Abstract
Satisfying nutrient requirement of corals is still a major constraint for maintaining corals in marine aquariums. Corals are polytrophic in nature. Heterotrophic feeding on zooplankton is one of the corals’ strategies to overcome nutrient deficiency. Artemia salina nauplii are commonly used as biocarriers for many fish larvae in aquaculture and can also serve as a biocarrier for coral in aquariums, provided coral acceptability, optimal feeding rate, and digestibility of the nauplii are well understood. Feeding rate and digestibility of coral fed on A. salina nauplii at 100, 2,000, 4,000, 6,000, and 10,000 ind. l−1 under light and dark conditions was assessed in this study. The maximum feeding rates of Galaxea fascicularis under light and dark conditions was 113.6 ind. polyp−1 h−1 and 76.9 ind. polyp−1 h−1, respectively. The daily feeding rates of G. fascicularis varies and depends on nauplii density. Light plays an important role in coral feeding. Nevertheless, the quantity of A. salina nauplii consumed by the coral under light and dark conditions was not significantly different (P > 0.05). A. salina nauplii are well accepted by G. fascicularis. Complete nauplii digestion was observed after 180 min. Digestibility of A. salina nauplii by G. fascicularis was positively correlated with digestion time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine ornamental industry is a multimillion dollar business (US $200–330 million year−1) (Wabnitz et al. 2003). Live coral is one of the most favorite marine organisms in the industry (Green 2003). Based on importers’ data (1997–2001) from the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), 99.7% of the total global trade in live corals is harvested from the wild, and only 0.3% is captive bred. Mortality rate of corals in the marine aquarium is high. Wabnitz et al. (2003) reported high mortality of Plerogyra spp. (54% mortality), Catalaphyllia spp. (60% mortality), Heliofungia spp. (100% mortality), Goniopora spp. (95% mortality), and Tubastrea spp. (100% mortality) in the aquarium. Harvesting coral from the wild is not a sustainable business because when corals are difficult to maintain in the aquarium, they must be replaced regularly (Arvedlund et al. 2003). Strong market demands imply a heavy harvesting pressure on coral reefs, and coral aquaculture is increasingly mentioned as a priority solution to reduce this pressure (Parks et al. 2003). Attempts to culture live coral in captivity have been carried out over the decades. However, reports of successful ex-situ cultivation of coral are sparse (Arvedlund et al. 2003). Satisfying the nutrient requirement of corals remains one of the major tasks in maintaining and reproducing the organisms under captivity.
Corals are notable for their symbiosis with the unicellular dinoflagellate algae zooxanthellae (Davies 1984). Corals protect zooxanthellae, which are enclosed in their endodermal cells. In return, zooxanthellae supply organic carbon to the host. Therefore, lighting is an essential component in marine aquariums. Corals are polytrophic in nature (Goreau et al. 1971). Heterotrophic feeding is essential for overcoming nutrient deficiency. Corals exhibit several modes of feeding and energy acquirement from multiple sources. Corals feed on plankton (Lewis 1992; Ferrier-Pages et al. 2003; Picciano and Ferrier-Pages 2007), bacteria (Ferrier-Pages et al. 1998), and particulate organic matter (Lasker 1981; Anthony 1999; Anthony and Fabricius 2000; Mills et al. 2004), and they even absorb dissolved nutrients from the water (Titlyanov et al. 2000; Ferrier 1991; Ferrier-Pages et al. 2003). Heterotrophic feeding of corals is one of the strategies to overcome nutrient deficiency. Heterotrophic feeding enhances coral growth, calcification rate, and photosynthesis rate and even affects the symbiotic zooxanthellae (Miller 1995; Titlyanov et al. 2001; Grottoli 2002; Ferrier-Pages et al. 2000, 2003; Houlbreque et al. 2003, 2004; Davy et al. 2006).
Corals in captivity should be fed with zooplankton to complete their nutrient requirements. However, harvesting zooplankton from the sea to feed corals in marine aquariums is neither sustainable nor economically viable. Brine shrimp, Artemia salina nauplii, may be the right candidate for feeding corals in captivity. A. salina is widely used as life food for culturing fish larvae in aquaculture (Lavens and Sorgeloos 2000). Dormant cysts of artemia can be stored for long periods, and it is commercially available. A. salina nauplii can also be enriched with different nutrients to meet the specific nutrient requirements of many organisms (Dhert et al. 1990; Olsen et al. 1999; Hanaee et al. 2005; Monroig et al. 2006; Olivotto et al. 2006). A. salina nauplii can be used as a biocarrier and life food for corals in captivity provided that acceptability, optimal feeding rate, and digestibility are well understood.
Materials and methods
Coral specimens and A. salina nauplii
About 30 colonies of the scleractinian zooxanthellate coral Galaxea fascicularis were collected from Pulau Bidong, Malaysia. The corals were maintained in a 150-l aquarium supplied with running seawater and illuminated with white fluorescent light at 870.0 ± 82.8 lux on a 12-h light/12-h dark cycle. The experiment was conducted under a constant environment at parameters 28°C, salinity 30 ppt, and pH 8, with dissolved oxygen maintained at 5–7 mg l−1. Coral colonies were then broken into similar-sized coral pieces using a hammer and screw driver. Each coral piece had about 30 polyps. The pieces were allowed to acclimatization for 5 days before the experiment. Pieces from the coral colonies were mixed and pooled and randomly selected from the pool for the experiments. A. salina nauplii (Royal Artemia, 99% hatching) were prepared 24 h prior to the experiments. Preparation of A. salina nauplii was based on the procedure described by Dhont and Stappen (2003).
Determination of optimal feeding rate
Feeding experiments were conducted based on the method described by Leversee (1976) and Dai and Lin (1993). The coral pieces were fed different densities of A. salina nauplii at 100, 2,000, 4,000, 6,000, and 10,000 ind. l−1. Three pieces used in each treatment were randomly selected from the pool. Each piece was transferred from the aquarium into a 2-l clear glass beaker 1 h before the experiment. The experiment was conducted in three replicates and started when most of the polyps were fully extended. The feeding experiments were conducted in the morning and repeated every 24 h up to 6 days. A. salina nauplii at the mentioned densities were added to the beaker, and the volume of seawater in the beaker was made up to 1 l to yield the desired nauplii density in each treatment. The coral colonies were allowed to feed for 1 h before they were carefully transferred back into the aquarium. Densities of the remaining A. salina nauplii were determined by counting 10-ml samples (five replicates) from each beaker. This gives the average number of nauplii in each beaker. The total number of A. salina nauplii consumed by the corals was determined by the difference of initial and final density of the nauplii. G. fascicularis feeding rate was determined as the number of A. salina nauplii ingested per coral polyp per hour:
where, density i = initial number of A. salina nauplii, density r = remaining A. salina nauplii after 1 h.
Effects of light on G. fascicularis feeding
To determine the effect of light provision on G. fascicularis feeding, the feeding rate of G. fascicularis was determined under light and dark conditions. Under light conditions, coral colonies were illuminated with white fluorescent light at 870 ± 82.8 lux, and under dark conditions, the colonies were fed in the dark. G. fascicularis feeding rates with and without light were determined, as described previously.
Digestion rate of A. salina nauplii by G. fascicularis
To determine the digestion rate of A. salina nauplii by G. fascicularis, coral specimens were fed with A. salina nauplii at 10,000 ind. l−1. After feeding A. salina nauplii to the coral, the gastrovascular cavity contents of five polyps were withdrawn using a 1-ml syringe (0.36 mm Ø; Micro-Fine®) at intervals of 10, 30, 60, 90, 120, 180, and 420 min. The gastrovascular cavity contents were then examined using a compound microscope (Motic) under 40× magnification. The experiment was conducted in three replicates. Gastrovascular content of starved coral were used as controls. A few drops of 10% formalin were added to the gastrovascular cavity contents for preservation. A scale from one to five [0–20% digested scale (1), 20–40% digested scale (2), 40–60% digested scale (3), 60–80% digested scale (4), and 80–100% digested scale (5)] was assigned to indicate digestibility of the specimen.
Statistical analysis
G. fascicularis feeding rate at different densities of A. salina nauplii were evaluated using nonlinear least squares regression (Michaelis–Menten models), as described by Anthony (1999). The curvilinear Michaelis–Menten model is:
where, F D is the feeding rate as a function of nauplii density (D), F max is the maximum feeding rate, and K is the nauplii density at which half-saturation occurs. The effect of feeding condition (with and without light provision) and rate of G. fascicularis was tested using a split plot design analysis of variance (ANOVA). Spearman’s rank order correlation was conducted to test the correlation between digestibility of A. salina nauplii and coral digestion time. All null hypotheses were rejected at P ≤ 0.05.
Results
G. fascicularis feeding rate
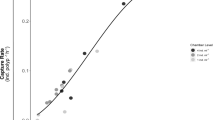
G. fascicularis feeding rate increased with increasing nauplii densities (Fig. 1). G. fascicularis feeding with and without light provision was significantly correlated with the curvilinear model (P values <0.0001 and 0.0015, respectively, Table 1). With light provision, the maximum feeding rate was 113.6 ind. polyp−1 h−1, whereas the maximum feeding rate under dark conditions was 76.9 ind. polyp−1 h−1 (Table 2). When G. fascicularis was fed at high nauplii density, it achieved its feeding saturation in a shorter time. The half-saturation constant (K) values of G. fascicularis with and without light provision were 1.25 × 104 ind. l−1 and 5.32 × 103 ind. l−1, respectively (Table 2). Corals pieces fed with A. salina nauplii retained a 100% survival rate for more than 10 months.
Feeding rates as a function of Artemia salina nauplii densities for Galaxea fascicularis under light and dark conditions. Markers ● and × are the means of n = 3 (with and without light provision, respectively). The solid line (with light provision) and dash line (without light provision) show the best-fit curve
Figure 2 shows that coral feeding has a rhythmic trend. Under light conditions, the daily feeding rate declined on day 2 but increased on day 4. However, the feeding rhythm extended up to day 3 under dark conditions. The daily feeding rate varied from day to day, and there was a significant interaction between light provision and feeding time (P < 0.05, Table 3). Nevertheless, the average feeding rate was not significantly different under light and dark conditions (P > 0.05, Table 3).
A. salina nauplii digestion by G. fascicularis
Table 4 shows the digestibility of A. salina nauplii by G. fascicularis. Complete A. salina nauplii digestion (scale 5) was observed after 180 min. Most gastrovascular cavity contents of the specimen showed minor digestion (scale 1) 10 min after feeding (Fig. 3). All major body parts were intact, but there was some minor degradation in the nauplii appendices. The modes of nauplii digestibility after 30 and 60 min were recorded at scales 2 and 3, respectively. Nauplii were partially digested at the time. After 90 and 120 min, most A. salina nauplii were further digested and recorded at scale 4. A. salina nauplii body shape could hardly be recognized at this time. After 180 and 420 min after feeding, only mucus was left in the contents. As for controls, only mucus was found in the gastrovascular cavity contents withdrawn from the coral polyps. Analysis of Spearman’s rank order correlation indicates that there was a significant correlation between A. salina nauplii digestibility and digestion time (r = 0.920, P = 0.014).
Discussion
G. fascicularis is one of the favorite organisms in the marine ornamental industry (Wabnitz et al. 2003). The scarcity of data in coral feeding and nutritional requirement renders it difficult to maintain corals in captivity. A. salina nauplii acting as a biocarrier may be useful to study nutrient requirements of corals. Our results revealed that G. fascicularis was able to feed on A. salina nauplii. Coral feeding capacity was positively correlated with nauplii availability. When nauplii are present at low density (1.0 × 102 ind. l−1), G. fascicularis feeding rate under light and dark conditions was 1.2 ind. polyp−1 h−1 and 0.8 ind. polyp−1 h−1, respectively. When nauplii were present at high density (1.0 × 104 ind. l−1), G. fascicularis feeding rate under light and dark conditions was 50.5 ind. polyp−1 h−1 and 46.6 ind. polyp−1 h−1, respectively. When the corals were fed with high nauplii density, their feeding capacity was about 50 times higher than those fed with low nauplii density. Similar results were documented by Lasker et al. (1982); Clayton and Lasker (1982); Ferrier-Pages et al. (2003); and Picciano and Ferrier-Pages (2007). Ferrier-Pages et al. (1998) reported that Stylophora pistillata, a zooxanthellate coral, increased its feeding rate by 2,000 times when the ciliates density increased from 200 to 800 ciliates ml−1. In another study, the feeding rate of a Mediterranean red coral, Corrallium rubrum, doubled, for a sixfold increase in prey concentration (Picciano and Ferrier-Pages 2007).
Feeding satiation and nauplii density was a typical curvilinear response. G. fascicularis feeding rate and nauplii density was well fitted into a saturation curve (Fig. 1). Feeding by coral polyps was analogous to the active sites as in the enzymatic kinetics. G. fascicularis feeding rate can be estimated using the Michaelis–Menten equation. The maximum G. fascicularis feeding rate with light provision was 113.6 ind. polyp−1 h−1, whereas the maximum G. fascicularis feeding rate without light provision was 76.9 ind. polyp−1 h−1. The half-saturation constant (K) of the coral’s feeding with and without light provision was 1.25 × 104 ind. l−1 and 5.32 × 103 ind. l−1, respectively. When the corals were fed with higher nauplii density than the half-saturation constant, there was no further increment in the coral feeding rate.
A plateau was observed in G. fascicularis when it was fed with high nauplii density. Ferrier-Pages et al. (1998) reported a feeding plateau in S. pistillata feeding after the coral ingested 4 × 104 ciliates. However, Clayton and Lasker (1982) and Picciano and Ferrier-Pages (2007) did not observed any feeding plateau over the range of prey densities fed to corals. Anthony (1999) reported the feeding rate of four coral species, Pocillopora damicornis, Montipora digitata, Acropora millepora, and Porites cylindrical, on fine suspended particulate matter. P. damicornis, M. digitata, and A. millepora ingestion rates increased linearly from 1 to 30 mg l−1 of the fine suspended particle, whereas P. cylindrica reached its plateau at 4–8 mg l−1 particle concentrations. The differences in feeding capability are due to differences in feeding mechanisms, morphology, variable sizes, number of tentacles and polyps, differences in coelenterates cnidom, prey type and size, water flow, depth, temperature, and light availability. Any changes of these parameters affect coral feeding rate and the plateau (Leversee 1976; Lasker 1981; Clayton and Lasker 1982; Okamura 1987; Dai and Lin 1993; Sebens et al. 1997, 1998; Piniak 2002; Tsounis et al. 2006; Picciano and Ferrier-Pages 2007).
Heterotrophic feeding of coral is affected by light. Ferrier-Pages et al. (1998) reported the ingestion rate of S. pistillata decreased when light intensity increased. Under dark conditions, S. pistillata polyps were fully expanded but remained closed most of the time under light conditions. Clayton and Lasker (1982) found that P. damicornis feeding capacity was not affected by short-term dark conditions. G. fascicularis feeding rate was not significantly different under light and dark conditions, as the coral was maintained in dark conditions for a few hours only. However, G. fascicularis feeding saturation occurred earlier under light conditions. With light provision, coral feeding rates decreased on day 2 and increased again on day 4 (Fig. 2). Feeding rates of coral fed under dark conditions achieved feeding satiation after 3 days of feeding, decreased after day 3, and increased again at day 6. G. fascicularis fed actively under light conditions. Polyp tentacles were fully extended to enhance prey capture. G. fascicularis consumes more nauplii density under light conditions for the first day, and feeding rates decreased gradually until day 4. Coral feeding exhibits a rhythmic trend. Lasker et al. (1982) indicated that Hydra viridis feeding rhythm was 4.8 h and the consumption reduced during the second feeding.
Dead nauplii were found throughout the feeding experiment, especially when the corals were fed at high nauplii density. G. fascicularis preyed on the nauplii with its extended tentacles (Fig. 4). When nauplii density was high, there were more frequent contacts between the nauplii and polyp tentacles. Not all captured nauplii were ingested by the corals. The nauplii may be stung by polyp tentacles, thus causing mortality.
The process by which Galaxea fascicularis preyed on Artemia salina nauplii by both tentacular and mucus. (a) G. fascicularis with extended polyp tentacles before feeding. (b) Polyps begin to prey on nauplii with tentacles when fed with nauplii. (c) Nauplii are wrapped with mucus and conveyed to the mouth. (d) Nauplii are drawn into the mouth and stomodeum
G. fascicularis produced mucus on its fully extended tentacles for better nauplii entrapment (Fig. 4). It has been observed that when corals are fed with high nauplii density, polyps produce greater mucus volume. A. salina nauplii were found entrapped by the mucus before being consumed. Ferrier-Pages et al. (1998) reported that mucus produced by polyps was one of the major mechanisms to enhance heterotrophy feeding of corals. The mucus produced by coral is a potential energy sources to the coral itself. It is high in nutritional value and contains wax esters, triglycerides, fatty acids, and other energy-rich compounds (Coffroth 1984).
A. salina nauplii digestibility by G. fascicularis is high. The digestion started 10 min after feeding and took about 180 min to complete (Fig. 5). Time for complete digestion was similar to that reported for gorgonian soft coral, Pseudoplexaura porosa. (Coffroth 1984). Coffroth (1984) reported that all ingested food was cleared from the gastrovascular gut within 3–4 h. Sebens and Koehl (1984) reported complete digestion of zooplankton by octocorals, Alcyonium siderium, was 4–6 h, whereas the hydrocoral Millepora complanata, was reported to have 91% digestion after 24 h (Lewis 1992). The discrepancies are due to the fact that coral’s digestion time is dependent on several factors, such as polyp size, prey type, and temperature (Rossi et al. 2004). There are few reports on A. salina nauplii digestibility by G. fascicularis, but digestibility of A. salina nauplii by other organisms is well documented. Martinussen and Bamstedt (1999) reported that Aurelia aurita digestion time with A. salina was 0.3–3.5 h. Morais et al. (2004) reported higher digestibility of Artemia sp. (60% after 24 h) by herring larvae, Clupea harengus.
Heterotrophic feeding is essential for corals to overcome any nutrient deficiency, as nitrogen, phosphate, vitamins, and other trace elements are not synthesized through autotrophic feeding. In this case, the amount of A. salina nauplii carbon that was transferred to G. fascicularis could be extrapolated based on nutrient content. Artemia sp. nauplii contained 0.93 μg C ind−1 and 0.20 μg N ind−1 (Evjemo and Olsen 1999). G. fascicularis was extrapolated to acquire 106 μg C polyp−1 and 23 μg N polyp−1 per 1-h feeding under light conditions, whereas under dark conditions, 72 μg C polyp−1 and 16 μg N polyp−1 was acquired by G. fascicularis after 1-h feeding. Carbon and nitrogen acquisition under light conditions was about double the level of carbon and nitrogen under dark conditions.
Conclusion
A. salina nauplii are well accepted by G. fascicularis (digestibility and feeding captivity). It is feasible to use them as biocarriers to supply specific nutrients to G. fascicularis. The maximum G. fascicularis feeding rate under light and dark conditions was 113.6 ind. polyp−1 h−1 and 76.9 ind. polyp−1 h−1, respectively. Average G. fascicularis feeding rate was not significantly different under light and dark conditions. However, coral fed under light conditions achieved earlier feeding satiation compared with coral fed under dark conditions. Based on the rhythmic trend of feeding satiation, A. salina nauplii can be fed to the coral in captivity once every 3 days. Complete A. salina nauplii digestion was observed 180 min after feeding.
Abbreviations
- ind. l−1 :
-
Individual of Artemia salina nauplii per liter
- ind. polyp−1 h−1 :
-
Individual of Artemia salina nauplii per polyp per hour
References
Anthony KRN (1999) Coral suspension feeding on fine particulate matter. J Exp Mar Biol Ecol 232:85–106. doi:10.1016/S0022-0981(98)00099-9
Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol 252:221–253. doi:10.1016/S0022-0981(00)00237-9
Arvedlund M, Craggs J, Pecoerlli J (2003) Coral culture—possible future trends and directions. In: Cato JC, Brown CL (eds) Marine ornamental species: collection, culture and conservation. Iowa State Press, Ames, pp 233–248
Clayton WS, Lasker HR (1982) Effects of light and dark treatments on feeding by the reef coral Pocillopora damicornis (Linnaeus). J Exp Mar Biol Ecol 63:269–279. doi:10.1016/0022-0981(82)90183-6
Coffroth MA (1984) Ingestion and incorporation of coral mucus aggregates by a gorgonian soft coral. Mar Ecol Prog Ser 17:193–199. doi:10.3354/meps017193
Dai CF, Lin MC (1993) The effect of flow on feeding of three gorgonians from southern Taiwan. J Exp Mar Biol Ecol 173:57–69. doi:10.1016/0022-0981(93)90207-5
Davies PS (1984) The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs 2:181–186
Davy SK, Withers KJT, Hinde R (2006) Effects of host nutritional status and seasonality on the nitrogen status of zooxanthellae in the temperate coral Plesiastrea versipora (Lamarck). J Exp Mar Biol Ecol 335:256–265. doi:10.1016/j.jembe.2006.03.019
Dhert P, Lavens P, Duray M, Sorgeloos P (1990) Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using ω3-HUFA-enriched live food. Aquaculture 90:63–74. doi:10.1016/0044-8486(90)90283-S
Dhont J, Stappen GV (2003) Biology, tank production and nutritional value of Artemia. In: Stottrup JG, McEvoy LA (eds) Live feeds in marine aquaculture. Blackwell, Oxford, pp 65–112
Evjemo JO, Olsen Y (1999) Effect of food concentration on the growth and production rate of Artemia franciscana feeding on algae (T. iso). J Exp Mar Biol Ecol 242:273–296. doi:10.1016/S0022-0981(99)00104-5
Ferrier MD (1991) Net uptake of dissolved free amino acids by four scleractinian corals. Coral Reefs 10:183–187. doi:10.1007/BF00336772
Ferrier-Pages C, Allemand D, Gattuso JP, Jaubert J, Rassoulzadegan F (1998) Microheterotrophy in the zooxanthellae coral Stylophora pistillata: effects of light and ciliate density. Limnol Oceanogr 43(7):1639–1648
Ferrier-Pages C, Gattuso JP, Dallot S, Jaubert J (2000) Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19:103–113. doi:10.1007/s003380000078
Ferrier-Pages C, Witting J, Tambutte E, Sebens KP (2003) Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs 22:229–240. doi:10.1007/s00338-003-0312-7
Goreau TF, Goreau NI, Yonge CM (1971) Reef corals: autrotrophs or heterotrophs? Biol Bull 141:247–260. doi:10.2307/1540115
Green E (2003) International trade in marine aquarium species: using the global marine aquarium database. In: Cato JC, Brown CL (eds) Marine ornamental species: collection culture and conservation. Iowa State Press, Ames, pp 31–47
Grottoli AG (2002) Effect of light and brine shrimp on skeletal δ13C in the Hawaiian coral Porites compressa: a tank experiment. Geochim Cosmochim Acta 66:1955–1967. doi:10.1016/S0016-7037(01)00901-2
Hanaee J, Agh N, Hanaee M, Delazar A, Sarker SD (2005) Studies on the enrichment of Artemia urmiana cysts for improving fish food value. Anim Feed Sci Technol 120:107–112. doi:10.1016/j.anifeedsci.2005.01.010
Houlbreque F, Tambutte E, Ferrier-Pages C (2003) Effect of zooplankton availability on the rates of photosynthesis, and tissue and skeletal growth in the scleractinian coral Stylophora pistillata. J Exp Mar Biol Ecol 296(2):145–166. doi:10.1016/S0022-0981(03)00259-4
Houlbreque F, Tambutte E, Allemand D, Ferrier-Pages C (2004) Interactions between zooplankton feeding, photosynthesis and skeletal growth in scleractinian coral Stylophora pistillata. J Exp Biol 207:1461–1469. doi:10.1242/jeb.00911
Lasker HR (1981) A comparison of the particulate feeding abilities of three species of gorgonian soft coral. Mar Ecol Prog Ser 5:61–67. doi:10.3354/meps005061
Lasker HR, Syron JA, Clayton WS Jr (1982) The feeding response of Hydra viridis: effects of prey density on capture rates. Biol Bull 162:290–298. doi:10.2307/1540984
Lavens P, Sorgeloos P (2000) The history, present status and prospects of the availability of Artemia cysts for aquaculture. Aquaculture 181:397–403. doi:10.1016/S0044-8486(99)00233-1
Leversee GJ (1976) Flow and feeding in fan-shaped colonies of the gorgonian coral, Leptogorgia. Biol Bull 151:344–356. doi:10.2307/1540667
Lewis JB (1992) Heterotrophy in corals: zooplankton predation by the hydrocoral Millepora complanata. Mar Ecol Prog Ser 90:251–256. doi:10.3354/meps090251
Martinussen MB, Bamstedt U (1999) Nutritional ecology of gelatinous planktonic predators. Digestion rate in relation to type and amount of prey. J Exp Mar Biol Ecol 232:61–84. doi:10.1016/S0022-0981(98)00101-4
Miller MW (1995) Growth of a temperate coral: effects of temperature, light, depth, and heterotrophy. Mar Ecol Prog Ser 122:217–225. doi:10.3354/meps122217
Mills MM, Lipschultz F, Sebens KP (2004) Particulate matter ingestion and associated nitrogen uptake by four species of scleractinian corals. Coral Reefs 23:311–323. doi:10.1007/s00338-004-0380-3
Monroig O, Navarro JC, Amat F, Gonzalez P, Bermejo A, Hontoria H (2006) Enrichment of Artemia nauplii in essential fatty acids with different types of liposomes and their use in the rearing of gilthead sea bream (Sparus aurata) larvae. Aquaculture 251:491–508. doi:10.1016/j.aquaculture.2005.06.026
Morais S, Conceicao LEC, Dinis MT, Rønnestad I (2004) A method for radiolabeling Artemia with applications in studies of food intake, digestibility, protein and amino acid metabolism in larval fish. Aquaculture 231:469–487. doi:10.1016/j.aquaculture.2003.09.005
Okamura B (1987) Particle size and flow velocity induced an inferred switch in bryozoan suspension-feeding behaviour. Biol Bull 173:222–229. doi:10.2307/1541874
Olivotto I, Rollo A, Sulpizio R, Avella M, Tosti L, Carnevali O (2006) Breeding and rearing the sunrise dottyback Pseudochromis flavivertex: the importance of live prey enrichment during larval development. Aquaculture 255:480–487. doi:10.1016/j.aquaculture.2006.01.007
Olsen AIBB, Attramadal Y, Jensen A, Olsen Y (1999) Influence of size and nutritional value of Artemia franciscana on growth and quality of halibut larvae (Hippoglossus hippoglossus) during the live feed period. Aquaculture 179:475–487. doi:10.1016/S0044-8486(99)00181-7
Parks JE, Pomeroy RS, Balboa CM (2003) The economic of live rock and live coral aquaculture. In: Cato JC, Brown CL (eds) Marine ornamental species: collection culture and conservation. Iowa State Press, Ames, pp 185–206
Picciano M, Ferrier-Pages C (2007) Ingestion of pico- and nanoplankton by the Mediterranean red coral Corallium rubrum. Mar Biol (Berl) 150:773–782. doi:10.1007/s00227-006-0415-0
Piniak GA (2002) Effects of symbiotic status, flow speed, and prey type on prey capture by the facultatively symbiotic temperate coral Oculina arbuscula. Mar Biol (Berl) 141:449–455. doi:10.1007/s00227-002-0825-6
Rossi S, Ribes M, Coma R, Gili JM (2004) Temporal variability in zooplankton prey capture rate of the passive suspension feeder Leptogorgia sarmentosa (Cnidaria: Octocorallia), a case study. Mar Biol (Berl) 144:89–99. doi:10.1007/s00227-003-1168-7
Sebens KP, Koehl MAR (1984) Predation on zooplankton by the benthic anthozoans Alcyonium siderium (Alcyonacea) and Metridium senile (Actiniaria) in the New England subtidal. Mar Biol (Berl) 81:255–271. doi:10.1007/BF00393220
Sebens KP, Witting J, Helmuth B (1997) Effects of water flow and branch spacing on particle capture by the reef coral Madracis mirabilis (Duchassaing and Michelotti). J Exp Mar Biol Ecol 211:1–28. doi:10.1016/S0022-0981(96)02636-6
Sebens KP, Grace SP, Helmuth B, Maney EJ Jr, Miles JS (1998) Water flow and prey capture by three scleractinian corals, Madracis mirabilis, Montastrea cavernosa and Porites porites, in a field enclosure. Mar Biol (Berl) 131:347–360. doi:10.1007/s002270050328
Titlyanov E, Bil K, Fomina I, Titlyanov T, Leletkin V, Eden N et al (2000) Effects of dissolved ammonium addition and host feeding with Artemia salina on photoacclimation of the hermatypic coral Stylophora pistillata. Mar Biol (Berl) 137:463–472. doi:10.1007/s002270000370
Titlyanov EA, Titlyanov TV, Yamazato K, Woesik RV (2001) Photo-acclimation of the hermatypic coral Stylophora pistillata while subjected to either starvation or food provisioning. J Exp Mar Biol Ecol 257(2):163–181. doi:10.1016/S0022-0981(00)00308-7
Tsounis G, Rossi S, Laudien J, Bramanti L, Fernandez N, Gili JM et al (2006) Diet and seasonal prey capture rates in the Mediterranean red coral (Corralium rubrum L.). Mar Biol (Berl) 149:313–325. doi:10.1007/s00227-005-0220-1
Wabnitz C, Taylor M, Green E, Razak T (2003) From ocean to aquarium. UNEP – WCMC, Cambridge, p 65
Acknowledgements
The authors thank the Ministry of Higher Education (MoHE), Malaysia, and the University Malaysia Terengganu (UMT) for funding this study. Special thanks to Dr. Chuah Tse Seng for his assistance in biostatistics and Choo Chee Kuang for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hii, YS., Soo, CL. & Liew, HC. Feeding of scleractinian coral, Galaxea fascicularis, on Artemia salina nauplii in captivity. Aquacult Int 17, 363–376 (2009). https://doi.org/10.1007/s10499-008-9208-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-008-9208-4