Abstract
This chapter aims to summarize the up-to-day evidence-based biomedical knowledge on serotonin-2A (5-HT2A) receptors and their role in pathophysiology and treatment of central nervous system (CNS) disorders, with a primary focus on depression. The first paragraph provides a brief introduction to serotonin (5-HT) system and 5-HT receptors, focusing on serotonin-2 (5-HT2) family and 5-HT2A receptor specifically. The second paragraph is focused on molecular genetics of 5-HT2A receptors, polymorphism of 5-HT2A receptor (5HT2AR) gene, 5HT2AR gene epigenetic mechanisms, such as DNA methylation, and post-translational modifications of 5HT2AR messenger ribonucleic acid (mRNA), such as alternative splicing. The molecular and cellular pharmacology and physiology of 5-HT2A receptors in normal and pathological conditions are discussed in the third paragraph. The 5-HT2A receptors-acting ligands are addresses. The fourth paragraph describes the role of 5-HT receptors in the interaction between 5-HT and other neurotransmitter systems in health and in CNS disorders. The fifth and the final paragraph specifically deals with the role of 5-HT2A receptor in pathophysiology and treatment of depression, focusing on the 5-HT2A receptor expressed in the hippocampus.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Serotonin-2A receptor (5HT2AR) gene polymorphism
- Deoxyribonucleic acid (DNA) methylation
- Messenger ribonucleic acid (mRNA) alternative splicing
- G-protein coupled receptors (GPCR)
- GαQ/Z-11 protein
- Phospholipase C (PLC)
- Inositol trisphosphate (IP3)
- Calcium signaling
- Antidepressant drugs
- Antipsychotic drugs
- Hippocampus
Serotonin-2A Receptor: An Introduction
The 5-HT2A receptors belong to the 5-HT2 family consists of two more subtypes: 5-HT2B and 5-HT2C receptors. These subtypes have similar molecular structure, amino acid sequence, and signaling properties. The 5-HT2B receptors have a restricted expression in CNS; they play an important role during the embryonic development [1]. The 5-HT2A and 5-HT2C receptors are widely distributed across the CNS and have multiple functions. All members of the 5-HT2 receptor family primarily couple to PLC on activation. Like other G-protein coupled receptors (GPCRs) , 5-HT2 functional regulation also involves sensitization and desensitization-regulatory processes that help prevent overstimulation and allow recuperation of signaling competence, respectively [2].
Serotonin-2 receptor subtypes have been cloned from various species and tissues. The 5-HT2A receptor from hamster, human, monkey, mouse, pig, rat, and sheep all have the same length of 471 amino acid. The 5-HT2B receptor from human, mouse and rat have a length of 481, 504, and 479 amino acids and the 5-HT2C receptor from human, mouse and rat have a length of 458, 459, and 460 amino acids, respectively [3]. The 5-HT2A and 5-HT2C receptors are glycosylated on multiple sites. The genes for the 5-HT2A and 5-HT2B receptor have 3 introns; the 5-HT2C receptor gene has two introns. In humans, the genes are located on chromosome 13q14-q21 for the 5-HT2A receptor, chromosome position 2q36.3–2q37.1 for the 5-HT2B receptor, and chromosome X q24 for the 5-HT2C receptor [1].
It has been shown that some GPCRs , including the 5-HT2A receptor, exhibit critical differences in some aspects of functional regulation from those seen in conventionally studied model GPCRs such as the β2-adrenergic receptor . This receptor couples to a number of intracellular signaling cascades, making it an important receptor to study. Therefore, the 5-HT2A receptor could well serve as an important alternate paradigm in the study of GPCR function [2].
Though the receptor has been studied largely in relation to its multiple functions in the CNS, high levels of receptor expression in other areas such as the intestine, platelets, and endothelial cells suggest that it could play crucial roles in other aspects of physiology, as well. They mediate contractile responses in many vascular smooth muscle preparations (e.g. bronchial, uterine and urinary smooth muscle), and part of the contractile effects of 5-HT in the guinea pig ileum. In addition, platelet aggregation and increased capillary permeability following exposure to 5-HT have been attributed to 5-HT2A receptor-mediated process. Moreover, 5-HT2 receptor agonists, in addition to precursors of 5-HT and 5-HT releasing agents, mediate certain behavioral syndromes in vivo (e.g. head twitching in mice, and wet-dog shakes and back muscle contractions in rats) [4]. Centrally, these receptors are principally located in the cortex, claustrum and basal ganglia. 5-HT2A receptor activation stimulates hormone secretion (e.g. ACTH, corticosterone, oxytocin, renin and prolactin) [5]. Considering the broad expression of 5-HT2A receptors across the brain and their involvement in multiple CNS functions, it is expected that these receptors will play a role pathophysiology of brain disorders . Indeed, the CNS disorders in which the 5-HT2A receptor seems to be involved range from schizophrenia, depression, obsessive compulsive disorder (OCD), and attention deficit–hyperactivity disorder (ADHD), to eating disorders such as anorexia nervosa, to autism spectrum disorders [2]. Implication of 5-HT2A receptors in mental disorders with complex etiologies is still not clearly understood. There are a large number of drugs targeted to this receptor.
Molecular Genetics and Epigenetics of Serotonin-2A Receptor
Serotonin-2A Gene Polymorphism
The 5-HT2A receptor, encoded by HTR2AR gene, is a widely-distributed post-synaptic target for 5-HT in the human brain. Serotonin-2A receptor heterogenity is affected by alternative polymorphisms and alternative splicing. The 5-HT2A receptor is a target for atypical antipsychotics and antidepressants. The role of genetic variants of HTR2AR in signaling modulation remains unclear, despite positive clinical associations [6]. Methods for detecting genetic polymorphisms are advancing rapidly and now allow simultaneous genotyping of several nucleotide polymorphisms. The Genetic Association Database [7] reports 346 unique association studies between single nucleotide polymorphisms (SNPs) in HTR2AR gene and human phenotypes and more than half of these studies find positive genotype-phenotype associations. Most are related to cognition or risk for neuropsychiatric disorders , supporting the presence of functional genetic variants in HTR2AR gene. Some of SNPs (e.g., T102C, C516T, A1438G) are silent mutations and do not cause a change in the protein. Other SNPs (e.g., W25S, I197V, S421F, A447V, H452Y) result in a change in an amino acid. Although the A1438G mutation is silent and does not result in alteration of the amino acid sequence of 5-HT2A receptor, it is located within promoter region of the gene. Thus was proposed that this mutation alters promoter activity and even so expression of 5-HT2A receptors [8]. Lower 5-HT2A receptor densities in some brain areas may cause another silent mutation, T102C [9]. On the other hand, mutation H452Y which caused change in protein has no effect on receptor expression, but reduces intracellular signaling capacity [10].
Numbers of studies have been conducted on the association between HTR2AR gene T102C polymorphism and major depressive disorder (MDD) [11,12,13]. To clarify the effects of HTR2AR gene T102C polymorphism on the risk of depression, Lin et al. [11] performed a meta-analysis in the Chinese population. Results have shown that HTR2AR gene T102C polymorphism is not associated with susceptibility to MDD in these population. Another study [14] demonstrated an association between T102C polymorphism of HTR2AR gene, lifespan, and the risk of age-related CNS disorders. Their results suggest that T102C is associated with mean life span, and thus this gene becomes a possible candidate for the group of adaptive genes to meat consumption proposed in the literature.
The 5HT2A receptor gene polymorphisms rs7997012 and rs6311 has been suggested to be involved in major depressive disorder. Htr2a knock-out mice (Htr2a−/−) displayed an increase in depressive-like behavior, compared to wild type, thus suggesting , that lowered 5-HT2A receptor transmission may favor the susceptibility and severity of major depressive episodes [15].
It is seems that genetic variants in the HTR2A gene affect the therapeutic effects of andtidepressant drugs but mechanism underlying the regulation of such response remains poorly described. According to study of Qesseveur et al. [16] the HTR2A gene may represent a relevant marker to predict the efficacy of antidepressant drugs . The effect of three HTR2A single nucleotide polymorphisms (SNPs- rs6313, rs6314 and rs7333412) was investigated. These three SNPs have potential functional consequences on 5-HT2A receptor, on response and remission rates after 3 months of antidepressant treatments. Their clinical data indicated that GG patients for the rs7333412 SNP were less prone to respond to antidepressant drugs than AA/AG patients.
T102C and A1438G polymorphisms were associated with risk for schizophrenia [17,18,19]. The T102C polymorphism is also related to tobacco use [20] and the A1438G polymorphism of HTR2AR gene is involved in the development of alcohol dependence [21]. Polymorphisms of the HTR2AR gene are associated with hallucinatory symptoms and delusions in demented and non-demented cohorts. The study of Craig et al. [22] examined the role of the HTR2AR gene T102C polymorphism in influencing psychotic symptoms in a large Northern Ireland Alzheimer’s disease (AD) population. No significant association was found either in frequency of genotype or allelic variation for either set of symptoms. On the other hand, Lam et al. [23] demonstrated significant association between neuropsychiatric symptoms in AD and HTR2AR gene polymorphisms.
Methylation
Differential DNA methylation has been suggested to contribute to differential activity of alleles C and T and thereby to genetic associations between the C/T(102) polymorphism in the HTR2AR gene and psychiatric disorders [24]. This study demonstrated methylation in two CpG sites, which are specific to allele C. The majority of allele C-specific CpG sites were methylated in human temporal cortex and peripheral leukocytes. Findings that methylation of allele C-specific CpG sites in the first exon correlated significantly with the expression of DNA methylase 1 but not S-adenosylhomocysteine hydrolase, support the hypothesis that allele-specific DNA methylation is involved in regulation of HTR2AR gene expression , influencing expression differences between alleles C and T.
De Luca et al. [25] developed an improved quantitative assay for the measurement of allele-specific methylation of the HTR2AR gene and genetic association between the HTR2AR gene T102C silent polymorphism and suicidality in patients with mood disorders and schizophrenia.
Falkenberg et al. [26] used functional and structural equation modeling (SEM) approaches to assess the contributions of the polymorphism (R6311S) to DNA methylation and HTR2AR gene expression in chronic fatigue syndrome (CFS) subjects from a population-based study. Their study suggests that the promoter polymorphism (rs6311) can affect both transcription factor binding and promoter methylation, and this along with an individual’s stress response can impact the rate of HTR2A transcription in a genotype and methylation-dependent manner.
Alternative Splicing
The first alternatively spliced isoform of 5-HT2A receptor was identified by Huang et al. [27] in the parasitic nematode species, Ascaris Suum. The 5-HT2A-s1 and 5-HT2A-s2 exhibited identical pharmacological profiles when stably expressed in human embryonic kidney (HEK) 293 cells . Both 5-HT2As isoforms had higher affinity for 5-HT than their closely related Caenorhabditis Elegans homolog (5-HT2C-e).
Guest et al. [28] identified an alternatively spliced HTR2AR gene transcript by PCR of human brain cDNA using degenerate oligonucleotide primers to transmembrane domains. PCR analysis showed that truncated (5HT2ARtr) and native HTR2AR genes were co-expressed in most brain tissues, with the highest levels being found in hippocampus, corpus callosum, amygdala, and caudate nucleus. Western blot analysis of HEK-293 cells transfected transiently with a 5HT2ARtr construct showed that a 30-kDa protein was expressed in cell membranes. Co-transfection studies showed no effect of the 5HT2ARtr variant on 3H-ketanserin binding to the native HTR2AR or on functional coupling of the HTR2AR to 5-HT-stimulated calcium influx.
Molecular Pharmacology of and Serotonin-2A Receptors
Signal Transduction Pathways of Serotonin-2A Receptor
The activation of 5-HT2A receptor leads to the dissociation of GαQ/Z protein into α and βγ subunits. The α subunit of GαQ/Z protein activates the phospholipase C (PLC) , which in turn catalyzes the dissociation of inositol 1,4,5-trisphosphate (IP3)-di-acylglycerol (DAG) complex into the IP3 and DAG. The DAG activates protein kinase C (PKC), and IP3 stimulates calcium (Ca2+) release from endoplasmic reticulum (ER) into the cytoplasm, a characteristic activation signature of many GPCRs [29, 30]. This cascade has been the most extensively studied and is perhaps the most important signal transduction pathway regulated by this receptor (Fig. 1).
Detailed signal transduction pathways of serotonin-2A receptors. Serotonin-2A (5-HT2A) receptor activates protein kinase Cβ (PLCβ). Protein kinase Cβ hydrolysis phosphatidylinositol 4,5 bisphosphate (PIP2) to diacylglycerol (DAG) which activates protein kinesis A (PKA) and inositol trisphosphate (IP3) which acts through inositol trisphosphate receptors (IP3R) localize on endoplasmic reticulum. Activation of this signaling pathway leads to increase in intracellular calcium concentration which affects ion channels, enzyme activity, and neurotransmission or gene expression. Intracellular calcium can also lead to activation of calmodulin which activates extracellular signal-regulated kinases (ERK) and activation of calcineurin leading to inhibition of voltage-dependent calcium channels. Activation of ERK signaling pathway suppresses 5-HT2A receptor signaling through RSK2 kinase. Extracellular signal-regulated kinases can be activated by TGFβ receptor signaling pathway involving Ras GTP-ases interacting with Raf kinases and mitogen-activated protein kinase kinases (MEK) which phosphorylates mitogen-activated protein kinase (MAPK)
Stimulation of the 5-HT2A receptor leads to the activation of at least three distinct signal transduction pathways: IP3/DAG-, arachidonic acid (AA)-, and 2-arachidonylglycerol (2-AG)-mediated. In addition to PLC, 5-HT2A receptors were also reported to activate phospholipase A2 (PLA2), so-called phospholipase B (PLB) [31].
Besides phospholipases-mediated calcium signaling , 5-HT2A receptor activation also induces extracellular signal-regulated kinase (ERK) phosphorylation via diverse intracellular signaling mechanisms [32]. Src and calmodulin (CaM) promote 5-HT2A receptor-mediated phosphorylation of ERK. In the PC12 cells, ERK phosphorylation by 5-HT2A receptor may not depend on PLC/PKC signaling, and instead requires an increase in intracellular calcium , and the activation of CaM and Src [33]. The ERK target p90 ribosomal S6 kinase 2 (RSK2) directly acts on the third intracellular (i3) loop of 5-HT2A receptor protein [34], leading to direct phosphorylation of the i3 loop at the conserved residue Ser-314 and to suppression of 5-HT2A receptor signaling.
In addition, RSK2 is required for tyrosine kinases, such as the epidermal growth factor receptor and the platelet-derived growth factor receptor, both of which have been demonstrated to attenuate 5-HT2A receptor functioning in primary cortical neurons [35, 36].
The 5-HT2A receptors, like other members of 5-HT2 family , couple preferentially via GαQ/Z-11 to the IP3/PKC/Ca2+ pathway, although inhibition of cyclic adenosine monophosphate (cAMP) production has been reported [37].
The 5-HT2A receptor also regulates the tyrosine kinase pathway activity [33]. Activation of neuronal 5-HT2A receptor activates transglutaminase which leads to transamidation of Rac1, a small G protein, resulting in constitutive activation of Rac1 [38]. Chronic treatment with olanzapine, an atypical antipsychotic drug, causes the desensitization of 5-HT2A receptor signaling. In rat frontal cortex, stimulation of the JAK-STAT pathway desensitizes the 5-HT2A receptor-mediated PLC activation induced by olanzapine [39]. Furthermore, constitutive activation of 5-HT2A receptor induces GαQ/Z-11 phosphorylation and desensitization (uncoupling) [40].
Functional Selectivity and Internalization of Serotonin-2A Receptors
Interestingly, different agonists of 5-HT2A receptors vary in the efficacy with which they stimulate individual signal transduction pathways [2, 41]. This phenomena is called functional selectivity and the 5-HT2A receptor was one of the first receptors for which this was described [29, 42]. This discovery was based of the observation that hallucinogenic effects of drugs such as LSD do not correlate with their activation of the IP3/DAG pathway [2].
It has been suggested that hallucinogen, but not nonhallucinogen, 5-HT2A receptor agonist induce phosphorylation of the 5-HT2A receptor at S280 located in the third intracellular loop. Importantly, these authors also demonstrated that pretreating cells with pertussis toxin (PTX) decreased PLC activation induced by the hallucinogens 2,5-Dimethoxy-4-iodoamphetamine (DOI) and LSD, whereas PTX treatment did not affect lisuride and ergotamine responses [43]. Jones et al. [44] discovered, that application of the 5-HT2A receptor agonist DOI to cultured cortical neurons induced phosphorylation of p21-activated kinase (PAK) via Rac guanine nucleotide exchange factor (RacGEF) kalirin-7 [44]. Taken together, these observations suggest that hallucinogens selectively activate GαI/O-dependent signaling , whereas non-hallucinogen 5-HT2A receptor agonists do not [45].
Both in vitro and studies in vivo have shown receptor redistribution in response to exposure to antagonists. The 5-HT2A receptor is internalized in response to both agonists and antagonists, adding a very interesting twist to its signaling properties [46, 47]. This feature of the 5-HT2A receptor may play important roles in its signaling and in the actions of antipsychotic medications. The antagonist-mediated internalization of the rat 5-HT2A receptor, unlike 5-HT-mediated internalization , is independent of protein kinase C (PKC) activation [47]. Bhatnagar and colleagues [46] examined the internalization process of this receptor in detail, demonstrating that both agonist- and antagonist-induced internalization of the 5-HT2A receptor were dynamin-dependent and via clathrin-mediated endocytosis. Activation of the 5-HT2A receptor by agonists, but not antagonists, induced greater translocation of arrestin-3 than arrestin-2 to the plasma membrane, and resulted in differential sorting of arrestin-2, arrestin-3, and 5-HT2A receptors into distinct plasma membrane and intracellular compartments. It is likely that these differences in distribution of the various signaling components induced by agonists and antagonists may be important in the “ligand-directed” of second messenger signals by the 5-HT2A receptor, depending upon which ligand is used to stimulate the receptor. Authors discovered, that in vitro knockdown of Caveolin-1 (Cav-1, a scaffolding protein) nearly abolished 5-HT2A receptor-mediated signal transduction as measured by calcium flux assays. Cav-1 appeared to modulate 5-HT2A receptor signaling by facilitating the interaction of 5-HT2A receptors with Gαq.
Serotonin-2A-Acting Drugs
Several drugs that have been developed for treatment of psychiatric disorders selectively bind to the 5-HT2A receptor and modulate its signaling pathways (Table 1). The antipsychotic drugs spiperone and methiothepin with antipsychotic properties are nonselective antagonists of 5-HT1 and 5-HT2 receptors. Both prevent the 5-HT-dependent PLC activation at 10 μM concentration. However, cyproheptadine (10 μM), another antagonist of 5-HT1 and 5-HT2 receptors, had no effect on PLC activity [48].
Brexpriprazole is an antagonist of 5-HT2A, 5-HT1A and D2 receptors, is approved for the clinical use as a main pharmacotherapy in schizophrenia and as an adjunct in antidepressant-resistant depression. This drug demonstrated robust antipsychotic, antidepressant-like and anxiolytic activities, and limited extrapyramidal symptom liability with pro-cognitive efficacy in animal models [49]. Accumulating evidence suggests that antipsychotic drugs act by promoting neurite outgrowth. In the study of Ishima and colleagues [50] authors examined whether brexpiprazole can affect neurite outgrowth in cell culture. They found that brexpiprazole significantly potentiated nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells, in a concentration dependent manner. Moreover, inhibitors of inositol IP3 receptors , xestospongin C and 2-aminoethoxydiphenyl borate (2-APB), significantly blocked the effects of brexpiprazole. These findings suggest that brexpiprazole-induced neurite outgrowth is mediated through 5-HT1A and 5-HT2A receptors , and subsequent Ca2+ signaling via IP3 receptors [50].
Role Serotonin-2A Receptors in the Regulation of CNS Circuits
Role of Serotonin-2A Receptors in the Interactions Between Serotonin and Glutamate and GABA Systems
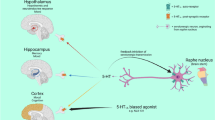
DOI (1-[2,5-dimethoxy-4-iodophenyl-2-aminopropane]) is a hallucinogen acting as agonist of 5-HT2A receptors, similarly to lysergic acid diethylamide (LSD) . It was reported that DOI causes a dose-related inhibition of 5-HT neuronal activity, with the highest dose reducing firing rates by >80%. Pretreatment with the 5-HT2 receptor antagonist ritanserin completely blocked the action of DOI [51]. Study of Quesseveur et al. [52] confirms this inhibitory effect of DOI on dorsal raphe (DR) nucleus 5-HT neuronal activity. DOI’s response is dependent on 5-HT2A receptors because it diminished in 5-HT2A receptors lacking mice. Possible way of DOI inhibitory effect on DR 5-HT neuronal activity is via increasing of GABA release in DR. Other study shows that activation of 5-HT2A receptors in the PFC by DOI increased the firing activity of DR 5-HT neurons. DOI administration also affected the firing rate of pyramidal neurons while most of them were excited, 11% were inhibited and rest was unaffected [53] In this case, excitatory and inhibitory actions of DOI on pyramidal cell firing are likely mediated by receptors located on pyramidal neurons and GABA interneurons, respectively. DOI also stimulates 5-HT release in the PFC, probably via a mechanism involving interaction between 5-HT2A and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors [54] (Fig. 2).
Interactions between 5-HT2A receptors and the other system. (a) Excitatory pyramidal neurons in the medial prefrontal cortex (mPFC) control activity of 5-HT neurons in dorsal raphe (DR) Fig. 2 (continued) through 3 different mechanisms: N-methyl-d-aspartate (NMDA) and 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionate (AMPA) receptors- mediated excitation; GABAA receptors- mediated inhibition; and 5-HT1A autoreceptors- mediated inhibition. (b) Regulation of the dopaminergic system through 5-HT2A receptors. In the ventral tegmental area (VTA) or in medial prefrontal cortex (mPFC), 5-HT2A receptors have also been identified in GABAergic interneurons. Their activation leads to the inhibition of dopaminergic activity. 5-HT2A receptors might also be expressed in dopaminergic neurons in VTA region and their activation would stimulate dopaminergic activity. (c) Locus coeruleus (LC) receives dense 5-HT projections coming from dorsal raphe (DR), which have an inhibitory effect on noradrenergic neurons. Increased 5-HT levels act also on excitatory 5-HT2A receptors on GABAergic neurons which lead to an inhibition of norepinephrine release
The PFC seems to play crucial role in depression. PFC is involved in higher brain functions and carries a control of brain functions through the processing and integration of signals from other brain areas, such as neocortex, several thalamic nuclei, and the brain stem. The apical and basal dendrites of pyramidal neurons of the PFC are highly enriched with 5-HT2A receptors. These receptors are present also on large and medium-sized GABAergic interneurons that control the activity of local microcircuits [55]. The mPFC in rodents innervates via long glutamatergic axons various brain areas involved in depression, such as nucleus accumbens (NAcc), amygdala, and PFC [56]. As well, activity of dopaminergic neurons in ventral tegmental area (VTA) is under the excitatory control of 5-HT2A receptors in mPFC. Neurons in mPFC excited through 5-HT2A receptors increase the firing rate and burst firing of dopaminergic neuron and dopamine release in VTA [57].
The 5-HT2A receptor activation located on thalamocortical afferents could increase glutamate release and increase spontaneous excitatory postsynaptic currents (EPSCs) through the activation of pyramidal AMPA receptors, however, this suggestion is based by the recent anatomical data indicating that the terminal 5-HT2A receptors are not located on glutamate axons [58].
Role of Serotonin-2A Receptors in the Interactions Between Serotonin and Dopamine Systems
The 5-HT2A receptor stimulation results in enhanced dopamine (DA) release in rat PFC, presumably via facilitation of 5-HT1A receptor stimulation. Ability of clozapine to increase DA release may be boosted by antagonism of 5-HT2A receptors [59].
The local infusion of DOI into the PFC dampened potassium (K+)-mediated DA release in a dose-dependent manner. Regular intracortical administration of MDL 100907 caused an increase in cortical DA efflux, suggesting that cortical 5-HT2A receptors potentiate the phasic release of DA [60]. The stimulatory effect of 5-HT on efflux of dopamine in the striatum is effective only when nigro-striatal DA transmission is elevated above basal levels [61]. Antagonism of 5-HT2A receptors may modulate the activity of dopamine neurons in different areas. For the nigro-striatal dopaminergic pathway was suggested a model in which blockade of these receptors led to increased output of dopaminergic neurons into the striatum [62].
Brexpiprazole has higher affinity to D2 than to the 5-HT2A receptors . While other antipsychotic drugs act as D2 antagonists, brexpiprazole is a partial agonist of the D2 receptors [63, 64]. The D2 receptor agonistic features could alter DA neurotransmission by stimulating D2 receptors when the levels of DA are lowered, while decreasing their activation when DA levels are increased [65].
Increase in 5-HT levels inhibits dopaminergic neurons as the lesion of 5-HT neurons results in an increase of dopaminergic neuronal activity in the VTA [66]. Thus, an increase in the availability of 5-HT cause by SSRIs might result in attenuation of the firing of dopaminergic neurons. Neuronal activity of dopaminergic neurons has a critical role in the VTA in motivation, hedonia and reward, so the inhibition of this firing might contribute to SSRI resistance in some patients [67].
Role of Serotonin 2A Receptors in the Interactions Between Serotonin and Norepinephrine Systems
The 5-HT2A receptor is likely to play an important role in the interaction between norepinephrine (NE) and serotonin (5-HT) systems [68]. Increased 5-HT levels act on excitatory 5-HT2A receptors on GABA neurons, thus leading to an inhibition of NE release [69].
Acute brexpiprazole administration reduced inhibition of two important interaction nodes between the 5-HT and NE systems. The blockade of 5-HT2A receptors revokes the tonic inhibition of NE neuronal firing activity, and the blocking of α2-adrenergic receptors on the nerve terminals of NE neurons stimulates NE release [70].
YM992 [(S)-2-[[(7-fluoro-4-indanyl)oxy]methyl]morpholine monohydrochloride] is a selective serotonin reuptake inhibitor (SSRI) and a potent 5-HT2A receptor antagonist. Acute injection of YM992 significantly decreased NE neuron firing activity and blocked the inhibitory effect of a subsequent injection of the 5-HT2 receptor agonist DOI. After 2-day treatment the firing activity was elevated even more significantly, however after 7-day and 21-day treatment a partial recovery was observed. This NE activity may be a result of 5-HT reuptake inhibition plus 5-HT2A receptor antagonism [69].
The activation of 5-HT2A and 5-HT1A receptors suppresses the firing of 5-HT and noradrenergic neurons of the locus coeruleus (LC). Serotoninergic neurons recover their firing rate with prolonged treatment, because of the desensitization of 5-HT1A autoreceptors , but the firing rate of noradrenergic neurons does not recover over time [68].
Role of Serotonin-HT2A in the Response to Antidepressant and Mood Stabilizing Drugs
Selective serotonin reuptake inhibitors (SSRIs) induce inhibition of NE neuron firing [71]. It was reported in several open-label and blind studies that antagonists of 5-HT2A receptors, such as atypical antipsychotic drugs, potentiate the therapeutic effect of SSRIs in patients with depression [72]. It is also reported that antidepressants induce down-regulation of 5-HT2A receptors after repeated treatment [55].
Risperidone is 5-HT2A and dopamine D2 receptor antagonist which is the only antagonist known to saturate the 5-HT2A receptors even at low doses (0.5–1 mg/day) [73]. It was reported that risperidone reverses SSRI-induced inhibition of NE neurons due to its 5-HT2A receptor antagonistic property [71]. Co-administration of risperidone with venlafaxine or fluoxetine may enhance their antidepressant effects. Addition of yohimibine to the combination of risperidone with venlafaxine or fluoxetine augmented the antidepressant-like action proposing an interaction of α2-adrenergic and 5-HT2A receptor in mediating their action [74]. Palperidone is the main metabolite of risperidone. Although they share the same receptor binding profile, it seems that they have different effects on 5-HT and NE firing in vivo. Co-administration of paliperidone did not interfere with the effect of SSRIs, but still managed to inhibit the NE firing inhibition induced by the SSRIs which leads to assumption that it may be an effective enhancement of the treatment [75].
Amibegron (SR58611A)—selective β3 adrenergic agonist [76] interacts with serotonergic system in the brain resulting in an antidepressant effect [77]. It increases the synthesis of 5-HT and tryptophan levels in several brain areas, such as hippocampus, cortex, hypothalamus and striatum. Amibegron did not modify noradrenaline synthesis and metabolism, but it did increase its release [78]. A 5-HT2A receptor antagonist ketanserin significantly reversed the effect of amibegron which leads to conclusion that these antidepressant-like effects are partially caused by the 5-HT2A receptor activation, more precisely by interaction with 5-HT1A, 5-HT2A/2C and 5-HT3 serotonin receptors [79, 80].
Function of cortical 5-HT2A receptors has a specific role in the modulation of conflict anxiety. Weisstaub et al. [81] demonstrated that global disruption of 5-HT2A receptor signaling in mice reduced inhibition in conflict anxiety paradigms without affecting fear-conditioned and depression-related behaviors. Selective restoration of 5HT2A receptor signaling to the cortex normalized conflict anxiety behaviors.
The serotonergic system appears to play a role in episodic memory which is affected in pathologies such as schizophrenia, Alzheimer and depression. The 5-HT2A receptors as one of the principal post-synaptic receptors for 5-HT in the brain are involved in neuropsychiatric and neurological disorders associated with memory deficits. Results of Morici et al. [82] showed that the 5-HT2A and also 5-HT1A receptors can be a novel target for drug development to improve episodic memory retrieval in psychiatric and neurological disorders.
Serotonin-2A Receptors in Pathophysiology and Treatment of Depression
Expression and Function of Serotonin-2A Receptors in the Hippocampus
Hippocampus is a brain structure which plays role in a spatial learning and declarative memory. It receives robust serotonergic innervation from medial and dorsal raphe nuclei. There is some evidence indicating role of 5-HT and its receptors in various aspects of cognitive functions including learning and memory. Nowadays, exact role of 5-HT in hippocampus is not fully understood. Results of functional studies are contradictory. One of possible explanation for these contradictory results is that 5-HT acts through different types of 5-HT receptors. The 5-HT2A receptor subtype is related to memory disorders [83] and several neurological diseases like Alzheimer disease [84, 85] and schizophrenia [86,87,88].
The presence of 5-HT2A receptors in hippocampus was demonstrated in different studies by multiple methods including immunohistochemistry, in situ hybridization, autoradiography and quantitative reverse transcription-polymerase chain reaction (RT-PCR) . Results from these studies are quite different and depending on methodology which was used. Minimal levels of 5-HT2A receptors were detected in human hippocampus by RT-PCR and autoradiography. They were barely detected in pyramidal cells in Cornu ammonis (CA) regions, and were not detected in dentate gyrus (DG) [89]. In rat hippocampus mRNA for 5-HT2A receptors was detected in both CA regions and in DG [90]. In CA area of rat hippocampus low levels of 5-HT2A receptors were detected by in situ hybridization and autoradiography methods. In ventral DG moderate levels of specific 5-HT2A receptors binding were detected [91]. Immunohistochemistry studies showed that 5-HT2A receptors expressed both excitatory glutamatergic and inhibitory GABAergic neurons [92,93,94,95]. Virtually all main hippocampal excitatory neurons (granular and pyramidal cells) expressed 5-HT2A receptors . Strong expression is localized in apical dendrites of pyramidal cells, where 5-HT receptors can increase excitatory postsynaptic currents (EPSP) [92, 94]. Electrophysiological studies demonstrated that outward current induced by 5-HT and α-methyl-serotonin (5-HT2A receptors agonist) in pyramidal cells of rat CA1 hippocampal area is blocked by ketanserin and spiperon (5-HT2A receptors antagonist) in dose dependent manner [96]. The 5-HT2A receptors are also expressed in mossy fiber in rat hippocampus [92]. Receptors localized on presynaptic side of mossy fibers could regulate excitatory neurotransmission and as result affect release of glutamate in hippocampus [97, 98]. On the other hand, colocalization analyses show that 5-HT2A receptors are expressed in GABAergic neurons located in different rat hippocampal regions . This colocalization is similar in different hippocampal areas: in DG, CA1, CA2 and CA3 field. In hippocampal CA areas are 5-HT2A receptors widespread in number of GABAergic interneurons distributed in pyramidal cell layer, in strata oriens, radiatum and lacunosum-moleculare.
The 5-HT2A receptors are expressed on 90% of GABAergic neurons in hippocampus [92]. Electrophysiology studies showed that activation of 5-HT2A receptors activate GABAergic neurons in rat DG [99] and in CA1 field [100]. High density of 5-HT2A receptor in deeper layers of granular cell layer corresponds with study demonstrating that 5-HT receptors can regulate neurogenesis in subgranular zone of DG [101]. Because GABA regulates progenitor turnover and integration of newly synthetized neurons in DG [102], it can be assumed that GABA neurons distributed in subgranular zone can be involved in hippocampal progenitor proliferation mediated by 5-HT2A receptors [103].
Function of 5-HT2A Receptors in Hippocampus in Health
Recent studies suggested that 5-HT2A receptors are included in several hippocampal functions although underlying mechanisms are still unclear. Activity of hippocampal pyramidal neurons can be modulated by 5-HT2A receptors in different ways: directly, by activation of 5-HT2A receptors in pyramidal cells, or indirectly, by activation of 5-HT2A receptors in GABA interneurons [96]. Serotonin 5-HT2A receptors can participate in information processing in hippocampus by participating in neurotransmission in different neuronal populations. Strong and widespread expression of 5-HT2A receptors in hippocampus is prerequisite for critical involvement of 5-HT receptors in number of brain functions including learning and memory [92]. It was shown that an application of M100907 (highly selective 5-HT2A receptors antagonist) to brain slices facilitates induction of long term potentiation (LTP) in CA1 field of rat hippocampus [104].
As a critical factor modulating brain plasticity is considered brain-derived neurotrophic factor (BDNF) . Hippocampal BDNF mRNA expression was induced by physical activity which positively regulated neurogenesis and induced LTP [105]. This factor can acutely influence synaptic efficiency of neurons. Some electrophysiological studies demonstrate that application of BDNF on hippocampal slices results in increase of synaptic strength [106,107,108,109,110]. In hippocampus 5-HT2A receptors participate in regulation of BDNF levels as their agonist DOI decreased the expression of BDNF mRNA in granular cell layer in DG, but not in CA regions. Effect of agonist was blocked by pretreatment with selective antagonist of 5-HT2A receptors. Same decrease of BDNF expression in hippocampus is observed during stress and it is possible that this effect is mediated by 5-HT2A receptors. This hypothesis is supported by an observation that pretreatment with ketaserin significantly blocked stress induced decrease in BDNF expression [111].
Involvement of 5-HT2A receptors in process of learning and memory is supported by study where systematic activation of 5-HT2A receptors with agonist (TCB-2) enhanced the consolidation of both fear memory and object memory [112]. The memory strengthening effect of TCB-2 was blocked by pretreatment with 5-HT2A receptors antagonist (MDL11,939). Local microinfusion of TCB-2 into CA1 field of dorsal hippocampus had similar effect on memory consolidation observed after systemic treatment [113]. Postsynaptic 5-HT2A receptors can modulate memory storage associated with object also by influencing on N-Methyl-d-Aspartate (NMDA) receptors . It is supported by fact that hippocampal 5-HT2A receptors are predominantly expressed in dendritic part of pyramidal neurons [93, 114] and dendrites which expressed 5-HT2A receptors expressed also NMDAR subunit NR1 and GluR2 [114]. Activation of 5-HT2A receptors causes an increase of intracellular Ca2+ concentration which in combination with NMDA receptor-mediated calcium influx can strengthen the synaptic plasticity. These observations suggest that an activation of 5-HT2A receptors induces facilitation of object memory storage and can result from potentiating of glutamate release in hippocampus, temporal dynamics of pyramidal neurons and critical post-training period. These receptors may serve as a drug target for pharmacological intervention in the treatment of memory disorders [115]. It is known that new neurons are generated in mammal DG. These new neurons are later during life integrated into hippocampal circuit. Serotonin belongs to important factors influencing neurogenesis. Among others 5-HT receptor subtypes (5-HT1A, 5-HT1B and 5-HT2C), activation of 5-HT2A receptors is involved in the positive regulation of adult neurogenesis in DG caused by regulation of cell proliferation in this region [103]. It was reported that some animal models of depression produce decrease in hippocampal cell proliferation and neurogenesis. Unlike the depression , chronic treatment with antidepressants, such as SSRIs, seem to have the positive effect on neurogenesis which is sufficient to reduce anxiety and depression-related behavior [116].
Role of Hippocampal Serotonin-2A Receptors in Pathophysiology and Treatment of Depression
The main effect of antidepressants is increasing of synaptic 5-HT levels. There is some evidence suggesting that hippocampus can be influenced by depression. It is known that hypercorticosolemia, an animal model of depression, results in the death of hippocampal neurons [117]. Change of serotonergic function in hippocampus is likely to be involved in defects of mood regulation associated with the major depressive disorder (MDD) . Serotonin 5-HT2A receptors play role in these changes. Postmortem studies in depressed suicide completers documented changes in 5-HT2A receptors binding in hippocampus [118, 119]. Magnetic resonance imaging (MRI) studies showed changes in 5-HT2A receptors binding potential in hippocampus in patients with MDD [120, 121]. Magnetic resonance imaging studies also demonstrated decrease of hippocampal volume in patients with MDD which correlated with duration of depression [120, 121]. However, decrease in 5-HT2A receptors binding potential is higher than volume loss and indicates that both conditions can coexist. Not only depression itself, but also the total number of days with depression inversely correlates with hippocampal volume [121, 122]. Serotonin 5-HT2A receptor binding is not influenced by depression phase. However, patients not previously treated for depression have lower 5-HT2A receptor binding than patients with previous medication treatment . It is possible that medication treatment provides compensatory upregulation of 5-HT2A receptors [123]. It is well established that decreased 5-HT2A receptor transmission is associated with depression [124]. It is also possible that decreased 5-HT2A receptor-mediated neurotransmission has special importance. Indeed, decreased 5-HT2A receptors binding was reported in patients with depression [123]. In addition, antidepressants treatment may cause changes in expression and binding of 5-HT2A receptors and these changes can persist for a long time after treatment [1, 125,126,127,128,129].
Nowadays, the role of astrocytes in depression has been intensively studied [130]. 5-HT2A receptors are expressed not only in hippocampal neurons, but also in astrocytes. This suggests the possibility that also 5-HT2A receptors express in astrocyte have functional implications in psychiatric disorders [95]. Beside their housekeeping functions, astrocytes are dynamic regulators of synaptogenesis, synaptic strength and control neurogenesis in the adult DG [131]. Astrocytes synthesize and release many neurotrophic factors vital for neuronal health such as BDNF, glial-derived neurotrophic factor (GDNF), nerve growth factor (NGF), and neurotrophins 3 and 4/5 [132, 133]. Brain-derivated neurotrophic factor blocks neurogenesis in depression which is opposite to healthy condition. Its function has been implicated in the neurogenesis hypothesis of depression in which the antidepressants enhance neurogenesis, and BDNF is a key regulator of this mechanism. Antidepressants (including SSRIs) induce the CREB phosphorylation, CREB binds to the BDNF 13 promoter and induces BDNF transcription. Moreover, stress can reduce the expression of BDNF in the hippocampus and this reduction can be prevented by long-term chronic antidepressant treatment [134, 135]. In vitro studies reported that SSRIs stimulate the expression of BDNF , GDNF and vascular endothelial growth factor (VEGF) in primary culture of astrocytes [136,137,138]. In vivo data showed that the specific over-expression of BDNF in hippocampal astrocytes produced antidepressant-like effect accompanied by an increase in cell proliferation, maturation and survival of new neurons by generated cells in the DG of the hippocampus [139]. It is possible that astrocytes contribute to the enhancement in neurotrophic support and associated augmentation in synaptic plasticity that may form the basis for antidepressant efficacy. Several reports suggested that fluoxetine and other drugs can modulate the structural plasticity of astrocytes. Following chronic administration of lithium and some antipsychotic drugs , increased numbers of glia have been reported in the hippocampi of rats and nonhuman primates [140, 141]. In another study fluoxetine prevented the stress-induced decrease on a number of hippocampal astrocytes , but had no effect in nonstressed animals [142]. It demonstrates that fluoxetine, a prominent member of the SSRI family, can significantly modify the structural plasticity of astrocytes, and it is very likely that these morphological alterations either reflect or induce functional changes within the glial–neuronal interaction [142]. In particular, it is well accepted that SSRIs activate 5-HT2A receptors and stimulate signaling intracellular cascades leading to the phosphorylation/activation of extracellular signal regulated kinases (ERK1/2). Hence, antidepressants may exert their therapeutic activity by stimulating this pathway. In the hippocampus ERK1/2 have been implicated in mood regulation [143] as suggested by their blunted activation and/or expression in both depressed patient [144] and animal models of depression [145].
Conclusion
The 5-HT2A receptors belong to the 5-HT2 receptor family, the only known group of 5-HT receptors which are coupled to GαQ/Z proteins . The primary signal transduction mechanism of 5-HT2A receptors involves activation of PLC and calcium signaling . However, 5-HT2A receptor-mediated alteration of cAMP levels has also been reported. The 5-HT2A receptor is a product of 5HT2AR gene. Genetic polymorphism of 5HT2AR gene, its epigenetic regulation, and post-translational modifications of 5HT2AR mRNA have been reported. Furthermore, pre- and post-translational 5HT2AR alterations correlate with certain CNS disorders, such as depression, schizophrenia, dementia, and alcohol and nicotine dependence. On the functional level, 5-HT2A receptors play a central role in the interaction between 5-HT and norepinephrine systems and they are also involved in 5-HT-glutamate, 5-HT-GABA, and 5-HT-dopamine interactions. In addition, 5-HT2A receptors are fundamental in the modulation of hippocampal neuronal circuits. These lines of evidence, taken together, indicate that 5-HT2A receptors are one of the primary targets for antidepressant and mood stabilizing drugs and other CNS medications. And indeed, atypical antidepressant drugs act as antagonist of 5-HT2A receptors.
References
Leysen JE (2004) 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord 3(1):11–26
Ishier R, Bhattacharya A, Panicker MM (2007) Serotonin-2A (5-HT2A) receptor function: ligand-dependent mechanisms and pathways. In: Chattopadhyay A (ed) Serotonin receptors in neurobiology. CRC, Boca Raton, FL, pp 105–132
Kroeze WK, Kristiansen K, Roth BL (2002) Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem 2(6):507–528
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71(4):533–554. doi:S0091305701007468 [pii]
Van de Kar LD, Javed A, Zhang Y et al (2001) 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci 21(10):3572–3579. doi:21/10/3572 [pii]
Kleinrock M (2011) The use of medicines in the United States: review of 2010. IMS Institute for Healthcare Informatics, Danbury, CT
Becker KG, Barnes KC, Bright TJ et al (2004) The genetic association database. Nat Genet 36(5):431–432. https://doi.org/10.1038/ng0504-431
Parsons MJ, D’Souza UM, Arranz MJ, Kerwin RW, Makoff AJ (2004) The -1438A/Gpolymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol Psychiatry 56(6):406–410
Serretti A, Drago A, De Ronchi D (2007) HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Curr Med Chem 14(19):2053–2069
Hazelwood LA, Sanders-Bush E (2004) His452Tyr polymorphism in the human 5-HT2A receptor destabilizes the signaling conformation. Mol Pharmacol 66(5):1293–1300
Lin CX, Hu Z, Yan ZM et al (2015) Association between HTR2A T102C polymorphism and major depressive disorder: a meta-analysis in the Chinese population. Int J Clin Exp Med 8(11):20897–20903
Tan J, Chen S, Su L et al (2014) Association of the T102C polymorphism in the HTR2A gene with major depressive disorder, bipolar disorder, and schizophrenia. Am J Med Genet B Neuropsychiatr Genet 165B(5):438–455. https://doi.org/10.1002/ajmg.b.32248
Zhao X, Sun L, Sun YH et al (2014) Association of HTR2A T102C and A-1438G polymorphisms with susceptibility to major depressive disorder: a meta-analysis. Neurol Sci 35(12):1857–1866. https://doi.org/10.1007/s10072-014-1970-7
Jobim PF, Prado-Lima PA, Schwanke CH et al (2008) The polymorphism of the serotonin-2A receptor T102C is associated with age. Braz J Med Biol Res 41(11):1018–1023. doi:S0100-879X2008005000045 [pii]
Petit AC, Quesseveur G, Gressier F et al (2014) Converging translational evidence for the involvement of the serotonin 2A receptor gene in major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 54:76–82. https://doi.org/10.1016/j.pnpbp.2014.04.013
Qesseveur G, Petit AC, Nguyen HT, Dahan L, Colle R, Rotenberg S, Seif I, Robert P, David D, Guilloux JP, Gardier AM, Verstuyft C, Becquemont L, Corruble E, Guiard BP (2016) Genetic dysfunction of serotonin 2A receptor hampers response to antidepressant drugs: a translational approach. Neuropharmacology 105:142–153. https://doi.org/10.1016/j.neuropharm.2015.12.022
Abdolmaleky HM, Faraone SV, Glatt SJ et al (2004) Meta-analysis of association between the T102C polymorphism of the 5HT2a receptor gene and schizophrenia. Schizophr Res 67(1):53–62. doi:S092099640300183X [pii]
Joober R, Benkelfat C, Brisebois K et al (1999) T102C polymorphism in the 5HT2A gene and schizophrenia: relation to phenotype and drug response variability. J Psychiatry Neurosci 24(2):141–146
Serretti A, Benedetti F, Mandelli L et al (2008) Association between GSK-3beta -50T/C polymorphism and personality and psychotic symptoms in mood disorders. Psychiatry Res 158(2):132–140. doi:S0165-1781(07)00199-0 [pii]
do Prado-Lima PA, Chatkin JM, Taufer M et al (2004) Polymorphism of 5HT2A serotonin receptor gene is implicated in smoking addiction. Am J Med Genet B Neuropsychiatr Genet 128B(1):90–93. https://doi.org/10.1002/ajmg.b.30004
Nakamura T, Matsushita S, Nishiguchi N et al (1999) Association of a polymorphism of the 5HT2A receptor gene promoter region with alcohol dependence. Mol Psychiatry 4(1):85–88
Craig D, Donnelly C, Hart D et al (2007) Analysis of the 5HT-2A T102C receptor polymorphism and psychotic symptoms in Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet 144B(1):126–128. https://doi.org/10.1002/ajmg.b.30409
Lam LC, Tang NL, Ma SL et al (2004) 5-HT2A T102C receptor polymorphism and neuropsychiatric symptoms in Alzheimer’s disease. Int J Geriatr Psychiatry 19(6):523–526. https://doi.org/10.1002/gps.1109
Polesskaya OO, Aston C, Sokolov BP (2006) Allele C-specific methylation of the 5-HT2A receptor gene: evidence for correlation with its expression and expression of DNA methylase DNMT1. J Neurosci Res 83(3):362–373. https://doi.org/10.1002/jnr.20732
De Luca V, Viggiano E, Dhoot R et al (2009) Methylation and QTDT analysis of the 5-HT2A receptor 102C allele: analysis of suicidality in major psychosis. J Psychiatr Res 43(5):532–537. https://doi.org/10.1016/j.jpsychires.2008.07.007
Falkenberg VR, Gurbaxani BM, Unger ER et al (2011) Functional genomics of serotonin receptor 2A (HTR2A): interaction of polymorphism, methylation, expression and disease association. Neuromol Med 13(1):66–76. https://doi.org/10.1007/s12017-010-8138-2
Huang X, Xiao H, Rex EB et al (2002) Functional characterization of alternatively spliced 5-HT2 receptor isoforms from the pharynx and muscle of the parasitic nematode, Ascaris suum. J Neurochem 83(2):249–258. doi:1067 [pii]
Guest PC, Salim K, Skynner HA et al (2000) Identification and characterization of a truncated variant of the 5-hydroxytryptamine(2A) receptor produced by alternative splicing. Brain Res 876(1–2):238–244. doi:S0006-8993(00)02664-0 [pii]
Berg KA, Maayani S, Goldfarb J et al (1998a) Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann N Y Acad Sci 861:104–110
Hoyer D, Clarke DE, Fozard JR et al (1994) International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev 46(2):157–203
Raymond JR, Mukhin YV, Gelasco A et al (2001) Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther 92(2-3):179–212. doi:S0163725801001693 [pii]
Gooz M, Gooz P, Luttrell LM et al (2006) 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem 281(30):21004–21012. doi:M512096200 [pii]
Quinn JC, Johnson-Farley NN, Yoon J et al (2002) Activation of extracellular-regulated kinase by 5-hydroxytryptamine(2A) receptors in PC12 cells is protein kinase C-independent and requires calmodulin and tyrosine kinases. J Pharmacol Exp Ther 303(2):746–752. https://doi.org/10.1124/jpet.102.038083
Sheffler DJ, Kroeze WK, Garcia BG et al (2006) p90 ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci U S A 103(12):4717–4722. doi:0600585103 [pii]
Strachan RT, Allen JA, Sheffler DJ et al (2010) p90 Ribosomal S6 kinase 2, a novel GPCR kinase, is required for growth factor-mediated attenuation of GPCR signaling. Biochemistry 49(12):2657-2671. https://doi.org/10.1021/bi901921k
Strachan RT, Sheffler DJ, Willard B et al (2009) Ribosomal S6 kinase 2 directly phosphorylates the 5-hydroxytryptamine 2A (5-HT2A) serotonin receptor, thereby modulating 5-HT2A signaling. J Biol Chem 284(9):5557–5573. https://doi.org/10.1074/jbc.M805705200
Arranz MJ, Munro J, Owen MJ et al (1998) Evidence for association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and response to clozapine. Mol Psychiatry 3(1):61–66
Dai Y, Dudek NL, Patel TB et al (2008) Transglutaminase-catalyzed transamidation: a novel mechanism for Rac1 activation by 5-hydroxytryptamine2A receptor stimulation. J Pharmacol Exp Ther 326(1):153–162. https://doi.org/10.1124/jpet.107.135046
Singh RK, Jia C, Garcia F et al (2010) Activation of the JAK-STAT pathway by olanzapine is necessary for desensitization of serotonin2A receptor-stimulated phospholipase C signaling in rat frontal cortex but not serotonin2A receptor-stimulated hormone release. J Psychopharmacol 24(7):1079–1088. https://doi.org/10.1177/0269881109103090
Shi J, Damjanoska KJ, Singh RK et al (2007) Agonist induced-phosphorylation of Galpha11 protein reduces coupling to 5-HT2A receptors. J Pharmacol Exp Ther 323(1):248–256. doi:jpet.107.122317 [pii]
Kurrasch-Orbaugh DM, Watts VJ, Barker EL et al (2003) Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther 304(1):229–237. https://doi.org/10.1124/jpet.102.042184
Berg KA, Maayani S, Goldfarb J et al (1998b) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54(1):94–104
Karaki S, Becamel C, Murat S et al (2014) Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteomics 13(5):1273–1285. https://doi.org/10.1074/mcp.M113.036558
Jones KA, Srivastava DP, Allen JA et al (2009) Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A 106(46):19575–19580. https://doi.org/10.1073/pnas.0905884106
Preedy VR (2016) Neuropathology of drug addictions and substance misuse, 1st edn. Elsevier B.V., Amsterdam, The Netherlands
Bhatnagar A, Willins DL, Gray JA et al (2001) The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem 276(11):8269–8277. https://doi.org/10.1074/jbc.M006968200
Bhattacharyya S (2005) Internalization and recycling of the serotonin 2A receptor in non-neuronal and neuronal cells, in National Centre for Biological Sciences. Manipal Academy of Higher Education of Bangalore, Bangalore
Barbas D, DesGroseillers L, Castellucci VF et al (2003) Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes. Learn Mem 10(5):373–386. https://doi.org/10.1101/lm.66103
Citrome L, Stensbol TB, Maeda K (2015) The preclinical profile of brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother 15(10):1219–1229. https://doi.org/10.1586/14737175.2015.1086269
Ishima T, Futamura T, Ohgi Y et al (2015) Potentiation of neurite outgrowth by brexpiprazole, a novel serotonin-dopamine activity modulator: a role for serotonin 5-HT1A and 5-HT2A receptors. Eur Neuropsychopharmacol 25(4):505–511. https://doi.org/10.1016/j.euroneuro.2015.01.014
Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol. 2003;139(5):998-1004. Erratum in: Br J Pharmacol. 2003;140(1):227–8.
Quesseveur G, Repérant C, David DJ, Gardier AM, Sanchez C, Guiard BP (2013) 5-HT2A receptor inactivation potentiates the acute antidepressant-like activity of escitalopram: involvement of the noradrenergic system. Exp Brain Res 226(2):285–295. https://doi.org/10.1007/s00221-013-3434-3
Puig MV, Celada P, Diaz-Mataix L et al (2003) In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex 13(8):870–882
Bortolozzi A, Amargos-Bosch M, Adell A et al (2003) In vivo modulation of 5-hydroxytryptamine release in mouse prefrontal cortex by local 5-HT(2A) receptors: effect of antipsychotic drugs. Eur J Neurosci 18(5):1235–1246. doi:2829 [pii]
Celada P, Puig MV, Casanovas JM et al (2001) Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci 21(24):9917–9929
Groenewegen HJ, Uylings HB (2000) The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res 126:3–28. doi:S0079-6123(00)26003-2 [pii]
Bortolozzi A, Díaz-Mataix L, Scorza MC, Celada P, Artigas F (2005) The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem 95(6):1597–1607
Miner LA, Backstrom JR, Sanders-Bush E et al (2003) Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 116(1):107–117. doi:S0306452202005808 [pii]
Ichikawa J, Dai J, Meltzer HY (2001) DOI, a 5-HT2A/2C receptor agonist, attenuates clozapine-induced cortical dopamine release. Brain Res 907(1-2):151–155. doi:S0006-8993(01)02596-3 [pii]
Pehek EA, McFarlane HG, Maguschak K et al (2001) M100,907, a selective 5-HT(2A) antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res 888(1):51–59. doi:S0006-8993(00)03004-3 [pii]
Lucas G, Spampinato U (2000) Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J Neurochem 74(2):693–701
Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Höschl C (2006) Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 20(5):389–409
Maeda K, Lerdrup L, Sugino H et al (2014a) Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 350(3):605–614. https://doi.org/10.1124/jpet.114.213819
Maeda K, Sugino H, Akazawa H et al (2014b) Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 350(3):589–604. https://doi.org/10.1124/jpet.114.213793
Burris KD, Molski TF, Xu C et al (2002) Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302(1):381–389
Guiard BP, El Mansari M, Merali Z, Blier P (2008) Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol 11(5):625–639. https://doi.org/10.1017/S1461145707008383
Dremencov E, El Mansari M, Blier P (2009) Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. J Psychiatry Neurosci 34(3):223–229
Szabo ST, Blier P (2001) Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur J Neurosci 13(11):2077–2087
Szabo ST, Blier P (2002) Effects of serotonin (5-hydroxytryptamine, 5-HT) reuptake inhibition plus 5-HT(2A) receptor antagonism on the firing activity of norepinephrine neurons. J Pharmacol Exp Ther 302(3):983–991
Oosterhof CA, El Mansari M, Blier P (2014) Acute effects of brexpiprazole on serotonin, dopamine, and norepinephrine systems: an in vivo electrophysiologic characterization. J Pharmacol Exp Ther 351(3):585–595. https://doi.org/10.1124/jpet.114.218578
Dremencov E, El Mansari M, Blier P (2007b) Noradrenergic augmentation of escitalopram response by risperidone: electrophysiologic studies in the rat brain. Biol Psychiatry 61(5):671–678. S0006-3223(06)00659-7 [pii]; https://doi.org/10.1016/j.biopsych.2006.05.015
Marek GJ, Carpenter LL, McDougle CJ et al (2003) Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology 28(2):402–412. https://doi.org/10.1038/sj.npp.1300057
Schotte A, Janssen PF, Gommeren W et al (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology 124(1-2):57–73
Dhir A, Kulkarni SK (2008) Risperidone, an atypical antipsychotic enhances the antidepressant-like effect of venlafaxine or fluoxetine: possible involvement of alpha-2 adrenergic receptors. Neurosci Lett 445(1):83–88. https://doi.org/10.1016/j.neulet.2008.08.074
Dremencov E, El Mansari M, Blier P (2007a) Distinct electrophysiological effects of paliperidone and risperidone on the firing activity of rat serotonin and norepinephrine neurons. Psychopharmacology. https://doi.org/10.1007/s00213-007-0818-8
Bianchetti A, Manara L (1990) In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical beta-adrenoceptors in rat colon. Br J Pharmacol 100(4):831–839
Overstreet DH, Stemmelin J, Griebel G (2008) Confirmation of antidepressantpotential of the selective beta3 adrenoceptor agonist amibegron in an animal model of depression. Pharmacol Biochem Behav 89(4):623–626. https://doi.org/10.1016/j.pbb.2008.02.020
Claustre Y, Leonetti M, Santucci V et al (2008) Effects of the beta3-adrenoceptor (Adrb3) agonist SR58611A (amibegron) on serotonergic and noradrenergic transmission in the rodent: relevance to its antidepressant/anxiolytic-like profile. Neuroscience 156(2):353–364. https://doi.org/10.1016/j.neuroscience.2008.07.011
Tanyeri P, Buyukokuroglu ME, Mutlu O et al (2013a) Evidence that the anxiolytic-like effects of the beta3 receptor agonist amibegron involve serotoninergic receptor activity. Pharmacol Biochem Behav 110:27–32. https://doi.org/10.1016/j.pbb.2013.05.017
Tanyeri P, Buyukokuroglu ME, Mutlu O et al (2013b) Involvement of serotonin receptor subtypes in the antidepressant-like effect of beta receptor agonist Amibegron (SR 58611A): an experimental study. Pharmacol Biochem Behav 105:12–16. https://doi.org/10.1016/j.pbb.2013.01.010
Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313(5786):536–540
Morici JF, Ciccia L, Malleret G, Gingrich JA, Bekinschtein P, Weisstaub NV (2015) Serotonin 2a receptor and Serotonin 1a receptor interact within the medial prefrontal cortex during recognition memory in mice. Front Pharmacol 6:298. https://doi.org/10.3389/fphar.2015.00298. eCollection 2015
de Quervain DJ, Henke K, Aerni A et al (2003) A functional genetic variation of the 5-HT2a receptor affects human memory. Nat Neurosci 6(11):1141–1142. https://doi.org/10.1038/nn1146
Lai MK, Tsang SW, Alder JT et al (2005) Loss of serotonin 5-HT2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer’s disease. Psychopharmacology 179(3):673–677. https://doi.org/10.1007/s00213-004-2077-2
Versijpt J, Van Laere KJ, Dumont F et al (2003) Imaging of the 5-HT2A system: age-, gender-, and Alzheimer’s disease-related findings. Neurobiol Aging 24(4):553-561. doi:S0197458002001379 [pii].
Dean B (2003) The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem 85(1):1–13. doi:1693 [pii]
Meltzer HY, Li Z, Kaneda Y et al (2003) Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 27(7):1159–1172. doi:S0278-5846(03)00223-9 [pii]
Roth BL, Hanizavareh SM, Blum AE (2004) Serotonin receptors represent highly favorable molecular targets for cognitive enhancement in schizophrenia and other disorders. Psychopharmacology 174(1):17–24. https://doi.org/10.1007/s00213-003-1683-8
Burnet PW, Eastwood SL, Harrison PJ (1994) Detection and quantitation of 5-HT1A and 5-HT2A receptor mRNAs in human hippocampus using a reverse transcriptase-polymerase chain reaction (RT-PCR) technique and their correlation with binding site densities and age. Neurosci Lett 178(1):85–89. doi:0304-3940(94)90296-8 [pii]
Wright DE, Seroogy KB, Lundgren KH et al (1995) Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol 351(3):357–373. https://doi.org/10.1002/cne.903510304
Pazos A, Cortes R, Palacios JM (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res 346(2):231–249. doi:0006-8993(85)90857-1 [pii]
Bombardi C (2012) Neuronal localization of 5-HT2A receptor immunoreactivity in the rat hippocampal region. Brain Res Bull 87(2-3):259–273. https://doi.org/10.1016/j.brainresbull.2011.11.006
Cornea-Hebert V, Riad M, Wu C et al (1999) Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409(2):187–209. https://doi.org/10.1002/(SICI)1096-9861(19990628)409:23.0.CO;2-P [pii].
Luttgen M, Ove Ogren S, Meister B (2004) Chemical identity of 5-HT2A receptor immunoreactive neurons of the rat septal complex and dorsal hippocampus. Brain Res 1010(1–2):156–165. https://doi.org/10.1016/j.brainres.2004.03.016
Xu T, Pandey SC (2000) Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Res Bull 51(6):499–505. doi:S0361-9230(99)00278-6 [pii]
Uneyama H, Munakata M, Akaike N (1992) 5-HT response of rat hippocampal pyramidal cell bodies. Neuroreport 3(7):633–636
Guo JD, Rainnie DG (2010) Presynaptic 5-HT(1B) receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience 165(4):1390–1401. https://doi.org/10.1016/j.neuroscience.2009.11.071
Hashimoto K, Kita H (2008) Serotonin activates presynaptic and postsynaptic receptors in rat globus pallidus. J Neurophysiol 99(4):1723–1732. https://doi.org/10.1152/jn.01143.2007
Piguet P, Galvan M (1994) Transient and long-lasting actions of 5-HT on rat dentate gyrus neurones in vitro. J Physiol 481(Pt 3):629–639
Shen RY, Andrade R (1998) 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther 285(2):805–812
Kulkarni VA, Jha S, Vaidya VA (2002) Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur J Neurosci 16(10):2008–2012
Ge S, Goh EL, Sailor KA et al (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439(7076):589–593. doi:nature04404 [pii]
Banasr M, Hery M, Printemps R et al (2004) Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29(3):450–460. https://doi.org/10.1038/sj.npp.1300320
Wang RY, Arvanov VL (1998) M100907, a highly selective 5-HT2A receptor antagonist and a potential atypical antipsychotic drug, facilitates induction of long-term potentiation in area CA1 of the rat hippocampal slice. Brain Res 779(1-2):309–313. doi:S0006-8993(97)01174-8 [pii]
Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR (2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124(1):71–79
Figurov A, Pozzo-Miller LD, Olafsson P et al (1996) Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381(6584):706–709. https://doi.org/10.1038/381706a0
Kang H, Schuman EM (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267(5204):1658–1662
Korte M, Carroll P, Wolf E et al (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92(19):8856–8860
Levine ES, Dreyfus CF, Black IB et al (1995) Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A 92(17):8074–8077
Patterson SL, Abel T, Deuel TA et al (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16(6):1137–1145. doi:S0896-6273(00)80140-3 [pii]
Vaidya VA, Marek GJ, Aghajanian GK et al (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17(8):2785–2795
Zhang G, Asgeirsdottir HN, Cohen SJ et al (2013) Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 64:403–413. https://doi.org/10.1016/j.neuropharm.2012.06.007
Zhang G, Stackman RW Jr (2015) The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol 6:225. https://doi.org/10.3389/fphar.2015.00225
Peddie CJ, Davies HA, Colyer FM et al (2008) Colocalisation of serotonin2A receptors with the glutamate receptor subunits NR1 and GluR2 in the dentate gyrus: an ultrastructural study of a modulatory role. Exp Neurol 211(2):561–573. https://doi.org/10.1016/j.expneurol.2008.03.003
Aghajanian GK, Marek GJ (1999) Serotonin and hallucinogens. Neuropsychopharmacology 21(2 Suppl):16S-23S. https://doi.org/10.1016/S0893-133X(98)00135-3
Hill AS, Sahay A, Hen R (2015) Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology. 40(10):2368–2378. https://doi.org/10.1038/npp.2015.85
Reagan LP, McEwen BS (1997) Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat 13(3):149–167. doi:S0891061897000318 [pii]
Cheetham SC, Crompton MR, Katona CL et al (1988) Brain 5-HT2 receptor binding sites in depressed suicide victims. Brain Res 443(1-2):272–280. doi:0006-8993(88)91621-6 [pii]
Rosel P, Arranz B, Vallejo J et al (1998) Variations in [3H]imipramine and 5-HT2A but not [3H]paroxetine binding sites in suicide brains. Psychiatry Res 82(3):161–170
MacQueen GM, Campbell S, McEwen BS et al (2003) Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A 100(3):1387–1392. https://doi.org/10.1073/pnas.0337481100
Sheline YI, Sanghavi M, Mintun MA et al (1999) Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19(12):5034–5043
Sheline YI, Wang PW, Gado MH et al (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 93(9):3908–3913
Mintun MA, Sheline YI, Moerlein SM et al (2004) Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: in vivo measurement with [18F]altanserin positron emission tomography. Biol Psychiatry 55(3):217–224. doi:S000632230300920X [pii]
Meltzer HY, Maes M (1995) Effect of pindolol pretreatment on MK-212-induced plasma cortisol and prolactin responses in normal men. Biol Psychiatry. 38(5):310–318
Massou JM, Trichard C, Attar-Levy D et al (1997) Frontal 5-HT2A receptors studied in depressive patients during chronic treatment by selective serotonin reuptake inhibitors. Psychopharmacology 133(1):99–101
Meyer JH, Kapur S, Eisfeld B et al (2001) The effect of paroxetine on 5-HT(2A) receptors in depression: an [(18)F]setoperone PET imaging study. Am J Psychiatry 158(1):78–85. https://doi.org/10.1176/appi.ajp.158.1.78
Meyer JH, Kapur S, Houle S et al (1999) Prefrontal cortex 5-HT2 receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry 156(7):1029–1034. https://doi.org/10.1176/ajp.156.7.1029
Yatham LN, Liddle PF, Shiah IS et al (2001) Effects of rapid tryptophan depletion on brain 5-HT(2) receptors: a PET study. Br J Psychiatry 178:448–453
Zanardi R, Artigas F, Moresco R et al (2001) Increased 5-hydroxytryptamine-2 receptor binding in the frontal cortex of depressed patients responding to paroxetine treatment: a positron emission tomography scan study. J Clin Psychopharmacol 21(1):53–58
Banasr M, Chowdhury GM, Terwilliger R et al (2010) Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15(5):501–511. https://doi.org/10.1038/mp.2008.106
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26(10):523–530. doi:S0166-2236(03)00266-2 [pii]
Althaus HH, Richter-Landsberg C (2000) Glial cells as targets and producers of neurotrophins. Int Rev Cytol 197:203–277
Friedman WJ, Black IB, Kaplan DR (1998) Distribution of the neurotrophins brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the postnatal rat brain: an immunocytochemical study. Neuroscience 84(1):101–114. doi:S0306-4522(97)00526-5 [pii]
Duman RS, Heninger GR, Nestler EJ (1997) A molecular and cellular theory of depression. Arch Gen Psychiatry 54(7):597–606
Russo-Neustadt AA, Chen MJ (2005) Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des 11(12):1495–1510
Allaman I, Belanger M, Magistretti PJ (2011) Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci 34(2):76–87. https://doi.org/10.1016/j.tins.2010.12.001
Mercier G, Lennon AM, Renouf B et al (2004) MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci 24(2):207–216. doi:JMN:24:2:207 [pii]
Tsuchioka M, Takebayashi M, Hisaoka K et al (2008) Serotonin (5-HT) induces glial cell line-derived neurotrophic factor (GDNF) mRNA expression via the transactivation of fibroblast growth factor receptor 2 (FGFR2) in rat C6 glioma cells. J Neurochem 106(1):244–257. https://doi.org/10.1111/j.1471-4159.2008.05357.x
David DJ, Wang J, Samuels BA et al (2010) Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist 16(5):578–591. https://doi.org/10.1177/1073858409360281
Rocha BA, Scearce-Levie K, Lucas JJ et al (1998) Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature 393(6681):175–178. https://doi.org/10.1038/30259
Selemon LD, Lidow MS, Goldman-Rakic PS (1999) Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry 46(2):161–172. doi:S0006-3223(99)00113-4 [pii]
Czeh B, Simon M, Schmelting B et al (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31(8):1616–1626. doi:1300982 [pii]
Galeotti N, Ghelardini C (2012) Regionally selective activation and differential regulation of ERK, JNK and p38 MAP kinase signalling pathway by protein kinase C in mood modulation. Int J Neuropsychopharmacol 15(6):781–793. https://doi.org/10.1017/S1461145711000897
Yuan P, Zhou R, Wang Y et al (2010) Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord 124(1–2):164–169. https://doi.org/10.1016/j.jad.2009.10.017
Gourley SL, Wu FJ, Kiraly DD et al (2008) Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry 63(4):353–359. doi:S0006-3223(07)00713-5 [pii]
Acknowledgements
The authors of this chapter were supported by the Slovak Academy of Sciences (SAS; 2013 Scholarship Award), by the Scientific Grant Agency of Ministry of Education of Slovak Republic and SAS (grants VEGA-2/0019/15 and VEGA-2/0024/15) and by the Slovak Research and Development Agency (Grants APVV-15-0388).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Moravčíková, L. et al. (2018). Role of Serotonin-2A Receptors in Pathophysiology and Treatment of Depression. In: Guiard, B., Di Giovanni, G. (eds) 5-HT2A Receptors in the Central Nervous System. The Receptors, vol 32. Humana Press, Cham. https://doi.org/10.1007/978-3-319-70474-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-70474-6_9
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-70472-2
Online ISBN: 978-3-319-70474-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)