Abstract
Coral mucus secreted by mucocytes provides a protective physicochemical and physiological barrier between coral tissue and external environmental threats. The biomolecules and nutrients of the secreted mucus are derived from endosymbionts, coral polyps and support coral functions, such as feeding, sediment clearing and protection, against numerous biotic and abiotic stressors. The surface mucus layer also houses a diverse community of beneficial microorganisms that defend against pathogens. Enzymes including peptidases, esterases, and glycosidases were observed and described in mucus. Most importantly, the presence of phenoloxidase, an intracellular enzyme in secretory mucus, generally triggers melanin synthesis, providing fast-acting components of invertebrate immunity in disease resistance. However, the purpose and the mechanism of mucus release, effects of mucus components on defense properties, and functional roles in intra- and interspecific interactions are not well described. Thus, the present review aims to understand the mucus components and the complex roles played by mucus in microbial associations, feeding, and resilience that influence coral health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to recent studies, 20% of coral reefs have already been irreparably damaged, and additionally 24% of the remaining reefs are facing serious threats due to anthropogenic stresses, such as overfishing and eutrophication (Sharma and Ravindran 2020; Galli et al. 2021). Biotic and abiotic stressor can cause coral bleaching, a process in which the algal symbionts are expelled from the host coral (Hafezi et al. 2020; Ravindran et al. 2022). The absence of the endosymbiont will weaken coral health, and mucus secretion will be affected (Ravindran et al. 2022). Environmental impacts, including temperature rise, on the secretion of mucus have not been studied in detail (Vaughan et al. 2021). For example, mucus release is enhanced during high sedimentation (Reynaud and Ferrier-Pages 2019), and when corals are exposed to air at low tide (Wild et al. 2004, 2005). In addition, mucus aggregations in the water column increase sedimentation and nutrient recycling (Wild et al. 2005; Plass-Johnson et al. 2015), which control microbial activities (Bythell and Wild 2011). Therefore, a better understanding of the changes in mucus caused by biological and physical stressors may help us to monitor coral health under different environmental conditions.
Mucus is a slimy substance produced by epithelial cells called mucocytes utilizing the organic carbon synthesized by photosynthetic endosymbiotic algae (Brown and Bythell 2005; Bakshani et al. 2018). Mucus production varies between and within coral species (Coffroth 1990; Hadaidi et al. 2019). It is mainly composed of proteolytic enzymes, microbes, mucins, proteins, polysaccharides, lipids and their substructures, such as monosaccharides, amino acids and mycosporines (Brown and Bythell 2005; Stabili 2019; Vilas Bhagwat et al. 2023). The beneficial microbes within the mucus form a barrier against potential coral pathogens, competing for nutrients and space or producing antibiotics (Ritchie 2006; Krediet et al. 2013; Raina et al. 2016; Irudayarajan et al. 2023). Coral mucus has several important functions, including protection against pathogens and UV radiation, capturing food, acting as an energy carrier, producing antibiotics, and serving as a nutrient source for coral-associated microbial communities (Bakshani et al. 2018; Bythell and Wild 2011; Vilas Bhagwat et al. 2023). Coral mucus contains up to 1000 times more bacteria than seawater (Nakajima et al. 2015). Vibrio shiloi, the causative agent of bleaching in the Mediterranean coral, and Oculina patagonica bind to the receptors of the surface mucus layer during temperature rise (Thompson et al. 2014). However, it has been demonstrated that beneficial probiotic bacteria enable O. patagonica to become resistant to V. shiloi infection (Reshef et al. 2006). Thus, studying the components of coral mucus is of great importance for a better understanding of the entire mucosal system, its interactions with other microorganisms (commensals and pathogens), and their involvement in coral immunity and health. Further, although the composition of coral mucus has been described in some studies (Coddeville et al. 2011; Hadaidi et al. 2019), it is limited to a few coral species and the sources of mucus components in mucus are not clearly understood. Therefore, the present study aims to describe the significant role of coral mucus in maintaining the health and functionality of coral reef ecosystems, especially the synthesis and constituents of coral mucus and their critical roles in protecting coral health.
Coral mucus synthesis and secretion
The mucocyte cells of the coral polyp synthesize mucus, with the major component being carbon-based carbohydrates, and are then secreted out as a coral surface layer (Brown and Bythell 2005). The coral-associated endosymbiotic algae are believed to provide the maximum carbon required for mucus synthesis although the source of the entire carbon requirement is not clear (Crossland 1987; Brown and Bythell 2005; Fang 2015) (Fig. 1). The other compounds required for mucus, such as amino acids, fatty acids, lipids, glycerol and nicotinamide adenine dinucleotide phosphate (NADPH), come from the coral diet (Hadaidi 2018). Only mucocytes contribute to mucus synthesis in corals, unlike in humans where multiple cell types are involved (Hadaidi et al. 2019; McShane et al. 2021). Some other gland cells are observed in coral tissues, but their roles are still unclear (Bruckner 2015). However, in vertebrates, the transformation of similar gland cells to mucus cells has been observed, which may be because these cells are either specialized secretory cells or developing mucocytes (Que 2015). The distribution of mucocytes varies in both abundance and location, not just within individual colonies but also between coral species (Baker et al. 2022). The production of coral mucus can be triggered by various factors, such as changes in light intensity, water flow, and the presence of predators or food particles (Zetsche et al. 2016). For example, an increase in the sea surface temperature increases the mucocyte density in host tissue; however, it decreases after bleaching (Piggot et al. 2009). In addition, more organic components are found in mucus from stressed than in healthy corals (Lee et al. 2016).
Coral mucus and properties. Coral mucus synthesis occurs in coral polyps from mucocyte cells. The mucus has a combination of nutrients provided by both the coral and endosymbiont. The released mucus acts as a first line of defense, with various properties against microbial pathogens, UV radiation and other stressors that influence the health of the corals
The synthesis of coral mucus is unique because it is derived from a significant portion of the photosynthetically fixed carbon (Hadaidi et al. 2019; Brown and Bythell 2005), and contains a high concentration of arabinose and xylose, which is not typically found in animal cells. Different coral species have varied rates of mucus production (Crossland et al. 1980; Kurihara et al. 2018), depending on the endosymbiont contribution of energy-rich glycerol, alanine, and glucose to the host (Burriesci et al. 2012; Gabay 2018) (Fig. 1). Excess photosynthetic carbon is eliminated by the coral through mucus release (Bakshani et al. 2018) along with lipids, such as wax esters and triglycerides for mucus synthesis (Krueger et al. 2018). Acropora spp. release up to 4.8 L of mucus from a 1m2 patch in one day and 56–80% of the mucus is dissolved in water (Wild et al. 2004), of which 20–40% of mucus forms strings and filaments (Stabili 2019). This mucus net filters seawater up to 100 ml cm2 of tissue per hour (Lewis 1976). Subsequently, heterotrophic coral reef organisms utilize the energy from the materials trapped in the coral mucus and the energy derived from zooxanthellae (Wild et al. 2004). Invertebrate mucus protects, lubricates, and helps in locomotion, mating, homing, and foraging, performing much more diverse functions than in vertebrates (Denny 1989). In addition, mucus released on the epithelial surface aims to attract particulate matter.
Mucus composition
Mucins and the importance of mucus rheology
The glycoprotein core, mucin, is made up of 20% polymers and 80% sugars and contains cysteine, which undergoes cleavage during mucus dispersion (Bakshani et al. 2018). O-glycosylation of mucin occurs on oligosaccharides in the core between serine and threonine and on the N-acetylgalactosamine carbohydrate chains, and N-glycosylation occurs on asparagine (Corfield 2015). The side chains of mucins are either branched or linear and up to 20 monosaccharides long and are linked with either β1 → 2 or β1 → 3 bonds (Krediet et al. 2009). The structural diversity of mucin is due to its carbohydrate composition (Thornton and Sheehan 2004). The ability of mucin to form a gel affects mucus function depending on its extensive glycosylation and polymerization ability (Younan et al. 1982). The von Willebrand D (VWD) domain is involved in the polymerization of mucin monomers via intermolecular disulfide bonds (Ambort et al. 2012). The outer layer of the epidermis of fishes continuously produces new goblet cells because they cannot produce mucus more than once. But coral mucus goblet cells can continuously produce mucus from the same cells (Subramanian et al. 2007).
Corals require relatively fluid mucus for their ciliary feeding and to prevent the desiccation of coral polyps by air exposure during low tide (Brown and Bythell 2005; Bessell-Browne et al. 2017). Based on rheology, coral mucus is distributed as fluid mucus, string, web or flocs, and mucus sheets (Bessell-Browne et al. 2017). Distinct mucin characteristics are present across various layers within the coral surface mucus layer (SML) (Guppy et al. 2019). The mucin concentration and gel-forming properties of mucus are necessary for its protective and lubricating functions (Thornton and Sheehan 2004). Human mucus becomes hydrated and mucus contents turn it into a gel (Piludu et al. 2003). As polymeric mucins have been detected in coral mucus, similar to vertebrates (Jatkar et al. 2010a), it is possible that coral mucus also contains different types of mucin molecules that convert it from gel to liquid and liquid to gel as needed (Horricks et al. 2019). Because coral mucus is rich in plant-associated sugar arabinose, it may have some carbohydrate chains that differ from those of mammalian mucins (Wild et al. 2005). Human mucus changes its rheology from viscoelastic gel to viscous fluid at high stress and returns to a viscous gel at low stress (Jatkar et al. 2010b) making it a lubricant and helping it move under various physiological conditions (Hill et al. 2022). A similar property may also be present in the coral SML, which remains to be investigated. However, corals can produce two types of mucus. The milked mucus has no gel-forming properties but can be converted to a gel by a higher concentration of mucin (Jatkar et al. 2010a), which is very similar to human respiratory mucus (Zetsche et al. 2016). Mucus sheets are sticky mucus layers derived from liquid mucus (Bessell-Browne et al. 2017). These are then transferred into the food web of the reef (Brown and Bythell 2005).
Carbohydrates
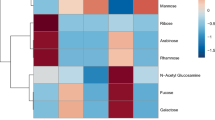
The relative concentration of carbohydrates varies among coral species, yet their composition remains constant, with certain sugars being shared across multiple species, and this variability is intricately linked to host phylogeny, microbial associations, and symbiont composition (Hadaidi et al. 2019). The mucosal sugars glucose, mannose, xylose, and galactose are found in all coral species that have been analyzed, whereas arabinose, N-acetylglucosamine, ribose, fucose, and rhamnose are present in only a few species (Table 1). Thermal stress has been shown to induce changes in both the enrichment and abundance of various carbohydrate pools, with a notable impact on galactose levels (Hillyer et al. 2017). For example, a significant decrease in glucose, galactose, and mannose was described under rising temperature-stress conditions (Lee et al. 2016). Thus, mannose, glucose, and galactose can serve as markers for temperature stress, while N-acetyl glucosamine exhibits anti-inflammatory properties and reduces the production of free radicals (Salvatore et al. 2000). N-acetyl galactosamine also helps in the O-glycosylation of coral mucin molecules. Xylose and arabinose are types of plant sugars commonly utilized as energy sources, indicating that they may have come from the endosymbiont (Teplitski et al. 2016). Arabinose is widespread in Acropora spp., suggesting that it is transferred from algal symbionts to the host (Wild et al. 2005, 2010; Lee et al. 2016).
The carbohydrate composition of mucus differs among the corals Acropora pharaonis, Porites verrucosa, and Porites lobata (Hadaidi et al. 2019). However, Stylophora pistillata and Pocillopora verrucosa, two pocilloporid corals, have similar carbohydrate (Neave et al. 2017) and zooxanthellae composition (LaJeunesse et al. 2018). Differences in mucus components could affect microbial community composition in coral reef habitats (Schottner et al. 2009). The most common mucus components observed in corals that occur across a large geographic range (e.g., A. muricata, Pachyseris speciosa, Fungia fungites, Stylophora sp., and Pocillopora sp.) are fucose, arabinose, glucose, galactose, the amino sugar N-acetylglucosamine, xylose, and mannose (Wild et al. 2005; Lee et al. 2016) (Table 1). Thus, mucus carbohydrates are essential substrates that support bacterial growth and contribute to the flux of dissolved organic matter (DOM) in seawater (Riemann and Azam 2002).
Fatty acids of mucus
The precise understanding of the fatty acid (FA) composition and its role in coral mucus is still inadequate and remains one of the least studied aspects of coral mucus. It was reported that the FA contents of Acropora and Porites species were almost the same, but the proportions were different not only in the mucus but also in comparison with the tissues (Ravindran et al. 2020). The most common and abundant FAs observed in the mucus of Acropora sp. in Discovery Bay, Jamaica, were palmitic acid and palmitoleic acid (Means and Sigleo 1986; Wild et al. 2005). These were also found more frequently in corals and zooxanthellae as structural and storage lipids (Patton et al. 1977; Means and Sigleo 1986). Other FAs, such as myristic acid and linoleic acid, have also been observed in mucus, but in lower amounts (Wild et al. 2005). The most abundant saturated FAs in marine organisms are palmitic acid (C16:0), palmitoleic acid (C16:1), and myristic acid (C14:0), similar to those found in certain unicellular marine algae (Uslu et al. 2021). Even though the presence of FAs in mucus has been reported, their origins have not been documented.
FAs in coral mucus are essential for the health and functioning of coral reefs, providing energy, supporting microbial communities, and aiding in defense mechanisms (Bachok et al. 2006). Polyunsaturated fatty acids (PUFAs), especially n−3 and n−6 PUFAs, regulate metabolism, including growth, respiration, energy generation, energy reserves, membrane constituents, photosynthesis, planula larvae and egg production, biomass formation and general enzyme activity, survival, and stress resistance (Bachok et al. 2006). C20:5n−3 FAs are essential for sperm maturation and C20:4n−6 is essential for egg maturation, and saturated (SAFA) and monounsaturated FAs (MUFA) are stored as triglycerides and wax esters (Grottoli et al. 2004; Tchernov et al. 2004; Treignier et al. 2008). In all of the previously studied coral mucus samples, higher concentrations of SAFAs were observed (Fig. 2). In addition, FAs in corals serve as markers for bacterial colonization and diversity, coral health, and food source tracking (Volkman et al. 1980). As the synthesis of FAs in corals and in coral mucus is directly coupled to photosynthesis, there is a significant correlation between dinoflagellate biomarkers and FA concentrations (Oku et al. 2003). Free fatty acids (FFAs) have been found to be involved in maintaining cell membrane stability and cell signaling (Binienda et al. 2013). FFAs are physiologically active as dietary components and participate in cell signaling (Zbigniew et al. 2013). FAs are also required for reproduction and membrane fluidity (Lin et al. 2012). However, the purpose and the function of FAs present in mucus are not well understood and need to be thoroughly investigated in various genera and species of corals based on their habitat and other environmental parameters.
Mucus lipids
Photosynthetic carbon is stored in the cytoplasm of zooxanthellae, in the form of lipids, which are then incorporated into the coral mucus, where it serves as an important source of energy for a variety of marine organisms (Crossland et al. 1980; Pasaribu et al. 2015). Many galactolipids synthesized by marine diatoms have been shown to induce apoptosis starting at micromolar concentrations in mammalian cells (Andrianasolo et al. 2008), suggesting the function of membrane lipids in regulating the symbiosis between corals and zooxanthellae. Coral mucus is composed of high-energy lipids which are also an important source of energy for fish (Wright et al. 2019). Upon thermal stress, corals may acquire more thermotolerant symbionts (Lajeunesse et al. 2010) having lipids with more SAFAs with a high melting point. This altered membrane lipid composition is observed in bleached corals, indicating differences in heat sensitivity among zooxanthellae lineages (Tchernov et al. 2004).
Zooxanthellae provides precursors and metabolites required for endogenous lipid synthesis in coral hosts mainly through photosynthesis (Oku et al. 2003; Iluz and Dubinsky 2015), and several studies have demonstrated the rapid conversion of photosynthetically fixed carbon to lipids (Radice et al. 2019). The presence of lipids (e.g., wax esters) in coral mucus has been demonstrated in significant amounts (Crossland 2021). Corals are rich in lipids, which are the biochemical link between the symbiotic mutualistic association of coral and zooxanthellae (Sikorskaya et al. 2022). Lipids are involved in coral physiological and biochemical processes and are an important source of long-term stored energy as mucus serves as an energy-rich source of nutrition for various organisms (Rodrigues et al. 2008). During bleaching, a change in FA composition and also a reduction in the rate of lipid production may affect the coral’s metabolic needs (Grottoli et al. 2004). This reduced energy reserve increases disease susceptibility and mortality (Smith et al. 2020), which may be due to a change in the microbial community in the damaged tissues.
Proteins/peptides of mucus
According to Benavides et al. (2017), a low C:N ratio in coral mucus indicates the presence of a large amount of protein. Previous studies on Palythoa sp. and Fungia sp. showed that their mucus contains 8–20% organic matter and 1–2% protein (Daumas and Thomassin 1977; Stabili et al. 2015). Coral mucus from Acropora, Fungia, and Sarcophyton species contains important enzymes, such as peptidases, esterases, and glycosidases (Vacelet and Thomassin 1991; Vilas Bhagwat et al. 2023). Hydroxyproline, found in coral mucus, is crucial for collagen synthesis and maintaining the thermodynamic stability of collagen's triple-helical conformation and associated tissues (Ortega et al. 2018). Fish mucus, on the other hand, is known for its high protein content and enzymes, such as lysozyme, alkaline phosphatase, complement, cathepsin B, transferrin, and C-reactive protein (Subramanian et al. 2007; Zhao et al. 2008). The mucus of the zoanthid Palythoa caribaeorum contains lectins and proteases (Guarnieri et al. 2018). However, phenoloxidase (PO), peroxidases (POD), and antioxidant enzymes like superoxide dismutase, catalase, and glutathione peroxidase, which have been reported from coral tissues (Anithajothi et al. 2014), have not yet been reported in coral mucus. Thus, mucus reported with antimicrobial properties has to be investigated for its potential peptides and proteins.
Amino acids
Corals and zooxanthellae can synthesize 20 different amino acids, with some produced by the coral polyps, others by the zooxanthellae, and yet others by both partners. However, different coral species have different amino acid synthesis abilities (Fitzgerald and Szmant 1997). Amino acids can also be taken up via plankton, detritus, and the water column. The acquired amino acids are used by the coral to synthesize proteins for tissue growth, calcification, and other processes (Grover et al. 2008; Shinzato et al. 2014). The production of methionine could be a result of the synergistic action of enzymes from both coral and zooxanthellae, indicating that these symbiotic partners have evolved to mutually benefit each other (Shinzato et al. 2014). Although corals acquire many amino acids, only 16 have been observed in the mucus, including aspartate, glutamine, serine, glycine, threonine, arginine, alanine, tyrosine, valine, phenylalanine, isoleucine, leucine, lysine, histidine and methionine, of several coral species (i.e., Acropora formosa, Pachyseris speciosa, Fungia fungites, Sarcophyton sp., Lemnalia sp., Cespitularia sp., Platygyra lamellina, Heteroxenia fuscesens, Oculina arbuscula (Meikle et al. 1988; Ducklow and Mitchell 1979; Means and Sigleo 1986; Coddeville et al. 2011).
Analysis of mucus produced by Palythoa and Fungia species has shown that the amino acids serine, aspartic acid, glutamic acid, and glycine are present in high abundance, and these play important roles in a variety of biological processes, including protein synthesis, cellular signaling, and energy metabolism (Daumas and Thomassin 1977). Glycine is the most abundant amino acid found in both coral tissues and mucus, particularly in species such as Montastraea cavernosa (Daumas and Thomassin 1977). Glutamic acid, which also acts as an antioxidant in many plants, animals, and bacteria, was more abundant in the mucus of Fungia sp., Acropora sp., Platygyra sp., Sarcophyton sp., and Heteroxenia sp., compared to serine, and methionine (Ducklow and Mitchell 1979). Alanine is generally observed in Porites divaricata, Acropora cervicornis, Orbicella faveolata, and the azooxanthellate corals, Astrangia poculata and Tubastraea coccinea (Fitzgerald and Szmant 1997). Similarly, Fungia scutaria was reported to utilize tyrosine, lysine, glycine and aspartic acid, from surrounding water (Stephens 1962). This suggests that corals acquire some amino acids through heterotrophic feeding from the surrounding water. In addition, both host and zooxanthellae can synthesize amino acids and this varies from species to species.
Mucus antioxidants against oxidative stress
A decrease in zooxanthellae density is due to oxidative stress causing coral bleaching (Downs et al. 2002; Weis 2008). Primarily, oxidative stress is generated during photosynthesis (Krieger-Liszkay 2005). Excessive production of reactive oxygen species (ROS) causes disorganization and rupture of the thylakoid membrane (Tchernov et al. 2004). Free radicals, such as hydrogen peroxide (H2O2) and hydroxyl radicals (–OH), degrade mucus glycoproteins at the histidine molecule, so that mucus property, molecular weight, and viscosity is affected (Dailah 2022). ROS imparts an adverse impact on the mucus defense properties which are then controlled by the mucus antioxidants (Suggett et al. 2008).
The presence of phenoloxidase (PO), an enzyme that activates the invertebrate defense system, was reported in coral secretory mucus (Rivera-Ortega and Thome 2018; Vilas Bhagwat et al. 2023). The mechanism of secretion of this enzyme into coral mucus is not known. However, the expulsion of similar molecules from tissue to the mucus was reported previously (Ritchie 2006). Thus, phenoloxidase is thought to be an intracellular enzyme, but similar activity was also observed in the mucus of other organisms, such as in lancelets (Zhang and Li 2000) and some insect cuticles (Arakane et al. 2016). The pro-phenoloxidase (proPO) activates PO, resulting in the synthesis of melanin which in turn activates innate immune responses. The proPO system itself is activated by tyrosine and phenylalanine, which is synthesized by both the coral host as well as zooxanthellae (Fitzgerald and Szmant 1997). To reduce the impact of toxic by-products, such as reactive oxygen (RO) and other free radicals, antioxidant enzymes are up-regulated to offset self-harm (Gardner et al. 2016). However, the source and the presence of such antioxidant enzymes and non-enzymatic antioxidants in coral mucus are not well studied (Vilas Bhagwat et al. 2023). Some enzymes, such as superoxide dismutase and glutathione peroxidase, have been reported as stress markers in corals (Gardner et al. 2016). Elevated PO activity triggers melanin production, serving as a signal for corals to react to pathogens, and concurrently functioning as a potent antioxidant against UV radiation (Palmer et al. 2011). The antioxidant capabilities of mucus against oxidative stress may determine the survival of the corals against bleaching and other stressors.
Antimicrobial activity of mucus and mucus-associated microbes
Coral mucus from different habitats is composed of unique components that alter the presence of specific microbes on the surface of different coral species (Guppy and Bythell 2006; McShane et al. 2021). The natural flora of healthy coral is presumably beneficial and any shift in this bacterial population may be an early indicator of coral stress, such as disease (Nithyanand et al. 2011). Therefore, the SML of coral is a selective medium determined by host–microbe–environment interactions, where the commensal microbiota act against potential pathogens (Ritchie 2006; Alagely et al. 2011; Krediet et al. 2013; Teplitski et al 2016; Irudayarajan et al. 2023). The microbial gene abundance in the coral mucus is known to be involved in stress resistance, virulence, chemotaxis and motility, utilization of FAs and lipids, sulfur and nitrogen metabolism, and secondary metabolism making the mucus-associated bacterium a first line of defense (Bythell and Wild 2011; Irudayarajan et al. 2023). Thus, some microbial communities in the SML are described as producing defensive antimicrobial chemicals (Orland and Kushmaro 2009; Irudayarajan et al. 2023). The Caribbean coral Acropora palmata demonstrated with antibacterial inhibition against the pathogen that causes white pox disease (Nissimov et al. 2009). Similarly, antibacterial activity was found in many cultivable bacteria of the coral mucus of six scleractinian corals from the Gulf of Eilat (Orland and Kushmaro 2009). Varied bacterial genera of both tissue and mucus of A. digitifera, including Vibrio sp. and Bacillus sp. have been elucidated for their antibacterial activity. Bacillus sp. synthesizes a significant peptide group of antibiotics including gramicidin, bacitracin and polymyxin B (Wiese et al. 2009). Thus, the highly specific mucus bacterial community is crucial for first line of defense of corals against diseases and other environmental stress factors (Kemp et al. 2015). Moreover, specific bacteriophages of the coral SML have been described to treat various bacterial infections as phage therapy (Nguyen et al. 2015; van Oppen 2019). The SML antimicrobial activity exhibited species specificity, while the mucus-associated beneficial bacteria, such as Pseudoalteromonas sp. and Roseobacter sp., have been described for their antibacterial activity against a number of coral pathogens, including V. shiloi (Ortega and Thome 2018). As a result, beneficial microorganisms of corals (BMCs) are used as probiotic drugs in order to increase coral resistance to environmental stress, and the rotifers, Brachionus plicatilis, are employed as carriers to impart BMCs into the coral Pocillopora damicornis (Assis et al. 2020).
The biochemicals present in the surface mucus of Acropora palmata and Pseudodiploria strigosa are responsible for regulating the coral-associated microbial community (Rivera-Ortega et al. 2018). Studies have shown that the mucus of A. palmata suppresses the growth of various bacterial strains, including Bacillus subtilis, Staphylococcus aureus, Salmonella typhimurium, and the white pox disease causative agent, Serratia marcescens (Ritchie 2006; Vilas Bhagwat et al. 2023). The mucus also exhibits antibacterial activity against both Gram-positive and Gram-negative bacteria (Ritchie 2006; Vilas Bhagwat et al. 2023). Similarly, healthy coral mucus obtained from P. strigosa has been found to possess antimicrobial activity against Serratia marcescens and Aurantimonas sp. pathogens (Ortega and Thome 2018). In addition to its antimicrobial properties, Galaxea fascicularis mucus has been found to have potential health benefits for humans, such as apoptotic and anticancer activity, DNase activity, and activity against Topoisomerase I and II enzymes (Ding et al. 1999; Khalesi 2015). Homarine, an organic compound with antibiotic potential, has been documented in corals for its ability to potentially inhibit the growth of a range of bacteria and fouling diatoms (Maldonado et al. 2016). Fish mucus contains a variety of components, including disulfides, glycoconjugates, antimicrobial peptides (AMPs), lysozyme, cathepsin B, complement, alkaline phosphatase, C-reactive protein, transferrin, and others, which act as effective protection (Meyer et al. 2007; Subramanian et al. 2007; Zhao et al. 2008). AMPs in fish mucus are effective antimicrobial agents against human pathogens, including algae, bacteria, fungi, viruses, or parasites (Komatsu et al. 2009; Fernandes et al. 2010). Although the antimicrobial properties of coral mucus and associated bacteria have been mentioned in numerous papers, the specific components responsible for these properties are not well documented (Wright et al. 2019; Wijayanti et al. 2020), and further research is needed to better understand the immunological properties of coral mucus and the mechanisms by which it provides protection.
Mucus as a sunscreen and effective antioxidant under heat stress
Mycosporine-like amino acids (MAAs) are responsible for shielding corals from the harmful effects of solar ultraviolet (UV) radiation by absorbing approximately 10% of UV radiation. This mechanism is critical for the survival of corals, as exposure to high levels of UV radiation can cause damage to coral DNA, resulting in a range of negative impacts on coral health and the overall health of the surrounding ecosystem. By providing this important protection against UV radiation, MAAs play a key role in maintaining the health and resilience of coral populations (Teai et al. 1998; Kageyama and Waditee-Sirisattha 2019). Mycosporine-glycine, mycosporine-2glycine, palythine, mycosporine-methylamine-serine, and mycosporine-methylamine-threonine, which were characterized from Lobophyllia, Acropora, Fungia, and Pocillopora species, have all been reported from coral mucus (Teai et al. 1998). In addition to their ability to combat oxidative stress, MAAs can protect many marine organisms from the harmful effects of light (Shick and Dunlap 2002). Similarly, mycosporine-glycine showed an additional antioxidant property in coral mucus along with sunscreen, probably protecting endosymbionts from oxidative damage produced through photosynthesis (Teai et al. 1998). Biologically harmful UV rays between 310 and 360 nm are absorbed by MAAs. Endosymbionts and the coral hosts are both protected from the harmful effects of UV radiation by mucus MAAs. It was reported that the UV-absorbing molecules in the coral mucus of Fungia fungites reduced with depth (Drollet et al. 1997). The reduction in UV radiation, light, and photosynthesis may be attributed to increased depth, as water absorbs and scatters UV radiation more effectively with increasing depth, leading to a decrease in UV radiation intensity.
Mycosporine-like amino acid concentrations vary depending on coral species and habitat. They have been found in both host tissues as well as in zooxanthellae (Shick and Dunlap 2002). MAAs are thermally stable, making them useful for scavenging reactive oxygen species under heat stress (Banaszak and Lesser 2009). MAAs play a role in cellular osmoregulation during salinity stress or in a high-salinity environment (Oren and Gunde-Cimerman 2007). The combination of mycosporine and glycine, the most common MAAs in coral hosts, protects the coral symbiosis from oxidative damage (Banaszak et al. 2006). MAA also serves as a nitrogen source within the cell, which is required for growth and proliferation (Oren and Gunde-Cimerman 2007).
Mucus as a source of nutrients for marine organisms
Mucus produced by coral is a valuable energy source with high glucose content. As glucose is readily available, it is a highly preferred energy source for microbes and other organisms (Ravindran et al. 2023). Thus, coral mucus is an important component of marine ecosystems, providing a rich source of energy for a wide group of organisms (Wild et al. 2010) (Table 2). Mucus energy levels are far greater than benthic photosynthetic algae. Further, a few soft corals have also been shown to feed on the mucus of other corals (Coffroth 1984). Similarly, fishes, such as blue sprat (Eckes et al. 2015), damselfishes (Coles and Strathmann 1973; McMohan et al. 2016), and other benthic organisms, such as an Acoelomorpha worm (Waminoa sp.), bivalves (Shafir and Loya 1983), shrimp (Coralliocaris superba) (Horka et al. 2016), coral crabs (Trapezia sp.) (Shmuel et al. 2022) and some planktonic organisms, such as crown-of-thorns starfish larvae (Nakajima et al. 2016), copepods (Acartia negligence) (Richman 1975), and mysids, (Mysidium integrum) (Gottfried and Roman 1983) also exhibit feeding on coral mucus. Coral mucus was found to be the best nutritional source with an C:N ratio of mucus fluid of 6.9 to 13.7 and mucus sheet of 4.8 to 5.9 (Coffroth 1990) (Table 2). Since mucus sheets that stay on the coral surface can trap organic materials, their nutritional value is higher than reef benthic algae (~ 1) and reef algal detritus (~ 1) (Wilson et al. 2003). When compared with the other forms of energy sources, reef fish feces contribute to a nutritional value of ~ 10 (Bailey and Robertson 1982), phytoplankton ~ 3.5 (Renaud et al. 1999), and zooplankton ~ 5.5 (Ventura 2006). Furthermore, coral mucus acts as a vehicle for nutrients to reach the mouth (Goldberg 2018). Small reef fish and copepods utilize wax esters and triglycerides as energy sources, both of which are major components of mucus (Ho 2017). Thus, coral mucus helps in the fulfillment of energy requirements as well as the cycling of nutrients (Wild et al. 2004).
Role of mucus in reef ecosystem nutrient cycling
When coral mucus is released into the water column, it is rapidly colonized by microorganisms that break down the organic compounds and release nutrients, such as nitrogen (N2) and phosphorus (Van Oppen et al. 2019). These nutrients are then available for uptake by other organisms in the ecosystem, such as phytoplankton and zooplankton. Corals obtain N2 through predation and from the water column and excrete ammonia (Fig. 3) (Siboni et al. 2008). Further, Archaea use this ammonia, which plays an important role in ocean nitrification. During daytime, when oxygen levels are high due to photosynthesis, certain types of Archaea convert ammonia into nitrite. Conversely, at night, when oxygen is low, different types of Archaea carry out denitrification to convert nitrite into nitrogen (Siboni et al. 2008, 2012). Nitrogen-cycling microbes play a vital role in coral–algae symbiosis, especially under nutrient-rich or -poor conditions for coral productivity (Radecker et al. 2015). Coral mucus contains high concentrations of dissolved organic nitrogen (DON), which can be readily taken up by bacteria for growth and metabolism. Some of these bacteria form symbiotic relationships with coral polyps, where they fix atmospheric nitrogen (N2) (Diaz 2017). Other bacteria in the mucus help us to recycle nitrogen within the holobiont by breaking down waste products and releasing ammonium (NH4+) and nitrate (NO3−) into the surrounding water. These forms of nitrogen can then be taken up by other organisms in the reef ecosystem (Wild et al. 2004).
Coral mucus in reef nutrient cycling. Corals release mucus rich in dissolved organic matter (DOM) to the surrounding water, which is utilized by reef organisms whose respiration returns carbon dioxide and other nutrients required for photosynthesis by the endosymbiont zooxanthellae. Also, endosymbiont derived organic compounds are released into the mucus and nutrient cycling is activated through various microbes. (DOM—dissolved organic matter, DON—dissolved organic nitrogen, DIP—dissolved inorganic phosphate, DOC—dissolved organic carbon, DOP—dissolved organic phosphate, NH4—Ammonia, NO2—nitrite, N2—nitrogen)
Up to 80% of mucus released by corals becomes dissolved organic carbon (DOC) (Wild et al. 2004). Sponges transfer dissolved organic matter (DOM) including DOC and DON to the next trophic level (Rix et al. 2017). Bacteria utilize mucus and break down organic compounds and release carbon dioxide (CO2) through respiration. This CO2 can then be taken up by photosynthetic organisms, such as algae and seagrasses, which use it to produce organic carbon through photosynthesis (Haas et al. 2016). This process helps us to maintain the balance of carbon in the reef ecosystem. Coral mucus also contains significant amounts of dissolved organic phosphorus (DOP), which can be taken up by bacteria and recycled within the holobiont (Nakajima et al. 2015). Some of these bacteria are capable of breaking down complex organic phosphorus compounds, such as phospholipids, to release inorganic phosphorus (PO43−) into the surrounding water (Bourne et al. 2013). This inorganic phosphorus can then be taken up by algae and other organisms in the reef ecosystem. Mucus functions as nutrient trapping, thus, higher ammonia, nitrate, and phosphate in mucus than in surrounding water suggest its role in nutrient adsorption. In addition, coral mucus transports nutrients into reef sediment (Wild et al. 2005). Overall, coral mucus plays a crucial role in maintaining the health and productivity of reef ecosystems by facilitating nutrient cycling and supporting the diverse array of organisms that inhabit these ecosystems. Recycling these nutrients within the holobiont and the surrounding water helps us to maintain a balance of nutrients and supports the ecosystem’s productivity. Additionally, the role of coral mucus in nutrient cycling highlights the importance of conserving coral reefs and protecting them from human activities that may disrupt this delicate balance.
Conclusion
The coral surface mucus serves as an interface between the coral epithelium and the seawater environment. It provides a primary line of defense and protects coral surfaces from a variety of physio-chemical and microbial invasions. It acts as a high-energy substrate for bacterial heterotrophic activity. As both host and endosymbiont provide mucus constituents, this symbiotic mucus synthesis is different from many other marine organisms. The exact mechanism of mucus synthesis, secretion, and sources of other important components present in mucus is unknown, and less information is available about the properties and rheological changes of mucus, which are not fully demonstrated. Only certain coral species have been studied for their mucus components, such as carbohydrates, fatty acids, amino acids, proteins, antioxidants, and antimicrobial compounds, but even among those, not all types have been thoroughly explored. Also, mucus defense properties, including antimicrobial peptides, antioxidants, and UV protectants (e.g., Mycosporine-like amino acids), and beneficial microbes, are not well described for their roles in supporting coral health in various stress conditions. Coral mucus serves multiple ecological roles with their associated microorganisms and the surrounding environment that need to be studied to understand reef ecosystem functioning. Thus, the present review aids in understanding the role of coral mucus in coral reef ecosystems; however, more detailed studies of coral mucus components and their roles in the coral reef ecosystem by developing appropriate biochemical and molecular approaches may provide insights into protecting and restoring these valuable ecosystems.
Data availability
All data analysis of the present study are drawn from numerous previous published articles.
References
Alagely A, Krediet CJ, Ritchie KB, Teplitski M (2011) Signaling-mediated cross-talk modulates swarming and biofilm formation in a coral pathogen Serratia marcescens. ISME J 5(10):1609–1620
Ambort D, Johansson ME, Gustafsson JK, Ermund A, Hansson GC (2012) Perspectives on mucus properties and formation-lessons from the biochemical world. Cold Spring Harb Perspect Med 2(11):014159
Andrianasolo EH, Haramaty L, Vardi A, White E, Lutz R, Falkowski P (2008) Apoptosis-inducing galactolipids from a cultured marine diatom, Phaeodactylum tricornutum. J Nat Prod 71(7):1197–1201
Anithajothi R, Duraikannu K, Umagowsalya G, Ramakritinan CM (2014) The presence of biomarker enzymes of selected scleractinian corals of Palk Bay, southeast coast of India. Biomed Res Int 2014:684874
Arakane Y, Noh MY, Asano T, Kramer KJ (2016) Tyrosine metabolism for insect cuticle pigmentation and sclerotization. In: Cohen E, Moussian B (eds) Extracellular composite matrices in arthropods. Springer, Berlin, pp 165–220
Assis JM, Abreu F, Villela HM, Barno A, Valle RF, Vieira R, Taveira I, Duarte G, Bourne DG, Høj L, Peixoto RS (2020) Delivering beneficial microorganisms for corals: rotifers as carriers of probiotic bacteria. Front Microbiol 15(11):608506
Bachok Z, Mfilinge P, Tsuchiya M (2006) Characterization of fatty acid composition in healthy and bleached corals from Okinawa. Japan Coral Reefs 25(4):545–554
Bailey TG, Robertson DR (1982) Organic and caloric levels of fish feces relative to its consumption by coprophagous reef fishes. Mar Biol 69(1):45–50
Baker LJ, Reich HG, Kitchen SA, Grace Klinges J, Koch HR, Baums IB, Muller EM, Thurber RV (2022) The coral symbiont Candidatus Aquarickettsia is variably abundant in threatened Caribbean acroporids and transmitted horizontally. ISME J 16(2):400–411
Bakshani CR, Morales-Garcia AL, Althaus M, Wilcox MD, Pearson JP, Bythell JC, Burgess JG (2018) Evolutionary conservation of the antimicrobial function of mucus: a first defense against infection. NPJ Biofilms Microbiomes 4(1):14
Banaszak AT, Barba Santos MG, LaJeunesse TC, Lesser MP (2006) The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean. J Exp Mar Biol Ecol 337(2):131–146
Banaszak AT, Lesser MP (2009) Effects of solar ultraviolet radiation on coral reef organisms. Photochem Photobiol Sci 8(9):1276–1294
Benavides M, Bednarz VN, Ferrier-Pagès C (2017) Diazotrophs: overlooked key players within the coral symbiosis and tropical reef ecosystems? Front Mar Sci 4:10
Bessell-Browne P, Fisher R, Duckworth A, Jones R (2017) Mucous sheet production in Porites: an effective bioindicator of sediment related pressures. Ecol Ind 77:276–285
Binienda ZK, Sarkar S, Silva-Ramirez S, Gonzalez C (2013) Role of free fatty acids in physiological conditions and mitochondrial dysfunction. Food Nutr Sci 04(9):6–15
Bourne DG, Dennis PG, Uthicke S, Soo RM, Tyson GW, Webster N (2013) Coral reef invertebrate microbiomes correlate with the presence of photosymbionts. ISME J 7(7):14521458
Brown BE, Bythell JC (2005) Perspectives on mucus secretion in reef corals. Mar Ecol Prog Ser 296:291–309
Bruckner AW (2015) White syndromes of western Atlantic reef-building corals. In: Woodley CM, Downs CA, Bruckner AW, Porter JW, Galloway SB (eds) Diseases of coral. Wiley, New York, pp 316–332
Burriesci MS, Raab TK, Pringle JR (2012) Evidence that glucose is the major transferred metabolite in dinoflagellate–cnidarian symbiosis. J Exp Biol 215(19):3467–3477
Bythell JC, Wild C (2011) Biology and ecology of coral mucus release. J Exp Mar Biol Ecol 408(1–2):88–93
Coddeville B, Maes E, Ferrier-Pages C, Guerardel Y (2011) Glycan profiling of gel forming mucus layer from the scleractinian symbiotic coral Oculina arbuscula. Biomacromol 12(6):2064–2073
Coffroth MA (1984) Ingestion and incorporation of coral mucus aggregates by a gorgonian soft coral. Mar Ecol Prog Ser 17(2):193–199
Coffroth MA (1990) Mucous sheet formation on poritid corals: an evaluation of coral mucus as a nutrient source on reefs. Mar Biol 105(1):39–49
Coles SL, Strathmann R (1973) Observations on coral mucus “flocs” and their potential trophic significance 1. Limnol Oceanogr 18(4):673–678
Corfield AP (2015) Mucins: a biologically relevant glycan barrier in mucosal protection. Biochem Biophys Acta 1850(1):236–252
Crossland CJ (1987) In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes. Coral Reefs 6(1):35–42
Crossland CJ (2021) Hermatypic coral, Acropora cf. acuminata. In: Endosymbiosis and cell biology: a synthesis of recent research. Proceedings of the International Colloquium on Endosymbiosis and Cell Research, Tübingen, April 1980, vol 1, pp 163. Walter de Gruyter GmbH & Co KG
Crossland CJ, Barnes DJ, Borowitzka MA (1980) Diurnal lipid and mucus production in the staghorn coral Acropora acuminata. Mar Biol 60(2–3):81–90
Dailah HG (2022) Therapeutic potential of small molecules targeting oxidative stress in the treatment of chronic obstructive pulmonary disease (COPD): a comprehensive review. Molecules 27(17):5542
Daumas R, Thomassin BA (1977) Protein fractions in coral and zoantharian mucus, possible evolution in coral reef environment. In: Proceedings of the (3rd) Int. Coral Reef Symp. (Miami), vol 1, pp 517–525
Denny MW (1989) Invertebrate mucous secretions—functional alternatives to vertebrate paradigms. Symp Soc Exp Biol 43:337–366
Diaz L (2017) Response of the coral associated nitrogen fixing bacteria toward elevated water temperature. J Water Resour Ocean Sci 6(6):98–109
Ding JL, Fung FMY, Ng GWS, Chou LM (1999) Novel bioactivities from a coral, Galaxea fascicularis: DNase-like activity and apoptotic activity against a multiple-drug-resistant leukemia cell line. Mar Biotechnol 1(4):328–336
Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM (2002) Oxidative stress and seasonal coral bleaching. Free Radical Biol Med 33(4):533–543
Drollet JH, Teai T, Faucon M, Martin PMV (1997) Field study of compensatory changes in UV-absorbing compounds in the mucus of the solitary coral Fungia repanda (Scleractinia: Fungiidae) in relation to solar UV radiation, sea-water temperature, and other coincident physico-chemical parameters. Mar Freshw Res 48(4):329–333
Ducklow HW, Mitchell R (1979) Bacterial populations and adaptations in the mucus layers on living corals 1. Limnol Oceanogr 24(4):715–725
Eckes M, Dove S, Siebeck UE, Grutter AS (2015) Fish mucus versus parasitic gnathiid isopods as sources of energy and sunscreens for a cleaner fish. Coral Reefs 34(3):823–833
Fang JHS (2015) Community analysis of coral mucus-associated bacteria and impact of temperature and CO2 changes on them (Doctoral dissertation. Swinburne University of Technology (Sarawak campus)
Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11(5):385–393
Fitzgerald LM, Szmant AM (1997) Biosynthesis of “essential” amino acids by scleractinian corals. Biochem J 322(1):213–221
Gabay Y (2018) The cellular and physiological basis of host-symbiont specificity in a model cnidarian-dinoflagellate symbiosis (Doctoral dissertation, Open Access Te Herenga Waka-Victoria University of Wellington)
Galli P, Montano S, Seveso D, Maggioni D (2021) Coral reef biodiversity of the Maldives. Atolls of the Maldives. Nissology and geography. Rowman and Littlefield, Lanham, pp 196–212
Gardner SG, Nielsen DA, Laczka O, Shimmon R, Beltran VH, Ralph PJ, Petrou K (2016) Dimethylsulfoniopropionate, superoxide dismutase and glutathione as stress response indicators in three corals under short-term hyposalinity stress. Proc Biol Sci 283(1824):20152418
Goldberg WM (2018) Coral food, feeding, nutrition, and secretion: a review. In: Kloc M, Kubiak JZ (eds) Marine organisms as model systems in biology and medicine. Springer, Berlin, pp 377–421
Gottfried M, Roman MR (1983) Ingestion and incorporation of coral-mucus detritus by reef zooplankton. Mar Biol 72(3):211–218
Grottoli AG, Rodrigues LJ, Juarez C (2004) Lipids and stable carbon isotopes in two species of Hawaiian corals. Porites compressa and Montipora verucosa, following a bleaching event. Mar Biol 145:621–631
Grover R, Maguer J-F, Allemand D, Ferrier-Pagès C (2008) Uptake of dissolved free amino acids (DFAA) by the scleractinian coral Stylophora pistillata. J Exp Biol 211:860–865
Guarnieri MC, de Albuquerque Modesto JC, Pérez CD, Ottaiano TF, Ferreira RDS, Batista FP, de Brito MV, Campos IHMP, Oliva MLV (2018) Zoanthid mucus as new source of useful biologically active proteins. Toxicon 143:96–107
Guppy R, Bythell JC (2006) Environmental effects on bacterial diversity in the surface mucus layer of the reef coral Montastraea faveolata. Mar Ecol Prog Ser 328:133–142
Guppy R, Brown B, Bythell JC (2019) Preserving the viscous coral surface mucus layer using low-acid glycol methacrylate (GMA) resin. Coral Reefs 38(3):521–526
Haas AF, Fairoz MFM, Kelly LW, Nelson CE, Dinsdale EA, Edwards RA et al (2016) Global microbialization of coral reefs. Nat Microbiol 1(7):16042
Hadaidi G, Gegner HM, Ziegler M, Voolstra CR (2019) Carbohydrate composition of mucus from scleractinian corals from the central Red Sea. Coral Reefs 38(1):21–27
Hadaidi GA (2018) Coral-associated bacterial community dynamics in healthy, bleached, and disease states, (Doctoral Dissertation), KAUST Research Repository. Doi: https://doi.org/10.25781/KAUST-0UN42
Hafezi M, Giffin AL, Alipour M, Sahin O, Stewart RA (2020) Mapping long-term coral reef ecosystems regime shifts: a small island developing state case study. Sci Total Environ 716:137024
Hill DB, Button B, Rubinstein M, Boucher RC (2022) Physiology and pathophysiology of human airway mucus. Physiol Rev 102(4):1757–1836
Hillyer KE, Dias DA, Lutz A, Wilkinson SP, Roessner U, Davy SK (2017) Metabolite profiling of symbiont and host during thermal stress and bleaching in the coral Acropora aspera. Coral Reefs 36:105–118
Ho TK (2017) Dredging land reclamation causing mucus development in massive spherical corals in the Spratly Islands, South China Sea: The effects on China’s fishing industry. Poster session presented at Virginia Commonwealth University Poster Symposium for Undergraduate Research and Creativity, Richmond, VA
Horka I, De Grave S, Fransen CH, Petrusek A, Ďuriš Z (2016) Multiple host switching events shape the evolution of symbiotic palaemonid shrimps (Crustacea: Decapoda). Sci Rep 6(1):26486
Horricks RA, Herbinger CM, Lillie BN, Taylor P, Lumsden JS (2019) Differential protein abundance during the first month of regeneration of the Caribbean star coral Montastraea cavernosa. Coral Reefs 38(1):45–61
Iluz D, Dubinsky Z (2015) Coral photobiology: new light on old views. Zoology 118(2):71–78
Irudayarajan L, Ravindran C, Raveendran HP (2023) Antimicrobial activity of coral-associated beneficial bacteria against coral disease-causing microbial pathogens. J Basic Microbiol. https://doi.org/10.1002/jobm.202300338
Jatkar AA, Brown BE, Bythell JC, Guppy R, Morris NJ, Pearson JP (2010a) Coral mucus: the properties of its constituent mucins. Biomacromol 11(4):883–888
Jatkar AA, Brown BE, Bythell JC, Guppy R, Morris NJ, Pearson JP (2010b) Measuring mucus thickness in reef corals using a technique devised for vertebrate applications. Mar Biol 157:261–267
Kageyama H, Waditee-Sirisattha R (2019) Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: molecular and cellular mechanisms in the protection of skin-aging. Mar Drugs 17(4):222
Kemp DW, Rivers AR, Kemp KM, Lipp EK, Porter JW, Wares JP (2015) Spatial homogeneity of bacterial communities associated with the surface mucus layer of the reef-building coral Acropora palmata. PLoS ONE 10(12):0143790
Khalesi MK (2015) Corals. Springer handbook of marine biotechnology. Springer, Berlin, pp 179–217
Komatsu K, Tsutsui S, Hino K, Araki K, Yoshiura Y, Yamamoto A, Nakamura O, Watanabe T (2009) Expression profiles of cytokines released in intestinal epithelial cells of the rainbow trout, Oncorhynchus mykiss, in response to bacterial infection. Dev Comp Immunol 33(4):499–506
Krediet CJ, Ritchie KB, Cohen M, Lipp EK, Sutherland KP, Teplitski M (2009) Utilization of mucus from the coral Acropora palmata by the pathogen Serratia marcescens and by environmental and coral commensal bacteria. Appl Environ Microbiol 75(12):3851–3858
Krediet CJ, Ritchie KB, Alagely A, Teplitski M (2013) Members of native coral microbiota inhibit glycosidases and thwart colonization of coral mucus by an opportunistic pathogen. ISME J 7(5):980–990
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56(411):337–346
Krueger T, Bodin J, Horwitz N, Loussert-Fonta C, Sakr A, Escrig S, Fine M, Meibom A (2018) Temperature and feeding induce tissue level changes in autotrophic and heterotrophic nutrient allocation in the coral symbiosis—a NanoSIMS study. Sci Rep 8(1):12710
Krupp DA (1982) The composition of the mucus from the mushroom coral Fungia scutaria. In: Proceedings of the 4th Int. Coral Reef Symp., vol 2, pp 9–73
Kurihara H, Ikeda N, Umezawa Y (2018) Diurnal and seasonal variation of particle and dissolved organic matter release by the coral Acropora tenuis. PeerJ 6:5728
LaJeunesse TC, Smith R, Walther M, Pinzón J, Pettay DT, McGinley M, Aschaffenburg M, Medina-Rosas P, Cupul-Magaña AL, Pérez AL, Reyes-Bonilla H, Warner ME (2010) Host–symbiont recombination versus natural selection in the response of coral–dinoflagellate symbioses to environmental disturbance. Proc R Soc B 277(1696):2925–2934
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28(16):2570–2580.e6. https://doi.org/10.1016/j.cub.2018.07.008
Lee ST, Dav SK, Tang SL, Kench PS (2016) Mucus sugar content shapes the bacterial community structure in thermally stressed Acropora muricata. Front Microbiol 7:371
Lewis RW (1976) Mucus globule membrane: an hypothesis concerning its role in determining the viscosity of mucus. J Theor Biol 61(1):21–25
Lin C, Wang LH, Fan TY, Kuo FW (2012) Lipid content and composition during the oocyte development of two gorgonian coral species in relation to low temperature preservation. PLoS ONE 7(7):38689
Maldonado A, Johnson A, Gochfeld D, Slattery M, Ostrander GK, Bingham JP, Schlenk D (2016) Hard coral (Porites lobata) extracts and homarine on cytochrome P450 expression in Hawaiian butterflyfishes with different feeding strategies. Comp Biochem Physiol c: Toxicol Pharmacol 179:57–63
McMahon KW, Thorrold SR, Houghton LA, Berumen ML (2016) Tracing carbon flow through coral reef food webs using a compound-specific stable isotope approach. Oecologia 180(3):809–821
McShane A, Bath J, Jaramillo AM, Ridley C, Walsh AA, Evans CM, Thornton DJ, Ribbeck K (2021) Mucus. Curr Biol 31(15):938–945
Means JC, Sigleo AC (1986) Contribution of coral reef mucus to the colloidal organic pool in the vicinity of Discovery Bay, Jamaica. WI Bull Mar Sci 39(1):110–118
Meikle P, Richards GN, Yellowlees D (1988) Structural investigations on the mucus from six species of coral. Mar Biol 99(2):187–193
Meyer W, Seegers U, Schnapper A, Neuhaus H, Himstedt W, Toepfer-Petersen E (2007) Possible antimicrobial defense by free sugars on the epidermal surface of aquatic vertebrates. Aquat Biol 1(2):167–175
Nakajima R, Nakatomi N, Kurihara H, Fox MD, Smith JE, Okaji K (2016) Crown-of-thorns starfish larvae can feed on organic matter released from corals. Diversity 8(4):18
Nakajima R, Tanaka Y, Yoshida T, Fujisawa T, Nakayama A, Fuchinoue Y, Othman BHR, Toda T (2015) High inorganic phosphate concentration in coral mucus and its utilization by heterotrophic bacteria in a Malaysian coral reef. Mar Ecol 36(3):835–841
Neave MJ, Rachmawati R, Xun L, Michell CT, Bourne DG, Apprill A, Voolstra CR (2017) Differential specificity between closely related corals and abundant Endozoicomonas endosymbionts across global scales. ISME J 11(1):186–200
Nguyen-Kim H, Bettarel Y, Bouvier T, Bouvier C, Doan-Nhu H, Nguyen-Ngoc L, Nguyen-Thanh T, Tran-Quang H, Brune J (2015) Coral mucus is a hot spot for viral infections. Appl Environ Microbiol 81(17):5773–5783
Nissimov J, Rosenberg E, Munn CB (2009) Antimicrobial properties of resident coral mucus bacteria of Oculina Patagonica. FEMS Microbiol Lett 292(2):210–215
Nithyanand P, Indhumathi T, Ravi AV, Pandian SK (2011) Culture independent characterization of bacteria associated with the mucus of the coral Acropora digitifera from the Gulf of Mannar. World J Microbiol Biotechnol 27(6):1399–1406
Oku H, Yamashiro H, Onaga K (2003) Lipid biosynthesis from [14C]-glucose in the coral Montipora digitata. Fish Sci 69(3):625–631
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269(1):1–10
Palmer CV, Traylor-Knowles NG, Willis BL, Bythell JC (2011) Corals use similar immune cells and wound-healing processes as those of higher organisms. PLoS ONE 6(8):23992
Pasaribu B, Weng LC, Lin IP, Camargo E, Tzen JT, Tsai CH, Ho SL, Lin MR, Wang LH, Chen CS, Jiang PL (2015) Morphological variability and distinct protein profiles of cultured and endosymbiotic Zooxanthellae cells isolated from Exaiptasia Pulchella. Sci Rep 5(1):15353
Patton JS, Abraham S, Benson AA (1977) Lipogenesis in the intact coral Pocillopora capitata and its isolated Zooxanthellae: evidence for a light-driven carbon cycle between symbiont and host. Mar Biol 44(3):235–247
Piggot AM, Fouke BW, Sivaguru M, Sanford RA, Gaskins HR (2009) Change in Zooxanthellae and mucocyte tissue density as an adaptive response to environmental stress by the coral. Montastraea Annularis Mar Biol 156(11):2379–2389
Piludu M, Rayment SA, Liu B, Offner GD, Oppenheim FG, Troxler RF, Hand AR (2003) Electron microscopic immunogold localization of salivary mucins MG1 and MG2 in human submandibular and sublingual glands. J Histochem Cytochem 51(1):69–79
Plass-Johnson JG, Cardini U, Van Hoytema N, Bayraktarov E, Burghardt I, Naumann MS, Wild C (2015) Coral bleaching. In: Armon RH, Hänninen O (eds) Environmental indicators. Springer, Dordrecht, pp 117–146
Que J (2015) The initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal–esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagus. Wiley Interdiscip Rev Dev Biol 4(4):419–430
Radecker N, Pogoreutz C, Voolstra CR, Wiedenmann J, Wild C (2015) Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol 23(8):490–497
Radice VZ, Brett MT, Fry B, Fox MD, Hoegh-Guldberg O, Dove SG (2019) Evaluating coral trophic strategies using fatty acid composition and indices. PLoS ONE 14(9):0222327
Raina JB, Tapiolas D, Motti CA, Foret S, Seemann T, Tebben J, Willis BL, Bourne DG (2016) Isolation of an antimicrobial compound produced by bacteria associated with reef-building corals. PeerJ 4:2275
Ravindran C, Lawrance I, Vasanth AJ (2022) Endosymbiotic Symbiodinium clades occurrence and influence on coral growth and resilience during stress. Symbiosis 86:261–272
Ravindran C, Bhagwat PV, Silveira KA, Shivaramu MS, Lele UP (2020) Characterization of coral associated ciliates and their interactions with disease lesion progression of Indian scleractinian corals. Microb Pathog 149:104472
Ravindran C, Irudayarajan L, Raveendran HP (2023) Possible beneficial interactions of ciliated protozoans with coral health and resilience. Appl Environ Microbiol 89:e0121723
Renaud SM, Thinh LV, Parry DL (1999) The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 170(2):147–159
Reshef L, Kore O, Loya Y, Zilber-Rosenberg I, Rosenberg E (2006) The coral probiotic hypothesis. Environ Microbiol 8:2068–2073
Reynaud S, Ferrier-Pagès C (2019) Biology and ecophysiology of Mediterranean cold–water corals. In: Orejas C, Jiménez C (eds) Mediterranean cold-water corals: past, present and future: understanding the deep-sea realms of coral. Springer, Cham, pp 391–404
Ritchie KB (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322:1–14
Rivera-Ortega J, Thomé PE (2018) Contrasting antibacterial capabilities of the surface mucus layer from three symbiotic cnidarians. Front Mar Sci 5:392
Rix L, de Goeij JM, van Oevelen D, Struck U, Al-Horani FA, Wild C, Naumann MS (2017) Differential recycling of coral and algal dissolved organic matter via the sponge loop. Funct Ecol 31(3):778–789
Rodrigues LJ, Grottoli AG, Pease TK (2008) Lipid class composition of bleached and recovering Porites compressa Dana 1846 and Montipora capitata Dana 1846 corals from Hawaii. J Exp Mar Biol Ecol 358(2):136–143. https://doi.org/10.1016/j.jembe.2008.02.004
Salvatore S, Heuschkel R, Tomlin S, Davies SE, Edwards S, Walker-Smith J, Murch SH (2000) A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment Pharmacol Ther 14(12):1567–1579
Schottner S, Hoffmann F, Wild C, Rapp HT, Boetius A, Ramette A (2009) Inter-and intra-habitat bacterial diversity associated with cold-water corals. ISME J 3(6):756–759
Shafir A, Loya A (1983) Consumption and assimilation of coral mucus by the burrowing mussel Lithophaga lessepsiana. Int Conf Mar Sci Red Sea 9:135–140
Sharma D, Ravindran C (2020) Diseases and pathogens of marine invertebrate corals in Indian reefs. J Invertebr Pathol 173:107373
Shick JM, Dunlap WC, Pearse JS, Pearse VB (2002) Mycosporine-like amino acid content in four species of sea anemones in the genus Anthopleura reflects phylogenetic but not environmental or symbiotic relationships. Biol Bull 203(3):315–330
Shinzato C, Inoue M, Kusakabe M (2014) A snapshot of a coral “holobiont”: a transcriptome assembly of the scleractinian coral, Porites, captures a wide variety of genes from both the host and symbiotic zooxanthellae. PLoS ONE 9(1):e85182
Shmuel Y, Ziv Y, Rinkevich B (2022) Coral-inhabiting Trapezia crabs forage on demersal plankton. Front Mar Sci 9:1652
Shnit-Orland M, Kushmaro A (2009) Coral mucus-associated bacteria: a possible first line of defense. FEMS Microbiol Ecol 67(3):371–380
Siboni N, Ben-Dov E, Sivan A, Kushmaro A (2008) Global distribution and diversity of coral associated Archaea and their possible role in the coral holobiont nitrogen cycle. Environ Microbiol 10(11):2979–2990
Siboni N, Ben-Dov E, Sivan A, Kushmaro A (2012) Geographic specific coral-associated ammonia-oxidizingarchaea in the northern Gulf of Eilat (Red Sea). Microb Ecol 64:18–24
Sikorskaya TV, Ermolenko EV, Efimova KV (2022) Lipids of Indo-Pacific gorgonian corals are modified under the influence of microbial associations. Coral Reefs 41(2):277–291
Smith HA, Conlan JA, Pollock FJ, Wada N, Shore A, Hung JYH, Aeby GS, Willis BL, Francis DS, Bourne DG (2020) Energy depletion and opportunistic microbial colonisation in white syndrome lesions from corals across the Indo-Pacific. Sci Rep 10(1):19990
Stabili L (2019) The mucus of marine invertebrates: Cnidarians, polychaetes, and echinoderms as case studies. In: Trincone A (ed) Enzymatic technologies for marine polysaccharides. CRC Press, Boca Raton, pp 151–162
Stabili L, Schirosi R, Parisi MG, Piraino S, Cammarata M (2015) The mucus of Actinia equina (Anthozoa, Cnidaria): an unexplored resource for potential applicative purposes. Mar Drugs 13(8):5276–5296
Stephens GC (1962) Uptake of organic material by aquatic invertebrates. I. Uptake of glucose by the solitary coral, Fungia scutaria. Biol Bull 123(3):648–659
Subramanian S, MacKinnon SL, Ross NW (2007) A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp Biochem Physiol Part B Biochem Mol Biol 148(3):256–263
Suggett DJ, Warner ME, Smith DJ, Davey P, Hennige S, Baker NR (2008) Photosynthesis and production of hydrogen peroxide by Zooxanthellae (pyrrhophyta) phylotypes with different thermal tolerances 1. J Phycol 44(4):948–956
Tchernov D, Gorbunov MY, De Vargas C, Narayan Yadav SN, Milligan AJ, Häggblom M, Falkowski PG (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA 101(37):13531–13535
Teai T, Drollet JH, Bianchini JP, Cambon A, Martin PMV (1998) Occurrence of ultraviolet radiation-absorbing mycosporine-like amino acids in coral mucus and whole corals of French Polynesia. Mar Freshw Res 49(2):127–132
Teplitski M, Krediet CJ, Meyer JL, Ritchie KB (2016) Microbial interactions on coral surfaces and within the coral holobiont. The Cnidaria, past, present and future: the world of medusa and her sisters, pp 331–346
Thompson JR, Rivera HE, Closek CJ, Medina M (2014) Microbes in the coral holobiont: partners through evolution, development, and ecological interactions. Front Cell Infect Microbiol 4:176
Thornton DJ, Sheehan JK (2004) From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc 1(1):54–61
Treignier C, Grover R, Ferrier-Pagés C, Tolosa I (2008) Effect of light and feeding on the fatty acid and sterol composition of Zooxanthellae and host tissue isolated from the scleractinian coral Turbinaria reniformis. Limnol Oceanogr 53(6):2702–2710
Uslu L, Sayin S, Naz M, Taskin E, Soyler O, Saygili I, Cetin Z, Dinler ZM, Isik O (2021) Proximate analysis and fatty acid profile of some brown macroalgae collected from the Northeastern Mediterranean coast. Fresenius Environ Bull 30:9433–9437
Vacelet E, Thomassin BA (1991) Microbial utilization of coral mucus in long term in situ incubation over a coral reef. Hydrobiologia 211(1):19–32
Van Oppen MJ, Blackall LL (2019) Coral microbiome dynamics, functions and design in a changing world. Nat Rev Microbiol 17(9):557–567
Vaughan EJ, Wilson SK, Howlett SJ, Parravicini V, Williams GJ, Graham NAJ (2021) Nitrogen enrichment in macroalgae following mass coral mortality. Coral Reefs 40(3):767–776
Ventura M (2006) Linking biochemical and elemental composition in freshwater and marine crustacean zooplankton. Mar Ecol Prog Ser 327:233–246
Vilas Bhagwat P, Ravindran C, Irudayarajan L (2023) Characterization of the defense properties of healthy and diseased coral mucus. J Invertebr Pathol 201:108001
Volkman JK, Johns RB, Gillan FT, Perry GJ, Bavor HJ Jr (1980) Microbial lipids of an intertidal sediment—I. Fatty acids and hydrocarbons. Geochemica Et Cosmochimica Acta 44(8):1133–1143
Weis VM (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211(19):3059–3066
Wiese J, Thiel V, Nagel K, Staufenberger T, Imhoff JF (2009) Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Mar Biotechnol 11(2):287–300
Wijayanti DP, Sabdono A, Dirgantara D, Widyananto PA, Sibero MT, Bhagooli R, Hidaka M (2020) Antibacterial activity of acroporid bacterial symbionts against White Patch Disease in Karimunjawa Archipelago, Indonesia. Egypt J Aquat Res 46(2):187–193
Wild C, Huettel M, Klueter A, Kremb SG, Rasheed MY, Jorgensen BB (2004) Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428(6978):66–77
Wild C, Naumann M, Niggl W, Haas A (2010) Carbohydrate composition of mucus released by scleractinian warm- and cold-water reef corals. Aquat Biol 10(1):41–45
Wild C, Woyt H, Huettel M (2005) Influence of coral mucus on nutrient fluxes in carbonate sands. Mar Ecol Prog Ser 287:87–98
Wilson SK, Bellwood DR, Choat JH, Furnas MJ (2003) Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr Mar Biol 41:279–310
Wright RM, Strader ME, Genuise HM, Matz M (2019) Effects of thermal stress on amount, composition, and antibacterial properties of coral mucus. Peer J 7:6849
Younan F, Pearson J, Allen A, Venables C (1982) Changes in the structure of the mucous gel on the mucosal surface of the stomach in association with peptic ulcer disease. Gastroenterology 82(5 Pt 1):827–831
Zetsche EM, Baussant T, Meysman FJ, Van Oevelen D (2016) Direct visualization of mucus production by the cold-water coral Lophelia pertusa with digital holographic microscopy. PLoS ONE 11(2):0146766
Zhang S, Li G (2000) Presence of phenoloxidase and prophenoloxidase in the epidermal cells and the epidermis mucus of the lancelet Branchiostoma belcheritsingtauense. Ophelia 52(3):207–212
Zhao X, Findly RC, Dickerson HW (2008) Cutaneous antibody-secreting cells and B cells in a teleost fish. Dev Comp Immunol 32(5):500–508
Acknowledgements
The authors thank the Director, CSIR-National Institute of Oceanography for the facilities, including Ministry of Environment, Forest and climate change (MoEFCC), Govt. of India, New Delhi and Forest Department, Principal Chief Conservator of Forests (PCCF) and Chief wildlife warden, Chennai-15, Tamil Nadu and Wildlife Warden, Gulf of Mannar Marine National Park, Tamil Nadu, India, for the entry and as well as for collecting the samples. his is NIO contribution # 7164.
Funding
This study was supported by the Department of Biotechnology (DBT), (Grant No. BT/PR15162/AAQ/3/752/2015) India to Dr. C.R.. PVB was supported by CSIR (UGC) (Grant No.20/12/2015(ii)EU-V).
Author information
Authors and Affiliations
Contributions
PVB wrote the original draft, performed data analysis. CR Conceptualization, fund acquisition, writing and editing the original draft, performed data analysis. LI wrote the original draft, performed data analysis. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests.
Ethical standards
Not applicable.
Additional information
Responsible Editor: D. Gochfeld.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhagwat, P.V., Ravindran, C. & Irudayarajan, L. Beneficial properties of mucus in coral adaptations and ecological interactions. Mar Biol 171, 46 (2024). https://doi.org/10.1007/s00227-023-04372-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04372-4