Abstract
Reports on the use of the real-time quantitative polymerase chain reaction (RQ-PCR) for childhood acute promyelocytic leukemia (APL) therapy are scarce. This study describes the prognostic significance of quantification of the PML-RARa transcript in children with APL. Since January 2004, we have analyzed 40 children treated with all-trans-retinoic acid ± arsenic trioxide in induction. Thirty-nine patients (97.5%) entered complete remission. The 5-year rates of disease-free survival (DFS) and overall survival in these patients were 73.1 and 91.4%, respectively. By employing a standardized RQ-PCR protocol for minimal residual disease (MRD) monitoring, we determined that less than 1 normalized copy number (NCN) after induction indicates higher probability of a more favorable treatment outcome. After induction therapy, thirteen out of 38 (34.2%) patients in hematologic remission showed a negative RQ-PCR result (less than 1 NCN), which was correlated with the lower probability of relapse (100 and 55.2% DFS at 5 years in the negative and positive RQ-PCR groups, respectively; P = 0.018). PML/RARa-based MRD monitoring by RQ-PCR may allow us to identify subgroups of patients at low risk of relapse after induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute promyelocytic leukemia (APL), a distinct subtype of acute myeloid leukemia (AML), is characterized by a translocation between chromosomes 15 and 17 in leukemia cells and the presence of a PML/RARa fusion gene product. The introduction of all-trans-retinoic acid (ATRA), arsenic trioxide (ATO), and high-dose cytarabine (Ara-c) has significantly improved disease-free and overall survival rates in APL patients. Only early death and relapse remain major impediments in the ability to cure all patients. Current efforts for the treatment of APL focus on reduction of the relapse rate [1–10].
APL is rare in children, comprising only 7–10% of all acute myeloid leukemia patients, and is characterized by a more frequent incidence of hyperleukocytosis, the microgranular M3 variant (M3v), and bcr2 and bcr3 isoforms of PML/RARa rearrangement than in adults [9–11]. When chemotherapy was the sole treatment option in APL, the outcome in children was equal to or less favorable than in adults because of a higher incidence of relapse in some series. It was confirmed that first-line treatment with ATRA combined with chemotherapy for induction provided just as favorable results in children with APL as in adults [9–12].
Minimal residual disease (MRD) monitoring based on the detection of PML/RARa transcripts employing PCR technology has clearly demonstrated a benefit in the diagnosis and follow-up of APL patients. In early studies, MRD monitoring was mainly performed utilizing the reverse transcription polymerase chain reaction (RT-PCR) [13–15]. Although RT-PCR assays have proved highly informative, they lack the capacity to distinguish between rising and falling numbers of fusion transcripts. Importantly, they also cannot reliably identify poor quality samples that could potentially give rise to false-negative results [16]. These problems have been overcome with the advent of real-time quantitative PCR (RQ-PCR), which facilitates the absolute quantification of leukemic targets and endogenous control gene transcripts, representing a significant advance towards the realization of MRD-directed therapy [17]. So far, RQ-PCR has been investigated to provide prognostic indexes for APL management in many adult studies [18–21]. However, there are still no data on the use of such assays for child APL therapy. Considering the difference between child and adult APL, RQ-PCR monitoring in children should be investigated.
The current study was conducted to clarify the relationship between the level and kinetics of MRD and outcome in children with newly diagnosed PML/RARa-positive APL, and to identify the subgroups at low risk of relapse.
Methods
Patients and samples
The diagnosis of APL was confirmed by clinical manifestations, or the presence of t (15; 17) or PML/RARa gene rearrangements in a patient’s sample, aside from the morphologic results. From January 2004 to February 2010, 40 patients younger than 16 years old were consecutively diagnosed with APL in our hospital. A total of 299 viable samples were analyzed, and the median was 7 follow-up MRD assessments per patient (range 1–15).
Treatment
Parents or legal guardians of all patients were informed of the study, and they provided signed informed consent. This study was approved by the Institutional Committee for Medical Care and Safety. The induction treatment regime was ATRA (20–45 mg/m2/day) alone or ATRA (20–45 mg/m2/day) + ATO (3–10 mg/day), which was maintained until complete remission (CR) before reaching a maximum of 60 days. Patients with an initial WBC count of less than 10 × 109/L received ATRA (45 mg/m2/day) at the beginning. If the patients complained of intolerable side effects, such as headache or bone ache, ATRA was discontinued or reduced to 20–25 mg/m2/day. Then, ATO was added. All determinations were made by the chief physician in our department. Patients with an initial WBC count of 10 × 109/L or more were treated with ATRA and ATO from the beginning. After achieving CR, at least two courses of consolidation chemotherapy were given sequentially. The treatment of consolidation chemotherapy was daunorubicin alone (45 mg/m2/day for 3 days). In the maintenance therapy stage, children were started on ATRA (30 mg/m2, 14 days) and chemotherapy with mercaptopurine (100 mg/m2/day, 14 days) and methotrexate (20 mg/m2/week, 2 times). The cycles were repeated every month. Maintenance treatment was planned for 2 years.
Definition and study endpoints
A hematological complete remission (HCR) and hematologic relapse were defined according to the report of Cheson et al. [22]. The PML/RARa fusion gene of bone marrow samples was tested using RT-PCR/RQ-PCR and fluorescence in situ hybridization (FISH) at the diagnosis and consolidation. After consolidation, bone marrow samples were tested every 3–6 months. Molecular remission and relapse were defined by the disappearance and reappearance of the PML/RARa fusion gene on one occasion. Early death (ED) was defined as death within the first 2 weeks of treatment.
Disease-free survival (DFS) was calculated from the date of HCR achievement to that of either the last follow-up or an event (relapse or death); the overall survival (OS) duration was calculated from the date of diagnosis to that of either the last follow-up or death.
RNA extraction and cDNA synthesis
Total RNA was isolated from BM samples using the acid guanidinium tyocyanate–phenol–chloroform extraction method [23]. Reverse transcription was performed using 1 μg total RNA according to a protocol, as previously described [24].
RT-PCR qualitative and RQ-PCR (real-time) assays
To amplify the PML/RARa fusion gene, a two-step qualitative RT-PCR analysis was performed, as described previously [24].
In the RQ-PCR method, established in our laboratory, based on cDNA, a dilution of the NB4 cell line reached a sensitivity of 1 × 10−5 for PML-RARa. The Abelson housekeeping gene (ABL) was selected as a control gene of RNA expression, as previously reported [25]. The different PML-RARa transcripts were quantified using the ABI PRISM 7500 DNA Sequence Detection System. Primers were designed as previously reported [20]. A valid result required an ABL Ct within a range of 22.4–28.5, with at least 2,000 copies of the ABL gene in the sample. All samples were tested in triplicate, and the results are reported as the normalized copy number (NCN), derived by multiplying the PML-RARa copy number/ABL copy number ratio by 10,000 [26]. A result of less than 1 NCN was reported as RQ-PCR-negative.
Statistical analyses
All statistical analyses were carried out using SPSS software version 16. Baseline characteristics of the patients were compared employing Fisher’s exact test for qualitative variables and Student’s t test for quantitative variables. DFS and OS were estimated using the Kaplan–Meier method and, for comparison, the log-rank test. The relationships of clinical features with the outcome were analyzed using the Cox regression proportional hazard model with a 95% CI. All P values are two-sided, with values of 0.05 or less indicating significance.
Results
Pretreatment characteristics
The pretreatment characteristics of the 40 patients are listed in Table 1. The fusion gene PML/RARa was positive in 39 patients. The other one was confirmed by the presence of t (15; 17). In our series, a total of 28 (71.8%) patients carried the bcr1 PML/RARa transcript, 3 (7.7%) patients carried the bcr2 PML/RARa transcript, and 8 (20.5%) patients carried the bcr3 PML/RARa transcript. The clinical characteristics, PML/RARa NCN, and relapse rate in the three PML/RARa transcripts showed no significant difference. Among the 40 patients, 39 (97.5%) children achieved hematologic CR. The cases who failed to enter CR died of intracerebral hemorrhage on day 1.
As2O3 was administered for a median of 18 days (range 2–35) during induction. The median dose of As2O3 in these patients was 0.25 mg/kg/day (range 0.20–0.33). No dose reduction occurred. The As2O3-related adverse reactions included one case of nausea (1/16, 6.2%), one of gastric pain (1/16, 6.2%), and three of infection-unrelated fever (3/16, 18.8%). As2O3 was discontinued (1/16, 6.2%) in one patient because of edema (grade III according to the National Cancer Institute’s common toxicity criteria). There was no sudden death due to a cardiac event in this series of patients. No adverse events involving the peripheral nervous system occurred.
The follow-up of patients was updated on April 2011 with a median of 47 months (range 0.01–88). Eight patients experienced relapse. The 5-year probabilities of DFS and OS were 73.1 ± 8.6 and 91.4 ± 6.3%, respectively. No parameters had a prognostic impact on the relapse risk. The 5-year DFS was 72.7 ± 9.8% in patients with WBC <10 × 109/L and 76.2 ± 14.8% in those with WBC ≥10 × 109/L (P = 0.812). The comparison of patients (WBC counts <10 × 109/L) treated with ATRA or ATRA + ATO is shown in Table 2. The initial characteristics of the two groups revealed no difference except for the platelet count. The 5-year DFS was 64.8 ± 12.0% in the ATRA group compared with 100% in the ATRA + ATO group (P = 0.160; Fig. 1). Six patients experienced relapse in the ATRA group compared with none in the ATRA + ATO group. We could not study the benefit of ATO in the patients with WBC counts ≥ 10 × 109/L due to their small numbers.
RQ-PCR in different phases of treatment and influence on survival
At diagnosis, 39 samples showed >2,000 NCN (median 8,448, range 2,451–17,847) by RQ-PCR. After induction therapy, the PML/RARa NCN decreased significantly (median 17, range 0–4,068). No significant difference was observed in the NCN by RQ-PCR between patients in continuous complete remission and those who relapsed (neither at diagnosis nor after induction).
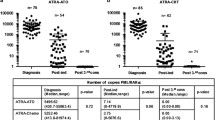
After induction therapy, thirteen out of 38 (34.2%) patients in hematologic remission displayed a negative RQ-PCR result, which was correlated with a lower probability of relapse (100 and 55.2% DFS at 5 years in the negative and positive RQ-PCR groups, respectively; P = 0.018; Fig. 2). Also of note, in the positive RQ-PCR group, the patients treated with ATRA + ATO in induction had a lower relapse rate when compared with those treated with ATRA alone (P = 0.03).
In addition, the kinetics of tumor burden reduction (log reduction in NCN between diagnosis and post-induction) were evaluated. Two cutoff points (2- and 3-log reduction) were evaluated to determine the prognostic value of the NCN on DFS. Indeed, no relationship between a PML/RARa NCN 2-log reduction after induction and the relapse risk was observed. However, a PML/RARa NCN 3-log reduction after induction indicated a better outcome (P = 0.047). In summary, there were 13 patients with negative RQ-PCR results and 25 patients with positive results after induction. Within the latter group, 21 patients showed a PML/RARa NCN reduction of less than 3-log, and the remaining 4 showed a reduction of more than 3-log. The DFS at 5 years was 100, 53.5, and 75% for the NCN-negative group (group-1), NCN reduction ≤ 3 log group (group-2), and NCN reduction >3 log but not negative group (group-3), respectively. There was no difference between group-2 and group-3 (P = 0.908). Similarly, there was no difference between group-1 and group-3 (P = 0.071). However, the difference between group-1 and group-2 was significant (P = 0.018).
In addition, in those with WBC <10 × 109/L, 20 patients were treated with ATRA alone in induction. After induction therapy, 8 out of 20 (40.0%) patients in hematologic remission displayed a negative RQ-PCR result, which was correlated with a lower probability of relapse (100 and 45.5% DFS at 5 years in the negative and positive RQ-PCR groups, respectively; P = 0.010). At the end of the third consolidation course, no patient was RQ-PCR-positive except for one who relapsed.
RQ-PCR monitoring in relapsed children
Eight patients relapsed during follow-up at a median time of 25.5 months (range 3–55). The characteristics of relapsed children are described in Table 3. One patient relapsed 3 times and died. The delivery of pre-emptive therapy with ATRA + ATO was successful in 2 of 8 patients, in whom MRD monitoring revealed molecular relapse at 10 and 29 months, respectively. The other five patients failed to be treated pre-emptively. Two patients showed molecular relapse and progressed to hematologic relapse 2 and 5 months later, respectively. Both of them failed to be treated pre-emptively because of a delay in returning to hospital. In three patients, the hematologic relapse was not predicted by molecular techniques (neither RQ-PCR nor RT-PCR). The final molecular analysis was performed 6, 7, and 32 months before relapse occurred, respectively.
Univariate analysis of prognostic factors
We conducted univariate analysis first, using the prognostic factors including the days needed to achieve CR, leukocyte count at present, different treatment, and PCR status after induction.
Patients who achieved CR at less than 42 days were considered as quick responders. Otherwise, they were slow responders. The median days to achieve CR was 30 days (range 28–41) in quick responders. The median days to achieve CR was 49 days (range 42–60) in slow responders. The 5-year estimate of DFS between quick and slow responders showed no difference (P = 0.208). The 5-year DFS of patients treated with ATRA alone was 64.5 ± 11.2%, and 90.9 ± 8.7% in patients treated with ATRA + ATO (P = 0.138). The 5-year DFS was 72.7 ± 9.8% in patients with WBC <10 × 109/L, and 76.2 ± 14.8% in patients with WBC ≥ 10 × 109/L (P = 0.812). In addition, the 5-year DFS was 100% in patients who were PCR-negative after induction, and 55.2 ± 13.4% in patients who were PCR-positive (P = 0.018).
Multivariate analysis
Multivariate analysis was performed to identify prognostic factors, including the WBC count, initial count of blasts in the marrow, induction treatment, and the status of RQ-PCR after induction, using Cox regression. Our multivariate study showed that only the negative status of RQ-PCR after induction was associated with DFS (P = 0.019) and no factors were associated with OS.
Discussion
In the present study, we analyzed the prognostic value of the standardized PML/RARa RQ-PCR assay in children with newly diagnosed APL. Our results indicated that patients with a negative RQ-PCR result after induction were correlated with a lower probability of relapse.
Over the last decade, relapse in APL has markedly reduced following the introduction of ATRA, ATO, and high-dose Ara-C into treatment. Furthermore, among patients who show relapse, a second CR (molecular) is achieved in almost 90% with ATO [1–5, 9]. Thus, Tallman MS questioned how important MRD was to detect relapse early, especially in the ATO era [6]. Molecular monitoring in APL may have become less important for the majority of patients. Therefore, the question of which subgroups are appropriate to restrict MRD assessment remains open.
The lack of a predictive value of the RT-PCR status post-induction is recognized in the International APL Guideline, which recommends that treatment is not modified on the basis of laboratory findings at this timepoint, because being PCR-positive in this early period may simply reflect delayed maturation instead of resistance [1]. However, the relationship between PCR negativity after induction and the relapse risk was not discussed. The detection of APL fusion transcripts after consolidation predicted an increased relapse risk [16]. However, the achievement of PCR negativity after consolidation completion does not represent a guaranteed cure because 90–95% of patients are PCR-negative at this stage.
Lee et al. [19] suggested, after the first consolidation, that patients with an MRD of less than 10−3 showed a better outcome. Then, patients at a high risk of relapse can be identified earlier during treatment. In our study, we observed a significant correlation between the MRD status after induction and outcome when PML/RARa NCN-negative and -positive results were considered. None of the 13 patients with a negative RQ-PCR result after induction relapsed, whereas 8 of the 25 patients with a positive RQ-PCR result underwent relapse (P = 0.034). However, our results contrast with those of Santamaría and Grimwade et al. [18, 20]. Such differences could be explained in part by the differences of induction therapy and ages of the patients. We suggest that PML/RARa NCN negativity after induction may reflect the sensitivity of leukemic clones to treatment and, thus, MRD monitoring by RQ-PCR might allow us to identify subgroups of patients at low risk of relapse earlier after induction in children. For such patients, MRD monitoring could be performed less frequently. However, for patients with a positive RQ-PCR result, especially those with a PML/RARa NCN reduction of less than 3-log, PML/RARa NCN should be monitored more frequently so as to modify treatment early to improve the outcome. Although the small sample and retrospective analysis may not have sufficient power to lead to a conclusion, further clinical research with large numbers of patients should be conducted.
In addition, if ATO were to be added to induction, the outcome would be improved, although PML/RARa NCN was positive (P = 0.03). Also of note, in patients with WBC <10 × 109/L, there was a 35% higher DFS rate for the ATRA + ATO group than ATRA group (P = 0.160). We confirmed that ATRA + ATO in induction was helpful to improve the outcome.
Recently, Grimwade and Tallman [6] suggested that monitoring MRD with a high-risk disease seemed reasonable, and monitoring MRD with a low- and intermediate-risk disease could be discontinued at the post-consolidation timepoint if the patient had achieved molecular remission. However, this notion could not be applied to our series. In our patients with WBC counts <10 × 109/L, the 5-year DFS was only 64.8% when ATRA was used alone in induction. In addition, there were 6 patients (6/16, 37.5%) who relapsed when PML/RARa was positive after induction. This discrepancy might be related to differences in the treatment protocols. We suggest that valuable subgroups for MRD monitoring might vary for different treatment protocols.
A key aim of MRD monitoring was to start pre-emptive therapy ahead of clinical relapse. However, this was not achieved in 5 of 8 patients. In two patients, the final molecular analysis was consistently performed 6 and 7 months before hematologic relapse occurred. It is regretful, we did not detect these patients every month, so we could not decide on the relationship between the PML/RARa NCN change and outcome. Also of note, their DFS was 26 and 27 months, respectively. This indicates that those patients should be monitored more frequently. The optimal schedule for detecting patients presenting with valuable subgroups for MRD monitoring should be the subject of further research.
Douer et al. [27] suggested that, in patients from Latin American, the rate of bcr1 PML/RARa subtype mRNA is significantly higher than the bcr1 rate reported in the literature among non-Latinos from the USA and Europe. Interestingly, in a small cohort of Chinese APL patients and in a Japanese group, the bcr1 or bcr1 + bcr2 rates were similar to the Latin American patients [28, 29]. They speculate that a non-European genetic factor might have migrated from East Asia through the Bering Straits into America approximately 12,000 years ago [27]. In Europe, de Botton et al. [12] reported that bcr2 and bcr3 isoforms were more frequently found in children (25 and 37.5%, respectively) than in adults (12 and 25%, respectively), but the difference was not significant (P = 0.09). However, in our patients, the bcr1 rate was higher than in those pediatric reports, and was similar to that which Douer et al. reported. Our series validated what Douer et al. speculated. In our study, we found no correlation between the bcr subtybe and clinical characteristics and PML/RARa NCN at diagnosis, although the small sample may not have sufficient power to detect such a relationship. Because of the small number of patients and differences in treatment, we could not investigate the association between the bcr subtypes and outcome.
Disadvantages of our study are the small number of patients and its retrospective nature, with patients not treated according to the same protocol. It is well known that APL is very rare in children. In addition, the recommendation for induction therapy in newly diagnosed APL remained the standard approach with ATRA in 2004, and we only added ATO to induction in patients with WBC counts ≥ 10 × 109/L or those who could not tolerate the side effects of ATRA in patients with WBC counts <10 × 109/L. Notwithstanding, in patients with WBC <10 × 109/L treated with ATRA alone in induction, a negative RQ-PCR result was still correlated with a lower probability of relapse (P = 0.010). Since February 2010, a prospective study has been performed in our study. More data will be available in the future.
In conclusion, PML/RARa-based MRD monitoring by RQ-PCR might allow us to identify subgroups of patients at low risk of relapse after induction, at least in the patients with similar regimens. The optimal schedule for MRD monitoring by RQ-PCR in child APL should be the subject of further research.
References
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91.
Zhang L, Zhu X, Zou Y, Chen Y, Chen X. Effect of arsenic trioxide on the treatment of children with newly diagnosed acute promyelocytic leukemia in China. Int J Hematol. 2011;93:199–205.
Kelaidi C, Chevret S, De Botton S, Raffoux E, Guerci A, Thomas X, et al. Improved outcome of acute promyelocytic leukemia with high WBC counts over the last 15 years: the European APL Group experience. J Clin Oncol. 2009;27:2668–76.
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2009;106:3342–7.
Adès L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115:1690–6.
Grimwade D, Tallman MS. Should minimal residual disease monitoring be the standard of care for all patients with acute promyelocytic leukemia? Leuk Res. 2011;35:3–7.
Testi AM, Biondi A, Lo Coco F, Moleti ML, Giona F, Vignetti M, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106:447–53.
Ortega JJ, Madero L, Martín G, Verdeguer A, García P, Parody R, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol. 2005;23:7632–40.
Imaizumi M, Tawa A, Hanada R, Tsuchida M, Tabuchi K, Kigasawa H, et al. Prospective study of a therapeutic regimen with all-trans retinoic acid and anthracyclines in combination of cytarabine in children with acute promyelocytic leukaemia: the Japanese childhood acute myeloid leukaemia cooperative study. Br J Haematol. 2011;152:89–98.
Creutzig U, Zimmermann M, Dworzak M, Urban C, Henze G, Kremens B, et al. Favourable outcome of patients with childhood acute promyelocytic leukaemia after treatment with reduced cumulative anthracycline doses. Br J Haematol. 2010;149:399–409.
Mantadakis E, Samonis G, Kalmanti M. A comprehensive review of acute promyelocytic leukemia in children. Acta Haematol. 2008;119:73–82.
de Botton S, Coiteux V, Chevret S, Rayon C, Vilmer E, Sanz M, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22:1404–12.
Diverio D, Rossi V, Avvisati G, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter AIDA trial. GIMEMA-AIEOP Multicenter AIDA Trial. Blood. 1998;92:784–9.
Diverio D, Rossi V, Avvisati G, De Santis S, Pistilli A, Pane F, et al. Molecular evaluation of residual disease as a predictor of relapse in acute promyelocytic leukaemia. Lancet. 1992;340:1437–8.
Grimwade D, Howe K, Langabeer S, Burnett A, Goldstone A, Solomon E. Minimal residual disease detection in acute promyelocytic leukemia by reverse-transcriptase PCR: evaluation of PML-RAR alpha and RAR alpha-PML assessment in patients who ultimately relapse. Leukemia. 1996;10:61–6.
Grimwade D. The significance of minimal residual disease in patients with t(15;17). Best Pract Res Clin Haematol. 2002;15:137–58.
Freeman SD, Jovanovic JV, Grimwade D. Development of minimal residual disease-directed therapy in acute myeloid leukemia. Semin Oncol. 2008;35:388–400.
Santamaría C, Chillón MC, Fernández C, Martín-Jiménez P, Balanzategui A, García Sanz R, et al. Using quantification of the PML-RARalpha transcript to stratify the risk of relapse in patients with acute promyelocytic leukemia. Haematologica. 2007;92:315–22.
Lee S, Kim YJ, Eom KS, Min CK, Kim HJ, Cho SG, et al. The significance of minimal residual disease kinetics in adults with newly diagnosed PML-RARalpha-positive acute promyelocytic leukemia: results of a prospective trial. Haematologica. 2006;91:671–4.
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27:3650–8.
Cassinat B, de Botton S, Kelaidi C, Ades L, Zassadowski F, Guillemot I, et al. When can real-time quantitative RT-PCR effectively define molecular relapse in acute promyelocytic leukemia patients? (Results of the French Belgian Swiss APL Group). Leuk Res. 2009;33:1178–82.
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9.
van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–28.
Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe against cancer program. Leukemia. 2003;17:2474–86.
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17:2318–57.
Douer D, Santillana S, Ramezani L, Samanez C, Slovak ML, Lee MS, et al. Acute promyelocytic leukaemia in patients originating in Latin America is associated with an increased frequency of the bcr1 subtype of the PML/RARalpha fusion gene. Br J Haematol. 2003;122:563–70.
Dong S, Geng JP, Tong JH, Wu Y, Cai JR, Sun GL, et al. Breakpoint clusters of the PML gene in acute promyelocytic leukemia: primary structure of the reciprocal products of the PML-RARA gene in a patient with t(15;17). Genes Chromosomes Cancer. 1993;6:133–9.
Fukutani H, Naoe T, Ohno R, Yoshida H, Miyawaki S, Shimazaki C, et al. Isoforms of PML-retinoic acid receptor alpha fused transcripts affect neither clinical features of acute promyelocytic leukemia nor prognosis after treatment with all-trans retinoic acid. The Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho). Leukemia. 1995;9:1478–82.
Acknowledgments
The authors would like to thank all patients and their parents for kindly participating in this study. This study was supported in part by a grant from the Science and Technology Support Key Program of Tianjin (09ZCZDSF03800), International Cooperation in Science and Technology (2010DFB30270) and The Natural Science Fund Foundation Project (08JCYBJC06500). Li Zhang and Zeng Cao performed the research and wrote the paper. Yao Zou and Min Ruan collected and analyzed the data. Qinghua Li performed the research. Xiaofan Zhu and Jianxiang Wang designed the research study.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Zhang and Z. Cao contributed equally to this work.
About this article
Cite this article
Zhang, L., Cao, Z., Zou, Y. et al. Quantification of PML/RARa transcript after induction predicts outcome in children with acute promyelocytic leukemia. Int J Hematol 95, 500–508 (2012). https://doi.org/10.1007/s12185-012-1034-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1034-9