Abstract
Age-related macular degeneration (AMD) is a leading cause of legal blindness in developed countries. Several new drugs are now available to reduce the sight threatening complications of this disease, however, all are useful in only a small fraction of patients and none of them prevents disease development. An understanding of the pathogenesis of the retinal and macular degeneration is the first step in developing preventive and fully effective treatment options for this condition. Lifelong oxidative stress seems to be an etiologic factor. In this study, we used cultured human retinal pigment epithelial cells to study the mechanism of cell death and survival in cells exposed to oxidative stress. Our studies demonstrate that valproic acid (VPA), an epigenetic factor, reduces apoptosis in hRPE cells that were subjected to hydrogen peroxide-induced oxidative injury by alteration in P38 kinase activity. Since VPA has been shown to have therapeutic use in other neuronal diseases, better understanding of the mechanism of this VPA anti-apoptotic activity may enhance its development as a therapeutic agent.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Retinal Pigment Epithelium
- Valproic Acid
- ARPE19 Cell
- Human Retinal Pigment Epithelial Cell
- Human Retinal Pigment Epithelium
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness in the industrial world. Lifelong oxidative stress of human retinal pigment epithelium (hRPE) has been implicated in the pathogenesis of AMD (Kothary et al. 2014) by production of reactive oxygen species (ROS), which can result in damage to hRPE.
The hRPE form a single layer of mitotically inactive cells that lie between the choroid and the neural retina. Pigment epithelial cells transport and store toxic nutrients for the photoreceptors and remove waste products such as shed photoreceptor segments. Damage to the RPE can affect the functioning of neurosensory retina.
Valproic acid (VPA) , an epigenetic factor, is a drug that is widely used to treat patients with epilepsy (Monti et al. 2009 and it also inhibits growth of some cancer cells. In addition, VPA has been shown to reduce cell death in ARPE19 cells that were subjected to oxidative injury. It is postulated that a cascade of signaling molecules may be involved in beneficial effect of VPA in the treatment of epilepsy and reduced cell death in ARPE 19 cells during oxidative stress.
MAP kinases are involved in cell proliferation and apoptosis (Wang et al. 1998; Kothary et al. 2008) . Previous studies have shown that extracellular signal-regulated kinase (ERK) is involved in proliferation where as P38 and STAT 3 (Kothary et al. 2004) are involved in cell death and cell survival (Gutierrez-Uzquiza et al. 2012) . In the present study, we have used hydrogen peroxide to induce oxidative stress in hRPE cells and investigated the effect of VPA on hRPE cell viability and P38 production, to determine if these factors may be involved in the molecular mechanisms related to cell survival.
2 Materials and Methods
2.1 Establishment and Maintenance of hRPE Cell Cultures
hRPE cells were collected from donor human eyes obtained from the Michigan Eye Bank, and differentiated primary cultures were established as described previously (Weng et al. 2009). In brief, cells were grown in an incubator at 37 °C in Ham’s F12 nutrient media until confluent, and then trypsinized and plated. The media in the cultured plates was changed every 3 days until experimental reagents were added.
2.2 Trypan Blue Exclusion Method
The procedure described in previous publication (Kothary et al. 2006) . Briefly, cell media was aspirated and cells were washed twice with F12. 3.0 mL Experimental reagents were added to each well. Plates were incubated at 37 °C for 48 h, and then media was aspirated. Cells were washed with 1 mL PBS and 1 mL Hank’s Buffer, and then 750 μL trypsin was added and mixed. After incubating 37 °C for 10 min, cell detachment was verified under a microscope and 10 uL trypan blue dye was added and mixed. Samples of cell mixture from each well were placed on a slide and transferred to a hemocytometer, where unstained and stained cells were counted in four different fields.
2.3 14 C-Methionine Assay
The procedure described in previous publication (Kothary et al. 2010) . Briefly, cell media was aspirated and cells were washed twice with F12. Experimental reagents were added, 0.5 mL to each well. After incubating at 37 °C for 1 h, 50 μL 14 C-methionine was added. Plates were incubated at 37°C for 24 h, then media was aspirated and cells were washed with 0.5 mL PBS and 200 μL Zwitteragent in 0.2 % BSA. Upon mixing, cells in Zwitteragent were transferred to microfuge tubes and 10 μL anti-P38 was added. Plates were refrigerated for 24 h, then 10 μL Protein A was added. After 1 h, tubes were centrifuged at 14,000 rpm for 5 min at 4 °C. The supernatant fluid was discarded, and 0.5 mL NaOH was added. Cells in NaOH were transferred to scintillation vials, and 10 mL Ecolite was added. After 1 h, 14 C-methionine incorporation was counted by a scintillation counter.
2.4 Nuclear Staining
Nuclear staining of hRPE cells after H2O2 and VPA treatment was performed by method described previously described by Weng et al 2009. Nuclear staining showed that H2O2 and VPA decreased the hRPE cell number (data not shown).
3 Results
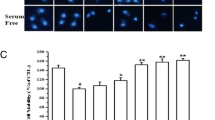
3.1 Effect of FBS on hRPE Cell Viability
Figure 102.1 shows hRPE cell proliferation is stimulated by increasing concentrations of FBS in a dose dependent manner.
3.2 Effect of H2O2 and VPA on hRPE Cell Viability
Figure 102.2a shows increasing concentrations of H2O2 decrease hRPE cell viability and proliferation to a limited extent.
Figure 102.2b shows increasing concentrations of VPA decrease hRPE cell proliferation.
3.3 Effect of VPA in Presence of H2O2 on hRPE Cell Viability
Figure 102.3 shows VPA (1 mM) eliminates the H2O2 (1 mM) reduction in hRPE cell proliferation.
3.4 Effect of VPA in 14 C-P38 Production
Figure 102.4 shows increasing concentrations of VPA increase 14 C-P38 synthesis in hRPE cells.
3.5 Effect of VPA in Presence of H2O2 on P38 Production
Figure 102.5 shows VPA (1 mM) eliminates the H2O2 (1 mM) induced increased 14 C-P38 synthesis in hRPE cells back to baseline.
4 Discussion
AMD affects millions of older people in the industrial world resulting in loss of central reading vision often to legal blindness. AMD is associated with progressive deterioration of the retinal pigment epithelium and lifelong oxidative stress seems to play a role. Therapeutically, invasive surgery e.g. laser photocoagulation of neovascular membranes, macular translocation surgery and recently discovered anti-VEGF medications have been used to treat these patients for stabilization of vision loss, but no successful preventive or fully restorative treatment has been discovered. Additional investigation of the molecular mechanism of this disease is required to develop better treatments. Therefore, our study aimed at understanding the role of the signaling molecule P38 MAPK in the survival of hRPE may aid in the development of pharmacological treatments for macular degeneration.
We have examined the nature of hydrogen peroxide induced oxidative stress in hRPE cells. Our goal was to determine the molecular expression of P38 in hRPE cells in presence and absence of hydrogen peroxide induced acute oxidative stress and the effect of adding VPA, a known inhibitor of oxidative damage, on P38 expression. We have shown that hRPE cells treated with H2O2 and VPA separately decreases hRPE cell proliferation and viability and increases P38 production. Xie et al. (2010) has shown that VPA increases P38 synthesis in microglia and that VPA induced microgia cell death is mediate by P38. Previously, we have shown that VPA treatment also increases caspase-3, a marker for apoptosis in hRPE cells.
We found that VPA reduces P38 synthesis and decreases cell death caused by H2O2 oxidative stress in cultured differentiated hRPE cells. Our data is in agreement with Gutierrez-Uzquiza et al. (2012) who showed P38 alpha mediates cell survival in response to oxidative stress. Others have shown that P38 activation may be linked mTOR inhibition (Chen et al. 2010; Pocrnich et al. 2009) . Further investigation of effect of VPA and H2O2 on mTOR expression may clarify the role of mTOR in P38 signaling. P38 may also be up regulating antioxidant gene expression, Gutierrez-Uzquiza et al. 2012) .
We conclude that VPA has a pro-survival function in H2O2 induced hRPE cell death because of its ability to down regulate P38. VPA is commonly used in the treatment of epilepsy, bipolar disease and cancers. These studies suggest that VPA may also have therapeutic value in the prevention or treatment of AMD as well.
References
Chen L, Xu B, Liu L et al (2010) Hydrogen peroxide inhibits mTOR signaling by activation of AMPK alpha leading to apoptosis of neuronal cells. Lab Invest 90:985–993
Gutierrez-Uzquiza A, Arechederra M, Bragado P et al (2012) p38a Mediates cell survival in response to oxidative stress via induction of antioxidant genes. Effect on the p70s6k pathway. J Biol Chem 287:2632–2642
Kothary PC, Del Monte MA (2008) A possible impaired signaling mechanism in human retinal pigment epithelial cells from patients with macular degeneration. Adv Exp Med Biol 613:269–275
Kothary PC, Badhwar J, Weng C et al (2010) Impaired intracellular signaling may allow up-regulation of CTGF-synthesis and secondary peri-retinal fibrosis in human retinal pigment epithelial cells from patients with age-related macular degeneration. Adv Exp Med Biol 664:419–428
Kothary PC, Lahiri R, Kee L et al (2006) Pigment epithelium-derived growth factor inhibits fetal bovine serum stimulated vascular endothelial growth factor synthesis in cultured human retinal pigment epithelial cells. Adv Exp Med Biol 572:513–518
Kothary PC, Lee P, Al-Khersan H, et al (2014) L-Ascorbic acid may protect against oxidative damage in hRPE cells by stimulating intracellular erythropoietin activity. Adv Med Biol 74:115–123
Kothary PC, Pauuw JD, Bansal AK, et al (2004) Inhibition of growth factor stimulated STAT3 by AG490 in human retinal pigment epithelial cells. In: Proceedings of 5th International Symposium on Ocular Pharmacology and Therapeutics, Medimond Srl Bologna, Italy, pp 237–241
Monti B, Polazzi E, Contestabile A (2009) Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol 2:95–109
Pocrnich CE, Liu H, Feng M et al (2009) p38 mitogen-activated protein kinase protects human retinal pigment epithelial cells exposed to oxidative stress. Can J Ophthalmol 44:431–436
Wang Y, Huang S, Sah VP et al (1998) Cardiac Muscle Cell Hypertrophy and Apoptosis Induced by Distinct Members of the p38 Mitogen-activated Protein Kinase Family. J Biol Chem 273:2161–2168
Weng CY, Kothary PC, Verkade AJ et al (2009) MAP kinase pathway is involved in IGF-1-stimulated proliferation of human retinal pigment epithelial cells (hRPE). Curr Eye Res 34:867–876
Xie N, Wang C, Lin Y, Li H et al (2010) The role of p38 MAPK in valproic acid induced microglia apoptosis. Neurosci Let 482:51–56
Acknowledgment
This study was supported by Skillman Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Kothary, P., Rossi, B., Del Monte, M. (2016). Valproic Acid Induced Human Retinal Pigment Epithelial Cell Death as Well as its Survival after Hydrogen Peroxide Damage is Mediated by P38 Kinase.. In: Bowes Rickman, C., LaVail, M., Anderson, R., Grimm, C., Hollyfield, J., Ash, J. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 854. Springer, Cham. https://doi.org/10.1007/978-3-319-17121-0_102
Download citation
DOI: https://doi.org/10.1007/978-3-319-17121-0_102
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17120-3
Online ISBN: 978-3-319-17121-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)