Abstract
Recent clinical trials based on human pluripotent stem cell-derived retinal pigment epithelium cells (hPSC-RPE cells) were clearly a success regarding safety outcomes. However the delivery strategy of a cell suspension, while being a smart implementation of a cell therapy, might not be sufficient to achieve the best results. More complex reconstructed tissue formulations are required, both to improve functionality and to target pathological conditions with altered Bruch’s membrane like age-related macular degeneration (AMD). Herein, we describe the various options regarding the stem cell source choices and the different strategies elaborated in the recent years to develop engineered RPE sheets amenable for regenerative therapies.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Human pluripotent stem cell
- Human-induced pluripotent stem cell
- Human embryonic stem cell
- Stem cell
- Tissue engineering
- Retinal pigment epithelium
- Age-related Macular degeneration
- Retinitis pigmentosa
- Cell therapy
- Regenerative medicine
1 Introduction
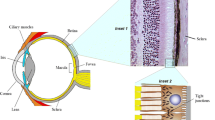
The retinal pigment epithelium (RPE) has a pivotal role in maintaining photoreceptor survival, integrity, and functionality (Strauss 2005; da Cruz et al. 2007; Ben M’Barek et al. 2015). RPE cells organize as monolayered epithelium in tight contact with the outer segment of photoreceptors (Strauss 2005). Dysfunctions or degeneration of RPE cells is associated with AMD and retinitis pigmentosa (RP). These diseases that might ultimately lead to blindness are estimated to affect about 30 millions of people worldwide (Gehrs et al. 2006). In addition, AMD disease burden will dramatically increase with a projection of 288 millions of people to be affected by 2040, due to prolonged life expectancies (Wong et al. 2014).
RP is a heterogeneous group of rare diseases caused by diverse genetic mutations (https://sph.uth.edu/retnet/disease.htm). Some of these mutations affect specifically RPE functions like the mutations in MERTK, RPE65, CRALBP, BEST1, or LRAT genes (Sparrow et al. 2010). MERTK, RPE65, CRALBP, and LRAT mutations correspond to about 5% of autosomal recessive RP (Hartong et al. 2006), and a prevalence of 1.5 case in 100,000 births for BEST1 gene mutations was estimated (Bitner et al. 2012). At the opposite, AMD is a complex pathology caused by the combination of genetic and environmental factors (Ambati et al. 2003). Two forms of AMD are typically described: a dry form (atrophic) that account for 90% of all cases and a wet form (neovascular). RPE degeneration and damages of the Bruch’s membrane associated with the formation of protein and lipid deposits, called drusen, are characteristics of the dry form. Wet AMD involves choroidal neovascularization with potential hemorrhages that are susceptible to damage the macula (Ambati et al. 2003).
Treatment options for AMD and RP are extremely limited (Gehrs et al. 2006). Only wet AMD benefits from the recent advance of anti-angiogenic treatments (anti-VEGF therapy). In light of this unmet medical need, regenerative medicine appears as an attractive therapeutic alternative to cure patients.
2 Stem Cell-Based RPE Production
Replacement of dead/dysfunctional RPE cells by new ones is a strategy that was first proposed in early 1980s (Gouras et al. 1985). Initially, RPE sources consisted of autologous RPE from the periphery, allogeneic RPE from cadavers, or fetal RPE (Binder et al. 2007; Radtke et al. 2008). These sources have a lot of limitations in terms of supply chain, donor to donor variability, and scale-up (limited cell amplification). With the emergence of hPSCs, efforts are now directed to stem cells sources (Thomson et al. 1998; Takahashi et al. 2007).
2.1 Sources
Three main sources for stem cell therapy are preferred regarding the following criteria: ability to differentiate into RPE cells, ease of culturing, and suitability for a clinical application.
The first one is the recently described human adult RPE stem cells (RPESCs) (Salero et al. 2012). About 2–3% of human RPE cells, harvested from human eye cadavers, could self-renew in vitro, a prominent characteristic of stem cells. Besides their capacity to differentiate into other lineages, they are able to generate new RPE cells that keep their epithelial morphology after transplantation (Stanzel et al. 2014). This source remains an option that will be tested in clinical trials but amplification and isolation remain difficult.
The second stem cell source is based on human embryonic stem cells (hESCs), which were first derived in 1998 (Thomson et al. 1998). These cells retain pluripotency, meaning that they can be differentiated in any cell type of the adult body (including RPE cells) if the required signaling cues are provided. In addition, they can be amplified for a virtually unlimited number of passages, allowing the possibility of industrialized manufacturing processes.
Human-induced pluripotent stem cells (hiPSCs) represent the third stem cell source (Takahashi et al. 2007). While not being completely identical, hiPSCs share the same pluripotency and self-renewal potentials than hESCs (Wu et al. 2016). hiPSCs differentiated into RPE cells are functional in vivo and restore visual functions of rodents with dystrophic retinas (Kamao et al. 2014) to a similar level than hESC-RPE (Riera et al. 2016).
2.2 Protocols for the Generation of RPE from hPSCs
hPSCs (hESC and hiPSC) are able to spontaneously differentiate into RPE cells in adherent cultures after FGF2 withdrawal (Klimanskaya et al. 2004; Lustremant et al. 2013; Leach and Clegg 2015). Protocol improvements were undertaken to reduce the culture duration or to improve the yield. Purity of RPE cells can be achieved by manual selection or using growth factors/small molecules (Bharti et al. 2011; Leach and Clegg 2015). These hPSC-RPE cells display characteristics similar to primary fetal RPE cells. In addition, hPSC-RPE cells could also be obtained concomitantly with the formation of self-forming neural retinas in vitro (Meyer et al. 2011; Reichman et al. 2014).
2.3 Manufacturing Strategy
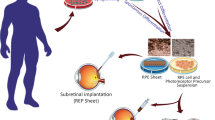
From an industrial perspective, clinical hPSCs should be manufactured in a good manufacturing practice (GMP) and compliant establishment and preserved as frozen cell banks (either a unique master cell bank (MCB) or a MCB and derived working cell banks). This MCB has to be characterized based on regulatory standards that include safety analysis (virology, fungi, mycoplasma, bacteria, and endotoxins), purity, potency (embryonic body formation and alkaline phosphatase activity), and stability. From this MCB of hPSCs, a bank of hPSCs-RPE is derived and banked according to GMP principles. Cryovials of this qualified hPSC-RPE bank could be sent straight to the surgical rooms in order to be thawed and transplanted as a cell suspension without prior additional culture in vitro (Schwartz et al. 2012, 2015). This formulation represents the most practical implementation for the handling and delivery to the surgical site with a simplified logistic. A formulation as a reconstructed epithelial sheet might require an additional culture period of hPSC-RPE cells from the bank in order to organize them as an epithelium. The graft should then be transferred in a closed and sterile packaging to the surgical room. The stability of the graft is therefore limited in time, and scheduling should be carefully prepared to have the graft ready for the day of the surgery. To overcome this limitation, freezing steps might be necessary.
3 Cell Delivery Strategy: Cell Suspension Versus Reconstructed Tissue
The formulation remains a crucial aspect depending on the nature of the pathology in particular when the Bruch’s membrane is altered. First approaches were based on cell suspension, but the field has moved to a more elaborated tissue formulation. The idea is that safety and clinical outcomes might be better as RPE functions depend also on their polarized epithelial organization (Stanzel et al. 2014; Hsiung et al. 2015; Nazari et al. 2015). In addition, the survival of the transplanted cells was improved when transplanted as a cell sheet (Diniz et al. 2013). This paradigm was recently demonstrated to improve the functionality of the graft in vivo in animal models (Ben M'Barek et al. 2017).
4 Types of Support for Tissue Reconstruction
The delivery of an already formed epithelium requires the use of a supportive matrix allowing the sheet to be safely removed from the culture plate to be then loaded on the transplantation device. In addition, this support might replace damaged Bruch’s membrane in patients. Thus specific qualities are required to support hPSC-RPE functions and survival: thickness, mechanical properties (flexibility, ease of handling), permeability, and eventually biodegradation (nontoxic by-products) (Hynes and Lavik 2010; Kador and Goldberg 2012; Nazari et al. 2015).
4.1 Synthetic Polymers
Synthetic polymers have the main advantage of being structurally and chemically precisely defined, with consistency, homogeneous properties, and a high potential for industrial processing (Diniz et al. 2013; Stanzel et al. 2014; Ilmarinen et al. 2015). Among others, ultrathin parylene substrates have proven their safety and efficacy (Hu et al. 2012; Diniz et al. 2013; Pennington and Clegg 2016). They will be tested in phase I/II clinical trial for dry AMD in the USA (clinical trial reference: NCT02590692). Poly-lactic-co-glycolic acid or PLGA is also an attractive substrate that is biodegradable (Song and Bharti 2016). hPSC-RPE can be cultured on such substrate and form an epithelium that can be transplanted (Bharti et al. 2014; Song and Bharti 2016). This combination will also be tested in a USA-based clinical trial (Song and Bharti 2016). Finally, a porous polyester scaffold is currently tested in the UK (NCT01691261) (Ramsden et al. 2013).
4.2 Biological Materials
Various biological scaffolds have been proposed like Descemet’s membranes (Thumann et al. 1997) or human amniotic membranes (hAMs) (Kiilgaard et al. 2012). hAMs, obtained from cesarean sections of normal births (Capeans et al. 2003), are well-tolerated in the subretinal space (Kiilgaard et al. 2012). Our group, in collaboration with the Institut de la Vision and St Louis Hospital (France), is developing the use of this scaffold for a phase I/II clinical trial targeting RP caused by a RPE dysfunction.
4.3 No Support
A Japanese group developed a strategy without additional scaffold (Kamao et al. 2014). RPE are allowed to form an epithelium on a collagen coating. Then the collagen coating is digested to liberate the sheet. So far, one patient has been transplanted using this strategy (Mandai et al. 2017).
5 Conclusion
First clinical trials have demonstrated the safety of hESC-RPE implantation and have paved the way for optimized systems. Future clinical trials with engineered tissue based on the various scaffolds described herein will give clues about the formulations that provide the best results to patients.
References
Ambati J, Ambati BK, Yoo SH et al (2003) Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 48:257–293
Ben M'Barek K, Regent F, Monville C (2015) Use of human pluripotent stem cells to study and treat retinopathies. World J Stem cells 7:596–604
Bharti K, Miller SS, Arnheiter H (2011) The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res 24:21–34
Bharti K, Rao M, Hull SC et al (2014) Developing cellular therapies for retinal degenerative diseases. Invest Ophthalmol Vis Sci 55:1191–1202
Binder S, Stanzel BV, Krebs I et al (2007) Transplantation of the RPE in AMD. Prog Retin Eye Res 26:516–554
Bitner H, Schatz P, Mizrahi-Meissonnier L et al (2012) Frequency, genotype, and clinical spectrum of best vitelliform macular dystrophy: data from a national center in Denmark. Am J Ophthalmol 154(403–412):e404
Ben M’Barek K, Habeler W, Plancheron A et al (2017) Human ESC-derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci Transl Med. 9(421)
Capeans C, Pineiro A, Pardo M et al (2003) Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol Scand 81:271–277
da Cruz L, Chen FK, Ahmado A et al (2007) RPE transplantation and its role in retinal disease. Prog Retin Eye Res 26:598–635
Diniz B, Thomas P, Thomas B et al (2013) Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci 54:5087–5096
Gehrs KM, Anderson DH, Johnson LV et al (2006) Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med 38:450–471
Gouras P, Flood MT, Kjedbye H et al (1985) Transplantation of cultured human retinal epithelium to Bruch's membrane of the owl monkey's eye. Curr Eye Res 4:253–265
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809
Hsiung J, Zhu D, Hinton DR (2015) Polarized human embryonic stem cell-derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress-induced cell death than nonpolarized cultures. Stem Cells Transl Med 4:10–20
Hu Y, Liu L, Lu B et al (2012) A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res 48:186–191
Hynes SR, Lavik EB (2010) A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 248:763–778
Ilmarinen T, Hiidenmaa H, Koobi P et al (2015) Ultrathin polyimide membrane as cell carrier for subretinal transplantation of human embryonic stem cell derived retinal pigment epithelium. PLoS One 10:e0143669
Kador KE, Goldberg JL (2012) Scaffolds and stem cells: delivery of cell transplants for retinal degenerations. Expert Rev Ophthalmol 7:459–470
Kamao H, Mandai M, Okamoto S et al (2014) Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep 2:205–218
Kiilgaard JF, Scherfig E, Prause JU et al (2012) Transplantation of amniotic membrane to the subretinal space in pigs. Stem Cells Int 2012:716968
Klimanskaya I, Hipp J, Rezai KA et al (2004) Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 6:217–245
Leach LL, Clegg DO (2015) Concise review: making stem cells retinal: methods for deriving retinal pigment epithelium and implications for patients with ocular disease. Stem Cells 33:2363–2373
Lustremant C, Habeler W, Plancheron A et al (2013) Human induced pluripotent stem cells as a tool to model a form of Leber congenital amaurosis. Cell Reprogram 15:233–246
Meyer JS, Howden SE, Wallace KA et al (2011) Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29:1206–1218
Mandai M, Watanabe A, Kurimoto Y et al (2017) Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med. 376:1038–1046
Nazari H, Zhang L, Zhu D et al (2015) Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res 48:1–39
Pennington BO, Clegg DO (2016) Pluripotent stem cell-based therapies in combination with substrate for the treatment of age-related macular degeneration. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther 32:261–271
Radtke ND, Aramant RB, Petry HM et al (2008) Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol 146:172–182
Ramsden CM, Powner MB, Carr AJ et al (2013) Stem cells in retinal regeneration: past, present and future. Development 140:2576–2585
Reichman S, Terray A, Slembrouck A et al (2014) From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A 111:8518–8523
Riera M, Fontrodona L, Albert S et al (2016) Comparative study of human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC) as a treatment for retinal dystrophies. Mol Ther Methods Clin Dev 3:16010
Salero E, Blenkinsop TA, Corneo B et al (2012) Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 10:88–95
Schwartz SD, Hubschman JP, Heilwell G et al (2012) Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379:713–720
Schwartz SD, Regillo CD, Lam BL et al (2015) Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385:509–516
Song MJ, Bharti K (2016) Looking into the future: using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling. Brain Res 1638:2–14
Sparrow JR, Hicks D, Hamel CP (2010) The retinal pigment epithelium in health and disease. Curr Mol Med 10:802–823
Stanzel BV, Liu Z, Somboonthanakij S et al (2014) Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Rep 2:64–77
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Thumann G, Schraermeyer U, Bartz-Schmidt KU et al (1997) Descemet's membrane as membranous support in RPE/IPE transplantation. Curr Eye Res 16:1236–1238
Wong WL, Su X, Li X et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106–e116
Wu J, Ocampo A, Izpisua Belmonte JC (2016) Cellular metabolism and induced pluripotency. Cell 166:1371–1385
Acknowledgments
I-Stem is supported by the AFM-Téléthon. This work was supported by grants from the Fondation pour la Recherche Medicale (Bio-engineering program - DBS20140930777), the LABEX REVIVE (ANR-10-LABX-73), NeurATRIS (Investissements d'Avenir - ANR-11-INBS-0011), INGESTEM: the National Infrastructure Engineering for Pluripotent and differentiated Stem cells (Investissements d'Avenir - ANR-11-INBS-000).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Ben M’Barek, K., Habeler, W., Monville, C. (2018). Stem Cell-Based RPE Therapy for Retinal Diseases: Engineering 3D Tissues Amenable for Regenerative Medicine. In: Ash, J., Anderson, R., LaVail, M., Bowes Rickman, C., Hollyfield, J., Grimm, C. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 1074. Springer, Cham. https://doi.org/10.1007/978-3-319-75402-4_76
Download citation
DOI: https://doi.org/10.1007/978-3-319-75402-4_76
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75401-7
Online ISBN: 978-3-319-75402-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)