly half of picoplankton production, which in turn is grazed by higher trophic levels, especially nanoplanktonic microflagellates (Ferrier-Pagès and Gattuso 1998; Ferrier-Pagès and Furla 2001). In addition, some autotrophic nanoflagellates are capable of supplementing their photosynthetic output by bacterial phagocytosis (Laybourn-Parry and Parry 2000).
Lastly, the importance of viruses in the microbial loop (Fig. 18.5) cannot be understated. At concentrations of approximately ten million per milliliter of surface seawater, viruses are the most abundant biological entities in the oceans (Breitbart 2012). The majority are phages that lyse their bacterial hosts and may be responsible for the turnover of 20–120% of the daily standing stock of bacteria and archaea depending on location. Likewise, there are also large populations of cyanophages that infect and lyse the smallest and most abundant members of the autotrophic picoplankton (Aylward et al. 2017). In addition, while eukaryotic viruses are of ecological importance everywhere, some of them infect important taxa of phytoplanktonic primary producers. Indeed, phytoplankton blooms appear to be curtailed in part due to large-scale viral infections that are transported by winds over long distances in marine aerosols (e.g., Sharoni et al. 2015). Conversely, these infective agents release cellular DOM and inorganic material into the seawater, which can serve as fuel for further microbial growth and nanoflagellate feeding (reviews by Suttle 2005; Thurber et al. 2017).
The importance of allochthonous particulate organic matter (POM) is increasingly apparent. Recent studies have demonstrated that POM may contribute significantly to reef nutrient budgets (Genin et al. 2009; Sorokin and Sorokin 2010; Wyatt et al. 2010), influence coral calcification (e.g., Ferrier-Pagès et al. 2003; Houlbrèque et al. 2004a), and increase resilience to stress (e.g., Grottoli et al. 2006; Ferrier-Pagès et al. 2010).
4 Benthic and Epibenthic Organic Matter Production and Consumption
The origins of marine POM (and DOM) on shallow coral reefs are varied and include primary producers such as macroalgae and turf algae (Purcell and Bellwood 2001; Wilson et al. 2003; Haas and Wild 2010; Haas et al. 2010; Haas et al. 2011). Seagrass and mangrove communities in reef-associated environments also export organic matter and augment these sources (e.g., Heck et al. 2008; Granek et al. 2009). POM may also originate from zooplanktonic and piscine fecal matter, its bacterial decay, and coprophagy by planktonic fishes (Robertson 1982; reviewed by Turner 2002; Smriga et al. 2010). Coprophagy continues in the sediments as benthic scrapers and shredders feed on settled fecal matter (Wotton and Malmqvist 2001). While organic particulates are typically carbon-rich, they may also contain organic and inorganic forms of phosphorus and nitrogen that may become sources of nutrition for corals in the water column and the sediments that surround them (e.g., Houlbrèque and Ferrier-Pagès 2009). These nutrients are further considered below.
4.1 Release of Mucus
Coral tissue is often heavily invested with cells that produce copious amounts of mucus (e.g., Marshall and Wright 1993; Fig. 18.6). Secretions from mucocytes have distinct functions including sediment cleansing, wound healing, and protection from invasive microbes, as well as a shielding from desiccation and UV damage (reviewed by Brown and Blythell 2005). Mucus on the surface of corals is also associated with a diverse and incompletely known core microbiota including viruses, bacteria, and archaea, some of which are thought to be species-specific (e.g., McKew et al. 2012; Nguyen-Kim et al. 2014; Rubio-Portillo et al. 2018). A highly diverse mucus microbiome is thought to perform vital services including nitrogen metabolism, sulfur cycling, and antimicrobial defense, among other functions (e.g., Thurber et al. 2017; Rohwer et al. 2002; Kellogg 2004). Stresses such as elevated temperature, pH, or UV radiation may cause a change in the composition of mucus (Lee et al. 2016), which may induce changes in the microbiome leading to the prevalence of potentially pathogenic species (e.g., Sato et al. 2009; Lee et al. 2015; Sweet and Bythell 2016; Glasl et al. 2016). This area of research is very important for an understanding of anthropogenic effects on corals and is the focus of much current research, but is not further considered here.
Left: Discharge of mucus from coral epidermis. Left: Darkly stained, intact mucocytes. Right: Discharge of mucus cell in response to food. Spirocyst (sp), microvilli (mv), kinocilium (k), after Goldberg (2002a)
Stable isotope signatures suggest that photosynthates translocated by endosymbiotic zooxanthellae represent the foundation of mucus production (Naumann et al. 2010). However, even though the energetic price is quite high at 20–45% of the daily net photosynthate produced (Davies 1984; Edmunds and Davies 1989; Brown and Blythell 2005), coral mucus is continually released into the water column and may contribute as much as half of the total mucus released by all of the benthic organisms (animal and vegetable) on coral reefs (Crossland 1987; Naumann et al. 2010). Acropora spp., the dominant scleractinian genus on the Great Barrier Reef, can exude up to 4.8 L of mucus per square meter of reef area per day (Wild et al. 2004a). High light levels can induce such elevated releases, which include both particulate mucus and dissolved organic matter, primarily organic carbon, DOC (Crossland 1987; Naumann et al. 2010). However, mucus release differs among tropical species and conditions. Tanaka et al. (2009), for example, found that only about 5% of the net daily photosynthetic production was released from Acropora pulchra. By contrast, Muscatine et al. (1984) found that 6–50% of newly fixed carbon was lost as DOC from Stylophora pistillata depending on irradiance levels. Tremblay et al. (2012a) found that the free availability of heterotrophic food as well as high light levels were required for the accumulation of autotrophic carbon, which was then released as DOC in S. pistillata. Conversely, release rates of 13C-labeled fixed carbon dropped under low light levels even when corals were fed (Tremblay et al. 2014). Mucus release appears to constitute a dominant form of organic matter generated in the reef ecosystem (e.g., Hatcher 1988; Ferrier-Pagès et al. 1998a; Bythell and Wild 2011) and represents the main pathway for coral primary production to enter the food web (Hatcher 1988). It is also an important energetic and ecological link between corals, reef waters, and carbonate sediments (reviewed by Garren and Azam 2011). Even cold-water corals release DOC in approximately the same quantities as their shallow-water counterparts (Wild et al. 2008).
Mucus is a complex of very large glycoproteins (mucins), which tend to be sulfated in coral exudates and form gel-like strands that have varying properties of viscosity, polymerization, and flow (Jatkar et al. 2010; Coddeville et al. 2011). Freshly released coral mucus differs among species due to variations in lipid, sugar, and amino acid composition (Ducklow and Mitchell 1979; Crossland 1987; Meikle et al. 1988; Wild et al. 2010b). Particulate mucus may also contain high levels of phosphate if mucus release is coincident with egestion of food from the coelenteron (Nakajima et al. 2015). The nitrogen content of mucus is also variable and may be influenced by planktonic food availability, by the colonization of picoplanktonic organisms, and by nitrogen-fixing bacteria as described below. In addition, mucus may contain zooxanthellae, nematocysts, and coral tissue (Crossland 1987; Wild et al. 2004a, b).
At one time, coral mucus was thought to be a negligible source of nutrients (e.g., Krupp 1984; Coffroth 1990). However, it is now widely accepted that strand or weblike mucus acts as a particle trap for sediment and microorganisms in which pico- and nanoplankton have been shown to increase five- to sixfold (Ferrier-Pagès et al. 2000; Wild et al. 2004a; Allers et al. 2008; Naumann et al. 2009). This growth is up to 50 times faster than in the open ocean (Sharon and Rosenberg 2008; Silveira et al. 2017). Cycles of mucus production, aging, and shedding are accompanied by an increase in total microbial abundance. It is now clear that this colonization enhances the food value of released mucus in terms of carbon (Ferrier-Pagès et al. 1998a; Nakajima et al. 2009) and nitrogen (Grover et al. 2014; Bednarz et al. 2017 and contained references). As this enriched exudate is concentrated or fragmented by currents and cast adrift across the reef, it becomes a downstream pelagic food source for mucus-feeding fishes (Benson and Muscatine 1974) and zooplankton (Gottfried and Roman 1983), and after sinking into the sediment, it is cycled to members of the benthic community (Wild et al. 2005; Huettel et al. 2006; Mayer and Wild 2010; Tanaka et al. 2011; Naumann et al. 2012). It is thus a mechanism by which the pelagic food supply is coupled to the benthos (Fig. 18.5; Naumann et al. 2009; Bythell and Wild 2011).
4.2 Mucus and Coral Feeding
Mucus sheets several hundred microns thick are thought to be a means by which certain scleractinian and gorgonian corals avoid fouling by trapping and then releasing particulate sediments (e.g., Duerden 1906; Coffroth 1990; Tseng et al. 2011; Bessell-Brown et al. 2017). However, mucus is also a major mechanism for the entrapment and ingestion of small nutritive particles (e.g., Carpenter 1910; Yonge 1930b). Its production can serve as an adjunct to more selective zooplankton feeding by the tentacles, or it can be an important or perhaps the primary mode of food acquisition. Lewis and Price (1975) showed that mucus secretion by the epidermis and from the mouth was a common response to the presence of food by corals within several groups and included the production of dense nets, thick strands, or thin filaments depending on the species and the vigor of the water flow. These secretions entangle visible zooplankton as well as fine particulate material (visible as aggregates in Fig. 18.7). Experiments with 3H-labeled ciliates and bacteria have shown that the label appears in the mucus before becoming incorporated into the tissues of Stylophora pistillata (Ferrier-Pagès et al. 1998b). Corals of various affinities produce and re-ingest their own secretions by guiding mucus toward the mouth with ciliary currents, in some cases whether or not the polyps are expanded (Muscatine 1973; Lewis and Price 1975, 1976; Schlichter and Brendelberger 1998). This mechanism of capture can thus be quite subtle.
The action of ciliary currents by themselves may also become a means of obtaining food. Shapiro et al. (2014) described tracts of ~12 μm-long cilia on the epidermal surface of corals that they examined using high-speed video microscopy. The cilia created strong counter-rotating vortices that extended up to 2 mm from the coral surface. These in turn drove food particles 5 μm or less parallel to the coral epidermis, suggesting a possible role of self-generated currents in nutrition, and less of a reliance on transport by ambient flows. The function of mucus in conjunction with such ciliary activity has yet to be evaluated.
4.3 Feeding by Cold-Water Corals
Cold- and deepwater corals rely on carbon input from the ocean surface. Food sources may include zooplankton, bacteria, POM, and DOM (Dodds et al. 2009; Mueller et al. 2014a; Orejas et al. 2016) from shallow-water phytoplankton, zooplankton feeding, and fecal production as well as viral-mediated lysis of bacteria and phytoplankton (Fig. 18.5). Particulate organic matter is also derived from shallow water, especially “marine snow,” primarily composed of sinking polymeric exudates colonized by bacteria, as well as by heterotrophic flagellates and zooplanktonic fecal pellets (Waite et al. 2000; Turner 2015). Coral mucus from shallow water may also be a nutrient source for cold-water corals (Huettel et al. 2006; Rix et al. 2016). This organic rain produced in the epipelagic undergoes dissolution and disaggregation as bacteria consume it, but sinking particulates may also aggregate (reviewed by Lee et al. 2004) and contribute to the food supply for deep-sea communities.
Corals in these environments are frequently reported from sites with relatively high concentrations of organic particulate material. These are transported vertically by internal waves, tidal currents, and the gradients presented by the continental slope or topographic high spots. Such locations may produce an optimal environment for suspension feeders despite the lack zooxanthellae (Roberts et al. 2006; Wheeler et al. 2007). One of the common reef-forming corals in the deep North Atlantic and elsewhere is Lophelia pertusa, a branched scleractinian that exhibits an opportunistic feeding strategy. While this species feeds readily on zooplankton in low-flow conditions (Purser et al. 2010; Naumann et al. 2011), it is thought to thrive mainly on POM judging from the lipid profiles, stable isotope signatures, and observations of localized mucus secretion in response to the presence of food particles (Mortensen 2001; Duineveldt et al. 2012; Zetsche et al. 2016). The particulates also include phytoplankton resuspended from the bottom by turbulence and that appears to be a preferred food (Mueller et al. 2014a; van Oevelen et al. 2016). In addition, L. pertusa is able to take up free amino acids (Gori et al. 2014; van Oevelen et al. 2016) and may also fix inorganic carbon through chemoautotrophic activity of its associated microbiome (Middleburg et al. 2015). Less is known of other species, but fatty acid profiles in two different cold-water corals from >460 m in the Mediterranean suggest that they consume living zooplankton rather than POM (Naumann et al. 2015).
5 Dissolved Organic and Inorganic Matter
5.1 Mucus and the Production of Dissolved Organic Carbon
Several authors have found that dissolved organic carbon (DOC) constitutes more than half to 80% of the organic content of freshly released mucus (Wild et al. 2004a; Nakajima et al. 2009; Fonvielle et al. 2015). Net release of DOC by corals on a daily basis has been reported for some species (e.g., Crossland 1987; Wild et al. 2010a; Naumann et al. 2010), in particular if the colonies had been fed (Ferrier-Pagès et al.1998a). Thus, corals are often thought to be sources of DOC rather than sinks (e.g., Hata et al. 2002; Nakajima et al. 2009, 2010). However, in other cases, corals took up more DOC than they released or produced more particulate organic carbon (POC) than DOC, that rapidly settled to the bottom (e.g., Tanaka et al. 2008; Naumann et al. 2010). Thus, the generalization that corals are net producers of DOC remains unclear. In addition, the amount of DOC production by corals under normal circumstances may vary with conditions of light, temperature, and the nutritional state of the colony (Wild et al. 2010b; Mueller et al. 2014b; Courtial et al. 2017). There may also be a delay in the time it takes to convert DOC to an assimilatable form (e.g., simple sugars described below). Dissolved organics that are decomposed within hours or days are referred to as labile, compared with semi-labile material that may require a month. Refractory DOC may persist for many months or longer while resisting bacterial growth, perhaps due to antibiotic properties, organic composition, or other factors that influence its bioavailability (Tanaka et al. 2008, 2011; Taniguchi et al. 2014).
Tropical coral reefs survive in oligotrophic waters by means of efficient recycling mechanisms (Wild et al. 2004b; Bourne et al. 2016) including the disposition of mucus. Mucus DOC that is hydrolyzed by microbial activity releases simple sugars, typically including arabinose, mannose, galactose, glucose, and N-acetyl-glucosamine, along with rhamnose, fucose, and xylose in some (Wild et al. 2005). The dissolved fraction may also contain amino acids (Wild et al. 2010b; Nelson et al. 2013). Coral-derived DOC is an excellent medium that induces a rapid increase in bacterial growth (Allers et al. 2008; Nakajima et al. 2015). It also influences the growth and abundance of heterotrophic nanoflagellates that are able to transfer carbon to higher trophic levels through the microbial loop (Nakajima et al. 2017b).
Although mucus DOC is thought to be predominantly recycled and remineralized by microbes in the sediment (e.g., Wild et al. 2004a), it is not clear how much of that process takes place in the water column above the corals (e.g., Walsh et al. 2017), in the coral mucus or tissues, in calcareous reef sands, or within the porous structure of the reef itself (Scheffers et al. 2004; Rusch et al. 2009; Schöttner et al. 2011). Indeed, while coral-associated bacteria may play a key role in the uptake of dissolved organic matter by the holobiont (e.g., Ferrier-Pagès et al. 1998a), coral tissues also appear well suited to that task. The epidermis of their column walls, tentacles, as well as their oral disk and pharynx are similar to the absorptive epithelia of the mammalian kidney and duodenum as Goreau et al. (1971) have pointed out.
Although the ability of corals to take up glucose has been documented (references in Houlbrèque and Ferrier-Pagès 2009), it can be metabolized by zooxanthellae and incorporated into lipids, an activity that is light enhanced (e.g., Oku et al. 2003). This is interesting because lipid production, while vital for growth and storage, is typically envisioned as a function of either photoautotrophy or heterotrophy by the capture of zooplankton (e.g., Baumann et al. 2014). Sorokin (1973) found that radiolabeled DOC constituted up to 20% of the total daily carbon assimilated by several important coral genera. Thus, while the production of DOC by corals is well known, DOC uptake appears to be relatively small compared with sponges, which take up >80% of their carbon as dissolved organic matter (e.g., de Goeij et al. 2008; Mueller et al. 2014c), an ability that may reflect the function of their associated microbiota (e.g., Hoer et al. 2018).
5.2 Sources of Dissolved Organic Nitrogen
Corals can obtain organic nitrogen from a variety of sources including protein from digested plankton (Ferrier-Pagès et al. 2003; Houlbrèque et al. 2004b) or ingestion of nitrogen-rich sediment particles (Mills and Sebens 2004; Mills et al. 2004a). Mucus production and release may represent a loss of dissolved organic nitrogen (DON) for corals that produce it, although the composition is quite variable, ranging from 5 to 59% by dry weight (Ducklow and Mitchell 1979; Meikle et al. 1988), and release rates are greatly influenced by colonial light and feeding history (Ferrier-Pagès et al. 1998a). Mucus nitrogen is enhanced by the activity of N2-fixing microbes, which can increase the nitrogen content 79-fold after only 3 h (e.g., Huettel et al. 2006; Grover et al. 2014). Other sources of DON (and DOC) include high molecular weight compounds released by benthic reef algae including proteins, and other forms of organic nitrogen (Haas and Wild 2010), as well as estuarine input of humic and fulvic acids (Jaffé et al. 2004), all of which are high molecular weight compounds that are unavailable as nutrients for corals without microbial intervention. Urea, C=O(NH2)2, is an important, low-molecular-weight source of nitrogen on coral reefs and is available from the excreta of various organisms including fishes (Meyer and Schultz 1985; Schantz et al. 2017) and seabirds (Lorrain et al. 2017), as well as from heterotrophic feeding by corals. Fishes and seabirds may create ephemeral hotspots of urea that can be up to 20 times the ambient concentration (Crandall and Teece 2012) even though the typical urea levels in seawater are 0–13 μmol−l (e.g., Suzuki and Casareto 2011). Zooxanthellae are capable of using nitrogen in this form, even in nanomolar concentrations (Houlbrèque and Ferrier-Pagès 2009), but some corals can assimilate urea at least fourfold over uptake by the algal fraction (Grover et al. 2006).
Free amino acids represent another form of nitrogen that is available to zooxanthellae (e.g., Sorokin 1973; Ferrier 1991). Grover et al. (2008) found that 15N-labeled amino acids are taken up within 2 h at 200–500 nmol, concentrations similar to those found in situ. Together with inorganic sources of nitrogen (considered below), dissolved free amino acids are capable of supplying nearly 100% of nitrogen required for tissue growth in Stylophora pistillata. Cold-water corals are also capable of taking up amino acids, albeit at lower rates than their tropical counterparts (Gori et al. 2014).
5.3 Dissolved Inorganic Nitrogen (DIN)
Nitrogen is ordinarily a limiting nutrient for coral growth and metabolism in oligotrophic tropical seas (Hatcher 1990). However, both the coral host and their zooxanthellae are able to assimilate DIN primarily in the form of ammonium (NH4+), and nitrate (NO3−) to a lesser extent, even at very low concentrations (Muscatine and D’Elia 1978; D’Elia et al. 1983; Grover et al. 2003). Ammonium is the favored form of nitrogen assimilation by corals, and it is most readily available from the digestion of prey in the coelenteron (Grover et al. 2002, 2008). Although the hosts have the ability to assimilate DIN, uptake is more rapid in algal cells, and they accumulate this form of nitrogen 14–23 times more quickly compared with host tissue (Grover et al. 2002; Pernice et al. 2012; Tanaka et al. 2015). Nitrogenous waste products from heterotrophic feeding by the host are either lost to the environment or recycled to zooxanthellae, which may store assimilated nitrogen in the form of uric acid crystals if conditions allow, or the symbionts may actively translocate simple N-compounds to their hosts (Reynard et al. 2009; Kopp et al. 2013).
Coral reefs are sources of fixed nitrogen, that is, conversion of elemental N2 to ammonium, and the process has been reported from corals (Lesser et al. 2004, 2007) as well as from other members of the benthic reef community (e.g., Charpy-Roubaud et al. 2001). While initially thought to be primarily the work of cyanobacteria, recent studies have shown that corals harbor diverse communities of heterotrophic bacteria and archaea that fix nitrogen and produce a distinctive diazotroph-derived nitrogen, DDN (e.g., Olson et al. 2009; Olson and Lesser 2013; Benavides et al. 2016, 2017). A large number of these microbes are found within the surface mucus layer where they produce 400 times the amount of ammonium in seawater, perhaps due to the enhanced organic carbon and inorganic phosphate content of the mucins. Phosphate is required for nitrogen fixation as described below. Corals may capture diazotrophs during feeding, although this activity appears to be dependent on the environment and the nutritional condition of the host, among other factors (Bednarz et al. 2017). Tropical symbiotic corals in shallow water are capable of DDN production, especially during summer months when inorganic nutrient concentrations are lowest (Cardini et al. 2015). However, symbiotic corals in shallow water take up little DDN, perhaps only 1% of their total nitrogen requirements. Those that depend more on heterotrophy (e.g., corals in low-light environments, corals that are bleached, and asymbiotic deepwater corals) assimilate 15-fold more DDN than their shallow-water counterparts (Cardini et al. 2014; Bednarz et al. 2017).
A majority of the N2-fixation gene sequences from several corals are related to Rhizobium, the diazotroph responsible for nitrogen nutrition in the roots of leguminous plants. Legume-associated rhizobia only fix nitrogen under oxygen-limited conditions; thus, it is possible that the process may be limited to nighttime. However, not all diazotrophs are rhizobia-like and oxygen levels do not limit all nitrogenases (reviewed by Benavides et al. 2017).
Other elements of the nitrogen cycle including nitrification, the oxidation of ammonium to nitrite (NO2−) or nitrate (NO3−), have been found associated with coral mucus, tissues, and the skeleton, and are abundant in the microbiomes of several species. The substrate appears to be ammonium derived from coral metabolism and may represent a mechanism of preventing nitrogen loss (reviewed by Rädecker et al. 2015). Likewise, nitrite reduction to ammonia, denitrification, and nitrification all appear to be active within carbonate reef sediments, but further work will be required to determine the ecological significance of these nitrogen cycle elements for corals and the reef ecosystem (Erler et al. 2014; Cook et al. 2017).
5.4 Dissolved Forms of Phosphorus
Dissolved organic phosphorus in open tropical and subtropical waters exhibit a distinct seasonality due to plankton blooms where dissolved organic phosphate (DOP) often accounts for >80% of the total dissolved phosphorus pool (Lomas et al. 2010). It is a key nutrient for phytoplankton growth, particularly in the production of nucleic acids and phospholipids, as well as for intracellular energy through the production of ATP. By contrast, oligotrophic surface reef waters are typically depleted of phosphorous with concentrations often well below 0.5 μmol l−1 (Ferrier-Pagès et al. 2016). Corals typically take up phosphorus as dissolved inorganic phosphate (DIP), in the form of orthophosphate, PO43−. Sources include plankton lysis within the microbial loop, runoff from rain, upwelling, sediment resuspension, and microbial degradation of coral mucus (e.g., Wild et al. 2005; Nakajima et al. 2015; den Haan et al. 2016). Zooxanthellae take up significant quantities of this nutrient and have a high affinity for it. They can store phosphorus (see below) when abundant, but it is most often rapidly metabolized and rendered undetectable (D’Elia 1977; Jackson and Yellowlees 1990; Yellowlees et al. 2008). While phosphorus cannot be fixed as nitrogen can, phosphorus is required for N2 fixation due to the considerable ATP demands of nitrogenase production by diazotrophs (Mills et al. 2004b; reviewed by Sohm et al. 2011). Thus, the two nutrients are linked, and the addition of small amounts of phosphorus enhances the uptake of nitrogen (Davy et al. 2012; Tanaka et al. 2015). Phosphate is critical for photosynthesis, likely from its multiple products in the Calvin cycle (e.g., Martin et al. 2000), although this has not been specifically examined in zoooxanthellae. Likewise, phospholipids are incorporated into the organic matrix of corals and may be vital for the control of nucleation and crystal growth of the skeleton (Ferrier-Pagès et al. 2016 and contained references). However, an undersupply of phosphate coupled to an increase in inorganic nitrogen disrupts photosynthesis and causes a loss of coral biomass, ultimately leading to coral disease and bleaching (Rosenberg et al. 2007; Wiedenmann et al. 2012; Rosset et al. 2017).
Even at low concentrations of DIP, uptake by the symbiotic association is both rapid and is typically light enhanced. Host symbiosome membranes that surround the zooxanthellae in hospite are associated with ATPase activity and perhaps represent an active transport mechanism for phosphate. This suggests a way to control the flux of nutrients across the symbiotic interface by the host (Godinot et al. 2009, 2011; Ferrier-Pagès et al. 2016). However, uptake is influenced by a variety of factors including the availability of external and internal reserves and feeding history (D’Elia 1977; Godinot et al. 2009; Ferrier-Pagès et al. 2016). Internal reserves may be different when comparing host to symbiont. Godinot et al. (2016) found that feeding increased intracellular phosphate in host tissue, but primarily in the form of phosphonates, compounds in which P is linked to carbon, along with smaller amounts of phosphate esters. The formation of these potential storage forms of phosphorus appears to be independent of zooxanthellae, which become enriched with intracellular phosphate when their culture media is augmented with inorganic phosphate. However, intracellular phosphate becomes depleted in the presence of organic phosphate sources, indicating that they are unable to use that form (Godinot et al. 2016).
Corals are able to obtain phosphate from feeding on plankton including picoplankton, presumably from digestion and reduction to DIP (Sorokin 1973). Indeed, heterotrophic and autotrophic picoplankton are capable of appreciable DOP uptake (Karl and Björkman 2015), suggesting that these organisms may be a rich source of source phosphate. Interestingly, some coral species, or perhaps their symbionts, apparently are able to assimilate DOP directly (Wijgerde et al. 2011). However, a balance sheet for phosphate uptake will have to be reconciled with the release of organic phosphates into the environment, which apparently occurs during both day and night (D’Elia 1977; Sorokin 1992). The formation of phosphorus compounds, their apportionment between host and symbiont, and environmental feedbacks that may control them are complex and currently are poorly understood.
6 Heterotrophic Feeding in the Octocorallia
Octocorals in general appear to feed on zooplankton, especially epibenthic copepods and particulate material. The survey undertaken by Lewis (1982) found that food was captured by tentacles in a raptorial manner and was then taken to the coelenteron by ciliary currents in the mouth and pharynx. No mucus or other capture devices (i.e., mesenterial filaments) were employed, as octocorals are not known to protrude them in pursuit of prey (e.g., Fabricius and Alderslade 2001). Octocorals include those with flexible skeletons (gorgonians) tend to orient their branches perpendicular to the prevailing current direction, thereby maximizing the volume of water passing the polyps (Wainwright and Dillon 1969). Whether on flexible skeletons or not (e.g., alcyonacean soft corals), the polyps are bent by moderate flows and likewise are able to feed downstream either at the tentacle tips or on the pinnules (Lasker 1981; Patterson 1991). As in all passive suspension feeders, feeding efficiency is flow-dependent, and differences may be related to polyp morphologies. Those octocorals with tall polyps encounter higher drag and are more easily deformed in currents with flow velocities > 10 cm sec−1, thus interfering with food capture. Conversely, octocorals with short polyps are less readily deformed in currents and feed in a wider range of flow forces, up to 40 cm sec−1 (Dai and Lin 1993).
Unlike the simple conical tentacles of scleractinians (Fig. 18.2), the arrangement of multiple polyps and their pinnate tentacles form a comb-like filter that can function efficiently as a passive plankton trap depending on pinnule length, shape, and density (Fig. 18.8).
Tentacles from two species of Dendronephthya, azooxanthellate soft corals from the Red Sea are shown, D. hemprichi (a, b) and D. sinaiensis (c, d). Pinnule density and tentacle length likely represent differences in capture efficiency. After Grossowicz and Benayahu (2012) courtesy of the authors
Denser pinnules have a greater chance of capturing small particles. Elongated pinnules have a larger surface area and thus have greater capture efficiencies (Lasker et al. 1983; Grossowicz and Benayahu 2012). Correspondingly, the octocoral diet differs from scleractinians, even though there may be differences among asymbiotic polar, temperate, and tropical species, and those within temperate and tropical regions that are symbiotic.
6.1 Alcyonacean Soft Corals
Alcyonaceans can dominate the shallow waters of the Indo-Pacific. They are typically zooxanthellate, and while some may exhibit low photosynthesis and respiration rates compared with scleractinians (Fabricius and Klumpp 1995), that is not always the case. The P:R ratio in most symbiotic alcyonaceans studied by Sorokin (1991) was more than 1 (1.4–2.7), perhaps suggesting that they are autotrophs. Likewise, xeniid octocorals are zooxanthellate, but unlike other corals, they actively pulsate, thrusting polyps upward into the boundary layer (Kremien et al. 2013). This movement increases the oxygen content of the surrounding water and enhances both net photosynthesis and feeding efficiency, the expenditure of energy in motion notwithstanding. Zooxanthellate alcyonaceans that have been studied are mixotrophic and deplete the water of particulate matter passing over them (Fabricius and Dommisse 2000). Examination of the gut contents from several species has shown that they feed on zooplankton, especially epibenthic copepods (Lewis 1982). However, using radiocarbon labeling, Sorokin (1991) concluded that most of the octocorals he examined appeared to be poor consumers of microzooplankton and fed instead on bacterioplankton and dissolved organic matter. Likewise, xeniids supplement their photoautotrophic nutrition with particulate organic carbon and nitrogen and DOM (Schlichter 1982; Bednarz et al. 2012). Thus, the diet and the degree to which these corals depend on zooplankton are not yet clear. Tropical soft corals without zooxanthellae have an eclectic diet. Those with a pinnule mesh of 60–80 μm capture weakly swimming invertebrate larvae, but >90% of the carbon demand was met by phytoplankton (Fabricius et al. 1995a, b). Following 14C-labeled diatoms and dinoflagellates, Widdig and Schlichter (2001) found that phytoplankton were digested in the coelenteron and were apparently transported there by ciliary activity of the mouth and pharynx without the assistance of mucus. The same authors noted that detritus was also part of the diet of this group. By contrast, tropical azooxanthellate soft corals studied by Sorokin (1991), as well as those in the genus Alcyonium (Migne and Davoult 2002), consumed nearly five times as much zooplankton as phytoplankton in temperate waters. An Alcyonium congener similarly appeared to show strong affinity for the capture of small zooplankton (~300 μm) and detritus particles, but not phytoplankton. This approximately corresponded with its 200–280 μm pinnule spacing, although the tentacle tips intercepted most particulate material (Sebens and Koehl 1984; Sebens 1984). Digestion has been little studied in this group although Pratt (1905) noted that Alcyonium digitatum incorporated carmine-stained fish macerate into digestive vacuoles of the mesenterial filaments. From there, food particles were then carried through the colony by amoeboid cells in the solenial canals.
6.2 Gorgonian Octocorals
Studies of feeding in zooxanthellate gorgonian octocorals have reported the capture and ingestion of particulate matter (Artemia cysts) as measured in flow tank experiments (e.g., Lasker 1981; Lasker et al. 1983). However, examination of the coelenteron has shown that zooplankton in the range of 100–700 μm also are captured, with larger prey items corresponding to species with larger polyps. In addition, microplankton and nanoplankton (diatoms, dinoflagellates, and ciliates) were consumed, but the contribution of these items to heterotrophically derived carbon was about half that of the larger zooplankton. Picoplankton was not a significant part of the diet (Ribes et al. 1998; Coma et al. 1998). Coffroth (1984) found that one tropical species readily ingested mucus. It appears that the carbon balance sheet for tropical symbiotic gorgonians has yet to be directly reconciled, although the consumption of particulate matter by nonsymbiotic species (see below) suggests an additional potential food source.
Temperate gorgonian species show distinct seasonal changes in their diet. The coelenteron contents of a totally heterotrophic species from the Mediterranean (Paramuricea clavata) indicate that zooplankton accounts for an important share of the diet. Nauplii, eggs from copepods and other invertebrates, and other small prey items of low motility (100–200 μm) account for 78% of prey items, though adult calanoid copepods (600–700 μm) were also captured. Phytoplankton (diatoms, dinoflagellates) and nanoeukaryotic flagellates and ciliates were also an important part of the diet, as was particulate organic matter with and without living cells, especially during the winter season when it accounted for 96% of the total ingested carbon (Ribes et al. 1999; Coma et al. 1994, 2001). Leptogorgia sarmentosa, another asymbiotic Mediterranean species, consumed a similar diet composed of microplantonic prey including invertebrate eggs and low-motility larvae, as well as nanoeukaryotes, dinoflagellates, diatoms, ciliates, and detrital POC (Ribes et al. 2003; Rossi et al. 2004). Isotopic signatures suggest a seasonal shift from zooplankton in summer to organic matter in winter (Cocito et al. 2013). As with other temperate gorgonians, picoplankton and DOM consumption was not significant (Coma et al. 1994; Ribes et al. 1999). Similarly, a congener from the temperate Atlantic region, L. virgulata, displayed a seasonal isotopic shift in its diet that signaled a change from heterotrophic nanoplankton to microplankton (Leal et al. 2014b).
Particulate organic matter was found throughout the year in the coelenteron of Corallium rubrum, another Mediterranean octocoral, and was the main source of dietary carbon along with secondary amounts of copepods, invertebrate eggs, and phytoplankton (Tsounis et al. 2006). There were distinct shifts in prey size and ingestion rate (smaller and lower respectively in winter) in this asymbiotic species. However, in addition Picciano and Ferrier-Pagès (2007) demonstrated that detrital organic particulates account for ~93% of their carbon requirements, along with phyto- and zooplanktonic nanoflagellates, and an apparently atypical consumption of picoplankton. A similar seasonal change in diet was noted in another temperate species (Eunicella singularis), a relationship with zooxanthellae notwithstanding (Ferrier-Pagès et al. 2015). Zooxanthellae in this species supply considerably less carbon to their hosts than scleractinians in the same habitat, but reduced respiration rates appear to compensate.
Antarctic octocorals seem to show the same trophic behavior as those studied in other latitudes by feeding primarily on a mixed diet of microplankton. Autotrophic nanoflagellates and small diatoms dominate the Southern Ocean in summer (e.g., Bathmann et al. 1997), and a corresponding menu should not be surprising. However, the evidence suggests that when summer sun and the plankton supply fade, the diet changes to heterotrophic nanoflagellates, dinoflagellates, and ciliates, which are present practically all year. In addition, several species supplement this diet either by feeding on sediment in a bent-over position or by feeding on resuspended bottom material. Stable isotopes suggest that winter meals include phytodetritus that accumulates in the winter as a “green carpet” and microzooplankton (Slattery et al. 1997; Orejas et al. 2003; Elias-Piera et al. 2013). Similar seasonal changes in diet have been noted in an Antarctic sea pen (Servetto et al. 2017). Likewise, fatty acid profiles and stable isotopes of carbon and nitrogen suggest a varied and perhaps opportunistic diet among deepwater octocorals. Specimens from the family Paragorgiidae on the continental slopes of Labrador (Newfoundland) and the Sea of Okhotsk likely feed on fresh phytodetritus, whereas primnoids among other gorgonian families appear to supplement their diet of POM by feeding on herbivorous zooplankton (Sherwood et al. 2008; Imbs et al. 2016).
7 Heterotrophic Feeding in the Antipatharian Corals
Limited observations suggest that antipatharian corals capture zooplankton as a major component of their diet (Lewis 1978; Wagner et al. 2012) with the use of tentacular nematocysts and spirocysts, which appear to be present in abundance (e.g., Goldberg and Taylor 1989a, b; Ocaña et al. 2006; Bo et al. 2008). The coelenteron contents indicate that large copepods, polychaetes, amphipods, ostracods, and chaetognaths are the most common prey (Tazoli et al. 2007). Likewise, the coelenteric cavities of four species were found with the remains of copepods and small zooplankton (Warner 1981; Carlier et al. 2009). Mucus nets are secreted all over the colony surface and likely entrap food particles, as do the mesenterial filaments (Lewis 1978). Stable isotope analyses likewise suggest that particulate organic matter may be an important source of nutrition for these animals (Williams and Grottoli 2010).
8 Questions for Future Research
Scleractinian corals have long been used as models of intracellular symbiosis and the mutual exchange of nutrients. However, further work will be required to determine which symbiotic species can upregulate heterotrophic feeding as a means of compensating for a reduction in photosynthesis. Likewise, the sources and use of nutrients by the holobiont, especially phosphorus, will require further work. The roles of nitrogen fixation in coral nutrition and the participation of the nitrogen cycle on reefs remain to be clarified. The manner in which nano- and picoplankton are captured and brought to the coelenteron is also unclear, however likely it may be that mucociliary mechanics are involved. Likewise, the production of mucus by scleractinian corals is prolific, but how much of either the dissolved or particulate forms of this secretory material they re-consume is still unresolved. Many scleractinians employ mesenterial filaments to procure food, either zooplanktonic or particulate. However, little is known of their functional morphology or chemistry. The tentacles possess spirocysts as the principal type of cnidae, but other than characterization as a glutinant, no studies have shown how they are used to obtain prey. The diet of octocorals is distinct from scleractinians, and less is known about zooxanthellate forms in the tropics than those that have been examined from cold-water and temperate environments. Likewise, the degree of reliance on particulate feeding by octocorals and mucus net feeding by anthipatharians would benefit from further study.
Acknowlegments
I am grateful to my colleagues Christine Ferrier-Pagès, Centre Scientifique de Monaco; Yehuda Benayahu, Tel-Aviv University; and an anonymous reviewer for comments and suggestions that improved the quality of this work.
References
Ainsworth TD, Thurber RV, Gates RD (2010) The future of coral reefs: a microbial perspective. Trends Ecol Evol 25:233–240
Alldredge A, Carlson C, Carpenter R (2013) Sources of organic carbon to coral reef flats. Oceanography 26:108–113
Allers E, Niesner C, Wild C, Pernthaler J (2008) Microbes enriched in seawater after addition of coral mucus. Appl Environ Microbiol 74:3274–3278
Al-Moghrabi S, Allemand D, Couret JM, Jaubert J (1995) Fatty acids of the scleractinian coral Galaxea fascicularis: effect of light and feeding. J Comp Physiol B 165:183–192
Anthony KRN (1999) Coral suspension feeding on fine particulate matter. J Exp Mar Biol Ecol 232:85–106
Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol 252:221–253
Aylward FO, Boeuf D, Mende DR et al (2017) Diel cycling and long-term persistence of viruses in the ocean’s euphotic zone. Proc Natl Acad Sci USA 114:11446–11451
Azam F, Malfatti F (2007) Microbial structuring of marine ecosystems. Nat Rev Microbiol 5:782–792
Azam F, Fenchel T, Field JG et al (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Bachar A, Achituv Y, Pasternak Z, Dubinsky Z (2007) Autotrophy versus heterotrophy: the origin of carbon determines its fate in a symbiotic sea anemone. J Exp Mar Biol Ecol 349:295–298
Bak RPM, Joenje M, de Jong I et al (1998) Bacterial suspension feeding by coral reef benthic organisms. Mar Ecol Prog Ser 175:285–288
Baker DM, Freeman CJ, Wong JCY et al (2018) Climate change promotes parasitism in a coral symbiosis. ISME J 12(3):921–930. https://doi.org/10.1038/s41396-018-0046-8
Bathmann UV, Scharek R, Klass C et al (1997) Spring development of phytoplankton biomass and composition in major water masses of the Atlantic sector of the Southern Ocean. Deep-Sea Res 44:51–57
Battey JF, Patton JS (1984) A reevaluation of the role of glycerol in carbon translocation in zooxanthellae-coelenterate symbiosis. Mar Biol 79:27–38
Baumann J, Grotolli AG, Hughes AD, Matsui Y (2014) Photoautotrophic and heterotrophic carbon in bleached and non-bleached coral lipid acquisition and storage. J Exp Mar Biol Ecol 461:469–478
Bednarz VN, Naumann MS, Niggl W, Wild C (2012) Inorganic nutrient availability affects organic matter fluxes and metabolic activity in the soft coral genus Xenia. J Exp Biol 215:3672–3679
Bednarz VN, Grover R, Maguer J-F et al (2017) The assimilation of diazotroph-derived nitrogen by scleractinian corals depends on their metabolic status. MBio 8(1):e02058-16 http://mbio.asm.org/content/8/1/e02058-16.full
Benavides M, Houlbrèque F, Camps M et al (2016) Diazotrophs: a non-negligible source of nitrogen for the tropical coral Stylophora pistillata. J Exp Biol 219:2608–2612
Benavides M, Bednarz VN, Ferrier-Pagès C (2017) Diazotrophs: overlooked key players within the coral symbiosis and tropical reef ecosystems? Front Mar Sci 4(10). https://doi.org/10.3389/fmars.2017.00010
Benson AA, Muscatine L (1974) Wax in coral mucus: energy transfer from corals to reef fishes. Limnol Oceanogr 19:810–814
Bessell-Brown P, Fisher R, Duckworth A, Jones R (2017) Mucous sheet production in Porites: and effective bioindicator of sediment-related pressures. Ecol Indicators 77:276–285
Bo M, Tazoli S, Spano BG (2008) Antipathella subpinnata (Antipatharia, Myriopathidae) in Italian seas. Ital J Zool 75:185–195
Boschma H (1925) On the feeding reactions and digestion in the coral polyp Astrangia danæ, with notes on its symbiosis with zoöxanthellæ. Biol Bull 49:407–439
Bourne DG, Garren M, Work TM et al (2009) Microbial disease and the coral holobiont. Trends Microbiol 17:554–562
Bourne DG, Morrow KM, Webster NS (2016) Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu Rev Microbiol 70:317–340
Breitbart M (2012) Marine viruses: truth or dare. Annu Rev Mar Sci 4:425–448
Briand MJ, Bonnet X, Goiran C et al (2015) Major sources of organic matter in a complex coral reef lagoon: identification from isotopic signatures (δ13C and δ15N). PLoS One 10(7): e0131555. doi:https://doi.org/10.1371/journal.pone.0131555
Brown BE, Blythell JC (2005) Perspectives on mucus secretion in reef corals. Mar Ecol Prog Ser 296:291–309
Browne NK, Precht E, Last K, Todd PA (2014) Photo-physiological costs associated with acute sediment stress events in three near-shore turbid water corals. Mar Ecol Prog Ser 502:129–143
Burriesci MS, Raab TK, Pringle JR (2012) Evidence that glucose is the major transferred metabolite in dinoflagellate-cnidarian symbiosis. J Exp Biol 215:3467–3477
Bythell JC, Wild C (2011) Biology and ecology of coral mucus release. J Exp Mar Biol Ecol 408:88–93
Calbet A, Saiz E (2005) The ciliate-copepod link in marine ecosystems. Aquat Microb Ecol 157:157–167
Cardini U, Bednarz VN, Foster RA et al (2014) Benthic N2 fixation in coral reefs and the potential effects of human-induced environmental change. Ecol Evol 4:1706–1727
Cardini U, Bednarz VN, Naumann MS et al (2015) Functional significance of dinitrogen fixation in sustaining coral productivity under oligotrophic conditions. Proc R Soc B 282:20152257. https://doi.org/10.1098/rspb.2015.2257
Carlier A, Le Guilloux E, Olu K et al (2009) Trophic relationships in a deep Mediterranean cold-water bank (Santa Maria di Leuca, Ionian Sea). Mar Ecol Prog Ser 397:125–137
Carlson CA, Hansell DA (2015) DOM sources, sinks, reactivity and budgets. In: Hansell DA, Carlson CA (eds) Biogeochemistry of marine dissolved organic matter, 2nd edn. Elsevier, Amsterdam, pp 65–126
Carpenter FW (1910) Feeding reactions of the rose coral (Isophyllia). Proc Am Acad Arts Sci 46:149–162
Charpy L, Blanchot J (1999) Picophytoplankton biomass, community structure and productivity in the Great Astrolabe Lagoon, Fiji. Coral Reefs 18:255–262
Charpy-Roubaud C, Charpy L, Larkum AWD (2001) Atmospheric dinitrogen fixation by benthic communities of Tikehau lagoon (Tuamotu Archipelago, French Polynesia) and its contribution to benthic primary production. Mar Biol 139:991–997
Clayton WS, Lasker HR (1982) Effects of light and dark treatments on feeding by the reef coral Pocillopora damicornis (Linnaeus). J Exp Mar Biol Ecol 63:269–279
Cocito S, Ferrier-Pagès C, Cupido R et al (2013) Nutrient acquisition in four Mediterranean gorgonian species. Mar Ecol Prog Ser 473:179–188
Coddeville B, Maes E, Ferrier-Pagès C, Guerardel Y (2011) Glycan profiling of gel forming mucus layer from the scleractinian symbiotic coral Oculina arbuscula. Biomolecules 12:2064–2073
Coffroth MA (1984) Ingestion and incorporation of coral mucus aggregates by a gorgonian soft coral. Mar Ecol Prog Ser 17:193–199
Coffroth MA (1990) Mucus sheet formation on poritid corals- an evaluation of mucus as a nutrient source on reefs. Mar Biol 105:39–49
Coma R, Gili J-M, Zabala M, Riera T (1994) Feeding and prey capture cycles in the aposymbiotic gorgonian Paramuricea clavata. Mar Ecol Prog Ser 155:257–270
Coma R, Ribes M, Gili JM, Zabala M (1998) An energetic approach to the study of life-history traits of two modular colonial benthic invertebrates. Mar Ecol Prog Ser 162:89–103
Coma R, Ribes M, Gili J-M, Hughes RN (2001) The ultimate opportunists: consumers of seston. Mar Ecol Prog Ser 219:305–308
Cook PLM, Kessler AJ, Eyre BD (2017) Does denitrification occur within porous carbonate grains? Biogeosciences 14:4061–4069
Cooper TF, Lai M, Ulstrup KE et al (2011) Symbiodinium genotypic and environmental controls on lipids in reef building corals. PLoS One 6(5):e20434. https://doi.org/10.1371/journal.pone.0020434
Courtial L, Ferrier-Pagès C, Jacquet S et al (2017) Effects of temperature and UVR on organic matter fluxes and the metabolic activity of Acropora muricata. Biol Open 6:1190–1199
Crandall JB, Teece MA (2012) Urea is a dynamic pool of available nitrogen on coral reefs. Coral Reefs 31:207–214
Crossland CJ (1987) In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes. Coral Reefs 6:35–42
Crossland CJ, Barnes DJ, Borowitzka MA (1980) Diurnal lipid and mucus production in the staghorn coral Acropora acuminata. Mar Biol 60:81–90
D’Elia CF (1977) The uptake and release of dissolved phosphorus by reef corals. Limnol Oceanogr 22:301–315
D’Elia CF, Domotor SL, Webb KL (1983) Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar Biol 75:157–167
Dai C-F, Lin M-C (1993) The effects of flow on feeding of three gorgonians from southern Taiwan. J Exp Mar Biol Ecol 173:57–69
Darwin C (1842) The structure and distribution of coral reefs. Smith, Elder and Co, London (Reprinted 1962, University of California Press)
Davies PL (1984) The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs 2:181–186
Davy SK, Allemand D, Weis VM (2012) Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol Rev 76:229–262
de Goeij J, Van den Berg H, Van Oostveen M et al (2008) Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar Ecol Prog Ser 357:139–151
den Haan J, Huisman J, Brocke HJ et al (2016) Nitrogen and phosphorous uptake rates from different species from a coral reef community after a nutrient pulse. Sci Rep 6:28821
Dodds LA, Black KD, Orr H, Roberts JM (2009) Lipid biomarkers reveal geographic differences in food supply to the cold-water coral Lophelia pertusa (Scleractinia). Mar Ecol Progr Ser 397:113–124
Dubinsky Z, Jokiel PL (1994) Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pac Sci 48:313–324
Ducklow HW, Mitchell R (1979) Composition of mucus released by reef coelenterates. Limnol Oceanogr 24:706–714
Duerden JE (1902) West Indian madreporarian polyps. Mem Natl Acad Sci 7:309–649
Duerden JE (1906) The role of mucus in corals. Quart J Microsc Sci 49:591–614
Duineveldt GCA, Jeffreys RM, Lavaleye MSS et al (2012) Mar Ecol Prog Ser 444:97–115
Edmunds PJ, Davies PS (1989) An energy budget for Porites porites (Scleractinia) growing in a stressed environment. Coral Reefs 8:37–43
Einbinder S, Mass T, Brokovich E et al (2009) Changes in morphology and diet of the coral Stylophora pistillata along a depth gradient. Mar Ecol Prog Ser 381:167–174
Elias-Piera F, Rossi S, Gili J-M, Orejas C (2013) Trophic ecology of seven Antarctic gorgonian species. Mar Ecol Prog Ser 477:93–106
Erler BD, Santos IR, Eyre D (2014) Inorganic transformations within permeable carbonate sands. Cont Shelf Res 77:69–80
Eyal G, Wiedenmann J, Grinblat M et al (2015) Spectral diversity and regulation of coral fluorescence in a mesophotic reef habitat in the Red Sea. PLoS One 10(6):e0128697. https://doi.org/10.1371/journal.pone.0128697
Fabricius K, Alderslade P (2001) Soft corals and sea fans. Australian Institute of Maine Sciences, Townsville
Fabricius KE, Dommisse M (2000) Depletion of suspended particulate matter over coastal reef communities dominated by zooxanthellate soft corals. Mar Ecol Prog Ser 196:157–167
Fabricius KE, Klumpp DW (1995) Widespread mixotrophy in reef-inhabiting soft corals: the influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser 125:195–204
Fabricius KE, Benayahu Y, Genin A (1995a) Herbivory in asymbiotic soft corals. Science 268:90–92
Fabricius KE, Genin A, Benayahu Y (1995b) Flow-dependent herbivory and growth in a zooxanthellae-free soft coral. Limnol Oceanogr 40:1290–1301
Falkowski PG, Dubinsky Z, Muscatine L, Porter JW (1984) Light and energetics of a symbiotic coral. Bioscience 34:705–709
Fenchel T (1982) Ecology of heterotrophic microflagellates. 1. Some important forms and their functional morphology. Mar Ecol Prog Ser 8:211–223
Ferrier MD (1991) Net uptake of dissolved free amino acids by four scleractinian corals. Coral Reefs 10:183–187
Ferrier-Pagès C, Furla P (2001) Pico- and nanoplankton biomass and production in the two largest atoll lagoons of French Polynesia. Mar Ecol Prog Ser 211:63–76
Ferrier-Pagès C, Gattuso J-P (1998) Biomass, production and grazing rates of pico- and nanoplankton in coral reef waters (Miyako Island, Japan). Microb Ecol 35:46–57
Ferrier-Pagès C, Gatusso J-P, Cauwet G et al (1998a) Release of dissolved organic carbon and nitrogen by the zooxanthellate coral Galaxea fascicularis. Mar Ecol Prog Ser 172:265–274
Ferrier-Pagès C, Allemand D, Gattuso J-P et al (1998b) Microheterotrophy in the zooxanthellate coral Stylophora pistillata: effects of light and ciliate density. Limnol Oceanogr 43:1639–1648
Ferrier-Pagès C, Leclercq N, Jaubert J, Pelegri SP (2000) Enhancement of pico- and nanoplankton growth by coral exudates. Aquat Microb Ecol 21:203–209
Ferrier-Pagès C, Witting J, Tambutté E, Sebens KP (2003) Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs 22:229–240
Ferrier-Pagès C, Rottier C, Beraud E, Levy O (2010) Experimental assessment of the feeding effort of three scleractinian coral species during a thermal stress: effect on the rates of photosynthesis. J Exp Mar Biol Ecol 390:118–124
Ferrier-Pagès C, Hoogenboom M, Houlbrèque F (2011a) The role of plankton in coral trophodynamics. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 215–229
Ferrier-Pagès C, Peirano A, Abbate M et al (2011b) Summer autotrophy in the temperate symbiotic coral Cladocora cespitosa. Limnol Oceanogr 56:1429–1438
Ferrier-Pagès C, Reynaud S, Beraud E et al (2015) Photophysiology and daily primary production of a temperate symbiotic gorgonian. Photosynth Res 123:95–1204
Ferrier-Pagès C, Godinot C, D’Angelo C (2016) Phosphorus metabolism of reef organisms with algal symbionts. Ecol Monogr 86:262–277
Fonvielle JA, Reynaud S, Jaquet S et al (2015) First evidence of an important organic matter trophic pathway between temperate corals and pelagic microbial communities. PLoS One 10(10):e0139175. https://doi.org/10.1371/journal.pone.0139175
Frazão B, Vasconcelos V, Antunes A (2012) Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: an overview. Mar Drugs 10(8):1812–1851. https://doi.org/10.3390/md10081812
Furnas MJ, Mitchell AW (1986) Phytoplankton dynamics in the central Great Barrier Reef-I. Seasonal changes in biomass and community structure and their relation to intrusive activity. Cont Shelf Res 6:363–384
Garren M, Azam F (2011) New directions in coral reef microbial ecology. Environ Microbiol 14:833–844
Gattuso J-P, Yellowlees D, Lesser M (1993) Depth- and light-dependent variation of carbon partitioning and utilization in the zooxanthellate coral Stylophora pistillata. Mar Ecol Prog Ser 92:267–276
Genin A, Monismith SG, Reidenbach MA et al (2009) Intense benthic grazing of phytoplankton in a coral reef. Limnol Oceanogr 54:938–951
Glasl B, Herndl GJ, Frade PR (2016) The microbiome of coral surface mucus has a key role in mediating holobiont health and survival upon disturbance. ISME J 10:2280–2292
Godinot C, Ferrier-Pagès C, Grover R (2009) Control of phosphate uptake by zooxanthellae and host cells in the scleractinian coral Stylophora pistilata. Limnol Oceanogr 54:1627–1633
Godinot C, Grover R, Allemand D, Ferrier-Pagès C (2011) High phosphate uptake requirements of the scleractinian coral Stylophora pistillata. J Exp Biol 214:2749–2754
Godinot C, Gaysinki M, Thomas OP et al (2016) On the use of 31P NMR for the quantification of hydrosoluble phosphorous-containing compounds in coral host tissues and cultured zooxanthellae. Sci Rep 6:21760
Goldberg WM (2002a) Feeding behavior, epidermal structure and mucus cytochemistry of the scleractinian Mycetophyllia reesi, a coral without tentacles. Tissue Cell 24:232–245
Goldberg WM (2002b) Gastrodermal structure and feeding responses in the scleractinian Mycetophyllia reesi, a coral without tentacles. Tissue Cell 34:246–261
Goldberg WM (2004) Epidermal structure of the scleractinian coral Mycetophyllia ferox: light-induced vesicles, copious mucocytes, and sporadic tentacles. Hydrobiologia 530:451–458
Goldberg WM, Taylor GT (1989a) Cellular structure and ultrastructure of the black coral Antipathes aperta: 1. Organization of the tentacular epidermis and nervous system. J Morphol 202:239–253
Goldberg WM, Taylor GT (1989b) Cellular structure and ultrastructure of the black coral Antipathes aperta: 2. The gastrodermis and its collar cells. J Morphol 202:255–269
Goldberg WM, Taylor GT (1996) Ultrastructure of the spirocyst tubule in black corals (Coelenterata: Antipatharia) and its taxonomic implications. Mar Biol 125:655–662
Goreau TF, Goreau NI, Yonge CM (1971) Reef corals: autotrophs or heterotrophs? Biol Bull 141:247–260
Gori A, Grover R, Orejas C et al (2014) Uptake of dissolved free amino acids by four cold-water coral species from the Mediterranean Sea. Deep Sea Res 99:42–50
Gottfried M, Roman MR (1983) Ingestion and incorporation of coral-mucus detritus by zooplankton. Mar Biol 72:211–218
Granek EF, Compton JE, Phillips DL (2009) Mangrove-exported nutrient incorporation by sessile coral reef invertebrates. Ecosystems 12:462–472
Grossowicz M, Benayahu Y (2012) Differential morphological features of two Dendronepthya soft coral species suggest differences in feeding niches. Mar Biodiv 42:65–72
Grottoli AG, Rodrigues LJ, Juarez C (2004) Lipids and stable carbon isotopes in two species of Hawaiian corals, Montipora verrucosa and Porites compressa, following a bleaching event. Mar Biol 145:621–631
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189
Grover R, Maguer J-F, Reynard-Vaganay S, Ferrier-Pagès C (2002) Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol Oceanogr 47:782–790
Grover R, Maguer J-F, Allemand D, Ferrier-Pagès C (2003) Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol Oceanogr 48:2266–2274
Grover R, Maguer J-F, Allemand D, Ferrier-Pagès C (2006) Urea uptake by the scleractinian coral Stylophora pistillata. J Exp Mar Biol Ecol 332:216–225
Grover R, Maguer JF, Allemand D, Ferrier-Pagès C (2008) Uptake of dissolved free amino acids by the scleractinian coral Stylophora pistillata. J Exp Biol 211:860–865
Grover R, Ferrier-Pagès C, Maguer J-F et al (2014) Nitrogen fixation in the mucus of Red Sea corals. J Exp Biol 217:3962–3963
Gustafsson MSM, Baird ME, Ralph PJ (2013) The interchangeability of autotrophic and heterotrophic nitrogen sources in scleractinian coral symbiotic relationships: a numerical study. Ecol Model 250:183–194
Haas AF, Wild C (2010) Composition analysis of organic matter released by cosmopolitan coral reef-associated green algae. Aquat Biol 10:131–138
Haas AF, Naumann MS, Struck U et al (2010) Organic matter release by coral reef associated benthic algae in the northern Red Sea. J Exp Mar Biol Ecol 389:53–60
Haas AF, Nelson CE, Wegley Kelly L et al (2011) Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS One 6:e27973
Hanson CE, McLaughlin MJ, Hyndes GA, Strzelecki J (2009) Selective uptake of prokaryotic picoplankton by a marine sponge (Callyspongia sp.) within an oligotrophic coastal system. Estuar Coast Shelf Sci 84:289–297
Hata H, Setsuko K, Yamano H et al (2002) Organic carbon flux in Shiraho coral reef (Ishigaki Island, Japan). Mar Ecol Prog Ser 232:129–140
Hatcher BG (1988) Coral reef primary productivity: a beggar’s banquet. Trends Ecol Evol 3:106–111
Hatcher BG (1990) Coral reef primary productivity. A hierarchy of pattern and process. Trends Ecol Evol 5:149–155
Heck KL, Carruthers TJB, Duarte CM et al (2008) Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial communities. Ecosystems 11:1198–1210
Heidelberg KB, Sebens KP, Purcell JE (2004) Composition and sources of near-reef zooplankton on a Jamaican reef along with implications for coral feeding. Coral Reefs 23:263–276
Hessinger DA (1988) Nematocyst venoms and toxins. In: Hessinger DA, Lenhoff HM (eds) The biology of nematocysts. Academic Press, New York, pp 333–368
Hii Y-S, Soo C, Liew H (2009) Feeding of scleractinian coral, Galaxea fascicularis, on Artemia salina nauplii in captivity. Aquacult Int 17:363–376
Hoer DR, Gibson PJ, Tommerdahl JP et al (2018) Consumption of dissolved organic carbon by Caribbean reef sponges. Limnol Oceanogr 63:337–351
Hoogenboom M, Rodolfo-Metalpa R, Ferrier-Pagès C (2010) Co-variation between autotrophy and heterotrophy in the Mediterranean coral Cladocora caespitosa. J Exp Biol 213:2399–2409
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17
Houlbrèque F, Tambutté E, Allemand D, Ferrier-Pagès C (2004a) Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J Exp Biol 207:1461–1469
Houlbrèque F, Tambutté E, Richard C, Ferrier-Pagès C (2004b) Importance of a micro-diet for scleractinian corals. Mar Ecol Prog Ser 282:151–160
Houlbrèque F, Delesalle B, Blanchot J et al (2006) Picoplankton removal by the coral reef community of La Prévoyante, Mayotte Island. Aquat Microb Ecol 44:59–70
Huettel M, Wild C, Gonelli S (2006) Mucus trap in coral reefs: formation and temporal evolution of particle aggregates caused by coral mucus. Mar Ecol Prog Ser 307:69–84
Hughes AD, Grottoli AG (2013) Heterotrophic compensation: a possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS One 8:e81172
Imbs AB, Yakovleva IM (2012) Dynamics of lipid and fatty acid composition of shallow-water corals under thermal stress: an experimental approach. Coral Reefs 31:41–53
Imbs AB, Latyshev NA, Dautova TN (2010) Distribution of lipids and fatty acids in corals by their taxonomic position and presence of zooxanthellae. Mar Ecol Prog Ser 409:65–75
Imbs AB, Demidkpva DA, Dautova TN (2016) Lipids and fatty acids of cold-water soft corals and hydrocorals: a comparison with tropical species and implications for coral nutrition. Mar Biol 163:202–213
Jackson AE, Yellowlees D (1990) Phosphate uptake by zooxanthellae isolated from corals. Proc R Soc B 242:201–204
Jacobi Y, Yahel G, Shenkar N (2017) Efficient filtration of micron and submicron particles by ascidians from oligotrophic waters. Limnol Oceanogr. https://doi.org/10.1002/lno.10736
Jaffé R, Boyer JN, Lu X et al (2004) Source characterization of dissolved organic matter in a subtropical mangrove-dominated estuary by fluorescence analysis. Mar Chem 84:195–210
Jatkar AA, Brown BE, Bythell JC et al (2010) Coral mucus: the properties of its constituent mucins. Biomacromolecules 11:883–888
Johnson AS, Sebens KP (1993) Consequences of a flattened morphology: effects of flow on feeding rates of the scleractinian coral Meandrina meandrites. Mar Ecol Prog Ser 99:99–114
Jouiaei M, Yanagihara AA, Madio B et al (2015) Ancient venom systems: a review on Cnidaria toxins. Toxins 7:2251–2271
Karl DM, Björkman KM (2015) Dynamics of dissolved organic phosphorus. In: Hansell DA, Carlson CA (eds) Dynamics of dissolved marine organic matter. Academic Press, Burlington, pp 233–334
Kellogg CA (2004) Tropical archaea: diversity associated with the surface microlayer of corals. Mar Ecol Prog Ser 273:81–88
Kopp C, Pernice M, Domart-Coulon I et al (2013) Highly dynamic cellular-level response of symbiotic coral to a sudden increase in environmental nitrogen. MBio 4:1–9
Kremien M, Shavit U, Mass T, Genin A (2013) Benefit of pulsation in soft corals. Proc Natl Acad Sci USA 110:8978–8983
Krupp DA (1984) Mucus production by corals exposed during an extreme low tide. Pac Sci 38:1–11
LaBarbara ML (1984) Feeding currents and particle capture mechanisms in suspension feeding animals. Am Zool 24:71–84
Lang JC (1973) Interspecific aggression by scleractinian corals. 2. Why the race is not only to the swift. Bull Mar Sci 23:260–279
Lang JC, Chornesky EA (1990) Competition between scleractinian reef corals-a review of mechanisms and effects. In: Dubinsky D (ed) Ecosystems of the world. Coral reefs, vol 25. Elsevier, Amsterdam, pp 209–252
Lasker HR (1981) A comparison of the particulate feeding abilities of three species of gorgonian soft coral. Mar Ecol Prog Ser 5:61–67
Lasker HR, Gottfried MD, Coffroth MA (1983) Effects of depth on feeding capabilities of two octocorals. Mar Biol 73:73–78
Laybourn-Parry JEM, Parry JD (2000) Flagellates and the microbial loop. In: Leadbeater SC, Green JC (eds) The flagellates, unity, diversity and evolution. Taylor and Francis, London, pp 216–239
Leal MC, Ferrier-Pagès C, Calado R et al (2014a) Trophic ecology of the facultative symbiotic coral Oculina arbuscula. Mar Ecol Prog Ser 504:171–179
Leal MC, Berger SA, Ferrier-Pagès C et al (2014b) Temporal changes in the tropic ecology of the asymbiotic gorgonian Leptogorgia virgulata. Mar Biol 161:2191–2197
Lee C, Wakeham S, Arnosti C (2004) Particulate organic matter in the sea: the composition conundrum. Ambio 33:565–575
Lee STM, Davy SK, Tang S-L et al (2015) Successive shifts in the microbial community of the surface mucus layer and tissues of the coral Acropora muricata under thermal stress. FEMS Microbiol Ecol 91:fiv142. https://doi.org/10.1093/femsec/fiv142
Lee STM, Davy SK, Tang S-L, Kench PS (2016) Mucus sugar content shapes the bacterial community structure in thermally stressed Acropora muricata. Front Microbiol 7:371. https://doi.org/10.3389/fmicb.2016.00371
Legendre L, Demers S, Delesalle B, Harnols C (1988) Biomass and phototrophic picoplankton in coral reef waters (Moorea, French Polynesia). Mar Ecol Prog Ser 47:153–160
Léger P, Bengtson DA, Sorgeloos P et al (1987) The nutritional value of Artemia: a review. In: Persoone G, Sorgeloos P, Roels O, Jaspers E (eds) The brine shrimp Artemia. Ecology, culturing, use in aquaculture, vol 3. Universa Press, Wetteren, pp 357–372
Lesser MP, Mazel CH, Gorbunov MY, Falkowski PG (2004) Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305:997–1000
Lesser MP, Falcón LI, Rodriguez-Román A et al (2007) Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar Ecol Prog Ser 346:143–152
Lesser MP, Slattery M Stat M et al (2010) Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology 91:990–1003
Levy O, Mizrahi L, Chadwick-Furman NE, Achituv Y (2000) Factors controlling the expansion behavior of Favia favus (Cnidaria:Scleractinia): effects of light, flow, and planktonic prey. Biol Bull 200:118–126
Levy O, Dubinsky Z, Achituv Y (2003) Photobehavior of stony corals: responses to light spectra and intensity. J Exp Biol 206:4041–4049
Levy O, Karako-Lampert S, Ben-Asher HW et al (2016) Molecular assessment of the effect of light and heterotrophy in the scleractinian coral Stylophora pistillata. Proc R Soc B 283:20153025
Lewis JB (1978) Feeding mechanisms in black corals (Antipatharia). J Zool 186:393–396
Lewis JB (1982) Feeding behavior and feeding ecology of the Octocorallia (Coelenterata: Anthozoa). J Zool 196:371–384
Lewis JB, Price WS (1975) Feeding mechanisms and feeding strategies of Atlantic reef corals. J Zool 176:527–544
Lewis JB, Price WS (1976) Patterns of ciliary currents in Atlantic reef corals and their functional significance. J Zool 178:77–89
Lomas MW, Burke AL, Bell DW et al (2010) Sargasso Sea phosphorus biogeochemistry: an important role for dissolved organic phosphorus. Biogeosciences 7:695–710
Longhurst A, Pauly D (1987) Ecology of tropical oceans. Academic Press, San Diego
Lorrain A, Houlbrèque F, Benzoni F et al (2017) Seabirds supply nitrogen to reef-building corals on remote Pacific islets. Sci Rep 7:3721. https://doi.org/10.1038/s41598-017-03781-y
Mariscal RN (1984) Cnidae. In: Bereiter-Hahn J, Matolsky AG, Richards KS (eds) Biology of the integument. Springer, Berlin, pp 57–111
Mariscal RN, Bigger CH (1977) Possible ecological significance of octocoral epithelia1 ultrastructure. Proc 3rd Intl Coral Reef Symp Miami 1:127–134
Mariscal RN, Lenhoff HM (1968) The chemical control of feeding behavior in Cyphastrea ocellina and in some other Hawaiian corals. J Exp Biol 49:689–699
Mariscal RN, McLean RB, Hand C (1977) The form and function of cnidarian spirocysts. 3. Ultrastructure of the thread and the function of spirocysts. Cell Tissue Res 178:427–433
Marshall AT, Wright OP (1993) Confocal laser scanning light microscopy of the extra-thecal epithelia of undecalcified scleractinian corals. Cell Tissue Res 272:533–543
Martin W, Scheibe R, Schnarrenberger C (2000) The Calvin cycle and its regulation. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis. Advances in photosynthesis and respiration, vol 9. Springer, Dordrecht, pp 9–51
Matthai G (1918) On reactions to stimuli in corals. Proc Camb Philos Soc 19:164–162
Mayer FW, Wild C (2010) Coral Mucus release and following particle trapping contribute to rapid nutrient recycling in a northern Red Sea fringing reef. Mar Freshw Res 61:1006–1014
McKew BA, Dumbrell AJ, Daud SD et al (2012) Characterization of geographically distinct bacterial communities associated with coral mucus produced by Acropora spp. and Porites spp. Appl Environ Microbiol 84:5229–5237
McMurray SE, Johnson ZI, Hunt DE et al (2016) Selective feeding by the giant barrel sponge enhances foraging efficiency. Limnol Oceanogr 61:1271–1286
Meikle P, Richards GN, Yellowlees D (1988) Structural investigations on the mucus from six species of coral. Mar Biol 99:187–193
Meyer JL, Schultz ET (1985) Migrating haemulid fishes as a source of nutrients and organic matter on coral reefs. Limnol Oceanogr 30:146–156
Middleburg JJ, Mueller CE, Veuger B et al (2015) Discovery of symbiotic nitrogen fixation and chemoautotrophy in cold-water corals. Sci Rep 5:17962. https://doi.org/10.1038/srep17962
Migne A, Davoult D (2002) Experimental nutrition in the soft coral Alcyonium digitatum (Cnidaria: Octocorallia): removal rate of phytoplankton and zooplankton. Cah Biol Mar 43:9–16
Mills MM, Sebens KP (2004) Ingestion and assimilation of nitrogen from benthic sediments by three species of coral. Mar Biol 145:1097–1106
Mills MM, Lipschultz F, Sebens KP (2004a) Particulate matter ingestion and associated nitrogen uptake by four species of scleractinian corals. Coral Reefs 23:311–323
Mills MM, Ridame C, Dave M (2004b) Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429:292–294
Møller EF, Thjor P, Nielsen TG (2003) Production of DOC by Calanis finmarchicus, C. glacialis and C. hyperboeus through sloppy feeding and leakage from fecal pellets. Mar Ecol Prog Ser 262:185–191
Moran Y, Genikhovich G, Gordon D et al (2012) Neurotoxin localization to ectodermal gland cells uncovers an alternative mechanism of venom delivery in sea anemones. Proc Biol Sci 279:1351–1358
Mortensen PB (2001) Aquarium observations on the deep-water coral Lophelia pertusa (L., 1758) (Scleractinia) and selected associated invertebrates. Ophelia 54:83–104
Mueller CE, Larsson AI, Veuger B et al (2014a) Opportunistic feeding on various organic food sources by the cold-water coral Lophelia pertusa. Biogeosciences 11:123–133
Mueller B, van der Zande RM, van Leent PJM et al (2014b) Effect of light availability on dissolved organic carbon release by Caribbean reef algae and corals. Bull Mar Sci 90:875–893
Mueller B, de Goeij JM, Vermeij MJA et al (2014c) Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC). PLoS One 9(2):e90152
Muscatine L (1973) Nutrition of corals. In: Jones OS, Endean R (eds) Biology and geology of coral reefs, vol 2., Biology. Academic Press, New York, pp 77–116
Muscatine L, D’Elia CF (1978) The uptake, retention, and release of ammonium by reef corals. Limnol Oceanogr 23:725–734
Muscatine L, Porter JW (1977) Reef corals: mutualistic symbiosis adapted to nutrient-poor environments. BioScience 27:454–459
Muscatine L, McCloskey LR, Marian RE (1981) Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr 26:601–611
Muscatine L, Falkowski PG, Porter JW et al (1984) Fate of photosynthetic fixed carbon in light-and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc B Biol Sci 222:181–202
Muscatine L, Falkowski PG, Dubinsky Z et al (1989) The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc R Soc B 236:311–324
Nakajima R, Yoshida T, Azman BAR et al (2009) In situ release of coral mucus by Acropora and its influence on the heterotrophic bacteria. Aquat Ecol 43:815–823
Nakajima R, Yoshida T, Fujita K et al (2010) Release of particulate and dissolved organic carbon by the scleractinian coral Acropora formosa. Bull Mar Sci 86:861–870
Nakajima R, Tanaka Y, Yoshida T et al (2015) High inorganic phosphate concentration in coral mucus and its utilization by heterotrophic bacteria in a Malaysian coral reef. Mar Ecol 36:835–841
Nakajima R, Yamazaki H, Lewis LS et al (2017a) Planktonic trophic structure in a coral reef ecosystem—grazing versus microbial food webs and the production of mesozooplankton. Progr Oceanogr 156:104–120
Nakajima R, Tanaka Y, Guillemette R, Kurihara H (2017b) Effects of coral-derived organic matter on the growth of bacterioplankton and heterotrophic nanoflagellates. Coral Reefs 36:1171–1179
Naumann MS, Richter C, el-Zibdah M, Wild C (2009) Coral mucus as an efficient trap for picoplanktonic cyanobacteria: implications for pelagic-benthic coupling in the reef ecosystem. Mar Ecol Prog Ser 385:65–76
Naumann MS, Haas A, Struck U et al (2010) Organic matter release by dominant hermatypic corals of the northern Red Sea. Coral Reefs 29:649–659
Naumann MS, Orejas C, Wild C, Ferrier-Pagès C (2011) First evidence for zooplankton feeding sustaining key physiological processes in a scleractinian cold-water coral. J Exp Biol 214:3570–3576
Naumann MS, Richter C, Mott C et al (2012) Budget of coral-derived organic carbon in a fringing coral reef in the Gulf of Aqaba. J Mar Syst 105–108:20–29
Naumann MS, Tolosa I, Taviani M (2015) Trophic ecology of two cold-water coral species from the Mediterranean Sea revealed by lipid biomarkers and compound-specific isotope analyses. Coral Reefs 34:1165–1175
Nelson CE, Goldberg SJ, Kelly LW (2013) Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. ISME J 7:962–979
Nguyen-Kim H, Bouvier T, Bouvier C et al (2014) High occurrence of viruses in the mucus of scleractinian corals. Environ Microbiol Rep 6:675–682
Ocaña O, Opresko DM, Brito A (2006) First record of the black coral Antipathella wollastoni (Anthozoa: Antipatharia) outside of Macaronesian waters. Rev Acad Can Cien 18:125–138
Oku H, Yamashiro H, Onaga K (2003) Lipid biosynthesis from 14C-glucose in the coral Montipora digitata. Fish Sci 69:625–631
Olson ND, Lesser MP (2013) Diazotrophic diversity in the Caribbean coral, Montastraea cavernosa. Arch Microbiol 195:853–859
Olson ND, Ainsworth TD, Gates M, Takabayashi M (2009) Diazotrophic bacteria associated with Hawaiian Montipora corals: diversity and abundance in correlation with symbiotic dinoflagellates. J Exp Mar Biol Ecol 371:140–146
Omori M, Ikeda T (1992) Methods in marine zooplankton ecology. Krieger Publishing, Malabar
Orejas C, Gili J-M, Arntz W (2003) Role of small-plankton communities in the diet of two Antarctic octocorals (Primnoisis Antarctica and Primnoella sp.). Mar Ecol Prog Ser 250:105–116
Orejas C, Gori A, Rad-Menéndez C et al (2016) The effect of flow speed and food size on the capture efficiency and feeding behavior of the cold-water coral Lophelia pertusa. J Exp Mar Biol Ecol 481:34–40
Palardy JE, Grottoli AG, Matthews KA (2005) Effects of upwelling, depth, morphology and polyp size on feeding in three species of Panamanian corals. Mar Ecol Prog Ser 300:79–89
Palardy JE, Grottoli A, Mathews KA (2006) Effect of naturally changing zooplankton concentrations on feeding rates of two coral species in the Eastern Pacific. J Exp Mar Biol Ecol 331:99–107
Palardy JE, Rodrigues LJ, Grottoli AG (2008) The importance of zooplankton to the daily requirements of healthy and bleached corals. J Exp Mar Biol Ecol 367:180–188
Partensky F, Hess WR, Vaulot D (1999) Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev 63:106–127
Passow U (2002) Transparent exopolymer particles (TEP) in aquatic environments. Prog Oceanogr 55:287–333
Patten N, Wyatt A, Lowe R, Waite A (2011) Uptake of picophytoplankton, bacterioplankton and virioplankton by a fringing coral reef community (Ningaloo Reef, Australia). Coral Reefs 30:555–567
Patterson MR (1991) The effects of flow on polyp-level prey capture in an octocoral, Alcyonium siderium. Biol Bull 180:93–102
Patton J, Abraham S, Benson AA (1977) Lipogenesis in the intact coral Pocillopora capitata and its isolated zooxanthellae. Evidence of a light-driven carbon cycle between symbiont and host. Mar Biol 44:235–247
Pernice M, Meier H, Van Den Heuvel A et al (2012) A single-cell view of ammonium assimilation in coral–dinoflagellate symbiosis. ISME J 6:1314–1324
Picciano M, Ferrier-Pagès C (2007) Ingestion of Pico- and nanoplankton by the Mediterranean red coral Corallium rubrum. Mar Biol 150:733–782
Piniak GA (2002) Effect of symbiotic status, flow speed, and prey type on prey capture by the facultatively symbiotic temperate coral Oculina arbuscula. Mar Biol 141:449–455
Piraino S, Ulianich L, Zupo V, Russo GF (1993) Cnidocyst morphology and distribution in Corallium rubrum (L.) (Cnidaria, Anthozoa). Oebalia 19:67–78
Porter JW (1974) Zooplankton feeding by the Caribbean reef- building coral Montastrea cavernosa. In: Cameron AM, Campbell BM, Cribb AB et al (eds) Proceedings of the second international coral reef symposium. The Great Barrier Reef Committee, Brisbane, pp 111–125
Pratt EM (1905) The digestive organs of the Alcyonaria and their relation to the mesogloeal cell plexus. Quart J Micr Sci 49:327–362
Purcell S, Bellwood D (2001) Spatial patterns of epilithic algal and detrital resources on a windward reef. Coral Reefs 20:117–125
Purser A, Larsson AI, Thomsen L, van Oevelen D (2010) The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. J Exp Mar Biol Ecol 395:55–62
Rädecker N, Pogoreutz C, Voolstra CR et al (2015) Nitrogen cycling in corals: the key to understanding holobiont functioning. Trends Microbiol 23:490–497
Raz-Bahat M, Douek J, Moiseeva E et al (2017) The digestive system of the stony coral Stylophora pistillata. Cell Tissue Res 368:311–323
Reynard S, Martinez P, Houlbrèque F et al (2009) Effect of light and feeding on the nitrogen isotopic composition of a zooxanthellate coral: role of nitrogen recycling. Mar Ecol Prog Ser 392:103–110
Ribes M, Coma R, Gili J-M (1998) Heterotrophic feeding by gorgonian corals with symbiotic zooxanthellae. Limnol Oceanogr 43:1170–1179
Ribes M, Coma R, Gili J-M (1999) Heterogeneous feeding in benthic suspension. feeders: the natural diet and grazing rate of the temperate gorgonian Paramuricea clavata (Cnidaria: Octocorallia) over a year cycle. Mar Ecol Prog Ser 183:125–137
Ribes M, Coma R, Rossi S (2003) Natural feeding of the temperate asymbiotic octocoral-gorgonian Leptogorgia sarmentosa (Cnidaria: Octocorallia). Mar Ecol Prog Ser 254:141–150
Ribes M, Coma R, Atkinson MJ, Kinzie RA III (2005) Sponges and ascidians control removal of particulate organic nitrogen from coral reef water. Limnol Oceanogr 50:1480–1489
Rix L, de Goeij JM, Mueller CE et al (2016) Coral mucus fuels the sponge loop in warm- and cold-water reef ecosystems. Sci Rep 6:18715
Roberts MJ, Wheeler AJ, Freiwald A (2006) Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312:543–547
Robertson DR (1982) Fish feces as fish food. Mar Ecol Prog Ser 7:253–265
Rodolfo-Metalpa R, Periano A, Houlbrèque F et al (2008) Effects of temperature, light and heterotrophy on the growth rate and budding of the temperate coral Cladocora. Coral Reefs 27:17–25
Rodrigues LJ, Grottoli AG (2007) Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol Oceanogr 52:1874–1882
Roff G, Dove SG, Dunn SR (2009) Mesenterial filaments make a clean sweep of substrates for coral growth. Coral Reefs 28:79
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10
Rosenberg E, Koren O, Efrony R, Zilber-Rosenberg I (2007) The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol 5:355–362
Roshan S, DeVries T (2017) Efficient dissolved organic carbon production and export in the oligotrophic ocean. Nat Commun 8:2036. https://doi.org/10.1038/s41467-017-02227-3
Rosset S, D’Angelo C, Wiedenmann J (2015) Ultrastructural biomarkers in symbiotic algae reflect the availability of dissolved inorganic nutrients and particulate food to the reef coral holobiont. Front Mar Sci 2:103. https://doi.org/10.3389/fmars.2015.00103
Rosset S, Wiedenmann J, Reed AJ, D’Angelo C (2017) Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates. Mar Pollut Bull 118:180–187
Rossi S, Ribes M, Coma R, Gili J-M (2004) Temporal variability in zooplankton prey capture rate of the passive suspension feeder Leptogorgia sarmentosa (Cnidaria: Octocorallia), a case study. Mar Biol 144:89–99
Rubio-Portillo E, Kersting DK, Linares C et al (2018) Biogeographic differences in the microbiome and pathobiome of the coral Cladocora caespitosa in the western Mediterranean Sea. Front Microbiol 9:22. https://doi.org/10.3389/fmicb.2018.00022
Rusch A, Hannides AK, Gaidos E (2009) Diverse communities of active bacteria and archaea along oxygen gradients in coral reef sediments. Coral Reefs 28:15–26
Sammarco PW, Coll JC (1992) Chemical adaptations in the Octocorallia—evolutionary considerations. Mar Ecol Prog Ser 88:93–104
Sammarco PW, Risk MJ, Schwarcz HP, Heikoop JM (1999) Cross-continental shelf trends in coral δ15N on the Great Barrier Reef: further consideration of the reef nutrient paradox. Mar Ecol Prog Ser 180:131–138
Sato Y, Willis BL, Bourne DG (2009) Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. ISME J 24:203–214
Schantz AA, Ladd MC, Schrack E, Burkepile DE (2017) Fish-derived nutrient hotspots shape coral reef benthic communities. Ecol Appl 25:2142–2152
Scheffers SR, Nieuwland G, Bak RPM, Van Duyl FC (2004) Removal of bacteria and nutrient dynamics within the coral reef framework of Curacao (Netherlands Antilles). Coral Reefs 23:413–422
Schlichter D (1982) Epidermal nutrition of the alcyonarian Heteroxenia fuscescens (Ehrib): absorption of dissolved organic material and lost endogenous photosynthates. Oecologia 53:40–49
Schlichter D, Brendelberger H (1998) Plasticity of the scleractinian body plan: functional morphology and trophic specialization of Mycedium elephantotus (Pallas, 1766). Facies 39:227–241
Schlichter D, Fricke HW, Weber W (1986) Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Mar Biol 91:403–407
Schmidt H, Moraw B (1982) The cnidogenesis of the Octocorallia (Anthozoa, Cnidaria) 2. maturation, migration and degeneration of cnidoblast and nematocyst. Helgol Meeresunters 35:97–118
Schöttner SI, Pfitzner B, Grünke S (2011) Drivers of bacterial diversity dynamics in permeable carbonate and silicate coral reef sands from the Red Sea. Environ Microbiol 13:1815–1826
Sebens KP (1984) Water flow and coral colony size: interhabitat comparisons of the octocoral Alcyonium siderium. Proc Natl Acad Sci USA 81:5473–5477
Sebens KP, Johnson AS (1991) Effect of water movement on prey capture and distribution of reef coral. Hydrobiologia 226:91–101
Sebens KP, Koehl MAR (1984) Predation on zooplankton by the benthic anthozoans Alcyonium siderium (Alcyonacea) and Metridium senile (Actiniaria) in the New England subtidal. Mar Biol 81:255–271
Sebens KP, Vandersall KS, Savina LA, Graham KR (1996) Zooplankton capture by two scleractinian corals, Madracis mirabilis and Montastraea cavernosa, in a field enclosure. Mar Biol 127:303–317
Sebens KP, Grace SP, Helmuth B et al (1998) Water flow and prey capture by three scleractinian corals, Madracis mirabilis, Montastraea cavernosa and Porites porites, in a field enclosure. Mar Biol 131:347–360
Seeman J (2013) The use of 13C and 15N isotope labeling techniques to assess heterotrophy of corals. J Exp Mar Biol Ecol 442:88–95
Servetto N, Rossi S, Fuentes V et al (2017) Seasonal trophic ecology of the dominant Antarctic coral Malacobelemnon daytoni (Octocorallia, Pennatulacea, Kophobelemnidae). Mar Environ Res 130:264–274
Shapiro OH, Fernandez VI, Garren M et al (2014) Vortical ciliary flows actively enhance mass transport in reef corals. Proc Natl Acad Sci USA 111:13391–13396
Sharon G, Rosenberg E (2008) Bacterial growth on coral mucus. Curr Microbiol 56:481–488
Sharoni S, Trainic M, Schatz D et al (2015) Infection of phytoplankton by aerosolized marine viruses. Proc Natl Acad Sci USA 112:6643–6647
Sherwood OA, Jamieson RE, Edinger EN et al (2008) Stable C and N isotopic composition of cold-water corals from Newfoundland and Labrador continental slope: examination of trophic, depth and spatial effects. Deep-Sea Res 55:1392–1402
Shick JM (1991) A functional biology of sea anemones. Springer Science+Business Media, Dordrecht
Sieburth JM, Smetacek V, Lenz J (1978) Pelagic ecosystem structure: heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnol Oceanogr 23:1256–1263
Silveira CB, Cavalcanti GS, Walter JM et al (2017) Microbial processes driving coral reef organic carbon flow. FEMS Microbiol Rev 41:575–595
Slattery M, McClintock JB, Bowser SS (1997) Deposit feeding: a novel mode of nutrition in the Antarctic colonial soft coral Gersemia antarctica. Mar Ecol Prog Ser 149:299–304
Smriga S, Sandin SA, Azam F (2010) Abundance, diversity, and activity of microbial assemblages associated with coral reef fish guts and feces. FEMS Microbiol Ecol 73:31–42
Sohm JA, Webb EA, Capone DG (2011) Emerging patterns of marine nitrogen fixation. Nat Rev Microbiol 9:499–508
Sokolow S (2009) Effects of a changing climate on the dynamics of coral infectious disease: a review of the evidence. Dis Aquat Organ 87:5–18
Sorokin Y (1973) On the feeding of some scleractinian corals with bacteria and dissolved organic matter. Limnol Oceanogr 18:380–385
Sorokin Y (1991) Biomass, metabolic rates and feeding of some common reef zoantharians and octocorals. Aust J Mar Freshwater Res 42:729–741
Sorokin YI (1992) Phosphorus metabolism in coral reef communities: Exchange between the water column and bottom biotopes. Hydrobiologia 242:105–114
Sorokin Y (1995) Coral reef ecology. Springer, Berlin
Sorokin YI, Sorokin PY (2010) Plankton of the central Great Barrier Reef: abundance, production and trophodynamic roles. J Mar Biol Assoc UK 90:1173–1187
Stimson JS (1987) Location, quantity and rate of change in quantity of lipids in tissue of Hawaiian hermatypic corals. Bull Mar Sci 41:889–904
Stocker JG (1988) Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnol Oceanogr 33:765–775
Stocker JG (2012) Marine microbes see a sea of gradients. Science 338:628–633
Strömberg SM, Östman C (2017) The cnidome and internal morphology of Lophelia pertusa (Linnaeus, 1758) (Cnidaria, Anthozoa). Acta Zool 98:191–213
Suttle CA (2005) Viruses in the sea. Nature 437:356–361
Suzuki Y, Casareto BE (2011) The role of dissolved organic nitrogen (DON) in coral biology and reef ecology. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer Sci Business Media BV, Dordrecht, pp 207–214
Sweet M, Bythell J (2016) The role of viruses in coral health and disease. J Invertebr Pathol 147:136–144
Tada K, Sakai K, Nakano Y et al (2003) Size-fractionated phytoplankton biomass in coral reef waters off Sesoko Island, Okinawa, Japan. J Plankton Res 25:991–997
Tagliafico A, Rudd D, Rangel MS et al (2017a) Lipid-enriched diets reduce the impacts of thermal stress in corals. Mar Ecol Prog Ser 573:129–141
Tagliafico A, Rangel S, Kelaher B, Christidis L (2017b) Optimizing heterotrophic feeding rates of three commercially important scleractinian corals. Aquaculture 483:96–101
Tanaka Y, Miyajima T, Isao K et al (2008) Production of dissolved and particulate organic matter by the reef-building corals Porites cylindrica and Acropora pulchra. Bull Mar Sci 82:237–245
Tanaka Y, Miyajima T, Umezawa Y (2009) Net release of dissolved organic matter by the scleractinian coral Acropora pulchra. J Exp Mar Biol Ecol 377:101–106
Tanaka Y, Ogawa H, Miyajima T (2011) Bacterial decomposition of coral mucus as evaluated by long-term and quantitative observation. Coral Reefs 30:443–449
Tanaka Y, Grottoli AG, Matsui Y et al (2015) Partitioning of nitrogen sources to algal endosymbionts of corals with long-term 15N-labelling and a mixing model. Ecol Model 309–310:163–169
Taniguchi A, Yoshida T, Eguchi M (2014) Bacterial production is enhanced by coral mucus in reef systems. J Exp Mar Biol Ecol 461:331–336
Tazoli S, Bo M, Boyer M et al (2007) Ecological observations of some common antipatharian corals in the marine park of Bunaken (North Sulawesi, Indonesia). Zool Stud 46:227–241
Tchernov D, Gorbunov MY, de Vargas C et al (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching. Proc Natl Acad Sci USA 101:13531–13535
Tezcan ÖD (2016) Unusual cnidarian envenomations. In: Goffredo S, Dubinsky Z (eds) The cnidaria, past, present and future. The world of medusa and her sisters. Springer, Switzerland, pp 609–622
Thorington GU, Hessinger DA (1996) Efferent mechanisms of discharging cnidae: I. Measurements of intrinsic adherence of cnidae discharged from tentacles of the sea anemone, Aiptasia pallida. Biol Bull 190:125–138
Thorington GU, Hessinger DA (1998) Efferent mechanisms of discharging cnidae: II. A nematocyst release response in the sea anemone tentacle. Biol Bull 195:145–155
Thorington GU, McAuley V, Hessinger DA (2010) Effects of satiation and starvation on nematocyst discharge, prey killing and ingestion in two species of sea anemone. Biol Bull 219:122–131
Thurber RV, Payet JP, Thurber AR, Correa AMS (2017) Virus-host interactions and their roles in coral reef health and disease. Nat Rev Microbiol 15:205–216
Treignier C, Grover R, Ferrier-Pagès C, Tolosa I (2008) Effect of light and feeding on the fatty acid and sterol composition of zooxanthellae and host tissue isolated from the scleractinian coral Turbinaria reniformis. Limnol Oceanogr 53:2702–2710
Tremblay P, Peirano A, Ferrier-Pagès C (2011) Heterotrophy in the Mediterranean symbiotic coral Cladocora caespitosa: comparison with two other scleractinian species. Mar Ecol Prog Ser 422:165–177
Tremblay P, Grover G, Maguer J-F et al (2012a) Autotrophic carbon budget in coral tissue: a new 13C-based model of photosynthate translocation. J Exp Biol 215:1384–1393
Tremblay P, Ferrier-Pagès C, Maguer JF et al (2012b) Controlling effects of irradiance and heterotrophy on carbon translocation in the temperate coral Cladocora caespitosa. PLoS One 7(9):e44672
Tremblay P, Grover R, Maguer JF (2014) Carbon translocation from symbiont to host depends on irradiance and food availability in the tropical coral Stylophora pistillata. Coral Reefs 33:1–13
Tremblay P, Maguer JF, Grover R, Ferrier-Pagès C (2015) Trophic dynamics of scleractinian corals: stable isotope evidence. J Exp Biol 218:1223–1243
Tseng LC, Dahma HU, Hsu NJ, Hwang JS (2011) Effects of sedimentation on the gorgonian Subergorgia suberosa (Pallas, 1766). Mar Biol 158:1301–1310
Tsounis G, Rossi S, Laudien J et al (2006) Diet and seasonal prey capture rates in the Mediterranean red coral (Corallium rubrum L.). Mar Biol 149:313–325
Turner JT (2002) Zooplankton fecal pellets, marine snow and sinking plankton blooms. Aquat Microb Ecol 27:57–102
Turner JT (2015) Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog Oceanogr 130:205–248
Ulstrup KE, van Oppen MJH, Kühl M, Ralph J (2007) Inter-polyp genetic and physiological characterization of Symbiodinium in an Acropora valida colony. Mar Biol 153:225–234
van Oevelen D, Mueller CE, Lundälv T, Middleberg JJ (2016) Food selectivity and processing by the cold-water coral Lophelia pertusa. Biogeosciences 13:5789–5798
Vanhaecke P, Sorgeloos P (1980) The biometrics of Artemia strains from different geographical origin. In: Persoone G, Sorgeloos P, Roels O, Jaspers E (eds) The brine shrimp Artemia, vol 3 Ecology, culturing, use in aquaculture. Universa Press, Wetteren, pp 393–405
Van-Praët M (1985) Nutrition of sea anemones. Adv Mar Biol 22:65–99
Wagner D, Luck DG, Toonen RJ (2012) The biology and ecology of black corals (Cnidaria:Anthozoa: Hexacorallia: Antipatharia). Adv Mar Biol 63:67–132
Wainwright SA, Dillon JR (1969) On the orientation of sea fans (genus Gorgonia). Bull Mar Sci 136:130–139
Waite AM, Safi K, Hall JA, Nodder SD (2000) Mass sedimentation of picoplankton embedded in organic aggregates. Limnol Oceanogr 45:87–97
Walsh K, Haggerty JM, Doane MP et al (2017) Aura-biomes are present in the water layer above coral reef macro-organisms. Peer J 5:e3666
Warner GF (1981) Species descriptions and ecological observations of black corals (Antipatharia) from Trinidad. Bull Mar Sci 31:147–163
Warner ME, LaJeunesse TC, Robison JD, Thur RM (2006) The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol Oceanogr 51:1887–1897
Watson GM, Mariscal RN (1983) The development of a sea anemone tentacle specialized for aggression: morphogenesis and regression of the catch tentacle of Haliplanella luciae (Cnidaria:Anthozoa). Biol Bull 164:506–517
Wheeler AJ, Beyer A, Freiwald A et al (2007) Morphology and environment of cold-water coral carbonate mounds on the NW European margin. Int J Earth Sci 96:37–56
Widdig A, Schlichter D (2001) Phytoplankton: a significant trophic source for soft corals? Helgoland Mar Res 55:198–211
Wiedenmann J, D’Angelo CD, Smith EG et al (2012) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3:160–164
Wijgerde T, Diantari R, Lewaru MW et al (2011) Extracoelenteric zooplankton feeding is the key mechanism of nutrient acquisition for the scleractinian coral Galaxea fascicularis. J Exp Biol 214:3351–3357
Wijgerde T, Spijkers P, Karruppannan E et al (2012) Water flow affects zooplankton feeding by the scleractinian coral Galaxea fascicularis on a polyp and colony level. J Mar Biol 854489 https://doi.org/10.1155/2012/854849
Wild C, Huettel M, Klueter A et al (2004a) Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428:66–70
Wild C, Rasheed MY, Werner U et al (2004b) Degradation and mineralization of coral mucus in reef environments. Mar Ecol Prog Ser 267:159–171
Wild C, Woyt H, Huettel M (2005) Influence of coral mucus on nutrient fluxes in carbonate sands. Mar Ecol Prog Ser 287:87–98
Wild C, Mayr C, Wehrmann L et al (2008) Organic matter release by cold-water corals and its implications for fauna-microbe interaction. Mar Ecol Prog Ser 272:67–75
Wild C, Naumann MS, Haas A (2010a) Organic matter release by Red Sea coral reef organisms- potential effects on microbial activity and in situ O2 availability. Mar Ecol Prog Ser 411:61–71
Wild C, Naumann M, Niggl W, Haas A (2010b) Composition of mucus released by scleractinian warm- and cold-water reef corals. Aquat Biol 10:41–45
Williams B, Grottoli AG (2010) Stable nitrogen and carbon isotope (δ15N and δ13C) variability in shallow tropical Pacific soft coral and black coral taxa and implications for paleoceanographic reconstructions. Geochim Cosmochim Acta 74:5280–5288
Wilson SK, Bellwood DR, Choat HJ, Furnas MJ (2003) Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr Mar Biol Annu Rev 41:279–309
Wooldridge SA (2017) Excess seawater nutrients, enlarged algal symbiont densities and bleaching sensitive reef locations: 1. Identifying thresholds of concern for the Great Barrier Reef, Australia. Mar Pollut Bull 114:343–354
Work T, Meteyer C (2014) To understand coral disease, look at coral cells. Ecohealth 11:610–618
Wotton RS, Malmqvist B (2001) Feces in aquatic ecosystems: feeding animals transform organic matter into fecal pellets, which sink or are transported horizontally by currents; these fluxes relocate organic matter in aquatic ecosystems. BioScience 51:537–544
Wyatt ASJ, Lowe RJ, Humphries S, Waite AM (2010) Particulate nutrient fluxes over a fringing coral reef: relevant scales of phytoplankton production and mechanisms of supply. Mar Ecol Prog Ser 405:113–130
Wyatt ASJ, Lowe RJ, Humphries S, Waite AM (2013) Particulate nutrient fluxes over a fringing reef: source-sink dynamics inferred from carbon to nitrogen ratios and stable isotopes. Limnol Oceanogr 58:409–427
Yahel R, Yahel G, Genin A (2005) Near-bottom depletion of zooplankton over coral reefs: I: diurnal dynamics and size distribution. Coral Reefs 24:75–85
Yamamuro M, Kayanne H, Minagawa M (1995) Carbon and nitrogen stable isotopes of primary producers in coral reef ecosystems. Limnol Oceanogr 40:617–621
Yamashiro H, Oku H, Higa H et al (1999) Composition of lipids, fatty acids and sterols in Okinawan corals. Comp Biochem Physiol B 122:397–407
Yellowlees D, Rees TAV, Leggat W (2008) Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ 31:679–694
Yoffe C, Lotan Y, Benayahu Y (2012) A modified view on octocorals: Heteroxenia fuscesans nematocysts are diverse, featuring both an ancestral and a novel type. PLoS One 7(2):e31902
Yonge CM (1930a) Studies on the physiology of corals 3. Assimilation and excretion. British Mus Nat Hist Gt Barrier Reef Exped 1928–1929 Sci Repts 1:83–92
Yonge CM (1930b) Studies on the physiology of corals 1. Feeding mechanisms and food. British Mus Nat Hist Gt Barrier Reef Exped 1928–1929 Sci Repts 1:13–57
Zetsche E-M, Baussant T, Meysman FJR, van Oevelen D (2016) Direct visualization of mucus production by the cold-water coral Lophelia pertusa with digital holographic microscopy. PLoS One 11:e0146766. https://doi.org/10.1371/journal.pone.0146766
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Goldberg, W.M. (2018). Coral Food, Feeding, Nutrition, and Secretion: A Review. In: Kloc, M., Kubiak, J. (eds) Marine Organisms as Model Systems in Biology and Medicine. Results and Problems in Cell Differentiation, vol 65. Springer, Cham. https://doi.org/10.1007/978-3-319-92486-1_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-92486-1_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92485-4
Online ISBN: 978-3-319-92486-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)