Abstract
Behavioural responses of organisms are frequently affected by variation in resource availability. For eusocial insects, the nutritional status of the colony can modulate responses to chemical cues determining intra- and inter-colonial aggressiveness. Species co-occurrence in termites seems to be modulated by resource availability. Here, we tested the effects of resource availability on acceptance of chemical cues and aggressive behaviour in the Neotropical termite Nasutitermes aff. coxipoensis (Termitidae: Nasutitermitinae). Nasutitermes aff. coxipoensis nests were transplanted into three plots in which resource availability was manipulated over 4 months. Experiments were carried out to evaluate: (i) colony response to internal chemical cues and those of neighbouring colonies reared under the same resource levels; (ii) the choice among chemical paths of colonies reared at different resource levels; and (iii) inter-colony aggression to nestmates and to neighbouring colonies reared under the same resource levels. Our results suggest that resource availability affects acceptance of chemical cues, path choice and aggression in N. aff. coxipoensis. Resource availability may thus modulate behavioural responses influencing coexistence between termite species and other taxa at different spatial scales.
Significance statement
Environmental resource availability is known to limit a range of traits in animals and plants. Here, we report that resource availability is also responsible for changes in behavioural responses of termites. The behavioural modifications found in the present study contribute to our comprehension of ecological patterns in this important ecological group. This work increases our understanding of mechanisms of co-occurrence and coexistence of termite species, as well as patterns of termite species richness in distinct biomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
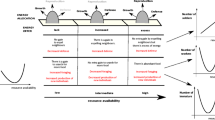
Variation in energy availability is often associated with changes in the behavioural strategies of organisms (Bélisle 2005). Such changes play an important role in tolerance and aggression in many animal groups (Gabor and Jaeger 1995; Sorvari and Hakkarainen 2004; Grover et al. 2007; Vogel and Janson 2007). In social insects, the ability to perceive and respond to chemical cues determines intra- (Liang and Silverman 2000; Liang et al. 2001) and inter-colony aggression (Bland et al. 2001; Florane et al. 2004). Moreover, the perception of chemical signals based on nutritional status is also known to improve a range of decision-making tasks in colonies, such as recruitment and food searching (Hangartner 1970; Grover et al. 2007; Molet et al. 2008; Sorvari et al. 2008), division of labour (Toth et al. 2005) and the choice of sexual partners (Hartke and Baer 2011). In addition, there is some evidence that chemical cues may be detected and exploited by foreign individuals (i.e. from neighbouring colonies), which can benefit from the information about food source location (Adams 1990), predators (Peake 2005), competitors (Evans et al. 2009; Lichtenberg et al. 2011; Binz et al. 2014) and other features of the local environment (Valone 2007). The nature of inter-colonial interactions may depend on colony nutritional status, which can determine aggression or tolerance as well as utilization of foreign chemical information. In a broader context, this process can interfere with species coexistence at different scales, both in the home range and in more intimate proximity (i.e. nest sharing between builder and inquiline species, or colony fusion).
Aggressive behaviour often occurs in phylogenetically closer species, which tend to have similar food requirements (Eltz et al. 2002). However, because aggression can be costly in terms of energy expenditure and risk of injury or death, it may depend on environmental context. In order to minimize energy expended when in close contact with other species, a wide range of taxa (e.g. insects, reptiles, birds, mammals) reduce the degree of aggression toward neighbours compared to strangers when food availability is low (“Dear Enemy Hypothesis”; see Fisher 1954). Studies on termites specifically have suggested that social insect aggressiveness can also be dependent on environmental context, including variation in resource availability (Kaib et al. 2002, 2004). Some evidence suggests that termites can exhibit territorial behaviour (Adams and Levings 1987; Korb and Linsenmair 1998), including regular spacing between colonies. Termite species also overlap in foraging range as a response to resource suitability (Araújo 2009), producing a pattern in which species co-occurrence is greater in low and high resource suitability but low in sites with intermediate resource suitability (“U-shaped” pattern). The mechanism proposed to explain this is the absence of territorial behaviours in areas with low and high resource suitability due to energy restriction and lack of necessity, respectively. Low range overlap under intermediate resource suitability arises from strong territorial behaviour, in which case home range defence may produce a net benefit. Colony nutritional status can thus influence the level of inter-colonial aggression or tolerance. However, to our knowledge, studies evaluating effects of resource availability on termite aggression and capacity to choose among colony signals have not yet been reported (but see Florane et al. 2004).
The present study evaluates effects of resource availability on acceptance and choice of chemical cues and propensity for aggressive behaviour in the Neotropical termite Nasutitermes aff. coxipoensis (Termitidae: Nasutitermitinae). This species occurs in Northeastern Brazil and was recently reported to forage at night utilizing a sophisticated labour system in which individuals explore the environment through trails that could be converted into tunnels (see Almeida et al. 2016). We predicted that (i) at low and high resource availability, colonies will be more receptive to chemical cues of neighbouring colonies and will exhibit low levels of aggression towards neighbours and, (ii) at intermediate resource availability, colonies will reject chemical cues of neighbouring colonies and exhibit high levels of aggression towards neighbours.
Materials and methods
Ethics statement
The permit for termite sampling was provided by ICMBio/IBAMA (no. 47652-1). No specific permits were required for the described laboratory studies, which have been carried out using a species that is neither endangered nor protected.
Study site and food resource manipulation in field
Termite colonies were reared in dune areas interspersed by sandbank (previously cleaned to remove vegetation) at the Santa Isabel Biological Reserve (10° 43′ 56″ S, 36° 50′ 36″ W) in Pirambu, Sergipe, Northeastern Brazil. Regional vegetation consists of grasslands (grasses and sedges) and post-beach, sandbank, palm trees, wetlands and marshes. The climate is characterized as humid megathermal and sub-humid, with annual rainfall between 1500 and 1800 mm and an average annual temperature of 26 °C (SEPLAN/SUPES 2009).
Nasutitermes aff. coxipoensis nests were removed in entirety from their original sites (grassland) and immediately transplanted arbitrarily among three plots, with one nest placed into each of three quadrants (5 m × 5 m) per plot (N = 9) (see Fig. 1a). Quadrants were spaced 0.5 m apart. Nests were placed into excavated holes (30 cm deep) in the centre of each quadrant, and nest bases were covered with substrate from the transplantation site (see more details in Almeida et al. 2016). Only visibly active nests were chosen for transplantation. Colonies were reared at different levels of resource availability for 4 months (from March to June 2015).
Scheme of the experimental design for food resource manipulation of N. aff. coxipoensis transplanted nests in the field. a Nests were placed into excavated holes (30 cm deep) in the centre of each quadrant, and nest bases were covered with substrate from the transplantation site (see more details in Almeida et al. 2016). b Resource availability was manipulated as follows: six concentric circumferences were delineated around each nest. Eight points per circumference were determined (one per cardinal direction), in which 15 × 4 × 2 cm sugarcane baits were distributed. Quadrants with low, intermediate and high resource availability were given 8, 16 and 48 baits (density = 0.32, 0.96 and 1.92 baits/m2), respectively. Increased availability by design included baits being placed in locations closer to the nests. Colonies reared in each one of the quadrant inside the plot with the same resource availability are referred in the text as “neighbours”. Colonies reared in quadrants from different plots (i.e. with different resource availability) are referred in the text as “strangers”. “Own colony” (control) is used to refer to the response of colony to own chemical signals and/or the aggressiveness among individuals from the same colony (i.e. nestmates)
Resource availability was manipulated as follows: six concentric circumferences were delineated around each nest. Eight points per circumference were determined (one per cardinal direction), in which 15 × 4 × 2-cm sugarcane baits were distributed. Quadrants with low, intermediate and high resource availability were given 8, 16 and 48 baits (density = 0.32, 0.96 and 1.92 baits/m2), respectively. Increased availability by design included baits being placed in locations closer to the nests (Fig. 1b). Colonies reared in each one of the quadrants inside the plot with the same resource availability are referred to hereafter as “neighbours”. Colonies reared in quadrants from different plots (i.e. with different resource availability) are referred to hereafter as “strangers”. “Own colony” (control) is used to refer to the response of colony to own chemical signals and/or the aggressiveness among individuals from the same colony (i.e. nestmates).
Specimens were previously identified by comparison with samples from the Termitology Museum at University of Brasília (UNB), where voucher specimens (no. UnB-10616, 10617, 10618, 10619, 10620, 10621) are deposited.
Behavioural bioassays
Chemical cue preference
Linear and Y-shaped trail-following bioassays were conducted in the laboratory to test whether resource availability modulates N. aff. coxipoensis preference for chemical cues. Whole body extracts were prepared by immersion of 100 freeze-killed workers from the same colony in ∼500 μl of hexane for 24 h. After 24 h, a second wash was done with approximately 100 μl of hexane, and both washes were merged.
Linear bioassays tested colony responses to their own chemical cues (control) and to chemical cues from workers of neighbouring colonies reared in the field under the same food levels (treatment). Control assays included exposure of workers to extract from their own nest, which was applied onto a linear 10-cm path. Treatment assays included exposure of workers to extracts from their own colonies applied onto a 6-cm linear path and extracts from neighbouring colonies applied along a 6-cm linear path arising from the opposite direction. These paths were organized such that they overlapped in the centre by 2 cm, forming a 10-cm-long trail (see more details in Cristaldo et al. 2014 and in Online Supplementary Material). All pairwise combinations of the three colonies at each resource level were tested, resulting in 9 combinations for own chemical cues (control) and 18 combinations for chemical cues from neighbouring colonies. We carried out 10 repetitions per paired combination, each using one (different) worker per nest, totalizing 270 assays and 27 true replicates (see the Online Supplementary Material for details).
Y-shaped bioassays were used to test N. aff. coxipoensis preference for paths established by colonies reared at resource levels different from their own. We tested the following combinations of rearing treatment (subjects) and choice of chemical cues: (i) workers reared with low resource availability choosing between chemical cues from intermediate vs. high resource level colonies; (ii) workers reared with intermediate resource availability choosing between chemical cues from low vs. high resource level colonies and (iii) workers reared with high resource availability choosing between chemical cues from low vs. intermediate resource level colonies (see the Online Supplementary Material for details). We carried out 10 repetitions per paired combination, each using one (different) worker per nest, totalling 90 assays and 9 true replicates (see the Online Supplementary Material for details).
All data were recorded using blinded methods in order to minimize observer bias.
Aggression and survival assays
Aggression and survival assays tested the colony response to workers and soldiers from their own colony (control) and to workers and soldiers from neighbouring colonies reared under the same food levels (treatment). Trials took place in Petri dishes (θ 6 cm) lined with filter paper to facilitate termite mobility. Twenty termites (10 individuals from each colony [2 soldiers and 8 workers] or 20 from the same colony [4 soldiers and 16 workers]) were placed at the same time on opposite sides of the dish. Termites were scanned every 30 s with intervals of 15 s, totalling five observations over 3.5 min. Worker behaviours were classified into one of followed categories: (i) antennation, (ii) grooming, (iii) trophalaxis (proctodeal and stomadeal), (iv) biting and (v) fighting (aggression resulting in death or severe injury). We calculated the mean Aggression Index (number of negative interactions [(iv) biting and (v) fighting]/total number of behaviours of five categories) ranging from 0 (no aggression) to 1 (intense aggression). All pairwise combinations of the three colonies at each resource level were tested, resulting in 9 combinations (three per resource level) for nestmates (controls) and 9 combinations for neighbouring colonies (treatment). We carried out three repetitions for all pairwise combination, each using different workers and soldiers per nest, totalizing 54 assays and 18 true replicates (see the Online Supplementary Material for details).
After the aggression bioassays, Petri dishes with all pairwise combinations were maintained in B.O.D. chamber (protected from light) to record the termite survival. The number of dead individuals was quantified at 30-min intervals between 7 a.m. and 9 p.m., until either 24 h had elapsed or all individuals were dead.
All behavioural data were recorded using blinded methods in order to minimize observer bias.
Statistical analyses
Data were analysed using generalized linear models (GLM), choosing error distribution according to the nature of the response variable, as described below. All models were tested by analysis of deviance (ANODEV) with F tests in R 3.2.2 statistical software (R Core Team 2015). Model simplification, when necessary, was conducted by extracting explanatory terms from the initial model and evaluating the subsequent change in deviance, as recommended by Crawley (2007). Residual analyses were performed to verify error distribution and model suitability, including tests for overdispersion. Statistical simplification among treatments was performed via t test using the multcomp package.
To test whether resource availability affects N. aff. coxipoensis receptivity to chemical cues of neighbouring colonies, data from linear bioassays were analysed in a single model under Normal error distribution with identity link. This model included, as response variable (y-var), the mean distance followed by workers, and “resource availability” (x-var1), “chemical cues” (x-var2; own colony cues vs. neighbour colonies cues) and their first-order interaction as explanatory variables.
To test N. aff. coxipoensis preference for paths established by colonies reared at resource levels different from their own, data from the Y-shaped bioassays were analysed in three separate models for each resource availability. All models included the proportion of choices by workers (number of choices between chemical cues/total observations) as response variable (y-var) under Binomial error distribution with logit-link. For colonies under low resource availability, the model included a categorical independent variable (x-var) with two levels: “intermediate” to represent chemical cues from colonies under intermediate resource availability and “high” to represent chemical cues from colonies under high resource availability. For colonies under intermediate resource availability, the model included a categorical independent variable (x-var) with two levels: “low” to represent chemical cues from colonies under low resource availability and “high” to represent chemical cues from colonies under high resource availability. For colonies under high resource availability, the model included a categorical independent variable (x-var) with two levels: “low” to represent chemical cues from colonies under low resource availability and “intermediate” to represent chemical cues from colonies under intermediate resource availability. Each of these models was run independently for each bioassay.
To test whether N. aff. coxipoensis aggression was modulated by resource availability, data from aggressive assays were analysed in a single model under Normal error distribution with identity link. This model included, as response variable (y-var), the mean aggressive index, and resource availability (x-var1), “opponent type” (x-var2; workers and soldiers from own colony (control) vs. workers and soldiers from neighbour colonies) and their first-order interaction as explanatory variables. The mean time to death was calculated by survival analysis with Weibull distribution using the survival package, as described in DeSouza et al. (2009). After that, data were analysed in a single model under Normal error distribution with identity link. This model included as response variable (y-var) the mean time to death and resource availability (x-var1), opponent type (x-var2; workers and soldiers from own colony (control) vs. workers and soldiers from neighbour colonies) and their first-order interaction as explanatory variables.
Results
Chemical cue preference
Receptivity to chemical cues of neighbouring colonies was significantly affected by resource availability (ANODEV: F [2,21] = 6.28, P = 0.006; Fig. 2). The trail distance followed depended on the interaction between resource availability and cues (own colony vs. neighbour colonies), rather than on resource availability alone (ANODEV: F [2,21] = 6.52, P = 0.02; Tab. 1). Colonies reared under low or intermediate resource levels followed the same distance on chemical cues from their own colonies and from neighbouring colonies. However, under high resource availability, colonies followed trails with chemical cues from their own colonies for a greater distance (t test; P < 0.001; Fig. 2) than when subjected to cues from neighbouring colonies.
Effects of resource availability and chemical cues (own colony cues vs. neighbouring colony cues) on mean trail distance followed by N. aff. coxipoensis (Termitidae: Nasutitermitinae) workers in linear bioassays using whole worker body extracts. See the Online Supplementary Material for details. Two asterisks means significant difference (P < 0.05) among treatments and n.s. means no significant difference among treatments (P > 0.05)
For Y-shaped bioassays, workers from colonies reared under low or high resource availability did not show a preference for paths of colonies reared under different resource availabilities (ANODEV; low: F [1,5] = 0.023, P = 0.886, Fig. 3a; high: F [1,5] = 5.80, P = 0.073, Fig. 3c). However, workers from colonies reared under intermediate resource availability significantly preferred paths of colonies reared at high resource availability compared to low resource availability (ANODEV: F [1,5] = 1.69, P < 0.001; Fig. 3b).
Mean proportion (± S.E.) of workers followed in a given arm of the Y-shaped bioassays (choice test). a N. aff. coxipoensis workers from colonies reared under low resource availability showed no significant preference between chemical cues from colonies reared under high or intermediate resource availability. b Workers from colonies reared under intermediate resource availability showed a significant preference for chemical cues from colonies reared under high resource availability compared to those reared under low resource availability. c N. aff coxipoensis workers from colonies reared under high resource availability showed no significant preference between chemical cues of colonies reared under low or intermediate resource availability. See the Online Supplementary Material for details. Asterisk means significant difference (P < 0.05) among treatments and n.s. means no significant difference among treatments (P > 0.05)
Aggression and mean time to death
The aggressive index (AI) was significantly affected by the interaction between resource availability and opponent type (ANODEV: F [2,12] = 146.76, P < 0.001; Table 1). Aggression towards workers and soldiers from neighbouring colonies was significantly higher in colonies reared with intermediate resource levels, followed by colonies reared with high and low resource levels, respectively (Fig. 4a). No significant differences in AI were observed towards workers and soldiers from own colony (control) among colonies reared with different resource levels (Fig. 4a; t test: P = 0.362 low × intermediate; P = 0.993 intermediate × high; P = 0.116 low × high).
Effects of resource availability and opponent type (workers and soldiers from own colony (control) vs. workers and soldiers from neighbour colonies) on a N. aff. coxipoensis Aggressive Index and b mean time to death after aggressive encounter. See the Online Supplementary Material for details. Three asterisks means significant difference (P < 0.05) among treatments
Mean time to death was significantly affected by the interaction between resource availability and opponent type (ANODEV: F [2,12] = 24.316, P < 0.001; Table 1). The mean time to death of workers and soldiers from neighbouring colonies was significantly affected by resource availability; neighbours that confronted workers and soldiers from intermediate and high resource colonies died faster than those that confronted workers and soldiers from low resource colonies. The mean time to death of workers and soldiers from own colony (control) did not differ among resource availability treatments (Fig. 4b; t test: P = 0.99 low × intermediate; P = 0.99 intermediate × high; P = 1.00 low × high).
Discussion
Numerous studies have demonstrated the importance of resource availability for tolerance and aggression (e.g. Gabor and Jaeger 1995; Sorvari and Hakkarainen 2004; Grover et al. 2007; Vogel and Janson 2007). Our results clearly show that resource availability modulates the response of termite colonies to chemical cues (Figs. 2 and 3) and degree of aggression towards neighbouring colonies (Fig. 4a).
Linear bioassays showed that workers from colonies reared with high resource availability avoided following chemical cues from neighbouring colonies and that workers from colonies reared with low and intermediate resource availability followed the same distance on trails with extracts from their own colonies compared to extracts from neighbouring colonies (Fig. 2). Such results indicate that colonies under low and intermediate resource densities are able to exploit olfactory cues (“colony label”) of neighbouring colonies. By doing so, colonies with poor resource availability can obtain information about food sources from colonies in the same spatial area. The ability to use conspecific or heterospecific chemical cues has been reported for bees (Boogert et al. 2006; Jarau 2009) and ants (Menzel et al. 2010). Mutual use of chemical cues for termites has only been reported, to the best of our knowledge, for those sharing a nest (Cristaldo et al. 2014). The significance of eavesdropping chemical cues for social insects seems to be obvious; they are “central-place” foragers, and many of their food resources are spatially and temporally aggregated. Consequently, the ability to obtain information about food sources from neighbouring colonies can benefit individuals and colonies under poor resource availability. Public information (sensu Wagner and Danchin (2010)—“any potential information that is accessible to others”) has been reported to permit faster and more accurate estimates of patch resource densities, allowing more effective foraging.
Interestingly, when given the choice (Y-shaped bioassays), only workers from colonies under intermediate resource availability exhibited a preference for chemical cues from colonies with better nutritional status (higher resource density; see Fig. 3b). The absence of preference of workers from colonies under low and high resource density (Fig. 3a, c) may be a consequence of nutritional status. When a colony has low food resources, any foreign trail is likely to lead to better resources, while for colonies under high resource availability, most resources discovered by following foreign trails will have the same or lower quality than what they already have. Only for colonies at intermediate levels of resource availability there is a payoff for discriminating between trails leading into high vs. low resource areas. Thus, the ability of colonies with intermediate resource availability to perceive and make an appropriate choice with regard to chemical cues can facilitate the exploitation of food sources in areas with high resource availability.
Colonies with low resource density were less aggressive towards workers and soldiers from neighbouring colonies compared with colonies with intermediate and high resource density (Fig. 4a). These results suggest that termite aggressiveness is context-dependent, which indicates that termite aggression should be modulated by the valuable resources to protect (i.e. food resource). This phenomenon may occur to minimize energy expended when in constant confrontation, especially with low availability of resources in the environment. Low levels of aggression and the ability to follow chemical cues from neighbouring colonies may also facilitate tolerance and coexistence among termite colonies in sites with scarce resources. In fact, low resource availability has been suggested to facilitate termite colony fusion (see Korb and Foster 2010; Korb and Roux 2012) and cooperative nest defence (Shellman-Reeve 1994). Reduced levels of aggression when resource density is low could be generated by lack of available energy in these colonies. However, the mean time to death of workers and soldiers from own colonies did not differ among colonies reared with different resource availabilities (Fig. 4b), which indicates that these colonies likely have similar survival probabilities. Colonies reared with low resource density may avoid confrontation (i) to save energy for other essential tasks (e.g. foraging) or (ii) because they perceive chemical cues from strangers, which indicates an opportunity to find and exploit better foraging sites (as suggested by our chemical bioassays; see Fig. 2). Previous studies have shown that the ability of termite inquiline to perceive the chemical cues from their host is one of the mechanisms responsible for nest sharing among termite species (see Cristaldo et al. 2014). Thus, in stressful environments (including sites with low availability of resources), there is a greater likelihood of tolerance between different colonies and also among different species, increasing the rate of facilitation and species coexistence. These results may also be the mechanisms generating the U-shaped pattern, in which sites with intermediate resource suitability have low species co-occurrence as a consequence of higher aggression.
In conclusion, our results show that resource availability affects termite behavioural responses to chemical cues and the degree of colony aggression. Chemical cues from neighbouring colonies can be detected by termites and may influence colony foraging choice. The highest aggression levels seem to occur in colonies with intermediate resource density followed by colonies with high and low resource density. The response to neighbouring colony cues and the degree of aggression coupled with resource density seem to have a strong impact on termite community structure, including the spatial distribution of foraging areas, species co-occurrence and species coexistence in a single nest (so-called “inquilinism” in termite literature).
References
Adams ES (1990) Interaction between the ants Zacryptocerus maculatus and Azteca trigona: interspecific parasitization of information. Biotropica 22:200–206
Adams ES, Levings SC (1987) Territory size and population limits in mangrove termites. Br Ecol Soc 56:1069–1081
Almeida CS, Cristaldo PF, Florencio DF et al (2016) Combined foraging strategies and soldier behaviour in Nasutitermes aff. coxipoensis (Blattodea: Termitoidea: Termitidae). Behav Process 126:76–81
Araújo APA (2009). Regulation of foraging areas and structuring of termite communities. PhD Thesis (Entomology), Federal University of Viçosa, Brazil.
Bélisle M (2005) Measuring landscape connectivity: the challenge of special feature. Ecology 86:1988–1995
Binz H, Foitzik S, Staab F, Menzel F (2014) The chemistry of competition: exploitation of heterospecific cues depends on the dominance rank in the community. Anim Behav 94:45–53. doi:10.1016/j.anbehav.2014.05.024
Bland JM, Osbrink WLA, Cornelius ML et al (2001) Solid-phase microextraction for the detection of termite cuticular hydrocarbons. J Chromatogr 932:119–127
Boogert NJ, Hofstede FE, Monge IA (2006) The use of food source scent marks by the stingless bee Trigona corvina (Hymenoptera: Apidae): the importance of the depositor’s identity. Apidologie 37:366–375. doi:10.1051/apido
Crawley MJ (2007) The R Book. Wiley, Chichester, 942 pp
Cristaldo PF, Desouza O, Krasulova J et al (2014) Mutual use of trail-following chemical cues by a termite host and its inquiline. Plos One 9:e85315. doi:10.1371/journal.pone.0085315
DeSouza O, Araújo APA, Reis-Jr R (2009) Trophic controls delaying foraging by termites: reasons for the ground being brown? Bull Entomol Res 99:603–609. doi:10.1017/S000748530900666X
Eltz T, Brühl CA, van der Kaars S, Linsenmair KE (2002) Determinants of stingless bee nest density in lowland dipterocarp forests of Sabah, Malaysia. Oecologia 131:27–34. doi:10.1007/s00442-001-0848-6
Evans TA, Inta R, Lai JCS et al (2009) Termites eavesdrop to avoid competitors. Proc R Soc B 276:4035–4041. doi:10.1098/rspb.2009.1147
Fisher J (1954) Evolution and bird sociality. In: Huxley J, Hardy AC, Ford EB (eds) Evolution as a process. Allen and Unwin, London, pp 71–83
Florane CB, Bland JM, Husseneder C, Raina AK (2004) Diet-mediated inter-colonial aggression in the Formosan subterranean termite Coptotermes formosanus. J Chem Ecol 30:2559–2574
Gabor CR, Jaeger RG (1995) Resource quality affects the agonistic behaviour of territorial salamanders. Anim Behav 49:71–79
Grover CD, Kay AD, Monson JA, et al (2007) Linking nutrition and behavioural dominance: carbohydrate scarcity limits aggression and activity in Argentine ants. Proc R Soc B 2951–2957. doi: 10.1098/rspb.2007.1065.
Hangartner W (1970) Control of pheromone quantity in odour trails of the ant Acanthomyops interjectus MAYR. Experientia 26:664–665
Hartke TR, Baer B (2011) The mating biology of termites: a comparative review. Anim Behav 82:927–936. doi:10.1016/j.anbehav.2011.07.022
Jarau S (2009) Chemical communication during food exploitation in stingless bees. In: Jarau S, Hrncir M (eds) Food exploitation by social insects: ecological. Behavioral and theoretical approaches. CRC Spress, Boca Raton, pp 223–249
Kaib M, Franke S, Francke W, Brandl R (2002) Cuticular hydrocarbons in a termite: phenotypes and a neighbour ± stranger effect. Physiol Entomol 27:189–198
Kaib M, Jmhasly P, Wilfert L et al (2004) Cuticular hydrocarbons and aggression in the termite Macrotermes subhyalinus. J Chem Ecol 30:365–385
Korb J, Foster KR (2010) Ecological competition favours cooperation in termite societies. Ecol Lett 13:754–760. doi:10.1111/j.1461-0248.2010.01471.x
Korb J, Linsenmair KE (1998) The effects of temperature on the architecture and distribution of Macrotermes bellicosus (Isoptera, Macrotermitinae) mounds in different habitats of a West African Guinea savanna. Insect Soc 45:51–65
Korb J, Roux EA (2012) Why join a neighbour: fitness consequences of colony fusions in termites. J Evol Biol 1–10. doi: 10.1111/j.1420-9101.2012.02617.x.
Liang D, Silverman J (2000) “You are what you eat”: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416
Liang D, Blomquist GJ, Silverman J (2001) Hydrocarbon-released nestmate aggression in the Argentine ant, Linepithema humile, following encounters with insect prey. Comp Biochem Physiol B 129:871–882. doi:10.1016/S1096-4959(01)00404-3
Lichtenberg EM, Hrncir M, Turatti IC, Nieh JC (2011) Olfactory eavesdropping between two competing stingless bee species. Behav Ecol Sociobiol 65:763–774. doi:10.1007/s00265-010-1080-3
Menzel F, Pokorny T, Blüthgen N, Schmitt T (2010) Trail-sharing among tropical ants: interspecific use of trail pheromones? Ecol Entomol 35:495–503. doi:10.1111/j.1365-2311.2010.01206.x
Molet M, Chittka L, Stelzer RJ et al (2008) Colony nutritional status modulates worker responses to foraging recruitment pheromone in the bumblebee Bombus terrestris. Behav Ecol Sociobiol 62:1919–1926. doi:10.1007/s00265-008-0623-3
Peake TM (2005) Eavesdropping in communication networks. In: Animal Communication Networks. pp 13–35.
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria
SEPLAN/SUPES (2009) Sergipe em dados. Aracaju: v.10 il. IOP Publishing PhysicWeb. http://www.seplag.se.gov.br/attachments/article/1385/sergipe_em_dados_2009.pdf. Acessed 15 december 2014
Shellman-Reeve JS (1994) Limited nutrients in a dampwood termite: nest preference, competition and cooperative nest defence. J Anim Ecol 63:921–932. doi:10.2307/5269
Sorvari J, Hakkarainen H (2004) Habitat-related aggressive behaviour between neighbouring colonies of the polydomous wood ant Formica aquilonia. Anim Behav 67:151–153. doi:10.1016/j.anbehav.2003.03.009
Sorvari J, Pascal T, Stefano T et al (2008) Food resources, chemical signalling, and nest mate recognition in the ant Formica aquilonia. Behav Ecol. doi:10.1093/beheco/arm160
Toth AL, Kantarovich S, Meisel AF, Robinson GE (2005) Nutritional status influences socially regulated ontogeny in honey bees nutritional status influences socially regulated foraging ontogeny in honey bees. J Exp Biol 208:4641–4649. doi:10.1242/jeb.01956
Valone TJ (2007) From eavesdropping on performance to copying the behavior of others: a review of public information use. Behav Ecol Sociobiol 62:1–14. doi:10.1007/s00265-007-0439-6
Vogel ER, Janson CH (2007) Predicting the frequency of food-related agonism in white-faced capuchin monkeys (Cebus capucinus), using a novel focal-tree method. Am J Primatol 550:533–550. doi:10.1002/ajp
Wagner RH, Danchin E (2010) A taxonomy of biological information. Oikos. doi:10.1111/j.1600-0706.2009.17315.x
Acknowledgments
We are grateful to Prof. Reginaldo Constantino (UnB) for species identification and to the staff reserves (REBIO/ICMBio) for logistic support. The Brazilian National Research Council (CNPq) support A.P.A.A (484823/2013-2), P.F.C, is supported by CNPq/FAPITEC-SE (302246/2014-2), and ODS holds a CNPq Fellowship (305736/2013-2). The others co-authors were supported by CAPES or CNPq grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The permit for termite sampling was provided by ICMBio/IBAMA (no. 47652-1). No specific permits were required for the described laboratory studies, which have been carried out using a species that is neither endangered nor protected.
Additional information
Communicated by W. Hughes
Paulo F. Cristaldo and Ana P. A. Araújo contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 358 kb)
Rights and permissions
About this article
Cite this article
Cristaldo, P.F., Araújo, A.P.A., Almeida, C.S. et al. Resource availability influences aggression and response to chemical cues in the Neotropical termite Nasutitermes aff. coxipoensis (Termitidae: Nasutitermitinae). Behav Ecol Sociobiol 70, 1257–1265 (2016). https://doi.org/10.1007/s00265-016-2134-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2134-y