Abstract
During the last two decades there has been an increasing interest in cardiac resynchronization therapy (CRT) in patients with heart failure. Major randomized clinical trials which tested the effectiveness of CRT included patients based on electrocardiographic (ECG) criteria amongst other features. However, there is an assumption that mechanical synchrony, as may be assessed by echocardiography, is the underlying mechanism that improves left ventricular function and finally the clinical outcome. Large multi-centre trials have failed to prove the usefulness of echocardiography in both selection of patients for CRT and identification of non-responders. On the contrary smaller trials from centres where there is high expertise in echo techniques have demonstrated that mechanical dyssynchrony can be assessed by echocardiography and can actually predict the response to CRT in a better way than traditional ECG criteria. In this manuscript, we have analysed current evidence from published data and attempted to provide insight to the field of cardiac dyssynchrony as assessed with echocardiography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The beneficial effect of cardiac resynchronization therapy (CRT) has been demonstrated in a series of randomized clinical trials. The first trials demonstrated that CRT improves symptoms, exercise capacity and LV structure and function (improvement in left ventricular ejection fraction [LVEF], reduction in left ventricular [LV] volumes and improvement of mitral regurgitation) [1–5]. Subsequently two more randomized trials (COMPANION and CARE-HF) investigated the effect of CRT on all-cause mortality and hospitalizations due to heart failure (HF) [6, 7]. A 24 % relative risk reduction in mortality associated with CRT-P was nearly statistically significant (P = 0.059) in COMPANION trial. In CARE-HF a 36 % relative reduction in the risk of death (p < 0.002) was demonstrated after a mean follow-up time of 29 months.
Definitions of Dyssynchrony
The above mentioned trials used prolonged QRS (usually ≥120 ms) as inclusion criterion. Most of the patients had left bundle branch block (LBBB) morphology. Prolongation of QRS is suggestive of ventricular conduction abnormality, thus considered a feature of electrical dyssynchrony. However dyssynchrony may have three different aetiologies [8]: (i) “electrical dyssynchrony”, this is considered an electrical conduction abnormality which causes uncoordinated timing of myocyte depolarization, (ii) abnormalities in excitation — contraction coupling and (iii) “mechanical dyssynchrony”, that is an abnormality in myocardial contractility leading to regional delay in contraction (onset of myocyte shortening or time to peak shortening). A typical example of electrical dyssynchrony is LBBB and of mechanical dyssynchrony is regional ischaemia or scar. Furthermore, cardiac dyssynchrony has been differentiated into atrio-ventricular (AV), inter-ventricular and intra-ventricular dyssynchrony [9]. Cardiac resynchronization therapy has an impact on all types of dyssynchrony.
Evidence from all randomized trials shows that only 60-80 % of patients benefit from CRT as evaluated by clinical or echocardiographic parameters [10]. Many possible causes have been investigated. One theory, as discussed above, is that electrical dyssynchrony is not always related to mechanical dyssynchrony. Indeed, it is true that QRS is not an accurate marker of LV dyssynchrony, thus patients with wide QRS complex may not demonstrate mechanical dyssynchrony as evaluated with echocardiography and patients with narrow QRS may actually have LV dyssynchrony [11–13]. That fact triggered attempts to relate mechanical dyssynchrony, as assessed by echocardiography, with both selection of patients for CRT and evaluation of outcome.
Mechanical Dyssynchrony in Patients with Significant Electrical Dyssynchrony
The first prospective non-randomized multi-centre clinical trial to assess echocardiographic parameters as predictors of clinical and echocardiographic response to CRT was PROSPECT [14]. The trial included 426 patients eligible for CRT based on guidelines provided indications (NYHA class III or IV heart failure, LVEF ≤ 35 %, QRS ≥ 130 ms and stable medical regimen). Echo data were analysed in three different core laboratories. Twelve echocardiographic parameters were used as predictors of positive response to CRT (Table 1). Response to CRT was defined using two separate primary outcomes: the heart failure clinical composite score and relative change in LVESV at 6 months.
Clinical response was predicted by three non-TDI parameters (interventricular mechanical delay [IVMD] ≥ 40 ms, p = 0.045; left ventricular filling time adjusted to cardiac cycle length [LVFT/RR] ≤ 40 %, p = 0.018; left ventricular pre-ejection interval [LPEI] ≥ 140 ms, p = 0.013) and one TDI-based method (maximum difference of time to onset of systolic velocity for six segments at basal level [Ts onset basal] ≥ 67 ms). Whereas the echocardiographic response was predicted by four non-TDI parameters (septal-posterior wall motion delay, IVMD, LVFT/RR and LPEI) and one TDI-based method (delay between time to peak systolic velocity in ejection phase at basal septal and basal lateral segments [Ts Lat-Sep] ≥ 60 ms, p = 0.005).
Despite the fact that several parameters predicted the response to CRT in terms of improvement in clinical and echocardiographic outcomes, the sensitivity and specificity were modest. However, there was a significant degree of interobserver and intraobserver variability that may account for the lack of predictive capabilities of echocardiographic parameters. A sub-analysis of PROSPECT trial [15] revealed that IVMD (p = 0.0002) and Ts Lat-Sep (p = 0.0022) were strongly associated with a larger reduction in LVESV at 6-month follow-up.
Subsequently, Kapetanakis et al. [16] utilized the potential advantages of 3D echocardiography to assess global LV dyssynchrony and predict response to CRT. The parameter they described and tested was the Systolic Dyssynchrony Index (SDI) which is the standard deviation of time to peak contraction (minimum volume) in each of the 16 LV sub-volumes normalized for the R-R duration and is expressed as a percentage of cardiac cycle duration (Figs. 1, 2). They included 147 patients, in two different centres, who fulfilled traditional criteria for CRT. Amongst them there were 24 patients with atrial fibrillation (AF), 23 patients with QRSd < 120 ms and 17 patients with paced rhythm who required upgrade to CRT. The outcomes they investigated were 1) clinical improvements assessed by reduction in NYHA functional class, 2) 20 % relative increase in EF and 3) LV reverse remodelling defined as 15 % reduction in LVESV. Predictive value of SDI was compared against QRS duration.
Responders who showed functional improvement had a significant difference in baseline SDI compared to non-responders (p < 0.0001), whereas QRSd was not different (QRSd: 137.5 ± 25.9 ms vs. 133.8 ± 25.9 ms, p = 0.43). The cutoff for SDI in predicting response was 10.4 % (sensitivity 90 % and specificity 67 %). In terms of improvement in EF, SDI was again significantly different between responders and non-responders (p < 0.0001). Similarly, baseline SDI was significantly different (p = 0.02) between patients who demonstrated LV reverse remodelling with optimal SDI cutoff of 11.4 %. QRSd did not show significant difference at baseline between the two groups for both echocardiographic outcomes (Fig. 3). The inter-observer variability showed excellent correlation for LVEDV, LVESV and LVEF. Quantification of dyssynchrony also showed very good agreement between operators based on variability of SDI. The study showed that 3D echo derived systolic dyssynchrony index is an excellent predictor of response to CRT, irrespective of QRS morphology and duration. Moreover, there is a sigmoid probability distribution for SDI (Fig. 4). For SDI > 10.4 % there is a high probability of responding that does not increase significantly with higher SDI and for SDI < 7.5 % there is a low probability of response, which does not reduce further with lower SDI. On the other hand the plot of probability of response predicted by QRS duration was nearly linear.
The Baseline QRS duration and SDI in responders and nonresponders. Top row: scatter plots for QRS duration in responders and nonresponders as defined by clinical and echocardiographic criteria. Bottom row: scatter plots for SDI in responders and nonresponders for the same criteria of response. NYHA response: response based on New York Heart Association functional class. EF20 response: Relative increase of ≥20 % in ejection fraction. ESV15 Response: ≥ 15 % increase in end-systolic volume. EF10 Response: Relative increase of ≥ 10 % in ejection fraction. SDI: Systolic dyssynchrony Index. (Published in JACC Cardiovasc Imaging, 2011;4(1), Kapetanakis S, Bhan A, Murgatroyd F, et al. “Real-time 3D echo in patient selection for cardiac resynchronization therapy” pages 16-26, Copyright © 2011 by the American College of Cardiology Foundation)
Probability of positive response to CRT predicted by QRS duration and SDI. Left panel: Poor prediction of response to CRT by QRS duration as probabilities are similar across the range of QRS values. Right panel: Sigmoid probability curve of SDI. Values >10.4 % correspond to a very high probability with little further increase at higher values. SDI < 7.5 % demonstrates low probability of response. For intermediate values between 7.5 and 10.4 % there are large changes in probability with small changes in SDI. These values represent a “grey zone” and therefore are less reliable. Histograms along each axis show the distribution of subjects. SDI: Systolic Dyssynchrony Index. (Published in JACC Cardiovasc Imaging, 2011;4(1), Kapetanakis S, Bhan A, Murgatroyd F, et al., “Real-time 3D echo in patient selection for cardiac resynchronization therapy” pages 16-26, Copyright © 2011 by the American College of Cardiology Foundation)
The value of 3D echocardiography and SDI was also outlined by Auger et al. [17] who studied 166 patients who fulfilled traditional criteria and were treated with CRT. They used two different echocardiographic criteria to define LV dyssynchrony: 1) Standard deviation (SD) of time to peak systolic velocity (Ts) in the 12 basal and mid-segments (Ts-SD-12) ≥ 33 ms and 2) systolic dyssynchrony index (SDI) ≥ 6.4 %. The investigators used a different software platform compared to Kapetanakis et al. [16] to calculate SDI, therefore there was a different cut-off value. Response to CRT was defined as ≥15 % decrease in 3D LV ESV in 6 months after CRT implantation.
In multi-variate analysis, Ts-SD-12 [Odds ratio (OR): 1.054, 95 % CI: 1.032-1.077, p < 0.001] and 3D echocardiographic SDI (OR: 1.468, 95 % CI: 1.216-1.772, p < 0.001) showed significant correlation with response to CRT. Patients who had significant LV dyssynchrony as assessed by triplane Ts-SD-12 and SDI had a high likelihood of response to CRT (86.3 %). On the contrary, patients who did not exhibit LV dyssynchrony had a high likelihood of non-response (97 %).
Szulik et al. [18] tested a novel echocardiographic measurement of LV dyssynchrony by analysing apical rocking. They used apical transverse motion (ATM) as a marker of apical motion perpendicular to the LV long-axis and this was calculated in each apical plane (A4C, A3C and A2C) as the average of the integrated longitudinal myocardial velocity curves from the two opposite apical segments. A true apex transverse motion (ATMloop) was reconstructed from the ATM curves of the three apical views and the main direction and amplitude of the ATMloop was measured (Fig. 5). They included 67 patients with measurable ATM who fulfilled conventional criteria for CRT based on current guidelines and they defined as response a 15 % or more decrease in LVESV.
a Calculation of the apical transverse motion (ATM) using the apical 4-chamber view. Using tissue Doppler (left) the longitudinal apical septal motion (dxsep) and the inverted lateral motion (dxlat) are plotted and averaged over time to produce the transverse apex motion in apical 4-chamber view (ATM4CV). The ECG shows left bundle branch block pattern. In that case it is typical to observe an early short septal motion of the apex followed by a lateral motion during ejection time (ET). b Calculation of the apical transverse motion loop (ATMloop) using the all three apical image planes. The polar plot on the right shows the transverse displacement of the apex in a single cardiac cycle. (Yellow: isovolumic contraction, Red: ejection time, Green: Isovolumic relaxation, Blue: diastole). 2CV: 2-chamber view, 3CV: 3-chamber view, 4CV: 4-chamber view. (Published in Eur J Echocardiogr, 2010;11(10), Szulik M, Tillekaerts M, Vangeel V, et al. “Assessment of apical rocking: a new, integrative approach for selection of candidates for cardiac resynchronization therapy”, pages 863-9. Published on behalf of the European Society of Cardiology, © The Author 2010)
The maximum amplitude of the reconstructed ATMloop was significantly higher in responders compared to non-responders (p = 0.002). A cut-off value of 2.8 mm predicted response with a sensitivity of 70 %, specificity of 90 % and accuracy of 76 %. ATM in the A4C view during ejection time (ATMA4C_ET) was the best predictor of CRT response with a cut-off value of 1.5 mm yielding a sensitivity of 75 %, specificity 96 % and accuracy of 83 %.
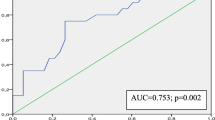
More recently, apical rocking as a marker of LV dyssynchrony to predict CRT response was also tested by Stankovic et al. [19]. They selected 58 patients based on current guidelines for CRT (LV ejection fraction ≤35 %, QRS duration >120 ms, NYHA functional class III or IV, and on optimized pharmacological therapy for at least 3 months before CRT) and they enrolled them for low-dose Dobutamine stress echo (DSE). They used ATM in the A4-C view during ejection time (ATMA4C_ET) and dyssynchrony was defined with a cut-off value of 1.5 mm. Calculation of ATM was possible in 55 patients (95 % feasibility). During low-dose DSE, the amplitude of apical rocking increased significantly in the group of responders (p = 0.001) but not in nonresponders (p = 0.074). The changes in the amplitude of apical rocking during low-dose DSE were also associated with CRT response (area under curve [AUC]: 0.89, 95 % CI 0.80–0.97, p = 0.001) defining response as >10 % reduction in LV ESV.
In a 12 ± 2 months follow-up assessment by echocardiography, the investigators demonstrated that the degree of reverse remodelling was correlated with the amplitude of apical rocking at rest (r = 0.5, p < 0.001) but more strongly with changes in apical rocking during low-dose DSE (r = 0.7, p < 0.001). Apical rocking at peak stress was also associated with improved long-term survival (log rank test p = 0.008).
Though it is known that CRT may not be effective in about 30 % of patients who fulfil current inclusion criteria [11] it is only recently that Auger et al. [20•] argued that CRT in those patients may actually be harmful. They studied 290 patients undergoing CRT based on current guideline criteria who did not have mechanical dyssynchrony before implantation. Dyssynchrony was defined as maximum delay between peak systolic velocities of the septal and lateral wall [Ts-Sep/Lat] using a cut-off value of ≥60 ms; 131 (45 %) patients had QRSd < 150 ms and the remaining 159 (55 %) had QRSd > 150 ms. They also included a matched control group of 290 with overt mechanical dyssynchrony before implantation. The first group was then divided in two groups according to the 48 hours post-CRT median value of LV dyssynchrony. Thus, patients who had Ts-Sep/Lat ≥ 40 ms were considered to have induced LV dyssynchrony with CRT and patients with Ts-Sep/Lat < 40 ms considered to remain in synchrony.
At 6-months follow-up the percentage of non-responders was significantly higher in the group of induced LV dyssynchrony (Ts-Sep/Lat ≥ 40 ms) compared to the group with no LV dyssynchrony (Ts-Sep/Lat < 40 ms); 93 % vs 51 %, p < 0.001. In a multivariate Cox regression analysis, induced LV dyssynchrony was an independent predictor of all-cause mortality (hazard ratio [HR]: 1.247, 95 % CI: 1.056 – 1.474, p = 0.009) in a median follow-up of 34 months. The importance of this study is that it demonstrates that CRT may induce mechanical dyssynchrony in patients with broad QRSd (>120 ms) who did not have LV dyssynchrony before implantation. This may not only account for a higher percentage of non-responders but may also cause adverse outcomes.
Mechanical Dyssynchrony in Patients with Intermediate Electrical Dyssynchrony
The DESIRE trial [21] tested the value of Doppler based echo measurements to predict response to CRT in a population with QRS duration less than 150 ms. It was a multi-centre prospective non-randomized trial that included 60 patients. The patients were assessed for atrioventricular, inter-ventricular and intra-ventricular dyssynchrony using four echocardiographic parameters (Table 2). Patients were divided in two groups, dyssynchronous group and non-dyssynchronous group, based on the presence of one or more of the tested criteria.
The primary combined end-point of the study included 1) all-cause mortality 2) HF hospitalizations and 3) NYHA class at 6 months. The patients were classified as improved, unchanged or worsened based on the above parameters. Mortality rate was similar in the two groups. A clinical improvement was observed in 19 of 27 dyssynchronous group patients (70 %) as opposed to 14 of 33 (42 %) non-dyssynchronous patients (p < 0.04). Based on the criteria used in this study, the post-implant measurements did not reveal improvement in dyssynchrony with CRT.
The RethinQ trial [22] was a double-blind controlled randomized clinical trial which enrolled and followed-up 156 patients with primary indication for ICD (ischaemic or non-ischaemic with EF ≤ 35 %) and additionally NYHA class III, QRS duration ≤130 ms and evidence of mechanical dyssynchrony on echocardiography. The echocardiographic measurements used to assess dyssynchrony were: 1) mechanical delay in the septal-to-posterior wall obtained by M-Mode ≥ 130 ms, 2) opposing wall delay between anteroseptal-to-posterior or septal-to-lateral wall ≥65 ms measured using tissue Doppler imaging. All patients received CRT-D and were randomized in two groups: CRT-on and CRT-off.
At 6 months the two groups did not differ significantly in the primary end point which was an increase in peak oxygen consumption of at least 1.0 ml per kg of body weight per minute during cardiopulmonary exercising test. Furthermore, there was no significant difference in quality-of-life scores, 6-minute walk test (6-MWT) and echocardiographic measurements. The only significant difference observed between the two groups was that the CRT-group had a significant improvement in NYHA class (54 %) compared to the control group (29 %, p = 0.006). Interestingly, a pre-specified subgroup analysis of patients with QRS duration between 120 and 130 ms, demonstrated that the CRT-group patients had significant improvement in peak oxygen consumption (p = 0.02) and NYHA class (p = 0.01) but not in quality of life and 6-minute walk distance.
Mechanical Dyssynchrony in Patients without Electrical Dyssynchrony
A non-randomized clinical trial performed by Yu et al. [23] included 102 patients with HF and EF < 40 %; 51 patients had QRS duration >120 ms and 51 had QRS duration <120 ms. Dyssynchrony was assessed by using TDI in apical 4-chamber, 2-chamber and 3-chamber views. Long-axis motion of the LV and the standard deviation of the time to peak velocity in the ejection phase of the 12 LV segments were measured [Ts-SD or asynchrony index (AI)]. The cut-off value was 32.6 ms. As predetermined, nearly half of the patients with narrow QRS (N = 27) had significant systolic dyssynchrony (AI ≥ 32.6 ms) and the remaining did not (AI < 32.6 ms).
The primary end-point of the study was LV reverse remodelling defined as 15 % or more reduction in LV ESV. In both groups, Ts-SD was the only independent predictor of LV reverse remodelling. In the group of patients with QRS duration <120 ms, those with significant mechanical dyssynchrony had a greater extend of reverse remodelling as defined by a change in LVESV, LVEDV and EF (all p < 0.001) compared to those without mechanical dyssynchrony. Furthermore, both groups of patients with narrow and broad QRS experienced significant improvement in NYHA functional class, 6-MWT and exercise capacity expressed in metabolic equivalents of task (METs). However, quality of life, as assessed by the Minnesota Living with Heart Failure Questionnaire, improved only in patients with wide QRS.
A more recent large multi-centre randomized clinical trial that attempted to assess the value of echocardiography in selection of patients with narrow QRS for CRT is the Echo-CRT trial [24•]. It included 809 patients who received CRT-D and were randomized into two arms: CRT on (404 patients) and CRT off (405 patients). Inclusion criteria were NYHA class III or IV, EF <35 %, QRS duration less than 130 ms and evidence of dyssynchrony on echocardiography. The assessment of dyssynchrony was made based on tissue Doppler imaging or speckle tracking radial strain. The primary end-point of the study was all-cause mortality and first hospitalization for heart failure. The primary end-point occurred in 28.7 % of patients in the CRT group and in 25.2 % of patients in the control group (hazard ratio [HR] with CRT: 1.20; 95 % confidence interval [CI]: 0.92-1.57; p = 0.15). Mortality in CRT group was 11.1 % and 6.4 % in the control group (HR: 1.81; CI: 1.11-2.93; p = 0.02). Cardiovascular death was more prevalent in CRT group (9.2 %,) as opposed to control group (4.2 %); HR: 1.74; CI: 0.80–3.81; p = 0.004. Changes from baseline to 6 months with respect to functional status and quality of life, as evaluated by NYHA class and the Minnesota Living with Heart Failure Questionnaire, did not differ significantly between the study groups. Interestingly, inappropriate shocks were more prevalent in CRT group compared to control group (20 patients [5.0 %] vs. 7 [1.7 %], p = 0.01). The authors argue that CRT induced pro-arrhythmia could account in part for the increased mortality among patients assigned to active therapy. In addition, unnecessary pacing may contribute to the development of heart failure.
The results of Echo-CRT are questioned by another prospective, single-blind multicentre trial, the NARROW-CRT study [25•]. It included candidates for ICD with NYHA class II and III, ischemic cardiomyopathy, ejection fraction of ≤35 % and QRS interval of ≤120 ms and evidence of mechanical dyssynchrony as assessed by echocardiography. The investigators used the difference in time to peak systolic velocities between the basal septal and lateral wall in tissue Doppler imaging and defined dyssynchrony using a cut-off value of 60 ms. The patients were randomized into CRT-D and only ICD arms. Primary end-point was the HF clinical composite score. Secondary end-point was the cumulative survival from HF hospitalization and HF death. 233 patients met eligibility criteria for ICD and 111 of them who demonstrated dyssynchrony on echocardiography were randomized and completed the 1-year follow-up.
Improvement in the HF clinical composite response was significantly higher in CRT-D group in 1 year (p = 0.004). Secondary end-point was higher in CRT-D group but did not meet statistical significance (p = 0.077). Another secondary end-point that was tested was the composite of HF death, HF hospitalization and spontaneous ventricular fibrillation. The survival from this additional end-point was significantly higher in CRT-group (p = 0.028). Echocardiographic parameters were also compared between groups. Increase in EF was significantly higher (p < 0.001) in the CRT-D group and LV reverse remodelling was also significantly higher (p = 0.003) in the same group as assessed by change in LVESV. Furthermore, the investigators studied the patients who did not demonstrate dyssynchrony on baseline echocardiography who were not randomized into the two arms. Kaplan-Meier estimates of secondary and additional secondary end-point were calculated for those patients and were compared with the estimates made in the CRT-D and ICD study arms. Interestingly the CRT-D group exhibited event rate comparable with that of the group with no evidence of mechanical dyssynchrony, but the ICD group had significantly worse outcome.
Discussion
The role of echocardiography for selection of patients for CRT remains unclear and controversial based on published data. While two large multi-centre clinical trials have failed to provide evidence of usefulness of echocardiography [14, 24•], several single or smaller multi-centre trials have demonstrated that in expert centres, echocardiography can be a very useful tool and can provide more robust predictors of CRT response compared to traditional criteria [16–18, 25•].
CRT as a method of delivering electrical impulse to different sites of the heart is considered a technique that restores or improves electrical dyssynchrony. However, there is a clear mechanical effect in terms of improving LV ejection fraction and promoting LV reverse remodelling [4, 7]. Is restoration of mechanical dyssynchrony the cause of structural and clinical improvement? Auger et al. [26] demonstrated with 2-D speckle tracking echocardiography that CRT responders have significant restoration of LV mechanical dyssynchrony as opposed to non-responders who remain dyssynchronous. That implies that mechanical resynchronization after electrical resynchronization with CRT is important for clinical and hemodynamic improvement. Kapetanakis et al. [16] showed that in patients with a ≥20 % improvement in LVEF with CRT there was a significant improvement in dyssynchrony index (ΔSDI: 5.9 ± 4.3 %, p = 0.0001, Fig. 6) with a good correlation between change in LVEF and change in SDI (linear correlation coefficient: 0.62). Similarly, patients with symptomatic improvement had a significant reduction in SDI (ΔSDI: 6.1 ± 0.66 %, p = 0.009) suggesting that restoration of mechanical synchrony is related to favourable outcome with CRT.
Change in SDI and left ventricular EF after CRT. Left: The change in EF illustrated as a scatter plot for responders and non-responders. Reduction of SDI (positive ΔSDI) is greater in responders, p < 0.001. Middle: Reduction in mechanical dyssynchrony as assessed by change in SDI (positive ΔSDI) is well correlated with increase in left ventricular ejection fraction. Right: The change in EF (ΔEF) is correlated well with SDI. Red line: linear correlation. Blue line: polynomial correlation. Dark blue area: confidence intervals for mean, line and polynomial fits. Light blue area: 95 % confidence limits for an individual predicted value. Δ = difference. Other abbreviations as in Fig. 3. (Published in JACC Cardiovasc Imaging, 2011;4(1), Kapetanakis S, Bhan A, Murgatroyd F, et al., “Real-time 3D echo in patient selection for cardiac resynchronization therapy” pages 16-26, Copyright © 2011 by the American College of Cardiology Foundation)
The two most recent multi-centre trials provide contradicting results in patients without electrical dyssynchrony who exhibit mechanical dyssynchrony in echocardiography. In NARROW-CRT study [25•] which investigated patients without electrical dyssynchrony (QRSd < 120 ms) the investigators demonstrated that clinical improvement was similar between patients without mechanical dyssynchrony and patients with mechanical dyssynchrony who received treatment (CRT). On the other hand patients who had mechanical dyssynchrony but did not receive treatment (randomized in the ICD arm) had worse outcome compared to patients who did not have mechanical dyssynchrony on baseline echocardiography. The above findings suggest that CRT is beneficial in patients without electrical dyssynchrony who have mechanical dyssynchrony on echocardiography. However the investigators did not test if CRT actually improved the dyssynchrony indices.
On the other hand Echo-CRT trial [22] failed to prove that CRT in patients without electrical dyssynchrony may be useful. On the contrary, the results suggest that it may be harmful. The authors argue that the lack of benefit with CRT may be attributed to the fact that the LV placement was not tailored to the mechanical abnormal substrate or the leads were placed in scar areas. Indeed, the role of mechanical restoration on the outcome of CRT was demonstrated by two randomized control trials TARGET [27] and STARTER [28] in which the LV lead placement was guided by echocardiography. Results suggest that LV lead position adjacent or at the latest contracting segment can improve the outcome of CRT.
The lack of consistency in results from different studies has given birth to several theories which try to explain the multifactorial phenomenon of dyssynchrony. Smiseth et al. [29] suggest that one reason that echocardiography has not been proven consistently to provide added value in selection of patients for CRT is due to suboptimal methodological approaches. They focus on the differentiation between electrical and non-electrical aetiologies of dyssynchrony and claim that the use of ejection-phase velocity indices may not be the most appropriate because peak ejection velocity has significant limitations as a marker of electrical conduction delay. They propose a non-invasive method to assess regional myocardial work by LV pressure-strain loop analysis and they demonstrated that the loops are entirely different for segments with electrical dyssynchrony compared to segments with impaired contractility due to ischaemia (Fig. 7). In electrical dyssynchrony the early-activated septum exhibits marked pre-ejection shortening, whereas at the same time the lateral wall reacts with pre-ejection lengthening. On the other hand, an ischaemic segment demonstrates pre-ejection lengthening without associated changes in the opposite wall. Furthermore, experimental data (Fig. 8) supports the fact that differences in timing to peak myocardial shortening velocity may reflect mechanical dyssynchrony but does not provide a strong relationship with electrical dyssynchrony. Thus, they suggest that electrical and non-electrical aetiologies of dyssynchrony should be differentiated and that investigators and clinicians need to consider potential ischaemia, scarring and other structural changes as contributors to dyssynchrony. The novel method they utilize (LV pressure – strain loop) to estimate timing of electrical activation needs to be further explored in future studies.
a LBBB pattern. Left ventricular pressure strain loops in the septum and lateral wall (animal experiment). Normal pattern of strain loop rotation in the lateral segment (counter-clockwise). The area in the loop reflects segmental work. Significantly diminished loop area in the septal segment suggestive of reduced segmental work. The red arrow indicates the pre-ejection septal shortening. b Experimental left anterior descending artery occlusion. On the left, the pressure strain graph of the anterior segment shows a loop area close to zero which suggests that this segment is not generating work (affected by ischaemia). As shown by a positive value strain during systole (black arrow) the anterior segment is stretched. On the right side there is a normal pressure strain loop of the posterior segment with counter-clockwise pattern. (Published in Eur Heart J Cardiovasc Imaging. 2012;13(1), Smiseth O, Russell K, Skulstad H. “The role of echocardiography in quantification of left ventricular dyssynchrony: state of the art and future directions”, pages 61-68. Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2011)
Experimental study in left bundle branch block pattern. Upper panel: Myocardial velocity recorded by sonomicrometry and regional electrical activation recorded by intramyocardial electromyograms (EMG) for the septal wall. Middle: Similar recordings for the lateral wall. Lower panel: Recording of left ventricular pressure and ECG. Comparison of the graphs shows that peak septal ejection velocity occurs after peak lateral wall velocity, opposite to electrical activation. AVC: aortic valve closure. AVO: aortic valve opening. IVC: isovolumic contraction. (Published in Eur Heart J Cardiovasc Imaging. 2012;13(1), Smiseth O, Russell K, Skulstad H. “The role of echocardiography in quantification of left ventricular dyssynchrony: state of the art and future directions”, pages 61-68. Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2011)
In a different approach, Auger et al. [17] combined two different echocardiographic indices to assess LV dyssynchrony. The tissue-Doppler based Ts-SD-12 evaluates time difference between peak velocities of different LV segments that occur in the early systolic phase. The 3D echo derived SDI represents time differences in regional end-systolic volumes. However, end-systolic volumes is the ultimate consequence of electromechanical LV activation (peak tissue velocities) occurring at the end of myocardial contraction. Thus, the combination of tri-plane tissue synchronization imaging (TSI) and real time 3D echocardiography (RT3DE) are two complimentary methods to evaluate LV dyssynchrony at different time points during LV systole. The authors also demonstrated the additive role of two techniques in predicting response to CRT.
There is strong evidence that patients with significant electrical dyssynchrony (QRSd > 150 ms) benefit from CRT and this has been shown in large multi-centre trials [6, 7, 30]. The Cardiology community remains sceptical about whether echocardiography can add to selecting and predicting those patients who will respond to CRT. Despite the fact that the question is very important, given the non-responder rate, current guidelines do not encourage denying CRT to patients with broad QRS (>150 ms) who do not demonstrate mechanical dyssynchrony in echocardiography. However, Auger et al. [29] argue that CRT in patients without mechanical dyssynchrony may induce dyssynchrony with adverse effects. Whether this is likely to change current clinical practice will only be discovered if larger trials are conducted in the future.
In patients with intermediate QRS duration (QRSd = 120 – 150 ms) the evidence of benefit from CRT is less robust. In this field echocardiography may have a role in selection of patients based in small trials [16–19, 21] which have shown that different dyssynchrony indices in patients with QRSd > 120 ms can have significant predictive value for CRT response. Furthermore, in the large CARE – HF trial [7], mechanical dyssynchrony, as assessed by echocardiography, was an inclusion criterion for patients with QRSd between 120 and 150 ms.
Finally, in patients with narrow QRS further evidence is needed to change current practice, as the largest randomized multi-centre trial [24•] has shown that in this group of patients, CRT guided by echocardiographically assessed mechanical dyssynchrony, is not useful and may carry unfavourable outcome.
Conclusion
In patients with electrical dyssynchrony not exhibiting LV mechanical dyssynchrony and patients without electrical dyssynchrony having clear evidence of mechanical dyssynchrony, it is reasonable to anticipate a significant role of echocardiography in identifying and stratifying those patients. The results of large multi-centre trials have failed to demonstrate usefulness of echocardiography in selection of patients for CRT and current guidelines [9] recommend using only QRS duration and morphology for selection of patients. There is general agreement that assessment of LV dyssynchrony with echocardiography is a demanding approach requiring specific training to obtain competency. A multi-centre trial has demonstrated significant inter-observer variability [14]. In addition, the large number of echo parameters that have been tested makes consensus difficult in terms of recognizing the indices which have the highest predictive yield, the highest correlation with CRT outcome and will be more reproducible in clinical practice. New insight in the multifactorial nature of dyssynchrony comes from direct correlation of myocyte electrical activation with mechanical contraction [29]. The encouraging results of small single or multi-centre trials will have clinical usefulness only when validated in prospective randomized multi-centre trials. Until then it is clinically and scientifically advisable to follow current guidelines regarding selection of patients for CRT.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53.
Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, Misier AR, et al. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003;42:2109–16.
Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80.
Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–9.
Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–94.
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49.
Otto A. The role of echocardiography in quantification of left ventricular dyssynchrony: state of the art and future directions. Eur Heart J Cardiovasc Imaging. 2012;13:61–8.
Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34(29):2281–329.
Bax JJ, Gorcsan III J. Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933–43.
Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–9.
Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol. 2002;39:194–201.
Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60.
Chung E, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16.
van Bommel RJ, Bax JJ, Abraham WT, et al. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30(20):2470–7.
Kapetanakis S, Bhan A, Murgatroyd F, et al. Real-time 3D echo in patient selection for cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2011;4(1):16–26.
Auger D, Bertini M, Marsan NA, et al. Prediction of response to cardiac resynchronization therapy combining two different three-dimensional analyses of left ventricular dyssynchrony. Am J Cardiol. 2011;108(5):711–7.
Szulik M, Tillekaerts M, Vangeel V, et al. Assessment of apical rocking: a new, integrative approach for selection of candidates for cardiac resynchronization therapy. Eur J Echocardiogr. 2010;11(10):863–9.
Stankovic I, Aarones M, Smith H-J, et al. Dynamic relationship of left-ventricular dyssynchrony and contractile reserve in patients undergoing cardiac resynchronization therapy. Eur Heart J. 2014;35(1):48–55.
Auger D, Bleeker GB, Bertini M, et al. Effect of cardiac resynchronization therapy in patients without left intraventricular dyssynchrony. Eur Heart J. 2012;33(7):913–20. This study suggests that CRT induced LV dyssynchrony in patients without baseline dyssynchrony may carry adverse outcome.
Cazeau SJ, Daubert J-C, Tavazzi L, Frohlig G, Paul V. Responders to cardiac resynchronization therapy with narrow or intermediate QRS complexes identified by simple echocardiographic indices of dyssynchrony: the DESIRE study. Eur J Heart Fail. 2008;10(3):273–80.
Beshai JF, Grimm R, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357(24):2461–71.
Yu C-M, Chan Y-S, Zhang Q, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48(11):2251–7.
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–405. Recent large multi-centre trial demonstrating that CRT in patients with narrow QRS who exhibit LV dyssynchrony on echocardiography is not beneficial and may carry unfavourable outcome.
Muto C, Solimene F, Gallo P, et al. A randomized study of cardiac resynchronization therapy defibrillator versus dual-chamber implantable cardioverter-defibrillator in ischemic cardiomyopathy with narrow QRS: the NARROW-CRT study. Circ Arrhythm Electrophysiol. 2013;6(3):538–45. Recent multi-centre trial which demonstrates that CRT in patients with narrow QRS and evidence of dyssynchrony is beneficial.
Auger D, Hoke U, Thijssen J, et al. Effect of cardiac resynchronization therapy on the sequence of mechanical activation assessed by two-dimensional radial strain imaging. Am J Cardiol. 2014;113(6):982–7.
Khan FZ, Virdee MS, Palmer CR, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59(17):1509–18.
Saba S, Marek J, Schwartzman D. Ventricular lead placement for cardiac resynchronization therapy results of the speckle tracking assisted resynchronization therapy for electrode region trial. Circ Heart Fail. 2013;6(3):427–34.
Smiseth O, Russell K, Skulstad H. The role of echocardiography in quantification of left ventricular dyssynchrony: state of the art and future directions. Eur Heart J Cardiovasc Imaging. 2012;13(1):61–8.
Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart failure events. N Engl J Med. 2009;361:1329–38.
Acknowledgements
The authors would like to thank Elsevier, Oxford University Press, the European Society of Cardiology, the American College of Cardiology Foundation and the authors Otto Armin Smiseth and Jens-Uwe Voigt for kindly giving permission to reproduce material from previously published articles.
Dr. Alexandros Papachristidis reviewed the literature and wrote the manuscript. Prof. Mark J Monaghan revised the manuscript and provided expert opinion throughout.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Prof. Mark J Monaghan has been granted research software from TomTec Imaging Systems GmbH and Speaker’s bureau for Koninklijke Philips N.V.
Dr. Alexandros Papachristidis' post was funded by Medtronic, Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Echocardiography
Rights and permissions
About this article
Cite this article
Papachristidis, A., Monaghan, M.J. Echocardiographic Assessment of Cardiac Dyssynchrony. Where do We Stand?. Curr Cardiovasc Imaging Rep 7, 9300 (2014). https://doi.org/10.1007/s12410-014-9300-2
Published:
DOI: https://doi.org/10.1007/s12410-014-9300-2