Abstract
Valproic acid (VPA) has been reported to inhibit cancer cell growth and has therapeutic use in retinal diseases. However, the mechanism of this action remains unclear. In order to explore this mechanism, primary human retinal pigment epithelial (hRPE) cell cultures were established. Cell viability was assessed by the trypan blue exclusion method (T), and the cell proliferation was measured by 3H–thymidine incorporation (3H–thy). P38 synthesis was quantitated by using 14C-methionine-labeled P38 (14C-P38) by using P38-specific antibody. SB203580 (SB), a selective inhibitor of p38 MAPK, was also used to test the specificity of P38 stimulation. Antinuclear staining (NS) studies were performed by DAPI. Statistical significance was established by student’s t-test. We observed that VPA (1 mM) inhibited 10% fetal bovine serum (FBS)-stimulated cell proliferation (1.75 ± 0.37 vs. 3.25 ± 0.68 cells per 1 μl ± SEM, p < 0.05, n = 4). VPA also stimulated 14C-P38 synthesis in a dose-dependent manner. SB (30 μM) inhibited VPA (4 mM)-stimulated 14C-P38 synthesis (197.74 ± 41.17 vs. 425.89 ± 59.17, CPM ± SEM, p < 0.05, n = 4) and increased hRPE cell proliferation (1.79 ± 0.45 vs. 4.93 ± 1.12 cells per 1 μl ± SEM, p < 0.05, n = 4); NS demonstrated VPA-induced cell damage. We conclude that VPA inhibits hRPE cell growth via P38 MAP mechanism and may be of therapeutic value in treating or preventing proliferative eye diseases.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The human retinal pigment epithelium (hRPE) is a mitotically inactive monolayer of cells in the adult eye located under the sensory retina which plays a critical role in proper visual function (Boulton and Dayhaw-Barker 2001). Derangements in the hRPE cell morphology and function are involved in the pathogenesis of several eye conditions. Central among these impairments is pathological RPE cell proliferation and metaplasia , a phenomenon which is present in diseases such as proliferative vitreoretinopathy and in tumors of the choroid and inner retina (Stern and Temple 2015). Currently, the leading treatment strategy for pathological RPE proliferation in these settings includes surgery, anti-inflammatory agents, and anti-growth factor therapies (Sadaka and Giuliari 2012). However the prognosis for diseases involving RPE cell proliferation remains unsatisfactory. Therefore, it is necessary to identify novel molecular targets and therapies to improve outcomes for patients suffering from these sight-threatening conditions.
Valproic acid (VPA) is an epigenetic factor that can readily cross the blood-brain barrier and has been used clinically to treat epilepsy (Monti et al. 2009). Recently, VPA has been reported to inhibit cellular proliferation in cancer cells and has also shown potential for use in some retinal diseases. However, the mechanistic role of VPA in treating proliferative eye disorders in the hRPE is not yet well understood. This led us to examine the mechanism of VPA action on the proliferation of hRPE cells in vitro to improve our understanding of the possible role of VPA in treating vitreoretinal diseases.
2 Materials and Methods
2.1 Establishment and Maintenance of hRPE Cell Culture
Primary hRPE cell cultures were established and maintained using techniques developed in our laboratory as previously described (Weng et al. 2009). Specimens were obtained from disease-free eyes that were obtained from Eversight Michigan within 24 h of patient death. Posterior segments of eyes were preserved and rinsed with Hank’s balanced salt solution and plated with Ham’s F-12 nutrient medium containing 16% FBS, 100 U/ml penicillin, 100 microgram/ml streptomycin, and 0.075% (wt/vol) sodium bicarbonate. HRPE cells were detached under direct observation with a dissecting microscope and then placed in 16 mm Primaria plates and incubated at 37C in a 95% humidified air/5% CO2 incubator. Medium was replaced every 72 h until confluent. Primary cultures were washed with Hank’s balanced salt solution and then subcultured with 0.5 g/100 mL trypsin and 0.2 g/100 mL EDTA in Hank’s solution (Sigma T-3924) at 37C for 10 min as previously described.
2.2 Cellular Proliferation Assays
Human RPE cells were trypsinized and plated at 2 × 104 cells/well in 16-mm wells of 24-well plates containing growth medium. In order to measure a dose-response effect of FBS, 0.1–10% FBS in serum-free Ham’s F12 nutrient medium was added for 24 h. To examine the effect of FBS on cellular proliferation, cells were combined with either F-12 alone, FBS 0.1% in F12, FBS 1% in F-12, FBS 5% in F-12, or FBS 10% in F-12; cells were then pulsed with 1uCi/100ul of [H]thymidine and incubated at 37C for 48 h. Cells were washed with 0.5 mL ice-cold phosphate-buffered saline (PBS, pH 7.4) and 0.5 mL ice-cold 5% trichloroacetic acid, and 0.5 mL of 0.1 M NaOH was then added to each well. Cell lysate was added to 10 mL of scintillation fluid (EcoLite™) and counted in a Beckman scintillation counter. In a separate experiment, the effect of VPA on cellular proliferation was determined by adding VPA to 10% FBS-stimulated hRPE cell culture. This was compared to a hRPE cells in F-12 and with hRPE cell culture treated with 10% FBS in F-12 medium. The number of cells was determined as mentioned above. Last, 30 mM of SB203580, a known MAPK inhibitor , was added to cell culture with 1 mM VPA and 10% FBS to determine the mechanism of action of VPA.
2.3 14C Immunoprecipitation Assay
Human RPE cells were trypsinized and plated at 2 × 104 cells/well in 16-mm wells of 24-well plates containing growth medium and grown to confluence. To measure a dose-response effect of VPA, 0–4 mM VPA in serum-free Ham’s F-12 nutrient medium was added for 2 days. To examine the effect of VPA on P38 synthesis, cells were incubated with either F-12 alone, VPA at 0.1 mM, VPA at 1 mM, VPA at 2 mM, or VPA at 4 mM or VPA (1 or 4 mM) + SB203580 (SB) (30 μM); cells were then pulsed with 1 microCi/100 micro L of [14C] methionine and incubated at 37C for 48 h. Cells were washed with 0.5 mL ice-cold phosphate-buffered saline (PBS, pH 7.4), and then 200 microL of Zwittergent in 0.2% bovine serum albumin (BSA) was added to each well for 5 min. Cell lysate was transferred to microfuge tubes and precipitated using 10 microL of anti-P38 antibody (diluted to 1:500 with PBS). Cells were then refrigerated for 24 h at 4C. 10ul of protein A was then added to each tube and allowed to incubate for 1 h at room temperature. Cell lysate in the microfuge tubes was then centrifuged and the supernatant was discarded, and 0.5 ml of NaOH was added to each tube to dissolve the invisible precipitate . Cell lysate was then transferred to 10 mL of scintillation fluid (EcoLite™) and counted in Beckman scintillation counter.
2.4 Nuclear Staining
Human RPE cells were grown to confluence on glass coverslips placed in 6-well plates. Forty-eight hours later, F12, FBS 1% in F-12, or VPA 1–4 mM in F-12 was added to each well. Plates were incubated at 37C for another 24 h. Cells were washed twice with ice-cold phosphate-buffered saline (PBS, pH 7.4) and then fixed with formaldehyde in PBS for 20 min at room temperature. After two additional 10 min washes with ice-cold PBS to prevent nonspecific binding, 2 mL of Zwittergent in 0.2% BSA was added for 1 h. Coverslips were subsequently washed twice with Zwittergent in 0.2% BSA and twice with PBS before incubating with rhodamine-conjugated anti-rabbit IgG (diluted 1:100 in Zwittergent) for 1 h at room temperature . Cells were then treated 1:100 concentration of anti-P38 antibody (this data not shown). Coverslips were washed twice with Zwittergent and twice with PBS before being mounted and then viewed on a Nikon Eclipse E800 epifluorescence microscope in TRITC setting, captured with a Nikon DXM1200 digital camera, and digitized to jpg format using ACT-1 Version 2.62. All images were captured at 20× or 40× magnification with a 3-second shutter speed.
2.5 Statistical Analysis
The results are expressed as mean +/− the standard error of the mean (SEM) . A p-value was determined by student t-test. A p-value of less than or equal to 0.05 was considered significant. *, ** = significantly different from each other as determined by student t-test.
3 Results
3.1 Effect of FBS on hRPE Cell Proliferation
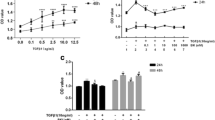
To create a model of HRPE cell proliferation in vitro for study, we treated hRPE cell cultures with FBS in a dose-dependent manner as previously described (Weng et al. 2009). FBS stimulated hRPE cell proliferation in a dose-dependent manner which became statistically significant at 10% FBS. In separate experiments FBS also stimulated 3H–thymidine incorporation in hRPE cells (data not shown) (Fig. 55.1).
3.2 Effect of VPA on hRPE Cell Proliferation
We sought to determine the effect of valproic acid in this FBS-stimulated hRPE cellular proliferation model. HRPE cells treated with 10% FBS in F-12 medium were also exposed to 1 mM VPA. VPA statistically and significantly inhibited hRPE cell proliferation in this FBS-stimulated hRPE cell model (Fig. 55.2).
3.3 Effect of VPA and SB on hRPE Cell Proliferation
SB203580, a known inhibitor of MAPK , was added to FBS-stimulated hRPE cells treated with VPA. VPA-stimulated inhibition of hRPE cellular proliferation was significantly reversed in cells treated with 30 μM SB203580. This suggests that the mechanism by which VPA inhibits FBS-stimulated hRPE cell proliferation involves the MAPK signaling pathway (Fig. 55.3).
3.4 Effect of VPA on Synthesis of p38 Protein
In order to ascertain the effect of VPA on p38 synthesis in hRPE cells, we treated hRPE cells with the increasing concentrations of VPA. Increasing concentrations of VPA increased immunoprecipitated p38 synthesis in hRPE cells in a dose-dependent manner (Fig. 55.4).
3.5 Effect of SB on Synthesis of Immunoprecipitated p38 Protein
To determine if MAPK p38 signaling was involved in the VPA stimulation of hRPE proliferation, we evaluated the effect of exposure to 30 μM SB203580, an inhibitor of MAPK p38 synthesis. SB decreased p38 immunoprecipitation in a statistically significant manner in hRPE cells (Fig. 55.5).
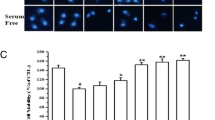
3.6 VPA Effect on the hRPE Cell Nucleus
To visualize the effect of VPA on hRPE cell morphology, nuclear staining studies were performed. In FBS-treated hRPE cells, hRPE cells remain intact, and there were very few damaged cells (Fig.55.6a). However, addition of VPA decreased the number of intact hRPE cells resulting in greater numbers of cells with damaged nuclei (Fig.55.6b).
4 Discussion
The therapeutic value of VPA in retinal disease is in a state of evolution. VPA has been shown to halt and reverse vision loss in patients with retinitis pigmentosa (Kumar et al. 2014; Iraha et al. 2016). The action and mechanism of VPA in treating atypical hRPE cellular proliferation however has not yet been fully understood.
Our experiments demonstrated that FBS-stimulated hRPE cell proliferation is significantly decreased with the addition of VPA, and fluorescence microscopy confirmed that cells treated with FBS alone proliferated in number and in viability in comparison to cells treated with VPA. In order to explore the mechanism of this action, we conducted several experiments using SB203580 , a known inhibitor of MAPK. We demonstrate that the administration of SB203580 significantly reversed the antiproliferative effects of VPA in hRPE. Additionally, we observed that with VPA alone, hRPE cells produced increased p38 synthesis. These experiments suggest that the mechanism by which VPA acts to reduce proliferation and induce apoptosis in hRPE cells involves stimulation of the MAPK signaling pathway with synthesis of p38 protein. Our results are supported by Xie et al. (2010) who demonstrate that VPA promotes p38 MAPK-mediated apoptosis in microglia.
Previously, our research group and others identified that VPA stimulates caspase-3, another known marker of apoptosis , in hRPE cells (Berner and Kleinman 2016). Our current study adds further evidence to the theory that VPA induces programmed cell death in RPE via several different mechanisms.
Conversely, our research group also previously demonstrated that VPA has a protective effect by downregulating P38 in hRPE cells exposed to oxidative damage, such as H2O2 (Kothary et al. 2016). These previous findings combined with our current study show that VPA may be acting by several different mechanisms to protect hRPE cell form and function, depending on the cellular environment .
These studies further support the possibility that VPA may be useful as a therapeutic agent to treat and/or even prevent the blinding complications in multiple vitreoretinal proliferative or neoplastic eye diseases . VPA has already been proven safe and useful as an anticonvulsant and in the treatment of cancer; however, studies like ours now highlight its potential future use in treating blinding retinal diseases as well.
References
Berner AK, Kleinman ME (2016) Therapeutic approaches to histone reprogramming in retinal degeneration. Adv Exp Med Biol 854:39–44
Boulton M, Dayhaw-Barker P (2001) The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 15:384–389
Iraha S, Hirami Y, Ota S, Sunagawa GA, Mandai M, Tanihara H, Takahashi M, Kurimoto Y (2016) Efficacy of valproic acid for retinitis pigmentosa patients: a pilot study. Clin Ophthalmol 10:1375–1384
Kothary PC, Rossi B, Del Monte MA (2016) Valproic acid induced human retinal pigment epithelial cell death as well as its survival after hydrogen peroxide damage is mediated by P38 Kinase. Adv Exp Med Biol 854:765–772
Kumar A, Midha N, Gogia V, Gupta S, Sehra S, Chohan A (2014) Efficacy of oral valproic acid in patients with retinitis pigmentosa. J Ocul Pharmacol Ther 30:580–586
Monti B, Polazzi E, Contestabile A (2009) Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol 2:95–109
Sadaka A, Giuliari GP (2012) Proliferative vitreoretinopathy: current and emerging treatments. Clin Ophthalmol 6:1325–1333
Stern J, Temple S (2015) Retinal pigment epithelial cell proliferation. Exp Biol Med (Maywood) 240:1079–1086
Weng CY, Kothary PC, Verkade AJ, Reed DM, Del Monte MA (2009) MAP kinase pathway is involved in IGF-1-stimulated proliferation of human retinal pigment epithelial cells (hRPE). Curr Eye Res 34:867–876
Xie N, Wang C, Lin Y, Li H, Chen L, Zhang T, Sun Y, Zhang Y, Yin D, Chi Z (2010) The role of p38 MAPK in valproic acid induced microglia apoptosis. Neurosci Lett 482:51–56
Acknowledgments
We thank Skillman Foundation for their support. This chapter is dedicated to late Dr. Sarla P. Kothary (wife of Dr. Piyush C Kothary). Sarla attended Retinal Degeneration (RD) meeting for two decades and forged friendship with many attendees and their spouses. PCK misses Sarla’s spirit of adventure. The next RD meetings will not be the same.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Anand, R., Kothary, P.C., Del Monte, M.A. (2018). Valproic Acid Inhibits Human Retinal Pigment Epithelial (hRPE) Cell Proliferation Via a P38 MAPK Signaling Mechanism. In: Ash, J., Anderson, R., LaVail, M., Bowes Rickman, C., Hollyfield, J., Grimm, C. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 1074. Springer, Cham. https://doi.org/10.1007/978-3-319-75402-4_55
Download citation
DOI: https://doi.org/10.1007/978-3-319-75402-4_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75401-7
Online ISBN: 978-3-319-75402-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)