Abstract
Arsenic trioxide (ATO) is an effective therapy for relapsed acute promyelocytic leukemia (APL) patients; however, the optimal treatment strategy remains unclear, and knowledge of the prognostic factors is still limited. We retrospectively analyzed the outcomes of 64 consecutive first relapsed APL patients (12 with molecular relapse and 52 with hematologic relapse). Patients received re-induction with intravenous ATO-based regimens. Patients who achieved a CR2 were offered further courses of alternating ATO/conventional chemotherapy with or without stem cell transplantation (SCT). With a median follow-up of 27 months (range, 6–57) in the molecular relapsed subgroup, the 3-year relapse-free survival (RFS) and overall survival (OS) rates were 81.5 % and 100 %, respectively. With a median follow-up of 38 months (range, 0–129) in the hematologic relapse group, the 3-year RFS and OS rates were 57.1 % and 72.1 %, respectively. Furthermore, in the hematologic relapse group, we compared the outcome between relapsed patients after previous ATO therapy (n = 20) with those who did not receive prior ATO therapy (n = 32). The CR2 rate was 80 % (16/20) vs. 93.8 % (30/32), (p = 0.189). However, the relapse rate was 68.8 % (11/16) vs. 33.3 % (10/30), (p = 0.03). The 4-year OS rate was 62.4 % vs. 71.2 %, (p = 0.816), and the 4-year RFS rate was 29.8 % vs. 66.2 % (p = 0.023). The results indicate that, irrespective of frontline therapy with ATO, salvage therapy with an ATO-based regimen remains effective. However, the long-term survival for those patients who received previous ATO-based treatment was inferior compared to those who did not receive prior ATO. In addition, the alternating ATO/chemotherapy strategy can be a post-remission treatment option in a subset of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incorporation of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) into the frontline treatment regimens of acute promyelocytic leukemia (APL) has led to markedly improved cure rates that exceed 85 % in clinical trials [1–5]. Despite these advances, early death and disease relapse remain the two major causes of treatment failure [6–8]. Currently, a common salvage strategy for relapsed APL includes the administration of ATO-based re-induction, followed by ATO and/or stem cell transplantation (SCT) [9–11]. Several studies demonstrated that approximately 80–85 % of relapsed patients following initial ATRA and chemotherapy treatment can achieve a second complete remission (CR2) [12–14]. However, limited data are available on the efficacy of ATO treatment in relapsed patients who had prior ATO-containing therapy. In addition, the optimal strategy for post-remission therapy after ATO induced CR2 remains controversial. The role of repeated courses of ATO and chemotherapy is unclear, particularly on those only molecular relapsed patients.
To address this issue and define the prognostic factors for relapsed APL, we performed a retrospective analysis over a 10-year period in first relapsed APL patients in our institution.
Patients and methods
Patients
We collected the data of all patients with first relapsed APL who registered in the database of our hospital from January 2002 to December 2012. We also included a few patients who were initially diagnosed and treated at other institutions but were then transferred to our hospital after relapse. We reviewed the medical records including clinical and laboratory information both at diagnosis and after relapse. Isolated extramedullary relapse was not included. The study was approved by the Institutional Review Board and in accordance with the Declaration of Helsinki.
Treatment strategies of newly diagnosed APL
All newly diagnosed APL patients were initially administered according to two protocols: ATRA plus ATO combination regimen for remission induction [15, 16], or ATRA-based regimen for induction [17]. In brief, the ATRA plus ATO protocol combines ATRA with ATO for remission induction followed by three courses of chemotherapy consolidation and ATRA/ATO maintenance. In the ATRA-based induction group, patients received ATRA (25 mg/m2/day) orally until CR. After CR achievement, patients were further divided into two subgroups according to the different regimens for post-remission therapy: ATRA/chemotherapy; or ATO/ATRA/chemotherapy. Details of the protocols have been described previously [16, 17].
Treatment strategies of relapsed APL patients
Re-induction and post-remission regimens were modified from the Shanghai protocol [14]. For remission re-induction, ATO was administered intravenously at a dose of 10 mg/day until bone marrow remission was observed or up to a cumulative maximum of 60 doses (for the molecular relapse subgroup, ATO was administered for 28 days). At the discretion of the treating physician, ATRA was administered at a dose of 20–25 mg/m2/day orally during ATO administration. Patient’s complete blood count, coagulation, renal/liver function and electrocardiography were routinely monitored. Patients received mini-dose chemotherapy (idarubicin 6 mg/m2/day for 2–5 days, or daunorubicin 40 mg/m2/day for 2–5 days, or mitoxantrone 2 mg/day for 7–10 days) once the white blood cell (WBC) count exceeded 10.0 × 109/L. After CR2 achievement, autologous (auto) or allogeneic (allo) SCT was generally recommended for patients younger than 60 years. Auto-SCT was restricted to individuals who achieved a molecular remission. Patients who did not receive the SCT procedure were offered consolidation with alternating courses of ATO (10 mg/day for 28 days) and conventional chemotherapy (daunorubicin 40–45 mg/m2/day on days 1 to 3, idarubicin 8–10 mg/m2/day on days 1 to 3, or homoharrgtonine 4 mg/m2/day, days 1 to 3; with cytarabine 100 mg/m2/day on days 1 to 7). The sequential therapy was administrated for a total of 6–8 courses with an interval of 4–5 weeks for each course. Central nervous system (CNS) prophylaxis was attained by means of intrathecal injection of methotrexate, cytarabine, and corticosteroids. Patients with cytological evidence of CNS leukemia received intrathecal injections twice a week and/or cranial irradiation, until complete clearance of leukemic cells in the cerebrospinal fluid (CSF). The PML-RARA transcripts were detected by a nested qualitative or real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis with a sensitivity of 10−4 [18]. Molecular monitoring was carried out after each treatment course. Patients who had a relapse again were further treated with ATO, ATRA and chemotherapy with or without SCT.
Definitions and statistical analysis
Hematologic relapse was confirmed morphologically (>5 % blasts or abnormal promyelocytes in the bone marrow) as well as molecularly (PML-RARA positive). Molecular relapse was defined as PML-RARA positivity in two subsequent consecutive tests and in the absence of overt hematologic relapse [19]. A hematologic CR was defined using conventional criteria, including bone marrow blasts and abnormal promyelocytes ≤5 %; with an absolute neutrophil count ≥1.0 × 109/l and a platelet count ≥100 × 109/l. A molecular CR was defined as a negative RT-PCR result from bone marrow cells [20]. Overall survival (OS) was defined as the time from first relapse to death or last follow-up. Relapse-free survival (RFS) was defined as the time from second remission to last follow-up or an event (relapse). The follow-up data were updated in May 2013.
Baseline parameters between groups were compared using chi-square test or the Fisher’s exact test for categorical variables and the Mann–Whitney test for continuous variables. A Kaplan–Meier survival analysis was performed to estimate the probabilities of OS and RFS, and differences between the curves were analyzed by the log-rank test. Factors associated with a p < 0.20 by univariate analysis were entered into Cox proportional hazard models for multivariate analysis. All P values were two-sided, with a p value of <0.05 indicating statistical significance. Statistical testing was performed using GraphPad Prism version 5 (San Diego, CA, USA) and SPSS statistical software version 20 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of relapsed patients
A total of 70 consecutive patients with first relapsed APL were identified at our institute in the study period. Six cases were excluded in this analysis: three patients received other treatment regimens that did not utilize ATO contained schemes (chemotherapy and/or ATRA), two patients were incomplete clinical records, and one patient died before treatment. Sixty-four patients were enrolled for analysis. We classified patients into two groups: patients presenting with molecular relapse (12 patients, including three who had received prior ATO treatment) or hematologic relapse (52 patients, including 20 who had received prior ATO treatment). Thirty-four (53.1 %) of first relapses occurred within 2 years of CR achievement, and 30 (46.9 %) patients relapsed after 2 years (late relapse). Simultaneous medullary and CNS relapse occurred in two patients. The baseline characteristics of the patients in the two groups are depicted in Table 1.
Response to salvage therapy of molecular relapse patients
Of the 12 patients presenting with molecular relapse, salvage induction consisted of a regimen with single-agent ATO in eight patients, or ATO plus ATRA in the remaining four patients. Eleven out of 12 cases obtained negative testing for PML-RARA after the first cycle of ATO-based re-induction. Use of ATO in this setting did not induce hyperleukocytosis during re-induction. An autologous SCT was performed in one patient in second molecular CR after one course of ATO consolidation. The remaining patients received post-remission therapy with ATO/chemotherapy courses. All patients achieved complete molecular response after two cycles of ATO. For the three molecular relapsed patients who had been treated with ATO as frontline therapy. One patient got a hematological relapse in the sixth month. The other two patients (including one received auto-SCT) were in molecular remission (followed by 9+, 14+ months).
Overall, after a median follow-up of 27 months (range 6–57 months), two relapse events occurred. One patient (previous with ATO treatment) experienced a hematological relapse in the sixth month, and the other patient experienced an isolated CNS relapse 8 months later. The median duration of CR2 was 24 months (5–56 months). The 3-year RFS and OS rates were 81.5 % and 100 %, respectively.
Response to salvage therapy of hematologic relapse
For 52 patients with hematologic relapse, ATRA was added for 18 patients during ATO re-induction. Mini-dose chemotherapy was added during the re-induction course for 12 patients. Following the first course of induction therapy, 46 (88.5 %) patients went into hematologic CR again. Six (11.5 %) patients failed to achieve CR (including three early deaths and three resistant patients). PML-RARA remained positive in all 46 patients after ATO-induced hematologic CR. However, 43 out of 46 patients hematologic CR patients became PCR negative at various time points (3–6 months) during the post-remission therapy.
Six CR2 patients received auto-SCT (n = 2) or allo-SCT (n = 4). Two patients underwent allo-SCT immediately after CR2 achievement. One patient underwent auto-SCT after one consolidation course with ATO. The remaining three patients underwent transplantation after two consolidation courses with ATO and chemotherapy. One patient who received auto-SCT relapsed at 9 months, she was given an allo-SCT during CR3 and remained in CR3. No other patients undergoing SCT therapy during CR2 relapsed. The remaining 40 patients in CR2 received the alternating ATO/chemotherapy regimens as post-remission therapy. Of these 40 patients, one patient died at the nadir during chemotherapy, 20 (50 %) experienced a second relapse after a median of 18 months (range, 6–39 months). Ten patients achieved CR3 by the ATO- and chemotherapy-based re-induction regimens. Two patients received allo-SCT in CR3, but one patient died from treatment-related mortality, and the other survived and remained in CR3.
Overall, a total of 21 (39.7 %) out of 46 patients relapsed during CR2 (medullary relapse, 18 patients; molecular relapse, two patients; isolated CNS relapse one patient). Twelve patients unfortunately died in the setting of disease progression at a median survival of 27 months (range 5–60 months). Thirty-three patients were alive at last follow-up, including 24 in continuous CR2, five in CR3, three in CR4, and 1 in CR5. After a median follow-up of 38 months (range, 0–129 months), the 3-year RFS and OS rates were 57.1 % and 72.1 %, respectively.
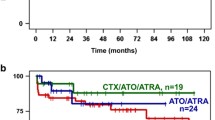
Furthermore, we compared the outcome between those relapsed after prior ATO therapy (n = 20) and those who had not previously received ATO therapy (n = 32). As shown in Table 2, there were no significant differences in the two subgroups with respect to demographics and clinicopathologic features. The CR2 rate was 80 % (16/20) vs. 93.8 % (30/32) (p = 0.189). The relapse rate was 68.8 % (11/16) vs. 33.3 % (10/30), (p = 0.030). The median duration of CR2 was 36 vs. 25 months, (p = 0.038). The 4-year Kaplan–Meier estimated OS and RFS were 62.4 % vs. 71.2 %, (p = 0.816), 29.8 % vs. 66.2 % (p = 0.023) in the two subgroups, respectively (Fig. 1).
Comparisons between the molecular and hematologic relapse groups
Kaplan–Meier curves for OS and RFS for molecular vs. hematologic relapse are shown (Fig. 2). Although there was no significant difference between the two groups in terms of OS and RFS, as expected, there was a trend toward favorable outcomes for the molecular group in terms of OS.
Analyses of prognostic factors
Clinically important variables, including gender, age, risk category, frontline therapy (ATO vs. without ATO), duration of CR1, type of relapse (molecular vs. hematologic), and salvage induction therapy (ATO vs. ATO plus ATRA) were analyzed on survival after relapse. Table 3 shows the results of univariate and multivariate analyses for OS and RFS after relapse. Multivariate analysis demonstrated the favorable impact on survival after relapse of younger age (≤40 years) and no prior ATO therapy. Moreover, the prior ATO treatment was shown to be independently associated with worse RFS.
Discussion
Although APL has a favorable prognosis with current treatment regimens, the treatment of relapsed APL remains challenging. Recently, several clinical trials have established the benefit of ATO in the frontline management of APL [2–4]. However, the salvage treatment of relapsed patients following previous ATO therapy remains to be determined. In this study, for hematologic relapsed patients with previous ATO treatment, the 80 % CR rate is particularly encouraging. These results suggest ATO-based re-induction regimens remain effective despite prior therapy with ATO. Thirugnanam et al. [21] reported that 13 out of 14 patients in the ATO-based primary treatment group achieved CR2. Similar findings have been noted by Au et al. [22, 23]. Thus, these studies demonstrate that initial ATO being first line therapy, is unlikely to prevent a successful ATO re-induction after relapse.
However, the 4-year RFS rate of 29.8 % was unsatisfactory for those with prior ATO treatment. In addition, the prior ATO therapy was shown to be an independent unfavorable factor in term of RFS and OS in multivariate analysis. It is possible that relapsed patients acquire additional molecular defects that account for this worsened survival rate [24]. Therefore, subsequent SCT procedures should be strongly recommended after the ATO induced CR2 for relapsing patients with prior treatment with ATO. Interestingly, the median duration of CR2 was up to 25 months suggesting that ATO and conventional chemotherapy being salvage therapy did win time for these patients to receive SCT.
On the other hand, the CR rate was 93.8 % for patients with hematologic relapse who never received prior ATO therapy. The CR rate was comparable with newly diagnosed APL patients. However, the 4-year RFS rate of 66.2 % was inferior compared to previous studies for newly diagnosed patients who were treated with ATO as the frontline therapy [3, 4]. This observation suggests that patients with relapsed APL with no previous ATO treatment are probably biologically different from newly diagnosed APL [25]. Similar findings have been reported previously by Thirugnanam et al. [21]. Further basic scientific studies are needed to address this issue.
Previous reports indicated that molecular monitoring of the PML-RARA transcript is useful to anticipate overt hematological relapse [19, 26, 27]. However, the optimal treatment protocol for APL patients with molecular relapse remains unclear. Grimwade et al. [28] reported the regimen of delivery of pre-emptive therapy with ATO re-induction followed by transplantation consolidation. This regimen prevented progression to frank hematologic relapse in ten out of 14 patients. The RFS rate at 1 year was 73 % [28]. Interestingly, the Italian group reported nine relapsed APL patients (eight in molecular relapse) who received prolonged therapy with combined ATO and ATRA without receiving a transplant. They showed 88 % of patients remained in prolonged molecular CR2 [29]. In our series, patients treated with ATO and chemotherapy (one with auto-SCT) achieved a 3-year RFS rate of 81.5 %. This suggests that ATO and chemotherapy could be an optimal approach for treating APL relapsed cases with early molecular relapse, particularly for those with no prior ATO therapy.
SCT remains a widely adopted strategy as a part of the salvage therapy for relapsed APL patients [6, 9, 11, 30, 31]. Recently, Pemmaraju et al. [32] reported retrospectively evaluated 40 patients with relapsed APL. The investigators found that treatment with auto-SCT demonstrates a trend toward better outcomes, however, no significant difference in clinical outcomes is seen in the three treatment approaches (auto-SCT, allo-SCT, and chemotherapy only) [32]. Fujita et al. [33] reported that the 5-year OS was significantly better in the non-SCT group than in the SCT group among older patients (age ≥40 years). It was suggested ATO in combination with conventional chemotherapy has previously shown to be an effective regimen for post-remission therapy in CR2 of APL [14]. However, the role of this regimen has not yet been determined. In our series, due to financial constraints, ineligibility for SCT, mobilization failure, or early relapse before planned SCT, only a small proportion of patients received SCT during CR2. Thus, we were able to perform this retrospective analysis of patients with relapsed APL who underwent post-remission therapy consisting of ATO and chemotherapy. This protocol results in the 4-year RFS of 66.2 % in patients with hematologic relapse who never had previous ATO treatment. The survival rate is in comparable with previous SCT data [9, 30]. Therefore, ATO and chemotherapy together can be a treatment alternative in selected patients with a second molecular remission and no prior ATO therapy who are not candidates for transplant.
The major limitation of this study is the retrospective nature in a single institution. The post-remission therapy consisted of ATO and chemotherapy with or without SCT, but no uniform treatment guidelines were available. Further prospective studies with larger patient numbers are required to confirm the impact of the combination of ATO and chemotherapy regimens on outcomes for patients with APL in CR2.
In summary, the results of this study suggest ATO-based regiments could be a feasible therapeutic option for patients with relapsed APL despite prior therapy with ATO. In selected patients who have a better prognosis (no previous ATO therapy or molecular relapse) or who are not candidates for transplant, treatment options like ATO in combination with conventional chemotherapy can be an alternative to transplantation.
References
Wang ZY, Chen Z (2008) Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111(5):2505–2515. doi:10.1182/blood-2007-07-102798
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M, Fioritoni G, Salih HR, Cazzola M, Melillo L, Carella AM, Brandts CH, Morra E, von Lilienfeld-Toal M, Hertenstein B, Wattad M, Lubbert M, Hanel M, Schmitz N, Link H, Kropp MG, Rambaldi A, La Nasa G, Luppi M, Ciceri F, Finizio O, Venditti A, Fabbiano F, Dohner K, Sauer M, Ganser A, Amadori S, Mandelli F, Dohner H, Ehninger G, Schlenk RF, Platzbecker U (2013) Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369(2):111–121. doi:10.1056/NEJMoa1300874
Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A, Reynolds J, Di Iulio J, Tiley C, Taylor K, Filshie R, Seldon M, Taper J, Szer J, Moore J, Bashford J, Seymour JF (2012) All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 120(8):1570–1580. doi:10.1182/blood-2012-02-410746, quiz 1752
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, Li JM, Tang W, Zhao WL, Wu W, Sun HP, Chen QS, Chen B, Zhou GB, Zelent A, Waxman S, Wang ZY, Chen SJ, Chen Z (2009) Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A 106(9):3342–3347. doi:10.1073/pnas.0813280106
Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S, Tournilhac O, de Botton S, Ifrah N, Cahn JY, Solary E, Gardin C, Fegeux N, Bordessoule D, Ferrant A, Meyer-Monard S, Vey N, Dombret H, Degos L, Chevret S, Fenaux P (2010) Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood 115(9):1690–1696. doi:10.1182/blood-2009-07-233387
Tallman MS (2007) Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol 20(1):57–65. doi:10.1016/j.beha.2006.11.002
McClellan JS, Kohrt HE, Coutre S, Gotlib JR, Majeti R, Alizadeh AA, Medeiros BC (2012) Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica 97(1):133–136. doi:10.3324/haematol.2011.046490
Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM, Tallman MS (2011) Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood 118(5):1248–1254. doi:10.1182/blood-2011-04-346437
Yanada M, Tsuzuki M, Fujita H, Fujimaki K, Fujisawa S, Sunami K, Taniwaki M, Ohwada A, Tsuboi K, Maeda A, Takeshita A, Ohtake S, Miyazaki Y, Atsuta Y, Kobayashi Y, Naoe T, Emi N (2013) Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood. doi:10.1182/blood-2012-11-466862
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Buchner T, Dohner H, Burnett AK, Lo-Coco F (2009) Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 113(9):1875–1891. doi:10.1182/blood-2008-04-150250
Ramadan SM, Di Veroli A, Camboni A, Breccia M, Iori AP, Aversa F, Cupelli L, Papayannidis C, Bacigalupo A, Arcese W, Lo-Coco F (2012) Allogeneic stem cell transplantation for advanced acute promyelocytic leukemia in the ATRA and ATO era. Haematologica 97(11):1731–1735. doi:10.3324/haematol.2012.065714
Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutre S, Dahlberg S, Ellison R, Warrell RP Jr (2001) United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 19(18):3852–3860
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP Jr (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 339(19):1341–1348. doi:10.1056/NEJM199811053391901
Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, Wu W, Zhang FQ, Chen Y, Zhou L, Li JM, Zeng XY, Yang RR, Yuan MM, Ren MY, Gu FY, Cao Q, Gu BW, Su XY, Chen GQ, Xiong SM, Zhang TD, Waxman S, Wang ZY, Chen Z, Hu J, Shen ZX, Chen SJ (1999) Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood 94(10):3315–3324
Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, Shi JY, Zheng PZ, Yan H, Liu YF, Chen Y, Shen Y, Wu W, Tang W, Waxman S, De The H, Wang ZY, Chen SJ, Chen Z (2004) All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A 101(15):5328–5335. doi:10.1073/pnas.0400053101
Lou Y, Qian W, Meng H, Mai W, Tong H, Tong Y, Huang J, Jin J (2013) High efficacy of arsenic trioxide plus all-trans retinoic acid based induction and maintenance therapy in newly diagnosed acute promyelocytic leukemia. Leuk Res 37(1):37–42. doi:10.1016/j.leukres.2012.09.004
Lou Y, Qian W, Meng H, Mai W, Tong H, Tong Y, Huang J, Jin J (2013) Long-term efficacy of low-dose all-trans retinoic acid plus minimal chemotherapy induction followed by the addition of intravenous arsenic trioxide post-remission therapy in newly diagnosed acute promyelocytic leukaemia. Hematol Oncol. doi:10.1002/hon.2076
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela JM, Cave H, Pane F, Aerts JL, De Micheli D, Thirion X, Pradel V, Gonzalez M, Viehmann S, Malec M, Saglio G, van Dongen JJ (2003) Standardization and quality control studies of 'real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia — a Europe Against Cancer program. Leukemia 17(12):2318–2357. doi:10.1038/sj.leu.2403135
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, Biondi A, Rossi G, Carlo-Stella C, Selleri C, Martino B, Specchia G, Mandelli F (1999) Therapy of molecular relapse in acute promyelocytic leukemia. Blood 94(7):2225–2229
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD (2003) Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 21(24):4642–4649. doi:10.1200/JCO.2003.04.036
Thirugnanam R, George B, Chendamarai E, Lakshmi KM, Balasubramanian P, Viswabandya A, Srivastava A, Chandy M, Mathews V (2009) Comparison of clinical outcomes of patients with relapsed acute promyelocytic leukemia induced with arsenic trioxide and consolidated with either an autologous stem cell transplant or an arsenic trioxide-based regimen. Biol Blood Marrow Transplant 15(11):1479–1484. doi:10.1016/j.bbmt.2009.07.010
Au WY, Chim CS, Lie AK, Liang R, Kwong YL (2002) Combined arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia recurring from previous relapses successfully treated using arsenic trioxide. Br J Haematol 117(1):130–132
Au WY, Lie AK, Chim CS, Liang R, Ma SK, Chan CH, Mak YK, Chen YT, So CC, Yeung YM, Yip SF, Wong LG, Chan JC, Liu SY, Kwong YL (2003) Arsenic trioxide in comparison with chemotherapy and bone marrow transplantation for the treatment of relapsed acute promyelocytic leukaemia. Ann Oncol 14(5):752–757
Tomita A, Kiyoi H, Naoe T (2013) Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) in acute promyelocytic leukemia. Int J Hematol 97(6):717–725. doi:10.1007/s12185-013-1354-4
Dimov ND, Medeiros LJ, Ravandi F, Bueso-Ramos CE (2010) Acute promyelocytic leukemia at time of relapse commonly demonstrates cytogenetic evidence of clonal evolution and variability in blast immunophenotypic features. Am J Clin Pathol 133(3):484–490. doi:10.1309/AJCPJ7K0AWMBHMAI
Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH (1999) Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the Randomized MRC Trial. Blood 93(12):4131–4143
Chendamarai E, Balasubramanian P, George B, Viswabandya A, Abraham A, Ahmed R, Alex AA, Ganesan S, Lakshmi KM, Sitaram U, Nair SC, Chandy M, Janet NB, Srivastava VM, Srivastava A, Mathews V (2012) Role of minimal residual disease monitoring in acute promyelocytic leukemia treated with arsenic trioxide in frontline therapy. Blood 119(15):3413–3419. doi:10.1182/blood-2011-11-393264
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, Diverio D, Jones K, Aslett H, Batson E, Rennie K, Angell R, Clark RE, Solomon E, Lo-Coco F, Wheatley K, Burnett AK (2009) Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol 27(22):3650–3658. doi:10.1200/JCO.2008.20.1533
Breccia M, Cicconi L, Minotti C, Latagliata R, Gianni L, Lo-Coco F (2011) Efficacy of prolonged therapy with combined arsenic trioxide and ATRA for relapse of acute promyelocytic leukemia. Haematologica 96(9):1390–1391. doi:10.3324/haematol.2011.045500
Sanz MA, Labopin M, Gorin NC, de la Rubia J, Arcese W, Meloni G, Bacigalupo A, Alessandrino P, Carreras E, Iriondo A, Novitzky N, Jacobs P, Bandini G, Lo-Coco F, Frassoni F, Rocha V (2007) Hematopoietic stem cell transplantation for adults with acute promyelocytic leukemia in the ATRA era: a survey of the European Cooperative Group for Blood and Marrow Transplantation. Bone Marrow Transplant 39(8):461–469. doi:10.1038/sj.bmt.1705620
Ferrara F, Finizio O, Izzo T, Riccardi C, Criscuolo C, Carbone A, Borlenghi E, Rossi G (2010) Autologous stem cell transplantation for patients with acute promyelocytic leukemia in second molecular remission. Anticancer Res 30(9):3845–3849
Pemmaraju N, Tanaka MF, Ravandi F, Lin H, Baladandayuthapani V, Rondon G, Giralt SA, Chen J, Pierce S, Cortes J, Kantarjian H, Champlin RE, De Lima M, Qazilbash MH (2013) Outcomes in patients with relapsed or refractory acute promyelocytic leukemia treated with or without autologous or allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk 13(4):485–492. doi:10.1016/j.clml.2013.02.023
Fujita H, Asou N, Iwanaga M, Hyo R, Nomura S, Kiyoi H, Okada M, Inaguma Y, Matsuda M, Yamauchi T, Ohtake S, Izumi T, Nakaseko C, Ishigatsubo Y, Shinagawa K, Takeshita A, Miyazaki Y, Ohnishi K, Miyawaki S, Naoe T (2013) Role of hematopoietic stem cell transplantation for relapsed acute promyelocytic leukemia: a retrospective analysis of JALSG-APL97. Cancer Sci 104(10):1339–1345. doi:10.1111/cas.12230
Acknowledgments
We thank all medical and nursing staff working in the department of hematology, the affiliated hospital of Zhejiang University for providing patients information. This work was supported in part by the National Public Health Grand Research Foundation (No. 201202017), the National High Technology Research and Development Program of China (863 Program) (2012AA02A505), Leukemia Research Innovative Team of Zhejiang Province (2011R50015), National TCM Project (JDZX 2012169) and the Medical Science Foundation for Excellent Youth Scholars by Zhejiang Province (No. 2008QN011).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, Y., Suo, S., Tong, Y. et al. Outcomes and prognostic factors of first relapsed acute promyelocytic leukemia patients undergoing salvage therapy with intravenous arsenic trioxide and chemotherapy. Ann Hematol 93, 941–948 (2014). https://doi.org/10.1007/s00277-013-2000-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-2000-1