Abstract
Cardiac resynchronization therapy (CRT) is an important therapeutic tool in the management of patients with heart failure and electrical dyssynchrony. In appropriately selected patients, landmark randomized controlled trials have demonstrated morbidity and mortality benefit beyond standard goal-directed medical therapy. Current guidelines emphasize the greatest clinical efficacy of CRT in patients with symptomatic heart failure, left bundle branch block, and wide QRS duration (> 150 ms). Other relevant considerations include the presence of atrial fibrillation, the presence of AV block, the etiology of cardiomyopathy, the presence of masked left-sided conduction delay, and the impact of comorbidities that might predict poor clinical response. At the time of CRT implantation, key considerations include targeting of the left ventricular (LV) lead to sites of greatest electrical and/or mechanical delay, the use of quadripolar versus bipolar LV pacing leads, evaluation of multiple pacing vectors to maximize electrical resynchronization, and in select instances pre-procedure imaging of the coronary venous anatomy to help guide decision-making at the time implant. Post-implant care includes the selective use of atrio-ventricular and inter-ventricular optimization algorithms, mitigation of right ventricular pacing, recognition, and treatment of suboptimal biventricular pacing, as well as management by a multi-disciplinary team of cardiovascular specialists. Emerging therapeutic strategies for patients eligible for CRT include the use of endocardial LV pacing, novel LV pacing options including multi-point pacing, His bundle pacing, and the integration of remote monitoring platforms that may identify patients at risk for clinical worsening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac resynchronization therapy (CRT) is a key therapeutic strategy for patients with symptomatic heart failure, depressed left ventricular function, and electrical dyssynchrony [1, 2]. Biventricular pacing therapy in appropriately selected patients is associated with favorable remodeling of the left ventricle, improvements in the efficiency of ventricular contraction, and ultimately improvements in clinical outcomes including quality of life, risk of heart failure hospitalization, and survival [3,4,5,6,7]. The evaluation and care of the patient undergoing CRT involves the coordinated cooperation of specialists in electrophysiology, advanced heart failure, and cardiovascular imaging [8].
Landmark trials in cardiac resynchronization therapy
Advanced heart failure
There are now numerous landmark trials establishing the efficacy of CRT therapy in patients with heart failure (Table 1). For patients with advanced heart failure (NYHA III/IV), the first double-blind randomized controlled comparison of CRT was the MIRACLE (Multicenter Insync Randomized Clinical Evaluation) trial in which 453 patients with advanced HF (NYHA III/IV), LVEF ≤ 35%, and QRS ≥ 130 ms were randomized to CRT or goal-directed medical therapy (GDMT) [3]. CRT was associated with left ventricular reverse remodeling (improved LVEF, decreased LV diastolic dimension), improved functional capacity, and a 40% decrease in the composite primary endpoint of heart failure hospitalization or death. Similar findings were reported in the CARE-HF (Cardiac Resynchronization on Morbidity and Mortality in Heart Failure) trial [5] which randomized patients with symptomatic heart failure (NYHA III/IV), LVEF ≤ 35%, and QRS > 120 ms to CRT pacing (CRT-P) or GDMT. CRT-P was associated with improvements improved functional capacity, LV reverse remodeling, and decreased HF hospitalization. In long-term follow-up, CRT-P was associated with a 36% reduction in all-cause mortality when compared to GDMT, which established a mortality benefit from CRT pacing in the absence of a defibrillator [9]. The COMPANION (Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure) trial compared a strategy of GDMT to that of CRT-P and CRT with ICD (CRT-D) in patients with LVEF ≤ 35%, NYHA III/IV, and QRS > 120 ms [4]. Both CRT strategies (CRT-P, CRT-D) reduced the risk of death or HF hospitalization compared to GDMT and both CRT strategies reduced the hazard for all-cause mortality (24% for CRT-P, 36% for CRT-D), although the reduction in mortality for CRT-P was only of borderline significance. To date, there remains no direct and appropriately powered randomized comparison of CRT-D versus CRT-P.
Mild heart failure
The efficacy of CRT therapy in patients with mildly symptomatic HF was demonstrated in the REVERSE-HF, MADIT-CRT, and RAFT trials. In REVERSE (Resynchronization Reverse Remodeling in Systolic Left Ventricular Dysfunction), patients with an LVEF <40%, QRS ≥ 120 ms, and mild HF (NYHA I/II) were randomized to CRT or GDMT [10]. CRT was associated with LV reverse remodeling as well as a 50% reduction in HF hospitalizations at 1 year follow-up. In the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial, 1820 patients with mild HF (NYHA I with ischemic etiology or NYHA II), QRS > 130 ms, and LVEF < 30% were randomized to CRT-D or ICD therapy alone [6]. CRT therapy was associated with a 34% reduction in the primary endpoint of death or symptomatic HF, driven primarily by a reduction in HF events. Finally, in the RAFT (Resynchronization/Defibrillation for Ambulatory Heart Failure Trial) study [7], CRT-D versus ICD therapy was compared in patients with mild HF (NYHA II/III), LVEF ≤ 30%, and QRS > 120 ms. CRT therapy was associated with a 25% reduction in the primary endpoint of death or HF hospitalization with significant reductions in both mortality (25% risk reduction) and HF hospitalization (30%). Of trials of CRT in patients with mild HF, RAFT was the only one to demonstrate a significant mortality benefit, which likely reflects the baseline mortality risk in the study (placebo group mortality of 21% in patients with NYHA II/III HF) versus trials that included NYHA I patients (MADIT-CRT, REVERSE) in which the mortality was significantly lower (placebo group mortality 2–7%) and follow-up time was shorter.
Chronic RV pacing
Right ventricular (RV) pacing is associated with asynchronous activation of the left ventricle and several previous studies have shown an association between RV pacing burden and an increased risk of heart failure hospitalization, atrial fibrillation, and left ventricular dysfunction [11,12,13]. In this context, current North American guidelines recommend biventricular pacing therapy in patients with LVEF ≤ 35% who are undergoing new or replacement device with anticipated requirement for significant (> 40%) ventricular pacing [2]. The BLOCK-HF (Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block) study examined the impact of CRT versus RV pacing alone in patients with an indication for pacing, LVEF ≤ 50%, and HF (NYHA I-III) [14]. CRT was associated with a 26% reduction in the primary endpoint of death or HF hospitalization, which was driven by a reduction in HF events. In contrast, the BIOPACE (Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization) study did not demonstrate a morbidity or mortality benefit of CRT versus RV pacing in patients with a preserved left ventricular ejection fraction [15].
Narrow QRS and mechanical dyssynchrony
In addition to patients with electrical dyssynchrony, investigators observed that some patients with a narrow QRS demonstrated mechanical LV dyssynchrony and initial studies suggested that patients with mechanical dyssynchrony and normal QRS duration demonstrated clinical improvement with CRT [16, 17]. These studies prompted the larger scale evaluation of CRT in this population including the RethinQ, LESSER-EARTH, and ECHO-CRT trials, all of which showed no benefit and in the case of LESSER-EARTH and ECHO-CRT, possible harm [18,19,20]. The RethinQ (Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS) study compared CRT-D to ICD therapy in patients with NYHA III HF, LVEF ≤35%, and QRS < 130 ms with mechanical dyssynchrony as assessed by tissue Doppler imaging (TDI) [18]. There was no improvement in peak oxygen consumption or LV remodeling parameters at 6 months. The LESSER-EARTH (Evaluation of Resynchronization Therapy for Heart Failure) study compared a CRT-On versus CRT-Off strategy in patients with symptomatic HF, LVEF ≤ 35%, and narrow QRS (< 120 ms) [20]. The study was terminated early for futility with evidence of a trend to increased HF hospitalization in the CRT-On arm. Finally, the ECHO-CRT (Echocardiography Guided Cardiac Resynchronization Therapy) study assessed the impact of CRT in patients with advanced HF (NYHA III/IV), LVEF ≤ 35% and QRS < 130 ms with mechanical dyssynchrony (tissue Doppler imaging or speckle-tracking radial strain delay) [19]. Similar to LESSER-EARTH, the study was terminated early in the context of a significant increase in mortality associated with CRT therapy (11 vs. 6.4%) at 20 month follow-up. Presently, we believe the available literature to date does not favor the use of CRT in patients with narrow QRS with or without mechanical dyssynchrony, in the absence of AV block necessitating ventricular pacing.
Defining response to CRT
To date, there remains no standardized definition of response to CRT. Historically, studies have used measures of left ventricular reverse remodeling (improvements in LVEF, decrease in LV volumes), improvement in functional capacity (6 min walk distance, peak oxygen consumption, quality of life scores), or reduction in clinical endpoints (worsening HF or mortality) [21]. In a seminal study examining the agreement amongst these response criteria, Fornwalt and colleagues applied 17 echocardiographic and clinical criteria for CRT response to a prospective registry of patients undergoing CRT [22]. Agreement amongst echocardiographic response criteria was poor, agreement amongst clinical response criteria was moderate, and agreement between echocardiographic and clinical criteria was generally no better than chance alone. Recent studies have shown that short-term LV reverse remodeling, and particularly ‘super-response’ (LVEF improvement > 10–15%, reduction in LV volume > 20–30%), is associated with improvements in long-term survival [23, 24]. In general, we believe that CRT response should be defined as a composite of short and intermediate-term (3–12 month) echocardiographic reverse remodeling as well as longer-term (years) clinical outcomes including survival and heart failure events.
Patient selection for CRT
In landmark trials of CRT efficacy, the presence of electrical dyssynchrony, defined by a wide QRS duration (> 120–130 ms), is a unifying feature of identifying patients who benefit from CRT. The salutary effects of CRT are related to reversal of this electrical dyssynchrony through effective fusion of electrical wavefronts generated by RV and LV pacing. In patients with a left bundle branch block (LBBB), the site of latest LV activation is generally in the basal posterior/postero-lateral segment of the LV [25], and targeting this region for LV pacing has been the standard approach during CRT implantation. By comparison, patients with a right bundle branch block (RBBB) or non-specific intraventricular conduction delay (IVCD) are not expected to have the same activation pattern and may therefore not be expected to derive the same benefit from CRT. Meta-analysis of previous randomized studies has found that the clinical benefits of CRT are generally restricted to patients with LBBB and not those with IVCD or RBBB [26]. Post hoc analysis of the MADIT-CRT study, which evaluated CRT therapy in patients with mild HF, identified an increased risk of HF hospitalization and death with randomization to CRT therapy in patients with a non-LBBB and relatively narrow QRS (≤ 134 ms) [27•]. By comparison, the same post hoc analysis found that CRT was associated with improved clinical outcomes in patients with non-LBBB, prolonged QRS duration (> 134 ms) and prolonged PR interval (PR > 230 ms). The benefit in this subgroup may have been related to improvements in atrio-ventricular synchrony. We would note that some patients with RBBB or IVCD, including those with very wide QRS duration (> 180 ms) may demonstrate prolonged LV activation times (i.e., masked left bundle branch delay) [28, 29] and in some instances, these patients may still derive benefit from CRT [30]. Overall, these data underlie the current North American guidelines for CRT therapy which identify a class I recommendation only in patients with symptomatic HF, LVEF ≤ 35%, LBBB, and QRS ≥ 150 ms [2].
Other considerations for patient selection beyond QRS morphology include the presence of atrial fibrillation, etiology of cardiomyopathy, and comorbidities. In patients with atrial fibrillation, there is often suboptimal effective biventricular pacing related to high and/or irregular ventricular rates causing fusion, pseudo-fusion, or lack of pacing. Previous meta-analyses of randomized trials have found decreased CRT benefit in patients with AF [31, 32] including an analysis of the RAFT study which found that CRT did reduce HF hospitalization but did not impact mortality in this population [33]. In patients with atrial fibrillation and suboptimal biventricular pacing, the adjunctive role of atrioventricular junction ablation is an important option to consider and has been associated with improved clinical outcomes [34].
Previous studies have also suggested that patients with non-ischemic cardiomyopathy are more likely to demonstrate LV reverse remodeling following CRT compared to patients with non-ischemic cardiomyopathy [35]. LV reverse remodeling after CRT is associated with a decreased risk of ventricular arrhythmias [36•] and patients with non-ischemic cardiomyopathy are at generally lower risk of ventricular arrhythmias compared to ischemic cardiomyopathy [37]. In this context, investigators have inquired as to whether ICD therapy might be foregone at the time of CRT implant for patients with non-ischemic cardiomoypathy and no previous history of ventricular arrhythmias. Recent observational work has suggested no difference in survival for non-ischemic patients undergoing CRT-P versus CRT-D [38]. The potential role of a CRT-P only strategy in patients with non-ischemic cardiomyopathy warrants prospective evaluation.
Finally, several medical comorbidities have been shown to be associated with decreased CRT efficacy including the presence of chronic renal insufficiency [39, 40], pre-capillary pulmonary hypertension [41], and non-revascularizable coronary artery disease [42, 43]. A recent sub-analysis of the MADIT-CRT study confirmed a relationship between increasing comorbidity burden and attenuated LV reverse remodeling, although the burden of comorbidity did not seem to impact the overall clinical benefits of CRT [44•].
Treatment
Pharmacologic
-
Patients being considered for CRT should be treated with guideline-recommended goal-directed medical therapy for heart failure and left ventricular dysfunction [45] including treatment with β-blockers as well as renin-angiotensin-aldosterone system antagonists such as angiotensin-converting enzyme (ACE) inhibitors and mineralocorticoid receptor antagonists (MRA).
Interventional procedures
Intra-procedural strategies
-
The standard approach to implantation of the left ventricular lead during CRT is to target the lateral or posterolateral branch of the coronary sinus veins. As detailed above, this strategy reflects the expected pattern of electrical dyssynchrony in patients with a LBBB. Previous studies have shown that placement of the LV lead can be constrained by absence of available venous branches, and the response to CRT can often be variable even in this traditional anatomic location [46]. This heterogeneous response may reflect variations in electrical dyssynchrony related the underlying myocardial substrate, the distribution of ventricular scar, as well as RV pacing-induced changes in LV activation. Post hoc analysis of the MADIT-CRT study showed that placement of the LV lead in an apical position was associated an increased risk of worse clinical outcome [47], and we believe that in general, pacing from an apical LV lead position should be avoided (noting that the critical aspect is the location of the LV electrode selected for pacing, rather than the tip of the LV lead).
-
Other strategies for LV lead targeting include positioning of the LV lead at the site of latest electrical or mechanical activation [48, 49] or employing lead positions which maximize hemodynamic improvement [50]. During implantation of the LV lead, electrical delay can be standardly assessed by assessing the delay between the surface QRS and the initial sensed LV lead electrogram (Q-LV) [51]. Lead placement at sites of increasing QLV is associated with greater rates of LV reverse remodeling and improvement in patient symptoms [51, 49]. The ongoing ENHANCE-CRT study (NCT01983293) is evaluating the efficacy of a QLV-based strategy in patients with non-LBBB undergoing CRT [52].
-
Other considerations at the time of LV lead implantation include avoiding LV lead targeting at areas of LV scar as this has been associated with poor response to CRT [42]. The routine role of pre-procedure LV imaging to guide LV lead implantation (e.g., to evaluate underlying LV scar) remains unknown.
-
Given limitations of coronary venous anatomy, contemporary LV leads now commonly employ multi-polar (quadripolar) LV lead pacing capabilities, employing a distal tip electrode and three ring electrodes. These quadripolar leads can be programmed to yield numerous possible pacing vectors and as such could theoretically overcome some of the limitations of lead stability and anatomy associated with standard bipolar LV leads. The selection of optimal pacing vector is often guided by the greatest narrowing of the QRS complex or maximizing the electrical delay at the LV pacing electrode. The recent MORE-CRT trial (NCT01510652) was a randomized comparison of standard bipolar CRT and quadripolar CRT in 1078 patients. Quadripolar pacing was associated with reduced LV lead-related events including lead instability, phrenic nerve stimulation, elevated pacing threshold, or dislodgement.
-
In addition to increased flexibility of pacing vectors, quadripolar LV leads additionally offer the ability for multi-site or multi-point pacing (MPP), when placed with MPP-compatible generators. The ability to pace from multiple sites yields a larger wavefront that may improve resynchronization over standard biventricular pacing [53]. Recent studies have reported improvement in acute hemodynamic response and short-term LV reverse remodeling with an MPP strategy compared to standard biventricular pacing [54•, 55]. Ongoing studies including the MultiPoint Pacing in CRT trial (NCT01786993) [56] and the MORE-CRT MPP study, NCT02006069) [57], are examining the role of MPP in patients undergoing CRT, including its use at the time of implant as well as in patients who demonstrate non-response to standard biventricular pacing.
Optimization algorithms and strategies
-
Previous studies in patients with atrio-ventricular pacemakers demonstrated that optimization of the atrio-ventricular delay can improve cardiac hemodynamics [58, 59]. In patients undergoing CRT, options for such atrio-ventricular optimization include echocardiographic-based optimization (i.e., using mitral inflow patterns) [60] or use of intrinsic device algorithms which integrate native A-V conduction [51, 61, 62]. Randomized studies of routine A-V optimization have not shown significant benefit [51, 61, 62], whereas such optimization may be of clinical use in patients demonstrating non-response [8]. Similarly, optimization of inter-ventricular timing has also been shown to improve hemodynamic response in patients undergoing CRT [63, 64]. While routine V-V optimization using either echocardiographic or intrinsic device algorithms has not shown benefit when applied routinely [59, 63, 62], such optimization may be most helpful in patients with heterogeneous LV activation patterns (e.g., LV scar from prior myocardial infarction). At least one previous study identified a clinical benefit to V-V optimization in CRT patients with ischemic as opposed to non-ischemic etiologies to cardiomyopathy [65].
-
An emerging investigational optimization algorithm has been the use of contractility sensors embedded in the right atrial lead to guide optimization of A-V and V-V timing (e.g., the SonR system). In the RESPOND-CRT (SonR tip lead and automatic AV-VV optimization algorithm in the paradym RF SonR) study, 998 patients were randomized to a contractility-based optimization algorithm versus standard echocardiographic optimization of A-V and V-V timing. At 12 month follow-up, there was a marginal but significantly higher rate of clinical response in the SonR arm compared to the echocardiographic optimization arm (75 vs. 70%).
-
As most patients undergoing CRT have normal right ventricular synchrony, recent device algorithms have attempted to facilitate intrinsic RV activation by timing LV-pacing to the sensed RV electrogram. The AdaptCRT algorithm (Medtronic Inc., Fridley, MN) is one such algorithm which has been shown to decrease RV pacing by nearly 44% [66] and was associated with improved clinical outcomes when compared to standard echocardiography-optimized biventricular pacing [67]. The ongoing AdaptResponse study (NCT02205359) will be a randomized comparison of the AdaptivCRT algorithm versus conventional CRT and evaluate clinical endpoints including all-cause mortality and HF events [68].
Post-implant care and remote monitoring
-
Contemporary implantable devices, including CRT implants, offer the ability to remotely measure a range of patient and device parameters including information regarding biventricular pacing percentage, patient activity, trans-thoracic impedance, and atrial or ventricular arrhythmias. In the recent MORE-CARE study, patients undergoing CRT were randomized to remote monitoring versus standard in-office evaluation [69•]. Over a median follow-up of 2 years, remote monitoring was associated with significant decreased use of healthcare resources but no difference in clinical outcomes including all-cause mortality or cardiovascular hospitalization.
-
Previous studies have suggested that a higher biventricular pacing percentage is associated with improved clinical outcomes, with at least one real-world study suggesting that biventricular pacing percentage > 98% was associated with improved survival [70]. Reasons for suboptimal biventricular pacing include the presence of atrial tachyarrhythmias (most commonly atrial fibrillation), inappropriately programmed sensed, and paced atrio-ventricular intervals, and premature ventricular contractions [71]. Appropriate recognition of these common mechanisms of suboptimal biventricular pacing is a key component to post-implant care.
-
Multidisciplinary care for the CRT patient is a critical component to improving clinical outcomes. Such care offers the opportunity to integrate expertise from electrophysiology, advanced heart failure, and cardiac imaging. In our experience, this multidisciplinary approach was associated with improved clinical outcome when compared to standard clinical follow-up [72].
Surgery
-
In patients for whom left ventricular lead placement via an endovascular approach cannot be achieved, LV leads can be implanted via a surgical epicardial approach [73]. A recent small randomized controlled trial of standard trans-venous versus epicardial LV lead placement for patients undergoing CRT [74] showed no clinical or LV reverse remodeling benefit to an upfront epicardial LV lead implant strategy. We believe that such an approach should be generally restricted to patients who have either failed an endocardial LV lead implant attempt or patients who qualify for CRT and are undergoing cardiothoracic surgery for another indication.
Emerging therapies
Endocardial LV pacing
-
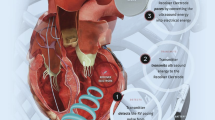
Given the potential challenges of trans-venous LV lead implantation including limitations of coronary sinus anatomy, high LV pacing threshold and/or phrenic nerve capture, there has been considerable interest in the role of an endocardial LV lead strategy in patients eligible for CRT [75, 46]. Pacing the LV endocardium reflects a more rapid and physiological activation of the left ventricle as compared to standard epicardial LV pacing, and previous studies have identified greater acute hemodynamic improvements with endocardial versus conventional LV pacing [76]. The ALSYNC (Alternate Site Cardiac Resynchronization) study demonstrated both safety and efficacy of LV endocardial pacing patients who either demonstrated CRT non-response or in for whom LV lead placement was not technically possible [77]. LV endocardial pacing in this study was associated with clinical and echocardiographic improvement in two-thirds of patients. It should be noted that anticoagulation was required in this study given permanent LV endocardial leads, and that thromboembolic events were detected in some patients in this study despite anticoagulation.
-
The WiSE-CRT (EBR Systems, Sunnyvale, CA) system employs a pacing system using a small leadless ultrasound-based electrode placed into the LV endocardial surface [75]. In the recent SELECT-LV (Safety and Performance of Electrodes implanted in the Left Ventricle) study, 35 patients who had failed conventional CRT underwent successful implant in 97% of cases [78••]. At 6 months, approximately two-thirds of patients demonstrated LV reverse remodeling (improved LVEF ≥ 5%) and 85% of patients demonstrated an improvement in clinical composite score. The soon to enroll SOLVE-CRT (Stimulation of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy in Non-Responders and Previously Untreatable Patients) study (NCT02922036) will be the first randomized comparison of an endocardial LV pacing strategy in CRT non-responders or those in whom a standard trans-venous LV lead implantation was not feasible.
His bundle pacing
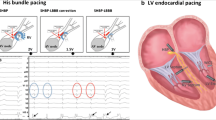
-
His bundle pacing (HBP) represents a theoretically ideal site for ventricular pacing as it retains activation of the intrinsic electrical conduction system [79]. Several limited case series have suggested that HBP may lead to resynchronization in CRT-eligible patients with LBBB [80••]. For example, in a recent series of 21 patients eligible for CRT, HBP was successfully implanted in 16 with evidence of electrical resynchronization (i.e., narrowing of QRS duration) in 76% and LV reverse remodeling overall (improved LVEF and decreased LV dimensions), although not all studies of HBP have demonstrated this high a rate of QRS narrowing in LBBB patients. The ongoing His-SYNC (His Bundle Pacing versus Coronary Sinus Pacing for Cardiac Resynchronization Therapy, NCT02700425) will be the first randomized comparison of HBP versus standard coronary sinus LV lead implantation in CRT-eligible patients. HBP is a clear alternative to traditional CRT in patients with a narrow QRS but AV block in which a narrow QRS can be maintained with HBP.
Integrated multisensor algorithms
-
The recent MultiSENSE (Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients) study examined the clinical impact of a multi-modal sensor strategy combining heart sounds, respiration, thoracic impedance, heart rate, and activity [81•]. Over a 1 year follow-up, the multi-sensor algorithm (HeartLogic) was able to identify a HF event with a lead time of 34 days. The routine use of such multi-modal sensor strategies to identify ‘at-risk’ CRT patients remains a point of future investigation.
Conclusion
In appropriately selected patients with heart failure and electrical dyssynchrony, CRT improves survival beyond standard goal-directed medical therapy. Important considerations in patient selection include the pattern and magnitude of electrical dyssynchrony, etiology of cardiomyopathy, as well as the presence of atrial arrhythmias and other medical comorbidities which may attenuate clinical response to CRT. Intra-procedural strategies that may optimize the delivery of CRT include targeting of the LV lead to sites of maximal electrical delay as well as the use of multi-site LV pacing. Post-implant considerations include the targeted use of atrio-ventricular and interventricular optimization, avoidance of right ventricular pacing, recognition and treatment of suboptimal biventricular pacing, and management by a multi-disciplinary cardiovascular care team. Emerging therapeutic strategies for patients who are candidates for CRT include the use of endocardial LV pacing, His bundle pacing, multi-point pacing strategies, and the integration of multi-modal remote monitoring into clinical care.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace : Eur Pacing, Arrhythmias, Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2013;15(8):1070–118. https://doi.org/10.1093/europace/eut206.
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6–75. https://doi.org/10.1016/j.jacc.2012.11.007.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–53. https://doi.org/10.1056/NEJMoa013168.
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–50. https://doi.org/10.1056/NEJMoa032423.
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–49. https://doi.org/10.1056/NEJMoa050496.
Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–38. https://doi.org/10.1056/NEJMoa0906431.
Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385–95. https://doi.org/10.1056/NEJMoa1009540.
Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53(9):765–73. https://doi.org/10.1016/j.jacc.2008.11.024.
Cleland JG, Freemantle N, Daubert JC, Toff WD, Leisch F, Tavazzi L. Long-term effect of cardiac resynchronisation in patients reporting mild symptoms of heart failure: a report from the CARE-HF study. Heart. 2008;94(3):278–83. https://doi.org/10.1136/hrt.2007.128991.
Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52(23):1834–43. https://doi.org/10.1016/j.jacc.2008.08.027.
Leclercq C, Cazeau S, Lellouche D, Fossati F, Anselme F, Davy JM, et al. Upgrading from single chamber right ventricular to biventricular pacing in permanently paced patients with worsening heart failure: the RD-CHF study. Pacing Clin Electrophysiol : PACE. 2007;30(Suppl 1):S23–30. https://doi.org/10.1111/j.1540-8159.2007.00598.x.
Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107(23):2932–7. https://doi.org/10.1161/01.cir.0000072769.17295.b1.
Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the dual chamber and VVI implantable defibrillator (DAVID) trial. JAMA. 2002;288(24):3115–23.
Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368(17):1585–93. https://doi.org/10.1056/NEJMoa1210356.
Funck RC, Blanc JJ, Mueller HH, Schade-Brittinger C, Bailleul C, Maisch B. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the ‘Biventricular pacing for atrioventricular block to prevent cardiac desynchronization (BioPace)’ study. Europace : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2006;8(8):629–35. https://doi.org/10.1093/europace/eul075.
Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, Alessi C, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42(12):2117–24. https://doi.org/10.1016/j.jacc.2003.08.024.
Yu CM, Chan YS, Zhang Q, Yip GW, Chan CK, Kum LC, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48(11):2251–7. https://doi.org/10.1016/j.jacc.2006.07.054.
Beshai JF, Grimm RA, Nagueh SF, Baker JH 2nd, Beau SL, Greenberg SM, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357(24):2461–71. https://doi.org/10.1056/NEJMoa0706695.
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369(15):1395–405. https://doi.org/10.1056/NEJMoa1306687.
Thibault B, Harel F, Ducharme A, White M, Ellenbogen KA, Frasure-Smith N, et al. Cardiac resynchronization therapy in patients with heart failure and a QRS complex <120 milliseconds: the evaluation of resynchronization therapy for heart failure (LESSER-EARTH) trial. Circulation. 2013;127(8):873–81. https://doi.org/10.1161/circulationaha.112.001239.
Kandala J, Altman RK, Park MY, Singh JP. Clinical, laboratory, and pacing predictors of CRT response. J Cardiovasc Transl Res. 2012;5(2):196–212. https://doi.org/10.1007/s12265-012-9352-0.
Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, et al. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121(18):1985–91. https://doi.org/10.1161/circulationaha.109.910778.
Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112(11):1580–6. https://doi.org/10.1161/circulationaha.105.538272.
Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, et al. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. J Am Coll Cardiol. 2012;59(25):2366–73. https://doi.org/10.1016/j.jacc.2012.01.065.
Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109(9):1133–9. https://doi.org/10.1161/01.cir.0000118502.91105.f6.
Sipahi I, Chou JC, Hyden M, Rowland DY, Simon DI, Fang JC. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Am Heart J. 2012;163(2):260–7.e3. https://doi.org/10.1016/j.ahj.2011.11.014.
• Biton Y, Kutyifa V, Cygankiewicz I, Goldenberg I, Klein H, McNitt S, et al. Relation of QRS duration to clinical benefit of cardiac resynchronization therapy in mild heart failure patients without left bundle branch block: the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy substudy. Circ Heart Fail. 2016;9(2):e002667. https://doi.org/10.1161/circheartfailure.115.002667. This study is a post-hoc analysis of the randomized MADIT-CRT trial and identifies a subset of patients with non-left bundle branch block who may benefit from CRT.
Fantoni C, Kawabata M, Massaro R, Regoli F, Raffa S, Arora V, et al. Right and left ventricular activation sequence in patients with heart failure and right bundle branch block: a detailed analysis using three-dimensional non-fluoroscopic electroanatomic mapping system. J Cardiovasc Electrophysiol. 2005;16(2):112–9; discussion 20-1. https://doi.org/10.1046/j.1540-8167.2005.40777.x.
Richman JL, Wolff L. Left bundle branch block masquerading as right bundle branch block. Am Heart J. 1954;47(3):383–93. https://doi.org/10.1016/0002-8703(54)90295-1.
Sundaram V, Sahadevan J, Waldo AL, Stukenborg GJ, Reddy YNV, Asirvatham SJ, et al. Implantable cardioverter-defibrillators with versus without resynchronization therapy in patients with a QRS duration >180 ms. J Am Coll Cardiol. 2017;69(16):2026–36. https://doi.org/10.1016/j.jacc.2017.02.042.
Upadhyay GA, Choudhry NK, Auricchio A, Ruskin J, Singh JP. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008;52(15):1239–46. https://doi.org/10.1016/j.jacc.2008.06.043.
Wilton SB, Leung AA, Ghali WA, Faris P, Exner DV. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm : Off J Heart Rhythm Soc. 2011;8(7):1088–94. https://doi.org/10.1016/j.hrthm.2011.02.014.
Healey JS, Hohnloser SH, Exner DV, Birnie DH, Parkash R, Connolly SJ, et al. Cardiac resynchronization therapy in patients with permanent atrial fibrillation: results from the resynchronization for ambulatory heart failure trial (RAFT). Circ Heart Fail. 2012;5(5):566–70. https://doi.org/10.1161/circheartfailure.112.968867.
Yin J, Hu H, Wang Y, Xue M, Li X, Cheng W, et al. Effects of atrioventricular nodal ablation on permanent atrial fibrillation patients with cardiac resynchronization therapy: a systematic review and meta-analysis. Clin Cardiol. 2014;37(11):707–15. https://doi.org/10.1002/clc.22312.
Barsheshet A, Goldenberg I, Moss AJ, Eldar M, Huang DT, McNitt S, et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur Heart J. 2011;32(13):1622–30. https://doi.org/10.1093/eurheartj/ehq407.
• Chatterjee NA, Roka A, Lubitz SA, Gold MR, Daubert C, Linde C, et al. Reduced appropriate implantable cardioverter-defibrillator therapy after cardiac resynchronization therapy-induced left ventricular function recovery: a meta-analysis and systematic review. Eur Heart J. 2015;36(41):2780–9. https://doi.org/10.1093/eurheartj/ehv373. This study demonstrates the impact of CRT-associated reverse remodeling on a decreased risk of ventricular arrhythmia.
Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375(13):1221–30. https://doi.org/10.1056/NEJMoa1608029.
Barra S, Boveda S, Providencia R, Sadoul N, Duehmke R, Reitan C, et al. Adding defibrillation therapy to cardiac resynchronization on the basis of the myocardial substrate. J Am Coll Cardiol. 2017;69(13):1669–78. https://doi.org/10.1016/j.jacc.2017.01.042.
Fung JW, Szeto CC, Chan JY, Zhang Q, Chan HC, Yip GW, et al. Prognostic value of renal function in patients with cardiac resynchronization therapy. Int J Cardiol. 2007;122(1):10–6. https://doi.org/10.1016/j.ijcard.2006.11.015.
Garg N, Thomas G, Jackson G, Rickard J, Nally JV Jr, Tang WH, et al. Cardiac resynchronization therapy in CKD: a systematic review. Clin J Am Soc Nephrol : CJASN. 2013;8(8):1293–303. https://doi.org/10.2215/cjn.00750113.
Chatterjee NA, Upadhyay GA, Singal G, Parks KA, Dec GW, Singh JP, et al. Pre-capillary pulmonary hypertension and right ventricular dilation predict clinical outcome in cardiac resynchronization therapy. JACC Heart Fail. 2014;2(3):230–7. https://doi.org/10.1016/j.jchf.2014.02.004.
Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153(1):105–12. https://doi.org/10.1016/j.ahj.2006.10.015.
Bilchick KC, Kuruvilla S, Hamirani YS, Ramachandran R, Clarke SA, Parker KM, et al. Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol. 2014;63(16):1657–66. https://doi.org/10.1016/j.jacc.2014.02.533.
• Zeitler EP, Friedman DJ, Daubert JP, Al-Khatib SM, Solomon SD, Biton Y, et al. Multiple comorbidities and response to cardiac resynchronization therapy: MADIT-CRT long-term follow-up. J Am Coll Cardiol. 2017;69(19):2369–79. https://doi.org/10.1016/j.jacc.2017.03.531. This study examines the impact of comorbidites on the efficacy of CRT.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. https://doi.org/10.1016/j.jacc.2017.04.025.
Derval N, Steendijk P, Gula LJ, Deplagne A, Laborderie J, Sacher F, et al. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J Am Coll Cardiol. 2010;55(6):566–75. https://doi.org/10.1016/j.jacc.2009.08.045.
Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011;123(11):1159–66. https://doi.org/10.1161/circulationaha.110.000646.
Gold MR, Yu Y, Singh JP, Stein KM, Birgersdotter-Green U, Meyer TE, et al. The effect of left ventricular electrical delay on AV optimization for cardiac resynchronization therapy. Heart Rhythm : Off J Heart Rhythm Soc. 2013;10(7):988–93. https://doi.org/10.1016/j.hrthm.2013.03.009.
Singh JP, Fan D, Heist EK, Alabiad CR, Taub C, Reddy V, et al. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm : Off J Heart Rhythm Soc. 2006;3(11):1285–92. https://doi.org/10.1016/j.hrthm.2006.07.034.
Duckett SG, Ginks M, Shetty AK, Bostock J, Gill JS, Hamid S, et al. Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. 2011;58(11):1128–36. https://doi.org/10.1016/j.jacc.2011.04.042.
Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122(25):2660–8. https://doi.org/10.1161/circulationaha.110.992552.
CRT Implant Strategy Using the Longest Electrical Delay for Non-left Bundle Branch Block Patients (ENHANCE CRT). Accessed 6 Oct 2014 at http://clinicaltrials.gov/show/NCT01983293.
Rinaldi CA, Burri H, Thibault B, Curnis A, Rao A, Gras D, et al. A review of multisite pacing to achieve cardiac resynchronization therapy. Europace: Eur Pacing Arrhythmias Cardiac Electrophysiol : J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2014; https://doi.org/10.1093/europace/euu197.
• Pappone C, Calovic Z, Vicedomini G, Cuko A, McSpadden LC, Ryu K, et al. Multipoint left ventricular pacing improves acute hemodynamic response assessed with pressure-volume loops in cardiac resynchronization therapy patients. Heart rhythm : Off J Heart Rhythm Soc. 2014;11(3):394–401. https://doi.org/10.1016/j.hrthm.2013.11.023. This study demonstrates the acute hemodynamic benefits of multi-point pacing in CRT.
Pappone C, Calovic Z, Vicedomini G, Cuko A, McSpadden LC, Ryu K, et al. Multipoint left ventricular pacing in a single coronary sinus branch improves mid-term echocardiographic and clinical response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2014;26(1):58–63. https://doi.org/10.1111/jce.12513.
Tomassoni G, Baker J 2nd, Corbisiero R, Love C, Martin D, Sheppard R, et al. Rationale and design of a randomized trial to assess the safety and efficacy of MultiPoint Pacing (MPP) in cardiac resynchronization therapy: The MPP Trial. Ann Noninvasive Electrocardiol : Off J Int Soc Holter Noninvasive Electrocardiol Inc. 2017; https://doi.org/10.1111/anec.12448.
MOre REsponse on Cardiac Resynchronization Therapy With MultiPoint Pacing (MORE-CRT MPP). http://clinicaltrials.gov/show/NCT02006069. Accessed 6 Oct 2014 at http://clinicaltrials.gov/show/NCT02006069.
Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The pacing therapies for congestive heart failure study group. The Guidant congestive heart failure research group. Circulation. 1999;99(23):2993–3001. https://doi.org/10.1161/01.CIR.99.23.2993.
Gras D, Gupta MS, Boulogne E, Guzzo L, Abraham WT. Optimization of AV and VV delays in the real-world CRT patient population: an international survey on current clinical practice. Pacing Clin Electrophysiol : PACE. 2009;32(Suppl 1):S236–9. https://doi.org/10.1111/j.1540-8159.2008.02294.x.
Barold SS, Ilercil A, Herweg B. Echocardiographic optimization of the atrioventricular and interventricular intervals during cardiac resynchronization. Europace : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2008;10(Suppl 3):iii88–95. https://doi.org/10.1093/europace/eun220.
Kamdar R, Frain E, Warburton F, Richmond L, Mullan V, Berriman T, et al. A prospective comparison of echocardiography and device algorithms for atrioventricular and interventricular interval optimization in cardiac resynchronization therapy. Europace : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2010;12(1):84–91. https://doi.org/10.1093/europace/eup337.
Singh JP, Abraham WT, Chung ES, Rogers T, Sambelashvili A, Coles JA Jr, et al. Clinical response with adaptive CRT algorithm compared with CRT with echocardiography-optimized atrioventricular delay: a retrospective analysis of multicentre trials. Europace : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Euro Soc Cardiol. 2013;15(11):1622–8. https://doi.org/10.1093/europace/eut107.
Boriani G, Biffi M, Muller CP, Seidl KH, Grove R, Vogt J, et al. A prospective randomized evaluation of VV delay optimization in CRT-D recipients: echocardiographic observations from the RHYTHM II ICD study. Pacing Clin Electrophysiol : PACE. 2009;32(Suppl 1):S120–5. https://doi.org/10.1111/j.1540-8159.2008.02267.x.
Leon AR, Abraham WT, Brozena S, Daubert JP, Fisher WG, Gurley JC, et al. Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol. 2005;46(12):2298–304. https://doi.org/10.1016/j.jacc.2005.08.032.
Marsan NA, Bleeker GB, Van Bommel RJ, Borleffs C, Bertini M, Holman ER, et al. Cardiac resynchronization therapy in patients with ischemic versus non-ischemic heart failure: differential effect of optimizing interventricular pacing interval. Am Heart J. 2009;158(5):769–76. https://doi.org/10.1016/j.ahj.2009.09.004.
Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm : Off J Heart Rhythm Soc. 2012;9(11):1807–14. https://doi.org/10.1016/j.hrthm.2012.07.009.
Birnie D, Lemke B, Aonuma K, Krum H, Lee KL, Gasparini M, et al. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm : Off J Heart Rhythm Soc. 2013;10(9):1368–74. https://doi.org/10.1016/j.hrthm.2013.07.007.
Filippatos G, Birnie D, Gold MR, Gerritse B, Hersi A, Jacobs S, et al. Rationale and design of the AdaptResponse trial: a prospective randomized study of cardiac resynchronization therapy with preferential adaptive left ventricular-only pacing. Eur J Heart Fail. 2017;19(7):950–7. https://doi.org/10.1002/ejhf.895.
• Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017;19(3):416–25. https://doi.org/10.1002/ejhf.626. This study examines the impact of remote monitoring platforms on healthcare utilization for patients undergoing CRT.
Hayes DL, Boehmer JP, Day JD, Gilliam FR 3rd, Heidenreich PA, Seth M, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm : Off J Heart Rhythm Soc. 2011;8(9):1469–75. https://doi.org/10.1016/j.hrthm.2011.04.015.
Cheng A, Landman SR, Stadler RW. Reasons for loss of cardiac resynchronization therapy pacing: insights from 32 844 patients. Circ Arrhythmia Electrophysiol. 2012;5(5):884–8. https://doi.org/10.1161/circep.112.973776.
Altman RK, Parks KA, Schlett CL, Orencole M, Park MY, Truong QA, et al. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J. 2012;33(17):2181–8. https://doi.org/10.1093/eurheartj/ehs107.
Kamath GS, Balaram S, Choi A, Kuteyeva O, Garikipati NV, Steinberg JS, et al. Long-term outcome of leads and patients following robotic epicardial left ventricular lead placement for cardiac resynchronization therapy. Pacing Clin Electrophysiol : PACE. 2011;34(2):235–40. https://doi.org/10.1111/j.1540-8159.2010.02943.x.
van Dijk VF, Fanggiday J, Balt JC, Wijffels M, Daeter EJ, Kelder JC, et al. Effects of epicardial versus transvenous left ventricular lead placement on left ventricular function and cardiac perfusion in cardiac resynchronization therapy: a randomized clinical trial. J Cardiovasc Electrophysiol. 2017;28(8):917–23. https://doi.org/10.1111/jce.13242.
Auricchio A, Delnoy PP, Butter C, Brachmann J, Van Erven L, Spitzer S, et al. Feasibility, safety, and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the wireless stimulation Endocardially for CRT (WiSE-CRT) study. Europace : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2014;16(5):681–8. https://doi.org/10.1093/europace/eut435.
Shetty AK, Sohal M, Chen Z, Ginks MR, Bostock J, Amraoui S, et al. A comparison of left ventricular endocardial, multisite, and multipolar epicardial cardiac resynchronization: an acute haemodynamic and electroanatomical study. Europace : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2014;16(6):873–9. https://doi.org/10.1093/europace/eut420.
ALternate Site Cardiac ReSYNChronization (ALSYNC) Study. http://clinicaltrials.gov/show/NCT01277783. Accessed 6 Oct 2014 at http://clinicaltrials.gov/show/NCT01277783.
•• Reddy VY, Miller MA, Neuzil P, Sogaard P, Butter C, Seifert M, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT-LV study. J Am Coll Cardiol. 2017;69(17):2119–29. https://doi.org/10.1016/j.jacc.2017.02.059. This study demonstrates the feasability of endocardial LV pacing in CRT-eligible patients.
Vijayaraman P, Bordachar P, Ellenbogen KA. The continued search for physiological pacing: where are we now? J Am Coll Cardiol. 2017;69(25):3099–114. https://doi.org/10.1016/j.jacc.2017.05.005.
•• Ajijola OA, Upadhyay GA, Macias C, Shivkumar K, Tung R. Permanent his-bundle pacing for cardiac resynchronization therapy: initial feasibility study in lieu of left ventricular lead. Heart Rhythm : Off J Heart Rhythm Soc. 2017;14(9):1353–61. https://doi.org/10.1016/j.hrthm.2017.04.003. This study examines the impact of his bundle pacing on CRT-eligible patients and demonstrates narrowing of left bundle branch block in a majority.
• Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart failure. 2017;5(3):216–25. https://doi.org/10.1016/j.jchf.2016.12.011. This study examines the impact of a multi-sensor algorithm to predict incident clinical worsening with a lead time of 30 days.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Neal A. Chatterjee declares no potential conflicts of interest.
E. Kevin Heist reports personal fees from Boston Scientific, grants and personal fees from Biotronik, grants and personal fees from St. Jude Medical, personal fees from Medtronic, grants from LivaNova, outside the submitted work. In addition, Dr. Heist has a patent Patented LV lead pacing design issued.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Arrhythmia
Rights and permissions
About this article
Cite this article
Chatterjee, N.A., Heist, E.K. Cardiac Resynchronization Therapy—Emerging Therapeutic Approaches. Curr Treat Options Cardio Med 20, 20 (2018). https://doi.org/10.1007/s11936-018-0614-2
Published:
DOI: https://doi.org/10.1007/s11936-018-0614-2