Abstract
Mood and behavior changes are part of the clinical presentation and/or course of the dementias. The treatment of these symptoms is complex, with significant side effects and/or paucity of data limiting evidence-based guidance for the treating physician. Non-pharmacological interventions are first-line treatment for the behavior and psychological symptoms of dementia (BPSD). However, severe mood and behavioral changes often warrant pharmacological intervention requiring detailed risk/benefit analysis regarding medication choice. This chapter focuses on the clinical treatment of BPSD pertinent for the treating neurologist, providing a synopsis of the evidence supporting medication use and practical guidance on treatment selection, monitoring, and discontinuation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Dementia, behavioral and psychological symptoms of dementia (BPSD)
- Neuropsychiatric symptoms of dementia
- Antipsychotic medication and dementia
- Antidepressant medications and dementia
Dementia is a term that includes multiple etiologies of cognitive impairment that significantly diminishes an individual’s level of daily functioning. Alzheimer’s disease (AD) is the most common form of dementia. AD is a progressive neurodegenerative disorder affecting memory and other cognitive domains interfering with daily functioning. Other dementias, such as vascular dementia, Lewy body dementia, and frontotemporal dementia, are less common but also cause considerable distress to patients and caregivers. With all dementias, behavior and mood changes often manifest as the neurodegenerative disorder progresses. These behavior and mood changes are either referred to as the behavioral and psychological symptoms of dementia (BPSD) or the neuropsychiatric symptoms of dementia (NPS) . This chapter focuses on the treatment of BPSD, as these symptoms frequently facilitate caregiver stress and nursing home placement [1, 2].
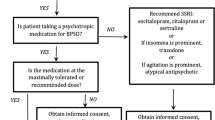
There are three main categories of BPSD, including agitation/aggression, psychosis, and mood disorders. Greater than 90% of patients with dementia develop at least one of these behavioral symptoms in a 5-year period, with 85% of the symptoms rising to the level of clinical significance [3]. Thus, it is imperative that physicians who frequently diagnose and treat patients with dementia are aware of the non-pharmacological and pharmacological treatments available for BPSD. This chapter will first present data for non-pharmacologic treatments of BPSD, as they are the recommended first-line interventions [4,5,6] (Figs. 7.1 and 7.2). However, the majority of the chapter will review the pharmacological options available to treat BPSD that fail to respond to non-pharmacological interventions.
Treatment of Agitation in Dementia
Agitation in individuals with dementia manifests in a variety of ways including restlessness, pacing, fidgeting, repetitive motor activities, and abnormal vocalizations. Physicians should first consider the etiology of the agitation; for example, does the patient have an infection such as a UTI and is the patient in pain, delirious, and frustrated by apraxia or word-finding difficulties? Addressing the etiology of the agitation is the most useful first step. However, the symptom of agitation itself may require direct treatment, while the underlying etiology is under investigation.
Non-pharmacological Interventions
There are few randomized, controlled trials examining non-pharmacological treatments of agitation in settings other than care home placement. A recent systematic meta-analysis of non-pharmacological interventions for agitation [7] found significant improvement with caregiver training . Specifically, improving communication, person-centered care, and dementia care mapping within the care home each significantly reduced agitation. Follow-up showed that improvement continued 3–6 months after intervention, demonstrating efficacy with paid caregiver training even months after the intervention ceased. Protocol-based activity and music therapy were also beneficial interventions. However, the benefits persisted only during the therapeutic intervention, with no sustained benefit at follow-up [7,8,9,10]. In contrast, interventions such as touch therapy were not beneficial, even during the therapeutic intervention [7]. Aromatherapy has shown some benefit in select studies, but the benefits were not replicated in blinded trials [7, 11, 12]. Similarly, there is insufficient evidence regarding the efficacy of exercise for agitation in dementia [7].
Pharmacological Interventions
Pharmacological interventions for agitation and aggression in dementia are at times necessary based on potential danger to the patient and/or caregivers, despite the lack of FDA-approved medications for these indications. In these situations, treating physicians must delicately weigh the risks versus benefits of available pharmacological interventions. Antipsychotic medications are commonly used to treat agitation and aggression in the elderly with dementia [13]. In 2005, the US Food and Drug Administration (FDA) released a warning that both typical and atypical antipsychotics are associated with increased mortality in the elderly with dementia. The FDA’s announcement was based on 17 placebo-controlled trials (15 positive trials) showing a 1.6- to 1.7-fold mortality increase in the elderly with behavioral disturbances and dementia. Increased mortality was related to both cardiac and noncardiac causes of death [14]. Currently, the American Geriatrics Society (AGS) Beers Criteria 2015 recommends that physicians “avoid antipsychotics for behavioral problems of dementia or delirium unless nonpharmacological options (e.g., behavioral interventions) have failed or are not possible and the older adult is threatening substantial harm to self or others” [15].
Medication Selection
Once physicians, patients, and/or surrogate decision-makers have jointly decided that an antipsychotic is necessary, which one should the prescriber choose? There is a large body of literature examining this question. A recent systematic review of meta-analyses addressing antipsychotic use in dementia provides considerable guidance for prescribing physicians [6]. One meta-analysis of typical antipsychotics for the treatment of BPSD concluded that typical antipsychotics were significantly more effective than placebo at treating symptoms of agitation in dementia, with a modest effect size (0.18). There was no difference in efficacy comparing haloperidol, thioridazine, or other typical antipsychotics [16]. However, other side effects, such as the anticholinergic side effects of thioridazine, must be considered in medication choice.
The 2016 review of meta-analyses included ten studies examining atypical antipsychotics for BPSD. The authors concluded that risperidone, olanzapine, and aripiprazole have significant benefit in treating agitation and aggression in dementia, with a modest effect size. Quetiapine did not significantly improve BPSD in dementia when compared to placebo [6]. Table 7.1 provides guidance on initiation, titration, and dosage recommendations for antipsychotic use in individuals with dementia, adapted from the APA’s practice guidelines for Alzheimer’s disease and other dementias [17] and guideline watch update from 2014 [18].
Adverse Events/Side Effects
Prescribing physicians must also consider the potential adverse effects related to antipsychotic use. Side effects such as akathisia, Parkinsonism, sedation, anticholinergic effects, cardiac conduction abnormalities, postural hypotension, urinary incontinence and urinary tract infections, and falls must be monitored in the elderly treated with antipsychotic medications [6, 17]. As discussed previously, increased risk of death, as well as an increased risk of cerebrovascular events, in elderly with dementia who are treated with antipsychotics (typical or atypical) is a significant risk that limits their use. Other serious adverse events include tardive dyskinesia, neuroleptic malignant syndrome, and metabolic syndrome [17].
Length of Treatment
How long should physicians prescribe an antipsychotic medication for agitation or aggression in individuals with dementia? Two meta-analyses [19, 20] are available to help guide this decision, as well as the American Psychiatric Association (APA) guideline on the use of antipsychotics for the treatment of agitation and psychosis in dementia [20]. Mortality rates were lower in individuals for whom antipsychotic discontinuation occurred, as opposed to those who continued treatment [20]. Discontinuation of antipsychotic medications can be well tolerated but has been shown to further aggravate BPSD in those with severe behavioral symptoms [6, 19, 20]. The APA recommends discontinuing antipsychotic medications after 4 weeks if there is limited efficacy at an adequate dose [21]. If there is clinically significant treatment efficacy, physicians should consider tapering and/or discontinuing the antipsychotic within 4 months of initiation, unless there has been a failed attempt at withdrawal. Symptoms should be monitored every month during the taper and for 4 months after discontinuing an antipsychotic medication. Another study looking specifically at subjects who had a significant clinical response to risperidone concluded that in those patients with dementia whose agitation and/or psychosis responded to risperidone which was continued for 4–8 months, discontinuation of the medication worsened relapse [22].
Are there other medication classes that are effective at treating agitation/aggression in individuals with dementia? Considering the increased risk of severe adverse events with antipsychotic medications, there is interest in identifying other medication classes with safer side-effect profiles for treatment of BPSD. Antidepressants have some data supporting their use. A Cochrane review of antidepressants for treating agitation and psychosis in dementia concluded that there is a paucity of high-quality data regarding efficacy of the antidepressants for this indication. They note that there is some evidence that sertraline, citalopram, and trazodone may help treat agitation and psychosis for some individuals with dementia [23]. The more recent Citalopram for Agitation in Alzheimer Disease Study (CitAD) , a randomized, double-blind, placebo-controlled, parallel study, examined the use of citalopram (10 mg titrated to a max of 30 mg daily) for BPSD in probable AD individuals. The authors concluded that in combination with a psychosocial intervention, citalopram showed a significant benefit in the treatment of agitation and reduced caregiver distress. However, there were significant cognitive side effects and prolonged QT intervals in the citalopram-treated individuals [24]. It is important to note that the FDA revised prescribing recommendation for citalopram in March 2012 to include the maximum recommended dosage for patients 60 years and older is now 20 mg daily, while the maximum dosage prescribed in the CitAD study was 30 mg daily [25]. Other medications, such as acetylcholinesterase inhibitors (e.g., galantamine 24 mg/day) and the NMDA receptor antagonist memantine, show very modest benefits in the treatment of agitation in dementia [26, 27]. There are other novel treatment investigations ongoing but remain investigational at this time [28]. The use of medications such as carbamazepine, valproate, beta blockers, and lithium for the treatment of agitation in dementia is not recommended at this time by the APA [17, 18].
In conclusion, the most data exists for using antipsychotic medications for the treatment of agitation and aggression in dementia. However, physicians, in consultation with patients and/or surrogate decision-makers, must carefully weigh the following options when non-pharmacological interventions are ineffective: the risks of treatment, the risks of untreated agitation/aggression, and the potential modest benefits of medication treatment. During this deliberation, other less-studied options, such as antidepressants, acetylcholinesterase inhibitors, and memantine, may be considered based on individual clinical factors.
Treatment of Psychosis in Dementia
Psychotic symptoms are common in the course of dementia, with 75% of patients experiencing some form of psychosis during the disease process [29]. Psychotic symptoms are part of the neurodegenerative progression of dementing illnesses and include delusions and hallucinations.
Similar to recommendations for agitation and aggression in dementia, pharmacological treatment of psychosis is appropriate if the symptoms are causing clinically significant distress and/or impacting the patient’s quality of life [21]. Although psychotic symptoms are common at some point in the course of dementia, if the symptoms are not causing significant consequences, they can be addressed with non-pharmacological interventions.
Medication Selection
It is helpful to consider the underlying type of dementia when tailoring interventions for individual patients. In patients with AD , antipsychotic medications are again the mainstay of treatment, with the most data supporting the use of risperidone for psychosis in dementia [6, 21]. Meta-analyses also demonstrate modest efficacy with the atypical antipsychotic medications, olanzapine and aripiprazole, with no significant efficacy seen with quetiapine [6]. Patients with Lewy body dementia or Parkinson’s dementia are more sensitive to the adverse effects of antipsychotic medications, in particular, extrapyramidal symptoms [30, 31]. Clinically, physicians most often prescribe quetiapine or clozapine in individuals with these particular types of dementia; however, there is very little evidence supporting this practice [21, 31]. Pimavanserin, a selective serotonin2A receptor inverse agonist, is a newer medication now FDA approved specifically for the treatment of hallucinations and delusions due to psychosis in patients with Parkinson’s disease. Further research and clinical experience will provide more information on the utility of this medication for the behavioral disturbances with specific dementias.
Adverse events and length of treatment for psychosis with antipsychotic medications are the same as discussed previously for agitation and aggression. There is not enough evidence supporting alternative medication classes for the treatment of psychosis in dementia [6, 32]. There is not sufficient evidence to support prescribing antidepressants specifically to address psychotic symptoms, despite the modest benefits noted when addressing agitation.
Treatment of Mood Disorders in Dementia
The relationship between mood disorders, specifically depression, and dementia is very complex. However, it is clear that depressive symptoms, which include mood changes, irritability, apathy, social isolation, thoughts of death, and suicidal ideation, are very common manifestations of neurodegenerative disorders. In the literature, rates of mood symptoms in individuals with dementia are between 10% and 86%, with 20–40% meeting full criteria for depression [33,34,35,36]. Thus, physicians commonly treating older adults with dementia must be familiar with the literature regarding current treatment for mood in this specific population.
Non-pharmacological Interventions
Non-pharmacological interventions for mood symptoms are also first line for patients with dementia. Unlike treatment of cognitively unimpaired older adults, beneficial therapeutic interventions require caregiver participation. Cognitive behavioral therapy (CBT) is an effective treatment modality for non-cognitively impaired older adults with depression and anxiety; however, adaptations are required for the cognitively impaired individual. For individuals with dementia, data support the efficacy of behavioral management techniques such as increased pleasant activities, behavioral problem-solving therapy, and structured life review [37,38,39,40]. For caregivers, CBT techniques targeting communication, caregiver stress, and reinforcement of the behavioral techniques helpful for the patient are effective psychosocial treatment modalities [7, 38, 40]. Thus, non-pharmacological interventions for mood disorders can be very effective, particularly when they incorporate caregivers into the treatment.
Pharmacological Interventions
Data for the efficacy of antidepressants in dementia show mixed results. However, SSRI antidepressants remain the mainstay of pharmacological treatment of depression in dementia with data supporting sertraline and citalopram [17, 41,42,43]. APA guidelines recommend avoiding cyclic antidepressants (such as amitriptyline) in the elderly with dementia, as they are less effective than other antidepressant medications and/or have more intolerable side effects. There is some evidence that psychostimulants such as methylphenidate (20 mg/day) can be an effective treatment for apathy in patients with dementia [17, 43,44,45]. Modafinil was not effective in treating apathy in a randomized, double-blind placebo-controlled study [46]. Electroconvulsive therapy (ECT) is another effective treatment strategy for elderly patients with depression, but there are limited data specifically studying ECT in elderly individuals with dementia [47,48,49]. Clinically, individuals with dementia and depression receive ECT if he or she has life-threatening symptoms (i.e., not eating) or has refractory depression to multiple antidepressant trials.
Adverse Events/Side Effects
SSRI antidepressants are the first-line pharmacological treatment in depressed elderly as they have less side effects than other antidepressant categories, such as the cyclic antidepressants and monoamine oxidase inhibitors (MAOIs), which have significant anticholinergic and cardiovascular side effects. Physicians prescribing SSRI antidepressants to elderly with dementia need to monitor for side effects including gastrointestinal side effects (nausea/vomiting), neurological side effects (Parkinsonism, akathisia), and sexual side effects, weight loss, and hyponatremia. There is an increased risk for falls in elderly individuals treated with SSRI medications [50]. Also, SSRIs and other antidepressants are metabolized through the cytochrome P450 system; thus, the prescriber must consider possible drug-drug interactions when prescribing antidepressants in the elderly [17].
Other antidepressants with less data in the elderly with dementia have specific side effects . Mirtazapine, a noradrenergic/specific serotonergic antidepressant, causes weight gain (which may be desirable in older adults with dementia) and sedation, with rare but serious side effects of liver toxicity and neutropenia. Venlafaxine, a serotonin-norepinephrine reuptake inhibitor (SNRI), can cause increased blood pressure during both treatment and discontinuation of the medication. Duloxetine, another SNRI, has no specific data supporting its use in the elderly population but is often used with depression and pain in older adults. Bupropion, a norepinephrine-dopamine reuptake inhibitor, lowers seizure threshold more than other antidepressants and would not be a good choice for older adults with known seizure disorder or other risk factors for seizures . Trazodone , a serotonin-2 antagonist/reuptake inhibitor, has common side effects of sedation and may be used at low doses for insomnia and/or agitation in this population; however, higher doses are required for depression and may cause sedation and orthostatic hypotension, increasing the risk of falls [17].
Length of Treatment
Based on the literature review while preparing this chapter, the recommendations were unclear regarding the length of treatment for depression in the elderly with dementia. According to the APA’s guidelines for the treatment of major depressive disorder in the elderly, the same length of treatment for the general adult population is recommended. Specifically, treatment with an effective antidepressant, at the full therapeutic dose, should be continued for 4–9 months, up to 12 months, after remission. If the individual has had three or more recurrent depressive episodes, or other factors that would warrant long-term treatment, medication should be continued indefinitely at the same dose that got the patient well. In the elderly population with dementia, mood symptoms can fluctuate; thus, determining the length of treatment depends on the specific clinical situation [17].
Case Report
Ms. A was a 51-year-old female with no past psychiatric history that self-presented with increased anxiety, irritability, and memory concerns. She reported using no alcohol or other illicit substances. Her family history was notable only for her maternal grandmother having AD and her mother having an unknown dementia in her 60s. Mental status exam was notable for a well-groomed female, good eye contact, very cooperative, increased psychomotor activity, very mild word-finding difficulty, logical thought process, no suicidal ideation, but perseverative concern with her memory and change in mood. She was visibly anxious, but alert and oriented, with good attention but poor recall, some repeating within 5–10 min. Labs (TSH, CBC, CMP, vitamin B12, folate, RPR) were within normal limits. We decided to first treat mood symptoms and then look at memory if not improved. We initiated fluoxetine 20 mg and CBT.
Visit 2: Ms. A’s anxiety and irritability were better but no change in memory. Her brain MRI was normal. We sent her for neuropsychological testing.
Visit 3: Ms. A developed myoclonic jerks while sleeping, possibly related to fluoxetine. Since she continued with some residual mood and anxiety symptoms, we changed fluoxetine to citalopram. Neuropsychological testing showed profound anterograde memory deficit, also visual memory and naming deficits. Her executive functioning was mildly impaired, though her anxiety did not fully account for the severity.
Ms. A was sent to a memory clinic, considering her very young age. PET imaging demonstrated biparietal hypometabolism, and CSF biomarkers were consistent with AD. She was diagnosed with Alzheimer’s disease and started on donepezil 10 mg daily. She was also continued on citalopram 20 mg daily. Her memory decline slowed over the next few months, and her mood and anxiety improved significantly.
At her last psychiatric visit, her AD was in moderate stage, so memantine was added, donepezil was continued, and her surrogate decision-maker requested a trial off citalopram (tapered 10 mg for 2 weeks, then discontinued). Her neuropsychiatric symptoms remained stable with this regimen.
Clinical Pearls
-
Behavioral and psychological symptoms of dementia occur in all dementia subtypes.
-
Non-pharmacological interventions are first-line treatments for BPSD.
-
Document a thorough risk/benefit discussion with the family in the patient’s chart if antipsychotics are the best option and if time permits (not emergency situation).
-
If medications are warranted, start at a low dose and titrate slowly, but the patient may require doses typical of other adults to have symptomatic relief.
-
Monitor carefully for side effects which are seen more frequently in the elderly with dementia.
-
At each visit, consider if the patient needs to continue BPSD medications.
Conclusion
Behavioral and psychological symptoms of dementia are common and part of the clinical presentation of any individual with dementia of any type. Physicians can best guide treatment strategies by considering the differential diagnosis of the emotion or behavior (i.e., is this agitation, aggression, psychosis, or a mood change?) and considering and treating any underlying etiology of that particular symptom. Non-pharmacological interventions should be first-line treatment for any BPSD. Pharmacological treatment initiation should ideally occur only after a full risk/benefit analysis is considered with the patient and/or surrogate decision-maker. When pharmacological treatment is warranted, close monitoring of side effects and regular consideration regarding the need for continued treatment are imperative.
References
Steele C, Rovner B, Chase BA, et al. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatr. 1990;147:1049–51.
Balestreri L, Grossberg A, Grossberg GT. Behavioral and psychological symptoms of dementia as a risk factor for nursing home placement. Int Psychogeriatr. 2000;12(Supplement 1):59–62.
Ballard CG, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5(5):245–55.
Kales H, Gitlin L, Lyketsos C. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62:762–9.
Kales H. Common sense: addressed to geriatric psychiatrists on the subject of behavioral and psychological symptoms of dementia. Am J Geriatr Psychiatry. 2015;23:1209–13.
Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis. 2016;7(5):229–45.
Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, Omar RZ, Katona C, Cooper C. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry. 2014;205(6):436–42.
Cooke ML, Moyle W, Shum DH, Harrison SD, Murfield JE. A randomized controlled trial exploring the effect of music on agitated behaviours and anxiety in older people with dementia. Aging Ment Health. 2010;14:905–16.
Lin Y, Chu H, Yang CY, Chen CH, Chen SG, Chang HJ, et al. Effectiveness of group music intervention against agitated behavior in elderly persons with dementia. Int J Geriatr Psychiatry. 2011;26:670–8.
Sung HC, Lee WL, Li TL, Watson R. A group music intervention using percussion instruments with familiar music to reduce anxiety and agitation of institutionalized older adults with dementia. Int J Geriatr Psychiatry. 2012;27:621–7.
Ballard CG, O’Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry. 2002;63:553–8.
Burns A, Perry E, Holmes C, Francis P, Morris J, Howes MJ, et al. A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31:158–64.
Zuidema S, Johansson A, Selbaek G, Murray M, Burns A, Ballard C, et al. A consensus guideline for antipsychotic drug use for dementia in care homes. Bridging the gap between scientific evidence and clinical practice. Int Psychogeriatr. 2015;27:1849–59.
FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. 2005. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed 20 Feb 2017.
By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46.
Schneider L, Pollock V, Lyness S. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38:553–63.
Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. American Psychiatric Association. 2007.
Guideline watch (October 2014): practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. American Psychiatric Association. 2014.
Declercq T, Petrovic M, Azermai M, Vander SR, De Sutter A, Van Driel M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
Pan Y, Wu C, Gau S, Chan H, Banerjee S. Antipsychotic discontinuation in patients with dementia: a systematic review and meta-analysis of published randomized controlled studies. Dement Geriatr Cogn Disord. 2014;37:125–40.
Reus VI, Fochtmann LJ, Evan Eyler A, Hilty DM, Horvitz-Lennon M, Jibson MD, Lopez OL, Mahoney J, Pasic J, Tan ZS, Wills CD, Rhoads R, Yager J. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatr. 2016;173(5):543–6.
Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497–507.
Seitz D, Adunuri N, Gill S, Gruneir A, Herrmann N, Rochon P. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;2:CD008191.
Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al., CitAD Research Group. Effect of citalopram on agitation in Alzheimer’s disease – the CitAD randomized controlled trial. JAMA: J Am Med Assoc. 2014;311(7):682–91.
FDA Drug Safety Communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. https://www.fda.gov/Drugs/DrugSafety/ucm297391.html. Accessed 27 Nov 2017.
Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1).
McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2).
Panza F, Solfrizzi V, Seripa D, Imbimbo B, Santamato A, Lozupone M, et al. Progresses in treating agitation: a major clinical challenge in Alzheimer’s disease. Expert Opin Pharmacother. 2015;16:2581–8.
Sadock BJ, Kaplan VA. Kaplan and Sadock’s concise textbook of clinical psychiatry. Philadelphia: Lippincott, Williams and Wilkins; 2008.
Aarsland D, Perry R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson’s disease and parkinsonian dementias. J Clin Psychiatry. 2005;66(5):633–7.
Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172(8):731–42.
Lochhead JD, Nelson MA, Maguire GA. The treatment of behavioral disturbances and psychosis associated with dementia. Psychiatr Pol. 2016;50(2):311–22.
Garre-Olmo J, López-Pousa S, Vilata-Franch J, Turon-Estrada A, Hernàndez-Ferràndiz M, Lozano-Gallego M, et al. Evolution of depressive symptoms in Alzheimer’s disease: one-year follow-up. Alzheimer Dis Assoc Disord. 2003;17(2):77–85.
Zubenko GS, Zubenko WN, McPherson S, Spoor E, Marin DB, Farlow MR, Sunderland T. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatr. 2003;160(5):857–66.
Lyketsos CG, Olin JT. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52(3):243–52.
García-Alberca JM. Cognitive-behavioral treatment for depressed patients with Alzheimer’s disease. An open trial. Arch Gerontol Geriatr. 2017;71:1–8.
Teri L, Logsdon RG, Uomoto J, McCurry SM. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci. 1997;52(4):159–66.
Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. J Am Med Assoc. 2003;290:2015–22.
Woods B, Spector A, Jones C, Orrell M, Davies S. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;18(2):CD001120.
Forstmeier S, Maercker A, Savaskan E, Roth T. Cognitive behavioural treatment for mild Alzheimer’s patients and their caregivers (CBTAC): study protocol for a randomized controlled trial. Trials. 2015;16:526.
Nyth AL, Gottfries CG, Lyby K, Smedegaard-Andersen L, Gylding-Sabroe J, Kristensen M, Refsum HE, Ofsti E, Eriksson S, Syversen S. A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatr Scand. 1992;86:138–45.
Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, Munro C, Baker AS, Sheppard JM, Frangakis C, Brandt J, Rabins PV. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60:737–46.
Rabins P, Rovner BW, Rummans T, et al. Guideline watch (October 2014): practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Am J Psychiatry. 2007 Dec;164(12 Suppl):5–56.
Galynker I, Ieronimo C, Miner C, Rosenblum J, Vilkas N, Rosenthal R. Methylphenidate treatment of negative symptoms in patients with dementia. J Neuropsychiatry Clin Neurosci. 1997;9:231–9.
Rosenberg PB, Lanctôt KL, Drye LT, Herrmann N, Scherer RW, Bachman DL, Mintzer JE. ADMET Investigators: safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74(8):810–6.
Frakey LL, Salloway S, Buelow M, Malloy P. A randomized, double-blind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer’s disease. J Clin Psychiatry. 2012;73(6):796–801.
Tew JD Jr, Mulsant BH, Haskett RF, Prudic J, Thase ME, Crowe RR, Dolata D, Begley AE, Reynolds CF III, Sackeim HA. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156:1865–70.
Rao V, Lyketsos CG. The benefits and risks of ECT for patients with primary dementia who also suffer from depression. Int J Geriatr Psychiatry. 2000;15:729–35.
O’Connor MK, Knapp R, Husain M, Rummans TA, Petrides G, Smith G, Mueller M, Snyder K, Bernstein H, Rush AJ, Fink M, Kellner C. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. report. Am J Geriatr Psychiatry. 2001;9:382–90.
Thapa PB, Gideon P, Cost TW, Milam AB, Ray WA. Antidepressants and the risk of falls among nursing home residents. N Engl J Med. 1998;339:875–82.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Newman, B.M. (2018). Treating the Behavioral Symptoms of Dementia. In: Grossberg, G., Kinsella, L. (eds) Clinical Psychopharmacology for Neurologists. Springer, Cham. https://doi.org/10.1007/978-3-319-74604-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-74604-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74602-9
Online ISBN: 978-3-319-74604-3
eBook Packages: MedicineMedicine (R0)