Abstract
Retinal pigment epithelium (RPE) is vitally important for supporting photoreceptor functions and welfare. RPE degeneration has a major role in pathogenesis of many retinal diseases including wet and dry forms of age-related macular degeneration (AMD). The loss of RPE cell functions leads to the degradation of photoreceptors and as a consequence to either partial or total loss of vision. Today, only cell replacement can restore RPE functionality, setting high demands for development of cell transplantation therapy. Transplantation of RPE cells derived from fetal, cadaveric, and stem cell sources have been extensively studied in different animal models and also in recent clinical trials. Based on current knowledge, human pluripotent stem cells (hPSC), including human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC), provide an exhaustless source of cells for cell-based therapies. Since 2004, numerous research groups have reported successful differentiation of functional RPE cells from hPSCs (hPSC-RPE) using a wide variety of methods. Two RPE cell transplantation strategies—each with their own pros and cons—are currently under development: injection of a single-cell suspension, and transplantation of intact RPE sheet with or without a biomaterial-based scaffold. So far, the ongoing clinical trials with hPSC-RPE have not raised any safety concerns, but additional proof of efficacy needs further trials. In light of the recent advances and the exponential activity in the field, this should be possible in the near future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Retinal pigment epithelium (RPE) is a highly polarized monolayer of cells located between the neural retina and the choroid (see Fig. 14.1). RPE has several vitally important functions as a part of the blood-retinal barrier and in supporting the neural retina: RPE cells provide nutrients for photoreceptors, phagocytose photoreceptor outer segments secrete important molecules including pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF), absorb stray light, and control regeneration of visual pigments, ion flow and oxidative damage. For a more extensive review of RPE functions and characteristics, the article by Strauss is highly recommended [1].
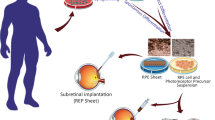
The hPSC-RPE transplantations. The hPSC-RPE cells can be efficiently differentiated and transplanted to the subretinal space between neural retina and choroid. Currently two different transplantation approaches are used (a) single-cell suspension injection and (b) transplantation of intact RPE cell sheet
Retinal degenerative diseases, such as age-related macular degeneration (AMD), retinitis pigmentosa, and Stargardt macular dystrophy, affect tens of millions of individuals worldwide. For example in AMD, the macula of the eye gradually degenerates, leading to the loss of central vision, and thus hindering tasks such as face recognition, reading, and driving—important cornerstones for individual independence and quality of life. With a steadily increasing life expectancy, the number of people suffering from AMD worldwide is predicted to increase to almost 200 million by the year 2020 and to over 280 million by 2040 [2]. There are two types of AMD: dry (atrophic) and wet (neovascular). The dry type accounts for approximately 85–90% of all AMD cases. In its advanced form, known as geographic atrophy (GA), dry AMD leads to RPE degeneration and subsequently photoreceptor death in the macula. The underlying cause of dry AMD remains unknown. Wet AMD is caused by abnormal neovascularization from the choriocapillaris beneath the macula. These new blood vessels tend to bleed, leak fluid, and scar, which damages the photoreceptors. Although there is no cure for AMD, repeated injections of anti-vascular drugs into the eye can slow down the neovascularization and progression of early-stage wet AMD [3,4,5]. On the other hand, no effective medical or surgical treatment is available for dry AMD, although dietary supplements have been suggested to slow its progression [6]. There is clearly a need to develop a therapy that could preserve or repopulate this important RPE cell layer.
Cell Based Therapy for RPE
One of the most promising future treatments for retinal degeneration is cell replacement therapy [7]. The eye in general is a very attractive target for tissue engineering and cell therapy for several reasons. Firstly, the eye offers easy access to well-developed surgical approaches and non-invasive follow-up methods including high resolution optical coherence tomography (OCT) [7, 8]. Secondly, a relatively small number of cells is sufficient for a cell replacement in the eye as compared to many other organs and tissues. Finally, the eye is generally less prone to immune rejection of transplanted cells, although this advantage may be compromised due to disease pathogenesis such as neovascularization of wet AMD [9, 10].
The single layer of RPE that lies on Bruch’s membrane between the photoreceptor outer segments and the choriocapillaris (Fig. 14.1) is a relatively easy target for cell replacement therapy, compared to highly complex neural retina with functional neural connections. Surgical attempts have been made to replace the RPE at the macula, either by moving the macula to the non-diseased periphery or by grafting new RPE under the macula [11]. These are difficult surgeries to perform and can lead to complications such as unplanned retinal detachment, cataract, and double vision [12]. Thus, it is unlikely that these procedures will prove cost effective in combatting the burden of AMD [11]. The earliest attempts of RPE cell transplantation in animal models provided evidence that cell replacement therapy could have potential in treating retinal degeneration. Although the success of these early studies was quite low [13], they encouraged further development of the technology. Later, clinical trials were conducted using fetal [14] or post-mortem adult RPE cells [15,16,17], as well as retina-RPE complex [18]. Unfortunately, visual acuity of the patients did not improve in the long term. Among these studies, Binder and colleagues were the first to report autologous RPE transplantation for AMD patients with promising outcomes [19]. Taken together, these and other early studies not mentioned here provided proof of concept that RPE cell replacement therapy is possible if a viable source of functional RPE cells is established.
As reviewed by da Cruz and colleagues [20] several cell sources for RPE transplantation have been considered: fetal, autologous, or allogeneic RPE, immortalized RPE cell lines, and stem cells. Among these, the use of fetal tissue is restricted mainly due to poor availability. Autologous RPE cells, on the other hand, may have genetic defects or be functionally impaired due to the disease. Immortalized cell lines likely contain mutations and genetic abnormalities. Thus, the most promising option is either allogeneic RPE cells or RPE cells differentiated from stem cells. Adult human RPE cells isolated from donated eyes can be activated in vitro into a stem cell state (RPE stem cells) which are polarized, express RPE markers and have the key physiological properties of native RPE cells making them a candidate for future cell replacement therapy [21, 22]. It is estimated that the macula harbors around 60,000 RPE cells that potentially need replacing. To achieve this, it is necessary to develop technologies to expand RPE stem cells in vitro, or to use their paracrine effects to trigger rejuvenation of native RPE in vivo [23]. Further preclinical studies are ongoing to develop adult RPE stem cell transplantations towards clinical trials [24]. In addition to the adult RPE stem cells, other stem cell types have been investigated as a source of RPE. Notably, human pluripotent stem cells (hPSC), with their excellent developmental and replicative capacity, can potentially provide an unlimited supply of RPE cells needed to treat the millions of patients suffering from retinal degeneration.
Human embryonic stem cells (hESCs) are usually isolated from surplus embryos of poor quality 4–6 days after in vitro fertilization [25]. Due to ethical issues and relatively low availability of these cells, as well as their immunogenic properties, the discovery of the possibility to reprogram human somatic cells to behave like hESCs offered even more exciting opportunities for regenerative medicine [26]. After the discovery of human induced pluripotent stem cells (hiPSC) in 2007, numerous non-integrating and non-viral reprogramming methods have been developed using various cell sources including skin fibroblasts, hair follicles, muscle, peripheral blood lymphocytes and urine [27]. In many respects, hiPSCs resemble hESCs, although epigenetic and genetic abnormalities in the hiPSC lines have been reported [28]. This has raised the issue whether epigenetic marks from the cell source may persist in the reprogrammed hiPSCs. This issue has been under critical evaluation as genomic instability in general is recognized as an important hurdle in the expanding field of stem cell-based therapies. According to the current knowledge, the epigenetic differences observed in some hiPSC lines compared to hESC lines seem to be caused mainly by the reprogramming method and diminish during passaging [29,30,31]. Further studies are needed to set a threshold for the acceptable level and genomic location of potential epigenetic and genetic changes in the stem cell product manufactured for clinical applications.
Differentiation of RPE from Human Pluripotent Stem Cells
So how can functional RPE cells be obtained from hPSC? During mammalian development, RPE and neural retina both develop from the optic neuroepithelium and share the same progenitors. The neuroepithelium near the anterior part of the neural tube evaginates laterally to form the optic vesicles. Invagination of the distal part of the optic vesicle leads to the formation of the optic cup. By the 6th or 7th week of gestation, the optic cup has differentiated into two epithelial layers. The distal layer then differentiates into the neural retina and the proximal layer develops into the RPE [32]. Since the pioneering work by Sasai and co-workers, hPSC-derived eye organoids mimicking the early retinal developmental steps have been extensively used for modeling eye development in vitro [33]. Similarly, in vitro differentiation of RPE cells from hPSCs follows the same developmental steps. In [34], Klimanskaya and co-workers were the first to report successful differentiation of RPE cells from hESCs [34]. Many research groups later demonstrated the same also with hiPSCs [35,36,37]. Since then, numerous research groups have developed methods for obtaining RPE cells from hPSCs, with varying efficiencies. Recent review describe the various differentiation methods in more detail [38], only the general approaches are presented here.
Human PSCs are typically cultured as colonies either on top of a layer of fibroblast feeder cells (mouse embryonic or human foreskin), or without feeder cells (feeder-free) on specific culture substrate in the presence of basic fibroblast growth factor (bFGF) [39,40,41]. There are two main approaches to initiate RPE differentiation: spontaneous RPE differentiation upon removal of bFGF from the culture medium or directed differentiation using growth factors, inhibitors and/or small molecules. In methods relying on spontaneous differentiation, RPE can be obtained through adherent over growth of hPSC cultures e.g. [34, 42, 43], or by growing embryoid body-like structures in suspension and later plating them down as adherent cultures e.g. [44, 45]. The more directed differentiation approaches attempt to replicate embryonic development by adding specific growth factors, inhibitors, or small molecules at appropriate time points [46,47,48,49,50]. As an example, we have established our own feeder cell-free culture and differentiation method for hPSC-RPE using both of these approaches [51].
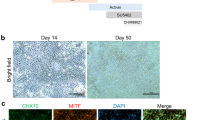
Figure 14.2 summarise the differentiation and characterisation approach of RPE cells. In general, depending on the method and cell lines used, pigmented foci usually appear in cultures within 1–4 weeks (see Fig. 14.2a). The pigmented areas are mechanically or enzymatically separated for RPE enrichment. Once separated, pigmented hPSC-RPE cells are seeded on substratum which resembles Bruch’s membrane or contains its extracellular matrix (ECM) components such as collagens and laminins [52]. After seeding, hPSC-RPE cells first lose their cobblestone morphology and pigmentation, but regain these characteristics within a few weeks [53] (also highly depending on culture conditions). However, if consecutively passaged, hPSC-RPE cells gradually lose their ability to re-establish RPE features [54]. This is problematic especially if multiple cell doublings are needed to obtain large enough quantities of pure RPE cells. Rho kinase (ROCK) inhibition during passaging may help extend hPSC-RPE passage [55], although it remains to be seen how this affects cell functionality and genetic stability. It is also well acknowledged that hPSC-RPE cells require an additional culture period of several weeks—preferably on permeable cell culture inserts—until they mature to a fully polarized and pigmented monolayer [51, 56, 57]. Furthermore, culture conditions such as the type of substratum and ECM protein coating highly affect hPSC-RPE maturation [52].
The general hPSC-RPE differentiation and characterisation approach. (a) hPSC-RPE differentiation can be divided in induction of differentiation, purification and expantion of pigmented cells, additional replating/passaging and cryopreservation of cells, and finally hPSC-RPE maturation and characterisations. Scale bar 100 μm. (b) hPSC-RPE characterized with expression and localization of Zonula Occludens-1 (ZO1), Na+/K+ ATPase, RPE65, MERTK proteins and phagocytosis of photoreceptor outer segments (OPSIN). Scale bar 10 μm
Different hPSC lines may respond very differently to exogenous signals, making it challenging to develop a universal RPE differentiation protocol. It is unlikely that 100% RPE differentiation efficiency will ever be achieved. Thus, further improvements are needed to increase the yield and purity of the RPE cultures in order to obtain sufficient amounts of mature cells with RPE characteristics for safe therapeutic use. Besides establishing more efficient hPSC-RPE differentiation methods, another strategy to improve the purity of RPE populations is to sort cells based on RPE specific marker expression [58]. As safety is a primary consideration for any clinical use of hPSC-RPE and due to the high risk of tumorigenicity, methods to detect any trace of pluripotent cells among the differentiated cells are critically important [59,60,61]. Another safety concern is the use of animal-derived material such as fetal bovine serum (FBS) and mouse feeder cells. These components are often used in establishment, culture and differentiation of hPSCs, and may transfer non-human pathogens to the patient and cause immune reactions [62, 63]. Finally, cell therapy applications require defined and reproducible conditions in accordance with Good Manufacturing Practice (GMP) during derivation and maintenance of hPSC lines and RPE differentiation [41, 45]. Overall, the variations in differentiation and culture methods influence hPSC-RPE characteristics, which is why critical consideration and planning is needed when aiming for clinical applications. But first, does hPSC-RPE resemble and behave like native human RPE?
Characterization of Stem Cell-Derived RPE Cells
As mentioned earlier, RPE cells have many vitally important characteristics and functions [1]. Thus, it is necessary to verify that hPSC-RPE cells possess these characteristics (See Fig. 14.2b). This is critically important for clinical use, but also for non-clinical applications where hPSC-RPE cells serve as a model of native human RPE. Since the very early studies, putative hPSC-RPE cells were proven to share many characteristics with authentic human RPE: they express RPE specific genes and proteins (e.g. Bestrophin, CRALBP, Na+/K+ATPase, MERTK, Zonula Occludens-1 and Claudin-19), have pigmented cobblestone-like morphology, form tight and highly polarized RPE with high transepithelial electrical resistance (TEER), phagocytose isolated photoreceptor outer segments, and secrete growth factors such as PEDF [34, 35, 45, 64,65,66,67]. Moreover, hPSC-RPE cells express many important transporters [68, 69] and aquaporin water channels [56], and possess other physiologically relevant functions [70]. For instance, AMD patient-derived hiPSC-RPE cells have decreased antioxidative defence compared to healthy hiPSC-RPE cells, providing proof of concept that their stress response properties are similar to that of native RPE [71]. RPE cells derived from hiPSCs are in many respects very similar to hESC-RPE cells [51, 72]. Furthermore, a recent comparison of hiPSC lines derived from different somatic cells suggests that hiPSC-RPE functions are more significantly affected by the genetic background of different donors than the epigenetic “memory” associated with the donor tissue [70]. In addition, there is always heterogeneity among hPSC-RPE cultures and cell characteristics and functionality may vary. Thus, it is important to define RPE identity [73, 74] and improve quantitative methods to identify different maturation stages of hPSC-RPE cells. There is ongoing discussion regarding hPSC-RPE characteristics and the various criteria these cells should fulfil in order to be considered authentic RPE cells [73]. For example, Buccholz and co-workers have suggested that the systematic characterization panel of hPSC-RPE should include at least gene and protein analyses, quantitative phagocytosis, TEER measurement, growth factor secretion analysis, retinoid metabolism assay, and functionality in an animal model [35]. To conclude, there is an extensive and ever increasing amount of studies supporting the current knowledge that hPSCs are a promising source of functional RPE cells. The main down-stream applications of these cells are for modelling native RPE for drug testing, for modelling retinal diseases [75], and for RPE cell replacement therapies—the focus of the following subchapters.
Human PSC-RPE Transplantation Studies in Animal Models
The efficacy of hPSC-RPE cell therapy has been extensively studied in different animal models and only some of these are mentioned here as examples. Although large-eyed animal models are preferred especially for the development of subretinal transplantation techniques [76,77,78,79], the Royal College of Surgeons (RCS) rat remains a widely used animal model. Its RPE is unable to phagocytose photoreceptor outer segments and therefore photoreceptors degenerate over a period of 3 months after birth [80,81,82]. The Food and Drug Administration (FDA) recommends the use of this animal model to demonstrate safety and efficacy of hPSC-RPE [74]. The hPSC-RPE transplantation studies follow one of two approaches: injection of a single-cell suspension into the subretinal space, or transplantation of an hPSC-RPE sheet with or without supportive biomaterial matrix (See Fig. 14.1). Both of these approaches have their own pros and cons which are briefly discussed next.
Injection-Based Transplantation
In the first RPE transplantation studies in 2004, primate ESC-derived RPE injected into the subretinal space of RCS rats helped recover retinal function [83]. It was later demonstrated that although single cell suspension injected hPSC-RPE cells survive and improve visual acuity in RCS rats, they rarely form tight epithelia after transplantation and gradually die within 10–15 weeks and cell survival for up to 20–30 weeks seems to be an exception [36, 42, 43, 47, 67, 84]. Besides RCS rats, hPSC-RPE cells have been studied in monkeys and nude rats with a similar outcome [85, 86]. The improvement of visual function has been assessed using electroretinography (ERG) and even using behavioral assays that measure eye or body movements in response to light (optokinetic responses) [36, 47]. The temporary improvements in visual acuity of the RCS rat are thought to be more due to trophic factors [87] secreted by the transplanted cells, or macrophages that might help to phagocytose photoreceptor outer segments [64]. It has been suggested that failure to maintain a long-term improvement may be due to the impaired survival of hPSC-RPE on diseased Bruch’s membrane [88,89,90]. However, injection-based transplantation is fast and technically less challenging than RPE sheet transplantation, which is why it was selected for the first clinical studies in human patients.
RPE Sheet Transplantation
RPE cells are sensitive to local extracellular substrates for anchoring and survival [91], so transplantation of a pre-formed, oriented, polarized monolayer with tight junctions could enhance cell viability and integration into the retina [50, 85]. Moreover, intact RPE monolayers have a higher resistance for oxidative stress and thus could survive better in diseased retina [50]. Finally, the required amount of cells for sheet transplantation is much lower than for subretinal injections [85]. Disadvantages of the transvitreal sheet transplantation are its invasiveness and demands of surgical procedure, although surgical techniques and specialized tools have been developed to ease RPE sheet transplantation into the back of the eye [76, 78].
Retinal degeneration often involves Bruch’s membrane—the dynamic, 2–4.7 μm thick, pentalaminar structure which mainly consists of collagens, elastins, laminins, and fibronectin. Its thickness and permeability varies with age, pathological stage, and retinal location [92, 93]. The aged and thickened submacular Bruch’s membrane does not support long-term survival and differentiation of transplanted RPE [94, 95]. Consequently, it could be beneficial to transplant hPSC-RPE sheet with a supportive biomaterial scaffold to simultaneously substitute RPE and Bruch’s membrane function. In order to best mimic the properties of Bruch’s membrane, biomaterial substrates for production and transplantation of hPSC-RPE cells should meet several requirements. First, the scaffold material should support formation of tight hPSC-RPE with proper apical-basal polarization as well as native RPE characteristics. Second, the substrate should be biocompatible, thin enough to fit the subretinal space, with mechanical properties suitable for handling of the cell sheet. Third, and most important, the material needs to enable integration of transplanted cells into the retina. Finally, permeability to fluids and biomolecules is a definite prerequisite for substrates to replace the lost functional role of the damaged Bruch’s membrane as a semipermeable barrier [96,97,98,99].
Many research groups are focusing on finding an optimal scaffold for RPE transplantation. Decellularized natural scaffolds, such as Bruch’s membrane, amniotic membrane, and anterior lens capsule, have been previously suggested as substrates in retinal transplantations [100,101,102]. These natural scaffolds provide a significant advantage in retaining the complex structure and molecular hierarchy of the ECM while possessing tissue-specific micro- and nanotopography [97, 103]. In addition, natural polymers such as collagen, alginate, and fibroin, provide biocompatible sources of polymers for retinal tissue engineering. Natural polymers also closely mimic the native ECM and possess innate biological activity [97, 104]. Still, biomaterials of natural origin have several disadvantages including poor mechanical properties, batch to batch variation, as well as concerns with immunogenicity, toxic by-products of biodegradation, and pathogen transfer.
Synthetic polymers have multiple attractive characteristics including controlled chemical and physical structure, predictable properties, mechanical durability, high degree of processing flexibility, and high reproducibility in commercial-scale manufacturing processes [97]. The most commonly used synthetic polymers are poly-α-hydroxy-acid-based polymers such as poly(L-lactide) (PLLA), poly(lactide-co-glycolide) (PLGA), poly(ε-caprolactone) (PCL), and combinations of these materials as co-polymers (e.g. PLCL) [97, 105, 106]. Even though synthetic polymers overcome the common drawbacks associated with natural polymers, they tend to be hydrophobic and lack cell binding ligands on the scaffold surface, which results in poor cell attachment without additional surface modifications [106]. Many synthetic biomaterial substrates with distinct architecture have been investigated as potential substrates for RPE [98, 107,108,109]. Finally, hybrid materials incorporate the beneficial aspects of both biologically active natural polymers and structurally flexible synthetic polymers such as combination of silk fibroin, gelatin and PCL [110]. Hybrid biomaterials show promise as potential Bruch’s membrane mimicking substrates for RPE [111, 112].
Most biomaterial studies use either primary RPE cells or immortalized cell lines, and studies of hPSC-RPE cell-biomaterial interactions have only recently gained popularity e.g. [65, 77]. The most disputed property of hPSC-RPE scaffold material is its biostability—are biodegradable scaffolds preferable over biologically inert/non-degradable materials? A biodegradable membrane would provide a temporary support for hPSC-RPE, until the cells remodel and replace it with new ECM layers. A biostable membrane would provide permanent support for the cells and at the same time improve integration of the transplant by providing better permeability, which is likely to be critical for retinal health [65, 113]. For instance, Parylene C (poly(para-xylene)) is a biostable and chemically inert polymer. When combined with Matrigel™ or human vitronectin, Parylene C was shown to support hPSC-RPE growth and functionality both in vitro and in vivo [114]. Our group has studied the performance of biologically inert polyimide (PI) for hPSC-RPE culture [115] and transplantation [108]. These approaches aim to overcome the disorganized fashion in which RPE cells adhere to Bruch’s membrane when injected as a suspension. The plastic polymer is also designed to act as a replacement for the aged and thickened Bruch’s membrane, and provides an anchor for the cells while aiding in surgical delivery [11]. It remains an important target for future preclinical and clinical studies to demonstrate whether the best efficacy for hPSC-RPE transplantation is achieved with the less invasive injection method or with the surgically challenging sheet transplantation. Alternatively, perhaps the transplantation method should be chosen based on individual patient and disease status. Finally, further clinical studies will need to demonstrate whether improved retinal function translates into improved cortical representation of images—an outcome which has been observed in humans after transplantation of adult RPE [20].
Clinical Trials with HPSC-RPE Cells
In 2012, 8 years after the first report of successful hESC-RPE differentiation, the first Phase I/II clinical trial using these cells was reported by Schwartz and co-workers, with indication of a good safety profile for patients with Stargardt disease and advanced dry AMD [116]. In the 3-year follow-up study with 18 participants, hESC-RPE cells were administered as a subretinal injection of three dosage cohorts (50,000 cells, 100,000 cells and 150,000 cells). In addition to the safety of the treatment, the authors also demonstrated improved vision in four out of nine AMD patients. Interestingly, only few, if any, pigmented hESC-RPE cells survived in the direct area of GA lesions. Instead, transplanted cells were detected in areas adjacent to the lesions, where they were deposited onto native RPE [117]. A Phase II study with more patients to assess efficacy is expected to report results imminently [23]. Other clinical trials with hPSC-RPE injections are ongoing in several countries including Israel, China, and Korea (for details see recent review [23] and https://clinicaltrials.gov). It remains to be seen if long-term survival and function of subretinally injected cells is achieved .
Unlike hESCs, hiPSCs can be obtained from the patients’ own somatic cells, offering the potential for immune-compatibility. To date, one AMD patient has been treated with autologous hiPSC-RPE cells manufactured and transplanted without an artificial scaffold [118]. RPE cells grown on a collagen gel were enzymatically lifted and transplanted as a sheet into the subretinal space of a patient with advanced wet AMD in Japan. It was reported that the patient did not experience any serious side effects and maintained visual acuity 1 year after surgery. The patient did not receive immunosuppressants and showed no signs of rejection. A second patient was recruited to the study, but was put on hold due to genetic mutations in hiPSC-RPE cells. [118, 119] Since then, the approach was modified towards using allogeneic hiPSCs and the clinical trial has resumed [23]
In a clinical trial carried out by The London Project to Cure Blindness in collaboration with Pfizer, two patients with wet AMD were treated with hESC-RPE monolayer immobilized on a polyester membrane [120, 121]. This is the first study demonstrating successful delivery and survival of hESC-RPE patch with a visual acuity gain of two patients treated. Similarly, a clinical trial led by Regenerative Patch Technologies (USA) is aiming to treat GA by transplanting hESC-RPE sheet on parylene C membrane and has very recently published first positive results with 4 out of 5 patients treated [122]. In both of these studies a non-degradable biomaterial with permanent support is used. In contrast, in a clinical trial planned by NEI/NIH, Bharti and co-workers aim to use a biodegradable matrix which will gradually dissolve after successful delivery of the hPSC-RPE cell sheet [38]. Perhaps the final outcomes of these trials will demonstrate what type of biomaterial substrates is better suited for hPSC-RPE delivery—biostable or biodegradable. Overall, it is very encouraging that studies using both cell delivery methods, and both hPSC types, appear to be safe in initial clinical studies.
Future Perspectives for RPE Cell Therapy
Although many clinical trials are ongoing, there are several open questions that need to be addressed and properly answered before safe and effective hPSC based cell therapies are widely available. One of the important and unanswered questions is whether to use hESC or hiPSC for cell replacement therapies. Although considered as functionally equivalent to hESCs, hiPSCs have been shown to harbor subtle differences in gene expression and DNA methylation [123]. Furthermore, there have been reports of point mutations and copy number variation in hiPSCs, which raises possible safety issues [118, 124].
The use of hPSC-RPE in clinical application faces multiple challenges, including manufacturing and characterization of clinical grade cells. The precise list of functional properties absolutely required prior to transplantation, and properties that the cells may or may not acquire once they are correctly integrated into the host tissue, is still missing [73].
Potential tumorigenicity of transplanted cells is a challenge that needs method development and increased understanding to guarantee safety and efficacy of transplanted cells. The pluripotent nature of hPSCs also raises the concern that if any undifferentiated hPSCs were left in the final clinical product, they could increase the risk of tumor or teratoma formation after transplantation. Another challenge is the immune-acceptance of transplanted cells. Although the subretinal space is relatively immune-privileged, damaged blood–retinal barrier, leaky blood vessels, and activated microglia may be present in diseased retina or induced by surgery. This can compromise the immune-privilege and cause cell rejection. Immunogenicity of allogeneic hPSC-RPE cells is therefore an issue [125, 126]. Autologous hiPSC-RPE cells offer minimal risk of cell rejection, but would not be a cost effective strategy on a large scale. Thus, a thorough understanding of the immunogenicity of hPSC-RPE and the optimal immunosuppression regime is essential for future clinical applications. In addition, international cell banking initiatives covering different human leukocyte antigen (HLA) types or universal hPSC lines with standardized cell banking and production methods are needed [127]. Otherwise there is a well-recognized risk that these treatments remain unaffordable for the majority of the patients.
Overall, multifactorial diseases such as AMD need further understanding of the disease pathogenesis, and most likely efficient treatment will require multidisciplinary approaches and personalized medicine. In addition, combination therapy of cell replacement, gene correction, supportive biomaterials and pharmaceuticals may be needed for certain diseases. First, as an example, strategies are being developed to genetically correct hiPSCs prior to differentiation and autologous transplantation [128]. Second, in addition to RPE and photoreceptor degeneration, Bruch’s membrane alterations including thickening and accumulation of drusen play an important role in AMD pathogenesis. Thus, further studies are needed to see if artificial scaffolds can replace Bruch’s membrane enabling proper RPE cell attachment in the diseased eye, or if novel methods are developed to improve attachment and polarization of injected RPE cells. Third, the critical cell type in early AMD seems to be RPE, while in more advanced cases, it may be necessary to replace neuronal cells either alone or together with RPE cells. Production and transplantation of photoreceptors or their progenitors has shown some promise, but is challenging due to the requirement for functional integration and synaptic contacts with the host neurons [129]. Last but not least, combination of effective medication and new therapeutic agents may be needed together with cell-based therapies to improve their efficacy.
In the end, it would be devastating for the whole field of regenerative medicine if any harm was caused to the patients in ongoing and upcoming clinical trials with hPSC-derived cells. Thus it is important to establish world-wide standards for the proper preclinical and clinical studies to minimize and hopefully avoid poor outcomes [130]. Several clinical trials are in progress around the world and the results will undoubtedly be exciting, but continued research and collaboration are needed to ensure above all safety, and then success of these ground-breaking approaches.
References
Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. https://doi.org/10.1152/physrev.00021.2004.
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014;2:2–e116. https://doi.org/10.1016/S2214-109X(13)70145-1.
Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. https://doi.org/10.1016/j.ophtha.2012.09.006.
Martin DF, Maguire MG, Ying G, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. https://doi.org/10.1056/NEJMoa1102673.
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. https://doi.org/10.1056/NEJMoa054481.
Group A. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA J Am Med Assoc. 2013;2:1–11. https://doi.org/10.1001/jama.2013.4997.
Zarbin M. Cell-based therapy for degenerative retinal disease. Trends Mol Med. 2016;22:115–34. https://doi.org/10.1016/j.molmed.2015.12.007.
Adhi M, Duker JS. Optical coherence tomography—current and future applications. Curr Opin Ophthalmol. 2013;24:213–21. https://doi.org/10.1097/ICU.0b013e32835f8bf8.
Stein-Streilein J. Mechanisms of immune privilege in the posterior eye. Int Rev Immunol. 2013;32:42–56. https://doi.org/10.3109/08830185.2012.740535.
Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep. 2010;2:pii: 3. https://doi.org/10.3410/B2-3.
Ramsden CM, Powner MB, Carr A-JF, et al. Stem cells in retinal regeneration: past, present and future. Development. 2013;140:2576–85. https://doi.org/10.1242/dev.092270.
Stanga PE, Kychenthal A, Fitzke FW, et al. Retinal pigment epithelium translocation after choroidal neovascular membrane removal in age-related macular degeneration. Ophthalmology. 2002;109:1492–8.
Jha BS, Bharti K. Regenerating retinal pigment epithelial cells to cure blindness: a road towards personalized artificial tissue. Curr Stem Cell Reports. 2015;1:79–91. https://doi.org/10.1007/s40778-015-0014-4.
Algvere PV, Berglin L, Gouras P, Sheng Y. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1994;232:707–16.
Del Priore LV, Kaplan HJ, Tezel TH, et al. Retinal pigment epithelial cell transplantation after subfoveal membranectomy in age-related macular degeneration: clinicopathologic correlation. Am J Ophthalmol. 2001;131:472–80.
Peyman GA, Blinder KJ, Paris CL, et al. A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg. 1991;22:102–8.
Tezel TH, Del Priore LV, Berger AS, Kaplan HJ. Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am J Ophthalmol. 2007;143:584–95. https://doi.org/10.1016/j.ajo.2006.12.007.
Radtke ND, Aramant RB, Petry HM, et al. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146:172–82. https://doi.org/10.1016/j.ajo.2008.04.009.
Binder S, Stolba U, Krebs I, et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: a pilot study. Am J Ophthalmol. 2002;133:215–25. https://doi.org/10.1016/S0002-9394(01)01373-3.
da Cruz L, Chen FK, Ahmado A, et al. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26:598–635. https://doi.org/10.1016/j.preteyeres.2007.07.001.
Blenkinsop TA, Saini JS, Maminishkis A, et al. Human adult retinal pigment epithelial stem cell-derived RPE monolayers exhibit key physiological characteristics of native tissue. Investig Ophthalmol Vis Sci. 2015;56:7085–99. https://doi.org/10.1167/iovs.14-16246.
Salero E, Blenkinsop TA, Corneo B, et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012;10:88–95. https://doi.org/10.1016/j.stem.2011.11.018.
Zhao C, Wang Q, Temple S. Stem cell therapies for retinal diseases: recapitulating development to replace degenerated cells. Development. 2017;144:1368–81. https://doi.org/10.1242/dev.133108.
Davis RJ, Blenkinsop TA, Campbell M, et al. Human RPE stem cell-derived RPE preserves photoreceptors in the Royal College of Surgeons rat: method for quantifying the area of photoreceptor sparing. J Ocul Pharmacol Ther. 2016;32:304–9. https://doi.org/10.1089/jop.2015.0162.
Skottman H. Derivation and characterization of three new human embryonic stem cell lines in Finland. In Vitro Cell Dev Biol Anim. 2010;46:206–9. https://doi.org/10.1007/s11626-010-9286-2.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;107:861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Brandl C, Grassmann F, Riolfi J, Weber B. Tapping stem cells to target AMD: challenges and prospects. J Clin Med. 2015;4:282–303. https://doi.org/10.3390/jcm4020282.
Huang K, Shen Y, Xue Z, et al. A panel of CpG methylation sites distinguishes human embryonic stem cells and induced pluripotent stem cells. Stem Cell Reports. 2014;2:36–43. https://doi.org/10.1016/j.stemcr.2013.11.003\rS2213-6711(13)00128-8. [pii]
Chin MH, Mason MJ, Xie W, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–23. https://doi.org/10.1016/j.stem.2009.06.008.
Nishino K, Toyoda M, Yamazaki-Inoue M, et al. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet. 2011;7:7. https://doi.org/10.1371/journal.pgen.1002085.
Polo JM, Liu S, Figueroa ME, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–55. https://doi.org/10.1038/nbt.1667.
Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res. 2014;123:141–50. https://doi.org/10.1016/j.exer.2013.09.003.
Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6. https://doi.org/10.1038/nature09941.
Klimanskaya I, Hipp J, Rezai KA, et al. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–45. https://doi.org/10.1089/clo.2004.6.217.
Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–34. https://doi.org/10.1002/stem.189.
Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:4. https://doi.org/10.1371/journal.pone.0008152.
Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–31. https://doi.org/10.1016/j.neulet.2009.04.035.
Song MJ, Bharti K. Looking into the future: using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling. Brain Res. 2016;1638:2–14. https://doi.org/10.1016/j.brainres.2015.12.011.
Crocco MC, Fratnz N, Bos-Mikich A. Substrates and supplements for hESCs: a critical review. J Assist Reprod Genet. 2013;30:315–23. https://doi.org/10.1007/s10815-012-9914-8.
Skottman H, Hovatta O. Culture conditions for human embryonic stem cells. Reproduction. 2006;132:691–8.
Unger C, Skottman H, Blomberg P, et al. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet. 2008;17:R48–53. https://doi.org/10.1093/hmg/ddn079.
Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–99. https://doi.org/10.1089/clo.2006.8.189.
Vugler A, Carr A-J, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214:347–61. https://doi.org/10.1016/j.expneurol.2008.09.007.
Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–703. https://doi.org/10.1073/pnas.0905245106.
Vaajasaari H, Ilmarinen T, Juuti-Uusitalo K, et al. Toward the defined and xeno-free differentiation of functional human pluripotent stem cell-derived retinal pigment epithelial cells. Mol Vis. 2011;17:558–75.
Buchholz DE, Pennington BO, Croze RH, et al. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med. 2013;2:384–93. https://doi.org/10.5966/sctm.2012-0163.
Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. https://doi.org/10.1016/j.stem.2009.07.002.
Maruotti J, Sripathi SR, Bharti K, et al. Small-molecule–directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc Natl Acad Sci. 2015;112:10950–5. https://doi.org/10.1073/pnas.1422818112.
Rowland TJ, Blaschke AJ, Buchholz DE, et al. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J Tissue Eng Regen Med. 2013;7:642–53. https://doi.org/10.1002/term.1458.
Zhu D, Deng X, Spee C, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Investig Ophthalmol Vis Sci. 2011;52:1573–85. https://doi.org/10.1167/iovs.10-6413.
Hongisto H, Ilmarinen T, Vattulainen M, et al. Xeno- and feeder-free differentiation of human pluripotent stem cells to two distinct ocular epithelial cell types using simple modifications of one method. Stem Cell Res Ther. 2017;8:291. https://doi.org/10.1186/s13287-017-0738-4.
Sorkio A, Hongisto H, Kaarniranta K, et al. Structure and barrier properties of human embryonic stem cell-derived retinal pigment epithelial cells are affected by extracellular matrix protein coating. Tissue Eng Part A. 2014;20:622–34. https://doi.org/10.1089/ten.TEA.2013.0049.
Abu Khamidakh AE, Dos Santos FC, Skottman H, et al. Semi-automatic method for Ca2+ imaging data analysis of maturing human embryonic stem cells-derived retinal pigment epithelium. Ann Biomed Eng. 2016;44:3408–20. https://doi.org/10.1007/s10439-016-1656-9.
Singh R, Phillips MJ, Kuai D, et al. Functional analysis of serially expanded human iPS cell-derived RPE cultures. Investig Ophthalmol Vis Sci. 2013;54:6767–78. https://doi.org/10.1167/iovs.13-11943.
Croze RH, Thi WJ, Clegg DO. ROCK inhibition promotes attachment, proliferation, and wound closure in human embryonic stem cell-derived retinal pigmented epithelium. Transl Vis Sci Technol. 2016;5(6):7. https://doi.org/10.1167/tvst.5.6.7.
Juuti-Uusitalo K, Delporte C, Gregoire F, et al. Aquaporin expression and function in human pluripotent stem cell-derived retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2013;54:3510–9. https://doi.org/10.1167/iovs.13-11800.
Juuti-Uusitalo K, Nieminen M, Treumer F, et al. Effects of cytokine activation and oxidative stress on the function of the human embryonic stem cell-derived retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:6265–74. https://doi.org/10.1167/iovs.15-17333.
Choudhary P, Whiting PJ. A strategy to ensure safety of stem cell-derived retinal pigment epithelium cells. Stem Cell Res Ther. 2016;7:127. https://doi.org/10.1186/s13287-016-0380-6.
Kanemura H, Go MJ, Shikamura M, et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One. 2014;9:1–11. https://doi.org/10.1371/journal.pone.0085336.
Kawamata S, Kanemura H, Sakai N, et al. Design of a tumorigenicity test for induced pluripotent stem cell (iPSC)-derived cell products. J Clin Med. 2015;4:159–71. https://doi.org/10.3390/jcm4010159.
Kuroda T, Yasuda S, Kusakawa S, et al. Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PLoS One. 2012;7:1–9. https://doi.org/10.1371/journal.pone.0037342.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–32. https://doi.org/10.1038/nm1181.
Sakamoto N, Tsuji K, Muul LM, et al. Bovine apolipoprotein B-100 is a dominant immunogen in therapeutic cell populations cultured in fetal calf serum in mice and humans. Blood. 2007;110:501–8. https://doi.org/10.1182/blood-2007-01-066522.
Carr A-J, Vugler A, Lawrence J, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–95.
Kamao H, Mandai M, Okamoto S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2:205–18. https://doi.org/10.1016/j.stemcr.2013.12.007.
Liao JL, Yu J, Huang K, et al. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. 2010;19:4229–38. https://doi.org/10.1093/hmg/ddq341.
Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–35. https://doi.org/10.1002/stem.149.
Juuti-Uusitalo K, Vaajasaari H, Ryhänen T, et al. Efflux protein expression in human stem cell-derived retinal pigment epithelial cells. PLoS One. 2012;7:e30089. https://doi.org/10.1371/journal.pone.0030089.
Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011;29:825–35. https://doi.org/10.1002/stem.635.
Miyagishima KJ, Wan Q, Corneo B, et al. In pursuit of authenticity: induced pluripotent stem cell-derived retinal pigment epithelium for clinical applications. Stem Cells Transl Med. 2016;5:1562–74. https://doi.org/10.5966/sctm.2016-0037.
Chang YC, Chang WC, Hung KH, et al. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front Aging Neurosci. 2014;6:191. https://doi.org/10.3389/fnagi.2014.00191.
Juuti-Uusitalo K, Delporte C, Grégoire F, et al. Aquaporin expression and function in human pluripotent stem cell–derived retinal pigmented epithelial cells. Invest Opthalmol Vis Sci. 2013;54:3510. https://doi.org/10.1167/iovs.13-11800.
Bharti K, Miller SS, Arnheiter H. The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011;24:21–34. https://doi.org/10.1111/j.1755-148X.2010.00772.x.
Bharti K, Rao M, Hull SC, et al. Developing cellular therapies for retinal degenerative diseases. Investig Ophthalmol Vis Sci. 2014;55:1191–201. https://doi.org/10.1167/iovs.13-13481.
Yvon C, Ramsden CM, Lane A, et al. Using stem cells to model diseases of the outer retina. Comput Struct Biotechnol J. 2015;13:382–9. https://doi.org/10.1016/j.csbj.2015.05.001.
Al-Nawaiseh S, Thieltges F, Liu Z, et al. A step by step protocol for subretinal surgery in rabbits. J Vis Exp. 2016;(115):53927. https://doi.org/10.3791/53927.
Brant Fernandes RA, Koss MJ, Falabella P, et al. An innovative surgical technique for subretinal transplantation of human embryonic stem cell-derived retinal pigmented epithelium in Yucatan mini pigs: preliminary results. Ophthalmic Surg Lasers Imaging Retina. 2016;47:342–51. https://doi.org/10.3928/23258160-20160324-07.
Stanzel BV, Liu Z, Brinken R, et al. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Invest Ophthalmol Vis Sci. 2012;53:490–500. https://doi.org/10.1167/iovs.11-8260.
Thieltges F, Liu Z, Brinken R, et al. Localized RPE removal with a novel instrument aided by viscoelastics in rabbits. Transl Vis Sci Technol. 2016;5:11. https://doi.org/10.1167/tvst.5.3.11.
Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109.
Edwards RB, Szamier RB. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 1977;197:1001–3.
Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192:799–801.
Haruta M, Sasai Y, Kawasaki H, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2004;45:1020–5.
Krohne TU, Westenskow PD, Kurihara T, et al. Generation of retinal pigment epithelial cells from small molecules and OCT4 reprogrammed human induced pluripotent stem cells. Stem Cells Transl Med. 2012;1:96–109. https://doi.org/10.5966/sctm.2011-0057.
Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 2013;54:5087–96. https://doi.org/10.1167/iovs.12-11239.
Kamao H, Mandai M, Ohashi W, et al. Evaluation of the surgical device and procedure for extracellular matrix–scaffold–supported human iPSC–derived retinal pigment epithelium cell sheet transplantation. Investig Opthalmol Vis Sci. 2017;58(1):211–20. https://doi.org/10.1167/iovs.16-19778.
Shi G, Maminishkis A, Banzon T, et al. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49:4620–30. https://doi.org/10.1167/iovs.08-1816.
Petrus-Reurer S, Bartuma H, Aronsson M, et al. Integration of subretinal suspension transplants of human embryonic stem cell-derived retinal pigment epithelial cells in a large-eyed model of geographic atrophy. Invest Ophthalmol Vis Sci. 2017;58(2):1314–22. https://doi.org/10.1167/iovs.16-20738doi.
Sugino IK, Gullapalli VK, Sun Q, et al. Cell-deposited matrix improves retinal pigment epithelium survival on aged submacular human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2011;52:1345–58. https://doi.org/10.1167/iovs.10-6112.
Sugino IK, Sun Q, Wang J, et al. Comparison of FRPE and human embryonic stem cell-derived RPE behavior on aged human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2011;52:4979–97. https://doi.org/10.1167/iovs.10-5386.
Tezel TH, Del Priore LV. Reattachment to a substrate prevents apoptosis of human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 1997;235:41–7.
Booij JC, Baas DC, Beisekeeva J, et al. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29:1–18. https://doi.org/10.1016/j.preteyeres.2009.08.003.
Ramrattan RS, van der Schaft TL, Mooy CM, et al. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–64.
Gullapalli VK, Sugino IK, Van Patten Y, et al. Impaired RPE survival on aged submacular human Bruch’s membrane. Exp Eye Res. 2005;80:235–48. https://doi.org/10.1016/j.exer.2004.09.006.
Sugino IK, Rapista A, Sun Q, et al. A method to enhance cell survival on Bruch’s membrane in eyes affected by age and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:9598–609. https://doi.org/10.1167/iovs.11-8400.
Binder S. Scaffolds for retinal pigment epithelium (RPE) replacement therapy. Br J Ophthalmol. 2011;95:441–2. https://doi.org/10.1136/bjo.2009.171926.
Hynes SR, Lavik EB. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch Clin Exp Ophthalmol. 2010;248:763–78. https://doi.org/10.1007/s00417-009-1263-7.
Pennington BO, Clegg DO. Pluripotent stem cell-based therapies in combination with substrate for the treatment of age-related macular degeneration. J Ocul Pharmacol Ther. 2016;32:261–71. https://doi.org/10.1089/jop.2015.0153.
Sorkio A, Haimi S, Verdoold V, et al. Poly(trimethylene carbonate) as an elastic biodegradable film for human embryonic stem cell-derived retinal pigment epithelial cells. J Tissue Eng Regen Med. 2017;11:3134–44. https://doi.org/10.1002/term.2221.
Akrami H, Soheili Z-S, Sadeghizadeh M, et al. Evaluation of RPE65, CRALBP, VEGF, CD68, and tyrosinase gene expression in human retinal pigment epithelial cells cultured on amniotic membrane. Biochem Genet. 2011;49:313–22. https://doi.org/10.1007/s10528-010-9409-1.
Kiilgaard JF, Scherfig E, Prause JU, la Cour M. Transplantation of amniotic membrane to the subretinal space in pigs. Stem Cells Int. 2012;2012:716968–5. https://doi.org/10.1155/2012/716968.
Nicolini J, Kiilgaard JF, Wiencke AK, et al. The anterior lens capsule used as support material in RPE cell-transplantation. Acta Ophthalmol Scand. 2000;78:527–31.
Walters NJ, Gentleman E. Evolving insights in cell-matrix interactions: elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater. 2015;11:3–16. https://doi.org/10.1016/j.actbio.2014.09.038.
Rahmany MB, Van Dyke M. Biomimetic approaches to modulate cellular adhesion in biomaterials: a review. Acta Biomater. 2013;9:5431–7. https://doi.org/10.1016/j.actbio.2012.11.019.
Lee J, Tae G, Kim YH, et al. The effect of gelatin incorporation into electrospun poly(L-lactide-co-epsilon-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials. 2008;29:1872–9. https://doi.org/10.1016/j.biomaterials.2007.12.029.
Sorkio A, Porter PJ, Juuti-Uusitalo K, et al. Surface modified biodegradable electrospun membranes as a carrier for human embryonic stem cell-derived retinal pigment epithelial cells. Tissue Eng Part A. 2015;21:2301–14. https://doi.org/10.1089/ten.tea.2014.0640.
Calejo MT, Ilmarinen T, Jongprasitkul H, et al. Honeycomb porous films as permeable scaffold materials for human embryonic stem cell-derived retinal pigment epithelium. J Biomed Mater Res A. 2016;104:1646–56. https://doi.org/10.1002/jbm.a.35690.
Ilmarinen T, Hiidenmaa H, Kööbi P, et al. Ultrathin polyimide membrane as cell carrier for subretinal transplantation of human embryonic stem cell derived retinal pigment epithelium. PLoS One. 2015;10:e0143669. https://doi.org/10.1371/journal.pone.0143669.
Sorkio AE, Vuorimaa-Laukkanen EP, Hakola HM, et al. Biomimetic collagen I and IV double layer Langmuir–Schaefer films as microenvironment for human pluripotent stem cell derived retinal pigment epithelial cells. Biomaterials. 2015;51:257–69. https://doi.org/10.1016/j.biomaterials.2015.02.005.
Wang C, Stewart RJ, Kopecek J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature. 1999;397:417–20. https://doi.org/10.1038/17092.
Warnke PH, Alamein M, Skabo S, et al. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013;9:9414–22. https://doi.org/10.1016/j.actbio.2013.07.029.
Xiang P, Wu K-C, Zhu Y, et al. A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials. 2014;35:9777–88. https://doi.org/10.1016/j.biomaterials.2014.08.040.
Stanzel BV, Liu Z, Somboonthanakij S, et al. Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Reports. 2014;2:64–77. https://doi.org/10.1016/j.stemcr.2013.11.005.
Koss MJ, Falabella P, Stefanini FR, et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatan minipigs. Graefes Arch Clin Exp Ophthalmol. 2016;254:1553–65. https://doi.org/10.1007/s00417-016-3386-y.
Subrizi A, Hiidenmaa H, Ilmarinen T, et al. Generation of hESC-derived retinal pigment epithelium on biopolymer coated polyimide membranes. Biomaterials. 2012;33:8047–54. https://doi.org/10.1016/j.biomaterials.2012.07.033.
Schwartz SD, Hubschman J-P, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–20. https://doi.org/10.1016/S0140-6736(12)60028-2.
Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16. https://doi.org/10.1016/S0140-6736(14)61376-3.
Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–46. https://doi.org/10.1056/NEJMoa1608368.
Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33:890–1. https://doi.org/10.1038/nbt0915-890.
Coffey P. Human embryonic stem cell derived retinal pigment epithelium transplantation in severe exudative age related macular degeneration: so far so visual. In: Annual ARVO 2017 meeting, Baltimore USA; 2017.
da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328–37. https://doi.org/10.1038/nbt.4114.
Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018;10:eaao4097. https://doi.org/10.1126/scitranslmed.aao4097.
Doi A, Park I-H, Wen B, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–3. https://doi.org/10.1038/ng.471.
Howden SE, Gore A, Li Z, et al. Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc Natl Acad Sci U S A. 2011;108:6537–42. https://doi.org/10.1073/pnas.1103388108.
Sugita S, Iwasaki Y, Makabe K, et al. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Reports. 2016;7:619–34. https://doi.org/10.1016/j.stemcr.2016.08.011.
Sugita S, Iwasaki Y, Makabe K, et al. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Reports. 2016;7:635–48. https://doi.org/10.1016/j.stemcr.2016.08.010.
Gornalusse GG, Hirata RK, Funk SE, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol. 2017;35:765–72. https://doi.org/10.1038/nbt.3860.
Meyer JS, Howden SE, Wallace KA, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–18. https://doi.org/10.1002/stem.674.
Singh MS, Balmer J, Barnard AR, et al. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat Commun. 2016;7:13537. https://doi.org/10.1038/ncomms13537.
Marks PW, Witten CM, Califf RM. Clarifying stem-cell therapy’s benefits and risks. N Engl J Med. 2017;376:1007–9. https://doi.org/10.1056/NEJMp1613723.
Acknowledgements
Heidi Hongisto, Tanja Ilmarinen and Outi Paloheimo are acknowledged for the artwork of Figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Skottman, H. (2020). RPE and Stem Cell Therapy. In: Klettner, A., Dithmar, S. (eds) Retinal Pigment Epithelium in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-28384-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-28384-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28383-4
Online ISBN: 978-3-030-28384-1
eBook Packages: MedicineMedicine (R0)