Abstract
Early onset scoliosis presents a challenging problem for both the patient and treating surgeon. Multiple techniques have been described to preserve growth and pulmonary development while managing the spinal deformity. A recent advancement has been the creation of magnetically controlled growing rods (MCGR). In this chapter we present the case of a patient with Prader-Willi syndrome and a 109° curve. He initially underwent treatment with traditional spine-based growing rods (TGR) and underwent conversion to MCGR when it became available in the United States. He has continued to be managed by magnetic lengthening and has not yet undergone final fusion. His curve has been maintained between 30 and 40° with the use of MCGR. He has had no complications to date.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Case Presentation
History and Physical Examination
The patient was a 6-month-old male with a known diagnosis of Prader-Willi syndrome . He presented with a thoracolumbar curvature of 31°. On serial examination, the curvature was noted to be increasing in magnitude. Despite custom TLSO bracing, it continued to progress over a 2-year time interval to 78°. At 2 years and 7 months, he was treated with Risser casts which corrected his curvature to 41° in cast. After 6 months, he could not tolerate casting any longer. He then returned to bracing and had continued progression of his kyphoscoliosis until the age of 4.
At the time of preoperative evaluation, the patient was 4 years and 6 months of age, 102 cm, and 20.3 kg and had a BMI of 19.1 kg/m2. On examination , he was hypotonic, developmentally delayed, nonverbal, and unable to ambulate. He had pronounced, fairly flexible thoracolumbar kyphosis , an asymmetric waist with a deep crease on the right, and a high left shoulder. Outside of his hypotonia and inability to cooperate with examination, he is otherwise neurovascularly intact.
Diagnostic Studies
Initial preoperative radiographic analysis prior to growing rod insertion demonstrated a left thoracolumbar curvature of 109° and kyphosis of 67° (Fig. 8.1). An MRI was completed with no evidence of spinal dysraphism . We typically do not encourage the use of MRI once the MCGR is implanted. Although studies of a 0.3 T MRI indicate that it may be possible, it is against manufacturer’s recommendations due to concerns of a higher-strength MRI’s effect on the rare earth magnets found within the MCGR [1].
Management Chosen
Surgical management for this patient was initiated prior to the availability of magnetically controlled growing rods in the United States. Secondary to his progression, lack of ability to tolerate more non-operative management, it was decided that his scoliosis would best be treated with “growth-friendly” spinal instrumentation . At the age of 4 years and 6 months, he had traditional spine-based growing rods inserted from T3–T4 to L5–L6 (with six lumbar vertebrae). He subsequently underwent open distraction five times over 2.5 years with no subsequent complications.
Three years after placement of the original growth-friendly construct, the patient underwent conversion to magnetically controlled growing rods. This was 4 months after the implant’s introduction to the United States. At the time of his conversion, the curvature was a 52° left thoracolumbar curve with 60° of kyphosis. The lead authors of this chapter believe that conversions are indicated in patients that still have potential for growth, in order to save subsequent surgeries and anesthetic events prior to final fusion. If the patient has reached or is close to maximum potential chest size and lung maturity, then we would recommend continuation of management with TGR until final fusion is recommended.
Surgical Procedure
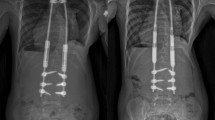
The patient was placed prone on the Jackson table in the standard fashion. No traction was applied during the procedure. Using prior incisions, localized to the upper thoracic levels (T3–T4) and the lumbar levels (L5–L6 with six lumbar vertebrae), we exposed and removed the prior traditional growing rods. The prior fusion masses and instrumentation at the upper and lower instrumented segments were explored and found to be stable. The magnetically controlled growing rods were measured, cut, and contoured appropriately. We tested each rod’s actuator individually prior to insertion to confirm their function. Of note, the contouring of the rods should be done prior to testing the actuator to be sure that the mechanism has not been damaged during contouring. The subfascial tunnels from the prior growing rods were utilized for placement of the new magnetically controlled growing rods into the previous fused proximal and distal level’s pedicle instrumentation. New set screws were placed. Gentle distraction was placed distally across the base after the proximal set screws were tightened. We then tightened the distal set screws and completed final tightening. A proximal cross connector was applied between the rods. Final irrigation, topical vancomycin powder, and a standard closure were then completed. No complications occurred. Pre- and postoperative images are provided in (Fig. 8.2).
Some technical points about the abovementioned procedure should be noted. The MCGR should not be used in patients less than 2 years of age or less than 25 lbs. (11.4 kg). Rod selection should be based off of the patient’s weight. Please consult the company’s insert for this detailed information. With contouring each rod, it is crucial that no contour be placed through the actuator or within 10 mm of the actuator on either side. This translates to maintaining a 90 mm flat segment of MCGR for the 70 mm actuator or 110 mm for the 90 mm actuator. By doing so it may damage the mechanism by which the MCGR distracts. It is important to note that when using a dual rod construct, the actuators should be placed at the same height. We recommend that at least one cross connector be used between the rods either proximally or distally if there is poor bone quality or question of foundation strength. Cross connectors are added to these constructs to help add rigidity to an inherently less stable construct than traditional pedicle-based posterior spinal instrumented fusion. We recommend doing this at the upper foundation. It is important to not use cross connectors proximally and distally, as this will lock the device and not allow distraction.
Subsequent Steps
Over 3 years following the MCGR insertion, 12 lengthenings were completed. During each office-based distraction, we gained 1–2.5 mm of length with each rod. It should be noted that earlier lengthenings yielded greater results.
During the lengthening visits, the magnetic portion of the MCGR is found by the use of ultrasound or a magnet locator. An electrically powered, motor driver called an external remote control (ERC) is held over the back with the patient prone on the exam table (Fig. 8.3). This position allows for maximum lengthening as it decreases the force required to lengthen the rod by removing the effect of gravity and weight of the upper thoracic cavity. If difficulties arise during lengthening, the first course of action is to reposition the patient. There are multiple positions that may allow for further lengthenings of the rod when other positions have failed. Some options to try include placing a pillow under the abdomen, traction, lateral decubitus position, or even sitting.
If none of these techniques work, the surgeon may need to wait a few months to attempt lengthening again as there may not have been adequate spinal growth in the interim to allow for distraction of the MCGR. The ERC activates the magnet within the rods causing them to rotate and lengthen the rod. If the magnet and the ERC are not at the same level, the ERC will not be able to engage the actuator, and no lengthening will occur. Lengthenings can be scheduled as often as desired by the treating surgeon. We personally recommend lengthenings every 2–3 months. To determine the amount of lengthening per distraction, calculations based on Dimeglio’s work should be performed. On average, T1–S1 increases 1 cm per year from the ages of 5–10 [2]. Then divide this by spinal segments included and the length of time between distractions. On average, this calculation ends up being 3 mm per lengthening if attempting lengthening in 3-month intervals. The preferred method for checking the magnet’s length pre- and post-lengthening is with the use of ultrasound (Fig. 8.4) [3]. If ultrasound is not available or radiographic imaging is needed, we prefer the use of microdose EOS to reduce overall radiation to the patient. If EOS were to be used, we would recommend taking one image per clinic visit prior to lengthening. We then compare the images from the subsequent visit to measure the length gained from the prior visit. This also helps to minimize radiation exposure to the patient.
Clinical Course and Outcome
Our patient has had no complications and no further operative interventions since the conversion to magnetically controlled growing rods. The obvious benefits to this method include more frequent, smaller lengthenings without the risk, morbidity, and cost of surgery. At last follow-up, the patient had a 30° curve from T10 to L2 and 46° of kyphosis from T2 to T7. Of note, the majority of the kyphosis is in the proximal portion of the construct. The T1–T12 and T1–S1 height gained from initial growing rod insertion to final magnetically controlled growing rod length was 5.6 and 11.9 cm, respectively. Please reference Table 8.1 for measurements completed at significant treatment intervals. During the last 3 years, there has been an increase in the axial rotation around the magnetically controlled growing rods as can be seen in Fig. 8.5. We are nearing the age of final fusion, which is typically after the age of 10 at our institution.
Clinical Pearls and Pitfalls
-
This technique should be used in young patients with increasing deformity, who have significant chest and lung development remaining.
-
Magnetically controlled growing rods are able to maintain deformity correction and be growth friendly through a noninvasive method.
-
Noninvasive lengthening procedures have decreased complication rates, avoidance of anesthetic events, and decreased overall cost, despite the up-front cost of implants.
-
Office-based lengthening helps the patient and family to avoid social stigma to decrease the pressures of missed school and work days and overall helps the patient from a psychological standpoint.
-
It is unclear if magnetically controlled growing rods will be subject to diminishing returns as seen with traditional spine-based growing rods.
-
There is a risk of high radiation exposure through serial imaging with this treatment as treatment intervals are typically shorter than traditional growing rods.
-
By using ultrasound technology to measure the magnet pre- and post-lengthening, the surgeon can monitor the distraction while minimizing the radiation risk.
Literature Review and Discussion
Early onset scoliosis is a challenge for both the patient and treating surgeon. The greatest issue is managing the magnitude of curvature, while attempting to allow the thoracic cavity and therefore pulmonary system to develop and grow [4]. This technique is one that should be considered in EOS patients younger than 10 years of age , with significant growth remaining and curve progression despite conservative measures. Extensive exposure, instrumentation, and fusion of the spine at this young age would result in a short trunk, decreased thoracic growth, and pulmonary insufficiency [5, 6]. Significantly reduced pulmonary function has been shown in comparison to a normal age-matched cohort, for patients that undergo posterior spinal fusion prior to the age of 9. Patients with fusion prior to this age had an average FVC of <60% of their age-matched cohort. The reason for this dramatic difference is that the alveolar number per terminal lung unit increases from approximately 1370 at 22 months of age to 2630 alveoli by age 10 [7]. It has been shown that a thoracic height, which can be correlated to lung maturity, of 18 cm is required to avoid severe respiratory insufficiency [8]. For this reason, it is recommended to strive for a thoracic height (T1–T12) of 22 cm. When this thoracic height is achieved, it is reasonable to proceed to final spinal fusion.
Multiple treatment strategies have been developed for this disease process. Harrington described attempting to preserve growth with internal fixation without fusion through subperiosteal dissection with rod placement against the bony posterior spinal elements. Moe described the first subcutaneous Harrington rod placement technique to control severe curves in young patients [9,10,11]. Since that time these techniques have been refined. TGR, Shilla, VEPTR, and now MCGR have been created as an attempt to improve growth preservation, curve control, and lung development and decrease complications [12].
The MCGR was created and initially marketed in Europe in October of 2009. By 2014, when the FDA approved the use of MCGR, it had been used in more than 500 patients in 20 countries worldwide [13]. In most published reports, it has shown favorable results with much less morbidity than traditional growing rod techniques. In 2014, Akbarnia et al. compared 12 matched patients who were treated with TGR (follow-up of 4.1 years) to 12 patients treated with the MCGR technique (follow-up of 2.5 years). There were 57 fewer operative procedures in the MCGR group, which is an average of 4.75 fewer procedures per patient [14]. Due to the replacement of surgical lengthenings with office-based lengthenings, it appears that surgical complications such as infection (3.7% vs. 11.1%), anesthetic complications, blood loss, and wound complications are all decreased with MCGR [15]. Despite this, the MGCR patient population is still at risk of infection, rod breakage, implant pullout, prominent implants, or junctional kyphosis inherent in patients with growth-friendly treatment [16,17,18]. One article found that all patients with single rod constructs required revision procedures and therefore concluded that these constructs should not be used [16]. Inaparthy et al. looked at a cohort of 21 patients with MCGR and showed the incidence of PJK (>10° increase of kyphosis) to be 28.6% at final follow-up of 33 months [17].
Aside from the diminished surgical complications and number of subsequent procedures, there are many other benefits that must be considered when discussing MCGR vs. TGR constructs. The most significant from a patient perspective is most likely psychosocial in nature [19]. With less surgery looming in the near future, there are significantly less psychosocial stressors, including decreased time out of school for the patient and time out of work for the parents. It can be psychologically difficult to be away from social networks and support as well as financially detrimental for the entire family unit. When taking all of these variables into consideration, it is apparent that having frequent surgery decreases quality of life.
Finally, treating surgeons need to evaluate and keep in mind the cost to the healthcare system. In a study by The National Institute for Health and Care Excellence in the United Kingdom, a cost analysis was completed. This research showed that after 6 years of treatment with MCGR vs. TGR , that overall cost savings per patient is around £12,000 (~$15,000) for patients treated with MCGR [20]. Since the United Kingdom is a socialized healthcare system, there may be doubts as to its applicability to a U.S. market. Polly et al. looked further into this issue in 2016 by constructing an economic model of cumulative costs of MCGR vs. TGR. They concluded that cost offsets accrue over time and that in order to achieve cost neutrality, the patient needs to be treated for 6 years [21]. In either model, it appears that the up-front cost of MCGR should not be a hindrance to its use, as it is at least economically neutral in the long term.
Early onset scoliosis is a difficult problem that has many potential treatment modalities. Magnetically controlled growing rods are a recent technological advancement and appear to have a promising future in the care for these complex patients. Deformity control , while permitting continued growth of the spine, is the goal of treating this disease process. With current short-term follow-up, MCGR has demonstrated that it can accomplish these goals without requiring serial operative procedures [10, 22,23,24,25,26].
References
Eroglu M, Demikiran G, Kocyigit IA, et al. Magnetic resonance imaging safety of magnetically controlled growing rods in an in vivo animal model. Spine. 2017;42(9):e504–8.
Canavese F, Dimeglio A. Normal and abnormal spine and thoracic cage development. World J Orthop. 2013;4(4):167–74.
Yoon WW, Chang AC, Tyler P, et al. The use of ultrasound in comparison to radiography in magnetically controlled growth rod lengthening measurement: a prospective study. Eur Spine J. 2015;24(7):1422–6.
Dimeglio A. Growth of the spine before age 5 years. J Pediatr Orthop. 1993;1(B):102–7.
Kesling KL, Lonstein JE, Denis F, et al. The crankshaft phenomenon after posterior spinal arthrodesis for congenital scoliosis: a review of 54 patients. Spine. 2003;28:267–71.
Winter RB, Moe JH. The results of spinal arthrodesis for congenital spinal deformity in patients younger than five years old. J Bone Joint Surg Am. 1982;64:419–32.
Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960;35(184):544–7.
Karol LA, Johnston C, Mladenov K, et al. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90:1272–81.
Akbarnia BA, Cheung K, Noordeen H, et al. Next generation of growth-sparing techniques. Spine. 2013;38(8):665–70.
Moe JH, Kharrat K, Winter RB, et al. Harrington instrumentation without fusion plus external orthotic support for the treatment of difficult curvature problems in young children. Clin Orthop Relat Res. 1984;185:35–45.
La Rosa G, Oggiano L, Ruzzini L. Magnetically controlled growing rods for the management of early-onset scoliosis: A preliminary report. J Pediatr Orthop. 2017;37(2):79–85.
Akbarnia BA, Marks DS, Boachie-Adjei O, et al. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine. 2005;30:S46–57.
Akbarnia B. Magnetically controlled growing rods (MCGR) for the treatment of progressive early-onset scoliosis (EOS). Spineuniverse Case Study Library. 2013. http://www.spineuniverse.com/professional/case-studies Accessed 21 Feb 2017.
Akbarnia BA, Pawelek JB, Cheung K, et al. Traditional growing rods versus magnetically controlled growing rods for the surgical treatment of early-onset scoliosis: a case-matched 2-year study. Spine Deform. 2014;2(6):493–7.
Choi E, Yaszay B, Mundis G et al. Implant complications after magnetically controlled growing rods for early onset scoliosis: a multicenter retrospective review. J Pediatr Orthop. (epub). 2016.
Teoh KH, Winson D, James S, et al. Magnetic controlled growing rods for early onset scoliosis: a 4-year follow up. Spine J. 2016;16(4):S34–9.
Inaparthy P, Queruz JC, Bhagawati D, et al. Incidence of proximal junctional kyphosis with magnetic expansion control rods in early onset scoliosis. Eur Spine J. 2016;25(10):3308–15.
Bess S, Akbarnia BA, Thompson GH, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am. 2010;92:2533–43.
Thompson W, Thakar C, Rolton DJ. The use of magnetically-controlled growing rods to treat children with early-onset scoliosis: early radiological results in 19 children. Bone Joint J. 2016;98-B(9):1240–7.
Jenks M, Craig J, Higgins J, et al. The MAGEC system for spinal lengthening in children with scoliosis: a NICE Medical Technology Guidance. Appl Health Econ Health Policy. 2014;12(6):587–99.
Polly DW, Ackerman SJ, Schneider K, et al. Cost analysis of magnetically controlled growing rods compared with traditional growing rods for early-onset scoliosis in the US: an integrated health care delivery system perspective. Clinicoecon Outcomes Res. 2016;8:457–65.
Hickey BA, Towriss C, Baxter G, et al. Early experience of MAGEC magnetic growing rods in the treatment of early onset scoliosis. Eur Spine J. 2014;23(S1):61–5.
Rolton D, Thakar C, Wilson-Macdonald J, Nnadi C. Radiological and clinical assessment of the distraction achieved with remotely expandable growing rods in early onset scoliosis. Eur Spine J. 2015;25(10):3371–6.
Cheung J, Cahill P, Yaszay B, et al. Special article: update on the magnetically controlled growing rod: tips and pitfalls. J Orthop Surg. 2015;23(3):383–90.
Cheung K, Man-Chee K, Pui-Yin Cheung J, et al. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet. 2012;379(9830):1967–74.
Lebon J, Batailler C, Wargny M, et al. Magnetically controlled growing rod in early onset scoliosis: a 30-case multicenter study. Eur Spine J. 2016. https://doi.org/10.1007/s00586-016-4929-y.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Fitzgerald, R.E., Rickert, K.D., Akbarnia, B.A., Yaszay, B. (2018). Early Onset Scoliosis Treated with Magnetically Controlled Growing Rods. In: El-Hawary, R., Eberson, C. (eds) Early Onset Scoliosis. Springer, Cham. https://doi.org/10.1007/978-3-319-71580-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-71580-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71579-7
Online ISBN: 978-3-319-71580-3
eBook Packages: MedicineMedicine (R0)