Abstract
Somatic embryogenesis (SE) technology has the potential to be the lowest-cost method to rapidly produce large numbers of high-value seedlings with desired characteristics for plantation forestry. SE is expected to play an important role in the future to increase forest productivity, sustainability and uniformity. SE technology has the advantages of: (1) shortening time to produce desired Planting stock, (2) allowing control of genetic variation, (3) permitting commercial hybrids, and (4) facilitating genetic engineering efforts for desirable traits. Conifer SE proceeds through four steps: initiation, multiplication, maturation and germination and cryopreservation when storage of cultures is desired. This report will focus on the initiation step. When research began, initiation rates for loblolly pine were often below 1%. Early improvements occurred through combinations of optimal embryo stages, half-strength P6 salts, ovule osmotic profile research, modeling activated carbon (AC) uptate of 2,4-D and research to understand the effect of pH and AC on mineral availability. Many improvements in loblolly pine initiation over the past 30 years have resulted from careful study of the developing seed and embryo. Medium supplements and environmental conditions are available to improve imitiation and somatic embryo development that have resulted from analytical studies of seed tissues, the seed environment and gene experssion in the megagametophyte, zygotic embryos and somatic embryos.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Somatic embryogenesis (SE) technology has the potential to be the lowest-cost method to rapidly produce large numbers of high-value seedlings with desired characteristics for plantation forestry . SE is expected to play an important role in the future to increase forest productivity , sustainability and uniformity. SE technology has the advantages of: (1) shortening time to produce desired planting stock, (2) allowing control of genetic variation, (3) permitting commercial production of hybrids, and (4) facilitating genetic engineering efforts for desirable traits.
Since the first reports of somatic embryogenesis in Picea abies and Larix decidua in 1985 (Chalupa 1985; Hackman and von Arnold 1985; Nagmani and Bonga 1985), many different coniferous species have shown the ability to produce embryogenic tissue . At least 27 Pinus species are reported to go through SE (Pullman and Bucalo 2011). However, it should be emphasized that SE only works well with a few species. Often, even for the most responsive species, initiation frequency is low, many desired seed sources are recalcitrant, culture survival is low and/or embryo maturation often stops prematurely resulting in slow initial growth and low germination percentages. These difficulties raise the costs of somatic seedlings produced from successfully initiated genotypes.
Loblolly pine (Pinus taeda L. ) is the most commercially important tree species in the Southeastern US and the second most common species in the US (Nix 2013). One to 1.5 billion trees are planted annually across the Southern USA (Schultz 1999). Since pine plantations in the South are expected to increase both in total area and silvicultural intensity, methods to provide the best planting stock will become increasingly important (Fox et al. 2007; Huggett et al. 2013).
Conifer SE proceeds through four steps: initiation , multiplication, maturation and germination and cryopreservation when storage of cultures is desired (Pullman et al. 2003a). This report will focus on the initiation step. The first report of SE in loblolly pine occurred in 1987 (Gupta and Durzan 1987). Since then many reports and patents on loblolly pine initiation have been published (Pullman and Webb 1994; Becwar and Pullman 1995; Pullman and Johnson 2002; Pullman et al. 2003a, c, d, 2005b, c, 2006, 2008, 2009, 2015; Pullman and Bucalo 2011; Pullman and Bucalo 2014).
As ET grows and somatic embryos develop in vitro, hormonal, nutritional and environmental conditions must be provided by the medium. Therefore, duplication of the seed hormonal, nutritional and environmental conditions found in vivo is likely to improve ET initiation or somatic embryo growth and development.
2 Natural and Somatic Embryogenesis
Natural zygotic embryogenesis starts with a fertilized egg and ends with a germinated plant (Gifford and Foster 1989). Conifer embryos arise from a single fertilization, creating a diploid embryo that develops in a haploid megagametophyte (Dogra 1967; Singh 1978; Nagmani et al. 1995). Conifer embryos grow and develop inside a megagametophyte ‘corrosion cavity ’, a space that enlarges as the suspensor lengthens and pushes the embryo deeper into the seed. Programmed death of cells adjacent to the embryo provides nutrients for growth (Durzan 2012).
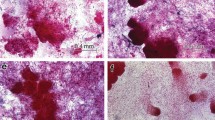
Multiple zygotic embryos usually occur in early-stage seeds of conifers and may form through two processes. In ‘simple embryony’ egg cells in different archegonia are fertilized by different pollen grains forming different genotypes. A process called ‘cleavage polyembryony ’ usually follows in Pinus , where the immature embryos are multiplied. Loblolly pine seeds have 1–4 archegonia, each containing an egg cell (Fig. 1a). Fertilization can occur in one or more archegonia (simple polyembryony). Fertilized embryos in the seed divide into four embryos (cleavage polyembryony ) so that up to 16 embryos may form within each seed (Fig. 1b). After simple or both types of embryony, one embryo becomes dominant and continues to grow (Fig. 1c). Subordinate embryos usually do not develop further but persist briefly in the ovule and appear to be the initiating material for SE in loblolly pine (Becwar et al. 1990, 1991; Becwar and Pullman 1995). MacKay et al. (2001) found that the number of zygotic embryos per seed may be a driver of initiation and could be a useful indicator of initiation potential.
Natural zygotic embryogenesis in Pinus taeda. a Megagametophyte with two archegonia visible shortly after fertilization. b Polyembryony several weeks after fertilization. Multiple early-stage zygotic embryos are visible resulting from simple and or cleavage polyembryony . Double-stained with acetocarmine and Evans blue (Gupta and Holmstrom 2005) . c As development continues, one embryo becomes dominant and the subordinate embryos slowly die. Tissue stained with acetocarmine and Evans blue. Reproduced from Pullman and Bucalo (2014) with permission from Springer
During SE somatic cells from the plant reprogram to form somatic embryos . Hormonal and nonhormonal inducers can be used to promote the somatic embryogenic transition (Fehr 2003). Nonhormonal inducers are often stress factors and include osmotic shock, culture medium dehydration, water stress, heavy metal ions, altered culture medium pH, heat or cold shock, hypoxia, antibiotics, ultraviolet radiation, and some mechanical or chemical treatments (Zavattieri et al. 2010, Fehr 2003). Stress, in particular oxidative stress, appears to be an important initiator of SE (Fehr 2003). 2,4-dichlorophenoxyacetic acid (2,4-D) which is one of the most effective and commonly used initiators of SE appears to function as an oxidative stress activator. 2,4-D may act by increasing auxin activity and simultaneously increasing stress responses (Fehr 2003).
3 Materials
-
A.
Seed (collected at specific developmental stages).
-
B.
Media for P. taeda: initiation (2785, 2880), capture and maintenance (1250). Components are shown in Table 1.
Table 1 Media components for loblolly pine initiation and capture -
C.
Sterilizing solutions: 10% Liqui-Nox with 0.2% Tween 20; 20% H2O2.
-
D.
Chemical reagents: reagent alcohol (70%).
-
E.
Consumable supplies: scalpel blades (sterile), pipettes (10, 50 mL), vacuum filters (0.2 mm, 250 mL), syringe filter (0.2, 13 mm) Costar #3526 Well Culture Cluster Plates and Parafilm.
4 Initiation of Embryogenic Tissue
4.1 Cone and Embryo Stage Collection
Somatic embryos can be grown from immature isolated zygotic embryo explants (Becwar et al. 1990), immature megagametophytes (Pullman and Bucalo 2011; Gupta 2016) or excised mature embryos (Tang et al. 2001). The most success has occurred with immature megagametophytes isolated from immature cones from breeding programs to initiate an ET culture or line. Open or control-pollinated cones are collected in early July when immature embryo stages inside the megagametophyte range from 2 to 4 (Pullman and Webb 1994; Cairney and Pullman 2007; Fig. 2). Cones are shipped on ice, received within 24–48 h and may be stored in plastic bags at 4–5 ℃ for several weeks until processed.
4.2 Initiation Medium Preparation
Medium is prepared, pH adjusted to 5.7 with KOH or HCl after addition of all ingredients except gelling agent and filter-sterilized materials then autoclaved at 121 ℃ for 20 min. Filter-sterilized solutions of L-glutamine, 24-epibrassinolide (E1641, Sigma-Aldrich, St. Louis, MO or E244, PhytoTechnology Laboratories, Showcase Mission, KS), and ABA are added to medium cooled to about 55 ℃. Acid-washed tissue culture tested activated carbon (AC) (C9157) is purchased from Sigma-Aldrich. Epibrassinolide stock solutions are prepared in absolute ethanol (Aaper Alcohol and Chemical Co.). Solubility is about 3 mg/ml and care should be taken to minimize ethanol medium content to avoid reduced ET growth.
4.3 Seed Sterilization and Explant Preparation
Cones are cut open, and seeds removed, washed in running cold tap water for 10 min, agitated in 10% Liqui-Nox (detergent) with 2 mL Tween 20/L for 10 min, and rinsed in running tap water for 30 min. Seeds are agitated aseptically in 20% H2O2 for 10 min and rinsed five times for 5 min with sterile deionized water (Pullman et al. 2005c, 2015).
4.4 Aseptic Dissection, Explant Placement and Liquid Overlay Addition
The seed coat, integuments and nucelli are removed. The megagametophyte containing the embryo(s) is placed onto 2 mL of initiation medium 2785 contained in individual wells of Costar #3526 Well Culture Cluster Plates. Plates are wrapped in two layers of Parafilm and incubated at 23–25 ℃ in the dark. After 14 days, 0.25 mL of medium 2880 (Table 1) is added (Pullman and Skryabina 2007; Pullman et al. 2015). The liquid overlay contains fresh medium, ABA , reduced NAA and functions to refresh medium contents, adjust pH, and expose extruding tissue to ABA .
4.5 Embryogenic Tissue Evaluation
Multiple points of ET initiation are often present on an explant. A typical sequence of initiation from immature zygotic embryos is shown in Fig. 3 and described in more detail by Becwar and Pullman (1995). P. taeda initiation occurs in three steps: extrusion at 1–4 weeks when most often subordinate zygotic embryos expand out of the megagametophyte micropylar end; cell proliferation and formation of a mass of ET (embryo suspensor mass). Initiation is evaluated after nine weeks and ET is transferred to medium 1250 (Table 1).
Typical sequence of embryogenic tissue initiation in loblolly pine . Reproduced from Pullman et al. (2003d) with permission from Springer
4.6 Embryogenic Tissue Capture and Maintenance
Tissue weights are tracked over three two-week subcultures on medium 1250, and an initiation is considered “captured” when it reaches 200 mg. A target weight of 200 mg was selected based on observations where captured cultures reaching this mass tended to continue growth while cultures of less weight had a greater chance of growth decline. About half of the new initiations reach 200 mg. The remaining 50% do not grow although ET formed but stopped growth within several months. During capture and maintenance transfers ET clumps are kept small (about 0.5 cm diameters) to maximize surface area where tissue grows most rapidly. Old, brown and dying ET in the center of larger clumps should be removed along with non-ET forming hard or green callus . This selection process is important to maintain ET as the culture ages.
5 Discussion
When research began, initiation rates for loblolly pine were often below 1%. Early improvements occurred through combinations of optimal embryo stages, half-strength P6 salts, ovule osmotic profile research, modeling AC uptake of 2,4-D and research to understand the effect of pH and AC on mineral availability (Teasdale et al. 1986, Pullman and Johnson 2002). Many improvements in loblolly pine initiation over the past 30 years have resulted from careful study of the developing seed and embryo (Pullman and Bucalo 2014, Xu et al. 1997, Cairney et al. 1999, 2000). Medium supplements and environmental conditions are available to improve ET initiation and somatic embryo development that have resulted from analytical studies of seed tissues, the seed environment and gene expression in the megagametophyte , zygotic embryos and somatic embryos .
Choice of explant. Immature and mature zygotic embryos have been used to initiate loblolly pine ET (Becwar et al. 1990; Becwar and Pullman 1995; Tang et al. 1998; Tang et al. 2001; Gupta 2016). However, initiation using whole megagametophytes containing optimum embryo stages has been the explant of choice due to ease of dissection. ET initiation has been found to correlate highly with the immature embryo stage within the megagametophyte and greatest initiation occurring from precotyledonary embryos at stages 2–4 (Becwar et al. 1990; Pullman and Johnson 2002; Pullman et al. 2003a, b, d). The staging system of Pullman and Webb (1994) is used to evaluate zygotic and somatic embryos . This system helps to understand variation in stage due to mother tree, location and time. Cone cold storage (4°) also can assist in obtaining target stages by allowing stage 1 embryos to slowly grow while in storage.
Initiation of embryogenic tissue. Extruded zygotic embryos have the same appearance as somatic embryos and cannot easily be distinguished except by observations of continued growth. Researchers have occasionally mistaken the zygotic extrusion process for ET and reported high initiation rates. Successful initiations will show ET forming a mass of proliferating cells and embryos that increase over time originating from the extruded zygotic embryos .
The ET frequently initiates from cell division and proliferation in the suspensor region near the interface of the suspensor cells and the embryo proper (sometimes called the embryo head) (Becwar et al. 1991; Becwar and Pullman 1995). The terms”embryonal suspensor masses” and “somatic polyembryogenesis” have been used to describe, respectively proliferating embryogenic cultures of loblolly pine and other conifers, and the in vitro embryo formation process in cultures (Gupta and Durzan 1987). Embryogenic tissue can be initiated from both dominant and subdominant zygotic embryos so that a culture may contain more than one genotype (Becwar et al. 1991).
Recently an interesting hypothesis was reported that ET from subordinate embryos undergoing cleavage embryony after the dominant embryo has formed may be inferior to ET developed prior to dominant embryo formation (Klimaszewska et al. 2007; Abrahamsson et al. 2017). ET lines from subordinate embryos may carry forward degeneration patterns resulting from the beginning of programmed cell death that cause abnormalities in subsequent cotyledonary embryo development . Gupta (2016) recently reported a method for initiation from megagametophytes prior to dominant embryo formation that showed high initiation rates and may overcome this problem. In this method megagametophytes collected shortly after fertilization prior to dominant embryo formation were simply dissected about one-eighth from the micropylar end and cultured on initiation medium. Further studies are needed to understand and compare the effects on embryo development of initiation and cleavage polyembryony from subordinate, predominant and dominant embryos.
Maternal and paternal effects on initiation. Paternal and maternal effects on initiation in loblolly pine were examined by performing a diallel mating and following the extrusion and initiation frequencies of the resulting families, compared with open pollinated families (MacKay et al. 2006). Using reciprocal crosses (A × B and B × A), both mother-tree and pollen parent had significant effects on initiation frequency (Mackay et al. 2006). One tree, which was recalcitrant in culture as a mother tree, produced high initiation rates when used as a pollen parent. Certain mother trees gave high initiation with all of the pollen parents. The work showed initiation could be increased 46% by careful selection of mother and father parent trees.
Plant hormones and plant growth regulators. Six groups of plant hormones and plant growth regulators (PGRs) that function together to regulate plant growth and development were examined. All are known to be present in conifer seed tissues during early seed development.
Abscisic acid. Abscisic acid (ABA ) is well known to regulate zygotic and somatic embryo maturation in both angiosperms and gymnosperms (Rai et al. 2011). ABA is produced by the megagametophyte and moves to the developing embryo. When the megagametophyte is absent in vitro, ABA must come from the medium. Kapik et al. (1995) measured ABA levels in loblolly pine zygotic tissues using an indirect ELISA method. When calculated on a micromole basis, peaks occurred in mid-development and during late embryo development . However, the presence of ABA throughout embryo development including early stages suggested ABA may improve ET initiation . Several research groups tested this hypothesis in P. taeda and other species and found increased initiation when ABA was added to the medium (Aitken-Christie and Parkes 1996; Handley 1997, 1999; Pullman and Skryabina 2007; Pullman et al. 2003c, 2009, 2016). The addition of 3.7 µM ABA , 20 µM AgNO3 (see ethylene section) and optimization of cytokinin levels almost tripled initiation across 32 seed families (Pullman et al. 2003c). ABA also increased loblolly pine ET growth in maintenance medium and after retrieval of cryopreserved cultures (Becwar and Krueger 2004; Pullman et al. 2005b).
Auxins and cytokinins. Optimal concentrations of auxins and cytokinins in the form of man-made PGRs are usually determined through empirical tests or adopted from the literature. NAA at 2 mg/l was found to work well for loblolly pine (Pullman and Johnson 2002). Cytokinin concentrations were optimized at 0.63 mg/l BAP and 0.61 mg/l kinetin in the presence of 50 mg/l AC (Pullman et al. 2003c).
Brassinosteroids . Brassinosteroids (BRs) were discovered recently and are involved in numerous plant processes. BRs in seeds have diverse tissue-specific and species-specific effects on cell elongation, division and differentiation, reproductive biology, senescence, the stimulation of ethylene production, and an increase in resistance to abiotic stress (Brosa 1999; Clouse and Sasse 1998; Clouse 2001). With analytical information that BRs are found in gymnosperms including seeds, tests found increased ET initiation when media was supplemented with brassinolide or 24-epibrassinolide (Pullman et al. 2003d; Malabadi and Nataraja 2007; Pullman et al. 2009; Ma et al. 2012; Pullman et al. 2016). Brassinolide at 0.1 µM improved initiation rates in loblolly pine from 15.0 to 30.1%, increased weight of loblolly pine ET tissue by 66% and stimulated initiation in recalcitrant families (Pullman et al. 2003d). Recently brassinolide has been difficult to obtain and 2.0 µM 24-epibrassinolide has been substituted (Pullman et al. 2015).
Ethylene. Ethylene can be produced by almost all parts of plants and is known to have significant effects on plant growth in vitro. Ethylene may act as either a growth promoter or inhibitor depending on the species (Biddington 1992). Ethylene was shown to reduce somatic ET growth in Picea glauca suspension cultures (Kumar et al. 1989). Preliminary analytical tests showed presence of ethylene in our culture containers (Pullman et al. 2003c). We therefore tested for effects of ethylene and ethylene inhibitors on loblolly pine initiation . Several reports have shown improved embryogenesis when silver nitrate , a strong ethylene action inhibitor, was added to the medium (Beyer 1976; Auboiron et al. 1990; Roustan et al. 1989, 1990; Li and Huang 1996). When 20 µM silver nitrate was added to the medium, loblolly pine ET initiation increased (Pullman et al. 2003c). It should also be noted that AC, also present in the medium, functions as an ethylene adsorbent (Thomas 2008).
Gibberellins. Gibberellins (GAs) are present in fruits and seeds and have been reported to both increase and decrease SE in angiosperms (Rademacher 2000; Rudus et al. 2000). Because GAs are known to be present in conifer seeds (Kong et al. 1997) we hypothesized that GAs may improve ET initiation for loblolly pine . In our first experiment the opposite occurred, GA3 decreased ET initiation . With reduced initiation from added GA3 and increased initiation with added ABA (Pullman et al. 2003c), we hypothesized reductions in endogenous GAs content to decrease the GA: ABA ratio would improve initiation . Paclobutrazol , an inhibitor of a reaction in the gibberellin synthesis pathway, improved ET initiation for loblolly pine , slash pine (Pinus elliottii), Douglas fir (Pseudotsuga menziesii) and Norway spruce (P. abies) (Pullman et al. 2005c). Using 0.33–1.0 mg/l paclobutrazol , initiation percentages in loblolly pine were improved from 37.7 to 44.2%. Other gibberellin inhibitors , effective at different points in the gibberellin pathway also showed statistically significant increases in ET initiation (Pullman et al. 2005c). Studies on meristem cells show GAs are excluded or kept low in meristem initials and may need to be low for formation of somatic embryos (Sakamoto et al. 2001). Paclobutrazol (95.8% active ingredients, Duchefa, Netherlands) stock solution slurries of 1 mg/ml are vortexed and rapidly added to the medium prior to autoclaving. Paclobutrazol has a solubility of 35 mg L−1. Paclobutrazol is not added to the current recommended initiation medium but may be useful for recalcitrant seed sources.
Nutritional components. The conifer embryo grows and develops within the megagametophyte in a corrosion cavity where secreted fluids nourish the embryo (Carman et al. 2005). Nutritional components of the megagametophyte or more finely, the embryo-megagametophyte interface, are of interest to help develop stage-specific SE growth media. The nutritional components and their stage-specific physiological concentrations are slowly becoming known.
Minerals. Teasdale et al. (1986) used mineral analysis of loblolly pine seed to formulate P6 medium for non-embryogenic suspension cultures of loblolly pine . The medium generally contained high concentrations of micronutrients and magnesium and low calcium. Hi iodide, borate and zinc were found to be beneficial to growth. Half-strength P6 salts has worked well for loblolly pine ET initiation and is used in our research. Pullman and Buchanan (2003) analyzed stage-specific P. taeda embryo and megagametophyte tissues for 14 key metals. The analytical data assisted in medium development for embryo maturation (Pullman et al. 2003b). Loblolly pine initiation has also been reported using other salt recipes: DCR (Becwar et al. 1990), WV5 (Coke 1996), LOB (Tang et al. 1998) and TX (Denchev et al. 2011).
Organic acids . Organic acids are important in plant metabolism and can occur in large amounts as free anions altering tissue water potential (Taiz and Zeiger 2010). Several organic acids are present in all plants in the citric acid cycle. When 26 organic acids were analyzed in loblolly pine seed tissues, five showed statistically significant increases in early-stage somatic embryo growth when added to medium at approximate physiological concentrations (Pullman and Buchanan 2006; Pullman et al. 2006). α-ketoglutaric acid, pyruvic acid and succinic acid improved ET initiation when alone or combined. The combination of these three amino acids and vitamins B12 and E showed 36.3% ET initiation across four loblolly pine seed sources compared to 27.3% initiation in a control medium.
Sugars. Carbohydrates play important roles providing energy and carbon for biosynthesis, as osmotic agents, in seed desiccation and cold tolerance, and as developmental regulators controlling gene expression (Iraqui and Tremblay 2001). Carbohydrates can accumulate in large amounts in seeds as deposited or dissolved free molecules. Pullman and Buchanan (2008) analyzed loblolly pine stage-specific embryo and megagametophyte tissues for starch and 18 sugars. When 17 sugars were screened at approximate physiological concentrations for effect on early-stage somatic embryo growth, D-xylose or D-chiro-inositol increased growth (Pullman et al. 2008). Medium supplementation with D-xylose or D-chiro-inositol increased loblolly pine initiation averages by +6.5% or +7.3%, respectively. Profiles of maltose showed high concentrations during early embryo development with a disappearance as a major shift in embryo development occurred after stage 9.1. This observation supported use of maltose as the main carbon source for initiation (Pullman and Johnson 2002). While not present in loblolly pine seeds, lactose increased ET culture initiation when used as a carbon source (Denchev et al. 2011).
Vitamins. Vitamins function as cofactors for essential metabolic reactions. Thiamine hydrochloride (Vitamin B1), pyridoxine (Vitamin B6), and nicotinic acid (niacin) are often present in plant tissue culture media and are common in conifer SE media. Benefits can occur from use of other vitamins including ascorbic acid (Vitamin C), biotin (Vitamin H), choline chloride (Vitamin B4), cyanocobalamin (Vitamin B12), folic acid (Vitamin M), pantothenic acid (Vitamin B5), para-aminobenzoic acid, riboflavin (Vitamin B2) or tocopherol (Vitamin E) (Bourgin and Nitsch 1967; Kao and Michayluk 1975; Dodds and Roberts 1995). When organic acids were profiled in seed tissues (Pullman and Buchanan 2006), ascorbic acid and nicotinic acid were also found in early embryo stages. With this observation, Pullman et al. (2005b, 2006) tested mixtures of biotin and folic acid or nine vitamins for effect on growth of early-stage somatic embryos . Biotin, folic acid, Vitamin B12 and Vitamin E alone or combined increased growth and prompted tests on ET initiation . These vitamins alone or combined increased ET initiation (Pullman et al. 2005b, 2006). Initiation increased from 22.5 to 38.5% using 12 loblolly pine families and medium supplemented with 2(n-morpholino)ethanesulphonic acid (MES, see pH section below), biotin and folic acid (Pullman et al. 2005b).
Duplication of physical seed conditions in vivo. Internal seed conditions other than nutrition and hormonal factors can influence embryo growth and development. Gas concentrations of O2, CO2 and ethylene, movement of water, H+ concentration (pH), redox potential and dynamics of nutrients, hormones and waste products are a few of the factors likely to affect seed and embryo development and ET initiation .
Water potential. Water potential (Ψ) conditions appear to control embryo development for many plant species (Bradford 1994). Ψ can be used to describe the tendency of water to move from areas of higher Ψ to areas of lower Ψ. While moving, water may carry dissolved nutritional components and thus regulate solute availability to the megagametophyte and developing embryos. Water relation parameters have been partially investigated for zygotic and somatic embryos of P. taeda (Dumont-BeBoux et al. 1996; Pullman 1997; Pullman and Johnson 2009b). These investigations showed that seed tissue Ψ values were much greater (measured in mmol/kg) than that measured in typical initiation media. This suggested that medium supplementation with osmoticants may improve initiation . Indeed, supplementation of initiation medium with 22.2 mM myo-inositol increased extrusion and proliferation (Li and Huang 1996) and 111 mM myo-inositol, raising medium osmolality about 120–130 mmol/kg, resulted in statistically significant increases in ET initiation (Pullman and Johnson 2002).
Activated carbon . AC is used in many tissue culture media. Benefits of AC are not well understood but may occur from adsorption of medium residual hormones, plant waste products, and toxic metabolites such as phenolic compounds, 5 hydroxy methyl-furfural and ethylene (Pan and van Staden 1998; Thomas 2008). Benefits may also occur from changes in medium nutrient and hormone dynamics as AC adsorbs component(s) or from change in endogenous hormones. Von Aderkas et al. (2002) quantified eight PGRs in ET of larch grown in media with or without 1% AC. AC caused a statistically significant increase in endogenous auxin.
Since AC may adsorb 95–99% of the hormones and PGRs present in medium, Pullman and Johnson (2002) tested initiation for loblolly pine on media with greatly increased PGRs combined with 2.5 g L−1 AC. Increased extrusion occurred when AC was added; however, only a few initiations resulted. Toering and Pullman (2005) tracked availability of radio-labeled 2,4-D in media. After adsorption, media with 2.5 g L−1 AC and 220 mg L−1 2,4-D still contained too much 2,4-D with 12–17 mg L−1 available during much of the initiation period. Two approaches were suggested to improve initiation : (1) lower 2,4-D from 220 to 110 mg L−1 with 2.5 g L−1 AC; or (2) greatly reduce AC and combine with standard or slightly raised PGR levels similar to levels in media without AC. The second approach worked well when 50 mg/l AC was used and CuSO4 5H2O was raised (Pullman and Johnson 2002). The high AC likely created a deficiency in Cu+2 by adsorbing most of the copper (Pullman and Johnson 2002; Van Winkle et al. 2003). Additional medium component adsorption studies have helped to develop effective media (Ebert and Taylor 1990; Nissen and Sutter 1990; Ebert et al. 1993; Pan and van Staden 1998; Van Winkle et al. 2003; Van Winkle and Pullman 2003, 2005; Toering and Pullman 2005; Pullman et al. 2005a).
pH. H+ concentration (pH) controls many chemical reactions. Pullman and Johnson (2009a) measured pH of loblolly pine seed tissues. Megagametophytes measured pH 5.5 shortly after fertilization, about 6.1 at mid-development and 6.3–6.5 during late development. In contrast, embryo pH remained nearly constant at 7.0. Based on a logarithmic scale, a pH difference of 1.0 equals a tenfold difference in H+ concentration. Measurements of pH 5.5 around the early-stage embryo suggested initiation media should target the same pH. Medium pH is known to change during tissue growth, dropping from ammonium usage and increasing from nitrate usage (Minocha 1987; Lulsdorf et al. 1992; Pullman et al. 2005b). Change in pH may also alter availability of ions and PGRs. Measurements of low medium pH at 4–4.5 during initiation suggested that maintaining the target pH may improve initiation . MES pH buffer agent and liquid medium added after 14 days provided pH control and increased initiation (Pullman et al. 2005b; Pullman and Skryabina 2007).
Liquid medium. Nutritional and hormonal components are delivered to the developing embryo by a surrounding aqueous film. Liquid medium advantages often include faster growth rates, lower variation, better visualization of tissues, and automation of cell suspension transfer. Adsorption of medium components differs in gelled vs. liquid media suggesting diffusion rates differ when medium is gelled (Ebert and Taylor 1990; Pullman et al. 2005a). Loblolly pine initiation media required reduction in NAA from 2 mg/l in gelled medium to 0.3 mg/l in liquid medium (Pullman and Skryabina 2007). Liquid overlays can be easily added to growing tissue to adjust pH, refresh components and/or add a new ingredient. When pH declined below target levels, liquid overlays added after 14 days containing 0.3 mg/l NAA brought pH back to desired levels and improved initiation +8.5% for high-value control-pollinated seed sources and +6.5 to +9.9% for open-pollinated and often recalcitrant seed sources (Pullman and Skryabina 2007).
Redox potential. Glutathione (GSH, reduced form)/glutathione disulfide (GSSG, oxidized form) and ascorbic acid (reduced form)/dehydroascorbate (oxidized form) are major redox pairs that control redox-state in a developing seed. Early-stage embryo development appears to occur best in a reducing environment while late-stage development occurs best in a more oxidizing environment (Stasolla 2010). Redox potential has been shown to modify embryo development in several plants including white spruce and the ratio of GSH: GSSG seems to be more important than the actual amounts of GSH and GSSG (Yeung et al. 2005).
Glutathione appears to be essential for SE, as silencing GSH biosynthetic pathways in wheat inhibited SE (Bossio et al. 2013). Expression of HBK3, a major embryogenesis control gene required for differentiation of proembryogenic masses in P. abies somatic embryos , was associated with ascorbate and glutathione metabolism (Belmonte and Stasolla 2009).
Pullman et al. (2015) found ASC and GSH in loblolly pine megagametophyte or zygotic embryos at low concentrations during stage 1, but DHA and GSSG were not present at all or were barely detectable. In vitro early-stage somatic embryo growth during ET initiation or maintenance may therefore benefit from addition of ASC, GSH or other non-toxic reducing agents.
Because high costs of GSH may prohibit its use, Pullman et al. (2015) investigated effects of low-cost anti-oxidants on ET growth or initiation . Sodium dithionite and sodium thiosulfate were effective reducing agents and increased early-stage somatic embryo growth and ET initiation for P. taeda and ET initiation for P. menziesii. Reducing agents increased loblolly pine initiation averages by 8–99% and P. menziesii initiation by 5–30% in trials over four years. Ascorbic acid, a combination of vitamins including the anti-oxidant tocopherol (vitamin E), or GSH increased P. glauca, P. taeda or Araucaria angustifolia ET proliferation or initiation (Stasolla and Yeung 1999; Pullman et al. 2006; Vieira et al. 2012).
5.1 Concluding Remarks
The loblolly pine initiation medium and practices presented were developed over 30 years. Many of the improvements were based on analytical studies of P. taeda developing seed, embryos and seed tissues. Most of the improvement concepts that have increased initiation in loblolly pine have also been shown to increase initiation for other species and therefore show promise for general use with coniferous species.
References
Abrahamsson M, Valladares S, Merino I, Larsson E, von Arnold S (2017) Degeneration pattern in somatic embryos of Pinus sylvestris L. In Vitro Cell Dev Biol-Plant 53:86–96
Aitken-Christie J, Parkes BD (1996) Improved embryogenesis process for initiation and maturation. International application under the patent cooperation treaty (PCT). WO 96/37096, International publication date: 28 November 1996
Auboiron E, Darron MP, Michaux-Ferriere N (1990) Influence of atmospheric gases, particularly ethylene, on somatic embryogenesis of Hevea brasiliensis. Plant Cell Tiss Org Cult 21:31–37
Becwar MR, Blush TD, Brown DW, Chesick EE (1991) Multiple paternal genotypes in embryogenic tissue derived from individual immature loblolly pine seeds. Plant Cell Tiss. Org. Cult. 26:37–44
Becwar MR, Krueger SA (2004) Recovering cryopreserved embryogenic cultures. US Patent 6,682,931, issued 27 Jan 2004
Becwar MR, Nagmani R, Wann SR (1990) Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda). Can J For Res 20:810–817
Becwar MR, Pullman GS (1995) Somatic embryogenesis in loblolly pine (Pinus taeda L.). In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants—Gymnosperms, vol 3. Dordrecht, The Netherlands, pp 287–301
Belmonte MF, Stasolla C (2009) Altered HBK3 expression affects glutathione and ascorbate metabolism during the early phases of Norway spruce (Picea abies) somatic embryogenesis. Plant Physiol Biochem 47:904–911
Beyer EM (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58:268–271
Biddington NL (1992) The influence of ethylene in plant tissue culture. Plant Growth Regul 11:173–187
Bossio E, Paleo AD, del Vas M, Baroli I, Acevedo A, Rios RD (2013) Silencing of the glutathione biosynthetic pathway inhibits somatic embryogenesis in wheat. Plant Cell Tiss Org Cult 112:239–248
Bourgin JP, Nitsch JP (1967) Obtention de Nicotiana haploids a partir d’etamines cultivees in vitro. Ann Physiol Veg 9:377–382
Bradford KJ (1994) Water stress and the water relations of seed development: a critical review. Crop Sci 34:1–11
Brosa D (1999) Biological effects of brassinosteroids. Crit Rev Biochem Mol Biol 34:339–358
Cairney J, Pullman GS (2007) The cellular and molecular biology of conifer embryogenesis. New Phytol 176:511–536
Cairney J, Xu N, Pullman GS, Ciavatta VT, Johns B (1999) Natural and somatic embryo development in loblolly pine: gene expression studies using differential display and cDNA arrays. Appl Biochem Biotechnol 77–79:5–17
Cairney J, Xu N, MacKay J, Pullman G (2000) Transcript profiling: a tool to assess the development of conifer embryos. In Vitro Cell Dev Biol-Plant 36:155–162
Carman JG, Reese G, Fuller RJ, Ghermay J, Timmis R (2005) Nutrient and hormone levels in Douglas-fir corrosion cavities, megagametophytes, and embryos during embryony. Can J For Res 35:2447–2456
Chalupa V (1985) Somatic embryogenesis and plantlet regeneration from cultured immature and mature embryos of Picea abies (L.) Karst. Commun Inst For Chech 14:57–63
Clouse SD (2001) Brassinosteroids. In: Somerville CR, Meyerowitz EM (eds) The arabidopsis book. American Society of Plant Biologists, Rockville, Md. http://www.aspb.org/publications/arabidopsis/
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49:427–451
Coke JE (1996) Basal nutrient medium for In Vitro cultures of loblolly pines. U.S. Patent 5,534,434, issued 9 July 1996
Denchev P, Attree SM, Kong L, Tsai C, Radley RA, Lobatcheva II (2011) Method for reproducing conifers by somatic embryogenesis using lactose as a carbon source. U.S. Patent 7,906,334, issued 15 Mar 2011
Dodds JH, Roberts LW (1995) Experiments in plant tissue culture, 3rd edn. Cambridge University Press, London
Dogra PD (1967) Seed sterility and disturbances in embryogeny in conifers with particular reference to seed testing and tree breeding in Pinaceae. Studia Forestalia Suecica 45:5–97
Dumont-BeBoux N, Mazari A, Livingston NJ, von Aderkas P, Becwar MR, Percy RE, Pond SE (1996) Water relations parameters and tissue development in somatic and zygotic embryos of three pinaceous conifers. Am J Bot 83:992–996
Durzan D (2012) Interpolated apomictic somatic embryogenesis, androsporogenesis, asexual heterospory, mitosporogenesis and genomic silencing in a gymnosperm artificial sporangium. In: Proceedings of the IUFRO working party 2.09.02 conference “Integrating vegetative propagation, biotechnologies and genetic improvement for tree production and sustainable forest management” 25–28 June 2012, Brno, Czech Republic, pp 3–36
Ebert A, Taylor HF (1990) Assessment of the changes of 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tis Org Cult 20:165–172
Ebert A, Taylor F, Blake J (1993) Changes of 6-benzylaminopurine and 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tiss Org Cult 33:157–162
Fehr A (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org Cult 74:201–228
Fox TR, Jokela EJ, Allen HL (2007) The development of pine plantation silviculture in the southern United States. J For 105:337–347
Gifford EM, Foster AS (1989) Morphology and evolution of vascular plants, 3rd edn. W.H. Freeman, New York, NY, USA
Gupta PK (2016) Methods of initiating plant somatic embryos. U.S. Patent 9374954, issued 28 June 2016
Gupta PK, Durzan DJ (1987) Biotechnology of somatic polyembryogenesis and plantlet regeneration in loblolly pine. Bio/Technol 5:147–151
Gupta PK, Holmstrom D (2005) Double staining technology for distinguishing embryogenic cultures. In: Jain SM, Gupta PK (eds) Protocol for somatic embryogenesis in woody plants. Springer, The Netherlands, pp 573–575
Hackman I, von Arnold S (1985) Plantlet regeneration through somatic embryogenesis in Picea abies (Norway spruce). J Plant Physiol 121:149–158
Handley L III (1997) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. U.S. Patent 5,677,185, issued 14 Oct 1997
Handley L III (1999) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. U.S. Patent 5,856,191, issued 5 Jan 1999
Huggett R, Wear DN, Li R, Coulston J, Liu S (2013) Forecasts of forest conditions. Ch. 5. In: Wear DN, Greis JG (eds) The southern forest futures project: technical report, USDA For. Serv. Gen. Tech. Rep. SRS-178, Southern Research Station, Asheville, NC
Iraqui D, Tremblay FM (2001) Analysis of carbohydrate metabolism enzymes and cellular contents of sugars and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J Exp Bot 52:2301–2311
Kao KN, Michayluk MR (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 126:105–110
Kapik RH, Dinus RJ, Dean JF (1995) Abscisic acid and zygotic embryogenesis in Pinus taeda. Tree Physiol 15(485–409):1995
Klimaszewska K, Trontin JF, Becwar MR, Devillard C, Park YS, Lelu-Walter MA (2007) Recent progress in somatic embryogenesis of four Pinus spp. Tree For Sci Biotechnol 1:11–25
Kong L, Attree SM, Fowke LC (1997) Changes in endogenous hormone levels in developing seeds, zygotic embryos and megagametophytes in Picea glauca. Physiol Plant 101:23–30
Kumar PP, Richard WJI, Thorp TA (1989) Ethylene and carbon dioxide accumulation, and growth of cell suspension cultures of Picea glauca (white spruce). J Plant Physiol 135:592–596
Li XY, Huang H (1996) Induction of somatic embryogenesis in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol-Plant 32:129–135
Lulsdorf MM, Tautorus TE, Kikcio SI, Dunstan DI (1992) Growth parameters of embryogenic suspension cultures of interior spruce (Picea glauca-engelmannii complex) and black spruce (Picea mariana Mill.). Plant Sci 82:227–234
Ma X, Bucalo K, Determann RO, Cruse-Sanders JM, Pullman GS (2012) Somatic embryogenesis, plant regeneration and cryopreservation for Torreya taxifolia, a highly endangered coniferous species. In Vitro Cell Dev Biol-Plant 48:324–334
MacKay J, Becwar M, Park Y, Perfetti C, Cordero J, Lockart L, Pullman GS (2006) Genetic control of somatic embryogenesis initiation in loblolly pine and implications for breeding. Tree Genet Genomes 2:1–9
MacKay J, Becwar M, Park YS, Perfetti C, Corderro J, Pullman GS, Lockhart L (2001) Genetics of somatic embryogenesis in loblolly pine. In: Dean JF (ed) Proceedings (Publ. No. 48) of the 26th southern forest tree improvement conference, University of Georgia, Athens, GA, USA, 26–29, June 2001, pp 40–47
Malabadi R, Nataraja K (2007) 24-Epibrassinolide induces somatic embryogenesis in Pinus wallichiana A. B. Jacks. J Plant Sci 2:171–178
Minocha SC (1987) PH of the medium and the growth and metabolism of cells in culture. In: Bonga JM, Durzan DJ (eds) Cell and tissue culture in forestry, vol 1. Martinus Nijhoff, Boston, pp 125–141
Nagmani R, Bonga JM (1985) Embryogenesis in subcultured callus of Larix decidua. Can J Res 15:1088–1091
Nagmani R, Diner AM, Garton S, Zipf AE (1995) Anatomical comparison of somatic and zygotic embryogeny in conifers. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 1. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 23–48
Nissen SJ, Sutter EG (1990) Stability of IAA and IBA in nutrient medium to several tissue culture procedures. HortScience 25:800–802
Nix S (2013) Ten most common trees in the United States. http://forestry.about.com/b/2012/07/21/ten-most-common-trees-in-the-united-states.htm. Accessed 12 Jan 2017
Pan MJ, van Staden J (1998) The use of charcoal in In Vitro culture—a review. Plant Growth Regul 26:155–163
Pullman GS (1997) Osmotic measurements of whole ovules during loblolly pine embryo development. In: TAPPI biological sciences symposium, San Francisco, CA, TAPPI Press, Atlanta, GA, 19–23 Oct 1997, pp 41–48
Pullman GS, Bucalo K (2011) Pine somatic embryogenesis using zygotic embryos as explants. In: Thorpe T, Yeung E (eds) Plant embryo culture: methods and protocols. Humana Press, New York, pp 267–291
Pullman GS, Bucalo K (2014) Pine somatic embryogenesis: analyses of seed tissue and medium to improve protocol development. New For. 45:353–377
Pullman GS, Buchanan M (2003) Loblolly pine (Pinus taeda L.): stage-specific elemental analyses of zygotic embryo and female gametophyte tissue. Plant Sci 164:943–954
Pullman GS, Buchanan M (2006) Identification and quantitative analysis of stage-specific organic acids in loblolly pine (Pinus taeda L.) zygotic embryo and female gametophyte. Plant Sci 170:634–647
Pullman GS, Buchanan M (2008) Identification and quantitative analysis of stage-specific carbohydrates in loblolly pine (Pinus taeda) zygotic embryo and female gametophyte tissues. Tree Physiol 28:985–996
Pullman GS, Johnson S (2002) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation rates. Ann For Sci 59:663–668
Pullman GS, Johnson S (2009a) Loblolly pine (Pinus taeda L.) female gametophyte and embryo pH changes during embryo and seed development. Tree Physiol 29:829–836
Pullman GS, Johnson S (2009b) Osmotic measurements in whole megagametophytes and embryos of loblolly pine (Pinus taeda L.) during embryo and seed development. Tree Physiol 29:819–827
Pullman GS, Skryabina A (2007) Liquid medium and liquid overlays improve embryogenic tissue initiation in conifers. Plant Cell Rep 26:873–887
Pullman GS, Webb DT (1994) An embryo staging system for comparison of zygotic and somatic embryo development. In: TAPPI R&D division biological sciences symposium, Minneapolis, MN, TAPPI Press, Atlanta, GA, 3–6 Oct 1994, pp 31–34 (ISBN 0-89852-930-1)
Pullman GS, Chase KM, Skryabina A, Bucalo K (2008) Conifer embryogenic tissue initiation: improvements by supplementation of medium with d-chiro-inositol and d-xylose. Tree Physiol 29:147–156
Pullman GS, Chopra R, Chase KM (2006) Loblolly pine (Pinus taeda L.) somatic embryogenesis: improvements in embryogenic tissue initiation by supplementation of medium with organic acids, Vitamins B12 and E. Plant Sci 170:648–658
Pullman GS, Gupta PK, Timmis R, Carpenter C, Kreitinger M, Welty E (2005a) Improved Norway spruce somatic embryo development through the use of abscisic acid combined with activated carbon. Plant Cell Rep 24:271–279
Pullman GS, Johnson S, Bucalo K (2009) Douglas fir embryogenic tissue initiation. Plant Cell Tiss Org Cult 96:75–84
Pullman GS, Johnson S, Peter G, Cairney J, Xu N (2003a) Improving loblolly pine somatic embryo maturation: comparison of somatic and zygotic embryo morphology, germination, and gene expression. Plant Cell Rep 21:747–758
Pullman GS, Montello P, Cairney J, Xu N, Feng X (2003b) Loblolly pine (Pinus taeda L.) somatic embryogenesis: maturation improvements by metal analyses of zygotic and somatic embryos. Plant Sci 164:955–969
Pullman GS, Namjoshi K, Zhang Y (2003c) Somatic embryogenesis in loblolly pine (Pinus taeda L.): Improving culture initiation with abscisic acid and silver nitrate. Plant Cell Rep 22:85–95
Pullman GS, Zhang Y, Phan B (2003d) Brassinolide improves embryogenic tissue initiation in conifers and rice. Plant Cell Rep 22:96–104
Pullman GS, Johnson S, Van Tassel S, Zhang Y (2005) Somatic embryogenesis in loblolly pine (Pinus taeda L.) and Douglas fir (Pseudotsuga menziesii): Improving culture initiation and growth with MES pH buffer, biotin, and folic acid. Plant Cell Tiss Org Cult 80:91–103
Pullman GS, Mein J, Johnson S, Zhang Y (2005) Gibberellin inhibitors improve embryogenic tissue initiation in conifers. Plant Cell Rep 23:596–605
Pullman GS, Olson K, Fischer T, Egertsdotter U, Frampton J, Bucalo K (2016) Fraser fir somatic embryogenesis: high frequency initiation, maintenance, embryo development, germination and cryopreservation. New For 47:453–480
Pullman GS, Zeng X, Copeland-Kemp B, Crockett J, Lucrezi J, May SW, Bucalo K (2015) Conifer somatic embryogenesis: improvements by supplementation of medium with oxidation-reduction agents. Tree Physiol 35:209–224
Rademacher W (2000) Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol 51:501–531
Rai MK, Shekhawat NS, Harish, Gupta AK, Phulwaria N, Ram K, Jaiswal U (2011) The role of abscisic acid in plant tissue culture: a review of recent progress. Plant Cell Tiss Org Cult 106:179–190
Roustan JP, Latche A, Fallot J (1989) Stimulation of Daucus carota somatic embryogenesis by inhibitors of ethylene synthesis: cobalt and nickel. Plant Cell Rep 8:182–185
Roustan JP, Latche A, Fallot J (1990) Control of carrot somatic embryogenesis by AgNO3, an inhibitor of ethylene action: effect on arginine decarboxylase. Plant Sci 67:89–95
Rudus I, Kepczynska E, Kepczynski J (2000) Regulation of Medicago sativa L. somatic embryogenesis by gibberellins. Plant Growth Regul 36:91–95
Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M (2001) KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev 15:581–590
Schultz RP (1999) Loblolly—the pine for the twenty-first century. New For 17:71–88
Singh H (1978) Embryology of gymnosperms. In: Handbuch der Pflanzenanatomie (Encyclopedia of Plant Anatomy), vol 10. Part 2. Gebruder Borntraeger, Berlin, Germany
Stasolla C (2010) Glutathione redox regulation of in vitro embryogenesis. Plant Physiol Biochem 48:319–327
Stasolla C, Yeung EC (1999) Ascorbic acid improves conversion of white spruce somatic embryos. In Vitro Cell Dev Biol-Plant 35:316–319
Taiz L, Zeiger E (2010) Plant physiology, 5th edn. Sinauer Associates Inc, Sunderland, MA, 782 p
Tang W, Ouyang F, Guo ZC (1998) Studies on embryogenic callus induction and plant regeneration in loblolly pine. Sci Silv Sin 34:115–119
Tang W, Guo ZC, Ouyang F (2001) Plant regeneration from embryogenic cultures initiated from mature loblolly pine zygotic embryos. In Vitro Cell Dev-Plant 37:558–563
Teasdale RD, Dawson PA, Woolhouse GW (1986) Mineral nutrient requirements of a loblolly pine (Pinus taeda) cell suspension culture. Plant Physiol 82:942–945
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
Toering A, Pullman GS (2005) Modeling available 2,4-dichlorophenoxyacetic acid in a tissue culture medium containing activated carbon. Plant Cell Tiss Org Cult 82:179–188
Van Winkle SC, Pullman GS (2003) The combined impact of pH and activated carbon on the elemental composition of plant tissue culture media. Plant Cell Rep 22:303–311
Van Winkle SC, Pullman GS (2005) Achieving desired plant growth regulator levels in liquid plant tissue culture media that include activated carbon. Plant Cell Rep 24:201–208
Van Winkle SC, Johnson S, Pullman GS (2003) The impact of Gelrite and activated carbon on the elemental composition of plant tissue culture media. Plant Cell Rep 21:1175–1182
Vieira LN, Santa-Catarina C, Fraga HPF, Santos ALW, Steinmacher DA, Schlogl PS, Silveira V, Steiner N, Floh EIS, Guerra MP (2012) Glutathione improves early somatic embryogenesis in Araucaria angustifolia (Bert) O. Kuntze by alteration in nitric oxide emission. Plant Sci 195:80–87
von Aderkas P, Label P, Lelu MA (2002) Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol 22:431–434
Xu N, Johns B, Pullman GS, Cairney J (1997) Rapid and reliable differential display from minute amounts of tissue: mass cloning and characterization of differentially expressed genes from loblolly pine embryos. Plant Mol Biol Rep 15:377–391
Yeung EC, Belmonte MF, Tu LTT, Stasolla C (2005) Glutathione modulation of in vitro development. Vitro Cell Dev Biol Plant 41:584–590
Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron J Biotechnol [online] 13(1) [11/14/13] 15 Jan 2010. http://www.scielo.cl/scielo.php?pid=S0717-34582010000100012&script=sci_arttext. Accessed 30 Mar 2017
Acknowledgements
I thank the Institute of Paper Science and Technology at Georgia Tech (Renewable Bioproducts Institute) and its member companies for providing funding, materials and supplies and a home for this research over the past 24 years. Without plant materials from forest companies including Arborgen, Boise Cascade, Georgia Pacific, MeadWestvaco Corporation, Union Camp, Westvaco and Weyerhaeuser NR Company this research could not have been done. I also thank the Georgia Institute of Technology, State of Georgia TIP3 Program, and the Consortium for Plant Biotechnology Research (DOE Prime Agreement No. DEFG36-02GO12026 and USEPA grant EM-83438801) along with member companies Arborgen, Monsanto Company and Weyerhaeuser Company for financial support. In addition, I am grateful for the valuable assistance of Michael Buchanan, Kylie Bucalo, Dr. John Cairney, Kelly-Marie Chase, Xiaorong Feng, Shannon Johnson, Dr. Sheldon W. May, Jonathan Mein, Paul Montello, Kavita Namjoshi, Anna Skryabina, Xiaoyan Zeng and Yalin Zhang.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Pullman, G.S. (2018). Embryogenic Tissue Initiation in Loblolly Pine (Pinus Taeda L.). In: Jain, S., Gupta, P. (eds) Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants. Forestry Sciences, vol 84. Springer, Cham. https://doi.org/10.1007/978-3-319-89483-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-89483-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89482-9
Online ISBN: 978-3-319-89483-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)