Abstract
Cardiac resynchronization therapy (CRT) is an established treatment of heart failure patients who remain symptomatic despite optimal medical treatment, have reduced left ventricular ejection fraction and show broad QRS on surface electrocardiogram. Selection of patients who will benefit from CRT remains challenging and is the focus of ongoing research. Cardiac imaging techniques have provided important pathophysiologic data on the mechanism of action of this therapy, and the factors associated with less benefit of CRT. Cardiac dyssynchrony, extent and location of transmural myocardial scar and position of the left ventricular pacing lead are the main determinants of CRT response. Furthermore, CRT pacing settings can be programmed to obtain the maximum benefit from the therapy. Although optimization of CRT with imaging techniques remains controversial, understanding how changes in CRT settings impact on cardiac hemodynamics may help us to select the most appropriate atrioventricular and interventricular delays. This book chapter provides an overview of the recent advances in imaging techniques to select heart failure patients for CRT and summarizes the echocardiographic techniques that can be used for CRT optimization.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cardiac resynchronization therapy (CRT) is an established therapy for heart failure patients with depressed left ventricular ejection fraction (LVEF) <35%, prolonged QRS >120 ms and mild-to-severe heart failure (HF) symptoms despite optimal pharmacological therapy [1]. Improvements in clinical symptoms, left ventricular (LV) function and mitral regurgitation as well as significant reductions in all-cause and cardiac mortality rates and heart failure rehospitalizations have been reported [2,3,4,5,6,7,8]. However, between 30% and 40% of patients do not improve after CRT and the reasons for these relatively high non-response rates to CRT remain still unclear [9, 10].
LV dyssynchrony is an independent determinant of response to CRT [11, 12]. However, definition of LV dyssynchrony is still highly debated. Recent guidelines have included QRS morphology as a criterion for CRT indication [1]. Based on previous studies, patients with left bundle branch block (LBBB) QRS morphology have benefited of CRT at larger extent than patients with right bundle branch block morphology [3, 8, 12, 13]. However, among patients with LBBB QRS morphology, LV activation may change considerably [14]. The presence of fixed or functional lines of block as assessed with 3-dimensional LV mapping may lead to distinct patterns of LV dyssynchrony that may also influence response to CRT [14]. Non-invasive imaging techniques permit characterization of LV activation pattern and quantification of LV dyssynchrony in heart failure patients with wide QRS and have provided several indices of LV dyssynchrony to predict response to CRT. The Predictors of Response to CRT (PROSPECT) trial was the first multicenter prospective trial to explore the role several echocardiographic dyssynchrony parameters to predict response to CRT [15]. With 498 enrolled patients, the trial demonstrated the modest accuracy of a dozen of echocardiographic LV dyssynchrony parameters to predict response to CRT [15]. However, the observational study design, the inclusion of patients with LVEF>35% or LV end-diastolic dimensions <65 mm, and technical related issues (different vendors, poor acoustic window) may have had significant impact on the results. Furthermore, several pathophysiological factors, such as myocardial scar and LV lead position were not considered in the interpretation of the results of the PROSPECT trial [16]. Subsequent studies have in fact shown that an integrative approach, including assessment of LV dyssynchrony, site of latest activation and presence of transmural scar at the segments targeted by the LV or amount of myocardial scar, may provide a more accurate selection of patients that will benefit from CRT [17,18,19]. Two-dimensional and 3-dimensional echocardiographic deformation imaging, cardiac magnetic resonance (CMR) and nuclear imaging are valuable tools to assess all these pathophysiological determinants of response to CRT. Furthermore, fusion imaging of positron emission tomography (PET) and computed tomography or CMR and fluoroscopy have permitted accurate visualization of cardiac vein anatomy overlaid on myocardial scar tissue providing an accurate guidance for LV lead delivery [20, 21].
Furthermore, after CRT implantation, the role of optimization of the device settings to improve clinical outcomes remains debatable [22]. Several echocardiographic approaches have been evaluated to optimize CRT settings.
The present chapter reviews the role of echocardiography to select heart failure patients who are candidate for CRT and the latest advances achieved after PROSPECT trial on multimodality imaging.
LV Dyssynchrony Assessment
Previous meta-analysis pooling data of more than 5000 heart failure patients included in CRT randomized controlled trials has shown that patients with a QRS duration ≥150 ms clearly benefit from CRT, whereas this benefit is more questioned in patients with a QRS duration between 120 and 149 ms [23]. Furthermore, the sub-analysis of the MADIT-CRT trial showed that the outcomes were significantly superior in patients treated with CRT who had LBBB QRS morphology than patients with other types of intraventricular conduction defects [12]. Accordingly, current European Society of Cardiology guidelines for diagnosis and management of heart failure patients have modified the inclusion criteria for CRT indication [1]. One quarter of heart failure patients have LBBB QRS morphology [24]. The delayed activation of the left ventricle due to LBBB is characterized by contraction of the interventricular septum against a lateral wall that is passively stretched and followed by late contraction of the lateral wall against the stretched interventricular septum leading to inefficient LV global contraction. However, this mechanical pattern may change depending on the LV substrate (i.e. areas of scar) and the effect of CRT may vary accordingly [14].
Several imaging techniques have provided multiple LV dyssynchrony indices that try to characterize the LV mechanical activation dispersion and to predict response to CRT. Table 26.1 summarizes the echocardiographic LV dyssynchrony parameters that were evaluated in the PROSPECT trial [15]. Although the results of the PROSPECT trial showed a modest accuracy of a dozen of M-mode and Doppler echocardiography and tissue Doppler imaging derived LV dyssynchrony parameters to predict response to CRT [15], subsequent analyses have shown that the presence of significant LV dyssynchrony assessed with tissue Doppler imaging for example is associated with increased likelihood of response to CRT and improved outcome [11, 25]. Therefore, analysis of LV dyssynchrony with imaging techniques provides incremental information in the selection of patients for CRT implantation.
However, in the last years the focus has been shifted towards evaluation of active mechanical deformation with speckle tracking echocardiography or tagged-CMR and towards global evaluation with 3-dimensional imaging techniques to characterize LV mechanical dispersion or dyssynchrony.
2D Speckle Tracking Echocardiography
Two-dimensional speckle tracking echocardiography analyzes multidirectional active myocardial deformation by tracking frame to frame the movement of the speckles (natural acoustic markers equally distributed within the myocardium) along the cardiac cycle. Applied to the LV short-axis view, LV dyssynchrony is frequently measured as the time difference between peak radial strain of the (antero)septal and (postero)lateral walls (Fig. 26.1) [26]. A time difference ≥ 130 ms has demonstrated to predict response to CRT with relative high accuracy (83% sensitivity and 80% specificity) and has been independently associated with improved outcome after CRT implantation [11, 27]. Furthermore, the assessment of LV mechanical dispersion with longitudinal strain speckle tracking echocardiography has also demonstrated good accuracy to predict response to CRT [28]. By calculating the standard deviation of the difference between peak and end-systolic longitudinal strain of 16 LV segments (in the apical 4-, 2- and 3-chamber views), Lim and coworkers derived the strain dyssynchrony index, an index that combines activation time and myocardial contractile reserve (Fig. 26.1) [28]. A cut-off value of strain dyssynchrony index ≥25% had a high accuracy to predict significant LV reverse remodeling after CRT (area under the curve: 0.94). Unlike tissue Doppler imaging techniques, the measurement of myocardial deformation with speckle tracking echocardiography is not influenced by the insonation angle of the ultrasound beam. However, some 2-dimensional speckle tracking echocardiography derived LV dyssynchrony parameters do not provide information on the global LV mechanical activation pattern and some parameters are computed based on the acquisition of several views during different cardiac cycles, introducing a potential beat-to-beat variability in its calculation [29]. In this regard, the development of 3-dimensional imaging techniques has enabled the assessment of global LV mechanical dyssynchrony.
Assessment of LV dyssynchrony with 2-dimensional speckle tracking echocardiography. Panel (a) shows an example of a patient with significant LV dyssynchrony as assessed with radial strain. The time difference between peak radial strain of the anteroseptal and posterior segments is 170 ms. Panel (b) shows the measurement of strain delay index based on longitudinal strain. This index evaluates the time difference and longitudinal strain magnitude between peak and end-systolic longitudinal strain of 16 segments. In this example, strain delay index indicates significant dyssynchrony (>25%). ES end-systolic
3-Dimensional Echocardiographic Techniques
One of the first LV dyssynchrony indices based on real-time 3-dimensional echocardiography was the systolic dyssynchrony index (Fig. 26.2). From a LV 3-dimensional full volume, the LV endocardial border is manually or semiautomated defined (depending on the post-processing software) at end-systole and end-diastole and a mathematical 3-dimensional model of the left ventricle is derived. This model is subsequently divided into 16 or 17 segments and the LV mechanical dyssynchrony is quantified by calculating the standard deviation of time to minimum regional volume of 16 or 17 LV subvolumes. A recent meta-analysis pooling data from 600 heart failure patients undergoing CRT implantation demonstrated a good accuracy of real-time 3-dimensional echocardiography to predict response to CRT [30]. A weighted mean systolic dyssynchrony index of 9.8% predicted response to CRT with a sensitivity of 93% and a specificity of 75% [30].
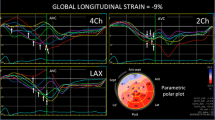
Three-dimensional echocardiographic technique s to evaluate LV dyssynchrony. Panel (a): LV dyssynchrony can be quantified from 3-dimensional full-volume datasets of the left ventricle by measuring the systolic dyssynchrony index (standard deviation of time to minimum systolic volume of 16 subvolumes [Tmsv16-SD]). The LV mechanical dispersion can be also visualized on color-coded polar maps, with the earliest activated regions coded in blue and the latest activated areas coded in orange-red. In this example, the patient shows significant LV dyssynchrony (Tmsv16-SD 20.05%) and the lateral and posterior LV regions as the most delayed activated areas. The time-volume curves of the 16 regional subvolumes are plotted in a graph providing also a visual estimation of LV dyssynchrony. Panel (b): Assessment of LV dyssynchrony with triplane tissue synchronization imaging. The polar map shows the time to peak systolic velocity of 12 basal and midventricular LV segments. LV dyssynchrony is calculated as the standard deviation of time to peak systolic velocity of the 12 segments. In this example, there is significant LV dyssynchrony (standard deviation: 47 ms) and the mid posterolateral segment is the most delayed activated area. Panel (c): With 3-dimensional speckle tracking, area strain index is derived by computing LV shortening in two directions (longitudinal and circumferential) and LV dyssynchrony (calculated as the standard deviation of time to peak strain of 16 segments). In this example, after defining the region of interest, the software derives 3-dimensional model of the left ventricle providing the value of area strain index (6.3%). This 3-dimensional model permits visualization of the latest activated areas (coded in red). The time to area strain curves for the 16 segments can be visually assessed in a graph. Adapted from Tatsumi et al. [34]
Furthermore, from triplane LV echocardiographic data, tissue Doppler imaging can be applied and LV dyssynchrony can be calculated as the standard deviation of time to peak velocity of 12 basal and mid ventricular segments [31]. Particularly, tissue synchronization imaging has provided a rapid and intuitive visualization of LV mechanical dyssynchrony providing color-coded polar map plots of the left ventricular activation. The earliest activated segments are color-coded in green whereas the latest activated segments are color-coded in orange (Fig. 26.2). Using this methodology, van de Veire et al. demonstrated that a standard deviation of time to peak systolic velocities ≥33 ms predicted response to CRT with 90% and 83% sensitivity and specificity, respectively [31].
Finally, the recent developed 3-dimensional speckle tracking has also permitted quantification of LV dyssynchrony. Unlike 2-dimensional speckle tracking echocardiography, 3-dimensional speckle tracking echocardiography permits analysis of myocardial deformation of the true full LV volume avoiding foreshortened images and it is not affected by the out-of-plane motion of the speckles. From a 3-dimensional LV full volume, the endocardial borders are manually traced and the software displays a region of interest including the myocardial wall and tracks the speckles along the cardiac cycle. Longitudinal, circumferential and radial strains are then calculated. In heart failure patients undergoing CRT implantation, the role of 3-dimensional speckle tracking to characterize LV dyssynchrony and predict response to CRT has been tested [32, 33]. LV dyssynchrony can be calculated as the standard deviation of time to peak strain for 16 LV segments. In a series of 54 heart failure patients treated with CRT, 3-dimensional speckle tracking demonstrated a larger LV dyssynchrony compared with healthy volunteers (124 ± 48 ms vs. 28 ± 11 ms, respectively; p < 0.001) [32]. Furthermore, the software provides an index of global LV performance (area strain) computing LV shortening in two directions (longitudinal and circumferential) and LV dyssynchrony (calculated as the standard deviation of time to peak strain of 16 segments) (Fig. 26.2). The accuracy of this 3-dimensional speckle tracking derived parameter to predict response to CRT has been recently tested in 14 heart failure patients [34]. A cutoff value of ≥3.8% predicted response to CRT with a sensitivity of 78% and specificity of 100% [34]. However, the low temporal resolution of 3-dimensional speckle tracking may be an important limitation in LV dyssynchrony assessment. Therefore, the results of published studies reporting the accuracy of this technique to predict response to CRT should be taken with caution since the proposed cut-off values of the LV dyssynchrony indices.
Magnetic Resonance Imaging
CMR is a valuable 3-dimensional imaging technique to characterize LV dyssynchrony. Using steady-state free-precession sequence, a stack of short-axis slices covering the entire left ventricle are acquired and the LV mechanical dyssynchrony can be analyzed by evaluating the regional radial wall motion. The standard deviation of the phase shift of the maximum radial wall motion of all LV segments provides the LV dyssynchrony index known as CMR-tissue synchronization index (Fig. 26.3). Using this technique, Chalil et al. demonstrated that CMR-tissue synchronization index was independently associated with long-term outcome of heart failure patients treated with CRT [35]. Patients with a CMR-tissue synchronization index ≥110 ms had fivefold increased mortality risk compared with patients with a CMR-tissue synchronization index <110 ms [35]. Therefore, this CMR-derived LV dyssynchrony parameter may be of importance in the patient selection for CRT. In addition, tagged CMR permits assessment of longitudinal and circumferential strain in 3 dimensions and provides data on LV mechanical activation that is operator independent [17]. Particularly, the measurement of LV mechanical dyssynchrony has been largely based on circumferential strain data provided by tagged-CMR. Applying harmonic phase method to LV short-axis tagged slices, circumferential strain is assessed and time to peak circumferential strain is measured to provide the LV mechanical index, the so-called circumferential uniformity ratio estimate (CURE) (Fig. 26.3) [17]. This LV dyssynchrony index ranges from 0 (pure dyssynchrony) to 1 (perfect synchronous). In 20 patients undergoing CRT implantation, Bilchick et al. demonstrated that a CURE index <0.75 had 90% accuracy to predict response to CRT (positive predictive value: 87% and negative predictive value: 100%) [17].
LV dyssynchrony assessment with cardiac magnetic resonance. Panel (a): Assessment of LV dyssynchrony with CMR based on evaluation of regional radial wall motion. From steady-state free-precession sequence, a stack of short-axis slices covering the entire left ventricle are acquired. The time to segmental radial wall motion can be displayed in a graph. The standard deviation of the phase shift of the maximum radial wall motion of all LV segments provides the LV dyssynchrony index known as CMR-tissue synchronization index. Panel (b): With tagged-CMR, circumferential strain can be measured at 12 LV segments (septal, posterior, lateral and anterior at pical, mid and basal levels of the left ventricle). Time to peak circumferential strain is measured providing the circumferential uniformity ratio estimate (CURE) index. This index ranges from 0 (dyssynchronous) to 1 (synchronous). Reproduced with permission from Chalil et al. [35] and Bilchick et al. [17]
Nuclear Imaging
Finally, LV dyssynchrony can be also assessed with nuclear imaging techniques. Quantitative ECG-gated single photon emission computed tomography (SPECT) characterizes LV dyssynchrony by calculating the phase angle of multiple LV samples and providing the phase angle distribution in a polar map or a histogram (Fig. 26.4). The bandwidth and the phase standard deviation are the LV dyssynchrony parameters. The proposed optimal cut-off value of the histogram bandwidth to predict response to CRT was 72.5° (sensitivity 83% and specificity 81%) whereas a phase standard deviation ≥19.6° predicted response to CRT with a sensitivity and specificity of 83% and 81%, respectively [36].
As many non-randomized single center trials published prior to the PROSPECT trial, the evidence provided by these new studies using novel technologies demonstrates and confirms that assessment of LV dyssynchrony plays an important role in selecting heart failure patients for CRT. However, subsequent studies following the PROSPECT trial have included in their analyses other pathophysiological factors that may influence the response to CRT such as myocardial scar or LV lead position and that were not evaluated in the PROSPECT trial.
LV Lead Position : Site of Latest Activation
Cannulation of the coronary sinus and placement of an LV lead in a suitable tributary that permits a stable position, avoids phrenic nerve stimulation and reduces LV dyssynchrony (as assessed with significant shortening of the QRS duration or with imaging techniques) is the most crucial aspect of CRT implantation. Lateral or posterolateral veins are the preferred locations for LV lead placement. However, several observational studies and two randomized trials have demonstrated that location of the LV pacing lead at the area of latest mechanical activation is associated with superior CRT outcomes [18, 37,38,39]. Assessment of the latest activated region of the left ventricle can be performed with echocardiographic and CMR techniques. Several echocardiographic techniques such as triplane tissue synchronization imaging, 3D speckle tracking or 3D echocardiography have demonstrated to accurately localize the site of latest activation. Two-dimensional speckle tracking echocardiography may be the imaging technique providing the largest evidence on the relevance of evaluating the site of latest activation and the impact of a concordant LV lead position [18, 37, 39]. With this technique, the latest activated areas are identified on the time-radial strain graphs as the segments with the most delayed peak radial strain. The first randomized trial evaluating the effect of LV lead position (coincident or not with the site of latest activation) on response to CRT and long-term outcome was the TARGET (Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy) trial [37]. A total of 220 patients treated with CRT were randomized to LV lead positioning according to 2-dimensional speckle tracking analysis results (concordant with the latest activated segments) or to conventional LV lead placement. At 6 months follow-up, the response rate to CRT (≥15% reduction in LV end-systolic volume) was significantly higher among patients with an LV lead coincident with the site of latest activation than in patients in whom the LV lead was placed conventionally (70% vs. 55%, p = 0.031). In addition, patients with an LV lead position concordant with the site of latest activation showed superior long-term outcome than patients with a discordant LV lead position (10% vs. 21% cumulative incidence of combined endpoint of HF hospitalizations or all-cause mortality; p < 0.001) [37].
Furthermore, noninvasive assessment of coronary venous anatomy may be helpful to plan the implantation strategy. In ischemic heart failure patients, particularly, the presence of a suitable lateral or posterolateral vein is less frequent and may challenge the LV lead positioning [40]. MDCT and CMR permit accurate non-invasive assessment of the coronary sinus and tributaries (Fig. 26.5). Evaluation of the coronary venous anatomy prior to CRT implantation has been associated with reduced procedural times, use of contrast volume and fluoroscopy times [41]. Furthermore, MDCT or CMR data can be integrated with fluoroscopy and provide an optimal guidance for LV lead positioning (Fig. 26.5).
Evaluation of coronary venous anatomy with MDCT and CMR. Prior to CRT implantation, MDCT permits accurate assessment of venous coronary anatomy. The example shows the posterior interventricular vein and the left lateral vein as tributaries of the coronary sinus (panel (a)). With CMR the coronary venous anatomy can be also evaluated and the data can be merged with real-time fluoroscopy permitting accurate guidance of LV lead positioning (panel (b)). Reprinted with permission from Duckett et al. [20]. CS coronary sinus, LLV left lateral vein, PIV posterior interventricular vein
Myocardial Scar
Coronary artery disease is the leading cause of heart failure. A subanalysis of the CARE-HF trial showed that patients with ischemic heart failure benefited from CRT at a lesser extent than patients with non-ischemic heart failure [42]. Similarly, a population-based study including almost 15,000 patients treated with CRT demonstrated that ischemic etiology was one of the strongest prognostic factors [9]. Observational studies have shown that placing the LV lead on an area of transmural myocardial scar is associated with high likelihood of non-response to CRT [18, 19]. In addition to the location of transmural scar, the extent or burden of myocardial scar has an important influence on the effects of CRT [43,44,45].
Delayed contrast-enhanced CMR and radionuclide myocardial perfusion imaging are considered the gold standard for assessment of myocardial scar (Fig. 26.6). Delayed contrast-enhanced CMR has higher spatial resolution than nuclear imaging techniques and permits exact visualization of the transmurality of myocardial scar. With this technique, Leyva et al. demonstrated that patients with an LV lead placed at myocardial areas with transmural scar had sixfold higher risk of cardiovascular death than patients without transmural scar (hazard ratio; 6.34, 95% confidence interval 3.64–11.0, p < 0.0001) [19]. Furthermore, using radionuclide myocardial perfusion imaging two large studies have demonstrated that global myocardial scar burden has an independent influence on long-term outcome of patients treated with CRT [43, 45]. In 213 heart failure patients treated with CRT who underwent pre-procedural PET (n = 46) or SPECT (n = 167) myocardial perfusion imaging, Xu et al. showed that an extent of myocardial scar >22% together with an age < 70 years were associated with superior long-term survival after CRT [45]. In addition, Adelstein et al. quantified myocardial scar burden with rest-redistribution 201Thallium-SPECT in 190 ischemic heart failure patients treated with CRT [43]. A summed rest score ≥ 27 identified patients with large myocardial scar burden. Patients with a summed rest score < 27 showed larger LV reverse remodeling and improvement in LVEF and had twofold higher survival free from cardiac transplant or LV assist device implantation as compared with patients with a summed rest score ≥ 27 [43].
Evaluation of location and extent of myocardial scar. Panel (a) shows the example of a patient with transmural myocardial scar on delayed contrast-enhanced cardiac magnetic resonance in the septum and anteroseptum of the left ventricle (arrows). Panel (b) shows the example of a patient with transmural scar in the inferoseptum and posterolateral regions (arrows). The position of the left ventricular lead in an area with transmural scar may lead to non-response to cardiac resynchronization therapy
Assessment of myocardial viability and scar with echocardiographic techniques is also feasible. Particularly, speckle tracking echocardiographic techniques have been validated against contrast-enhanced CMR to identify transmural myocardial scar [46]. Using speckle tracking radial strain echocardiography, a value of peak radial strain <16.5% has been proposed to identify regions of transmural myocardial scar [46]. In 397 ischemic heart failure patients the presence of transmural scar as assessed with speckle tracking radial strain echocardiography at the area targeted by the LV lead was independently associated with poor outcome [18]. The addition of myocardial scar in the segment targeted by the LV lead had incremental prognostic value over LV dyssynchrony, LV lead position and other well-known clinical prognostic markers [18].
This evidence suggests that integration of several pathophysiological determinants of CRT efficacy may be more important in patient selection rather than relying on one single selection criterion.
CRT Optimization
Simultaneous right and left ventricular pacing with a sensed atrioventricular (AV) delay programmed at 110–120 ms is the most common mode of CRT pacing [47]. However, these empirically programmed settings may not provide optimal LV filling or result in the most synchronous LV contraction in every patient. Previous study has shown that non-optimal AV delay was the cause of non-response to CRT in 45% of heart failure patients whereas suboptimal interventricular (VV) delay was less frequently the cause of suboptimal clinical response [48]. Optimization of CRT settings aims at achieving 100% biventricular pacing, maximum contribution of the left atrial contraction to the LV filling and at eliminating residual LV dyssynchrony. However, randomized and non-randomized trials evaluating the effects of AV and/or VV delay optimization on clinical outcomes have shown controversial results. Recent meta-analysis pooling data of 4356 patients treated with CRT showed no differences in clinical or echocardiographic outcomes between patients who underwent AV and/or VV delay optimization and patients with empirically programmed settings [22]. The lack of standardized methodologies to optimize the CRT device and the inclusion of heterogeneous populations in clinical trials (responders and non-responders to CRT) may explain the controversial results of CRT optimization across the different studies. Several echocardiographic methods have been largely used for CRT optimization (Table 26.2) [49].
Heart failure patients may have a conduction delay between the atrium and the ventricle (too long AV delay) leading to a short LV filling time. On echocardiography, this situation is recognized by a fusion of the early (E) and late (A) wave velocities on pulsed wave Doppler recordings of the transmitral inflow. In addition, diastolic mitral regurgitation can be observed if the pressures in the LV exceed the pressures of the left atrium before the LV contraction starts, leading to a further reduced LV preload and stroke volume. By shortening the AV delay , the E and A waves separate thereby increasing the LV filling time and leading to higher preload of the LV (Fig. 26.7). Optimization of the AV delay seeks for the shortest AV delay without truncation of the A wave. Table 26.2 summarizes the different echocardiographic methods to optimize the AV delay . Among them, the iterative method is the most frequently used. This method is based on pulsed wave Doppler recordings of the mitral inflow. From a long AV delay, the pulsed wave Doppler of the mitral inflow is recorded, and subsequently the AV delay is shortened in 20 ms steps until the A wave is truncated by the LV contraction on the spectral Doppler signal. Then, the AV delay is lengthened by steps of 10 ms until obtaining the optimal LV filling time. This method was used in the SMART-AV trial [50].
Optimization of the atrioventricular delay following the iterative method. From a long atrioventricular (AV) delay, where the early (E) and late (A) diastolic waves are fused, the AV delay is shortened by 20 ms steps until the A wave is truncated. From that time, the AV delay is lengthened to obtain the shortest AV delay without truncation of the A wave. Reproduced with permission from Bertini et al. [49]
Optimization of the VV delay may help to improve the response of patients with a non-optimal LV lead position. Residual LV dyssynchrony or more pronounced LV dyssynchrony after CRT implantation may occur leading to suboptimal clinical outcomes. By adjusting further the VV delay, a more physiological activation of the LV may be achieved. Several echocardiographic methods have been proposed to optimize the VV delay and are based on assessment of surrogates of stroke volume or assessment of mechanical dyssynchrony (Table 26.2). From pulsed wave Doppler recordings of the LV outflow, the stroke volume can be calculated by measuring the velocity time integral of the spectral signal. Using this method, the effect of several VV delay configurations on stroke volume can be assessed. Stroke volume is assessed during RV pre-stimulation (from +60 to +20 ms), simultaneous stimulation (0 ms) and LV pre-stimulation (from −20 to −60 ms). The VV delay that yields the largest velocity time integral and stroke volume would be considered the optimal delay. In addition, other studies have shown the value of tissue Doppler imaging to assess LV dyssynchrony across the several VV delay settings [49, 51, 52].
Future Steps: Imaging Integration
Technological advances have permitted integration or fusion of imaging techniques to provide information on several pathophysiological factors that may influence CRT response. One of the first experiences evaluating LV dyssynchrony and LV lead position in relation to areas of myocardial scar used 3-dimensional imaging fusion with 18F-FDG-PET/CT [21]. In 14 candidates for CRT, LV dyssynchrony was assessed with LV phase analysis of data obtained with ECG-gated PET [21]. Histogram bandwidth, phase angle standard deviation and global phase entropy (reflecting uniformity of movement) were provided. In addition, myocardial scar was detected from LV 18F-FDG polar maps normalized to the highest glucose uptake. Areas with <50% uptake identified non-viable areas (myocardial scar). Finally, integrating18F-FDG-PET and low-dose CT images, the LV lead tip was localized and assigned to viable or nonviable (scar) myocardium (Fig. 26.7). Responders to CRT showed more LV dyssynchronous contraction, less burden of myocardial scar and less frequently LV leads were placed in areas with nonviable myocardium [21]. In addition, Duckett et al. demonstrated the usefulness of integrating MDCT or delayed contrast-enhanced CMR with fluorosocopy to guide CRT implantation [20]. Specific post-processing softwares permit segmentation of the coronary venous anatomy and myocardial scar from delayed contrast-enhanced CMR data and overlay these data onto real-time fluoroscopy providing further guidance to CRT implantation [20]. In addition, Doring et al. showed the value of integrating LV mechanical activation data obtained with 3D transesophageal echocardiography with 3D data of the coronary sinus and venous tributaries obtained with rotational angiography (Fig. 26.8). The sites of latest activation are color-coded in red while the earliest activated segments are color-coded in blue. By interpolating the 3D model of the cardiac venous anatomy and the LV 3D activation map, the position of the LV lead can be guided to target the site of latest activation [52]. Recently, the role of fusion imaging on CRT optimization has also been evaluated. The polar maps of LV activation obtained with 3D real-time transesophageal echocardiography are fused with 3D models of the venous anatomy and position of the LV lead obtained with rotational angiography. In patients with suboptimal position of the LV lead (not coinciding with the site of latest activation), changes in the polar maps across the several VV delay settings can be evaluated and the VV delay that leads to the more synchronous activation can be selected (Fig. 26.8) [53]. These new areas of research on image integration may in the future contribute to improved patient selection for CRT.
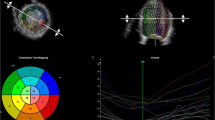
Fusion imaging in cardiac resynchronization therapy. Panel (a): On fluoroscopic rotation scan, the course of the right and left ventricular leads is registered. The right anterior oblique (RAO) and left anterior oblique (LAO) views are shown. Panel (b): Reconstruction of the 3-dimensional (3D) left ventricular (LV) model obtained with transesophageal echocardiography. The polar map with the time to minimum regional volume is provided. Panel (c): The 3D LV model with the time to minimum regional volume for all LV segments is displayed and fused with the angiographic rotation scan data indicating the position of the LV lead relative to the site of latest activation (color-coded in red). Panel (d): several VV delays are tested and the standard deviation of time from onset of QRS to minimum volume of 16–17 segments is measured (SDI). The optimal VV delay provides the lowest SDI and highest LV ejection fraction (LVEF). In this example, 30 ms pre-stimulation of the LV may be the preferred VV delay. Reproduced with permission from Nitsche et al. [53]. LAO left anterior oblique, LV left ventricle, RAO right anterior oblique, RV right ventricle, SDI standard deviation of time to minimum regional volume, SLMA site of latest mechanical activation, VV interventricular
Conclusions
Cardiac resynchronization therapy is one of the main therapeutic breakthroughs in heart failure of the last decades. Published data has demonstrated 60–70% efficacy of this therapy in improving LV function, inducing LV remodeling and improving long-term outcome of heart failure patients. However, the associated costs and risks demand an accurate selection of heart failure patients to maximize the results. The PROSPECT trials showed us that single evaluation of LV dyssynchrony may not be sufficient to identify the patients who will benefit from CRT. Many observational studies have demonstrated that LV dyssynchrony, LV lead position and location and extent of myocardial scar are independent determinants of response to CRT and need to be evaluated prior to CRT implantation. Currently, echocardiography, with a high availability and cost-effectiveness, is the method of choice to evaluate most of the pathophysiological determinants of response to CRT. Particularly, speckle tracking echocardiography has been demonstrated to be a valuable tool to assess LV dyssynchrony, site of latest activation and myocardial scar. However, assessment of myocardial scar may be preferably performed with delayed contrast-enhanced CMR or radionuclide imaging techniques. In this regard, CMR is a comprehensive imaging tool to assess LV dyssynchrony, location and extent of myocardial scar and coronary venous anatomy. Furthermore, optimization of CRT device settings in patients with suboptimal response may be guided by echocardiographic techniques. Although the results of several trials have shown inconclusive, current recommendations consider optimization of CRT device with echocardiography as a bailout methodology to improve the outcomes of heart failure patients with minimal improvement in symptoms after device implantation. Finally, post-processing imaging tools permit overlay of segmented CMR 3-dimensional datasets on real-time fluoroscopy, facilitating implantation and optimization of CRT devices. However, the availability is limited and in patients with pacemaker devices or with severe renal dysfunction the use of this technique may be contraindicated. Additional randomized studies selecting candidates for CRT based on current recommendations, taking these pathophysiological factors into account, may further establish the role of imaging techniques in patient selection for CRT.
References
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847.
Abraham WT, Hayes DL. Cardiac resynchronization therapy for heart failure. Circulation. 2003;108(21):2596–603.
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De MT, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–50.
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–49.
Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (resynchronization reverses Remodeling in systolic left ventricular dysfunction) trial. J Am Coll Cardiol. 2009;54(20):1837–46.
Linde C, Abraham WT, Gold MR, St John SM, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52(23):1834–43.
Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–38.
Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385–95.
Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation. 2010;122(20):2022–30.
Delgado V, Bax JJ. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is clinically useful. Circulation. 2011;123(6):640–55.
Gorcsan J III, Oyenuga O, Habib PJ, Tanaka H, Adelstein EC, Hara H, et al. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation. 2010;122(19):1910–8.
Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Circulation. 2011;123(10):1061–72.
Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen A, et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11(7):699–705.
Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109(9):1133–9.
Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–16.
Bax JJ, Gorcsan J III. Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (predictors of response to cardiac resynchronization therapy) study in perspective. J Am Coll Cardiol. 2009;53(21):1933–43.
Bilchick KC, Dimaano V, Wu KC, Helm RH, Weiss RG, Lima JA, et al. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2008;1(5):561–8.
Delgado V, Van Bommel RJ, Bertini M, Borleffs CJ, Marsan NA, Arnold CT, et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123(1):70–8.
Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:29.
Duckett SG, Ginks MR, Knowles BR, Ma Y, Shetty A, Bostock J, et al. Advanced image fusion to overlay coronary sinus anatomy with real-time fluoroscopy to facilitate left ventricular lead implantation in CRT. Pacing Clin Electrophysiol. 2011;34(2):226–34.
Uebleis C, Ulbrich M, Tegtmeyer R, Schuessler F, Haserueck N, Siebermair J, et al. Electrocardiogram-gated 18F-FDG PET/CT hybrid imaging in patients with unsatisfactory response to cardiac resynchronization therapy: initial clinical results. J Nucl Med. 2011;52(1):67–71.
Auger D, Hoke U, Bax JJ, Boersma E, Delgado V. Effect of atrioventricular and ventriculoventricular delay optimization on clinical and echocardiographic outcomes of patients treated with cardiac resynchronization therapy: a meta-analysis. Am Heart J. 2013;166(1):20–9.
Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med. 2011;171(16):1454–62.
Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143(3):398–405.
Shanks M, Delgado V, Ng AC, Auger D, Mooyaart EA, Bertini M, et al. Clinical and echocardiographic predictors of nonresponse to cardiac resynchronization therapy. Am Heart J. 2011;161(3):552–7.
Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J III. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113(7):960–8.
Delgado V, Ypenburg C, Van Bommel RJ, Tops LF, Mollema SA, Marsan NA, et al. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging. Comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51(20):1944–52.
Lim P, Buakhamsri A, Popovic ZB, Greenberg NL, Patel D, Thomas JD, et al. Longitudinal strain delay index by speckle tracking imaging: a new marker of response to cardiac resynchronization therapy. Circulation. 2008;118(11):1130–7.
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24(3):277–313.
Kleijn SA, Aly MF, Knol DL, Terwee CB, Jansma EP, Abd El-Hady YA, et al. A meta-analysis of left ventricular dyssynchrony assessment and prediction of response to cardiac resynchronization therapy by three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13(9):763–75.
van de Veire NR, Bleeker GB, De SJ, Ypenburg C, Holman ER, van der Wall EE, et al. Tissue synchronisation imaging accurately measures left ventricular dyssynchrony and predicts response to cardiac resynchronisation therapy. Heart. 2007;93(9):1034–9.
Tanaka H, Hara H, Saba S, Gorcsan J III. Usefulness of three-dimensional speckle tracking strain to quantify dyssynchrony and the site of latest mechanical activation. Am J Cardiol. 2010;105(2):235–42.
Thebault C, Donal E, Bernard A, Moreau O, Schnell F, Mabo P, et al. Real-time three-dimensional speckle tracking echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Eur J Echocardiogr. 2011;12(1):26–32.
Tatsumi K, Tanaka H, Tsuji T, Kaneko A, Ryo K, Yamawaki K, et al. Strain dyssynchrony index determined by three-dimensional speckle area tracking can predict response to cardiac resynchronization therapy. Cardiovasc Ultrasound. 2011;9:11.
Chalil S, Stegemann B, Muhyaldeen S, Khadjooi K, Smith RE, Jordan PJ, et al. Intraventricular dyssynchrony predicts mortality and morbidity after cardiac resynchronization therapy: a study using cardiovascular magnetic resonance tissue synchronization imaging. J Am Coll Cardiol. 2007;50(3):243–52.
Boogers MM, Van Kriekinge SD, Henneman MM, Ypenburg C, Van Bommel RJ, Boersma E, et al. Quantitative gated SPECT-derived phase analysis on gated myocardial perfusion SPECT detects left ventricular Dyssynchrony and predicts response to cardiac resynchronization therapy. J Nucl Med. 2009;50(5):718–25.
Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59(17):1509–18.
Saba S, Marek J, Schwartzman D, Jain S, Adelstein E, White P, et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the speckle tracking assisted resynchronization therapy for electrode region (STARTER) trial. Circ Heart Fail. 2013;6(3):8.
Ypenburg C, Van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, et al. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. 2008;52(17):1402–9.
van de Veire NR, Schuijf JD, De SJ, Devos D, Bleeker GB, de RA, et al. Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J Am Coll Cardiol. 2006;48(9):1832–8.
Girsky MJ, Shinbane JS, Ahmadi N, Mao S, Flores F, Budoff MJ. Prospective randomized trial of venous cardiac computed tomographic angiography for facilitation of cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2010;33(10):1182–7.
Wikstrom G, Blomstrom-Lundqvist C, Andren B, Lonnerholm S, Blomstrom P, Freemantle N, et al. The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE-HF trial. Eur Heart J. 2009;30(7):782–8.
Adelstein EC, Tanaka H, Soman P, Miske G, Haberman SC, Saba SF, et al. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J. 2011;32(1):93–103.
Marsan NA, Westenberg JJ, Ypenburg C, Van Bommel RJ, Roes S, Delgado V, et al. Magnetic resonance imaging and response to cardiac resynchronization therapy: relative merits of left ventricular dyssynchrony and scar tissue. Eur Heart J. 2009;30(19):2360–7.
Xu YZ, Cha YM, Feng D, Powell BD, Wiste HJ, Hua W, et al. Impact of myocardial scarring on outcomes of cardiac resynchronization therapy: extent or location? J Nucl Med. 2012;53(1):47–54.
Becker M, Hoffmann R, Kuhl HP, Grawe H, Katoh M, Kramann R, et al. Analysis of myocardial deformation based on ultrasonic pixel tracking to determine transmurality in chronic myocardial infarction. Eur Heart J. 2006;27(21):2560–6.
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European heart rhythm association (EHRA). Eur Heart J. 2013;34:24.
Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53(9):765–73.
Bertini M, Delgado V, Bax JJ, van de Veire NR. Why, how and when do we need to optimize the setting of cardiac resynchronization therapy? Europace. 2009;11(Suppl 5):v46–57.
Ellenbogen KA, Gold MR, Meyer TE, Fernndez LI, Mittal S, Waggoner AD, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122(25):2660–8.
Vidal B, Sitges M, Marigliano A, Delgado V, Diaz-Infante E, Azqueta M, et al. Optimizing the programation of cardiac resynchronization therapy devices in patients with heart failure and left bundle branch block. Am J Cardiol. 2007;100(6):1002–6.
Doring M, Braunschweig F, Eitel C, Gaspar T, Wetzel U, Nitsche B, et al. Individually tailored left ventricular lead placement: lessons from multimodality integration between three-dimensional echocardiography and coronary sinus angiogram. Europace. 2013;15(5):718–27.
Nitsche B, Eitel C, Bode K, Wetzel U, Richter S, Doring M, et al. Left ventricular wall motion analysis to guide management of CRT non-responders. Europace. 2015;17(5):778–86.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Höke, U., Bax, J.J., Ajmone Marsan, N., Delgado, V. (2018). Echocardiography in Cardiac Resynchronization Therapy. In: Nihoyannopoulos, P., Kisslo, J. (eds) Echocardiography. Springer, Cham. https://doi.org/10.1007/978-3-319-71617-6_26
Download citation
DOI: https://doi.org/10.1007/978-3-319-71617-6_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71615-2
Online ISBN: 978-3-319-71617-6
eBook Packages: MedicineMedicine (R0)