Abstract
Surgical fracture fixation is an important part of modern orthopedic care. Implants are designed by engineers, and selected and applied by surgeons, with careful consideration of clinical, biological, biomechanical, and biomaterials principles. Clinically, a large variety of screws, plates, intramedullary nails, and external fixation devices are used. Fracture healing is a biologically complex process that may proceed down one of multiple possible pathways. Successful fracture healing, as well as implant survival, is dependent on three-dimensional biomechanics as the patient resumes activity. These biomechanics are dependent on patient variables as well as the fracture fixation construct chosen by the surgeon. Implant biomaterials must satisfy stringent biomechanical and biocompatibility requirements. Experimental and computational models enable advances in implant design, as well as our understanding of how surgeons may best apply these implants for each patient.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Fracture fixation
- Orthopedic biomechanics
- Implant mechanics
- Bone healing
- Fatigue
- Failure analysis
- Implant materials
- Screw fixation
- Biocompatibility
- Computational modeling
- Finite element method
- Intramedullary nail
- Internal plating
- External fixation
- Translational research
1 Clinical Aspects
1.1 Introduction
Fracture management constitutes a very large portion of modern orthopedic care . Many fractures can be managed by non–surgical methods such as splinting, casting, and functional bracing. This type of fracture management is particularly applicable in nondisplaced or minimally displaced fractures – often in the pediatric population whose healing times are quite short and the remodeling potential after healing can correct residual deformity. Nonetheless, operative fracture care is being used increasingly as it has been shown to reduce disability, improve outcome, and improve the quality of life following a significant fracture. There are three areas in which surgical facture care has been shown to make a major impact on patient recovery from injury, and is pivotal in the restoration of function and quality of life.
Displaced fractures of the femur and tibia in the adult are now usually treated surgically as it has been well-demonstrated that this method is safe, promotes the rapid return to function including ambulation, preserves joint function, and avoids the condition associated with non-operative treatment previously coined “fracture disease” which is a complex of problems such as joint stiffness, pressure ulcers, disuse osteopenia, formation of deep vein thrombosis, and muscle atrophy.
The second area in which operative fracture care has resulted in dramatic improvement in clinical outcomes is the fracture of the articular surfaces of joints . The surfaces of most joints (e.g. hip, knee, ankle, and elbow) are highly congruent with each other such that distortion of the shape of either side of a joint from a fracture can dramatically alter the load transmission across the surface and raise cartilage contact pressures to the point that the cartilage will breakdown and lead to post-traumatic degenerative arthritis. Often, this will result in joint destruction within several years of the injury. Modern orthopedic care of the injured joint rests on the concept of restoration of the shape of bones on both sides of the joint as well as restoration of axial alignment and ligamentous stability of the joint. This approach restores the normal pressures across the joint during functional use of the limb. A joint that has been restored surgically in this way can undergo very early motion which prevents stiffness and optimally promotes recovery of the injured articular cartilage.
The final arena in which surgical treatment of fractures has made a dramatic improvement in outcome is the multiple injury trauma patient. In this group, rapid stabilization of long bone and pelvis fractures is viewed as part of the overall resuscitation of the patient since it helps control bleeding, pain, and facilitates overall management of the multisystem injuries. Often, this is accomplished initially using external fixation in a “damage control” mode . Then, after the patient is stable and through the initial period of bleeding, these external fixation frames are replaced with more definitive internal fixation to allow progressive mobilization and ambulation.
1.2 Types of Implants
The common element in the entire spectrum of surgical fracture care is the use of orthopedic implants to restore skeletal stability that has been lost by the fracture or joint dislocation. It is the thoughtful application of these devices that allows the modern fracture surgeon to create conditions at the fracture site that will promote healing as well as allow functional use of the extremity during the period of healing (Fig. 1).
Four basic types of implants are used in the surgical treatment of fractures: screws, plates, nails, and external fixators. The most basic type of orthopedic implant is the bone screw (Fig. 2). Modern orthopedic bone screws are available in a vast variety of sizes (1–8 mm diameter) and thread designs which allow use in a wide variety of clinical situations. Screws can be used as stand-alone fracture fixation devices which are placed across fracture fragments to generate compression and create stability and promote fracture union. These “compression screws” employ two different design techniques to achieve compression. The partially threaded bone screw gains purchase in the bone fragment away from where the screw is placed with no screw threads in the near fragment. Compression is generated when the head of the screw contacts the near bone fragment and the threads engage the far bone fragment in essence “pulling” them together. A fully threaded bone screw can also generate compression by the method of application (compression by technique). A hole is drilled in the near bone fragment that is slightly larger than the outer diameter of the screw (gliding hole) and the far bone fragment is drilled with a size equal to the core diameter of the screw (threaded hole). When the screw is placed, the threads gain purchase only in the far fragment and as the screw head contacts the bone surface, compression is generated. The same fully threaded screw can be placed across bone fragments without the use of a gliding hole. In this situation, the screw engages threads on both sides of the fracture. Compression cannot be generated, and this construct is termed a “static” or “position” screw.
Plates are placed on the outer aspect of the bone surface and are mechanically linked to the bone via the use of screws. These bone screws can be mechanically joined to the plate either by a frictional method (non-locked), or designed in such a way to allow the screw head to engage the holes in the plate via a series of threads in the head of the screw (locked) such that movement of the screw-plate interface is highly constrained. A plate designed to stabilize a fracture must fit within the local anatomy such that muscles and tendons and the adjacent joints can function normally while the plate is in place (Figs. 3 and 4a).
The third major type of fixation device is the intramedullary (IM) nail (Fig. 4b–d ). These devices are placed into the medullary cavities of diaphyseal bones (e.g. femur, tibia, and humerus), and act as internal splints across the fractures. IM nails can span the length of the bone and be large devices, up to 50 cm. in length. Modern IM nails allow screws to be placed perpendicular to the nail through the ends of the bone and the ends of the nail on either side of the fracture to control motion and displacement. This type of device is termed an “interlocked” nail and is the workhorse device in the treatment of adult femur and tibia fracture.
The fourth category of fixation device is the external fixator (Fig. 5). In this type of device, threaded bone pins (3–6 mm in diameter) are percutaneously placed into the bone on either side of the fracture and then connected outside the body using clamps and bars. External fixation can be constructed across an entire spectrum of stability (under the surgeon’s control), to solve many clinical problems in orthopedic fracture care up to the creation of stability to allow definitive fracture healing. By its very design, external fixation is temporary and will have to be removed. Screws, plates and intramedullary nails do not have to be removed per se, but may be at a secondary procedure for a large variety of clinical reasons.
We are currently conducting a clinical study in which the forces in vertical struts of ring-type external fixators are measured. Force data from the struts, as well as foot forces, are transmitted wirelessly (a) during the patient’s gait. A decrease over time in normalized strut forces was observed in this patient (b), and in other patients, consistent with offloading due to bone healing. (In (b), ‘W37D’ indicates dynamization of the frame.) (Image from our medical center)
Variations in fixation implant design and surgical application may enhance overall mechanics and promote healing. Features such as improved stability, reduced anatomical interference, and more robust fixation to bone may be achieved through implant design modification, and careful planning by the surgeon.
1.3 Anatomical Constraints
The biomechanical and anatomic design constraints on orthopedic fracture implants are quite rigorous. They must be biologically compatible, and meet the biomechanical requirements of the situation in which they are used. Since they are used to support the bone and not replace it during the period of healing, the implants must fit the local anatomy which varies among patients. IM nails must fit within the intramedullary canal. Plates must fit on the external surface of bone and not impede important muscles, tendons, and ligaments surrounding them. Implants are also designed with consideration of the surgical exposure needed to apply them. Although many plates and screws are applied with an open approach that exposes the bone fragments, IM nails, external fixators and certain plates can be applied with a minimally invasive approach that avoids disturbing the soft tissues surrounding the fracture that are important for blood supply and the healing process. Plates designed to reduce the area of contact with bone via geometric modification of the contact surface are termed minimum contact plates. Reducing contact area at the bone-plate interface may enhance fracture healing by mitigating disruption to the periosteum and bone blood supply [1].
As a result of the aforementioned stringent geometric design requirements, fracture fixation implants typically do not have an infinite fatigue life. Since the fixation device may initially (at the time of the acute fracture and before bone healing) be load bearing to allow the patient to begin functional use of the extremity or ambulation, there will be accumulated damage in the microstructure of the implant. It is well understood by both orthopedic clinicians and implant designers that there is a “race” between bone healing and implant fatigue fracture . The clinical presentation of a fatigue fracture of an orthopedic implant may imply some pathology of bone healing.
2 Fracture Healing Biology
2.1 Fracture Healing
Fracture healing is a complex biomechanical process with multiple possible endpoints. The most desirable endpoint, termed ‘union’ in the medical vernacular, is complete fusion of the fractured components into one continuous osseous structure. A union may be achieved through one of two well-described mechanisms [2]. Primary or intramembranous bone healing occurs through Haversian remodeling which requires absolute stability between bony fragments. On a cellular level, primary healing involves direct healing of bone without the presence of intermediary tissues. A very stable interface between two bone fragments is necessary because there is creation of bridging capillaries as part of the Haversian remodeling which is intolerant of shear forces due to its mostly linear morphology [3]. Primary healing also requires contact between the fracture fragments, or only a very small gap (on the order of 1 mm). The degree of stability required for primary bone healing can usually only be achieved via compression between fragments. Thus, primary bone healing usually only occurs for simpler fractures in which the fragments can readily be fixed back in their anatomical position, usually with plates and/or lag screws.
Secondary healing or enchondral ossification is characterized by formation and evolution of progressively stiffening intermediary tissues in a relative stability mechanical environment. Secondary fracture healing progresses through three biological stages. The inflammation phase begins the moment fracture occurs and can last up to 5 days. This first phase is characterized by an immune response in which damaged, necrotic tissues are scavenged by immune cells and the fibrinous hematoma formed during injury is organized into granulation tissue. Fibroblasts produce type III collagen. Inflammatory mediators are released resulting in pain, swelling, and an increase in local blood flow. A chondroid stage then begins enhancing the vascularity and cellularity of the granulation tissue via mesenchymal stem cells from the nearby periosteum. The chondroid matrix is then replaced by osteoblasts creating type I collagen. The replacement and removal of the cartilage intermediate is the hallmark of endochondral ossification. At this point in the healing process, mechanical loads can be transmitted from one fragment to the other, but there is no internal organization of the callus. As normal loading returns, the woven bone characteristic of early callus is remodeled into lamellar bone in response to the loading pattern. This remodeling process can take months to years and is accompanied by a decrease in the size of the callus mass often closely re-approximating the diameter of the normal surrounding bone. Coincident with the decrease in size during remodeling, is an optimization of the mechanical properties of the bone.
The biological stages of secondary bone healing are intimately linked to the local mechanical environment. The interfragmentary strain theory , first described by Stephen Perren in 1991 [4], states that a tissue type cannot exist in a region in which the mechanical forces exceed its strain tolerance. The elegant interplay of biology and mechanics described in the strain theory provides the fracture surgeon and implant designer a framework to understand these seemingly unrelated concepts. For example, recent research on bone healing has found immature myeloid cell-mediated angiogenic cascade to enhance bone healing in a mouse model [5].
By definition, a fracture involves damage to the local blood supply of the bone often extending to the local tissue. As a result, bleeding occurs around the bone ends creating a fracture hematoma . As might be expected, conditions within this clotted hematoma are acidotic, hypercarbic, and have a low oxygen tension. The first tissue to appear histologically in this region is type III collagen made by fibroblasts. The syncytia of loosely organized collagen fibers and fibroblasts within the clotted fracture hematoma is called granulation tissue. Mechanically, granulation tissue has a strain tolerance of about 100%. Metabolically, granulation tissue survives in this region because it has a low oxygen requirement which can be met via diffusion. The granulation tissue organizes into an early fracture callus and conditions for the second stage of fracture healing are created. The second tissue type to appear is cartilage, which is composed of type II collagen, is made by chondroblasts. The presence of chondrocytes implies that the region has become mechanically stiffer as the strain tolerance of cartilage is about 10%. At this time, the local blood supply is improving in response to the release of angiogenic pyrogens. This larger more organized callus composed of granulation tissue and cartilage further stiffens the region to the point where enchondral ossification can occur. In regions of exceptionally low strain, osteoblasts will differentiate from mesenchymal stem cells and begin production of an osteoid. The histological appearance of osteoblasts implies significant mechanical stability has been achieved, as this tissue type has a strain tolerance of only about 2%. An important concept is that osteoblasts are quite metabolically active and have a high oxygen requirement. This requires the co-development of a rich capillary network. Bone cannot form in regions of high strain because the supplying capillaries cannot survive. The strain theory informs that each tissue type that appears in a healing fracture prepares the region both mechanically and biologically for the next tissue. Bone formation implies that stability to maintain a capillary network has been achieved. A histological hallmark of fracture healing is capillaries crossing the fracture gap. In the case of primary bone healing, a surgical procedure has created stability between bone fragments such that a capillary can cross the gap without the appearance of precursor tissues.
The different stability requirements between primary and secondary fracture healing are functions of the strain tolerance of the local biological tissues. Although the strain tolerances for relative stability are less defined, secondary healing has been shown to be induced by interfragmentary motion on the order of millimeters [6].
Impaired fracture healing can have biological and mechanical origins. Focusing on mechanical causes, nonunions or delayed unions are generally a result of insufficient stability at the healing site. Excessive strain during healing can rupture nascent capillaries and prevent nutrient transport to the metabolically active healing site. Poor stability can have multiple origins including inadequate initial surgery, implant hardware failure, or an unforeseen traumatic mechanical event. Biologically, a patient may have decreased bone density that will affect the stability that can be achieved at surgery. Tissue damage, infection [7], medications, smoking [8], nutrition, and genetic factors [9] influence the healing response following a fracture. Surgically, a risk of nonunion is created if the local tissues are damaged by the procedure especially if secondary bone healing is relied upon. In this situation, callus formation is impaired as a result of the tissue damage, and the healing may not be able to proceed to union. In nonunion cases, stresses on the implant are never relieved by bone unions, eventually resulting in a fatigue failure of the implant.
Residual bone deformity can result from a failed fracture fixation or improper bone healing. Residual deformity can occur when the fracture is not reduced to its original anatomical position or orientation, bone migrates through partial implant loosening, or implants partially fail through yield. Most common in comminuted and bone loss fractures, residual deformity can leave a fracture patient with pain, joint stiffness, limb-length discrepancies, and posttraumatic arthritis (in addition to the deformity) associated with insufficient reduction of fractures affecting a joint. Residual deformity outcomes may require medical or even surgical attention and reduce a patient’s quality of life. The close relationship of local mechanics to successful bone healing necessitates that proper care and consideration be given to all fractures.
2.2 Infection
Infection is a devastating complication following surgery, and implanted materials lead to greater risk of infection. Open fractures where the skin is broken increase the risk for infection. This risk can reach 30% in certain high grade open fractures with severe contamination and damage to muscle and bone. Surgery further increases the risk for infection, although most infection rates during surgery on non-immunocompromised patients are below 1%. Diabetes mellitus, HIV, and rheumatoid arthritis are common examples of chronic conditions which can also increase the risk for infection during fracture fixation. Additionally, lifestyle characteristics such as smoking, obesity, and poor nutrition can also predispose patients to higher infection risks. Novel approaches for reducing bacterial colonization and biofilm formation on fracture fixation biomaterials are an active area of research.
3 Biomechanics
The biomechanics of the fracture fixation construct are integral to understanding how to develop an optimal implant and surgical approach to fracture repair. The term ‘implant’ herein will refer to the large fixation component that stabilizes the fracture often via attachment with screws. The term ‘construct ’ refers to the entire system encompassing the implant, screws, fracture site, bone, and healing tissues. ‘Configuration’ will refer to surgical variables, i.e. number of screws used, implant position, type of screw fixation, etc.
3.1 Implant Loading
The initial stress born by a fracture implant following surgery is quite variable across patients. At one end is the scenario in which bone fragments have been anatomically reduced and compressed (absolute stability). Load transmission will occur from one bone fragment to the other directly and result in low implant stresses. At the other end of the stability spectrum is the situation in which there is no initial contact between bone fragments and the implant carries the entire initial load of the construct. A bridge plating across a large zone of comminution would be an example of this.
Fracture fixation constructs are subjected to a variety of loading conditions ranging from singular high force loading, such as due to an accidental patient fall, to repeated functional activities. These load patterns are classified as either static or cyclic, respectively, and may lead to either material yield or fatigue failure mechanisms. Orthopedic implants can be subject to surprisingly large mechanical loads within the body. For example, instrumented arthroplasty implant studies show that implant reaction forces in the knee, hip, and spine can reach nearly three times the bodyweight during simple gait [10]. Furthermore, instrumented fracture fixation implant studies can show the strain in an implant and use strain data as a surrogate for fracture healing over time [11]. A study using an instrumented femoral nail demonstrated that the load in that implant was almost exclusively parallel to the implant’s primary axis in four different postures [12].
Before a fractured bone has begun healing, the fixation construct may be responsible for up to all of the load transferred across the fracture gap. This is particularly true in situations in which there is no contact between the major bone fragments of the fracture. As time progresses, intermediate bone tissues form across the fracture site which gradually reduce the load carried by the implant. The construct must maintain mechanical integrity during the course of bone healing as the mechanical environment at the fracture site plays a large role in achieving union. As discussed previously, excessive motion at the fracture gap can result in local tissue strain that exceeds the tolerance of nascent capillary networks that are attempting to bridge these gaps [3]. Thus, we must understand the mechanical responses of fixation constructs to physiological loads within the scope of the stability tolerance required for a biological fracture healing process.
Bone, as a living material, adapts its composition and shape over time in response to its mechanical stimulus history. Stress shielding refers to decreased bone density in regions that are subjected to lower stress levels due to loads borne by the implant. The fixation implant effectively offloads some of the forces that normally pass through the bone. Such a decrease in stress is believed to engage biomechanical signals and either stimulate bone resorption or inhibit bone formation.
Although limited or non-weightbearing is often appropriate in the early stages of fracture healing, sometimes to protect the implant itself, prolonged limited weightbearing in a patient can result in disuse osteopenia. Disuse osteopenia is a decrease in bone mineral density as a result of prolonged periods of unloading in the bone. Bone remodeling processes, perhaps with sensing by osteocyte cells, detect the relatively low loading profile of bone and favor resorption over deposition, resulting in a bone density that decreases with time. Osteopenia decreases bone mineral density and increases the risk of implant-bone loosening or bone fracture during activity [13, 14].
3.2 Implant Stress and Failure
Stress is a measure of internal forces in a localized region of material and has units of force divided by area. Most often in orthopedic biomaterials stresses are generated in an implant in response to applied external loads. Stress causes a material to deform, creating strain which is a measure of internal displacement in a localized region of material. Strain is a unitless ratio of final length to initial length. Basic yield and fatigue failure theories for engineering materials such as orthopedic implants primarily utilize stress to predict failure. Most current fracture fixation implants are usually considered as linear elastic materials, meaning that while they undergo deformation below failure thresholds, a linear relationship between stress and strain is maintained. Young’s Modulus or elastic modulus is equivalent to the ratio of stress to strain. Examples of linear elastic materials include steel, carbon fiber, and glass. Once the yield stress is reached, the material begins to fail plastically and the Young’s Modulus no longer accurately describes the stress-strain relationship.
Stress in a fracture fixation construct, like in other mechanical applications, is subject to concentration effects. Stress concentration describes the localized magnification of stress due to geometric features such as holes and sharp corners. Changes in cross sectional area are common along fracture fixation implants and are frequently caused by variable implant design, holes in the implant intended for screw placement, tapers, fillets, and edge characteristics. Most fracture fixation plates have unique underside geometries that limit areas of contact with the periosteum in order to reduce damage to blood supply. Variable cross sections are introduced in some cases to avoid interference with anatomical structures and reduce the weight of the implant. In most modern plates, the removal of material under the plate to limit bone contact area is done in such a way as to keep the cross-sectional moment area of inertia of the implant the same throughout the plate length. This allows the surgeon to easily bend the plate if needed during the surgical procedure.
Fracture fixation construct failure can occur within the implant. If the failure occurs under a static load, yield can occur at the location of maximum shear stress in ductile materials. The yield strengths for common fracture fixation implant materials such as cold-worked stainless steel, hot-forged CoCr alloy, and forged, heat treated titanium are 792, 1600, and 1034 MPa, respectively. Bone screws are typically made of annealed stainless steel which has a yield strength of 331 MPa. Typically, shear stress is maximized in the implant region near an unsupported fracture gap where there are fewer points of fixation and where the largest bending moments are likely to occur. When the applied load is not sufficient to cause yield failure, the implant may still accumulate damage in the form of microcracks. Cyclic loading at sub-yield stress levels causes fatigue failure via progressive crack propagation and eventual brittle fracture within the implant. At stresses above a material’s endurance limit (a cyclic stress amplitude below which failure will never occur), one can calculate the number of fully reversed loading cycles required to reach fatigue failure. The resulting data are plotted on an S-N curve to characterize the material’s response to cyclic loading. A Goodman Diagram may be used to consider the fact that implant mechanical loading is often not fully reversed, and is instead repeated with a non-zero mean stress.
However, the amplitude of cyclic loading of fracture fixation implants is often variable, dependent on a variety of functional activities (e.g. walking, climbing stairs, jumping, etc.). Under Miner’s rule, the total damage is defined as the sum of incremental damages calculated for each load amplitude [15]. Each incremental damage can be calculated as the ratio of the number of cycles at a given stress or strain range to the total number of cycles required for failure at that range. Some commercial finite element software include more advanced algorithms for predicting fatigue, e.g. ‘fe-safe’, a durability finite element software suite from Dassault Systemes. Fixation and contact points between the implant, screws, and bone are of special interest for biomechanical characterization as these locations are common sites of construct failure. Screw head shearing has been documented to occur. In this case, screw fractures typically occur near the screw-implant-bone junction (Fig. 6). Shear stresses in this region can be influenced by decreasing the distance between the fracture gap and the closest screws (working length), increasing the screw count, and biological stiffening across the fracture site via healing [16]. Local bone micro-fracture at the screw-bone interface can also occur, typically leading to screw pullout from the bone [17].
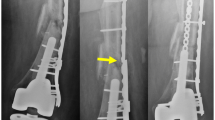
Careful considerations of biomechanics is needed when treating difficult fractures. Surgeons attempt to create conditions at the fracture site that will promote healing while allowing functional use of the extremity. Radiographs from our center show a loss of fixation, plate, and/or screw failure in the (left) proximal tibia, (middle) proximal humerus, and (right) distal dibia. (Images from our medical center)
3.3 Fracture Gap Strain
Bone is remarkable in its ability to regenerate following a fracture. Unlike most other tissues which produce scar tissue, a fractured bone, adequately stabilized and with sufficient vascularization and other biological factors, is able to ultimately regain its near-original form [18]. Nonoperative treatment relies on some degree of stability from soft tissues including muscles, ligaments, fat, and skin, whereas operative treatment increases stability through implant fixation.
Various theories have been proposed for how mechanical stimulus affects differentiation and adaptation of healing tissues. These theories are based on mechanical stimulus in the form of hydrostatic pressure, deviatoric stress, and even fluid flow velocity, the latter viewing the tissue as a poroelastic material.
Interfragmentary displacement or strain (displacement divided by fracture gap width) measures provide a useful and intuitive means of characterizing mechanics at the fracture gap. Based on available evidence, moderate strains of approximately 10–50% have been proposed as being beneficial to healing [19, 20]. This strain is often split into two components: axial or longitudinal, acting along the long bone axis, and shear, acting perpendicular to the long bone axis. There is some consensus that shear strains can be detrimental to fracture healing, whereas moderate axial strains are beneficial [21, 22]. Dynamized intramedullary nails and certain new plate technologies promote additional axial strain [23]. Excessively high strains are inhibitory to fracture healing and may result in nonunions [24,25,26]. Compressive interfragmentary displacements are superior to distractive displacements in creating callus [27]. Bone formation is linked with vascularization, and interfragmentary movements early in fracture healing to promote revascularization, but in the later stages of healing can inhibit blood flow [24, 28]. Some investigators have demonstrated clinically the influence of biomechanical and biomaterial choices on fracture healing and callus formation [29]. However much of the evidence supporting these theories are from studies in animals, especially sheep. In these studies, fractures, often in the tibia, were simulated with osteotomy cuts leaving a gap. The fractures were stabilized with external fixators. In one such study [21], five sheep were fixed with a device that allowed only axial interfragmentary displacements, and five sheep were fixed with a device that allowed only shear displacements. Displacement magnitudes were 1.5 mm with a 3 mm fracture gap (50% strain) in both groups. The group with shear displacements experienced significantly delayed healing of the osteotomies, with one third stiffness of the healing site after 8 weeks.
Although there is some consensus that axial and shear strain influence fracture healing differently, unfortunately control of these two strain components by changes in fracture fixation construct are not always intuitive. For example, increasing the working length (or bridge span) between the inner-most screws in a plate construct seems that it would mostly affect axial interfragmentary strain; however recent predictions from finite element models of 66 supracondylar femoral fracture fixations in patients (with supporting data from synthetic bone experiments) revealed that increasing working length primarily affected interfragmentary shear motions, not axial motions [22]. Changing the plate from stainless steel to titanium increased both types of motion. Callus formation in the patients was not associated with comorbidities including smoking or diabetes, but was promoted by longitudinal motions and inhibited by shear motions at the fracture site [22]. Additionally, improvements in one aspect of biomechanics may lead to concerns in another aspect; the scenario of large longitudinal strains combined with small shear strains may be achieved with smaller plate bridge spans, but unfortunately these smaller spans lead to larger plate stresses and a risk of plate failure [6, 22].
3.4 Biomechanical Variables
Bone strain and displacement at the fracture gap are influenced by the stiffness of the construct, interfragmentary contact, and load transfer at the fracture gap. The stiffness of a fixation construct is its resistance to deformation in response to applied loads. Stiffness is a function of several construct variables: design, orientation, material composition, working length, screw count, and biological material properties in the fracture gap. Construct stiffness can be measured in multiple modes: bending, torsion, and compression. The bending stiffness for plate constructs in long bones often dictates most of the displacement at the fracture gap, whereas circular-type external constructs rely more on the compression stiffness mode. Torsional stiffness can become important clinically in all types of fracture repair constructs (plate, intramedullary nails, and external fixation ).
Various fracture fixation devices provide stiffness to the fracture. For example, a bridge plate is a construct in which a plate implant acts as an extramedullary splint spanning a complex fracture and bends and compresses in response to axial loads on the bone. The bending stiffness of a bridge plate can be calculated as an applied bending moment divided by lateral deflection. Similarly, the compressive stiffness of the bridge plate can be determined by dividing the applied axial load by axial displacement. All loading modes (compression, bending, and torsion) work in unison to dictate the bony displacement and strain at the fracture site, critical for fracture healing, under a given load scenario.
Graded-stiffness and composite material compositions can modify construct stiffness although currently they do not see much clinical use. The working length of a fixation construct is the distance between the two fixation points closest to the fracture gap. The stiffness of the construct can be approximately inversely proportional to the working length [30]. Surgical decisions such as screw placement can cause construct stiffness to be too extreme in either direction (too stiff or too compliant) to promote healing.
Use of either locking or nonlocking screws will influence the fracture fixation biomechanics. Locking screws maintain a fixed angle with the plate and do not enable motion at the screw-plate interface. Nonlocking screws compress the plate and the bone together creating a frictional interface between them. Over time, especially in unicortical screw fixation, normal stresses in the cancellous bone surrounding screws may cause loosening at the bone-screw interface, allowing the screws to toggle at the implant-screw junction. Thus, the biomechanics of nonlocking screws allow for some motion at the screw-plate interface, potentially resulting in a decreasing construct stiffness over time [31].
4 Biomaterials
4.1 Stainless Steel Vs. Titanium alloys & Other Materials
Although a vast array of metallic alloys are available for titanium and stainless steel, the mechanical, chemical, and biocompatibility properties dictate that a small subset of these alloys are appropriate for use in fracture fixation applications. Metallurgical alloying can influence multiple material properties of the base metal. Specifically relevant to fracture fixation are the toughness, manufacturability, oxidative potential, corrosion resistance, and biocompatibility. Most modern implants are manufactured through forging [32].
The current most common stainless steel alloy used in orthopedic fracture fixation applications is designated as 316L. This low carbon content alloy is manufactured to follow ASTM F138 and F139 standards which specify: ≤0.03% carbon, ≤2% manganese, ≤0.03% phosphorous, ≤0.75% silicon, 17–20% chromium, 12–14% nickel, 2–4% molybdenum, with the remainder being iron. The carbon and chromium content contribute to corrosion resistance while nickel and molybdenum improve the mechanical toughness of the alloy. 316L is amenable to cold-working strengthening which can potentially increase the yield strength from 330 MPa to a maximum of 1200 MPa [32]. The elastic modulus for stainless steel is 190 GPa.
Titanium and titanium alloys have an elastic modulus of 110 GPa, considerably lower than that of stainless steel and closer to that of bone, which explains why titanium alloy implants mitigate the risk of bone loss associated with stress shielding. Titanium is commonly alloyed with aluminum and vanadium, the most common being Ti-6AL-4V (5.5–6.5% aluminum and 3.5–4.5% vanadium).
4.2 Biocompatibility
All components of internal fracture fixation implants and percutaneous components of external implants must be biocompatible. Because fracture healing typically occurs on the order of weeks to months, biocompatibility must be maintained long after the materials have been implanted. Although a complex topic, the biocompatibility of an implant refers to the local and systemic physiological changes undertaken by host tissues in response to the implant’s presence. Potential biocompatibility hazards of orthopedic implants include toxicity, immunogenicity, mutagenicity, and infection propensity. Most assessments of biocompatibility of fracture fixation constructs focus on immune responses to the implant. Immune responses can be triggered by material composition, surface topography, as well as size, depending on the local implant environment, and can activate traditional innate, complement, and humoral immunological pathways.
The implant surface-tissue interface is the single most important driver of biocompatibility in metallic implants [33]. Morphological features such as porosity, surface modification, and smoothness as well as chemical characteristics such as hydrophobicity, wettability, surface charge, and polarity influence an implant’s immunogenicity [34]. Furthermore, corrosion (see below) may modify an implant’s morphology, produce small particles from the implant, and ultimately reduce long term biocompatibility. Soluble corrosion products can be immunogenic and can also be associated with local cytotoxic and tissue toxicity reactions.
Biomaterials may be classified as inert, interactive, or viable [35]. Inert biomaterials such as cobalt-chromium alloys generate little or no biological response in the absence of wear and corrosion. Titanium and stainless steel are classified as inert biomaterials. Titanium and titanium alloys typically exceed the biocompatibility of stainless steel [36] as well as have lower infection rates over steel [37]. Both titanium and stainless steel alloys commonly used in fracture fixation have very good biocompatibility modulated by an inert, insoluble oxide layer that forms on the surface and is chemically impermeable.
Interactive biomaterials differ in that they are designed to trigger biological responses such as osteoinduction or osteointegration. Viable biomaterials contain a biological component and may be resorbed or biodegraded. Fracture fixation implants are currently most commonly comprised of inert biomaterials, but a construct that can adapt its mechanical properties as a fracture heals may be desired. Early investigation into resorbable plates and screws shows potential in a rising field of research [38, 39].
Biodegradable polymeric biomaterials, such as synthetic polyesters, are emerging as candidates for orthopedic implant applications as biodegradation may eliminate the need for surgical removal of implants. Degradation of synthetic polymeric biomaterials causes foreign-body reactions that are measured using histopathology and currently their immunogenic hazards have little clinical significance [40]. Current research finds biomaterials such as polyetheretherketone (PEEK) to have good strength and radiolucent properties that enable improved visualization of fracture healing by computed tomography and standard radiographs. However, PEEK suffers from inferior fixation strength and stability compared to titanium [41]. Advances in polymer manufacturing and fracture fixation technology may yet have clinical applications in fracture fixation.
4.3 Corrosion
Metallic materials used for fracture fixation in the body are susceptible to chemical attack, termed corrosion. Corrosion is a collection of degradative processes resulting from metals reacting with charged particles in solution. An oxidation reaction in which the metal loses electrons to the surrounding solution starts a corrosive process. Metallic surface particles then either dissolve or form an oxide on the material surface. Corrosion initiation is driven by the thermodynamics of redox reactions and inhibited by kinetic barriers. For a given implant material, the thermodynamics of reduction/oxidation reactions are fixed within a biological milieu. Therefore, a metallic implant’s defense against corrosion is through development of kinetic barriers through passivation. Passivation typically characterizes formation of a passive metal-oxide layer on the outer surface of the metal. A continuous passive film presents a physical obstacle for the ion transfer necessary to begin a corrosive process.
Broadly, corrosion can occur through several main mechanisms described as uniform attack, galvanic corrosion, intergranular corrosion, crevice corrosion, and fretting corrosion [32]. Fracture fixation implants, however, are typically susceptible to galvanic, crevice, and fretting corrosion pathways.
Galvanic corrosion is driven by an electrochemical potential gradient of metals in contact with each other. While a larger problem for modular implants, galvanic corrosion can occur in fracture fixation implants, for example, if the screws are made of a metal with a different electrochemical potential than that of the implant metal. For this reason it is uncommon for a fracture fixation construct to vary in composition between screws and implant.
Stress corrosion cracking is a subset of crevice corrosion in which pits grow on the surface of the material. The ionic microenvironment in pits serves as a barrier between the local implant surface and the surrounding tissues. The net effect of the pit microenvironment is to facilitate corrosion through ionic exchange between the implant metal and nearby tissue. Additionally pit geometry concentrates stress and promotes crack formation. When the stress intensity in a pit reaches the critical value for crack propagation under corrosive conditions, a crack can propagate and cause component failure. Unfortunately, the critical value for crack propagation under corrosive conditions is typically lower than the fracture toughness of the material, making stress corrosion cracking difficult to predict.
Fretting corrosion is corrosive damage resulting from cyclic motion between two opposing surfaces. In this mechanism, grooves and oxide debris develop on the surface as a result of toggling between contacting components. Fretting corrosion exposes the material beneath the passive oxide layer. For example, nonlocking fracture fixation designs are at risk for fretting corrosion whereas locking screws do not experience motion at the screw-plate interface and are less susceptible.
5 Experimental and Computational Modeling of Fracture Fixation Mechanics
The growth of orthopedic fracture knowledge facilitates a shift from hypothesis-based medicine to evidence based medicine. This change in approach accompanies technological developments in computer science, manufacturing, materials research, and mathematical methods which combine to create more accurate laboratory models of interactions between biology and medical devices than past iterations. Medical decisions should be made based on the best known available models which, sometimes, are still clinically observation-based. As research efforts expand to meet the evidence needs of modern fracture fixation, two distinct modalities emerge. Experimental research in this context will refer to physical experiments, often involving mechanical loading, kinematics, kinetics, or chemical phenomena. Computational experiments will encompass those experiments conducted predominantly in silica, although experimental research accompanies them as validation frequently.
Mechanical and biological environments involved in clinical fracture fixation are very complex. Despite substantial advances in lab-based experimental and computational models, clinical studies remain a vital part of modern investigation.
5.1 Experimental
Most lab-based experimental research in fracture fixation biomechanics and biomaterials focuses on mechanical testing and/or bone healing. Mechanical testing involves a direct application of load or displacement and is valuable because it enables direct measurement of a construct’s response. The mechanical testing setup includes a loading apparatus, implant hardware, a fixation mechanism, and a bone model. The most commonly used loading machines are either uniaxial or biaxial standard mechanical testing frames retrofitted with fixtures to accommodate a fracture construct. Implant hardware used during testing is commercially available hardware or, in the case of testing new devices, custom made. Regardless of hardware, most fixation mechanisms selected for testing are those that would be also used in clinical cases analogous to the experimental model. Depending on the research question, the bone model can range in complexity from cylindrical plastic pipe to compound synthetics to human cadaveric specimens . Such studies are typically focused on the ‘time-zero’ mechanical response before any fracture healing occurs, unless the fracture callus or bone healing is artificially simulated. Advanced composite fiber glass, epoxy resin, and polyurethane synthetic bones can adequately represent certain bone mechanical properties, but adequacy in representation of screw or implant fixation and loosening is still controversial. A question regarding specific implant stresses and strains may be answered without the need for expensive cadaveric bone specimens but a question focused on screw-bone fixation or involving a complex fracture pattern may be best answered by recreating the fracture in a true bone in the laboratory setting. These cadaver bones are typically stored frozen, not formalin-fixed because of potential changes in mechanical properties associated with chemical preservation.
Experimental research can be used to test fracture construct performance during functional activities or to simulate traumatic construct yield events. Generally of interest are failure scenarios in the implant, the fixation mechanism, or in the bone. Static loading patterns are effective ways to test yield failure modes, whereas cyclic loads can be applied to simulate fatigue failure. Both failure modes are usually possible in a patient; yield if a patient has an accidental fall or other high load event, and fatigue associated with long term physiological loading, especially if the fracture does not heal quickly and the implants bear larger loads for longer time (Fig. 7). Outcomes such as construct stiffness and strength are commonly measured under loading conditions including axial compression, torsion, bending, or a physiological combination thereof [42]. Motion of bone fragments or implant components during mechanical testing can be measured using infrared cameras, DVRTs, or simple optical measurement methods using a digital camera.
Lab-based experimental research aimed at fracture healing is often conducted in animal models. Animal models can be used to assess the impact of novel fixation constructs or biological therapies on the healing response. For example, a recent study demonstrates a faster and stronger healing response with active plating over conventional compression plating in sheep [43]. Although larger animal models are costly and time consuming they have the ability to simulate a biological environment with similarities to that of a fractured human bone. Animal models can be exposed to loading protocols in custom loading devices to simulate the mechanics of activities with ties to human function [44, 45]. Furthermore, studies in smaller species enable the use of genetic modification to understand mechanotransduction of fracture healing on a cellular and molecular level [46, 47], although the metabolism and anatomy of these animals is often very different than humans, a hurdle to clinical translation.
5.2 Computational
Computational fracture fixation research aims to simulate the mechanical environment of a fixation construct. Due to limitations in computational power and modern characterizations of biological materials it is not yet possible to accurately model all biological, chemical, and physical properties of implant hardware and biological tissue, however these features may have limited impact on specific aspects of construct behavior. Therefore, major assumptions are made to simplify development and run time of computational models of fracture fixation. The most commonly used computational approach is the finite element method .
Computational models from previous studies focus on the effects of varying implant design [48], implant material composition [49,50,51], implant alignment and positioning [52, 53], implant fixation [54,55,56], and bone and fracture characteristics [57,58,59]. Similar to experimental models, computational models often focus on predicting implant failure, fracture gap strain, and implant load transmission. Computational models have an important advantage of being able to predict displacement, stress, and strain throughout all 3D points of a construct, whereas experimental measurements are often limited to selected external points of a construct. Computational models may also simulate the time-dependent effects of fracture healing on local mechanics, a challenging scenario to recreate in an experimental model. Computational models enable comparison of results associated with variation in a single model parameter, which may not be easily facilitated by experimental models, especially if expensive biological specimens are involved. Computational models allow for multiple levels of data interrogation while the physical nature of experimental models limit analysis to specifically measured outcomes. However, computational models depend on user-defined inputs to run simulations whereas experimental models allow for direct measurement of a physical system. A computational model developer must understand and justify all inputs to the model. Experimental models generally can provide better realism, especially when using cadaver tissues, and are thus often used to validate computer models. In some cases though, experimental setups are less accurate such as when measurements are sensitive to boundary conditions that are difficult to precisely control experimentally but not computationally. Both computational and experimental modeling modes can be very time consuming depending on setup and research question.
6 Internal Plating
Internal plating is a common method of treatment for many types of fractures. Plate fixation can provide stability by transferring all of the load across a fracture or by sharing some of the load burden with bone. Depending on the technique of application and design, plates can be used for multiple functions: compression, bridging, buttress, protection, and tension band [60].
Screw fixation between the plate and the bone must have sufficient strength to resist failure. Screws can be locked or compression, uni- or bi- cortical, fixed angle or variable angle, and can be inserted into as many or as few screw holes as desired. Locking screws are threaded into the implant as well as the bone to maintain a gap between the two, whereas conventional screws rely on frictional compression to hold the plate to the bone. Unicortical screws anchor in the cortex closest to the implant and into any trabecular bone present beyond the cortex. Unicortical fixation is necessary around articular joints and pre-existing prostheses, but is more susceptible to loosening over time and may lead to construct failure via screw pullout from the bone [61]. Bicortical fixation is more rigid because the screws pass through the other dense cortex after crossing trabecular bone. Variable angle locking screw holes allow for user-directed angulation of the screw relative to the plate up to ~17°. Varying screw angle can allow the surgeon to accommodate for local anatomy and other implants, or to avoid regions of poor bone quality for screw fixation. Unfortunately variable angle locking plate-screw interfaces are not as strong as standard threaded locking mechanisms [62], presenting an opportunity for additional improvements in plate systems. Additionally, screw holes within a plate can be elastically suspended to enable dynamization of the fracture in what are called active plates. Furthermore, the screw holes in the implant may be designed to be slots instead of circular holes. Slot-shaped screw holes allow the plate to slide around the screw which, like active plating, enables motion at the fracture.
Internal fixation plates are most commonly made of either stainless steel or titanium alloys . It has been shown that stainless plates create a stiffer construct with a longer fatigue life than titanium plates for applications at the distal femur [63]. Other plate materials such as carbon fiber PEEK composites have been shown to have comparable torsional stiffness to stainless steel but inferior failure characteristics for distal fibula fractures [64]. New hybrid materials such as glass/flax/epoxy composites demonstrated higher ultimate strengths than conventional metals in tension, compression, and bending while also having a lower axial stiffness [65], however, they are not yet commonly used in the clinic.
Considering some of the subjective variables involved in internal plating construct implantation (plate length, plate material, number of screws, screw fixation, fracture shape, plate working length, etc.), it is evident that an optimization scheme could facilitate mechanically-informed surgical decisions [66]. Parametric models that iterate construct design variables have been employed to observe the 3D mechanical effects of design changes under axial as well as combined axial, torsional, and bending loads (Fig. 8) [16, 67]. Although the number of possible design variable combinations is exponentially large, consideration of the surgical environment and local anatomy and biology can focus the solution space and eliminate analysis of unlikely or impossible construct designs such as one in which no screws are implanted. Model outputs such as implant and screw stresses and fracture gap strain can also be used to predict construct failure and to observe the mechanical influences of simulated healing. Thus, the utility of such models extends beyond surgical decision support and preoperative planning into training applications for clinicians and implant designers.
Examples of finite element models of subtrochanteric fracture fixation with a lateral plate and screws subject to axial loading, showing effects of screw configuration and fracture gap size on resulting implant stresses (colors) and fracture gap strains. (Right) Callus was represented with springs having various stiffness. Blue represents low stress, red represents high stress. (Data from our laboratory)
7 Intramedullary Nailing
Intramedullary (IM) nailing is a common method of treatment for adult shaft fractures in diaphyseal bones. Nails are commonly used to provide relative stability for secondary bone healing for fractures in the femur, tibia, and sometimes even the humerus. IM nailing entails multiple decision points which may influence the healing course and even success of a fixation construct. For example, the nail implant diameter can be selected to achieve a tight or loose fit within the medullary canal. Additionally, the canal can be reamed to accommodate a larger nail diameter and increase the contact area between the nail and the bone. IM nails may be solid or cannulated. Solid nails were shown to have a lower risk of infection in rabbits [68], whereas the more common cannulated nails may be surgically guided by wire via their cannula during installation. IM nail length can dictate whether or not the nail engages bone in the distal metaphysis for stability. IM nails are often constrained relative to the bone both proximal and distal to the fracture with screws (static locking). Screws confer rotational as well as longitudinal stiffness to the construct.
Some femur nail designs accommodate two screws both proximal and distal to the fracture . A common design to stabilize a proximal femur fracture incorporates a helical blade which embeds within cancellous bone in the femoral head. Another design feature common to femur nails is a dynamic interlocking screw slot. A dynamic screw slot allows for guided translation of the fracture gap along the direction of the slot (inducing desirable axial interfragmentary strain) while still conferring torsional shear stability with bicortical screw fixation. Sometimes screws may not be installed on one side of the fracture to allow for dynamic compression at the fracture site, although care must be taken in these cases to ensure the nail does not perforate the bone on the unfixed side.
The first IM nail implants were made of stainless steel for their strength and biocompatibility. However, stainless steel nails were too stiff to accommodate shape mismatch between the nail and bone. These features were enhanced by a change to a Ti-6AL-7NB titanium alloy which is now standard as a nail material. Titanium nails demonstrate less slipping, more even stress distributions, and increased contact area within the medullary canal over stainless steel nails in a computational model [49]. Furthermore, titanium nails reduce interfragmentary shear motion to better promote fracture healing over stainless steel nails [69]. Another study found that titanium nails are more stable than stainless steel in torsion and axial compression, although both nail materials resisted failure at non-weight-bearing loads [70].
The mechanics of IM nails spanning a fracture are complex. The type and comminution of the fracture dictates how much of the load applied through the bone must pass through the nail. A fully comminuted fracture will require the nail to transfer all of the load across the gap via the locking screws and represents the worst-case scenario in terms of implant load bearing. Other factors that influence nail construct mechanics are the material properties of the nail and screws, the cross sectional shape and anterior bow of the nail, nail diameter, nail length, medullary canal reaming, and screw configuration. For example, it has been shown that longer nails can generate higher contact stresses with the bone medullary canal surface than shorter nails, and that the contact stresses can be mitigated by increasing the flexibility of the distal end of the nail [71]. An experimental model in cadavers indicated that distal screws significantly increase maximum rotational load to failure in unstable intertrochanteric fractures and recommended their use due to the improved torsional strength [72].
Contact within the medullary cavity is a significant mechanism for nail load transfer [73]. The location and area of contact are influenced by nail size and shape, canal reaming, and implant position and orientation. If the contact area is small, the load transferred to the bone is concentrated and can generate high stress. This is thought to be a primary cause of bone pain which some patients report after IM nail implantation. Increasing the area of contact between the implant and the bone may reduce pain through design of the nail cross section and radius of curvature, selecting an appropriate diameter nail for the bone, and canal reaming. It has been shown that an 11 mm-diameter nail with static interlocking reduces motion at the fracture site up to 59% compared with a 9 mm-diameter nail [74]. Nail implant modifications such as diameter, material, and cannulation as well as screw material and area have been shown to reduce interfragmentary shear motion up to 54% [69]. Prior work combining computational and experimental modeling has validated finite element models of an IM nailed femur at four stages of gait and suggested future improvements in implant design and surgical implantation [75].
Research in our laboratory using the finite element method has demonstrated some effects of surgical and implant alterations on IM nail biomechanics (Figure 9). These models utilize idealized bone and implant geometries . Additionally, more realistic bone and IM nail geometries have been modeled for the femur. The femur model was built using Mimics software suite from a patient CT scan. Separate materials are defined to represent cortical bone and adjacent cancellous bone. The nail geometry is generated in Solidworks software using the manufacturer’s design specifications. The nail implant is aligned with the bone to represent surgical positioning and then the bone canal is reamed. The implant and bone models are then imported into Abaqus software for finite element analysis. The models are meshed and boundary conditions, contact constraints, and applied loads are defined. Screws elements are positioned to align with screw holes in the nail and are embedded within the surrounding bone mesh. A fracture can then be modeled by defining a cutting plane and removing elements that intersect the plane. A complex 3D model with physiological boundary conditions and applied loads can provide insight into the location and magnitude of stresses in the implant, screws, and bone (Figure 10). Surgical variables such as nail positioning, bone quality, type and number of fixation screws, applied loads, and fracture type may all be varied to view the influence of these features on resultant stresses.
8 Perspective
Advances in fracture fixation rely heavily on the intersecting disciplines of biomaterials, mechanics, biology, and clinical sciences. The twenty-first century holds promise for advancement in personalized medicine for the field. As computational technologies become more accessible and advanced for medical applications, researchers identify novel challenges that can drive improvements in patient care. Developed computational methods can be used to iterate implant design concepts and suggest geometric, positional, and material variations to achieve better optimization of construct mechanics. Furthermore, the detailed, accurate approximations of mechanics now available from simulated fracture fixation experiments can be used in conjunction with fabrication techniques such as additive manufacturing to create implant concepts that were previously impractical to develop. As the one-implant-fits-all approach loses traction in lieu of case-specific construct designs, it is expected that patient outcomes will continue to improve, resulting in reduced time required for fixation, enhanced healed bone mechanics, and fewer negative outcomes such as residual deformity and implant or fixation failure.
References
Perren S, Buchanan J. Basic concepts relevant to the design and development of the point contact fixator (PC-fix). Injury. 1995;26:1–4.
Runyan C, Gabrick K. Biology of bone formation, fracture healing, and distraction osteogenesis. J Craniofac Surg. 2017;28:1380–9.
Maggiano I, et al. Three-dimensional reconstruction of haversian systems in human cortical bone using synchotron radiation-based micro-CT: morphology and quantification of branching and transverse connections across age. J Anat. 2016;228:719–32.
Cheal EJ, Mansmann KA, DiGioia AM, Hayes WC, Perren SM. Role of interfragmentary strain in fracture healing: ovine model of a healing osteotomy. J Orthop Res Off Publ Orthop Res Soc. 1991;9:131–42.
Levy S, et al. Immature myeloid cells are critical for enhancing bone fracture healing through angiogenic cascade. Bone. 2016;93:113–24.
Duda GN, et al. Interfragmentary motion in tibial osteotomies stabilized with ring fixators. Clin Orthop. 2002;396:163–72.
Garnavos C. Treatment of aseptic non-union after intramedullary nailing without removal of the nail. Injury. 2017;48:S76–81.
Santiago H, Zamarioli A, Neto M, Volpon J. Exposure to secondhand smoke impairs fracture healing in rats. Clin Orthop. 2017;475:894–902.
Sathyendra V, et al. Single nucleotide polymorphisms in osteogenic genes in atrophic delayed fracture-healing: a preliminary investigation. J Bone Joint Surg Am. 2014;96:1242–8.
Damm P, Kutzner I, Bergmann G, Rohlmann A, Schmidt H. Comparison of in vivo measured loads in knee, hip and spinal implants during level walking. J Biomech. 2017;51:128–32.
Kienast B, et al. An electronically instrumented internal fixator for the assessment of bone healing. Bone Jt Res. 2016;5:191–7.
Schneider E, et al. Loads acting in an intramedullary nail during fracture healing in the human femur. J Biomech. 2001;34:849–57.
Schlecht SH, Pinto DC, Agnew AM, Stout SD. Brief communication: the effects of disuse on the mechanical properties of bone: what unloading tells us about the adaptive nature of skeletal tissue. Am J Phys Anthropol. 2012;149:599–605.
Akhter MP, Alvarez GK, Cullen DM, Recker RR. Disuse-related decline in trabecular bone structure. Biomech Model Mechanobiol. 2011;10:423–9.
Miner MA. Cumulative damage in fatigue. J Appl Mech. 1945;12:A159–64.
Wee H, Reid JS, Chinchilli VM, Lewis GS. Finite element-derived surrogate models of locked plate fracture fixation biomechanics. Ann Biomed Eng. 2017;45:668–80.
Lenz M, Perren SM, Gueorguiev B, Höntzsch D, Windolf M. Mechanical behavior of fixation components for periprosthetic fracture surgery. Clin Biomech Bristol Avon. 2013;28:988–93.
Fratzl P, Weinkamer R. Nature’s hierarchical materials. Prog Mater Sci. 2007;52:1263–334.
Cowin S. Pathology of functional adaptation of bone remodeling and repair in vivo. In Bone mechanics handbook. Taylor and Francis Group; 2001.
Comiskey DP, MacDonald BJ, McCartney WT, Synnott K, O' Byrne J. The role of interfragmentary strain on the rate of bone healing: a new interpretation and mathematical model. J Biomech. 2010;43:2830–4.
Augat P, et al. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J Orthop Res. 2003;21:1011–7.
Elkins J, et al. Motion predicts clinical callus formation: construct-specific finite element analysis of supracondylar femoral fractures. J Bone Jt Surg. 2016;98:276–84.
Bottlang M, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92:1652–60.
Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–43.
Augat P, et al. Early, full weightbearing with flexible fixation delays fracture healing. Clin Orthop. 1996;328:194–202.
Schell H, et al. Mechanical induction of critically delayed bone healing in sheep: radiological and biomechanical results. J Biomech. 2008;41:3066–72.
Hente R, Füchtmeier B, Schlegel U, Ernstberger A, Perren SM. The influence of cyclic compression and distraction on the healing of experimental tibial fractures. J Orthop Res. 2004;22:709–15.
Claes L, Eckert-Hübner K, Augat P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J Orthop Res. 2002;20:1099–105.
Lujan TJ, et al. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24:156–62.
Nassiri M, Macdonald B, O’Byrne JM. Computational modelling of long bone fractures fixed with locking plates - how can the risk of implant failure be reduced? J Orthop. 2013;10:29–37.
Beltran MJ, Collinge CA, Gardner MJ. Stress modulation of fracture fixation implants: J. Am Acad Orthop Surg. 2016;24:711–9.
Pruitt LA, Chakravartula AM. Mechanics of biomaterials: fundamental principles for implant design. Cambridge University Press, 2011.
Morehead J, Holt G. Soft-tissue response to synthetic biomaterials. Otolaryngol Clin N Am. 1994;27:195–201.
Nuss KM, von Rechenberg B. Biocompatibility issues with modern implants in bone—a review for clinical orthopedics. Open Orthop J. 2008;2:66–78.
Wright T, Maher S. Biomaterials. In: Orthopaedic basic science 65–85 American Academy of Orthopaedic Surgeons, 2007.
Ungersboeck A, Geret V, Pohler O, Schuetz M, Wuest W. Tissue reaction to bone plates made of pure titanium: a prospective, quantitative clinical study. J Mater Sci Mater Med. 1995;6:223–9.
Arens S, et al. Influence of materials for fixation implants on local infection. J Bone Jt Surg Br. 1996;78:647–51.
Uckan S, Veziroglu F, Soydan SS, Uckan E. Comparison of stability of resorbable and titanium fixation systems by finite element analysis after maxillary advancement surgery. J Craniofac Surg. 2009;20:775–9.
Marasco SF, Liovic P, Šutalo ID. Structural integrity of intramedullary rib fixation using a single bioresorbable screw. J Trauma Acute Care Surg. 2012;73:668–73.
Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21:2615–21.
Schliemann B, et al. PEEK versus titanium locking plates for proximal humerus fracture fixation: a comparative biomechanical study in two- and three-part fractures. Arch Orthop Trauma Surg. 2017;137:63–71.
Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Jt Surg Am. 2009;91:1985–94.
Bottlang M, et al. Dynamic stabilization of simple fractures with active plates delivers stronger healing than conventional compression plating. J Orthop Trauma. 2017;31:71–7.
Piccinini M, Cugnoni J, Botsis J, Ammann P, Wiskott A. Influence of gait loads on implant integration in rat tibiae: experimental and numerical analysis. J Biomech. 2014;47:3255–63.
Gardner MJ, Ricciardi BF, Wright TM, Bostrom MP, van der Meulen MCH. Pause insertions during cyclic in vivo loading affect bone healing. Clin Orthop. 2008;466:1232–8.
Tsuji K, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–9.
McBride-Gagyi SH, McKenzie JA, Buettmann EG, Gardner MJ, Silva MJ. BMP2 conditional knockout in osteoblasts and endothelial cells does not impair bone formation after injury or mechanical loading in adult mice. Bone. 2015;81:533–43.
Helwig P, et al. Finite element analysis of four different implants inserted in different positions to stabilize an idealized trochanteric femoral fracture. Injury. 2009;40:288–95.
Perez A, Mahar A, Negus C, Newton P, Impelluso T. A computational evaluation of the effect of intramedullary nail material properties on the stabilization of simulated femoral shaft fractures. Med Eng Phys. 2008;30:755–60.
Fouad H. Assessment of function-graded materials as fracture fixation bone-plates under combined loading conditions using finite element modelling. Med Eng Phys. 2011;33:456–63.
Feerick EM, Kennedy J, Mullett H, FitzPatrick D, McGarry P. Investigation of metallic and carbon fibre PEEK fracture fixation devices for three-part proximal humeral fractures. Med Eng Phys. 2013;35:712–22.
Favre P, Kloen P, Helfet DL, Werner CML. Superior versus anteroinferior plating of the clavicle: a finite element study. J Orthop Trauma. 2011;25:661–5.
Tupis TM, Altman GT, Altman DT, Cook HA, Miller MC. Femoral bone strains during antegrade nailing: a comparison of two entry points with identical nails using finite element analysis. Clin Biomech Bristol Avon. 2012;27:354–9.
Shih K-S, Hsu C-C, Hsu T-P. A biomechanical investigation of the effects of static fixation and dynamization after interlocking femoral nailing: a finite element study. J Trauma Acute Care Surg. 2012;72:E46–53.
Brown CJ, Sinclair RA, Day A, Hess B, Procter P. An approximate model for cancellous bone screw fixation. Comput Methods Biomech Biomed Engin. 2013;16:443–50.
MacLeod AR, Pankaj P, Simpson AHRW. Does screw-bone interface modelling matter in finite element analyses? J Biomech. 2012;45:1712–6.
Wang H, et al. Accuracy of individual trabecula segmentation based plate and rod finite element models in idealized trabecular bone microstructure. J Biomech Eng. 2013;135:044502.
Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: a comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(1175–1183):1175–83.
Leonidou A, et al. The biomechanical effect of bone quality and fracture topography on locking plate fixation in periprosthetic femoral fractures. Injury. 2015;46:213–7.
Ruedi T, Buckley R, Moran C. AO principles of fracture management. 1. In: AO publishing; 2007.
Lewis G, et al. Tangential biocortical locked fixation improves stability in Vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29:e364–e370.
Tidwell JE, et al. The biomechanical cost of variable angle locking screws. Injury. 2016;47:1624–30.
Kandemir U, et al. Implant material, type of fixation at the shaft and position of plate modify biomechanics of distal femur plate Osteosynthesis. J Orthop Trauma. 2017;1:e241–6. https://doi.org/10.1097/BOT.0000000000000860.
Wilson WK, Morris RP, Ward AJ, Carayannopoulos NL, Panchbhavi VK. Torsional failure of carbon Fiber composite plates versus stainless steel plates for comminuted distal fibula fractures. Foot Ankle Int. 2016;37:548–53.
Manteghi S, Mahboob Z, Fawaz Z, Bougherara H. Investigation of the mechanical properties and failure modes of hybrid natural fiber composites for potential bone fracture fixation plates. J Mech Behav Biomed Mater. 2017;65:306–16.
Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. JBJS. 2007;89:2298–307.
Wee H, Reid J, Lewis G. Parametric and surrogate modeling of internal fixation of femur fractures demonstrate influence of surgical and patient variables. Ann Biomed Eng. 2016;44:3719–49.
Horn J, Schlegel U, Krettek C, Ito K. Infection resistance of unreamed solid, hollow slotted and cannulated intramedullary nails: an in-vivo experimental comparison. J Orthop Res. 2005;23:810–5.
Wehner T, Penzkofer R, Augat P, Claes L, Simon U. Improvement of the shear fixation stability of intramedullary nailing. Clin Biomech Bristol Avon. 2011;26:147–51.
Mahar AT, Lee SS, Lalonde FD, Impelluso T, Newton PO. Biomechanical comparison of stainless steel and titanium nails for fixation of simulated femoral fractures. J Pediatr Orthop. 2004;24:638–41.
Wang CJ, Brown CJ, Yettram AL, Procter P. Intramedullary nails: some design features of the distal end. Med Eng Phys. 2003;25:789–94.
Gallagher D, et al. Is distal locking necessary? A biomechanical investigation of intramedullary nailing constructs for intertrochanteric fractures. J Orthop Trauma. 2013;27:373–8.
Simpson DJ, Brown CJ, Yettram AL, Procter P, Andrew GJ. Finite element analysis of intramedullary devices: the effect of the gap between the implant and the bone. Proc Inst Mech Eng H. 2008;222(333–345):333–45.
Penzkofer R, et al. Influence of intramedullary nail diameter and locking mode on the stability of tibial shaft fracture fixation. Arch Orthop Trauma Surg. 2009;129:525–31.
Cheung G, Zalzal P, Bhandari M, Spelt J, Papini M. Finite element analysis of a femoral retrograde intramedullary nail subject to gait loading. Med Eng Phys. 2004;26:93–108.
Acknowledgments
The authors gratefully acknowledge support from the AO Foundation, Switzerland (Project S-15-196 L), and the National Science Foundation/Penn State Center for Health Organization Transformation. Hwabok Wee, PhD performed many of the finite element simulations shown in figures. We also acknowledge contribution from April D. Armstrong, MD.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Tucker, S.M., Reid, J.S., Lewis, G.S. (2018). Fracture Fixation Biomechanics and Biomaterials. In: Li, B., Webster, T. (eds) Orthopedic Biomaterials . Springer, Cham. https://doi.org/10.1007/978-3-319-89542-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-89542-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89541-3
Online ISBN: 978-3-319-89542-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)