Abstract
Gastroesophageal reflux disease (GERD) is “a condition that develops when stomach contents cause troublesome symptoms and/or complications.” The pathophysiology of GERD is multifactorial and complex and determined by interactions among multiple aggressive mechanisms and defensive factors. The esophagogastric junction (EGJ), that plays a central role because it is the main defense against GERD, anatomically consists of the Lower Esophageal Sphincter (LES), the crural diaphragm, and the anatomical flap valve, and acts as an antireflux barrier. Barrett’s esophagus (BE) is an acquired condition due to GERD, leading to the replacement of the normal squamous epithelium of the lower esophagus with a columnar lined mucosa.
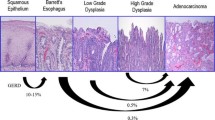
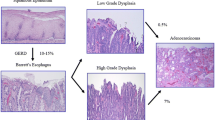
Natural history of BE is different in non-dysplastic BE, in BE indefinite for dysplasia, in BE with low grade and high grade dysplasia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastroesophageal reflux disease
- Esophagogastric junction
- Lower Esophageal Sphincter
- Crural diaphragm
- Barrett’s esophagus
- Transient lower esophageal Sphincter Relaxation
- Hiatus hernia
- Obesity
- Quality of life
- Endoscopy
- Low-grade dysplasia
- High grade dysplasia
- Esophageal adenocarcinoma
1 Gastroesophageal Reflux Disease
The most widely accepted definition of gastroesophageal reflux disease (GERD) is “a condition that develops when stomach contents cause troublesome symptoms and/or complications” [1].
The pathophysiology of GERD is multifactorial and complex and determined by interactions among multiple aggressive mechanisms and defensive factors.
Some mechanisms play a role in the provocation of GERD, schematically at the level of the esophagogastric junction (EGJ) such as Transient Lower Esophageal Sphincter Relaxations (TLESRs, low LES pressure, hiatus hernia (HH), and increased distensibility of the EGJ), above the EGJ in the esophageal body such as prolonged esophageal clearance, and below the EGJ such as acid pocket, delayed gastric emptying, and increased intragastric pressure. A number of other factors, indeed, may influence the intensity-frequency of GERD symptoms, including the phenomenon of peripheral (primary afferents) and central (spinal dorsal horn neurons) sensitization, as well as the characteristics of refluxate (acidity, the presence of gas, the presence of bile acids, the proximal extent), the longitudinal muscle contraction, and the mucosal integrity.
The EGJ, that play a central role because it is the main defense against GERD, anatomically consists of the LES, the crural diaphragm, and the anatomical flap valve, and acts as an antireflux barrier. LES, a short segment of tonically contracted smooth muscle at the very end of the esophagus, is considered the intrinsic sphincter and is surrounded by oblique gastric fibers that are fastened to the striated muscle of the crural diaphragm by the phreno-esophageal ligament, while the right crus of the diaphragm forms a sling that surrounds the distal esophagus (the extrinsic sphincter) augmenting the high-pressure zone of the LES [2, 3]. When these protective components are compromised, the deleterious effects are additive, resulting in abnormal esophageal reflux exposure.
TLESRs [4] by definition are characterized by complete prolonged (>10 s) LES relaxations not caused by swallowing and accompanied by inhibition of the crural diaphragm (CD) [5].
The dominant trigger for TLESR is distension of the proximal stomach, which stimulates afferent vagal innervation that input to the nucleus tractus solitarii in the brainstem and subsequently to the dorsal motor nuclei of the vagus. Dorsal motor nucleus neurons send motor outputs of the reflex circuit to inhibitory neurons localized into the myenteric plexus of the distal esophagus and an integrated motor response involving LES relaxation through reflex inhibitory responses, longitudinal muscle contraction that reduces EGJ obstruction through tension-mediated LES relaxation and repositioning of the LES above the crura, crural diaphragmatic inhibition, and contraction of the costal diaphragm as the final effector state of the TLESR reflex [6]. Compelling evidence has demonstrated that TLESR is the most frequent mechanism associated with gastroesophageal reflux episodes and essentially the only operant mechanisms during period of normal LES pressure (>10 mmHg) [4, 7]. Although TLESR is a major mechanism of gastroesophageal reflux, patients with GERD do not have more frequent TLESRs than controls. However, the frequency of acid reflux during TLESRs has been reported to be greater in GERD patients [8, 9]. It is therefore not the frequency of TLESRs which cause GERD but alterations in the complex processes comprising TLESRs.
LES pressure measured by conventional esophageal manometry (including mean basal and end-expiratory LES pressures) continues to be reported and utilized [10]. However, LES pressure has been described consistently low only in small number of GERD patients [11, 12]. These parameters are descriptive terms and cannot be used to evaluate the LES and CD separately, and so cannot provide an accurate picture of EGJ function. In fact, the resting tone varies also in healthy individuals during the day reaching lower pressure in the postprandial state [13]. LES tone is under regulation of multiple myogenic and neurogenic factors that are modified by gastric distension, intraabdominal pressure, hormones, and medication [14]. Recently, the new technique of high resolution manometry (HRM) introduced new parameters that incorporate EGJ length and respiratory variability such as LES pressure integral [15] or EGJ contractile integral [16] and it has been demonstrated that these parameters are significantly decreased in GERD patients. The EGJ-CI is evaluated using the distal contractile integral (DCI) value at the EGJ during three complete respiratory cycles with a threshold of 2 mmHg above the gastric baseline, and the recorded value is divided by the respiratory cycle duration. The magnitude of contractility can be integrated over time and EGJ length, which are fundamental for EGJ barrier function. The clinical utility of the EGJ-CI has been further investigated to diagnose GERD [17] and recently it has been suggested its clinical utility after surgical intervention in patients with GERD and achalasia [18].
A HH is present when a spatial dissociation of the antireflux barrier at the EGJ into the intrinsic sphincter and extrinsic sphincter crural diaphragm exists [19], which is likely caused by the weakening or rupture of the phreno-esophageal ligament [20]. Old studies in animal models demonstrated that severing the phreno-esophageal ligament reduced the LES pressure, and that the subsequent repair of the ligament restored the LES pressure to levels similar to baseline [21].
By using HRM it is now easily seen the spatial dissociation of the antireflux barrier into intrinsic sphincter and hiatal canal pressure components, and it has been demonstrated that each of these components was of lower magnitude than the EGJ pressure of a comparator group of healthy individuals [22].
It has been accurately established that patients with HH have more reflux episodes and greater esophageal acid exposure than patients without HH [23, 24]. When the LES lies constantly above the diaphragm, the swallow-associated re-reflux from the hiatal sac impairs esophageal clearance [25], allowing a prolonged acid exposure of the mucosa. In fact, patients with large HH show a severe alteration in the clearance of refluxate [26]. Furthermore, prolonged acid clearance correlates with the severity of esophagitis and the presence of Barrett’s metaplasia [27, 28]. However, the threshold for these responses and complications vary and is likely to be influenced by the integrity of the epithelium [27, 28].
Traditionally the assessment of esophageal clearance has therefore focused on pH measures, and esophageal acid clearance time is determined by the time where the esophageal lumen is acidified to a pH of less than 4 after a reflux event. Recently, new methodologies such as impedance analyze bolus presence and clearance [29] and other parameters such as post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters have been demonstrated to improve the diagnostic yield of impedance-pH monitoring and might identify subpopulations that will respond to acid suppression [30].
Peristaltic dysfunction is considered a potential cause of prolonged reflux exposure and acid clearance and an important contributor to the severity of esophagitis.
Delayed gastric emptying and altered motor function of the proximal stomach have often been described in GERD. It is uncertain, however, how much these alterations contribute to the increased gastroesophageal reflux [31]. The acid pocket was firstly described in 2001 as layer of unbuffered acidic, gastric juice sits on top of the meal, close to the cardia, ready to reflux in the postprandial period [32]. The acid pocket is present in GERD patients and controls. However, alterations in its location and/or distribution may favor acid reflux from the pocket and partially explain the difference between GERD patients and controls [33].
Central obesity increases the intragastric pressure [34] and the gastroesophageal pressure gradient. Both of them favor reflux of gastric content [35] and are also predictors of HH development [36]. Interestingly, it has been demonstrated that small decrease in weight (approximately 10–15 lbs) can reduce GERD symptoms, possibly reducing the pressure gradient and burden on the antireflux barrier [37].
The severity of esophageal acid exposure to gastric content does not directly correlate to the severity of symptoms. It is a matter of fact that patients with non-erosive reflux disease (NERD) and patients with PPI refractory symptoms are hypersensitive to minor stimuli while patients with severe esophagitis and Barrett are often hyposensitive or even asymptomatic [38]. In the last decade, a number of patient-related factors such as peripheral and central sensitization, impaired mucosal barrier function and possibly genetic factors together with the acidity, the proximal extent, the presence of bile acids and the presence of gas in the refluxate have been taken into account to explain the differences in perception in specific subtypes of GERD symptoms [39]. At peripheral level, nerve endings in the submucosal layer are belived that mediate the sensitivity to refluxed gastric contents. A loss of mucosal barrier function has been demonstrated in GERD that might allow components of refluxate can easily reach nerve endings and increase symptoms [40, 41].
Dilated intercellular spaces studied by histology or, most accurately, by electron microscopy of esophageal biopsies have been demonstrated in GERD as expressions of the loss of mucosal barrier integrity [42]. Recently, another technique, baseline impedance, has been used to evaluate the mucosal integrity [43, 44].
Acid and bile reflux have been involved in causing the dilation of intercellular spaces and increase in mucosal permeability suggesting that luminal aggressive refluxate activate receptors on submucosal nerve endings, then inducing symptoms [40, 45]. In last decade it has been suggested that refluxed gastric material stimulated esophageal epithelial cells to secrete cytokines that attracted immune cells, which ultimately damaged the mucosa [46] and, in 2016 a preliminary study of 12 patients with severe esophagitis successfully treated with PPI therapy, the interruption of PPI results in submucosal infiltration by T cells and dilated intercellular spaces in the basal layer without loss of surface cells [47].
The presence and density of reflux-sensing receptors such as transient receptor potential vanilloid type-1 (TRPV1), acid-sensitive ion channels, and the protease-activated receptor 2 (PAR2), which are all expressed in human esophageal mucosa might also determine symptom occurrence during reflux events and possibly determining esophageal hypersensitivity to reflux [48, 49].
The pathogenesis of esophageal hypersensitivity, however, could involve also central mechanisms that increase sensitivity of incoming signals from the esophagus affecting spinal cord excitability and altered descending modulation of nociceptive processing. These are regulated by factors that affect central mechanisms, such as stress, anxiety, and personality traits [50]. Stress is often presumed to alter central processing of afferent signals, such as heartburn, but animal studies showed that acute stress led to dilation of intercellular spaces in the esophagus, which could also account for the increased sensitivity to reflux [51].
GERD patients show reduced quality of life [52]. In presence of depression and especially anxiety, GERD patients have more symptoms and lower quality of life compared to GERD patients without anxiety and similar reflux parameters [53].
However, more studies are mandatory to explain how intensity-frequency of GERD symptoms could be modulated.
2 Natural History of Barrett’s Esophagus
Following the original description in 1950 by Normal Barrett of the condition that came to bear his name [54], it has been subsequently established from animal studies that Barrett’s oesophagus (BO) is an acquired condition due to gastroesophageal reflux, leading to the replacement of the normal squamous epithelium of the lower esophagus with a columnar lined mucosa [55].
BO is commonly defined endoscopically as an esophagus in which a variable proportion of the distal normal squamous epithelial lining has been replaced by visible metaplastic columnar epithelium above the gastroesophageal junction, usually defined by the proximal margin of the gastric folds, and confirmed histologically on endoscopic biopsy [56].
In 1952, a patient was reported who developed an adenocarcinoma in an esophageal mucosa of intestinal type goblet cells, consistent with BO [57]. Given the poor prognosis of many patients presenting with symptomatic esophageal adenocarcinoma, endoscopic screening of patients with BO has been widely practiced over the past three decades, though not without some controversy.
Endoscopic surveillance of BO is based on the histological progression in subjects with esophageal adenocarcinoma from non-dysplastic to low grade dysplasia and then high grade dysplasia before developing adenocarcinoma. Detecting dysplasia potentially allows endoscopic intervention with endoscopic mucosal resection or radio-frequency ablation to prevent progression to adenocarcinoma.
3 Natural History of Non-dysplastic Barrett’s Esophagus
The annual risk of developing esophageal adenocarcinoma in non-dysplastic BO is a key factor in determining the cost-effectiveness of endoscopic surveillance for BO and the recommendations we make to our patients concerning its value. A recent meta-analysis of the risk of esophageal adenocarcinoma in BO without dysplasia included 57 cohort studies involving over 11,000 BO subjects and over 58,000 years of follow-up and suggested that the annual esophageal adenocarcinoma risk in BO is 0.33% [58]. However, although there was no evidence of publication bias reported in this meta-analysis, many of the case series included are prone to a number of other biases, particularly selection bias. Two subsequent large population based studies suggest that the annual esophageal adenocarcinoma risk in BO is significantly lower [59, 60]. In a population based study of 8522 BO subjects in Northern Ireland with no dysplasia at study entry, the annual risk of esophageal adenocarcinoma was 0.1% [59]. In a nationwide study based on histopathology records of 11,028 Danish BO subjects with no dysplasia at study entry, the annual risk of EAC was 0.1% [60].
It is important to point out that other risk factors for esophageal adenocarcinoma in BO need to be taken into account when assessing the individual annual risk of esophageal adenocarcinoma, in particular the length of the Barrett’s segment. In a Dutch cohort study of 713 BO subjects, the risk of progression to esophageal adenocarcinoma increased by a factor of 1.11 for each extra centimeter of BO segment length [61]. The length of BO segment was only available in 20% of subjects in the Northern Ireland population based study and in none of the Danish population based study, since it was based on histopathology records [59, 60]. In Northern Irish subjects with short segments less than 3 cm of BO, the annual risk of esophageal adenocarcinoma was 0.07% and was 0.22% in longer segment Barrett’s [60]. Both population based studies are likely to have included a majority of BO subjects with short segments, since they are more common than long segment BO, thereby lowering their estimates of the annual risk of esophageal adenocarcinoma compared with cohort studies largely from surveillance programs, which would predominantly include subjects with longer BO segments.
In conclusion, subjects with BO with no dysplasia at index endoscopy are at lower overall risk of developing EAC than previously thought, with two recent population based studies suggesting this is around 0.1% per year.
4 Natural History of Barrett’s Esophagus Indefinite for Dysplasia
Indefinite for dysplasia is used for BO cases where the morphological features differentiating between true dysplasia and regenerative/inflammatory atypia are not clear [62]. The degree of agreement among histopathologists for a diagnosis of indefinite for dysplasia has been reported to be very poor and even lower than the known poor agreement for low grade dysplasia, with kappa values of 0.18 and 0.35, respectively [63]. There is very limited data on the natural history of BO subjects indefinite for dysplasia. The rate of progression to esophageal adenocarcinoma in BO subjects indefinite for dysplasia has been reported to be similar to non-dysplastic BO patients, unless the areas that were indefinite for dysplasia were multifocal, when the rate of progression to carcinoma seemed as high as in patients with low grade dysplasia [64].
It has therefore been recommended that patients with a diagnosis of indefinite for dysplasia should be managed with optimization of their medical therapy and a further surveillance endoscopy in 6 months [56]. If no dysplasia is found on biopsies at that stage, then the patient’s risk is thought to be the same as non-dysplastic BO, with the need and timing of future surveillance determined by segment length and patient factors such as age and co-morbidity.
5 The Natural History of Barrett’s Esophagus with Low Grade Dysplasia
Despite the recognized limitations of a diagnosis of low grade dysplasia, with the poor inter-observer agreement among histopathologists described above, recognition of low grade dysplasia appears of critical importance to the management of BO and prevention of esophageal adenocarcinoma. In cohort studies and two large population based studies it was the most important predictor of subsequently developing esophageal adenocarcinoma, with BO subjects with low grade dysplasia five times more likely to develop esophageal adenocarcinoma than subjects without dysplasia [60, 61, 65]. In contrast, BO subjects who do not have low grade dysplasia at baseline and do not develop it during endoscopic follow-up appear to be at much lower risk of esophageal adenocarcinoma. Among a cohort of 3515 BO subjects, the risk of esophageal adenocarcinoma progressively fell in subjects who had an increasing number of surveillance endoscopies up to five that did not reveal dysplasia, from 0.32% per year to 0.11% per year [66].
Important data on the risk of progression of low grade dysplasia to esophageal adenocarcinoma has been provided by a retrospective analysis of 293 subjects with low grade dysplasia diagnosed at a number of community hospitals [67, 68]. Following a new consensus review, the original diagnosis of low grade dysplasia was confirmed in 27% of cases and downgraded to no dysplasia or indefinite for dysplasia in the others. Subjects with confirmed low grade dysplasia had a high risk of progression to high grade dysplasia or esophageal adenocarcinoma of 9.1% per year. Subjects whose diagnosis was down-staged, to either non-dysplastic BO or indefinite for dysplasia, had much lower rates of progression to high grade dysplasia or esophageal adenocarcinoma of 0.6 and 0.9% per year, respectively. This is supported by the results of a meta-analysis that reported that cohort studies with a lower ratio of low grade dysplasia to non-dysplastic BO cases, suggesting more stringent criteria for low grade dysplasia, reported a higher annual incidence of esophageal adenocarcinoma (0.76%) compared to studies with higher ratios (0.32%) [69]. Furthermore, in a trial of radio-frequency ablation for low grade dysplasia in BO, over a 3 year period 26.5% of subjects undergoing surveillance only progressed to high grade dysplasia or esophageal adenocarcinoma, compared with only 1% in the radio-frequency ablation arm (p < 0.001) [70]. To be eligible for inclusion in this study, the diagnosis of low grade dysplasia had to be confirmed by a central pathologist with extensive experience in BO.
Due to the variability among histopathologists in diagnosing low grade dysplasia, it is recommended that low grade dysplasia should be diagnosed on at least two endoscopies and confirmed by a histopathologist with expertise in BO. BO patients with low grade dysplasia should therefore have a repeat endoscopy in 6 months. If low grade dysplasia is reported at any follow-up endoscopy and confirmed by an expert gastrointestinal histopathologist, the patient should be offered endoscopic ablation therapy following multi-disciplinary team discussion given the high risk of progression to adenocarcinoma under these circumstances [70, 71].
6 The Natural History of Barrett’s Esophagus with High Grade Dysplasia
Historically, high grade dysplasia was regarded as an indication for esophagectomy, given the high rate of carcinoma reported in resection specimens in surgical series for high grade dysplasia [72]. However, careful intensive endoscopic follow-up tempered this approach. The reported esophageal adenocarcinoma risk in subjects with high grade dysplasia seems to be highly variable between different series and is likely to reflect that patients are not usually just followed up when high grade dysplasia has been diagnosed, that there is variation in histopathological diagnosis of high grade dysplasia between different centers and that the endoscopic appearances of early esophageal adenocarcinoma were not as well appreciated in published studies as they are today.
In a cohort study in 50 BO subjects, 5 out of 8 high grade dysplasia subjects developed adenocarcinoma on repeat endoscopies within 1 year [73]. Among a cohort of 1099 subjects, 79 initially had high grade dysplasia or subsequently developed it, without evidence of adenocarcinoma. Of the 75 high grade dysplasia subjects who remained without detectable adenocarcinoma after 1 year of intensive searching, 12 developed cancer (16%) during a mean 7.3-year surveillance period [74]. In a recent multi-center, sham-controlled study of radio-frequency ablation in BO with dysplasia, 21 subjects with high grade dysplasia underwent sham treatment and were followed up for 12 months with endoscopy and biopsies at 3-month intervals. Progression to adenocarcinoma occurred in 19% of subjects, while disappearance of HGD was seen in 19%, implying that even in the carefully controlled conditions of a clinical trial, high grade dysplasia can still regress to lower grades or no dysplasia, as well as progress to adenocarcinoma [75].
Although there may be visible abnormalities at endoscopy in high grade dysplasia, these can often be subtle and overlooked. It is therefore essential that there is an immediate repeat high quality endoscopy to look for any visible lesions suitable for endoscopic mucosal resection and map the extent of any dysplastic changes prior to any management decisions [56]. More than 80% of patients referred for further management of HGD or early esophageal adenocarcinoma, apparently without visible abnormalities, have at least one visible lesion detected in their BO segment on expert endoscopic assessment [76]. All patients with high grade dysplasia for which therapy is being considered, should be discussed at the specialist MDT for esophagogastric cancer [56]. This team should include an interventional endoscopist, upper GI cancer surgeon, radiologist, and a GI pathologist. Increasingly endoscopic therapy with endoscopic mucosal resection and radio-frequency ablation is the mainstay of treatment in patients with high grade dysplasia. A systematic review showed a mortality of 1.2% in the esophagectomy group compared with 0.04% in the endoscopic group [77] and radio-frequency ablation has been shown to be more cost-effective than esophagectomy for high grade dysplasia [78].
References
Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20.
Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–32.
van Herwaarden MA, Samsom M, Smout AJ. The role of hiatus hernia in gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2004;16:831–5.
Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–10.
Roman S, Holloway R, Keller J, Herbella F, Zerbib F, Xiao Y, Bernard L, Bredenoord AJ, Bruley des Varannes S, Chen M, Fox M, Kahrilas PJ, Mittal RK, Penagini R, Savarino E, Sifrim D, Wu J, Decullier E, Pandolfino JE, Mion F. Validation of criteria for the definition of transient lower esophageal sphincter relaxations using high-resolution manometry. Neurogastroenterol Motil. 2017;29(2):e12920.
Tack J, Pandolfino JE. Pathophysiology of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):277–88. https://doi.org/10.1053/j.gastro.2017.09.047.
Dent J, Holloway RH, Toouli J, et al. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut. 1988;29:1020–8.
Trudgill NJ, Riley SA. Transient lower esophageal sphincter relaxations are no more frequent in patients with gastroesophageal reflux disease than in asymptomatic volunteers. Am J Gastroenterol. 2001;96:2569–74.
Sifrim D, Holloway R, Silny J, Tack J, Lerut A, Janssens J. Composition of the postprandial refluxate in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:647–55.
Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–24.
De Giorgi F, Palmiero M, Esposito I, Mosca F, Cuomo R. Pathophysiology of gastro-oesophageal reflux disease. Acta Otorhinolaryngol Ital. 2006;26(5):241–6.
Iovino P, Mohammed I, Anggiansah A, Anggiansah R, Cherkas LF, Spector TD, Trudgill NJ. A study of pathophysiological factors associated with gastro-esophageal reflux disease in twins discordant for gastro-esophageal reflux symptoms. Neurogastroenterol Motil. 2013;25(8):650–6. https://doi.org/10.1111/nmo.12137. Epub 2013 May 26.
Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256–67.
Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975;55:1119–26.
Hoshino M, Sundaram A, Mittal SK. Role of the lower esophageal sphincter on acid exposure revisited with high-resolution manometry. J Am Coll Surg. 2011;213:743–50.
Nicodeme F, Pipa-Muniz M, Khanna K, Kahrilas PJ, Pandolfino JE. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-contractile integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil. 2014;26:353–60.
Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil. 2015;27:1423–31.
Wang D, Patel A, Mello M, et al. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol Motil. 2016;28(5):639–46. NGM 2015.
Sloan S, Rademaker AW, Kahrilas PJ. Determinants of gastroesophageal junction incompetence: hiatal hernia, lower esophageal sphincter, or both? Ann Intern Med. 1992;117(12):977–82.
Curci JA, Melman LM, Thompson RW, Soper NJ, Matthews BD. Elastic fiber depletion in the supporting ligaments of the gastroesophageal junction: a structural basis for the development of hiatal hernia. J Am Coll Surg. 2008;207:191–6.
Michelson E, Siegel C. The role of the phrenico-esophageal ligament in the lower esophageal sphincter. Surg Gynecol Obstet. 1964;118:1291–129.
Kahrilas PJ, Lin S, Manka M, et al. Esophagogastric junction pressure topography after fundoplication. Surgery. 2000;127:200–8.
Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–63.
Patti MG, Goldberg HI, Arcerito M, Bortolasi L, Tong J, Way LW. Hiatal hernia size affects lower esophageal sphincter function, esophageal acid exposure, and the degree of mucosal injury. Am J Surg. 1996;171:182–6.
Sloan S, Kahrilas PJ. Impairment of esophageal emptying with hiatal hernia. Gastroenterology. 1991;100:596–605.
Jones MP, Sloan SS, Jovanovic B, Kahrilas PJ. Impaired egress rather than increased access: an important independent predictor of erosive oesophagitis. Neurogastroenterol Motil. 2002;14:625–31.
Gillen P, Keeling P, Byrne PJ, et al. Barrett’s oesophagus: pH profile. Br J Surg. 1987;74:774–6.
Karvelis KC, Drane WE, Johnson DA, et al. Barrett esophagus: decreased esophageal clearance shown by radionuclide esophageal scintigraphy. Radiology. 1987;162:97–9.
Frazzoni L, Frazzoni M, de Bortoli N, Tolone S, Furnari M, Martinucci I, Bertani H, Marchi S, Conigliaro R, Fuccio L, Savarino V, Savarino E. Postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance can link PPI-responsive heartburn to reflux better than acid exposure time. Neurogastroenterol Motil. 2017;29(11):e13116. https://doi.org/10.1111/nmo.13116.
Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol. 2016;14:40–6.
Penagini R, Bravi I. The role of delayed gastric emptying and impaired oesophageal body motility. Best Pract Res Clin Gastroenterol. 2010;24:831–45.
Fletcher J, Wirz A, Young J, et al. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology. 2001;121:775–83.
Beaumont H, Bennink RJ, de Jong J, et al. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut. 2010;59:441–51.
Iovino P, Angrisani L, Galloro G, Consalvo D, Tremolaterra F, Pascariello A, Ciacci C. Proximal stomach function in obesity with normal or abnormal oesophageal acid exposure. Neurogastroenterol Motil. 2006;18(6):425–32.
Iovino P, Angrisani L, Tremolaterra F, Nirchio E, Ciannella M, Borrelli V, Sabbatini F, Mazzacca G, Ciacci C. Abnormal esophageal acid exposure is common in morbidly obese patients and improves after a successful lap-band system implantation. Surg Endosc. 2002;16(11):1631–5.
de Vries DR, van Herwaarden MA, Smout AJ, Samsom M. Gastroesophageal pressure gradients in gastroesophageal reflux disease: relations with hiatal hernia, body mass index, and esophageal acid exposure. Am J Gastroenterol. 2008;103:1349–54.
Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–8.
Savarino E, Tutuian R, Zentilin P, Dulbecco P, Pohl D, Marabotto E, Parodi A, Sammito G, Gemignani L, Bodini G, Savarino V. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and nonerosive reflux disease: study using combined impedance-pH off therapy. Am J Gastroenterol. 2010;105(5):1053–61.
Weijenborg PW, Bredenoord AJ. How reflux causes symptoms: reflux perception in gastroesophageal reflux disease. Best Pract Res Clin Gastroenterol. 2013;27:353–64.
Barlow WJ, Orlando RC. The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology. 2005;128(3):771–8.
van Malenstein H, Farré R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol. 2008;103(4):1021–8.
Solcia E, Villani L, Luinetti O, Trespi E, Strada E, Tinelli C, Fiocca R. Altered intercellular glycoconjugates and dilated intercellular spaces of esophageal epithelium in reflux disease. Virchows Arch. 2000;436(3):207–16.
Farré R, Blondeau K, Clement D, Vicario M, Cardozo L, Vieth M, Mertens V, Pauwels A, Silny J, Jimenez M, Tack J, Sifrim D. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60(7):885–92.
Frazzoni M, de Bortoli N, Frazzoni L, Furnari M, Martinucci I, Tolone S, Farioli A, Marchi S, Fuccio L, Savarino V, Savarino E. Impairment of chemical clearance and mucosal integrity distinguishes hypersensitive esophagus from functional heartburn. J Gastroenterol. 2017;52(4):444–51.
Farré R, van Malenstein H, De Vos R, Geboes K, Depoortere I, Vanden Berghe P, Fornari F, Blondeau K, Mertens V, Tack J, Sifrim D. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut. 2008;57(10):1366–74.
Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137(5):1776–84.
Dunbar KB, Agoston AT, Odze RD, Huo X, Pham TH, Cipher DJ, Castell DO, Genta RM, Souza RF, Spechler SJ. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315(19):2104–12.
Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16(9):897–902.
Kandulski A, Wex T, Mönkemüller K, Kuester D, Fry LC, Roessner A, Malfertheiner P. Proteinase-activated receptor-2 in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2010;105(9):1934–43.
Knowles CH, Aziz Q. Visceral hypersensitivity in non-erosive reflux disease. Gut. 2008;57(5):674–83.
Farré R, De Vos R, Geboes K, Verbecke K, Vanden Berghe P, Depoortere I, Blondeau K, Tack J, Sifrim D. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut. 2007;56(9):1191–7.
Iovino P, Pascariello A, Limongelli P, Tremolaterra F, Consalvo D, Sabbatini F, Amato G, Ciacci C. The prevalence of sexual behavior disorders in patients with treated and untreated gastroesophageal reflux disease. Surg Endosc. 2007;21(7):1104–10.
Kessing BF, Bredenoord AJ, Saleh CM, Smout AJ. Effects of anxiety and depression in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2015;13(6):1089–95.e1.
Barrett NR. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg. 1950;38(150):175–82.
Bremner CG, Lynch VP, Ellis FH Jr. Barrett’s esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery. 1970;68:209–16.
Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42.
Morson BC, Belcher JR. Adenocarcinoma of the esophagus and ectopic gastric mucosa. Br J Cancer. 1952;6:127–30.
Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970–6.
Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103(13):1049–57.
Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–83.
Sikkema M, Looman CW, Steyerberg EW, et al. Predictors for neoplastic progression in patients with Barrett’s esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106(7):1231–8.
Odze RD. Barrett esophagus: histology and pathology for the clinician. Nat Rev Gastroenterol Hepatol. 2009;6:478–90.
Sonwalkar SA, Rotimi O, Scott N, et al. A study of indefinite for dysplasia in Barrett’s oesophagus: reproducibility of diagnosis, clinical outcomes and predicting progression with AMACR (alpha-methylacyl-CoA-racemase). Histopathology. 2010;56:900–7.
Younes M, Lauwers GY, Ertan A, et al. The significance of “indefinite for dysplasia” grading in Barrett metaplasia. Arch Pathol Lab Med. 2011;135:430–2.
Theron BT, Padmanabhan H, Aladin H, Smith P, Campbell E, Nightingale P, Cooper BT, Trudgill NJ. The risk of oesophageal adenocarcinoma in a prospectively recruited Barrett’s oesophagus cohort. United European Gastroenterol J. 2016;4(6):754–61.
Gaddam S, Singh M, Balasubramanian G, et al. Persistence of nondysplastic Barrett’s esophagus identifies patients at lower risk for esophageal adenocarcinoma: results from a large multicenter cohort. Gastroenterology. 2013;145(3):548–53.
Curvers WL, ten Kate FJ, et al. Low-grade dysplasia in Barrett’s esophagus: over diagnosed and underestimated. Am J Gastroenterol. 2010;105(7):1523–30.
Duits LC, Phoa KN, Curvers WL, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64(5):700–6.
Singh S, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79(6):897–909.
Phoa KN, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311(12):1209–17.
di Pietro M, Fitzgerald RC; BSG Barrett’s guidelines working group. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. Gut. 2018;67(2):392–3. doi: https://doi.org/10.1136/gutjnl-2017-314135. Epub 2017 Apr 7
Dar MS, Goldblum JR, et al. Can extent of high grade dysplasia in Barrett’s oesophagus predict the presence of adenocarcinoma at oesophagectomy? Gut. 2003;52(4):486–9.
Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96(5):1249–56.
Schnell TG, Sontag SJ, et al. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120(7):1607–19.
Shaheen NJ, Sharma P, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–88.
Curvers WL, Singh R, Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167–72.
Menon D, Stafinski T, Wu H, et al. Endoscopic treatments for Barrett’s esophagus a systematic review of safety and effectiveness compared to esophagectomy. BMC Gastroenterol. 2010;10:111.
Boger PC, Turner D, Roderick P, et al. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s oesophagus. Aliment Pharmacol Ther. 2010;32:1332–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Iovino, P., Santonicola, A., Trudgill, N.J. (2019). Pathophysiology of Gastroesophageal Reflux Disease and Natural History of Barrett’s Esophagus. In: Galloro, G. (eds) Revisiting Barrett's Esophagus. Springer, Cham. https://doi.org/10.1007/978-3-319-92093-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-92093-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92092-4
Online ISBN: 978-3-319-92093-1
eBook Packages: MedicineMedicine (R0)