Abstract
Dysnatremias are the most common electrolyte disorders, especially in critically ill and surgical patients. Brief notions of pathophysiology focused on the mechanisms and regulation of intracellular volume are needed to analyze dysnatremias. Such disorders may induce severe organ dysfunctions, especially cerebral dysfunction, and cause death. The practical diagnosis and therapeutic approach of hyponatremias and hypernatremias must follow safety rules of management to prevent iatrogenic complications. Because this book is dedicated to critically ill situations, we will focus essentially on acute and severe dysnatremias, especially for the treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Dysnatremias are the most common electrolyte disorders, especially in critically ill and surgical patients. Brief notions of pathophysiology focused on the mechanisms and regulation of intracellular volume are needed to analyze dysnatremias. Such disorders may induce severe organ dysfunctions, especially cerebral dysfunction, and cause death. The practical diagnostic and therapeutic approach of hyponatremias and hypernatremias must follow safety rules of management to prevent iatrogenic complications. Because this book is dedicated to critically ill situations, we will focus essentially on acute and severe dysnatremias, especially for the treatment.
2 Pathophysiology Definitions
This paragraph is voluntary summarized because it is largely detailed in another chapter (see “Water and Sodium Balance” chapter).
2.1 Body Compartments
Total body water (TBW) is the most compound of total body weight in an adult (50–70%). TBW distributes for two-thirds in the intracellular volume (ICV), and the remaining one-third in the extracellular volume (ECV) [1,2,3,4,5,6]. ICV and ECV are separated by cell membranes. ECV is divided into the plasma or the effective arterial blood volume (EABV) (25–30%) and the interstitial volume (70–75%). EABV is normally composed of 93% water that contains dissociated and non-dissociated solutes. Seven percent of the plasma volume is occupied by non-dissociated molecules (lipids and proteins) without water. As cell membranes are semipermeable, water crosses freely between the ICV and the ECV according to the osmotic transmembrane gradient [2, 7, 8]: water moves from the low to the high osmotic compartment until reaching the osmotic equilibrium. Therefore, ICV depends on the solute concentrations between both compartments. Only effective or impermeant solutes are able to create such an osmotic gradient across cell membranes, leading to water movements and changes in cell volume. Among them, sodium (Na+) is the major impermeant solute of the ECV and potassium (K+) of the ICV: thanks to the Na+-K+-ATPase pump located on cell membranes, Na+ is restricted to the ECV, whereas K+ is essentially located in the ICV. Therefore, total body sodium content (pool) is the major determinant of arterial pressure, while serum sodium concentration and its associated cations play a major role in determining plasma osmolality. Diffusive or ineffective solutes, i.e., urea and alcohols (ethanol, methanol, ethylene glycol), cross freely to the cell membrane and is distributed equally in the ICV and the ECV. Therefore, they are unable to create any change in cell volume. The osmotic effect of glucose depends on the nature of tissues: for non-insulin-mediated ones, glucose behaves as an ineffective solute; for insulin-mediated tissues in the presence of insulin, glucose remains a noneffective solute, but in case of insulinopenia or insulin resistance, glucose becomes an effective impermeant solute. At last, mannitol and glycerol, non-physiological solutes, are also extracellular effective solutes. Based on its osmotic properties, mannitol is one of the most popular treatments of cerebral edema (osmotherapy).
2.2 Osmolarities and Plasma Tonicity
Total plasma osmolarity is defined as the concentration of all osmotic solutes in a liter of plasma (mosm/L). Plasma osmolality is also the concentration of all solutes but in a kilogram of plasma water (mosm/kg). In normal conditions, both are very close as water contributes to 93% of 1 liter of plasma, but in case of severe hyperlipidemia or hyperprotidemia, the amount of plasma water decreases leading to an artificial decreased serum sodium concentration (see chapter “Plasma Tonicity and Hyponatremia”). Plasma osmolarity can be approached in different ways [1,2,3, 7,8,9]. The measured total plasma osmolality (mPosm [mosm/kg]), which is performed in the laboratory using the delta cryoscopic method, provides a global value of all osmoles present in the plasma, regardless of their normal or abnormal presence and their transmembrane diffusive properties. Posm can be easily calculated at bedside (cPosm [mosm/L]) considering the major electrolytes contained in plasma by the following formula: cPosm [mosm/L] = ([Na+ × 2] + glycemia + urea) (mmol/L) = 280–295 mosm/L. Because this calculation overrides abnormal (not usually measured) and minor plasma osmoles, mPosm is slightly higher than cPosm. The difference between these two parameters is known as the osmolar gap (OG = mPosm - cPosm), and its value is around 10 mosm/L. Plasma tonicity (or effective osmolarity) refers to only major effective osmoles and is calculated using the following formula: P tonicity = [Na+ × 2) + glycemia] (mmol/L) = 270–285 mosm/L. P tonicity is therefore the best practical parameter for evaluating accurately the ICV [2, 4, 7, 8]: a hypotonic stress always indicates an increased ICV (cell edema), whereas a hypertonic stress is always associated with a decreased ICV (cell shrinkage).
2.3 Body Water Balance and Its Regulation
Briefly, in physiological conditions, water intake and output are closely equilibrated, aiming to control TBW and consequently extracellular tonicity. This phenomenon allows to maintain a stable ICV and to avoid any changes in cell volume. Preservation of cell volume is fundamental to maintain cell functions and avoid cell death. Due to its essential contribution in plasma tonicity, serum sodium concentration, i.e., natremia, is the major parameter participating in TBW and cell volume. On the other hand, because body sodium is mainly extracellular, total body sodium amount determines ECV regulation.
TBW is controlled by three neurohormonal mechanisms: vasopressin (VP) or antidiuretic hormone (ADH), thirst, and the capacity of the kidney to concentrate or dilute urines. In physiological situations, thanks to these mechanisms, plasma tonicity remains stable despite wide daily variations in water intake or excretion [2,3,4, 8, 10]. VP and thirst are mainly triggered via an osmotic stimulus [1, 10, 11]. VP is synthetized by nuclei of the anterior hypothalamus, stored and released by the posterior pituitary. Tonicity is closely perceived by special neurons mainly located in the subfornical (SFO) and the organum vasculosum of the lamina terminalis (OVLT) of the circumventricular organs [3, 4, 6]. Such neurons are perfect osmoreceptors able to detect very low changes in plasma tonicity and cell volume. Modification in cell volume triggers the activation (cell shrinkage) or inhibition (cell edema) of some cationic protein channels, the transient receptor potential vanilloid (TRVP) of these osmoreceptors, leading finally to activate or inhibit VP release and thirst sensation [12,13,14]. Because any modification in cell volume is poorly tolerated (especially for the brain), VP release is modified for tonicity changes as small as 1–2%. In humans, above a threshold around 280 mosm/kg, VP secretion increases linearly with an increasing osmolality (from 280 to 330 mosm/kg); under this threshold, VP concentration remains undetectable in plasma [2, 15]. The threshold of thirst seems to be very close from that of VP, and its upper limit is very high depending on the total osmoles to be excreted (up to 25 l of urine output is possible with normal kidneys). VP release and thirst are also triggered by changes in arterial pressure and volemia via an activation of peripheral baro-/voloreceptors which are mainly located on the sino-aortic vascular walls [16, 17]. When both changes in osmolality and arterial pressure/volemia stimulate VP and thirst, there is an amplification of the phenomenon (e.g., hypotension and hyperosmolality). However, in case of opposite stimulus, the resulting effect depends on the severity of modification in volemia: only severe hypovolemia (of at least 5–10%) overrides the osmoregulation allowing extremely high VP concentrations [16, 17]. Other non-osmotic, non-volumic stimuli enable to activate VP release and thirst such as pain, morphinics, nausea, vomitings, and hypoxia.
Renal water excretion is mainly controlled by VP which promotes water renal reabsorption in the collecting tube. VP binds to its V2 receptors (V2R) which are located on the basal cell membrane [18]. The complex VP-V2R triggers a cascade of reactions, resulting finally in the expression and activation of water channels, i.e., aquaporins [5, 11, 19,20,21]. Aquaporin-2 activation allows high volume of water reabsorption by kidneys. In the absence of VP, urine is diluted with a maximum decrease in urine osmolarity of 50–100 mosm/L. The linear increase in VP concentration induces a linear increase in urine concentration with a maximal urine osmolarity of 1200 mosm/L. Above this value and despite a persistent increase in VP concentration, urine cannot concentrate more.
In summary: usually, thanks to VP and thirst mainly, TBW is maintained constant allowing to control plasma tonicity and consequently cell volume. The kidney is the central organ which regulates urine concentration or dilution according to plasma VP concentration and water intake. Thirst is the second major mechanism which allows to prevent the development of severe hypertonicity. Therefore, inappropriate secretion of ADH (SIADH) may be responsible for inappropriate water reabsorption by the kidney, leading to hypotonic hyponatremia. On the other hand, because thirst has no real upper limit, hypertonicity is theoretically impossible, except in case of abnormal thirst behavior (elderly patients) or difficulties to drink (prolonged gastric suctioning, coma, etc.) [18, 22].
2.4 Cell Volume Regulation-Osmoregulation
Cell volume modifications are poorly tolerated and a constant cell volume is essential to prevent cell damages and dysfunction. Cell edema secondary to a hypotonic stress can cause cell rupture; hypertonicity induces cell shrinkage which can promotes damages of the cytoskeleton, breaks in DNA, and apoptosis [13]. Because the brain is maintained in a non-extensible skull, brain swelling or shrinkage exposes to lethal brain injury, especially when changes in volume are rapid. Hypotonic-induced cerebral edema can be complicated by refractory intracranial hypertension and ultimately by brain death; hypertonic-induced brain shrinkage can cause intracerebral hemorrhage with poor neurological outcome or death too. This explains why clinical manifestations of hyponatremia and hypernatremia are primarily neurologic and life-threatening but nonspecific.
Fortunately, all but almost cerebral cells are not really perfect osmometers (except those responsible for VP stimulation located in the circumventricular organs). Indeed, brain cells enable to limit their volume changes related to an osmotic stress. Such protective effects, i.e., cerebral osmoregulation, result from an adjustment of the intracellular solute content to the extracellular one [1,2,3, 10, 13, 23]. This phenomenon aims to limit the development of a transmembrane osmotic gradient and consequently cell volume modifications. Cell volume regulation consists in a regulatory volume decrease (RVD) in response to hypotonic-induced cell swelling and of a regulatory volume increase (RVI) in response to hypertonic-induced cell shrinkage [24,25,26,27,28,29,30]. Two types of effective solutes, i.e., osmoprotective molecules, are implicated in this regulation: the inorganic are electrolytes (mainly Na, K, Cl), and the organic are idiogenic osmoles (or osmolytes) and consist in amino acids, polyols, and triethylamines. Chloride via its voltage-dependent channels (ClC) and its sodium (NCC), potassium (KCC), sodium/potassium (NKCC) cotransporters is also essential for regulating brain volume [31, 32]. NCC and NKCC favor sodium, potassium, and chloride entry in the cell and are inhibited by an increased intracellular chloride concentration. Plasma hypotonicity activates KCC3 leading to extrude potassium and chloride from the cell and to attenuate cerebral edema (RVD). On contrary, plasma hypertonicity activates NKCC1 which induces the entry of sodium, potassium, and chloride in the cell and finally decreases cell shrinkage (RVI). An acute hypotonicity triggers quickly in some minutes to 2–4 h an extrusion of inorganic osmoles. This phenomenon protects rapidly but moderately and incompletely the brain cell from volume changes. Only prolonged (chronic on 24–48 h) hypotonicity enables to strongly blunt the osmotic gradient and avoid relevant cell volume changes, thanks to the excretion of osmolytes. This mechanism takes a longer time required to obtain synthesis or metabolism of these organic solutes by cells. In this situation, cerebral osmoregulation is delayed but is strongly efficient and complete and restores the brain volume (Fig. 2.1). Cerebral osmoregulation induced by a hypertonic stress consists in opposite shifts of electrolytes and organic osmoles, leading also to control cerebral volume.
Mechanisms of brain volume regulation during plasma hypotonic stress (osmoregulation). (a): Normal brain volume: water and effective osmole contents are 100% (b): Immediate plasma hypotonicity: plasma hypotonicity induces water extrusion from brain cells because the amount of intracellular effective osmoles cannot change immediately. The resulting high transmembrane osmotic gradient provokes brain edema. (c): Acute plasma hypotonicity: after 2–3 h of plasma hypotonicity, brain cells enable to reduce their amount of effective osmolytes by extruding electrolytes (inorganic osmoles including potassium and chloride essentially) and attenuate the transmembrane osmotic gradient. As a result moderate and incomplete brain edema develops. (d): Chronic plasma hypotonicity: after 24 h or more prolonged plasma hypotonicity, a substantial extrusion of organic effective osmoles (osmolytes such as polyols and amino acids) from brain cells blunts the transmembrane osmotic gradient. As a result, cerebral osmoregulation appears quasi-complete and the brain recovers a subnormal volume
Aquaporin-4 channels (AQP4) are also largely involved in cerebral cell volume changes [25, 33, 34]. They are strongly present in the brain, located on glial cells which are close from arterial vessels and subarachnoid spaces, and in the supraoptic nucleus. Indeed, during hyponatremia, knockout animals for AQP4 show an intense reduction in cerebral edema and in mortality as compared with the control [35]. Hypertonic saline seems to induce its antiedematous effect, thanks to these perivascular AQP4 [34]. However, AQP4 are also responsible for a reabsorption of water in vasogenic edema related to brain tumor [36].
Non-osmotic stress as ischemia-reperfusion, hypothermia, or acidosis enables to trigger cell volume changes [29]. Chloride, potassium channels, and sodium-potassium-chloride (NKCC) cotransporters seem to be involved in this phenomenon which is controlled by the intracrine renin-angiotensin system.
In summary, the efficiency of cerebral osmoregulation depends strongly on the time of development and the duration of the osmotic disorder. Because of an incomplete cerebral osmoregulation, rapid dysnatremias are more often symptomatic and life-threatening and require an emergent aggressive treatment. Classically, longer development of hyponatremias, which is commonly associated with a quasi-complete cerebral volume regulation, is more usually asymptomatic or poorly symptomatic and thus does not require an emergent treatment. Moreover, due to a downregulation of transporters, the recovery of modifications in brain osmoles takes a longer time when cerebral osmoregulation is complete. Therefore, a rapid treatment becomes an osmolar stress which exposes to a risk of overcorrection with an inverse osmotic gradient. Such a risk is particularly well known in patients presenting chronic hyponatremia which expose to the risk of osmotic demyelination syndrome if natremia normalizes too rapidly. Efficiency of osmoregulation is also affected by hypoxia and sex, female being less protected than male [37, 38].
3 Epidemiology: Prognosis
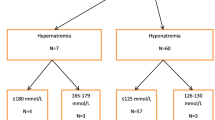
Dysnatremias are the most common electrolyte disorders [39,40,41,42,43]. Most studies reported a frequency between 25 and 50%, depending on the patient’s conditions, the threshold of abnormal values, and on the delay of appearance of dysnatremia. Hoorn et al. [39] showed that 15–30% of hospitalized patients experienced moderate (130–135 mmol/L) and 3% severe (<125 mmol/L) hyponatremia. Among the severe one, 36% were symptomatic and only 20% of them survived. Most frequent causes of hyponatremia are the syndrome of inappropriate secretion of antidiuretic hormone (SIADH), surgical patients (especially neurosurgical patients), and drug-induced hyponatremia, while hypernatremia is frequently iatrogenic. Numerous studies found that dysnatremias, even moderate, are independently associated with an increased risk of in-hospital mortality [39, 41, 44]. A recent meta-analysis including 81 studies found that 17.4% of patients (n = 147,948) experienced a moderate hyponatremia (125–135 mmol/L), which was significantly associated with an increased risk of overall mortality (RR = 2.6) and morbidity [41]. Moreover, mortality was inversely correlated with the depth of hyponatremia.
Several recent studies are focused on dysnatremias in critically ill patients [45,46,47,48,49]. Most of them considered only dysnatremias at admission in intensive care unit (ICU) and showed that hyponatremia was more frequent than hypernatremia (11–26% vs. 2.5–9%, respectively). In a retrospective study performed in 77 ICUs over a period of 10 years, Funk et al. [47] showed that 24% of patients presented dysnatremias at admission, 17.3% were hyponatremic and 6.9% were hypernatremic. According to the level of serum sodium concentration, patients were distributed as follows: slight, moderate, and severe hyponatremia in 13.8%, 2.7%, and 1.2%, respectively, and slight, moderate, and severe hypernatremia in 5.1%, 1.2% and 0.6%, respectively. Hypo- and hypernatremia were independent risk factors of mortality and poor outcome. The risk is raised with the importance of serum concentration abnormality (OR from 1.32 to 1.81 for hyponatremia and from 1.48 to 3.64 for hypernatremia). The global incidence of ICU-acquired dysnatremias varies from 30 to 40% of patients, and hypernatremia seems to have twice the incidence of hyponatremia [46, 48]. Globally, hyponatremia developed and persisted within 1–3 days and hypernatremia within 1–5 days. The impact of ICU-acquired dysnatremias on morbi-mortality in critically ill patients remains controversial [45, 46, 48]. In a recent large cohort prospective observational study, hyponatremia at admission in ICU was found in 34.3% of patients, and 36.2% of them were caused by a SIADH [50]. Patients were diagnosed as euvolemic in 58.9%, hypervolemic in 26.3%, and hypovolemic in 14.8% of cases. The authors confirmed that hyponatremia was an independent risk factor for an increased mortality (HR = 1.61) and morbidity (longer length of stay and mechanical ventilation).
Finally, none of these studies proves the causality between dysnatremias and the increased morbi-mortality. Indeed, in a retrospective observational study, Chawla et al. [43] reported that more patients presenting a moderate hyponatremia (<120 mmol/L) died than those with severe hyponatremia (<110 mmol/L), with a peak of mortality between 120 to 124 mmol/L. The authors hypothesized that severe hyponatremias were essentially due to a drug-associated effect, while moderate ones were observed in patients with numerous comorbidities and severe illnesses. Therefore at this time, it is not possible to conclude if dysnatremia is a simple marker or the direct cause of death.
4 Hyponatremias
4.1 Hyponatremia and Plasma Tonicity
Hyponatremia not always reflects plasma hypotonicity with its related risk of brain edema. Therefore, the first step in the management of hyponatremia is to eliminate non-hypotonic hyponatremias [2, 9, 13, 23, 51, 52]:
-
Pseudohyponatremias occur in case of a marked increase in lipid or protein plasma concentration. Hyponatremia is the result of a laboratory artifact due to the high volume occupied by excessive lipids or proteins in plasma and to the sample dilution before measurements [53]. In this situation, the total number of solute particle in the water phase of plasma is unchanged. Therefore, the direct measurement of serum osmolality (mosm/kg), which is performed on undiluted sample, confirms that hyponatremia is isotonic (Fig. 2.2). As a practical consequence, no shift of water occurs and there is no risk of brain edema.
-
False hyponatremias are caused by the abnormal accumulation of effective solutes other than sodium in the ECV. The resulting plasma hyperosmolality causes an osmotic shift of water from the ICV to the ECV that induces in turn a dilutional hyponatremia. This hyperosmolar hyponatremia can be isotonic or hypertonic, depending on the severity of excessive effective osmoles and on the osmotic-induced polyuria. When associated with hypertonicity, hyponatremia conducts to cell shrinkage which requires rehydration, whereas isotonic hyponatremia has no impact on cell volume. The most common cause of hypertonic hyponatremia is hyperglycemia [1, 2, 9, 23, 30, 54, 55]. In this situation, calculation of plasma tonicity remains the easier and most accurate tool to estimate possible changes in ICV, regardless the actual serum sodium concentration. Alternatively, natremia can be corrected considering the glucose elevation. Corrected natremia [cNa+], which estimates what would be natremia with normoglycemia, can be calculated using the following formula: [cNa+] = (measured [Na+] + [glycemia × 0.45 [mmol/L) (for every 5.5 mmol/L increase in glycemia, add 2.4 mmol/L to measured natremia) [54]. Other effective osmoles may accumulate in plasma and cause hypertonic hyponatremia: mannitol, glycerol/glycine surgical irrigant solutions, hyperosmolar radiocontrast media, histidine-tryptophan-ketoglutarate, and maltose. Due to the context, such situations are usually easily recognized. If necessary, an elevated osmolar gap, which is calculated by the difference between the measured and the calculated plasma osmolarity, will confirm the presence of excessive abnormal plasma effective osmoles.
-
Hypotonic hyponatremias are those at risk of cerebral edema and require a specific treatment.
Plasma sodium concentration in normal and increased fraction of solid plasma particles. (a): Normal repartition of solid and water plasma phases: 1 l of plasma distributes normally into 7% of solid phase (lipids and proteins) and 93% of water phase (containing electrolytes, especially sodium). Therefore, 1 kg of plasma water is very close from 1 l of plasma and normal plasma sodium concentration per kg, or per liter of plasma is also very close (140 ± 2 mmol/L). (b): Increased solid plasma phase (hyperlipidemia or hyperprotidemia): the increase in solid phase decreases in proportion of plasma water phase, and 1 kg of water plasma differs from 1 l of plasma. Therefore, if ion measurements assumed a constant distribution between the water and solid phase, sodium concentration per liter of plasma will be underestimated (related to a dilution effect), while sodium concentration per kilogram of plasma water remains normal: this is the isotonic pseudohyponatremia. Gray circles represent sodium particles
The following paragraphs will be focused on the sole hypotonic hyponatremias. The confirmation of hypotonicity is a prerequisite and the first step of the diagnosis of hyponatremia [2, 9, 13, 23, 30]. Therefore, the European clinical practice guidelines (ECPG) [9] and the American guidelines [23] recommend firstly to exclude hyperglycemic hyponatremias by measuring serum glucose concentration. Hyponatremia associated with a low measured osmolality always reflects hypotonicity. However, because it is not available everywhere and every time, such a measurement cannot be recommended. On the other hand, plasma tonicity which can be performed easily at bedside is accurate enough to diagnose hyponatremias at risk of brain edema and must be calculated in all situations associated with hyponatremia.
4.2 Definitions
Hyponatremia is consensually defined as a serum sodium concentration <135 mmol/L. The management of hyponatremic patients depends on its severity. Consequently, it is essential to define “severe” hyponatremia. Unfortunately, many definitions are reported in the literature [9, 13, 23, 30, 56, 57]. Hyponatremia below 120–125 mmol/L is usually considered as severe [13, 23, 55, 56]. The speed of development of hyponatremia is also a classical parameter of severity. Acute hyponatremia is defined by a rate of development <48 hours and is considered as severe due to the risk of brain edema, by opposition with chronic hyponatremia developed in more than 48 h. This classification is consistent with the pathophysiology of brain regulation and consequently with therapeutic strategies: acute hyponatremia requires an aggressive immediate treatment to limit brain edema, while chronic hyponatremia needs a slow correction aiming to avoid osmotic demyelination. Unfortunately, the speed of development of hyponatremia is not often known, especially in critical situations. However, some conditions and drugs are particularly associated with an acute decrease in natremia [9, 13] (Table 2.1). Among them psychotic polydipsia, exercise-induced hyponatremia, postoperative period, and intracranial injuries are frequent. After eliminating these situations, hyponatremia should be presumed as chronic despite no precise speed of its development.
The depth of hyponatremia as well as its delay of development is not only responsible for the efficiency of cerebral osmoregulation. Indeed, sex, age, hypoxia, and individual variations contribute also to this regulation [57]. For this reason, the ECPG [9] decided to provide three definitions of hyponatremia based on the (1) biochemical severity, (2) time of development, (3) and symptoms and their severity which is the first parameter to consider for the treatment (Table 2.2). These definitions were elaborated to avoid usual confusion by physicians and to recommend hierarchical therapeutic guidelines with successive steps.
4.3 Pathophysiology and Classification
Hyponatremia is primarily a disorder of water balance indicating a relative excess of body water to body solute (sodium): water intake (or infusion) exceeds kidney free water excretion. In most clinical situations, both mechanisms, i.e., excessive water intake and impaired and inappropriate urine dilution, are associated. Nevertheless, based on the major mechanism of development, hyponatremias are classified into three categories that are associated with three ECV status [2, 23, 30, 56, 58,59,60]:
-
Euvolemic hyponatremias are due to an absolute body water excess without change in total body amount. This is classically observed in case of inappropriate kidney water reabsorption (SIADH) or in case of excessive water intake (psychotic polydipsia).
-
Hypovolemic hyponatremias are due to excessive sodium losses which cause volume depletion and in turn increase VP secretion creating ultimately water retention with hyponatremia despite plasma hypotonicity [58]. Such conditions may be provoked by gastrointestinal kidneys or skin disorders.
-
Hypervolemic hyponatremias are due to excessive renal sodium and water reabsorption. In these situations, the effective arterial blood volume (EABV) is usually low, while the interstitial one is increased (as expressed clinically by peripheral edema). Effective hypovolemia triggers both VP and renin-angiotensin secretions, leading to renal water and sodium reabsorption, respectively. In such patients, both body water and sodium amount are elevated as observed in various congestive conditions (heart and liver failures, nephrotic syndrome, kidney diseases).
Such a classification is conceptually useful to understand major mechanisms according to the underlying cause of hyponatremia and in turn to select most appropriate therapies according to the ECV presentation. But in clinical practice, ECV assessment at bedside remains very difficult (except the increased ECV expressed by edema). Moreover, such clinical differentiations are not so clear-cut and depend on kidney function, drugs, and patient’s conditions. For example, a patient with SIADH can be treated concomitantly with diuretics that cause effective hypovolemia. Nevertheless, causes of hyponatremia remain commonly classified considering the theoretical volume of the extracellular compartment (see paragraph “etiologic diagnosis”).
5 Diagnosis
5.1 Clinical Symptoms
Because hypotonicity can induce cerebral edema, most clinical signs of hyponatremia are neurologic, but not specific [2, 10, 13, 23, 30, 56, 59]. The severity of hyponatremic encephalopathy depends on the importance of brain edema and its consequence, intracranial hypertension. Therefore, the severity of symptoms varies according to the efficiency of cerebral osmoregulation. Most symptoms are summarized in Table 2.2. In acute hyponatremia, various parameters are associated with an increased risk of cerebral edema expressed by clinical signs of encephalopathy: female (risk multiplied by 28), children, old female treated with thiazides, psychiatric polydipsia, and hypoxia [25, 26]. In all cases, the history and the context must be rigorously identified in order to confirm the causal relationship between clinical signs and hyponatremia. Complementary exams can be needed to eliminate other causes of encephalopathy (CT scan, EEG). Some studies reported that neurological signs associated with hyponatremia might be related not only to brain edema but also to alterations in brain excitability induced by a hypotonic-related neurotransmitter exocytosis (glutamate) [26].
For a long time, chronic hyponatremia has been thought to be asymptomatic and without any deleterious consequences. Since a decade, growing data show that mild chronic hyponatremia is an independent risk factor of various side effects including falls, gait instability, falls-related fractures, impaired attention, and death. These symptoms are independent from age and sex [61,62,63,64,65]. Kinsella et al. [63] found that the incidence of hyponatremia was higher in women presenting fractures than in those without fractures (8.7 vs. 3.2% or 2.25). Hoorn et al. [64] confirmed in elderly patients that chronic hyponatremia was an independent risk of fractures. Cognitive impairment may favor falls and fractures. Chronic hyponatremia is also responsible for a direct alteration in bone density. Indeed, bone, cartilage, and connective tissues serve as a sodium reservoir. Chronic hyponatremia stimulates directly the osteoclast activity, leading to a decreased bone mineral activity [63, 65, 66]. Moreover, decreased bone density correlates closely with the depth of hyponatremia.
In summary: chronic hyponatremia is highly more frequent than the acute one, but the latter is usually associated with moderate or severe neurological symptoms which need an emergent aggressive treatment aiming to prevent or reduce brain edema by increasing rapidly natremia. Many data suggest nowadays that mild chronic hyponatremia is not totally asymptomatic and is associated with cognitive impairment, falls, and fracture. This probably justifies to increase serum sodium levels to subnormal values, but always carefully at a slow rate to avoid osmotic demyelination syndrome (ODS).
5.2 Etiologic Diagnosis
The determination of the underlying cause of hypotonic hyponatremia is crucial, but may be impossible at admission and should not delay the emergent treatment of acute hyponatremias. In practice, such a diagnosis can be performed concomitantly with the treatment. In chronic asymptomatic/mild symptomatic hyponatremia, the determination of the underlying cause is needed and precedes the specific treatment. Therefore, the first step in the management of symptomatic acute hypotonic hyponatremia consists in increasing urgently serum sodium concentration, while the determination of the underlying cause is needed before beginning any treatment of chronic hypotonic hyponatremia [9]. For practical reasons, we decided to detail the etiologic approach in a paragraph before treatment, even not the order advised in practical algorithms.
Questions focused on treatments and history of the patient are crucial to approach the underlying cause of hyponatremia. The most common etiologic categorization is still based on the pathogenetic modifications in the ECV. But, despite a thorough clinical examination, this strategy is not realistic in clinical practice. The assessment of ECV is critical and not accurate (low sensitivity and specificity) leading to misclassifications [67]. Regardless of the parameters used, the diagnosis performance is always better with an algorithm than without [44, 68]. Both urine osmolality (Uosm) and sodium concentration (UNa) are needed, but the place of these parameters in the etiologic algorithm varies according to recommendations (Fig. 2.3) [9, 23].
A Uosm ≤100 mosm/L clearly indicates an excessive water intake. UNa with a threshold of 30 mmol/L has a good sensitivity and specificity to distinguish hypovolemia from euvolemia and hypervolemia. However, diuretics are responsible for an elevated UNa regardless of the ECV status [44, 68,69,70,71]. For an accurate interpretation, urine measurements must be performed as soon as possible (before any treatment if possible) on a spot urine sample taken simultaneously with a blood sample. Despite such recommendations, Uosm and UNa are measured in only 10% and 27%, respectively, leading to an absence of determination of the underlying cause of hyponatremia [44]. Other laboratory tests do not have to be performed systematically, but may be useful: serum copeptin, urea and acid uric concentrations, and fractional sodium and uric acid excretion [69, 72, 73]. Among them, fractional uric acid excretion <12% seems to be the most accurate to differentiate low effective arterial blood volume (EABV) from euvolemic hyponatremias. Fractional uric acid excretion might be used for ambiguous conditions such as diuretic treatments or differential diagnosis between SIADH and cerebral salt wasting syndrome. Diuretic treatment does not exclude the contribution of other causes of hyponatremia, especially if hyponatremia persists despite stopping diuretics. For all these reasons, the ECPG [9] who wanted a very pragmatic approach, decided to propose an etiologic algorithm based on the determination of Uosm, followed by UNa on priority before the ECV assessment (Fig. 2.3).
5.2.1 Urine Osmolality ≤100 mosm/kg
These situations are due to excessive water intake and low solute intake. The ECV is normal and VP secretion absent as expressed by the low Uosm which is the marker of the appropriate response of kidney: maximal dilution of urines in response to excessive water intake. Hyponatremia develops only when the kidney reaches its maximal possibility for diluting urines. Thus, such conditions require very high volume and rapid water intake (20–30 l per day), usually associated with an impairment in solutes and free water renal loss [9, 10, 49]. Most causes are psychotic polydipsia or self-water intoxication, beer potomania, and low solute intake. Polydipsia-related hyponatremia occurs in 60% of psychiatric patients.
5.2.2 Urine Osmolality >100 mosm/kg
Major causes of hyponatremias with a high urine osmolality are SIADH (30–40%) and diuretic treatments, especially thiazides (20%) [23, 44, 72, 74,75,76,77]. The determination of the underlying cause requires a further UNa determination which allows to distinguish situations with low UNa (≤30 mmolL) from those with high UNa (>30 mmol/L).
5.2.2.1 UNa ≤30 mmol/L
A low UNa strongly suggests a low EABV, even in patients on diuretics. Hypovolemic hyponatremia refers to patients with reduced global ECV. Clinical signs such as orthostatic hypotension, tachycardia, arterial hypotension, and dry mucus membrane suggest hypovolemia. Biological parameters of functional acute kidney injury can be helpful, but are neither sensitive nor specific, and can be altered by other factors. Correction of low volemia with 0.9% NaCl will correct concomitantly hyponatremia by stopping VP non-osmotic vasopressin secretion:
-
Hyponatremia with low ECV (hypovolemic) can be caused by sodium losses issued from the gastrointestinal tract. In case of substantial vomitings, hyponatremia is classically accompanied by metabolic alkalosis (concomitant losses of chloride). By contrast, profuse diarrhea induces hyponatremia associated with metabolic acidosis. Large cutaneous sodium losses represent another cause of hypovolemic hyponatremia as observed in extensive burned patients. At least, hypovolemic hyponatremia can be caused by prolonged administration of diuretics and an excessive water intake/infusion that is frequently associated and which contributes to worsen hyponatremia.
-
Hyponatremia with high ECV (hypervolemic) but low EABV is present in 25% of hyponatremic critically ill patients and is independently associated with an increased risk of morbi-mortality [78]. Modifications of ECV are complex, characterized by a high global ECV related to the increased interstitial compartment while the EABV is reduced. Congestive heart failure and cirrhosis are classical causes of hypervolemic hyponatremia [49, 56, 60, 79,80,81]. In these conditions, the decrease in EABV is due to the reduction in cardiac output and vasoplegia. These modifications activate the sympathetic nervous system, the renin-angiotensin-aldosterone axis, and VP release, leading to sodium and water renal reabsorption and finally hyponatremia. Therefore, patients present signs of volume overload: pulmonary edema, ascites, and peripheral edema. The diagnosis is usually easy to make on the context. The reduced EABV is responsible for a secondary hyperaldosteronism with the low UNa, except for the frequent situation of patients receiving diuretics [49, 56, 60, 79]. In nephrotic syndrome, the reduction in EABV is usually attributed to hypoalbuminemia and the resulting low oncotic pressure and vasoplegia, leading to non-osmotic VP release. Hypervolemic hyponatremia is commonly worsened by diuretics and fluid absorption and infusion.
5.2.2.2 UNa >30 mmol/L
When UNa is elevated, a very pragmatic approach is to confirm or eliminate a possible treatment by diuretics or kidney disease, both conditions that frequently affect natriuresis independently of ECV and may conduct to erroneous diagnosis [76, 82]. Moreover, both situations do not eliminate possible other causes of hyponatremia. On the other hand, UNa can also be reduced despite diuretics (especially with long-term treatment) and kidney disease (Fig. 2.3). Therefore, regardless of diuretics or kidney disease, hyponatremia associated with a UNa >30 mmol/L can be classified according to the ECV which can be reduced or normal:
-
Hypovolemic hyponatremia can be caused by gastrointestinal or renal losses. Vomitings are usual causes. Renal losses can be due to various causes. Cerebral salt wasting syndrome (CSW) is well documented in patients with intracranial injury such as subarachnoid hemorrhage, traumatic brain injury, brain tumors or infections, and neurosurgical postoperative period [49, 83,84,85]. Abnormal salt wasting may be related to kidney dysfunction (renal wasting syndrome, RWS) [82]. For some authors, regardless of the primary organ dysfunction, i.e., the brain or kidney, this is a unique syndrome, and the distinction between CSW and RSW is a simple semantic differentiation of a similar disorder which occurs in two different contexts [85, 86]. Primary adrenal mineralocorticoid deficiency (Addison disease) can cause hypovolemic hyponatremia associated with hyperkalemia due to renal sodium losses and potassium reabsorption.
-
Euvolemic hyponatremia. The SIADH is the most frequent cause of euvolemic hyponatremia. The inappropriate antidiuresis may result from an inappropriate secretion of ADH from the pituitary gland or an ectopic site. It may also result from vasopressin receptors or aquaporin abnormalities (genetic or acquired mutations). Therefore, SIADH is one clinical setting of the large conditions of the syndrome of the inappropriate antidiuresis (SIAD) [23, 49, 72, 74, 87, 88]. ECV is usually normal because after an initial phase of increased total body water, renal sodium excretion is stimulated in response to natriuretic factors. Consequently, despite a persisting high plasma concentration in ADH, patients present hypotonic urines related to a decreased number of V2R and of AQP2 expression on the collecting tube: this is the “vasopressin escape phenomenon.” Additional abnormalities of thirst sensation can contribute to worsen hyponatremia. The diagnosis of SIAD(H) is based on classical absolute (or essential) criteria that includes (1) hypotonic hyponatremia (plasma tonicity <275 mosm/kg), (2) urine osmolality >100 mosm/kg despite a decreased plasma tonicity (abnormal antidiuresis), (3) clinical euvolemia, (4) urine sodium concentration > 30 mmol/L with normal sodium and water intake and no diuretics, and (5) absence of adrenal, thyroid, pituitary, and renal insufficiency (Table 2.3). Relative (or supplemental) criteria include (1) (partial) correction of hyponatremia with fluid restriction, (2) failure to correct hyponatremia with 0.9% saline infusion, (3) serum uric acid <0.24 mmol/L, (4) serum urea <3.6 mmol/l, (5) fractional sodium excretion (FeNa) > 0.5%, (6) fractional urea excretion (Fe urea) >55%, and (7) fractional uric acid excretion(FeUA) > 12%. Calculation of fractional excretion is based on the following formula: Fe X (%) = (UX/PX) × (Pcreat/U creat) × 100, x being the substance and UX, PX, P creat, and Ucreat being the urine and plasma concentrations of X and creatinine, respectively. Plasma VP measurement does not frequently contribute to the diagnosis. Indeed, four situations have been observed in patients with SIADH receiving 0.9% NaCl: (1) type A or “random” SIADH, present in 30 to 40% of patients, is characterized by a constant ectopic raised VP plasma concentration, regardless of plasma tonicity; (2) type B or “leak” SIADH found in 30% of patients is characterized by a raised basal VP concentration which increases normally with plasma hypertonicity; (3) type C or “reset osmostat,” also present in 30% of patients, is characterized by a decreased threshold of VP secretion leading to high VP plasma concentration despite low plasma tonicity; and (4) type D in 10% of patients is characterized by a normal VP secretion: this is the antidiuresis nephrotic syndrome which is observed in children and is due to abnormal activation of V2R (gene mutations) [17]. In clinical practice, because of difficulties to assess ECV and because SIADH and CSW appear in similar contexts, it is recommended to consider additional biological parameters such as FeNa and FeUA to distinguish them (Table 2.4). In all cases, SIAD(H) remains a diagnosis of exclusion. There are numerous causes of SIAD(H) (Table 2.5), including essentially cancers (digestive and pulmonary), central nervous system disorders, and pulmonary diseases [9, 70, 87, 88]. The perioperative period is considered to be at high risk of SIAD because VP secretion is triggered by several non-osmotic stimuli (nausea, vomitings, pain, hypoxia, morphinics, hypovolemia) (see paragraph “perioperative hyponatremia”). A lot of drugs enable to induce SIADH such as selective serotonin reuptake inhibitors, antidiabetics, and thiazides which are responsible of SIADH in 10–30% of treated patients [75, 77, 89,90,91]. Table 2.5 summarizes most drugs at risk for developing hypotonic hyponatremia with SIADH. Finally, the cause of SIAD(H) remains frequently unidentified [70].
-
Endocrinological diseases: secondary adrenal insufficiency/glucocorticoid deficiency [49, 72, 87] because of low concentration of cortisol fails to suppress corticotrophin release factor leading to an increased release in ACTH and VP. Renal water excretion is also altered independently of VP [92]. Only severe hypothyroidism (myxedema) could cause hyponatremia because of a reduced cardiac output and glomerular filtration rate.
-
5.2.3 Special Conditions Associated with Hyponatremia
- Exercise-associated hyponatremia (EAH) [93,94,95,96]: EAH occurs in 5–28% of intense and prolonged physical activity (marathon). Risk factors involved in such disorder are combined: excessive water intake (hypotonic solutions) associated with a non-osmotic inappropriate secretion of ADH, long race duration (>4–6 h), and extreme temperatures. The production of IL6 (related to muscle glycogen depletion), stress, and nausea with hypovolemia triggers ADH secretion. Hyponatremia can be hyper-, hypo-, and euvolemic. Clinical presentation has no specific aspect and appears as an acute moderately or severely symptomatic life-threatening condition:
-
Postoperative hyponatremia: it is the third cause of acute in-hospital acquired hyponatremia. It develops preferentially in children and women [39, 49, 84, 97]. Hyponatremia results from a non-osmotic stimulation of ADH and is precipitated by hypotonic fluid infusions and perioperative impairment in renal urine dilution [97,98,99]. Postoperative hyponatremia may reach 30% of patients in surgeries at risk such as neurosurgery and orthopedic and gynecologic surgeries. However, only 1–2% of them are severe. The incidence of hyponatremia in neurosurgical patients is elevated, depending on the underlying cause: 10–20% in patients undergoing surgery for brain hematoma, hypophysectomy, intracranial tumors, and traumatic brain injury; it reaches 50% in subarachnoid hemorrhage [49, 83, 100, 101]. Hyponatremia develops acutely within the 2–4 days following surgery and is frequently symptomatic. Clinical presentation may be amplified by a preexistent brain edema or injury. The mechanism of hyponatremia is complex, and the distinction between SIADH and CSW remains difficult [102, 103]. ACTH deficiency is an additional confusing factor. In this setting, the North American guidelines recommend to treat hyponatremia as soon as its concentration is below 131 mmol/L [83].
-
Transurethral resection of prostate (TURP) syndrome: the irrigating solution for endoscopic surgery can be responsible of acute hyponatremia [104,105,106]. Large volume of hypotonic glycocolle solutions may be absorbed directly intravascularly or through the interstitial tissue. At the initial phase, hyponatremia is “translocational,” i.e., hypertonic (due to glycocolle), and is associated with a transitory hypervolemia which may cause arterial hypertension and cardiac failure. Hypotonic hyponatremia occurs later when glycocolle is metabolized leading to an excessive volume of free water in the vessels. Neurologic symptoms are related to brain edema but also to a direct cerebral toxicity of glycocolle metabolites (ammoniac, glutamate, serine) [106, 107]. This syndrome was firstly described after prostatic surgery but can develop after endoscopic gynecologic or arthroscopic surgeries. The preventive strategy consists in accurate assessment of fluid volumes irrigated during the procedure, a close monitoring of sodium concentration within the intra- and postoperative period, and the use of 0.9% NaCl irrigating solutions with a bipolar electrode device [108].
-
Diuretic-induced hyponatremia: all diuretics may cause hypotonic hyponatremia, but thiazides remain the most frequently implicated [75,76,77, 90, 91]. Leung et al. [91] have shown that 30% of the exposed patients to thiazide diuretics develop hyponatremia and that thiazides multiply the risk of hyponatremia by five. Female, black, age >70 years increase this risk. Hyponatremia develops rapidly within the 10–14 days following the first prescription, but the risk persists within the ten following years. Hyponatremia is usually accompanied by a severe and reversible hypokalemia within 2–5 days after stopping thiazides.
6 Treatment
The optimal specific treatment of hypotonic hyponatremia is still debated because of the risk of life-threatening cerebral edema in case of undercorrection of hyponatremia balanced by the risk of osmotic demyelination syndrome (ODS) provoked by a too excessive and rapid treatment (overcorrection) (Table 2.6) [9, 13, 23, 51, 58, 60, 109]. Four expert guidelines published within the last 3 years are available in the literature: North America [23], Spain [110], Europe [9], and the United Kingdom [88].
6.1 General Strategies
Major principles and strategies of treatment of hypotonic hyponatremia are consensual. However, some conflicting recommendations exist among countries due to various reasons: methodology (interpretation of the evidence), nature of the expert group, few strong evidence, recent data on chronic hyponatremia, and development of vaptans (different approvals among countries).
The first principle of treatment is to distinguish situations requiring an immediate and aggressive treatment aiming a rapid raised in natremia from that needing a slow increased natremia and favoring the etiologic treatment. This strategy is strongly supported by the risk of fatal brain swelling caused by “severe” hyponatremia, which overtakes on the induction of ODS. Acute hyponatremia which is defined by a speed of development in less than 48 h is usually accepted as being severe [23, 87, 110], by opposition to chronic hyponatremia developed in more than 48 h. However, from a practical point of view, the essential point is to identify patients with severe or moderate symptoms indicating brain edema, regardless whether hyponatremia is acute or chronic which is frequently difficult to determine [9, 23]. Moreover, it is helpful to know conditions that contribute to exacerbate cerebral edema [9, 10] (Table 2.6).
The second principle of treatment consists to identify patients at risk of ODS. Usually asymptomatic patients or those with mild symptoms may develop ODS, especially in case of chronic hyponatremia. Contrary to the previous conditions, an overcorrection or too rapid correction of these hyponatremia must be avoided, and the treatment consists firstly to stop and treat the underlying cause of hyponatremia as soon as possible, and consider alternative treatments if not sufficient.
The third principle consists in determining the most efficient and safety rate of correction depending on the symptoms and the rate of development of hyponatremia. In all cases, guidelines must define targets which are the expected goals and limits which do not have to be exceeded (Fig. 2.4) [9, 13, 51, 88].
Finally, the strategy can be elaborated considering (1) first the presence of symptoms and their severity and (2) second the acute or chronic development of hyponatremia. This leads to three situations: (1) hyponatremia with severe or moderate symptoms regardless of the speed of development of hyponatremia, (2) acute hyponatremia without severe or moderate symptoms, and (3) chronic hyponatremia.
6.2 Hyponatremia with Severe or Moderate Symptoms
The management of such patients requires an intensive care unit (ICU) or at least an environment where close biochemical and clinical monitoring can be provided. Regardless of whether hyponatremia is acute or chronic, the aim of the treatment is to raise rapidly serum sodium concentration by 4–6 mmol/L which is sufficient to reverse or avoid most serious complications (brain herniation). This can be achieved with a 100–150 mL (2 ml/kg) intravenous (i.v.) bolus infusion of 3% hypertonic saline (or equivalent 3–4.5 g) over 15–20 min [9, 10, 13, 23, 111–113]. The next step of the treatment is guided by the evolution of the patient. If severe symptoms improve or natremia increases of 4–6 mmol/L within the following hours, it is recommended to infuse 0.9% saline (smallest volume) until the underlying cause treatment is started. An additional 3% saline i.v. can be repeated two or three times if there is not enough or no improvement. In all cases, serum sodium concentration must be closely checked 15–20 min after each bolus and every 4–6 h daily after stopping hypertonic saline bolus and until natremia stabilized. The safe limit that does not have to be surpassed is a total increase in natremia of 6–10 mmol/L during the first 24 h and 8 mmol/L during every 24 h thereafter. As soon as serum sodium concentration reaches 130 mmol/L, stop hypertonic saline infusion.
While treating the patient, it is recommended to perform additional explorations to establish the underlying cause and administer its specific treatment. Guidelines do not recommend the use of any predictive formulas which overestimate the speed of correction [9, 13, 113]. Indeed, formulas are static and do not integrate the fact that kidney capacity of dilution and the possible avoidance of a specific cause may vary during time, leading to unpredictable modifications in natremia. The clinical judgment, the history of the patient, the context, and the follow-up remain essential parameters needed to guide the strategy after the initiation of the emergent administration of hypertonic saline. In this way, additional hypertonic saline boluses must be very carefully indicated in rapidly reversible acute hyponatremia when the cause is stopped, such as in self-induced water intoxication or in the postoperative period after stopping hypotonic fluid infusion, EAH [23].
6.3 Acute Hyponatremia Without Severe or Moderate Symptoms
The first step is to eliminate an error in serum sodium concentration measurement. After confirming hypotonic hyponatremia, the absence of severe or moderate symptoms allows to recommend firstly a cause-specific treatment and to stop all factors that contribute or provoke hyponatremia (hypotonic fluids, drugs). A bolus of 3% hypertonic saline can be suggested in case of profound acute decrease in natremia exceeding 10 mmol/L always associated with a close monitoring of natremia [9].
6.4 Chronic Hyponatremia Without Severe or Moderate Symptoms
6.4.1 Principles and Risks
Loss of organic osmolytes during chronic hyponatremia protects the brain from edema but exposes to osmotic demyelination lesions when the increase in serum sodium concentration is too rapid. Indeed, the recovery of osmolyte content is not immediate and takes several days. This explains why rapid correction of hyponatremia behaves as a hypertonic stress leading to various injuries (apoptosis, blood-brain barrier disruption, and demyelination). There is a consensus to recommend against the administration of hypertonic saline in these patients. The strategy consists firstly to stop all factors (fluids, water intake, drugs) that contribute or provoke hyponatremia and to give a cause-specific treatment [9, 10, 13, 51, 87]. Aiming a raise in natremia is only recommended for profound (<125 mmol/L) or moderate (125–129 mmol/L) chronic hyponatremia [9]. The recommended goal is an increase of 4–6 mmol/24 h and an upper limit increase of <10 mmol/L during the first day and <8 mmol/L during the following day. Lower limits around 8 mmol/L can be advised in patients at risk of ODS (Table 2.6). ODS has been reported when speed correction of chronic hyponatremia exceeded 10–12 mmol/L per 24 h. At last, raising serum sodium concentration should not be aimed in mild chronic hyponatremia (<130 mmol/L).
Despite these recommendations, overcorrection commonly occurs and current guidelines recommend to relower serum sodium concentration if the limit is exceeded [114,115,116,117]. This goal can be reached using desmopressin (DDAVP) or electrolyte-free water. Such a strategy may be performed as a curative or rescue therapy of excessive serum sodium correction by the administration of DDAVP alone or in association with glucose solutions or as preventive administration of DDAVP combined with hypertonic saline. The lack of strong evidence does not allow to recommend a unique preferred strategy. DDAVP is administered initially with a dose of 1–4 μg (intravenously or parenterally), followed by repeated boluses according to the response on neurological function, urine output, and serum sodium concentration change. The interval between two doses should not be less than 6–8 h. The administration of hypotonic fluids is an alternative strategy which consists in a 3–10 mL/kg infusion [9, 23]. The preventive attitude is less consensual than the curative one, because DDAVP seems to be unlogical and inefficient in patients presenting SIADH [58]. Moreover, a recent meta-analysis has shown that there is only limited data and low evidence concerning such a strategy [114]. At last, the optimal timing, dose, and duration of DDAVP administration remain to be determined. Therefore, considering the risk and the clinical experience to relower natremia, the ECPG group [9] recommends to refer or consult an expert for this patient management.
ODS, also named central pontine myelinolysis (CPM), is characterized by a demyelination located on central pontine and extrapontine structures. For a long time, this syndrome has been attributed exclusively to a too rapid correction or overcorrection of chronic hyponatremia. However, other conditions have been reported to provoke or favor CPM: chronic alcoholism, malnutrition, hypokalemia, and burn patients [118,119,120]. Regardless of the cause, blood-brain barrier is altered and becomes permeable allowing an abnormal cerebral entry of cytokines and lymphocytes and finally demyelination. Clinically, the patient presents a classical biphasic evolution: the initial neurological improvement obtained with the correction of hyponatremia, is followed by a worsening neurological status within the following 1–8 days. Manifestations consist in nonspecific signs of encephalopathy including behavioral abnormalities, seizures, pseudobulbar palsy, quadriparesis, locked-in syndrome, permanent disability, or death. Brain resonance magnetic imaging enables to confirm the diagnosis, thanks to the identification of demyelination injury after a delay of 1 or 2 weeks. Prevention of ODS is commonly based on the respect of speed correction of hyponatremia. Uremia has been reported to prevent demyelination by accelerating the recovery of cerebral amount of organic osmolytes [121]. Myoinositol and minocycline enabled to improve rat survival following rapid correction of chronic hyponatremia [122, 123].
6.4.2 Additional Specific Treatments According to the Underlying Cause
Additional non-emergent treatment depends on the underlying cause and on the ECV status.
Besides the specific cause therapy, the first-line treatment of chronic hypovolemic hyponatremia consists in restoring ECV with an i.v. infusion of crystalloids (0.9% saline or balanced solutions) at a rate of 0.5 to 1 mL/kg.
Despite limited data, fluid restriction (800–1200 mL) is suggested in moderate or profound hyper- and euvolemic chronic hyponatremia as a first-line therapy. This can be complemented by the administration of a loop diuretic (furosemide), especially when urines are concentrated [9, 74, 87, 124]. Both demeclocycline and lithium may increase serum sodium concentration by inducing a nephrogenic insipidus diabetes [9, 74, 87, 124]. However, their beneficial effect is inconstant, unpredictable, and associated with important adverse effects (neurotoxicity, nephrotoxicity). Consequently, most experts do not recommend these drugs in SIADH.
Major conflicting recommendations refer to urea and vaptan therapies. Urea has been successfully administered in moderate and profound chronic hyponatremia without real side effects [107, 125, 126]. The ECPG suggests to administer a daily dose of 0.25–0.50 g/day urea as a second-line treatment (after fluid restriction) [9]. But urea is not commercialized and requires a pharmaceutical preparation with sucrose and sparkling water to avoid the bitter taste. Vaptans are non-peptidic antagonists of vasopressin receptors [23, 109, 127]. While VP binds at a superficial site, vaptans penetrate deeply into the membrane. Their main effect consists in an increase in solute-free water excretion by kidneys. Regardless of their affinity on vasopressin receptors, they increase urine output. V2R antagonists exert only renal effects and are called “aquaretics.” V1a-V2R antagonists combine two properties, i.e., renal and vascular vasodilating effects. Currently, two molecules are commercialized and available in most countries: tolvaptan (Samsca®) is a selective V2R antagonist and conivaptan (Vaprisol®) is a mixed V1a-V2R antagonist. Pharmacokinetic characteristics are similar for both: high level of protein bound, half-life of 6–10 h, and metabolization by the hepatic cytochrome P450. Conivaptan may be administered intravenously at a dose of 40 mg daily for a maximum of 4 days because of its potential hepatotoxicity. Tolvaptan can only be prescribed orally at a dose of 15–60 mg daily. Indications of vaptans as a treatment of asymptomatic or mild symptomatic chronic hyponatremia remain a source of debate. Such a conflicting strategy can be explained by the difference in vaptan approval by agencies among countries, paucity of data with high level of proof, divergent interpretation of evidence-based medicine (efficiency vs. adverse events), expert’s disclosure, and industry sponsorship (editorial dependence) [128, 129]. The FDA and the Canadian agencies state that vaptans may be indicated as a first-line therapy for hyper- and euvolemic chronic hyponatremia. This strategy is based on several arguments. There is no doubt that vaptans enable to increase natremia of 4–6 mmol/L in these patients [130,131,132,133,134]. These results have been confirmed in two recent meta-analyses [133, 134]. Rozen-Zvi et al. [133] included 15 randomized controlled studies comparing vaptans vs. placebo or no treatment (± associated with fluid restriction). They found that vaptans increased serum sodium concentration by 5.27 mmol/L early (after 1–7 days) and up to 1 month. Similar results from 11 randomized controlled studies were reported in a second meta-analysis with an increased natremia of 5.7 mmol/L at day 5 of treatment [134]. More than 3000 hyper- and euvolemic asymptomatic/mild symptomatic hyponatremic patients were collected in an international registry [135]. The authors confirmed that tolvaptan successfully increased natremia by a median of 4 mmol/L for up to 4 years. The last meta-analysis has been performed by the ECPG group including additional trials published between 2010 and 2013. The update data with 20 randomized controlled studies (2009 patients) also confirmed that vaptans enable to increase serum sodium concentration by 4.3 mmol/L within 3–7 days as compared with placebo [9]. No real difference in adverse or serious adverse events (CPM) related to a rapid increase in natremia has been reported [9, 133,134,135]. Moreover, fluid restriction which is still considered as the first-line alternative treatment can be ineffective and difficult to maintain in numerous patients [129]. Failure of fluid restriction to increase natremia commonly occurs in patients with high urine osmolality (>500 mosm/kg), low urine output (<1500 ml/day), and low increase in sodium after 2 days of treatment (<2 mmol/L). Because tolvaptan was recently reported to be associated with liver toxicity, the FDA recommends limiting its use to 30 days and not to use it in patients with liver disease (hypervolemic hyponatremia) [136]. Tolvaptan is not indicated in hypovolemic chronic hyponatremia because of a high risk of worsening vasodilating-related hypotension. Current recommendations in North America indicate that vaptans can be considered as an optional treatment in asymptomatic or mild symptomatic chronic non-hypovolemic hyponatremia, except for patients with liver disease [109]. Short-term indication is safe and simple in patients presenting hyponatremia <125 mmol/L, and as soon as natremia raises of 6–8 mmolL, water intake must be matched with urine output to prevent an excessive elevation in natremia. Vaptans may be indicated as a long-term optional strategy in patients with irreversible mild symptomatic euvolemic hyponatremia (<130 mmol/L) who fail or resist to fluid restriction. Tolvaptan must be initiated with low daily dose (15 mg) and progressively increased (to 60 mg daily) until reaching serum sodium concentration ≥135 mmol/L within 1 week. Natremia must be checked at least once a week at the beginning and monthly thereafter. Tolvaptan requires regular liver enzyme monitoring and must be stopped in case of elevated values (except for patients waiting for liver transplantation). European experts do not recommend the use of vaptans considering that the quality of the evidence is reduced due to the absence of comparative studies with alternative treatment and the absence of evaluation of major endpoints such as mortality or severe adverse events [9, 87]. Indeed, meta-analysis does not report any beneficial effects of vaptans on long-term survival or quality of life [9]. Moreover, the risk for a rapid sodium concentration increase is present (RR = 1.61) and has been pointed by drug agencies [9, 137,138,139], especially when vaptans are combined with hypertonic saline administration. Despite no published report of ODS, neurological injury has been indicated in patients receiving tolvaptan due to an excessive correction of hyponatremia [137]. Vaptans also enable to induce adverse events such as polyuria, thirst, mouth dryness, constipation, and hepatotoxicity [109, 140]. At last, patients presenting concentrated urines have a resistance to vaptans (as observed with fluid restriction): patients with high level of VP (some SIADH), with hypervolemic heart failure or cirrhosis because of the low EABV, excessive water intake related to “reset osmostat” SIADH, or nephrogenic syndrome of inappropriate antidiuresis. Therefore, the ECPG group considered that there is no proven beneficial effect of vaptans apart the increase in natremia while safety remains questioned [9]. This group recommended against vasopressin receptor antagonists in treating hyper- and euvolemic chronic hyponatremic patients and recommends fluid restriction as a first-line strategy for these patients [9].
In summary: only severely and moderately symptomatic hyponatremic patients require an immediate and aggressive treatment with i.v. hypertonic saline, aiming to prevent or avoid urgently life-threatening cerebral edema. The first therapy recommended for treating mild symptomatic chronic hyponatremia is to stop drugs and reversible situations that provoke hyponatremia (thiazides, excessive water intake), before considering vaptans which require a long delay of action. For these latter patients, the cause-specific treatment is the most appropriate and increasing natremia should not be the sole goal. Hypovolemic hyponatremia requires volume expansion. Alternative treatments of hyper- and euvolemic chronic hyponatremia remain controversial: fluid restriction firstly and no vaptans in Europe and vaptans firstly in North America. In case vaptans are indicated, an expert management is preferable with a regular monitoring of serum sodium concentration and liver enzyme levels because of both risks of overcorrection of natremia and hepatotoxicity. Vaptans and hypertonic saline should never be associated.
7 Hypernatremia
7.1 Definition: Pathophysiology
Hypernatremia is defined by a serum sodium concentration >145 mmol/L. Hypernatremia is always associated with plasma hypertonicity and consequently responsible for cell shrinkage (dehydration). Hypernatremias may be induced by three mechanisms which conduct to classify them according to the resulting changes in ECV (Fig. 2.5) [13, 141]:
-
Euvolemic hypernatremias are due to “pure water” losses. Total body sodium content remains unchanged and maintains a normal ECV.
-
Hypovolemic hypernatremias are due to hypotonic losses leading to a combination of cell shrinkage and a reduced ECV.
-
Hypervolemic hypernatremias are due to an absolute sodium retention in the ECV which conducts to combine cell shrinkage and hypervolemia.
7.2 Diagnosis
7.2.1 Clinical Symptoms
Similarly with hyponatremia, severity of hypernatremia is essentially related to its impact on brain volume. Therefore, clinical manifestations are primarily neurologic but not specific, and their severity depends on the speed of development of the trouble. Acute hypertonic stress resulting from acute hypernatremia (<48 h) induces cerebral vascular injury and sudden brain shrinkage which induces intracranial hemorrhage, while symptoms are mild or absent in case of chronic hypernatremia. Severity of hypernatremia depends on the severity of neurological signs and on the presence of hypovolemia. The interpretation of symptoms must be integrated in the history, treatments of the patient, and the context which can be useful to distinguish acute symptomatic from chronic asymptomatic hypernatremia. Clinical signs are related to cell dehydration [13, 141]:
-
Neurological signs are those of nonspecific encephalopathy including consciousness impairment, ataxia, nystagmus, hypertonia, stupor, seizures, profound coma, or those related to intracranial hemorrhage (hemiplegia) or thrombosis of dural sinus. Acute hypernatremia may lead finally to death.
-
Thirst may be absent in case of hypodipsia and consciousness alteration.
-
Reduced body weight allows to assess body water deficit, but it may remain stable or elevates when plasma hypertonicity is associated with an increased ECV.
-
Other signs are fever, dyspnea, and rhabdomyolysis. However, it is commonly difficult to prove the causal relationship between those signs and hypernatremia.
In summary, there is no specific clinical sign, and treatment is delayed, explaining the high rate of morbi- mortality during hypernatremia which is also frequently associated with severe underlying causes [141].
7.2.2 Etiologic Diagnosis (Fig. 2.5)
Euvolemic hypernatremia: They include three major causes:
-
Diabetes insipidus [142]: this syndrome is characterized by a common polyuria and polydipsia. Urines are abnormally diluted. Central diabetes insipidus is caused by a lack of ADH secretion which is due to central nervous system alterations. However, in most cases, this is also combined with an impairment in thirst sensation which results from alterations in receptors of thirst. Therefore, hypernatremia can develop only if both abnormalities are associated or in case of impossible water intake. Central and nephrogenic diabetes insipidus can be distinguished, thanks to an administration of desmopressin that will increase urine osmolality of 50% only in central diabetes insipidus. Numerous nervous central system lesions may induce central diabetes insipidus (trauma, stroke, hemorrhage, tumors, neurosurgery, etc.). Nephrogenic diabetes insipidus may be due to gene mutations of V2R VP-receptors or of aquaporin-2. It may be caused by acquired troubles such as hypokalemia, hypercalcemia, or some drugs (lithium, antifungic molecules, antibiotics, antiviral drugs, or antimitotics).
-
Primary hypodipsia refers to an impairment in thirst behavioral while its stimulation is normal. Urine osmolality and density are elevated showing an appropriate response of kidneys to plasma hypertonicity.
-
Essential hypernatremia is due to an abnormally elevated threshold of ADH and thirst secretion. Urine osmolality in relation with plasma hypertonicity is too low:
-
Hypovolemic hypernatremia: the diagnosis of the underlying cause is orientated by the context, electrolyte blood measurements, and above all Uosm. A low Uosm indicates losses from the kidney which are caused by osmotic polyuria due to the presence of glucose or urea in urines or to diuretic administration. A high Uosm is present when losses are caused by gastrointestinal or cutaneous alterations.
-
Hypervolemic hypernatremia: they are usually due to therapeutic errors or voluntary self-poisoning. Most frequent causes are infusions of hypertonic saline or concentrated sodium bicarbonate infusions. The clinical presentation consists in severe signs of cell dehydration due the acute speed of development of hypernatremia. The frequent pulmonary edema or congestive heart failure manifestations are related to the rapid extracellular fluid overload.
-
Perioperative hypernatremia: age, infection, and diuretics have a major role in the development of hypernatremia whatever the context and independently of the type of surgery. Indeed, the risk of perioperative hypernatremia strongly increased in elderly patients which present an impairment in thirst sensation and urine concentration capacity, frequently associated with severe comorbidities or dementia. Hypernatremia occurs in 22% of cases following neurosurgery and abdominal surgeries. In half of cases, it appears within the 5 days following surgery. Digestive surgery is at risk of excessive hypotonic losses from the gastrointestinal tract: preoperative digestive losses (vomitings, diarrhea), intraoperative water losses, and postoperative digestive losses (gastric aspiration, digestive fistulas) which are commonly insufficiently supplemented. About 18% of central diabetes insipidus are observed after neurosurgery or in traumatic brain injury and appear early within the 12 or 24 postoperative hours and disappear after 5–7 days.
-
7.3 Treatment
Both preventive and curative including the underlying cause treatments are needed. As recommended for hyponatremia, the appropriate strategy for reducing hypernatremia depends on the severity of symptoms, the ECV alteration, the speed of development of hypernatremia, and the reversibility of the cause. Therefore, the therapy aims to:
-
1.
Maintain or restore the ECV especially in case of hypovolemia to avoid shock or tissue hypoperfusion (crystalloids). This is the first priority that must be reached before any correction of natremia [13, 141].
-
2.
Correct plasma hypertonicity/hypernatremia with hypotonic solutions (5% dextrose or water when possible). In case of acute symptomatic hypernatremia, a rapid decrease in serum sodium concentration is needed to prevent or treat brain shrinkage and its consequences. This can be achieved with a rapid i.v. 5% dextrose infusion or renal removal therapy especially when patient presents kidney insufficiency. Oral water intake is insufficient and frequently impossible due to central nervous alteration. Despite the real proof of side effects of an excessive correction of hypernatremia, it is commonly advised to not exceed a reduction in plasma tonicity of 5 mosm/L/h or in natremia 2–2.5 mmol/L. Therefore, serum sodium levels must be closely and frequently (every 4–6 h) checked, and a clinical monitoring is also required. Such a treatment is maintained until severe neurological signs disappear and natremia is 145 mmol/L [13]. Asymptomatic chronic hypernatremia requires the replacement of water losses, the cause-specific treatment, and if necessary, a reduction of natremia that will not exceed 10 mmol/L per day. Oral water intake may be useful because it allows a safe regular and progressive decrease in serum sodium concentration, allowing to prevent overcorrection and the risk of brain edema. The speed of correction of hypernatremia must be lower in patients at risk such as elderly patients, children, and patients at risk of brain edema or intracranial hypertension.
Conclusion
Cell volume depends on the transmembrane osmotic gradient, i.e., on plasma tonicity which is determined by the sole effective plasma osmoles. Plasma hypertonicity always induces cell shrinkage and conversely plasma hypotonicity always induces cell edema. ADH and thirst are both major mechanisms that regulate closely plasma tonicity and consequently cell volume. Because of its location in the inextensible skull, modifications in brain volume caused by dysnatremias are responsible for the common neurological signs. Therefore, dysnatremias must be considered when severe as life-threatening conditions.
Hyponatremia can be associated with plasma hypertonicity (in case of hyperglycemia) and isotonicity (pseudohyponatremia in case of hyperprotidemia or hyperlipidemia), but only hypotonic hyponatremia can induce brain edema. Due to cerebral osmoregulation, the risk of hypotonic hyponatremia depends on the efficiency of this regulation. Therefore, the most pragmatic strategy to treat hypotonic hyponatremia is to consider the severity of neurological signs. Patients presenting severe neurological signs require an immediate and aggressive treatment aiming to increase rapidly natremia of 4–6 mmol/L using an i.v. infusion of 3% hypertonic saline and to prevent or reverse brain edema. Increasing serum sodium in asymptomatic or mild symptomatic chronic hyponatremic patients is not a real goal, and the treatment is essentially based on the cause-specific treatment. Besides such treatment, the raise of chronic hyponatremia can be reached using fluid restriction and the use of vaptans remains controversial. Overcorrection of chronic hyponatremia exposes to the risk of osmotic demyelination.
Hypernatremia is always associated with plasma hypertonicity and consequently with cell shrinkage. Therefore, hypernatremic patients commonly present central nervous system impairments. Treatment of hypernatremia depends also on the severity of symptoms. Besides, the underlying cause treatment and severe symptomatic hypernatremic patients require a rapid decrease in serum sodium concentrations using i.v. hypotonic solutions, whereas asymptomatic patients can be treated using oral water intake aiming a slow and progressive reduction in natremia.
References
Mount DB (2010) Hyponatremia. Semin Nephrol 29:1–317
Orban JC, Ichai C (2012) Hyponatrémies en réanimation. Encycl Med Chir (Anesthésie-Réanimation) 36-860-A-05:16
Danziger J, Zeidel ML (2014) Osmotic homeostasis. Clin J Am Soc Nephrol 10:852–862
Antunes-Rodrigues J, Ruginsk SG, Mecawi AS et al (2014) Neuroendocrinology of Hydromineral homeostasis. In: De Luca LA Jr, Menani JV, Johnson AK (eds) Neurobiology of body fluid homeostasis: transduction and integration. CRC, Boca Raton, FL. Chapter 3
Noda Y (2014) Dynamic regulation and dysregulation of the water channel aquaporin-2: a common cause of and promising therapeutic target for water balance disorders. Clin Exp Nephrol 18:558–570
Ferguson AV (2014) Circumventricular organs: integrators of circulating signals controlling hydration, energy balance, and immune function. In: De Luca LA Jr, Menani JV, Johnson AK (eds) Neurobiology of body fluid homeostasis: transduction and integration. CRC, Boca Raton, FL. Chapter 2
Harring TR, Deal NS, Kuo DC (2014) Disorders of sodium and water balance. Emerg Med Clin North Am 32:379–401
Petitclerc T (2013) Disorders in sodium-water balance. Nephrol Ther 9:38–49
Spasovski G, Vanholder R, Allolio R et al (2014) Hyponatraemia guideline development group. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med 40:320–331
Adrogue HJ, Madias NE (2014) Diagnosis and treatment of hyponatremia. Am J Kidney Dis 64:681–684
Knepper MA, Tae-Hwan K, Nielsen S (2015) Molecular physiology of water balance. N Engl J Med 372:1349–1358
Prager-Khoutorsky M, Bourque CW (2015) Mechanical basis of osmosensory transduction in magnocellular neurosecretory neurons of the rat supraoptic nucleus. J Neuroendocrinol 27:507–515
Sterns RH (2015) Disorders of plasma sodium: causes, consequences and correction. N Engl J Med 372:55–65
Ciura S, Liedtke W, Bourque CW (2011) Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J Neurosci 31:14669–14676
Bourque CW (2008) Central mechanisms of osmosensation and systemic osmoregulation. Neuroscience 9:519–531
Daniels D (2014) Diverse role of angiotensin receptor intracellular pathways in the control of water and salt intake. In: De Luca LA Jr, Menani JV, Johnson AK (eds) Neurobiology of body fluid homeostasis: transduction and integration. CRC, Boca Raton, FL. Chapter 5
Robertson GL, Athat S (1976) The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J Clin Endocrinol Metab 42:613–620
Juul KV, Bichet DG, Nielsen S, NØrgaard JP (2014) The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol 306:F931–F941
Huber VJ, Tsujita M, Nakada T (2012) Aquaporins in drug discovery and pharmacotherapy. Mol Asp Med 33:691–703
Kortenoeven ML, Frenton RA (2014) Renal aquaporins and water balance disorders. Biochim Biophys Acta 1840:1533–1549
Wilson JL, Miranda CA, Knepper NA (2013) Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17:751–764
Holmes RP (2012) The role of renal water channels in health and disease. Mol Asp Med 33:547–552
Verbalis JG, Goldsmith SR, Greenberg A et al (2013) Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 126:S1–42
Bhardwaj A (2006) Neurological impact of vasopressin dysregulation and hyponatremia. Ann Neurol 59:229–236
Ayus JC, Achinger SG, Arieff A (2008) Brain cell volume regulation: role of sex, age, vasopressin and hypoxia. Am J Physiol Renal Physiol 295:F619–F624
Pasantes-Morales H, Lezama RA, Ramos-Mandujano G et al (2006) Mechanisms of cell volume regulation in hypo-osmolality. Am J Med 119:S4–11
Sterns R, Silver SM (2006) Brain volume regulation in response to hypo-osmolality and its correction. Am J Med 119(Suppl 7A):S12–S16
Sardini A, Amey JS, Weyland KH et al (2003) Cell volume regulation and swelling-activated channels. Biochem Biophys Acta 1618:153–162
De Mello W (2014) Regulation of cell volume and water transport – an old fundamental role of the renin-angiotensin aldosterone system components ate the cellular level. Peptides 58:74–77
Sterns RH, Hix JK, Silver SM (2013) Management of hyponatremia in the ICU. Chest 144:672–679
Verkman AS, Galietta LJV (2009) Chloride channels as drugs targets. Nature 8:153–171
Kahle KT, Staley KJ, Naheb BV et al (2008) Roles of the cation-chloride cotransporters in neurological disease. Nature Clin Parct 4:490–503
Badaut J, Fukuda AM, Jullienne A, Petry KG (2014) Aquaporin and brain diseases. Biochim Biophys Acta 1840:1554–1565
Zeynalov E, Chen CH, Froehener SC et al (2008) The perivascular pool of aquaporin-4 mediates the effect of osmotherapy in postischemic cerebral edema. Crit Care Med 36:2634–2640
Manley GT, Fukimura M, Ma T et al (2000) Aquaporin-4 depletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Papadopoulos MC, Manley GT, Krishna S, Verkman AS (2004) Aquaporin-4 facilitates reabsoprtion of excess fluid in vasogenic brain edema. FASEB 18:1291–1293
Wenner MM, Stachenfeld NS (2012) Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol 590:5949–5961
Sladek CD, Somponpun SJ (2008) Estrogen receptors: their roles in regulation of vasopressin release for maintenance of fluid and electrolyte homeostasis. Front Neuroendocrinol 29:114–127
Hoorn EJ, Lindemans J, Zietse R (2006) Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant 21:70–76
Thompson CJ (2010) Hyponatraemia: new associations and new treatments. Eur J Endocrinol 162:S1–S3
Corona G, Giuliani C, Parenti G et al (2013) Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PLoS One 8:e80451
Sherlock M, Sullivan EO, Agha A et al (2009) Incidence and pathophysiology of severe hyponatraemia in neurosurgical patients. Postgrad Med J 85:171–175
Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD (2011) Mortality and serum sodium: do patients die from or with hyponatremia? Clin J Am Soc Nephrol 6:960–965
Huda MS, Boyd A, Skagen K et al (2009) Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J 82:216–219
Darmon M, Pichon M, Schwebel C et al (2014) Influence of early Dysnatraemia correction on survival of critically ill patients. Shock 41:394–399
Stelfox HT, Ahmed SB, Khandwala F et al (2008) The epidemiology of intensive care unit-acquired hyponatremia and hypernatremia in medical-surgical intensive care units. Crit Care 12:R162
Funk GC, Lindner G, Drumml W et al (2010) Incidence and prognosis of dysnatraemias present on ICU admission. Intensive Care Med 36:304–311
Sakr Y, Rother S, Mendoca Pires Ferreira A et al (2013) Fluctuations in serum sodium level are associated with an increased risk of death in surgical ICU patients. Crit Care Med 41:133–142
Palmer BF (2009) Hyponatremia in the intensive care unit. Semin Nephrol 29:257–270
Padhi R, Panda BN, Jagati S, Patra SC (2014) Hyponatremia in critically ill patients. Ind J Crit Care Med 18:83–87
Sterns RH, Silver SM (2016) Complications and management of hyponatremia. Curr Opin Nephrol Hypertens 25:114–119
Thompson C, Berl T, Tejedor A, Johannsson G (2012) Differential diagnosis of hyponatraemia. Best Pract Res Clin Endocr Metab 26(suppl1):S1–S6
Turchin A, Seifter JL, Seely EW (2003) Clinical problem-solving. Mind the gap. N Engl J Med 349:1465–1469
Hillier TA, Abbott RD, Barrett EJ (1999) Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med 106:399–403
Sterns RH, Hix JK, Silver S (2010) Treating profound hyponatremia: a strategy for controlled correction. Am J Kidney Dis 56:774–779
Schrier RW, Bansal S (2008) Diagnosis and management of hyponatremia in acute illness. Curr Opin Crit Care 14:627–634
Arieff AI (2006) Influence of hypoxia and sex on hyponatremic encephalopathy. Am J Med 119(Suppl 7A):S59–S64
Adrogué HJ, Madias NE (2012) The challenge of hyponatremia. J Am Soc Nephrol 23:1140–1148
Thompson C, Hoorn EL (2012) Hyponatraemia: an overview of frequency, clinical presentation and complications. Best Pract Res Clin Endocr Metab 26(suppl1):S7–15
Lee JJ, Kilonzo K, Nistico A, Yeates K (2014) Management of hyponatremia. CMAJ 186:E281–E286
Ayus JC, Negri AL, Kalantar-Zadeh K, Moritz ML (2012) Is chronic hyponatremia a novel risk factor for hip fracture in the elderly? Nephrol Dial Transplant 27:3725–3731
Zaino CJ, Maheshwari AV, Goldfarb DS (2013) Impact of mild hyponatremia on falls, fractures, osteoporosis, and death. Am J Orthop 42:522–527
Kinsella S, Moran S, Sullivan MO et al (2010) Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol 5:275–280
Hoorn EJ, Rivadeneira F, van Meurs JB et al (2011) Mild hyponatremia as a risk factor for fractures: the Rotterdam study. J Bone Miner Res 26:1822–1828
Verbalis JG, Barsony J, Sugimura Y et al (2010) Hyponatremia-induced osteoporosis. J Bone Miner Res 25:554–563
Hannon MJ, Verbalis JG (2014) Sodium homeostasis and bone. Curr Opin Nephrol Hypertens 23:370–376
Chung HM, Kluge R, Schrier RW, Anderson RJ (1987) Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med 83:905–908
Fenske W, Maier SK, Blechschmidt A et al (2010) Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med 123:652–657
Fenkse W, Störk S, Koschker AC et al (2008) Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab 93:2991–2997
Hsu CY, Chen CL, Huang WC et al (2014) Retrospective evaluation of standard diagnostic procedures in identification of the causes of new-onset syndrome of inappropriate antidiuresis. Int J Med Sci 11:192–198
Musch W, Decaux G (2001) Utility and limitations of biochemical parameters in the evaluation of hyponatremia in elderly. Int Urology Nephrol 32:475–493
Decaux G (2009) The syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Semin Nephrol 29:239–256
Fenkse W, Störk S, Bleschschmidt A, Maier SG, Morgenthaler NG, Allolio B (2009) Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab 94:123–129
Peri A, Giuliani C (2014) Management of euvolemic hyponatremia attributed to SIADH in the hospital setting. Minerva Endocrinol 39:33–41
Ramos-Levi AM, Duran Rodriguez-Hervada A, Mendez-Bailon M, Marco-Martinez J (2014) Drug-induced hyponatremia: an update review. Minerva Endocrinol 39:1–12
Hix JK, Silver S, Sterns RH (2011) Diuretic-associated hyponatremia. Semin Nephrol 31:553–566
Rodenburg EM, Hoorn EJ, Ruiter R et al (2013) Thiazide-associated hyponatremia: a population-based study. Am J Kidney Dis 62:67–72
Bennani SL, Abouqal R, Zeggwagh AA et al (2003) Incidence, étiologies et facteurs pronostiques de l'hyponatrémie en réanimation. Rev Med Interne 24:224–229
Ichai C, Lena D (2008) Hyponatremia in the setting of acute heart failure syndrome. In: Mebazaa A, Gheorghiade M, Zannad FM, Parrillo JE (eds) Acute heart failure. Springer, London, pp 786–796
Angeli P, Wong F, Watson H, Ginès, Investigators CAPPS (2006) Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 44:1535–1542
Gianotti RJ, Cardenas A (2014) Hyponatremia and cirrhosis. Gastroenterol Rep (Oxf) 2:21–26
Combs S, Berl T (2014) Dysnatremias in patients with kidney disease. Am J Kidney Dis 63:294–303
Rahman M, Friedman WA (2009) Hyponatremia in neurosurgical patients: clinical guidelines development. Neurosurgery 65:925–935
Kirkman MA (2014) Managing hyponatremia in neurosurgical patients. Minerva Endocrinol 39:13–26
Verbalis JG (2014) Hyponatremia with intracranial disease: not often cerebral salt wasting. J Clin Endocrinol Metab 99:59–62
Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E (2009) Is it cerebral or renal salt wasting? Kidney Int 76:934–938
Hannon MJ, Thompson J (2010) The syndrome of inappropriate antidiuretic hormone: prevalence, causes and consequences. J Endocrinol Metab 162:S5–12
Grant P, Ayuk J, Bouloux PM et al (2015) The diagnosis and management of inpatient hyponatremia and SIADH. Eur J Clin Investig 45:888–894
Liamis G, Milionis H, Elisaf A (2008) A review of drug-induced hyponatremia. Am J Kideny Dis 52:144–153
van Blijderveen JC, Straus SM, Rodenburg EM et al (2014) Risk of hyponatremia with diuretics: chlortalidone versus hydrochlorothiazide. Am J Med 127:763–771
Leung AA, Wright A, Pazo V et al (2011) Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med 124:1064–1072
Schrier R (2008) Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin Nephrol 28:289–296
Armed Forces Health Surveillance Center (AFHSC) (2014) Update: Exertional hyponatremia, active component, U. S. armed forces, 1999-2014. MSMR 21:18–21
Hew-Butler T, Rosner MH, Fowkes-Godek S et al (2015) Statement of the 3rd international exercise-associated Hyponatremia consensus development conference, Carlsbad, California. Br J Sports Med 49:1432–1446
Urso C, Brucculeri S, Caimi G (2014) Physiopathological, epidemiological, clinical and therapeutic aspects of exercise-associated Hyponatremia. J Clin Med 3:1258–1275
Severac M, Orban JC, Leplatois T, Ichai C (2013) A near -fatal case of exercise-associated hyponatremia. Am J Emerg Med 813:e1–e2
Chung HM, Kluge R, Schrier RW, Anderson RJ (1986) Postoperative hyponatremia. A prospective study. Arch Intern Med 146:333–336
Gardner LB, Preston RA (2000) University of Miami Division of clinical pharmacology therapeutic rounds: the water-intolerant patient and perioperative hyponatremia. Am J Ther 7:23–30
Harris B, Schopflin C, Khaghani C et al (2015) Perioperative intravenous fluid prescribing: a multi-centre audit. Crit Care 4:15
Hannon MJ, Finucane FM, Sherlock M et al (2012) Clinical review: disorders of water homeostasis in neurosurgical patients. J Clin Endocrinol Metab 97:1423–1433
Hannon MJ, Thompson CJ (2014) Neurosurgical hyponatremia. J Clin Med 14:1084–1104
Kleindienst A, Hannon MJ, Buchfelder M, Verbalis JG (2015) Hyponatremia in neurotrauma: the role of vasopressin. J Neurotrauma 33(7):615–624
Gritti P, Lanterna LA, Rotasperti L et al (2014) Clinical evaluation of hyponatremia and hypovolemia in critically ill adult neurologic patients: contribution of the use of cumulative balance of sodium. J Anesth 28:687–695
Hahn RG (2006) Fluid absorption in endoscopic surgery. Br J Anaesth 96:8–20
Cooper JM, Brady RM (2000) Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin N Am 27:347–366
Ichai C, Ciais JF, Roussel LJ et al (1996) Intravascular absorption of glycine irrigating solution during shoulder arthroscopy: a case report and follow-up study. Anesthesiology 85:1481–1485
Decaux G, Andres C, Gankam KF, Kengne F, Soupart A (2010) Treatment of euvolemic hyponatremia in the intensive care unit by urea. Crit Care 14:R184
Tamman AE, Ahmed HH, Abdella AH, Taha SA (2015) Comparative study between monopolar electrodes and bipolar electrodes in hysteroscopic surgery. J Clin Diagn Res 9:QC11–QC13
Berl T (2015) Vasopressin antagonists. N Engl J Med 372:2207–2216
Runkle I, Navarro A, Pose A et al (2013) El tratamiento de la hiponatremia secundaria al sındrome de secrecion inadecuada de la hormona antidiuretica. Med Clin (Barc) 141:507.e1–507e10
Moritz ML, Ayus JC (2010) 100 cc 3% sodium chloride bolus: a novel treatment for hyponatremic encephalopathy. Metab Brain Dis 25:91–96
Rosner MH (2015) Preventing deaths due to exercise-associated hyponatremia. Clin J Sport Med 25:301–302
Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH (2007) Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. J Am Soc Nephrol 2:1110–1117
MacMillan TE, Tang T, Cavalcanti RB (2015) Desmopressin to prevent rapid sodium correction in severe hyponatremia: a systematic review. Am J Med 12:1362.e15–1362.e24
Gharaibeh KA, Cgraig MJ, Koch CA et al (2013) Desmopressin is an effective adjunct treatment for reversing excessive hyponatremia overcorrection. World J Clin Cases 16:155–158
Rafat C, Schortgen F, Gaudry S et al (2014) Use of desmopressin acetate in severe hyponatremia in the intensive care unit. Clin J Am Soc Nephrol 9:229–237
Sood L, Sterns RH, Hix JK, Silver SM, Chen L (2013) Hypertonic saline and desmopressin: a simple strategy for safe correction of severe hyponatremia. Am J Kidney Dis 61:571–578
Singh TD, Fugate JE, Rabinstein AA (2014) Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 21:1443–1450
Norenberg MD (2010) Central pontine myelinolysis: historical and mechanistic considerations. Metab Brain Dis 25:97–106
Murase T, Sugimura Y, Takefuji S, Oiso Y, Murata Y (2006) Mechanisms and therapy of osmotic demyelination. Am J Med 119(suppl7A):S69–S73
Gankam-Kengne F, Couturier BS, Soupart A, Decaux G (2015) Urea minimizes brain complications following rapid correction of chronic hyponatremia compared with vasopressin antagonists or hypertonic saline. Kidney Int 87:323–331
Takagi H, Sugimura Y, Suzuki H et al (2014) Minocycline prevents osmotic demyelination associated with aquaresis. Kidney Int 86:954–964
Silver SM, Schroeder BM, Sterns RH, Rojiani AM (2006) Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats. Exp Neurol 65:37–44
Moritz ML, Ayus JC (2014) Management of hyponatremia in various clinical situations. Curr Treat Options Neurol 16:310
Soupart A, Coffernils M, Couturier B et al (2012) Efficacy and tolerance of urea compared with vaptans for long-term treatment of patients with SIADH. Clin J Am Soc Nephrol 7:742–747
Coussement J, Danguy C, Zouaoui-Boudjeltia K et al (2012) Treatment of the syndrome of inappropriate secretion of antidiuretic hormone with urea in critically ill patients. Am J Nephrol 35:265–270
Palmer BF (2013) The role of V2 receptor antagonists in the treatment of hyponatremia. Electrolyte Blood Press 11:1–8
Nagler EV, Vanmassenhove J, van der Veer SN et al (2014) Diagnosis and treatment of hyponatremia: a systematic review of clinical practice guidelines and consensus statements. BMC Med 12:231
Verbalis JG, Grossman A, Höybye C, Runkle I (2014) Review and analysis of differing regulatory indications and expert panel guidelines for the treatment of hyponatremia. Curr Med Res Opin 30:1201–1207
Verbalis JG, Adler S, Schrier RW, Berl T, Zhao Q, Czerwiec FS, For the SALT Investigators (2011) Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol 164:725–732
Udelson JE, Bilsker M, Hauptman PJ et al (2011) A multicenter, randomized, double-blind, placebo-controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. J Card Fail 17:973–981
Hauptman PJ, Burnett JC Jr, Gheorghiade M et al (2013) Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail 19:390–397
Rozen-Zvi B, Yahav D, Gheorghiade M, Korzets A, Leibovici L, Gafter U (2010) Vasopressin receptor antagonists for the treatment of hyponatremia: systematic review and meta-analysis. Am J Kidney Dis 56:325–337
Jaber BL, Almarzouqi L, Borgi L, Seabra VF, Balk EM, Madias NE (2010) Short-term efficacy and safety of vasopressin receptor antagonists for treatment of hyponatremia. Am J Med 124:977.e1–977.e9
Greenberg A, Verbalis J, Amin A et al (2015) Hyponatremia: current treatment practice and outcomes report of the hyponatremia registry: an observational multicenter international study. Kidney Int 88:167–177
Food and Drug Administration. FDA Safety Communications (2013) FDA limits duration and usage of Samsca (tolvaptan) due to possible liver injury leading to organ transplant or death. http://www.fda.gov/Drugs/DrugSafety/ucm350062.htm
Di Benedetto G, See M (2012) Direct Health-care Professional Communication on the risk of increases in serum sodium with tolvaptan (Samsca) which are too rapid. http://www.mhra.gov.uk/home/groups/comms-ic/documents/websiteresources/con146921.pdf. Accessed 26 March 2011
Malhotra I, Gopinath S, Janca KC et al (2014) Unpredictable nature of tolvaptan in treatment of hypervolemic hyponatremia: case review on role of vaptans. Case Rep Endocrinol 2014:807054
Lehrich RW, Ortiz-Melo DI, Patel MB, Greenberg A (2013) Role of vaptans in the management of hyponatremia. Am J Kidney Dis 62:364–372
Gross P (2014) Panel recommendations on hyponatremia. Am J Med 127:e29
Adrogue HJ, Madias NE (2000) Hypernatremia. N Engl J Med 342:1493–1499
Garofeanu CG, Weir M, Rosas-Arellano MP et al (2005) Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis 45:626–637
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Ichai, C., Orban, JC. (2018). Sodium Disorders. In: Ichai, C., Quintard, H., Orban, JC. (eds) Metabolic Disorders and Critically Ill Patients. Springer, Cham. https://doi.org/10.1007/978-3-319-64010-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-64010-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-64008-2
Online ISBN: 978-3-319-64010-5
eBook Packages: MedicineMedicine (R0)