Abstract
Termites use a range of semiochemicals to maintain the organization and integrity in their colonies. Among these semiochemicals, the trail pheromone is responsible for the orientation and recruitment of nestmates from the nest to the food sources. Trail pheromones in termites are secreted by a unique exocrine gland source, the sternal gland present in the abdominal sternites of all termite castes. In the majority of termite species, trail pheromone comprises a single compound. However, in the most advanced species, trail pheromone comprises two or, in one exception case, a blend of three compounds. In general, there is a clear difference between composition of trail pheromone in termite species from basal families and from those of more advanced families. Distinct responses of workers and soldiers to trail pheromone are observed as well as the response to trail pheromone from neighbouring colonies. The present chapter outlines the current states of knowledge of trail pheromones in termites.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Insect societies (termites, ants, some bees and wasps) have been calling attention over years due to their ecological role, social evolution, collective behaviour and the efficient and spectacular ways of communication. Most of the activities in social insects are chemically mediated, which allow to maintain the organization and integrity in their colonies. Chemical communication is even more evident in termite species since they are blind, and semiochemicals are involved in almost all activities of their life. Among these activities, it is the exploration of environment in search of food resources (e.g. foraging process). Such collective behaviour is coordinated by trail pheromones, and in some termite species, for example, Constrictotermes cyphergaster (Termitidae: Nasutitermitinae), it is responsible for the establishment of impressive columns with thousand individuals (Fig. 7.1). The ecology and behavioural aspects of foraging in termites have been reviewed by Traniello and Robson (1995) and Traniello and Leuthold (2000). Recently, Almeida et al. (2016) have showed a new behavioural repertoire of foraging in termites.
Trail pheromone is secreted by sternal glands and is deposited when a termite presses its abdomen against the substrate (Fig. 7.2). Such pheromone stimulates foragers to leave the nest and orient them to the food source (Stuart 1961, 1981). Foraging seems to begin with an explanatory phase of searching for food, proceeding by the recruitment of the nestmate to the food source. In the explanatory phase, trail pheromone deposited appears less attractive than that deposited in the recruitment and foraging trails (Reinhard and Kaib 2001). Trail pheromone is responsible to trail-following behaviour. It is a decisive communication channel to those termite species that acquire their food resource outside of their nests (“separate” life type; Hodotermitidae, some Rhinotermitidae and almost all Termitidae) and also for species that consume a resource in which they nest or also those that move from one nesting site to another (“intermediate” life type; e.g. Mastotermitidae, Rhinotermitidae and some Termitidae). However, in “one-piece” termite species (e.g. Termopsidae and Kalotermitidae), trail pheromone is used to locate areas of disturbance and to colonize new food sources (Traniello and Leuthold 2000), because such species does not need orientation mechanisms to foraging.
According to Sillam-Dusses (2010), trail pheromone in “one-piece” termite species may be involved in the alarm communication, in which a trail laid by an alarmed termite from the point of disturbance leads the nestmates to defend and repair the disturbance point. Recently, Cristaldo et al. (2014) suggested that the obligatory inquiline Inquilinitermes microcerus (Termitidae, Termitinae) should use trail pheromone in similar way that in “one-piece” termite species, because such species seems not to forage outside from their host nest (Florencio et al. 2013; Barbosa-Silva et al. 2016). In this species, trail pheromone must be used to avoid contact with host colonies, inside the shared nest (Cristaldo et al. 2014).
In the last decades, numerous studies have shown the chemical nature of trail pheromones in different termites (see Costa-Leonardo et al. 2009; Sillam-Dusses 2010; Bordereau and Pasteels 2011). This chapter outlines the current status of knowledge of termite trail pheromones.
7.2 The Glandular Source of Trail Pheromone: The Sternal Gland
The sternal gland located in the abdominal sternites is the sole glandular source of trail pheromone in termites (Noirot 1969) (Fig. 7.2). The sternal gland has been observed in all castes and secretes not only the trail pheromone but also the sex pheromone (Noirot 1969; Bordereau and Pasteels 2011).
The number and position of sternal glands are variables across termite families (see Noirot 1995; Quennedey et al. 2008). In Mastotermitidae (Mastotermes darwiniensis), the most basal termite family, three separate sternal glands are present in the middle of the 3rd, 4th and 5th abdominal sternites. Although M. darwiniensis have three active sternal glands, their efficiency varies considerably. According to Sillam-Dusses et al. (2007), the trail pheromone secreted by the sternal gland present in the 4th abdominal sternites induces a higher activity, compared to that of the sternal gland present in the 3rd abdominal sternites. However, the trail pheromone produced by sternal glands from 5th abdominal sternites was almost inactive.
In other termite families, a single sternal gland is observed. In Termopsidae and Hodotermitidae species, the sternal gland is found in the margin of the 4th abdominal sternites. In Kalotermitidae, Rhinotermitidae, Serritermitidae and Termitidae, a single sternal gland is observed in the margin of the 5th abdominal sternite (Noirot 1995; Quennedey et al. 2008).
A decrease in the size of sternal glands is also observed among termite families, varying from the large one found in Mastotermitidae (200/300 μm × 450/800 μm × 15/25 μm) (length × width × height) to the very small sternal gland of Nasutitermitinae species (100 μm × 195 μm × 25 μm) (Quennedey et al. 2008).
Another interesting point is the presence of different cell classes in the sternal glands across termite families: class 1 and 2 cells are found in all termite species, while class 3 cells are found only in Termopsidae, Serritermitidae and Rhinotermitidae species (see details in Quennedey et al. 2008). The presence of different class cells can be related with semiochemicals secreted in the sternal glands that can have another role than the trail-following behaviour. In the dampwood termite Zootermopsis angusticollis (Archotermopsidae), for example, sternal gland secretions have been reported to have fungistatic properties (Rosengaus et al. 2004).
7.3 Chemistry of Trail Pheromones in Termites
7.3.1 Composition
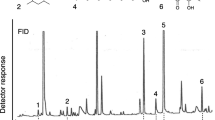
The trail pheromones have been identified in 66 species across 7 termite families (Table 7.1). Their chemical diversity is very low, with only nine compounds identified (Fig. 7.3; Table 7.1), belonging to four chemical classes (alcohols, aldehydes, hydrocarbons and ketones). In most of the termite species, the trail pheromone is made of a single compound while in others, particularly in Nasutitermitinae species, it is made of two compounds. The presence of three compounds acting as trail pheromone was described only in Nasutitermes corniger (see Sillam-Dusses et al. 2010).
The trail pheromone seems to be very conserved across termite families. The (E)-2,6,10-trimethyl-5,9-undecadien-1-ol (trimethylundecadienol; Fig. 7.3a) is the compound acting as trail pheromone in Mastotermes darwiniensis (Mastotermitidae) and also in two species of Termopsidae, Porotermes adamsoni (Porotermitinae) and Stolotermes victoriensis (Stolotermitinae) (Sillam-Dusses et al. 2007). In the family Archotermopsidae, syn 4,6-dimethyldodecanal (dimethyldodecanal; Fig. 7.3b) is the trail pheromone in Zootermopsis angusticollis and Z. nevadensis (Bordereau et al. 2010). However, in Hodotermopsis sjoestedti, the trail pheromone is composed of syn 4,6-dimethylundecan-1-ol (dimethylundecanol; Fig. 7.3c) and traces of dimethyldodecanal (Lacey et al. 2011). In Kalotermitidae, all species studied so far have (Z)-dodec-3-en-1-ol (dodecenol; Fig. 7.3d) as trail pheromone (Sillam-Dusses et al. 2009b). The (3Z,6Z,8E)-dodeca-3,6,8-trien-1-ol (dodecatrienol; Fig. 7.3g) is the trail pheromone in almost all species from Rhinotermitidae family (see Table 7.1 for references). The exception is the Prorhinotermes sp. that has (1E,5E,9E,12R)-1,5,9-trimethyl-12-(1-methylethenyl)-1,5,9-cyclotetradecatriene (neocembrene; Fig. 7.3h) or the mixture of neocembrene and dodecatrienol as trail pheromone (Sillam-Dusses et al. 2005, 2009a). In Serritermitidae family, it has been identified the (10Z,13Z)-nonadeca-10,13-dien-2-one (nonadecadienone; Fig. 7.3f) as trail pheromone of Glossotermes oculatus (Hanus et al. 2012).
In the advanced termite species (Termitidae), trail pheromone differs among subfamilies. Dodecenol (Fig. 7.3d) was identified in Macrotermitinae as trail pheromone of Macrotermes sp., Odontotermes hainanensis and O. maesodensis (Peppuy et al. 2001a), while dodecadienol (Fig. 7.3e) was identified as trail pheromone of Ancistrotermes pakistanicus (Robert et al. 2004) and O. formosanus (Deng et al. 2002). Dodecatrienol was identified as trail pheromone for Pseudacanthotermes militaris and P. spiniger (Bordereau et al. 1993).
Among Termitinae sp., dodecatrienol was identified as trail pheromone in Cubitermes sp., Drepanotermes perniger and Termes hispaniolae (Sillam-Dusses et al. 2006). The mixture of dodecatrienol and neocembrene was identified as trail pheromone in Amitermes evuncifer (Kotoklo et al. 2010) and Inquilinitermes microcerus (Cristaldo et al. 2014). Dodecatrienol is the trail pheromone in almost all species of Syntermitinae studied so far (Sillam-Dusses et al. 2006; Bordereau and Pasteels 2011). However, the mixture of dodecatrienol and neocembrene was identified as trail pheromone in Silvestritermes euamignatus (Bordereau and Pasteels 2011). In Nasutitermitinae species, the mixture of dodecatrienol and neocembrene was identified as trail pheromone in Constrictotermes cyphergaster (Sillam-Dusses et al. 2010; Cristaldo et al. 2014), some species of Nasutitermes genus and also in Trinervitermes geminatus and T. trinervoides (Sillam-Dusses et al. 2010). In N. corniger, the compound trinervitatriene (Fig. 7.3i) was also identified as trail pheromone, additionally to the mixture of dodecatrienol and neocembrene (Sillam-Dusses et al. 2010). Neocembrene was reported as single component of trail pheromones in N. exitiosus, N. graveolus, N. walker (Moore 1966; Birch et al. 1972) and in T. bettonianus (McDowell and Oloo 1984). The trail pheromone of termites from Apicotermitonae subfamily remains unidentified.
In general, there is a clear difference between trail pheromone from species of basal termites (from Mastotermitidae to Stolotermitidae) and those from more advanced families (from Kalotermitidae to Termitidae): basal termite species have trail pheromone made of C14 alcohol or a C14 or C18 aldehyde, while advanced termite species have trail pheromones made of C12 alcohols or C20 diterpenes. Such distinction seems to be related to their ecology and life style (Sillam-Dusses 2010).
7.3.2 Activity Threshold of Trail Pheromones
The trail pheromone activity threshold varies from 1 ng/cm in the most basal termite M. darwiniensis (Sillam-Dusses et al. 2007) to 10−8 ng/cm in Reticulitermes hesperus (Saran et al. 2007). Optimal activity threshold ranges, in most species, between 10−2 and 1 ng of pheromone per cm of trail (Bordereau and Pasteels 2011). Above 1 ng of trail pheromone, trail-following behaviour is generally reduced once chemoreceptors are saturated and termite is unable to follow the trails.
7.4 Responses of Workers and Soldiers to Trail Pheromone Signals
Different responses of termite castes (workers and soldiers) to the trail pheromone have been reported in the literature. In almost all termite species studied, the trail pheromone seems to be deposited only by workers during the recruitment phase (Costa-Leonardo et al. 2009). However, soldiers have been shown to be responsible for the initial exploration of foraging area, proceeded by the recruitment of workers in some Rhinotermitidae (R. santonensis (Reinhard and Kaib 2001), Heterotermes tenuis (Casarin et al. 2008) and Coptotermes intermedius (Olugbemi 2011)) and also in Nasutitermitinae species (N. corniger (Traniello 1981; Traniello and Busher 1985), Longipeditermes longipes (Miura and Matsumoto 1998), Constrictotermes cyphergaster (Moura et al. 2006) and N. aff. coxipoensis (Almeida et al. 2016)). In Coptotermes gestroi, workers initiate the foraging, but soldiers are recruited after discovery of the food source (Arab et al. 2012). Workers and soldiers have been reported to initiate the exploration of new areas in Velocitermes heteropterus (Haifig et al. 2015). According to Almeida et al. (2016), the participation of soldiers in the initial exploration of areas, with continued participation in trail construction and escorting services during tunnel construction, must be common among the Nasutitermitinae species.
The precise role of emission of trail pheromone and responses to these signals by the termite soldiers are still poorly explored. However, in Nasutitermes species, soldiers have been shown to deposit trails more attractive than those deposited by workers (see Arab et al. 2006; Almeida et al. 2016). In N. corniger, trails laid by soldiers were observed to recruit only soldiers, but those laid by workers recruited both workers and soldiers (Traniello 1981). These observed differences in the responses of castes to trail pheromone must be related to qualitative or quantitative differences between workers and soldiers, as already hypothesized by Arab et al. (2006) and Almeida et al. (2016).
Different responses to pheromone trails was also observed among small workers in M. bellicosus, preferentially following trails established by small workers than those established by large ones (Gessner and Leuthold 2001).
7.5 Detection of Trail Pheromones by Neighbouring Colonies
Although the ecological success of any organism depends on the specificity of its communication channels, perception of heterospecific signals by other individuals is widely observed in nature (Danchin et al. 2004; Valone 2007; Evans et al. 2009; Cristaldo et al. 2016b). The ability to perceive and respond to trail pheromones from neighbouring colonies can benefit the exploiter about food source location.
In termites, perception and response to trail pheromone from neighbouring colonies have been observed among neighbouring colonies of the same as well as of different species. Oloo (1981) observed that workers of Trinervitermes bettonianus do not show a significant preference to trails from their own colony compared with those from neighbouring colonies of the same species. However, T. bettonianus as well as the sympatric species M. michaelseni and Odontotermes sp. was not able to follow trail from neighbouring colonies of sympatric species. In another study, allopatric populations of T. bettonianus were able to follow alien as well as their own trails (Oloo and McDowell 1982). Similar results were also reported for R. grassei and R. santonensis (Wobst et al. 1999) and in P. spiniger and P. militaris (Bordereau and Pasteels 2011). According to Peppuy et al. (2001b), specific signals of trail pheromone must be more marked among species from different genera than among species from the same ones.
Recently, Cristaldo et al. (2016a) have shown that resource availability can modulate the perception and response of neighbouring colonies in Nasutitermes aff. coxipoensis. Workers from colonies reared under low and intermediate resource availability followed the same distance on trails with extracts from their own colonies, compared to extracts from neighbouring colonies. However, workers from colonies reared with high resource availability avoided the following chemical cues from neighbouring colonies. Such results indicate that chemical cues from neighbouring colonies can be detected by termites and may influence colony foraging choice.
The perception and response of chemical cues from neighbouring colonies should have a strong impact on termite community structure, including the spatial distribution of foraging areas, species co-occurrence and species coexistence in a single nest (termite inquilinism) (see details in Cristaldo et al. 2016a; Araújo et al. 2017). In fact, the perception and avoidance of trail cues from host nest by inquilines seem to ease the cohabitation among different termite species, in a single nest (see Cristaldo et al. 2014).
7.6 Conclusion
The study of trail pheromone in termites has been increased in the last decades; however, its composition in species of Apicotermitinae subfamily has not been studied yet. In general, the termite trail pheromone seems to be very conserved among families, with only nine compounds identified as trail pheromone. There is a clear difference between trail pheromone from basal termite species and that from more advanced families. Such distinction seems to be related with their ecological life style. In Nasutitermitinae species, trail pheromone deposited by soldiers plays a key role in the recruitment of workers, from the nest to the food source. Although few studies have reported the ability of termites to follow chemical cues from neighbouring colonies, such behaviour possibly has a strong impact on termite community structure.
References
Almeida, C. S., Cristaldo, P. F., Florencio, D. F., Cruz, N. G., Santos, A. A., Oliveira, A. P., Santana, A. S., Ribeiro, E. J. M., Lima, A. P. S., Bacci, L., & Araújo, A. P. A. (2016). Combined foraging strategy and soldier behaviour in Nasutitermes aff. coxipoensis (Blattodea: Termitoidea: Termitidae). Behavioural Processes, 126, 76–81.
Arab, A., Issa, S., Alfonzo, D., & Jaffe, K. (2006). Caste, colony, and species specificity of the trail pheromone in two sympatric Nasutitermitinae. Sociobiology, 47, 345–351.
Arab, A., Carollo Blanco, Y., & Costa-Leonardo, A. M. (2012). Dynamics of foraging and recruitment behavior in the Asian subterranean termite Coptotermes gestroi (Rhinotermitidae). Psyche, 2012, 7.
Araújo, A. P. A., Cristaldo, P. F., Florencio, D. F., Araújo, F. S., & DeSouza, O. (2017). Resource suitability modulating spatial co-occurrence of soil-forager termites (Blattodea: Termitoidea). Austral Entomology, 56, 235–243.
Barbosa-Silva, A. M., Farias, M. A. A., Mello, A. P., Souza, A. E. F., Garcia, H. H. M., & Bezerra-Gusmão, M. A. (2016). Lignocellulosic fungi in nests and food content of Constrictotermes cyphergaster and Inquilinitermes fur (Isoptera, Termitidae) from the semiarid region of Brazil. Fungal Ecology, 20, 75–78.
Birch, A. J., Brown, W. V., Corrie, J. E. T., & Moore, B. P. (1972). Neocembrene-A, a termite trail pheromone. Journal of the Chemical Society, Perkin Transactions, 1, 2653–2658.
Bordereau, C., & Pasteels, J. M. (2011). Pheromones and chemical ecology of dispersal and foraging in termites. In E. D. Bignell, Y. Roisin, & N. Lo (Eds.), Biology of termites: A modern synthesis (pp. 279–320). Dordrecht: Springer.
Bordereau, C., Robert, A., Laduguie, N., Bonnard, O., Le Quéré, J. L., & Yamaoka, R. (1993). Detection du (Z,Z,E)-3,6,8-dodecatrien-1-ol par les ouvriers et les essaimants de deux especes de termites champignonnistes: Pseudacanthotermes spiniger et P. militaris (Termitidae, Macrotermininae). Actes des Colloques Insectes Sociaux, 8, 145–149.
Bordereau, C., Lacey, M., Sémon, E., Braekman, J. C., Ghostin, J., Robert, A., Sherman, J. S., & Sillam-Dussès, D. (2010). Sex pheromones and trail-following pheromone in the basal termites Zootermopsis nevadensis (Hagen) and Z. angusticollis (Hagen) (Isoptera: Termopsidae: Termopsinae). Biological Journal of the Linnean Society, 100, 519–530.
Casarin, F. E., Costa-Leonardo, A. M., & Arab, A. (2008). Soldiers initiate foraging activities in the subterranean termite, Heterotermes tenuis. Journal of Insect Science, 8, 1–5.
Costa-Leonardo, A. M., Casarin, F. E., & Lima, J. T. (2009). Chemical communication in isoptera. Neotropical Entomology, 38, 747–752.
Cristaldo, P. F., DeSouza, O., Krasulová, J., Jirošová, A., Kutalová, K., Lima, E. R., Šobotník, J., & Sillam-Dussès, D. (2014). Mutual use of trail-following chemical cues by a termite host and its inquiline. PLoS One, 9, e85315.
Cristaldo, P. F., Araujo, A. P. A., Almeida, C. S., Cruz, N. G., Ribeiro, E. J. M., Rocha, M. L. C., Santana, A. S., Santos, A. A., Passos, A., DeSouza, O., & Florencio, D. F. (2016a). Resource availability influences aggression and response to chemical cues in the Neotropical termite Nasutitermes aff. coxipoensis (Termitidae: Nasutitermitinae). Behavioral Ecology and Sociobiology, 70, 1257–1265.
Cristaldo, P. F., Rodrigues, V. B., Elliot, S. L., Araújo, A. P. A., & DeSouza, O. (2016b). Heterospecific detection of host alarm cues by an inquiline termite species (Blattodea: Isoptera: Termitidae). Animal Behaviour, 120, 43–49.
Danchin, E., Giraldeau, L.-A., Valone, T. J., & Wagner, R. H. (2004). Public information: From nosy neighbors to cultural evolution. Science, 305, 487–491.
Deng, X. J., Zhang, J. M., JF, H., Yang, J. F., YY, H., & Zheng, Q. (2002). Biological activity of a synthetic trail-pheromone analogue of the black-winged subterranean termite, Odontotermes formosanus Shiraki. Acta Entomologica Sinica, 45, 739–742.
Evans, T. A., Inta, R., Lai, J. C. S., Prueger, S., Foo, N. W., Fu, E. W., & Lenz, M. (2009). Termites eavesdrop to avoid competitors. Proceedings of the Biological Sciences, 276, 4035–4041.
Florencio, D. F., Marins, A., Rosa, C. S., Cristaldo, P. F., Araújo, A. P. A., Silva, I. R., & DeSouza, O. (2013). Diet segregation between cohabiting builder and inquiline termite species. PLoS One, 8, e665.
Gessner, S., & Leuthold, H. R. (2001). Caste-specificity of pheromone trails in the termite Macrotermes bellicosus. Insectes Sociaux, 48, 238–244.
Haifig, I., Jost, C., Fourcassié, V., Zana, Y., & Costa-Leonardo, A. M. (2015). Dynamics of foraging trails in the Neotropical termite Velocitermes heteropterus (Isoptera: Termitidae). Behavioural Processes, 118, 123–129.
Hanus, R., Šobotník, J., Krasulová, J., Jiroš, P., Žáček, M., Dolejšová, K., Cvačka, J., Bourguigon, T., Roisin, Y., Lacey, M. J., & Sillam-Dussès, D. (2012). Nonadecadienone, a new termite trail-following pheromone identified in Glossotermes oculatus (Serritermitidae). Chemical Senses, 37, 55–63.
Kotoklo, E. A., Sillam-Dusses, D., & Ketoh, G. (2010). Identification of the trail-following pheromone of the pest termite Amitermes evuncifer (Isoptera: Termitidae). Sociobiology, 55, 1–10.
Lacey, M. J., Sémon, E., Krasulová, J., Sillam-Dussés, D., Robert, A., Cornette, R., Hoskovec, M., Žáček, M., Valterová, I., & Bordereau, C. (2011). Chemical communication in termites: Syn-4,6-dimethylundecan-1-ol as trail-following pheromone, syn-4,6-dimethylundecanal and (5E)-2,6,10-trimethylundeca-5,9-dienal as the respective male and female sex pheromones in Hodotermopsis sjoestedti (Isoptera). Journal of Insect Physiology, 57, 1585–1591.
Laduguie, N., Robert, A., Bonnard, O., Vieau, F., Le Quere, J. L., Sémon, E., & Bordereau, C. (1994). Isolation and identification of (3Z,6Z,8E)-3,6,8-Dodecatrien-1-ol in Reticulitermes santonensis Feytaud (Isoptera, Rhinotermitidae): Roles in worker trail-following and in alate sex-attraction behavior. Journal of Insect Physiology, 40, 781–787.
Matsumura, F., Coppel, H., & Tai, A. (1968). Isolation and identification of termite trail-following pheromone. Nature, 219, 963–964.
McDowell, P. G., & Oloo, G. W. (1984). Isolation, identification, and biological activity of trail-following pheromone of termite Trinervitermes bettonianus (Sjostedt) (Termitidae: Nasutitermitinae). Journal of Chemical Ecology, 10, 835–851.
Miura, T., & Matsumoto, T. (1998). Open-air litter foraging in the nasute termite Longipeditermes longipes (Isoptera: Termitidae). Journal of Insect Behavior, 11, 179–189.
Moore, B. P. (1966). Isolation of the scent-trail pheromone of an Australian termite. Nature, 211, 746–747.
Moura, F. M. S., Vasconcellos, A., Araujo, V. F. P., & Bandeira, A. G. (2006). Seasonality in foraging behaviour of Constrictotermes cyphergaster (Termitidae, Nasutitermitinae) in the Caatinga of Northeastern Brazil. Insectes Sociaux, 53, 472–479.
Noirot, C. (1969). Glands and secretions. In K. Krishna & F. Weesner (Eds.), Biology of termites (Vol. 1, pp. 89–123). New York: Academic Press.
Noirot, C. (1995). The sternal glands of termites: Segmental pattern, phylogenetic implications. Insectes Sociaux, 42, 321–323.
Oloo, G. W. (1981). Specificity of termite trails: Analysis of natural trails of Trinervitermes, Macrotermes and Odontotermes from sympatric populations. Entomologia Experimentalis et Applicata, 29, 162–168.
Oloo, G. W., & McDowell, P. G. (1982). Interspecific trail-following and evidence of similarity of trails of Trinervitermes species from different habitats. International Journal of Tropical Insect Science, 3, 157–161.
Olugbemi, B. O. (2011). Exploratory and recruitment phases in soldier-mediated foraging activities in the termite, Coptotermes intermedius Silvestri (Rhinotermitidae: Coptotermitinae). Bulletin of Entomological Research, 101, 423–427.
Peppuy, A., Robert, A., Semon, E., Ginies, C., Lettere, M., Bonnard, O., & Bordereau, C. (2001a). (Z)-dodec-3-en-1-ol, a novel termite trail pheromone identified after solid phase microextraction from Macrotermes annandalei. Journal of Insect Physiology, 47, 445–453.
Peppuy, A., Robert, A., Semon, E., Bonnard, O., Truong Son, N., & Bordereau, C. (2001b). Species specificity of trail pheromones of fungus-growing termites from northern Vietnam. Insectes Sociaux, 48, 245–250.
Quennedey, A., Sillam-Dusses, D., Robert, A., & Bordereau, C. (2008). The fine structural organization of sternal glands of pseudergates and workers in termites (Isoptera): A comparative survey. Arthropod Structure & Development, 37, 168–185.
Reinhard, J., & Kaib, M. (2001). Trail communication during foraging and recruitment in the subterranean termite Reticulitermes santonensis De Feytaud (Isoptera, Rhinotermitidae). Journal of Insect Behavior, 14, 157–171.
Robert, A., Peppuy, A., Semon, E., Boyer, F. D., Lacey, M. J., & Bordereau, C. (2004). A new C12 alcohol identified as a sex pheromone and a trail-following pheromone in termites: The diene (Z,Z)-dodeca-3,6-dien-1-ol. Naturwissenschaften, 91, 34–39.
Rosengaus, R. B., Traniello, J. F. A., Lefebvre, M. L., & Maxmen, A. B. (2004). Fungistatic activity of the sternal gland secretion of the dampwood termite Zootermopsis angusticollis. Insectes Sociaux, 51, 259–264.
Saran, R. K., Millar, J. G., & Rust, M. K. (2007). Role of (3Z,6Z,8E)-Dodecatrien-1-ol in trail following, feeding, and mating behavior of Reticulitermes hesperus. Journal of Chemical Ecology, 33, 369–389.
Sillam-Dussès, D. (2010). Trail pheromones and sex pheromones in termites. New York: Nova Science Publishers/Novinka.
Sillam-Dussès, D., Semon, E., Moreau, C., Valterová, I., Šobotník, J., Robert, A., & Bordereau, C. (2005). Neocembrene A, a major component of the trail-following pheromone in the genus Prorhinotermes (Insecta, Isoptera, Rhinotermitidae). Chemoecology, 15, 1–6.
Sillam-Dussès, D., Robert, A., Semon, E., Lacey, M., & Bordereau, C. (2006). Trail-following pheromones and phylogeny in termites. In: Proc XV Congr IUSSI. Washington DC, pp 100–101.
Sillam-Dussès, D., Semon, E., Lacey, M. J., Robert, A., Lenz, M., & Bordereau, C. (2007). Trail-following pheromones in basal termites, with special reference to Mastotermes darwiniensis. Journal of Chemical Ecology, 33, 1960–1977.
Sillam-Dussès, D., Kalinova, B., Jiros, P., Brezinová, A., Cvacka, J., Hanus, R., Šobotník, J., Bordereau, C., & Valterová, I. (2009a). Identification by GC-EAD of the two-component trail-following pheromone of Prorhinotermes simplex (Isoptera, Rhinotermitidae, Prorhinotermitinae). Journal of Insect Physiology, 55, 751–757.
Sillam-Dussès, D., Semon, E., Robert, A., & Bordereau, C. (2009b). (Z)-Dodec-3-en-1-ol, a common major component of the trail-following pheromone in the termites Kalotermitidae. Chemoecology, 19, 103–108.
Sillam-Dussès, D., Semon, E., Robert, A., Cancello, E., Lenz, M., Valterová, I., & Bordereau, C. (2010). Identification of multi-component trail pheromones in the most evolutionarily derived termites, the Nasutitermitinae (Termitidae). Biological Journal of the Linnean Society, 99, 20–27.
Sillam-Dussès, D., Hanus, R., El-Latif, A. O., Jiroš, P., Krasulová, J., Kalinová, B., Valterová, I., & Šobotník, J. (2011). Sex pheromone and trail pheromone of the sand termite Psammotermes hybostoma. Journal of Chemical Ecology, 37, 179–188.
Stuart, A. M. (1961). Mechanism of trail-laying in two species of termites. Nature, 189, 419.
Stuart, A. M. (1981). The role of pheromones in the initiation of foraging, recruitment and defence by the soldiers of a tropical termite, Nasutitermes corniger (Motschulsky). Chemical Senses, 6, 409–420.
Tai, A., Matsumura, F., & Coppel, H. C. (1969). Chemical identification of the trail-following pheromone for a southern subterranean termite. The Journal of Organic Chemistry, 34, 2180–2182.
Tokoro, M., Takahashi, M., Tsunoda, K., & Yamaoka, R. (1989). Isolation and primary structure of trail pheromone of the termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). Wood Research, 29–38.
Tokoro, M., Takahashi, M., Tsunoda, K., Yamaoka, R., & Hayashiya, K. (1991). Isolation and identification of the trail pheromone of the subterranean termite Reticulitemes speratus (Kolbe) (Isoptera: Rhinotermitidae). Wood Research, 78, 1–14.
Traniello, J. F. A. (1981). Enemy deterrence in the recruitment strategy of a termite: Soldier-organized foraging in Nasutitermes costalis. Proceedings of the National Academy of Sciences of the United States of America, 78, 1976–1979.
Traniello, J. F. A., & Busher, C. (1985). Chemical regulation of polyethism during foraging in the neotropical termite Nasutitermes costalis. Journal of Chemical Ecology, 11, 319–332.
Traniello, J. F. A., & Leuthold, R. H. (2000). Behavior and ecology of foraging in termites. In T. Abe, D. Bignell, & M. Higashi (Eds.), Termites: Evolution, sociality, symbioses, ecology (pp. 141–168). Dordrecht: Kluwer Academic Publishers.
Traniello, J. F. A., & Robson, S. K. (1995). Trail and territorial communication in social insects. In R. T. Carde & W. J. Bell (Eds.), Chemical ecology of insects 2 (pp. 241–286). Boston: Springer.
Valone, T. (2007). From eavesdropping on performance to copying the behaviour of others: A review of public information use. Behavioral Ecology and Sociobiology, 62, 1–14.
Wen, P., Ji, B. Z., & Sillam-Dussès, D. (2014). Trail communication regulated by two trail pheromone components in the fungus-growing termite Odontotermes formosanus (Shiraki). PLoS One, 9, e90906.
Wobst, B., Farine, J.-P., Ginies, C., Sémon, E., Robert, A., Bonnard, O., Connétable, S., & Bordereau, C. (1999). (Z,Z,E)-3,6,8-Dodecatrien-1-ol, a major component of trail-following pheromone in two sympatric termite species Reticulitermes lucifugus grassei and R. santonensis. Journal of Chemical Ecology, 25, 1305–1318.
Acknowledgements
PF. Cristaldo thanks CNPq/FAPITEC (#302246/2014-2) for his financial support, Dr. David Sillam-Dussès for his encouragement to study trail pheromones in termites and Dr. Ana Paula Albano Araújo for her help during the preparation of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Cristaldo, P.F. (2018). Trail Pheromones in Termites. In: Khan, M., Ahmad, W. (eds) Termites and Sustainable Management. Sustainability in Plant and Crop Protection. Springer, Cham. https://doi.org/10.1007/978-3-319-72110-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-72110-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72109-5
Online ISBN: 978-3-319-72110-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)