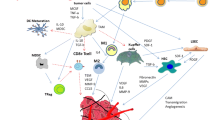
Abstract
A hallmark of aggressive hepatocellular carcinomas (HCCs) is the ability to metastasize. Metastatic lesions are difficult to manage in clinical practice as the extent of disease typically precludes curative resection and resistance to systemic treatments is common [1, 2]. Metastasis is a multistage process in which cancer cells (1) delaminate from the primary site and locally invade the host stroma (initiation), (2) enter into blood and/or lymphatic vasculature (intravasation), (3) survive and exit the circulation into distant sites (extravasation), and (4) colonize the new microenvironment and proliferate to form a macroscopic secondary tumor (colonization) [3, 4]. This simplified model provides a framework for a sequence of biological properties that must be acquired during cancer dissemination. Different malignancies, however, demonstrate unique regulatory events in this process largely governed by the complex microenvironment in which the metastatic cells originate and that of the distant location(s) where the metastasis is established [5]. Given the anatomic and physiologic complexity of the liver, the HCC microenvironment is one of the most difficult to reproduce experimentally and, consequently, understand in its totality. In the context of this chapter, HCC metastasis is discussed, with an emphasis on the role of the hepatic microenvironment and the role of circulating cancer cells in the metastatic process.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Circulating Tumor Cells (CTCs)
- Hepatic Microenvironment
- Tumor-associated Fibroblasts (TAFs)
- Hepatic Stellate Cells (HSCs)
- Cancer Stem Cells (CSCs)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
A hallmark of aggressive hepatocellular carcinomas (HCCs) is the ability to metastasize. Metastatic lesions are difficult to manage in clinical practice as the extent of disease typically precludes curative resection and resistance to systemic treatments is common [1, 2]. Metastasis is a multistage process in which cancer cells (1) delaminate from the primary site and locally invade the host stroma (initiation), (2) enter into blood and/or lymphatic vasculature (intravasation), (3) survive and exit the circulation into distant sites (extravasation), and (4) colonize the new microenvironment and proliferate to form a macroscopic secondary tumor (colonization) [3, 4]. This simplified model provides a framework for a sequence of biological properties that must be acquired during cancer dissemination. Different malignancies, however, demonstrate unique regulatory events in this process largely governed by the complex microenvironment in which the metastatic cells originate and that of the distant location(s) where the metastasis is established [5]. Given the anatomic and physiologic complexity of the liver, the HCC microenvironment is one of the most difficult to reproduce experimentally and, consequently, understand in its totality. In the context of this chapter, HCC metastasis is discussed, with an emphasis on the role of the hepatic microenvironment and the role of circulating cancer cells in the metastatic process.
9.2 The Dynamic Interaction Between Tumor Cells and the Tumor Microenvironment in HCC
Hepatocellular carcinoma is typically observed clinically as a consequence of chronic inflammation associated with cirrhosis. The etiology of cirrhosis is commonly due to alcoholism, HBV/HCV infection, or metabolic disorders, all of which create a tumor-permissive milieu [6]. Hepatocellular carcinoma is an extraordinarily unique malignancy, in which tumorigenesis and progression are significantly regulated not only by the intrinsic properties of tumor cells but also by constant communication with a heterogeneous microenvironment. Cumulative evidence suggests that the dynamic interaction between tumor cells and their surrounding milieu plays fundamental roles in the initiation of metastatic phenotypes at the primary site [7,8,9,10]. This interaction is dynamic, constantly evolving with tumor development. For example, the microenvironment may exert inhibitory effects in early stages. When tumor cells reach a certain point during their progression, they can circumvent these inhibitory signals and actually exploit surrounding nonmalignant cells to support metastasis [5]. The surrounding milieu within the HCC microenvironment may consist of (1) hepatic stellate cells, stromal cells, endothelial cells, and immune cells and (2) growth factors, inflammatory cytokines, and extracellular matrix proteins [11,12,13,14]. In this review, the intermingled contribution of the tumor microenvironment to HCC metastasis is emphasized from the perspective of tumor-associated inflammation and immune responses.
9.2.1 Contribution of Distinct Inflammatory Components to the Progression of HCC Metastasis
Direct evidence of the interplay between the hepatic microenvironment and HCC metastasis is evidenced by a comprehensive analysis of global gene expression profiling from the National Cancer Institute [15,16,17]. In this investigation, the gene expression profiles of nonmalignant hepatic tissue surrounding HCC tumors from patients with intra- or extrahepatic metastases were compared to those with no detectable metastasis. Peripheral stroma associated with HCC metastasis demonstrated a unique gene expression profile when compared to tissue associated with isolated HCC lesions. Importantly, this profile is also significantly different from the intratumoral signature. More specifically, the pattern of inflammatory cytokine expression was also unique in HCC patients with venous metastasis, suggesting that cytokines may contribute to the metastatic process. These data strongly suggest that the metastatic potential HCC may be influenced by the inflammatory response of the host microenvironment.
9.2.1.1 Hepatic Stellate Cells
The hepatic stellate cell (HSC), first described by Kupffer in the nineteenth century, has emerged in the past 25 years as a remarkably versatile mesenchymal cell with vital functions not only in liver injury but also in hepatic development, regeneration, xenobiotic responses, metabolism, and immune regulation [13, 18,19,20]. Equally intriguing is the remarkable plasticity of stellate cells. Stellate cells can be viewed as the nexus in a complex sinusoidal milieu that requires tightly regulated autocrine and paracrine cross talk, rapid responses to evolving extracellular matrix content, and exquisite responsiveness to the metabolic needs imposed by liver growth and repair [21, 22]. Moreover, stellate cells maintain systemic homeostasis through storage and mobilization of retinoids, antigen presentation and the induction of immune tolerance, as well as an emerging relationship with bone marrow-derived cells [22, 23]. In the tumor milieu, HSCs undergo a transition from the “quiescent” to “activated” state. Upon activation, HSCs infiltrate malignant hepatic tissue and localize around tumor sinusoids, adjacent fibrous parenchyma, and the tumor capsule [24, 25]. Activated HSCs have also been identified in the periphery of dysplastic hepatic nodules [26]. For tumor-associated HSCs, the restricted control of their function in regulating fibrotic matrix decomposition and degradation is disrupted, leading to the uninhibited production of extracellular matrix (ECM) proteins [20, 23, 27]. As a major source of ECM proteins, HSCs may therefore stimulate HCC metastasis via the regulation of tumor-stroma during the epithelial-to-mesenchymal transition, a process required for metastasis.
Although HSCs are considered central to the stimulation of a pro-metastatic microenvironment in HCC, the molecular mechanisms underlying this modulation are poorly understood. Unsupervised genome-wide expression profiling confirmed that the genes associated with cross talk between tumor cells and HSCs were significantly enriched in cirrhotic tissues from patients with metastasis [25]. These gene expression profiles, which are discussed in detail in subsequent sections, have the capability to activate inflammatory programs, which in turn contribute to tumor progression and metastasis.
Transforming growth factor-β (TGF-β) is secreted by both HSCs and hepatocytes and plays a multifunctional role in HCC pathogenesis [28, 29]. Tumor suppressor functions are observed in the early stages of liver damage and regeneration. Alternatively, during cancer progression, TGF-β may stimulate tumor invasiveness and metastatic behavior [30, 31]. TGF-β modulates the malignant properties of HCC not only through its own canonical signaling cascade but also via cross talk with many other growth factor pathways. Data from murine HCC models and three-dimensional, micro-organoid in vitro models reported by van Zijl et al. suggest a crucial role for the TGF-β/PDGF signaling axis in guiding epithelial-to-mesenchymal transition at the invasive front [32]. In a recent study, Park et al. identified tissue inhibitor of metalloproteinases-1 (TIMP-1) as one of the secreted proteins of HSCs and a key component of TGF-β-mediated cross talk between HSC and HCC cells. TGF-β stimulation led to increased expression of TIMP-1, which activated focal adhesion kinase (FAK) signaling via its interaction with CD63. Inhibition of TGF-β signaling using EW-7197, a small-molecule inhibitor of the TGF-β type I receptor kinase, abrogated TGF-β-mediated epithelial-to-mesenchymal transition in vitro using HCC cell lines and attenuated intrahepatic metastasis of HCC in an orthotopic xenograft mouse model using SK-HEP1-Luc cells [33].
Integrins, consisting of an α- and β-subunit, belong to a family of transmembrane receptors that integrate the extracellular and intracellular environment through binding both the ECM and the cytoskeleton [34]. Via transduction of signals between the internal and external cellular domains, integrins regulate cell adhesion, spreading, migration, proliferation, and differentiation as well as ECM deposition and remodeling [35]. In activated HSC, downstream integrin signaling, via the focal adhesion kinase (FAK)-phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway, promotes ECM deposition [36]. Integrin subunits α6 and β1 expression in human HCC tissue demonstrated a positive correlation metastasis [37]. These integrins can coordinate with other key signaling components, including but not limited to SERPINA5, CD151, PI3K-Akt, and TGF-β, to facilitate tumor invasion and metastasis properly via epithelial-to-mesenchymal transition [38,39,40,41].
A significant increase in Th2 cytokines (e.g., IL-4, IL-8, IL-10, and IL-5) and a concomitant decrease in the pro-inflammatory Th1 cytokines (e.g., IL-1α, IL-1β, IL-2, IL-12p35, IL-12p40, IL-15, and non-ILs, e.g., TNF-α and IFN-γ) were also found in livers associated with metastatic HCC, compared to normal samples. Such a profound Th1 to Th2 profile switch is unique to hepatic tissues from patients with HCC metastasis. This change is not related to the degree of viral hepatitis or cirrhosis, but it is a consequence of tumor burden [9, 15]. The findings strongly imply that an anti-inflammatory cascade, which is likely initiated/upregulated by HSCs, presents and promotes HCC metastasis.
9.2.1.2 Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) reside predominantly in the bone marrow, although they are not of hematopoietic origin. MSCs are multipotent cells that differentiate into osteoblasts, chondrocytes, adipocytes, and other cells of mesenchymal origin. In response to inflammation, MSCs are recruited to sites of tissue injury to participate in tissue remodeling and wound healing. The chronic inflammation observed in HCC leads to the local accumulation of MSCs in the liver. Current evidence suggests that tumor-infiltrating MSCs may influence the behavior of neighboring cancer cells [14]. The specific role of MSCs in HCC metastasis remains unclear. Upon co-culture with conditioned medium from TGF-β1-overexpressing MSCs with HCC cell lines having high (MHCC97-H) or low (MHCC97-L) metastatic potential, Li et al. showed that MSCs promote the proliferation of HCC cells and TGF-β1 signaling and inhibit cell migration and thus decrease metastatic potential. Inhibition of metastasis in this manner may be explained by the downregulation of osteopontin (OPN) in HCC cells after co-culture with TGF-β1 overexpressing MSC-conditioned medium [42]. Alternatively, MSCs pretreated with pro-inflammatory cytokines (IFN-γ and TNF-α) facilitate epithelial-to-mesenchymal transition of HCC cells, possibly through upregulation of TGF-β1 [43]. These findings reinforce the complexity associated with tumor-stromal signaling and illustrate the influence stromal signaling can have over tumor metastasis.
9.2.1.3 Tumor-Associated Fibroblasts
Tumor-associated fibroblasts (TAFs) are the prominent cell type in HCC microenvironment and play a critical role in tumor-stroma interaction. The origin of TAFs remains unclear. TAFs specifically promote tumor growth, angiogenesis, and metastasis, in part through secretion of high levels of stromal cell-derived factor 1 (SDF1 or CXCL12), properties that render these cells unique from normal fibroblasts [44]. Mazzocca and coworkers showed that HCC cell growth, intravasation, and metastatic spread are dependent upon the presence of CAFs, and HCC cells reciprocally stimulate proliferation of CAFs, suggesting a key role for CAFs in tumor-stromal interaction [40]. There is a complex cross talk between TAFs and tumor cells. For instance, both can secrete PDGF and TGF-β, which leads to stellate cell activation and consequently ECM deposition and also enhances the growth and migration of cancer cells [40]. TAFs interact with the microvasculature by secreting VEGF and MMPs as well as several hepatocyte growth factors such as HGF [45]. TAFs also secrete immune-modulatory cytokines (IFN-γ, IL-6, and TNF) that can mobilize lymphocytes, natural killer cells, and tumor-associated myeloid cells [46,47,48].
9.2.2 The Signature Roles of Immune Components to the Facilitation of HCC Metastasis
It is widely accepted that immune cells are recruited to the tumor site in response to the chronic inflammatory microenvironment of HCC [9, 11, 49]. In response to the local inflammatory response, at some point, cancer cells evolve mechanisms of immune escape. Evidence continues to accumulate implicating the local immune microenvironment of HCC as one of tolerance [50]. Specifically, a defined expression signature containing 17 immune genes (12 cytokines, HLA-DR, HLA-DPA, ANXA1, PRG1, and CSF1) has recently been validated to evaluate local immune suppression [15, 16]. This set of genes serves as a key orchestrator of the intricate dialogue between infiltrating immune cells and cancer cells. Budhu et al. demonstrated that this immune-related gene panel could successfully predict both venous metastases and extrahepatic metastases by follow-up with more than 92% accuracy. The prognostic performance of this signature was superior to and independent of any clinicopathologic variables, including age, tumor size, microvascular invasion, level of α-fetoprotein and/or albumin, Child-Pugh score for cirrhosis mortality, as well as several staging systems (TNM, CLIP, BCLC, and Okuda) [15]. In the following section, the orchestrated action of these inflammatory genes in regulating HCC metastasis is discussed with specific emphasis on tumor-infiltrating lymphocytes.
9.2.2.1 T Cells
The majority of tumor-infiltrated lymphocytes in solid tumors are of CD3+ T cells. They can be further stratified into CD4+ helper T cells; among this subset is the CD4+ regulatory T cell (Treg) and CD8+ cytotoxic T cells. T cells can exert either or both tumor-suppressive and tumor-promoting properties [51, 52]. Pathologic skewing of T cells in the tumor microenvironment can suppress antitumor immune responses and is defined as another key regulator in HCC progression. Accounting for 5–10% of all CD4+ T cells, Tregs are thought to be protumorigenic via the suppression of antitumor immune responses [53]. In HCC, tumor-infiltrating CD4+CD25+forkhead box P3+ (FoxP3) Tregs impair the cytotoxic activity of CD8+ T cells while suppressing the proliferation of IFN-γ secretingCD4+CD25− T cells [54]. In other study, Gao et al. showed that the ratio of intratumoral CD45RO+ to peritumoral CD57+ (memory/senescent) T cells serves as a negative predictor of HCC extrahepatic metastasis [55, 56].
Although the role of T cells in HCC metastasis continues to be investigated, a likely contribution is the array of inflammatory mediators secreted by activated lymphocytes. For example, serum levels of IL-6 were high in metastatic HCC [57] and were able to distinguish primary or metastatic liver tumors from benign HCC lesions [58]. Furthermore, serum levels of the pro-inflammatory cytokines TNF-α and IL-1β were high in HCC prior to resection compared with healthy individuals [59, 60]. In another study, higher levels of IL-1β and TNF-α were found in the tissue surrounding hepatic metastases than within the primary HCC tumor [11]. High expression of IL-8 (or CXCL8), a chemokine with angiogenic action, in malignant hepatic tissue was also associated with a higher frequency of portal vein, venous, and bile duct invasion in HCC patients undergoing operative resection and may therefore be important in invasion and metastasis [15]. Interestingly, Wang et al. demonstrated type I interferon-mediated angiogenesis inhibition by downregulating vascular endothelial growth factor (VEGF) and thus inhibiting metastasis in an HCC xenograft model with high metastatic potential [61].
9.2.2.2 Tumor-Associated Macrophages
In addition to T cells, tumor-associated macrophages (TAMs) are commonly found in the tumor microenvironment of many types of cancer. TAMs play a major role in mediating the cross talk between cancer and stromal cells, promoting tumor cell proliferation, and stimulating angiogenesis, invasion, and metastasis [62]. In HCC, TAMs are recruited to the tumor milieu, residing predominately in the peritumoral region, by a cascade of growth factors and chemokines secreted by cancer cells [63]. Soluble mediators promoting TAM recruitment include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), transformation growth factor-β (TGF-β), chemokine (C-C motif) ligand (CCL2), and macrophage colony-stimulating factor (M-CSF) [64, 65]. The expression of glypican-3 (GPC-3) on the surface of HCC cells may also promote TAM recruitment [66]. In human HCC, the majority of TAMs are polarized toward an M2 phenotype, characterized by poor antigen-presenting capability and the secretion of a distinct set of cytokines/chemokines (e.g., IL-10, TGF-β, CCL17, CCL22, CCL24, etc.) that interact with their receptors expressed mainly by Th2 and Treg cells, promoting the recruitment of these ineffective T cell subsets [67]. In the context of HCC metastasis, extensive macrophage infiltration and increased levels of M-CSF have been associated with intrahepatic metastasis and recurrence [68, 69]. Moreover, pharmacological approaches to directly target TAMs, via knocking out CCL2 or other TAM-specific chemokines, reduced migration and invasion of HCC cell lines [70].
The cross talk between tumor-associated macrophages and cancer cells in mediating HCC metastasis is conducted through various TAM-secreted factors and signaling pathways. Increased expression of CXCL12 and its corresponding receptor CXCR4, a particularly well-studied chemokine signaling axis, is associated with lymphatic metastasis in HCC patients [71]. This CXCL12/CXCR4 axis stimulates the growth, invasion, and metastasis of HCC cell lines, in part through enhancing the secretion of matrix metalloproteases (MMPs) 2 and 9 [72]. Upregulation of TAM-secreted IL-8 and its receptor CXCR2 have also been associated with intrahepatic metastasis of HCC [73]. In addition to chemokine signaling, TAM-derived growth factors also play a role in metastasis. Among these, TGF-β is well known for its role in tumor growth and metastasis. In HCC, TGF-β induces epithelial-to-mesenchymal transition through stimulation of the E- to N-cadherin switch, a signature event required for EMT, by upregulating Snail, an E-cadherin repressor, and PDGF signaling pathway [32, 74]. TGF-β can also affect α3β1 integrin, SMAD-2, and focal adhesion kinase (FAK), all of which are known to regulate tumor invasiveness [75]. All of these functions are regulated through TGF-β cross talk with other signaling cascades, such as FAK, PDGFR, STAT, HIF, etc. In addition to TGF-β signaling, osteopontin (OPN), a phosphorylated acidic glycoprotein which was found to be expressed in macrophages after liver injury [76], also contributes to HCC invasion and metastasis via the interaction with integrins [76]. OPN plasma levels were found increased in HCC patients and were associated with reduced liver function and worse prognosis [77]. Neutralizing OPN by anti-OPN antibodies resulted in strong inhibition of invasion and metastasis of HCC cells in vitro and in vivo [78].
9.3 Circulating Tumor Cells: The Foundation of Cancer Dissemination in HCC Metastasis
A highly heterogeneous subpopulation of cancer cells, termed circulating tumor cells (CTCs), is able to physically translocate from the primary tumor site to the peripheral circulation [79,80,81]. Historically, the presence of tumor cells in the peripheral circulation of cancer patients was first reported by Thomas Ashworth in 1869 [82]. Not long after, in 1889, Stephen Paget proposed the “seed and soil” hypothesis to explain the nonrandom pattern of metastasis to visceral organs and bones [83]. The presence of CTCs in the bloodstream of patients with epithelial cancers fits very well with this theory and has contributed to our understanding of cancer pathogenesis and metastasis [81]. Circulating tumor cells have frequently been observed in patients undergoing surgical resection or liver transplantation for HCC [84]. Although it remains unclear as to where these cells fall on the spectrum of primary to metastatic tumor cells, CTCs do appear to represent the link between a localized primary tumor and metastatic lesion. During the metastatic process, cancer cells must acquire traits to degrade and invade through the extracellular matrix of the surrounding tissue toward blood and lymphatic vessels [4]. The presence of CTCs correlates with the extent of metastatic burden, aggressive disease, and reduced time to progression [85]. An investigation by Vona et al. in 2004 detected CTCs in 52% of blood samples using the ISET (Isolation by Size of Epithelial/Throphoblastic Tumor cells) platform, from 44 HCC [86]. In other independent study, Xu et al. showed that CTCs were detected in more than 80% of HCC patients but not in healthy person or patients with benign liver diseases [87]. Fan et al. further demonstrated that CTCs were reduced in the peripheral blood of HCC patients after resection. Moreover, patients with greater than 0.01% CTCs in their blood were at a significantly elevated risk of disease progression and metastasis [88]. Taken together, these findings suggest that CTCs serve as a major contributor to HCC recurrence and metastasis.
Physically, circulating tumor cells 20–30 μm in diameter are far too large to pass through pulmonary capillary beds [86]. Theoretically, within minutes of being released by primary tumors into the venous circulation, CTCs should be trapped in these capillaries during their first pass through the heart. In addition, CTCs have a limited half-life (1–2.4 h) in the circulation, possibly due to circulatory shear stress or the loss of matrix-derived survival signals (anoikis) [89]. Based on these points, it would be logical to assume that only exceptionally small or physically plastic CTCs can transition through pulmonary microvasculature, thereby successfully surviving in the circulation to colonize a particular organ [3, 90]. Molecular analyses of CTCs demonstrate a heterogeneous gene expression pattern. CTCs have been generally recognized as negative for CD45 (a hematopoietic marker) and positive for extracellular epithelial cell adhesion molecule (EpCAM) and intracellular cytokeratins specific to epithelial cells (CK8/18/19), all of which have become “gold standard” markers for the detection of CTCs with an epithelial phenotype in patients with cancer [91, 92]. The reliance on epithelial markers to detect CTCs poses another challenge, in that tumor cells tend to lose epithelial characteristics and acquire mesenchymal features in order to metastasize [93]. Indeed, recent studies have shown that CTCs also expressed mesenchymal markers, such as Vimentin and N-cadherin [94,95,96]. The discrepancy in CTC marker expression suggests that CTCs represent a heterogeneous population that includes cells that have lost or are losing epithelial markers and that have undergone or are undergoing the EMT. It is reasonable to suggest that this is a dynamic event during which cells gradually lose their epithelial features to acquire other features that enable them to extravasate into circulation and then undergo the reverse process, which allows the cells to travel to the site of metastasis [81].
The lack of specific surface hepatic markers challenges CTC detection in HCC, which in turn limits investigation of this cell type. To date, there are only a few studies that have been conducted to identify and characterize these cells. Similar to CTCs derived from other tumors, HCC-derived CTCs were detected with mesenchymal as well as epithelial markers [97,98,99]. Compared with epithelial CTCs detected by the conventional markers EpCAM and cytokeratin, the high rates of EMT-associated CTCs correlated with poor prognosis in patients with hepatocellular carcinoma [84, 100,101,102]. A prospective study of 46 patients with liver cancer showed the EMT markers TWIST1 and Vimentin in 84.8% and 80.4% of those patients’ CTC samples, respectively [103]; tumor progression correlated with the presence of mesenchymal CTCs in those patients. In addition, some markers for cancer stem cells, such as CD44, CD133, CD90, or ICAM, were also detected in HCC CTCs [104]. Altogether these findings raise the possibility that significant overlap may exist between CTCs, cancer stem cells, and HCC cells that underwent EMT. Despite these complexities in the molecular expression of CTCs, a thorough understanding of the biology of CTCs in the metastatic process nonetheless offers the prospect of creating a highly useful diagnostic and prognostic surrogate measure. In this review, the discussion of CTCs emphasizes two major aspects: (1) the relationship between circulating tumor and cancer stem cells and (2) the molecular linkage between these subpopulations – the epithelial-to-mesenchymal transition.
9.3.1 Relationship Between Circulating Tumor Cells and Cancer Stem Cells
Found in many types of cancer, cancer stem cells (CSCs) are a rare subpopulation that possesses the ability to generate new tumors that consistently recapitulate the morphology of original tumors. Following the hierarchical hypothesis of CSC clonal evolution, these cells are able to self-renew and generate differentiated in a similar manner to normal stem cells [105,106,107]. CTCs appear to have a critical role in metastasis. The motility, invasiveness, and heightened resistance to apoptosis are instrumental for metastatic initiation, while the self-renewal and tumor-initiating ability are critical for successful metastatic establishment [108].
9.3.1.1 The Expression of CSC Markers in CTCs
Although it may not be specific for CSCs of HCC origin, commonly reported markers include EpCAM, CD133, CD90, CD44, and CD24 [84, 102, 109,110,111]. Most of these markers are expressed in normal hepatic progenitors and are known as oncofetal markers [107]. Tumor cells expressing these markers have demonstrated heightened tumorigenicity and metastasis using HCC cell lines and primary HCC models [98, 112]. These common CSC markers were also detected in the CTC subpopulation within the peripheral blood of HCC patients [87]. A recent prospective study by Fan et al. revealed a strong correlation between the number of cancer stem cells (CD45−/CD90+/CD44+) in the blood and post-hepatectomy intrahepatic recurrence and lower recurrence-free survival of HCC. Further, circulating CSCs >0.01%, tumor stage, and tumor size were all independent risk factors in predicting recurrence-free survival [88]. In another investigation, the Cell Search System was developed to examine preoperative blood samples from 123 HCC patients. Here, Sun et al. detected at least one EpCAM+ CTC in 66.7% of the samples, among which 41.5% had at least two EpCAM+ CTCs, and further determined that preoperative detection of at least two CTCs was an independent risk factor for postoperative recurrence [101]. The same group also reported expression of the CSC biomarkers CD133 and ABCG2 in EpCAM+ CTCs from 82 patients with HCC. A study by Schulze et al. reported at least one EpCAM+ CTC in 30.5% of HCC patients and in 5.3% of patients with cirrhosis, demonstrating a strong correlation between EpCAM+ CTCs and survival [100]. Furthermore, Liu et al. identified 30 out of 60 patients with greater than 0.157% circulating CD45− ICAM-1+ tumor cells. Again, these patients demonstrated significantly shorter disease-free and overall survival [113]. These investigations suggest that CTCs with stem cell-like phenotypes may represent a subset of CTCs with a more aggressive phenotype, leading to early recurrence, metastasis, and reduced overall survival.
9.3.1.2 Tumor Self-Seeding: A CSC Trait of CTCs
CTCs can be detected within the bloodstream of the overwhelming majority of carcinoma patients, including those who develop few, if any, overt metastases; however, less than 0.01% of tumor cells that enter into systemic circulation ultimately develop into macroscopic metastases [114, 115]. It has been suggested that CTCs may exist in the latent form in a state of pre-metastasis, defined as the time between primary tumor diagnosis and clinically detectable metastasis [116]. In breast cancer, malignant cells that disseminate early can reside as single cells or as micro-metastatic clusters, as shown in studies of bone marrow samples from patients without overt metastatic disease [117, 118]. These CTCs either lack the ability to colonize or are successful colonization is prevented, possibly by host environmental factors. In order to acquire a micro- to macro-metastatic switch, latent CTCs must have the capability to reinitiate a tumor. The development of macro-metastases is a manifestation of a so-called tumor self-seeding process. This unique cancer stem cell phenotype has been well demonstrated by Kim et al. in experimental mouse models of breast carcinoma, colon carcinoma, and malignant melanoma [119]. In these models, tumor masses become readily seeded by CTCs derived from separate tumors, metastatic lesions, or direct inoculation. Tumor self-seeding selects for highly aggressive CTCs, as evidenced by the consistent observation that metastatic cells are more efficient as seeders than their parental populations. Interestingly, tumor self-seeding in mice carrying high numbers of CTCs was not associated with de novo tumor formation in orthotopic sites, suggesting that self-seeding requires tumor-derived signals. Kim et al. further implicate IL-6 and IL-8 as tumor-derived attractants of CTCs in breast carcinoma and melanoma models, in agreement with other models of tumor cell chemotaxis and metastasis. High serum levels of IL-6 correlate with poor prognosis in breast, colon, and lung cancer [120,121,122], and high expression of IL-8 in metastatic melanoma is associated with tumor burden [123, 124]. Inflammatory cells recruited to the tumor site can also be sources IL-6 [125, 126]. Thus, stromal and cancer cell-derived factors may contribute to tumor self-seeding process of circulating tumor cells [119].
9.3.1.3 The Origin of CTCs
Despite advances in our understanding of the relationship between circulating tumor cells and clinical outcomes, the mechanistic role CTCs play in metastatic dissemination remains unclear. This is due, in part, to the limited sensitivity associated with current technical approaches as well as the lack of efficient in vitro and/or in vivo models to detect and characterize this rare subpopulation. One of the most fundamental, yet unanswered, questions relates to the origin of CTCs. Both molecular expressing patterns and functional properties of CSCs within CTC subpopulations point to possible overlap, implying that CTCs may actually represent CSCs in the circulation. Functionally, however, not all CTCs are able to form ectopic metastatic lesions, as would be expected of CSCs. In mouse models involving portal vein cell injections, only 2.5% of CTCs were able to establish metastatic foci, and 1% of micro-metastases progressed to a macro-metastatic tumor at day 13 after injection [127]. Therefore, among the heterogeneous population of CTCs, only a small subset is capable of successfully metastasizing. This subpopulation of CTCs associated with CSC properties has recently been defined as “circulating tumor stem cells” (CTSCs). The origin of circulating tumor stem cells has not been established to date. In a recent review, Yang et al. proposed two non-exclusive hypotheses for the derivation of CTSCs. First, circulating and thus metastatic cancer stem cells already arise in the primary tumor as cancer stem cells with additional features rendering them capable of evading the primary tumor, surviving in the bloodstream, and subsequently initiating metastasis. Second, circulating cancer stem cells may actually arise from previously disseminated tumor cells, e.g., out of a state of dormancy at a distant site after escape from the primary tumor. Importantly, CTCs must survive the hostile environment of the peripheral blood, evade immune surveillance, and extravasate at a distant location. These features are certainly not present in all CTCs [128]. While both hypotheses are reasonable, neither has been validated conclusively to date. Consistent with the hypothesis that circulating cancer stem cells are already present in primary tumors, primary tumor cells bearing CSC markers are able to form distant metastases when transplanted into a secondary host [129, 130]. As cancer stem cells are also associated with the functional plasticity required to transition between mesenchymal-like and epithelial-like states, these cells are a likely source of metastasis.
9.3.2 Epithelial-to-Mesenchymal Transition: A Hallmark of Metastasis That Links Circulating Tumor Cells and Cancer Stem Cells
Recent investigations have suggested that circulating tumor cells (CTCs) manifest phenotypes associated with both CSCs and epithelial-mesenchymal transition (EMT). Compelling evidence indicates that cancer cells are endowed with invasive characteristics through the EMT, which is a complex process leading to the loss of epithelial features and gain of mesenchymal traits via cellular rearrangement of junctional proteins and eventually the loss of cell adhesion. This transition enables the tumor cells to acquire migratory and invasive abilities, which facilitates metastasis through intravasation from the primary tumor site to the vascular system and extravasation to the secondary location [3, 43, 131]. However, EMT is often transient and reversible. Reestablishment of micro-metastasis in the distant sites requires a reversal process, termed the mesenchymal-to-epithelial transition (MET), by which cells regain epithelial characteristics necessary for further colonization [3]. Thus, the EMT-MET transition processes are critical to metastasis in all cancer cells.
9.3.2.1 The Evidences of EMT Phenotype in CTCs
The expression of EMT-related proteins (Vimentin, N-cadherin, and TWIST1) has been documented in CTCs obtained from patients with breast, lung, colon cancer as well as hepatocellular carcinoma [97, 132,133,134,135]. Li et al. isolated CTCs from 46 of 60 (76.7%) HCC patients, and immunofluorescence staining detected TWIST1 and Vimentin expression in CTCs obtained from 39 (84.8%) and 37 (80.4%) of the 46 patients, respectively. The expression of both TWIST1 and Vimentin in CTCs significantly correlated with portal vein tumor thrombus. Co-expression of TWIST1 and Vimentin in CTCs could be detected in 32 (69.6%) of the 46 patients and correlated strongly with portal vein tumor thrombus, TNM classification, and tumor size. Western blot analysis revealed that the expression levels of E-cadherin, Vimentin, and TWIST1 in primary HCC tumors were correlated with marker positivity in isolated CTCs (P = 0.013, P = 0.012, P = 0.009, respectively). However, there was no significant difference in ZEB1, ZEB2, snail, and slug expression levels in CTCs, primary HCC tumors, and adjacent hepatic tissue across samples with regard to clinicopathological parameters [136].
The contribution of EMT to CTC properties may be regulated through inducers of EMT and/or the mechanisms of signal transduction that facilitate this process. For example, TGF-β1 induces EMT in HCC by activating a cascade of signaling mechanisms including the platelet-derived growth factor (PDGF), cyclooxygenase-2, and phosphatidylinositol-2/Akt (PI3K/Akt) signaling pathways [30, 38, 131, 137]. Additionally, Ogunwobi et al. demonstrated that CTCs display phenotypic evidence of having undergone EMT, which appears to be inducible by HGF, using murine CTC cell lines established from an HCC model of BNL 1MEA.7R.1. CTCs are highly enriched for the expression of HGF and its receptor c-Met compared to the parental 1MEA cells. Moreover, upregulation of HGF and c-Met may be the consequence of a loss of DNA methylation in six CpG islands at the c-Met promoter [138].
Lee et al. reported an interesting role for TM4SF5, a transmembrane 4 L six family member 5, which is highly expressed in hepatic cancers and stimulates metastasis by enhancing cellular migration. Here, a novel TM4SF5/CD44 interaction mediated self-renewal and circulating tumor cell (CTC) capacities. TM4SF5-dependent sphere growth correlated with CD133+, CD24−, and ALDH activity and a physical association between CD44 and TM4SF5. In serial xenografts of less than 5000 cells per injection, TM4SF5-positive tumors exhibited increased CD44 expression, suggesting tumor cell differentiation. TM4SF5-positive cells were identified circulating in the blood 4–6 weeks after orthotopic liver injection. Anti-TM4SF reagents reduced metastasis. Such TM4SF5-mediated properties were supported by CD133/TM4SF5/CD44 (bound to TM4SF5)/c-Src/STAT3/TWIST1/Bmi-1 signaling pathways. Suppression of CD133, TM4SF5, or CD44 or disruption of the interaction between TM4SF5 and CD44 abolished the self-renewal and circulating tumor cell properties [139]. This study therefore elucidated a critical link between EMT, CSCs, and CTCs in hepatocellular carcinoma.
9.3.2.2 Dynamic Role of EMT in Postulating Cancer Stem Cell Phenotype of CTCs
Recent clinicopathologic and experimental evidence support the coexistence of both EMT and CSC phenotypes in CTC subpopulations of patients with metastasis [132]. The reciprocal action of these three components, however, is still not fully understood. EMT has been suggested to play a key role in the formation of CTCs, which can eventually form metastatic tumors. EMT may propagate or, in some instances, even generate neoplastic epithelial cells through the acquisition of stemlike characteristics [129, 136, 140,141,142]. Indeed, Mani et al. first demonstrated that EMT was sufficient to induce a population of cells with characteristics of stem cells bearing migratory and invasive capabilities [142]. Moreover, the overexpression of EMT markers on CTCs was often accompanied by the presence of the stem cell markers, including but not limited to ALDH1, CD133, and CD44 [95, 132]. Given the fact that EMT can induce non-CSCs to enter a CSC-like state, tumor cells may resemble CSCs through this process and transition to circulating tumor stem cells with high metastatic potential. On the other hand, several studies suggested that CTCs that are “frozen” in a mesenchymal phenotype seem to be unable to form metastases [143], as tumor cell lines that are arrested in a mesenchymal state by expression of EMT-inducing proteins such as Snail, TWIST1, or ZEB1 are more invasive and easily enter the bloodstream, but they are unable to form overt metastases after homing to distant organs [93, 144,145,146]. These cells may not be able to undergo the reverse process of mesenchymal-to-epithelial transition to establish micro-metastasis.
These observations are not contradictory. They indeed provide evidence for a dynamic regulation of EMT toward CTCs in a reversible manner. Early in the metastatic cascade, EMT is responsible for inducing mesenchymal and cancer stem cell phenotypes. These are obligatory properties required for intravasation from the primary tumor and extravasation to the metastatic site of CTSCs. At a late metastatic phase after CTSC homing, EMT may be switched to MET, by which cells regain epithelial characteristics necessary for colonization and establish macro-metastasis. Despite a profound contribution to the success of metastasis, the master controller(s) of the switch between EMT and MET is not yet understood. It is possible that this EMT/MET balance is driven by intrinsic properties of tumor cells as well as extrinsic components from both primary and secondary microenvironments.
Conclusion
Metastasis formation represents the dominant rate-limiting step of the invasion-metastasis cascade. In spite of this gross inefficiency of metastatic establishment, a small minority of disseminated carcinoma cells undergoes gradual genetic and/or epigenetic evolution to acquire the adaptive traits required for metastatic colonization. The tumor cells could not execute this complicated transformation alone but through their extensive communication with the microenvironment of the primary tumor and the metastatic site. Concomitant to anatomical and hemodynamical features of the liver, hepatocellular carcinoma represents one of the most complicated tumor microenvironments with a high degree of heterogeneity in cellular and molecular components, which contributes another layer of complexity to the metastatic process. The milieu of HCC is composed of a wide variety of inflammatory cell types, all of which interact with each other and tumor cells, directly or indirectly through factors that they secrete, in order to acquire a pathologic phenotype and alter the function of tumor cells. This mutual interaction between cancer cells and host components is dynamic, constantly evolving with tumor progression. Under microenvironmental signals, tumor cells can be transformed into metastatic circulating tumor cells through the process of epithelial-to-mesenchymal transition. This intriguing group of so-called circulating tumor stem cells can acquire unique stemlike characteristics simultaneously, a prerequisite for the success of metastasis. Better understanding of the biology behind these processes is critical to develop effective strategies for early cancer detection and novel treatment approaches that can be translated into clinical practice.
References
Yeung YP, Lo CM, Liu CL, Wong BC, Fan ST, Wong J. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100(9):1995–2004.
Paschos KA, Bird NC. Liver regeneration and its impact on post-hepatectomy metastatic tumour recurrence. Anticancer Res. 2010;30(6):2161–70.
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64.
Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–84.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52.
Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma—epidemiological trends and risk factors. Dig Dis. 2009;27(2):80–92.
Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144(3):512–27.
Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Hoyer-Hansen G, et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73(7):2031–43.
Qin LX. Inflammatory immune responses in tumor microenvironment and metastasis of hepatocellular carcinoma. Cancer Microenviron. 2012;5(3):203–9.
Tu T, Budzinska MA, Maczurek AE, Cheng R, Di Bartolomeo A, Warner FJ, et al. Novel aspects of the liver microenvironment in hepatocellular carcinoma pathogenesis and development. Int J Mol Sci. 2014;15:9422–58.
Wang H, Chen L. Tumor microenvironment and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol. 2013;28(Suppl 1):43–8.
Schrader J, Iredale JP. The inflammatory microenvironment of HCC—the plot becomes complex. J Hepatol. 2011;54:853–5. England.
Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21(1):35–43.
Heindryckx F, Gerwins P. Targeting the tumor stroma in hepatocellular carcinoma. World J Hepatol. 2015;7(2):165–76.
Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111.
Budhu AS, Zipser B, Forgues M, Ye QH, Sun Z, Wang XW. The molecular signature of metastases of human hepatocellular carcinoma. Oncology. 2005;69(Suppl 1):23–7.
Roessler S, Jia H-L, Budhu A, Forgues M, Ye Q-H, Lee J-S, et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70(24):10202–12.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401.
Vidal-Vanaclocha F. The prometastatic microenvironment of the liver. Cancer Microenviron. 2008;1(1):113–29.
Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–72.
Faouzi S, Le Bail B, Neaud V, Boussarie L, Saric J, Bioulac-Sage P, et al. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol. 1999;30(2):275–84.
Dubuisson L, Lepreux S, Bioulac-Sage P, Balabaud C, Costa AM, Rosenbaum J, et al. Expression and cellular localization of fibrillin-1 in normal and pathological human liver. J Hepatol. 2001;34(4):514–22.
Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275(4):2247–50.
Thompson AI, Conroy KP, Henderson NC. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol. 2015;15:63.
Coulouarn C, Corlu A, Glaise D, Guénon I, Thorgeirsson SS, Clément B. Hepatocyte–stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 2012;72(10):2533–42.
Park YN, Yang CP, Cubukcu O, Thung SN, Theise ND. Hepatic stellate cell activation in dysplastic nodules: evidence for an alternate hypothesis concerning human hepatocarcinogenesis. Liver. 1997;17(6):271–4.
Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. 2014;34(6):834–43.
Yoshida K, Murata M, Yamaguchi T, Matsuzaki K. TGF-beta/Smad signaling during hepatic fibro-carcinogenesis (review). Int J Oncol. 2014;45(4):1363–71.
Dooley S, Weng H, Mertens PR. Hypotheses on the role of transforming growth factor-beta in the onset and progression of hepatocellular carcinoma. Dig Dis. 2009;27(2):93–101.
Papageorgis P. TGFbeta signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015:587193.
Geng ZM, Li QH, Li WZ, Zheng JB, Shah V. Activated human hepatic stellate cells promote growth of human hepatocellular carcinoma in a subcutaneous xenograft nude mouse model. Cell Biochem Biophys. 2014;70(1):337–47.
van Zijl F, Mair M, Csiszar A, Schneller D, Zulehner G, Huber H, et al. Hepatic tumor-stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene. 2009;28(45):4022–33.
Park SA, Kim MJ, Park SY, Kim JS, Lim W, Nam JS, et al. TIMP-1 mediates TGF-beta-dependent crosstalk between hepatic stellate and cancer cells via FAK signaling. Sci Rep. 2015;5:16492.
Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23(4):397–418.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22.
Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, et al. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278(10):8083–90.
Zhao G, Cui J, Qin Q, Zhang J, Liu L, Deng S, et al. Mechanical stiffness of liver tissues in relation to integrin beta1 expression may influence the development of hepatic cirrhosis and hepatocellular carcinoma. J Surg Oncol. 2010;102(5):482–9.
Ke AW, Shi GM, Zhou J, Huang XY, Shi YH, Ding ZB, et al. CD151 amplifies signaling by integrin alpha6beta1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells. Gastroenterology. 2011;140(5):1629–41.e15.
Giannelli G, Fransvea E, Marinosci F, Bergamini C, Colucci S, Schiraldi O, et al. Transforming growth factor-beta1 triggers hepatocellular carcinoma invasiveness via alpha3beta1 integrin. Am J Pathol. 2002;161(1):183–93.
Fransvea E, Mazzocca A, Antonaci S, Giannelli G. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology. 2009;49(3):839–50.
Jing Y, Jia D, Wong CM, Oi-Lin Ng I, Zhang Z, Liu L, et al. SERPINA5 inhibits tumor cell migration by modulating the fibronectin-integrin beta1 signaling pathway in hepatocellular carcinoma. Mol Oncol. 2014;8(2):366–77.
Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren N, et al. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97-H cell line. Cancer Sci. 2010;101(12):2546–53.
Jing Y, Han Z, Liu Y, Sun K, Zhang S, Jiang G, et al. Mesenchymal stem cells in inflammation microenvironment accelerates hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition. PLoS One. 2012;7(8):e43272.
Song T, Dou C, Jia Y, Tu K, Zheng X. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget. 2015;6(14):12061–79.
Kaminski A, Hahne JC, Haddouti e-M, Florin A, Wellmann A, Wernert N. Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int J Mol Med. 2006;18(5):941–50.
Lin ZY, Chuang WL. Hepatocellular carcinoma cells cause different responses in expressions of cancer-promoting genes in different cancer-associated fibroblasts. Kaohsiung J Med Sci. 2013;29(6):312–8.
Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–31.
Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–7.
Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674–87.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22.
DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102.
Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50.
Yakirevich E, Resnick MB. Regulatory T lymphocytes: pivotal components of the host antitumor response. J Clin Oncol. 2007;25:2506–8. United States.
Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6(9):e24671.
Gao Q, Zhou J, Wang XY, Qiu SJ, Song K, Huang XW, et al. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Ann Surg Oncol. 2012;19(2):455–66.
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–93.
Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103(1):264–74.
Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–35.
Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 Suppl 1):S45–53.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801.
Wang L, Wu WZ, Sun HC, Wu XF, Qin LX, Liu YK, et al. Mechanism of interferon alpha on inhibition of metastasis and angiogenesis of hepatocellular carcinoma after curative resection in nude mice. J Gastrointest Surg. 2003;7(5):587–94.
Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–39.
Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42(1):1–7.
Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214(9–10):761–77.
Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013.
Takai H, Kato A, Kato C, Watanabe T, Matsubara K, Suzuki M, et al. The expression profile of glypican-3 and its relation to macrophage population in human hepatocellular carcinoma. Liver Int. 2009;29(7):1056–64.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96.
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26(16):2707–16.
Jia JB, Wang WQ, Sun HC, Zhu XD, Liu L, Zhuang PY, et al. High expression of macrophage colony-stimulating factor-1 receptor in peritumoral liver tissue is associated with poor outcome in hepatocellular carcinoma after curative resection. Oncologist. 2010;15(7):732–43.
Chen TA, Wang JL, Hung SW, Chu CL, Cheng YC, Liang SM. Recombinant VP1, an Akt inhibitor, suppresses progression of hepatocellular carcinoma by inducing apoptosis and modulation of CCL2 production. PLoS One. 2011;6(8):e23317.
Schimanski CC, Bahre R, Gockel I, Muller A, Frerichs K, Horner V, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95(2):210–7.
Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int J Biochem Cell Biol. 2007;39(1):197–205.
Liu Z, Yang L, Xu J, Zhang X, Wang B. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J Surg Res. 2011;166(2):241–6.
Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129(5):1375–83.
Fransvea E, Mazzocca A, Santamato A, Azzariti A, Antonaci S, Giannelli G. Kinase activation profile associated with TGF-beta-dependent migration of HCC cells: a preclinical study. Cancer Chemother Pharmacol. 2011;68(1):79–86.
Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci. 2008;103(1):4–13.
Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101(9):2051–9.
Ye Q-H, Qin L-X, Forgues M, He P, Kim JW, Peng AC, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9(4):416–23.
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8.
Lin EH, Jiang Y, Deng Y, Lapsiwala R, Lin T, Blau CA. Cancer stem cells, endothelial progenitors, and mesenchymal stem cells: “seed and soil” theory revisited. Gastrointest Cancer Res. 2008;2(4):169–74.
Scatena R, Bottoni P, Giardina B. Circulating tumour cells and cancer stem cells: a role for proteomics in defining the interrelationships between function, phenotype and differentiation with potential clinical applications. Biochim Biophys Acta. 2013;1835(2):129–43.
Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146.
Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3.
Zhang Y, Shi ZL, Yang X, Yin ZF. Targeting of circulating hepatocellular carcinoma cells to prevent postoperative recurrence and metastasis. World J Gastroenterol. 2014;20(1):142–7.
Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20(10):2553–68.
Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156(1):57–63.
Xu W, Cao L, Chen L, Li J, Zhang XF, Qian HH, et al. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res. 2011;17(11):3783–93.
Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg. 2011;254(4):569–76.
Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2(25):25ra3.
Tseng JY, Yang CY, Liang SC, Liu RS, Jiang JK, Lin CH. Dynamic changes in numbers and properties of circulating tumor cells and their potential applications. Cancers (Basel). 2014;6:2369–86.
Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF, et al. Molecular biomarker analyses using circulating tumor cells. PLoS One. 2010;5(9):e12517.
Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192(3):373–82.
Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623–31.
Velpula KK, Dasari VR, Tsung AJ, Dinh DH, Rao JS. Cord blood stem cells revert glioma stem cell EMT by down regulating transcriptional activation of Sox2 and Twist1. Oncotarget. 2011;2(12):1028–42.
Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9(8):997–1007.
Yang J, Eddy JA, Pan Y, Hategan A, Tabus I, Wang Y, et al. Integrated proteomics and genomics analysis reveals a novel mesenchymal to epithelial reverting transition in leiomyosarcoma through regulation of slug*. Mol Cell Proteomics. 2010;9:2405–13.
Burak KW, Kneteman NM. An evidence-based miltidisciplinary approach to the management of hepatocellular carcinoma (HCC): The Alberta HCC algorithm. Can J Gastroenterol. 2010;24(11):643–50.
Chow AK, Ng L, Lam CS, Wong SK, Wan TM, Cheng NS, et al. The enhanced metastatic potential of hepatocellular carcinoma (HCC) cells with sorafenib resistance. PLoS One. 2013;8(11):e78675.
Li YM, Xu SC, Li J, Han KQ, Pi HF, Zheng L, et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013;4:e831.
Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133(9):2165–71.
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57(4):1458–68.
Zhang Y, Li J, Cao L, Xu W, Yin Z. Circulating tumor cells in hepatocellular carcinoma: detection techniques, clinical implications, and future perspectives. Semin Oncol. 2012;39(4):449–60.
Christofori G. New signals from the invasive front. Nature. 2006;441(7092):444–50.
Nel I, David P, Gerken GG, Schlaak JF, Hoffmann AC. Role of circulating tumor cells and cancer stem cells in hepatocellular carcinoma. Hepatol Int. 2014;8(3):321–9.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61.
Trumpp A, Wiestler OD. Mechanisms of Disease: cancer stem cells—targeting the evil twin. Nat Clin Pract Oncol. 2008;5(6):337–47.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11.
Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–40.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73.
Woodward WA, Sulman EP. Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev. 2008;27(3):459–70.
Zhu CP, Wang AQ, Zhang HH, Wan XS, Yang XB, Chen SG, et al. Research progress and prospects of markers for liver cancer stem cells. World J Gastroenterol. 2015;21(42):12190–6.
Ma S. Biology and clinical implications of CD133(+) liver cancer stem cells. Exp Cell Res. 2013;319(2):126–32.
Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J, et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology. 2013;144(5):1031–41.e10.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92.
Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72.
Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–56.
Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68.
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802.
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–26.
Esfandi F, Mohammadzadeh Ghobadloo S, Basati G. Interleukin-6 level in patients with colorectal cancer. Cancer Lett. 2006;244(1):76–8.
Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007;102(2):129–35.
Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117(12):3660–3.
Scheibenbogen C, Mohler T, Haefele J, Hunstein W, Keilholz U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995;5(3):179–81.
Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19(2):577–83.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13.
Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153(3):865–73.
Yang MH, Imrali A, Heeschen C. Circulating cancer stem cells: the importance to select. Chin J Cancer Res. 2015;27(5):437–49.
Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14(1):29–43.
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–9.
Ogunwobi OO, Liu C. Therapeutic and prognostic importance of epithelial-mesenchymal transition in liver cancers: insights from experimental models. Crit Rev Oncol Hematol. 2012;83(3):319–28.
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46.
Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012;14(1):R15.
Krawczyk N, Meier-Stiegen F, Banys M, Neubauer H, Ruckhaeberle E, Fehm T, et al. Expression of stem cell and epithelial-mesenchymal transition markers in circulating tumor cells of breast cancer patients. Biomed Res Int. 2014;2014:415721.
Raimondi C, Gradilone A, Naso G, Vincenzi B, Petracca A, Nicolazzo C, et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011;130(2):449–55.
Gunasinghe NP, Wells A, Thompson EW, Hugo HJ. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012;31(3–4):469–78.
Olorunseun OO, Wang T, Zhang L, Liu C. COX-2 and Akt mediate multiple growth factor-induced epithelial-mesenchymal transition in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27(3):566–78.
Ogunwobi OO, Puszyk W, Dong HJ, Liu C. Epigenetic upregulation of HGF and c-Met drives metastasis in hepatocellular carcinoma. PLoS One. 2013;8(5):e63765.
Lee D, Lee JW. Self-renewal and circulating capacities of metastatic hepatocarcinoma cells required for collaboration between TM4SF5 and CD44. BMB Rep. 2015;48(3):127–8.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Książkiewicz M, Markiewicz A, Zaczek AJ. Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79(4):195–208.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23(5):573–81.
Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22(6):709–24.
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–36.
Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68(24):10377–86.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Pham, K., Delitto, D., Liu, C. (2018). Hepatocellular Carcinoma Metastasis and Circulating Tumor Cells. In: Liu, C. (eds) Precision Molecular Pathology of Liver Cancer. Molecular Pathology Library. Springer, Cham. https://doi.org/10.1007/978-3-319-68082-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-68082-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68080-4
Online ISBN: 978-3-319-68082-8
eBook Packages: MedicineMedicine (R0)