Abstract
Atresia has been poorly examined in cephalopods. We here provide a histological description of this process along the whole ovary development for Octopus vulgaris. Additionally, we related its occurrence to morphometric parameters, and its seasonal cycle was further analysed. Atresia occurred all year round in immature and mature females and in previtellogenic and vitellogenic oocytes. However, more mature females were more prone of being atretic. This occurred mainly in spring when females had atretic previtellogenic oocytes in mature macrostages. By contrast, vitellogenic atresia occurred mainly from spawning to post-spawning females. Furthermore, two types of phagocytic cells were identified as responsible for the reabsorption during atresia. The phagocytic follicle cells only occurred in yolk-bearing oocytes; and within the two haemocyte populations only the smaller ones seemed to be involved in engulfing atretic oocytes. Additionally, advanced atresia in post-spawning females showed yellow–brown bodies as a possible result of follicle cell apoptosis and highlighting the end of the reproductive cycle. Given the pattern of atresia, the reproductive strategy of this species is based on an asynchronic ovary development and a synchronous ovulation during spawning. We further suggest that potential fecundity for this species should be measured on late vitellogenic oocytes in pre-spawning females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian atresia is a degenerative and resorptive process whereby oocytes and post-ovulatory follicles are reabsorbed from the ovary. It regulates egg production reducing potential fecundity and allowing females to recover part of the energy invested in the formation of oocytes (Guraya 1986). The study of this phenomenon allows (i) the estimation of crucial reproductive traits such as fecundity and spawning biomass (Ganias et al. 2003) and (ii) the assessment of the physiological condition due to external factors. Thus, since ovarian atresia affects fertility rates, its determination allows to differentiate between potential and total fecundity and to identify at what stage of sexual maturity fecundity is reduced (Boyle and Chevis 1992). In the case of iteroparous species such as fishes, defining atretic stages and the subsequent assignment of females to different spawning status (e.g. active, inactive/immature) are of great importance for later estimation of spawning biomass (Hunter and Macewicz 1985; Hunter and Lo 1997). Moreover, the study of prevalence and intensity of histological stages of the atretic oocytes allows predicting the cessation of spawning for a given population (Kurita et al. 2003; Ganias et al. 2003). Furthermore, atresia is essential for the maintenance of ovarian homoeostasis; however, a number of factors have been described as potential causes of increased ovarian atresia such as marine pollution or reduced food supply (Cabrera-Páez et al. 2009; Ortiz-Zarragoitia et al. 2011; Yamamoto et al. 2011). Thus, besides indicating a poorer physiological condition for reproduction, elevated atretic indices could also reflect an environmental impact.
Gonadal atresia can appear at any stage of oocyte development; however, it is mainly described in vitellogenic oocytes in both marine vertebrates and invertebrates. By contrast, atresia in previtellogenic oocytes is less evident because oocytes at this stage are smaller and unyolked. In general, atresia has been widely studied in marine fishes (Valdebenito et al. 2011); however, little is known for other marine organisms. The study of this process is particularly poor in marine invertebrates such as cephalopod species, and only in a few cases, detailed histological analyses have been used in order to identify and/or describe the atretic oocytes. Examples include studies for octopods such as Octopus rubescens, Octopus hubbsorum and Octopus ocellatus (López-Peraza et al. 2013; Alejo-Plata and Gómez-Márquez 2015; Wang et al. 2015); loliginids such as Loligo gahi and Lolliguncula panamensis (Laptikhovsky and Arkhipkin 2001; Arizmendi-Rodríguez et al. 2012); chranchiids such as Galiteuthis glacialis (Nesis et al. 1998), lycoteuthids such as Lycoteuthis lorigera (Hoving et al. 2014), and ommastrephids such as Dosidicus gigas (Hernández-Muñoz et al. 2016).
Two structures, post-ovulatory follicles (POFs) and haemocytes, are associated with the ovarian atresia. POFs are generated as a consequence of ovulation when oocytes are released to the ovarian cavity with their innermost layer, the chorion. Meanwhile, the follicular envelopes of those oocytes, now called POFs, continue attached to the connective tissue strand. They start to degenerate in a similar way as atretic oocytes do, although more rapidly, being easily confounded with atretic oocytes in advanced resorptive stages. The classification of POFs in deteriorated stages over time has allowed to estimate spawning frequency in fishes (e.g. Ganias 2012) and, to a lesser extent, in cephalopods (e.g. Melo and Sauer 2007). For any considered species, the degree of POFs deterioration decreases with decreasing temperatures increasing the time that POFs can be detected within the ovary (Ganias et al. 2007; Laptikhovsky 2013). POFs have been histologically identified in several cephalopod species; however, POF staging has only been determined in the loliginid species Loligo reynaudii (Melo and Sauer 2007) and Doryteuthis opalescens (Macewicz et al. 2004).
On other hand, haemocytes are haemolymph circulating cells that are involved in several functions such as wound repair, nutrient digestion, transport and excretion. Moreover, in some molluscs, they have an important role as a defence cells against pathogens (Cheng 1975). Furthermore, haemocytes are essential for the resorption of the atretic oocytes through phagocytosis. This process has been identified in several marine organisms such as bivalve molluscs (Le Pennec et al. 1991; Suárez-Alonso et al. 2007; Camacho-Mondragón et al. 2012), crustaceans (Zara et al. 2013) and fishes, where these immune cells are called granulocytes (Bruslé-Sicard et al. 1992; Besseau and Faliex 1994; Miranda et al. 1999). Regarding cephalopods, haemocytes, as well as their phagocytic activity, have been detected in the hemolymph of Eledone cirrhosa (Malham et al. 1997), Sepia officinalis (Le Pabic et al. 2014), Euprymna scolopes (Nyholm et al. 2009) and Octopus vulgaris (Rodríguez-Domínguez et al. 2006). However, the identified cell types vary among these species, and in some cephalopods’ ovary development studies, other terms such as amoebocytes, granulocytes or lymphoid cells have been previously used instead of haemocyte. Buckley (1977) described the presence of occasional amoebocytes in the blood vessels towards the end of vitellogenesis and massive amoebocytes immediately prior to and afterwards egg laying in common octopus. Melo and Sauer (1998) suggested the presence of lymphoid cells and observed occasional granulocytes in the thecal stroma, both occurring in atretic previtellogenic oocytes of Loligo reynaudii. However, these authors did not attribute any phagocytic role to those cells during ovarian atresia.
The common octopus (Octopus vulgaris Cuvier, 1797) is one of the most commercially important cephalopods worldwide and, especially, in European waters (Pierce et al. 2010). As for most cephalopods, it has a short life cycle of less than 2 years; it grows rapidly to maturity, spawns once, often seasonally, at the end of its life and is an ecological opportunist with labile populations (Guerra 2006). Earlier described as a simultaneous terminal spawner with a synchronous ovulation (Rocha et al. 2001), its reproductive strategy has been recently reconsidered through detailed histological analyses of ovary development (Gonçalves et al. 2002; Cuccu et al. 2013; Sieiro et al. 2014). These works suggest that oogenesis is an asynchronic process. However, ovulation and spawning patterns, though presumably also asynchronic, remain unresolved. The study of gonadal atresia as a normal process in the ovary development would help to determine which maturity microstages are affected, and therefore, would allow further understanding of its reproductive strategy. Though previous studies have already identified the presence of atretic oocytes in the species (Di Cosmo et al. 2001; Cuccu et al. 2013), there is no other detailed information such as the morphological changes that occur in the atretic oocytes, their classification, the occurrence of other cell structures such as (atretic) POFs and haemocytes, or the relationship between atresia and morphometric variability.
Therefore, the objectives of this work were (i) to describe gonadal atresia in female common octopus according to different degenerative oocytes (i.e. different atretic microstages) found throughout the whole reproductive cycle and its categorization based on previtellogenesis and vitellogenesis phases; (ii) to identify haemocytes as phagocytic cells, together with follicle cells, executing the atretic process; (iii) to relate the presence of atresia with morphometric parameters; and (iv) to analyse its seasonal cycle.
Materials and methods
Morphometrical and histological analyses
A total of 359 females of Octopus vulgaris from the creel fishery were sampled at three ports in Galicia (NW Spain) from 2004 to 2007. In order to sample all maturity stages, 5 wild individuals were below the minimum legal catch size (1 kg), and 26 specimens were spawning females obtained from cage ongrowing (see the cages display in Chapela et al. 2006). All females were used for subsequent morphometric and histological analyses. Maturation and reproduction were assessed using a macroscopic maturity scale proposed by Inejih (2000) and six reproductive measurements. These included: body weight (BW), digestive gland weight (DGW), ovary weight (OW), oviducal complex weight (OCW), and longitudinal (LDOG) and transversal (TDOG) diameters of the oviducal gland. A suite of three morphological indices were also used: the gonadosomatic (GSI), Hayashi (HI) and digestive gland (DGI) index (see Otero et al. 2007 for calculations). Based on histological analyses, maturation was further assessed using a microscopic scale and a histological maturity index (HMI) (see further details in Sieiro et al. 2014).
Ovarian preparations were evaluated using a light microscope (Leica DM5500 B; 12.5–1000 × magnification) coupled with a Leica DFC 310 FX digital video camera. The software used was Leica Microsystems CMS GmbH, LAS v. 4.1 (Build 1264) (© 2003–2012). The histological analyses consisted of, first, a microscopic staging of ovarian maturity based on Sieiro et al. (2014). Second, ovaries were further classified according to the presence or absence of atresia based on the following criteria: (1) the arrangement and hypertrophy of follicular envelope, mainly follicle cells; (2) the presence of chromatin condensation; (3) the identification of haemocytes; (4) the phagocytosis of yolk; and (5) the degree of vascularisation and presence of yellow–brown bodies. If present, atresia was further subcategorised in previtellogenic, vitellogenic, or the presence of both types. In some cases, fluorescence reaction was used to differ between previtellogenic and vitellogenic atretic oocytes, as well as between POFs and atretic oocytes, using a fluorescence narrow bandwidth filter set (©Leica GFP-Plant; 470/40 nm excitation filter; 495 nm dichromatic beam splitter; and 525/50 nm barrier filter). Third, in atretic ovaries, haemocytes were morphologically identified following Castellanos-Martínez et al. (2014). Measures of total diameter, nucleus diameter and the ratio nucleus/cytoplasm (N/C) were taken from a sample of 303 haemocytes found in blood vessels of the ovarian stroma, and from 95 haemocytes found in initial atretic oocytes.
Statistical analyses
Generalized Linear Models (GLMs) were used to relate the presence of atresia to the set of morphometric variables described above. A binomial distribution with a logit link was used. To study the seasonal cycle of atresia, a generalized additive model was fit to the data. A penalized cyclic cubic regression spline was used. Differences in average values taken from within the population of haemocytes were evaluated using an analysis of variance (ANOVA).
Results
Morphological characterization of ovarian atresia
Based on observed relevant morphological changes in the oocytes, we have identified three atretic histological stages in both previtellogenic and vitellogenic oocytes. These stages are named and described as follows:
-
1.
Initial stage (Fig. 1). We have observed two substages:
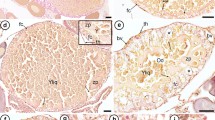
Fig. 1 Initial atresia. Irruption of haemocytes in the follicular envelope as well as in the ooplasm causing a generalized disorganization of the inner cell layer that is more apparent near the folds in the FO microstage (a, b). c The disorganized layer and the haemocytes in the SO microstage. cc cuboidal cells, fc flat cells, f fold, h haemocytes, N nucleus, Nl nucleolus
-
1.1.
There is an apparent increase of the area between the cell layers of the follicular envelope, that is, the outer flattened and elongated cells (outer cells) and inner cuboidal cells (follicle cells). There is a generalized disorganization of the inner cell layer losing its peculiar linearity. This is more obvious from the folding oocytes (FO) microstage onwards (see Sieiro et al. 2014), in the proximity of foldings where large blood vessels are formed between both cell layers.
-
1.2.
Through these blood vessels, haemocytes appear between cells of the follicular envelope mainly near the foldings. Haemocytes push the follicle cells to the ooplasm and invade it. If the chorion was previously formed (late vitellogenic microstage, LV, Sieiro et al. 2014), it is disintegrated into fragments that are also displaced inwards.
-
1.1.
-
2.
Medium stage (Fig. 2). We have observed two substages:
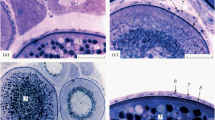
Fig. 2 Medium stage of atresia in previtellogenic and vitellogenic oocytes. a–d Haemocytes with pyknotic and basophilic nuclei that appear hypertrophied compared to follicle cells (inner cuboidal cells) in previtellogenic oocytes. At this stage chromatin condensation and nucleus disintegration starts. a, b The medium-atretic SO microstage with visible chromatin condensation, whereas c and d illustrate the medium-atretic FO microstage. e–g Haemocytes and follicular cells disposed over the yolk forming patches in medium-atretic vitellogenic oocytes. f, g The chorion and yolk under fluorescence reaction in a normal (LV microstage) and atretic (FV microstage) oocyte, respectively. Arrows in e indicate atretic oocytes. ch chorion, Chr chromatin condensation, y yolk; the other abbreviations as in Fig. 1
-
2.1.
The ooplasm appears to be invaded by phagocytic cells, that is, haemocytes and follicle cells, whereas the outer cells remain as an organized layer. Phagocytosis occurs in the same way as haemocytes invade the ooplasm, from the outer to the inner side of it. As a consequence, many of these cells are larger than in the previous phase with pyknotic and basophilic nuclei mainly regarding to previtellogenic atretic oocytes (Fig. 2a–d). The arrangement of both types of cells is different in vitellogenic atretic oocytes, where patches of haemocytes and follicle cells are found over the yolk, and no hypertrophied haemocytes occur (Fig. 2e–g). The oocyte nucleus remains intact.
-
2.2.
As cytoplasmic material is being reabsorbed, the nuclear chromatin condenses and the nucleus disintegration begins. Some yolk granules and chorion fragments remain in the ooplasm in full vitellogenic (FV) and LV atretic oocytes (see Sieiro et al. 2014), respectively. Some size reduction in outer cells and in the whole follicle can be appreciated.
-
2.1.
-
3.
Advanced stage (Fig. 3). We have observed three substages:
Fig. 3 Advanced atresia in previtellogenic (a–c) and vitellogenic oocytes (d–i). a, b Superficial cuts of atretic oocytes arranged as a mixture of cells from the follicular envelope with haemocytes. Some haemocytes are still hypertrophied. c An atretic oocyte showing a lumen with few cells and numerous blood vessels. d–f Atretic oocytes in spawning females with chorion fragments (e) and yellow–brown bodies (f). g Yellow–brown bodies and many wide blood vessels in a post-spawning female. h, i The final reproductive cycle of a post-spawning female with many wide blood vessels in the connective tissue (arrows in h) and the last stages of atresia (arrowheads in h). This stage displays a large lumen, few cells inside (presumably haemocytes) and indistinguishable flat cells (i). bv blood vessel, ch chorion, OV ovulated oocyte, yb yellow–brown bodies; the other abbreviations as in Fig. 1
-
3.1.
The follicle shrinks, mainly across its transversal section, acquiring an increasingly amorphous appearance. The nucleus has been completely disintegrated, and the cytoplasmic material is scarcely observed being replaced by numerous blood vessels. This blood network reduces the oocyte lumen and is arranged surrounding the haemocytes (Fig. 3a–e).
-
3.2.
A progressive decrease in the number and size of phagocytic cells occurs; the outer cells are hardly distinguishable. An increasing number of yellow–brown bodies appear in the ooplasm of vitellogenic oocytes (Fig. 3f, g).
-
3.3.
At this stage, the atretic oocytes begin to be easily confounded with ovarian connective tissue, remaining like a scarce in the ovary. In the case of atretic vitellogenic oocytes, they appear with a great lumen and small haemocytes, all surrounded by an indistinguishable outer layer. Neither yellow–brown bodies nor follicle cells can be seen (Fig. 3h, i).
-
3.1.
Haemocyte analyses
Different morphological characteristics were observed between haemocytes in blood vessels and in initial atretic oocytes prior to the onset of phagocytosis. Haemocytes in initial atretic oocytes were round, had a basophilic and centric nuclei, and granules in their cytoplasm were not apparent (Fig. 4a–c), whereas haemocytes in blood vessels were U-shaped, had a basophilic and eccentric nuclei, and basophilic granules occurred in the cytoplasm (Fig. 4d–f). Population sizes for haemocytes sampled in both initial atretic oocytes and in blood vessels were unimodal (Fig. 5a). Haemocytes in initial atretic oocytes had an average diameter of 5.12 ± 0.93 µm (range 3.40–7.40), while haemocytes in blood vessels averaged 7.26 ± 1.03 µm (range 4.18–10.51). This difference was statistically significant (p < 0.0001) (Fig. 5b). In addition, the ratio N/C was also unimodal in both locations (Fig. 5c) with an average value of 0.68 ± 0.1 µm (range 0.45–0.91) and 0.64 ± 0.09 µm (range 0.41–0.95) in initial atretic oocytes and blood vessels, respectively. The slightly larger ratio in haemocytes sampled within initial atretic oocytes was statistically significant (p < 0.0001) (Fig. 5d).
Haemocytes within atretic oocytes (a–c) and blood vessels (d–f). a Haemocytes within an atretic previtellogenic oocyte (arrows); b, c Haemocytes in atretic vitellogenic oocytes forming patches over the yolk (arrows); d, e Haemocytes within blood vessels of the ovarian connective tissue showing the U-shaped nuclei (arrows). In f, haemocytes occur within a blood vessel of the oviducal gland in a spawning female showing basophilic granules inside (arrows). y yolk; the other abbreviations as in Fig. 1
Occurrence of females with atretic oocytes and its seasonal cycle
The maturity stage of the ovaries ranged from macrostages I to V and from microstages SO (Secondary Oocytes) to OV (Ovulated Oocytes). There were no ovaries classified as either OO (Oogonia) or PO (Primary Oocytes). Almost 52 % of the sampled ovaries showed atresia, and this process occurred in previtellogenic (74.31 %), vitellogenic (13.19 %) or both types of oocytes (12.50 %) within the same ovary. The percentage of atresia, understood as the percentage of females with ovarian atresia, increased progressively from immature (macrostage I) to spawning females (macrostage V), reaching a 100 % in macrostage V (not shown). Similarly, regarding maturity microstages, atresia increased from SO to OV ovaries reaching virtually a 100 % in LV ones (Fig. 6a). All microstages were affected by atresia, from PO to LV, with the exception of OO and OV. When considering the type of atresia, there was a predominance of previtellogenic atresia throughout the ovarian development with the exception of OV microstage (Fig. 6b). However, atretic vitellogenic oocytes firstly occurred in microstage FV reaching a maximum at the spawning stage, that is, microstage OV (Fig. 6b). Finally, both types of atresia only occurred at microstages FV and LV (Fig. 6b).
The probability of occurrence of atresia, regardless of previtellogenic or vitellogenic type, showed a significant seasonal cycle with a peak at the end of March, beginning of April and a tough in July (Fig. 7).
Relationship between atresia and morphometric parameters
The probability of occurrence of atresia, regardless of the type, was related to virtually all morphometric measurements and indices. With the exception of DGI, the presence of atresia increased with octopus weight, reproductive organs’ size and DGW (Table 1). The probability of atresia occurrence further increased with morphological indices. In particular, the presence of atresia strongly increased with GSI (Fig. 8a) and HMI (Fig. 8b).
Discussion
Morphological description of ovarian atresia in common octopus
Atresia has been observed in several cephalopod species, though in most cases neither using histological techniques nor throughout ovary development. Regarding Octopus vulgaris, previous studies have suggested that the number of folds and the presence of a scarce yolk in the oocytes could be a morphological indicator of atresia (Di Cosmo et al. 2001). However, those facts might be confused given that the number of folds (and their depth) depends on the oocyte orientation during the histological sample preparation and cutting. In fact, in a previous study, we only observed a maximum of four foldings in fresh samples (Sieiro et al. 2014). Moreover, the scarce yolk could be a symptom of an early vitellogenesis more than the yolk resorption if no other morphological change is observed such as a follicular envelope disorganization. Furthermore, Cuccu et al. (2013) inferred the presence of atretic oocytes as structures with disorganized follicular epithelium and chorion fragments. However, this work neither provides a detailed description nor a classification of atresia for this species. Therefore, given the lack of a baseline for common octopus atresia, our proposed classification was based on histological characteristics as was similarly done in the seminal work in the chokka squid Loligo reynaudii by Melo and Sauer (1998) and other marine species such as fishes (e.g. Hunter and Macewicz 1985; Guraya 1986; Miranda et al. 1999).
Phagocytic cells: haemocytes and follicle cells
Two morphological and functional populations were previously characterized in O. vulgaris (Novoa et al. 2002; Castellanos-Martínez et al. 2014; Troncone et al. 2015), though usually using differing names and cell diameters among those studies. In our case, we also identified two haemocyte populations showing morphological characteristics very similar to the above cited works. These would be hyalinocytes and granulocytes as called in Troncone et al. (2015) and large and small granulocytes as named in Castellanos-Martínez et al. (2014). Moreover, our mean haemocyte diameters and N/C ratios were in agreement with the ranges shown by Troncone et al. (2015) and Castellanos-Martínez et al. (2014), respectively. The apparent differences might be due to distinct sample processing techniques.
Castellanos-Martínez et al. (2014) and Troncone et al. (2015) found phagocytic activity in both haemocyte types, being higher in the larger ones. Complementary to this, we showed that haemocytes occurred in both atretic previtellogenic and vitellogenic oocytes and were massive in those ovaries and oviducal glands of spawning and post-spawning females. Thus, we could conclude that these cells would digest the ooplasm content of atretic oocytes in O. vulgaris. This is one of the roles proposed for these cells in other marine organisms such as molluscs, crustaceans and fishes (see Introduction). Additionally, Troncone et al. (2015) postulated that the role of granulocytes in phagocytosis would be linked to their capability for intracellular killing. This could explain why we found small haemocytes within atretic oocytes and larger haemocytes within blood vessels of the ovarian stroma. Both cells could then play different roles, that is, an immune response to invading pathogens for the larger haemocytes and a phagocytic activity in gonadal atresia for the smaller ones.
Follicle cells have been classically involved in atresia through morphological changes such as hypertrophy, pyknosis and arrangement around the yolk (e.g. Linares-Casenave et al. 2002). In common octopus, only the inner cells (called here follicle cells) within the follicular envelope seemed to have a phagocytic role during gonadal atresia, whereas the outer cells experimented hypotrophy once atresia progresses in vitellogenic oocytes. On the other hand, the different aspect observed for follicle cells between reabsorbing previtellogenic and vitellogenic oocytes suggests a different function. Thus, follicle cells hardly increased in size unlike pyknotic haemocytes observed within atretic previtellogenic oocytes. In contrast, both enlarged follicle cells and haemocytes showed pyknotic nuclei and were arranged over the yolk forming basophilic patches in vitellogenic oocytes. This indicates that haemocytes have a clear phagocytic role in all atretic oocytes, while it seems that follicle cells are only involved in the phagocytic process if yolk is present. This could reinforce the hypothesis that small haemocytes are implicated in the atretic process, and their phagocytic capability is enhanced by follicle cells in vitellogenic atresia. Melo and Sauer (1998) assigned the same phagocytic role to follicle cells in atretic previtellogenic and vitellogenic oocytes for the chokka squid. However, they found thickened and hyperplasic outer cells invading the follicle cells in degenerating previtellogenic and vitellogenic oocytes. Regarding this topic, there is not a consensus in fishes (e.g. Saidapur 1978; Guraya 1998; McMillan 2007; Morais et al. 2012; Sharma and Bhat 2014).
Yellow–brown bodies
The occurrence of yellow–brown bodies in the ooplasm of atretic oocytes points out the end of the atresia in various marine taxa such as fishes, echinoderms and molluscs (e.g. Hunter and Macewicz 1985; Blazer 2002; Schäfer and Köhler 2009; Flores-Quintana et al. 2012; Cuevas et al. 2015). These pigmentary clusters are characterized as chromolipoids, mainly lipofuscins/ceroids, as a result of the degenerative process of proteins and lipids during atresia. However, their origin remains unclear. In fish studies, they seem to originate from oocyte, follicle envelope or granulocyte degeneration (e.g. Besseau and Faliex 1994; Miranda et al. 1999). In other cases, they were particularly observed within the remaining granulosa cells at the end of the atretic process (Santos et al. 2005). By contrast, these structures have not yet been identified in cephalopod atretic ovaries. We have observed them within the high vascularised ooplasm and near the haemocytes, coinciding with the disappearance of follicle cells. Taking into account that these bodies are only observed in atretic ovaries of spawning females and that there is no ovary regeneration after breeding, we suggest that follicle cell apoptosis results in the appearance of yellow–brown bodies that are ultimately phagocytised by haemocytes at the end of atresia in post-spawning females. In fact, in other marine organisms such as teleost fishes and other aquatic oviparous vertebrates, follicular cells degenerate once yolk resorption is completed during atresia (Wood and Van Der Kraak 2001). The cell remnants after follicular cell apoptosis in fish atretic oocytes and also POFs could be engulfed by normal follicle cells and/or granulocytes (Drummond et al. 2000; Santos et al. 2008; Üçüncü and Çakici 2009). In any case, future works should be addressed to confirm the apoptosis occurrence in follicle cells once yolk resorption ends in spawning females of common octopus using for instance TUNEL assay as a molecular technique for DNA fragmentation in these cells.
Postovulatory follicles (POFs)
Cuccu et al. (2013) ascribed the occurrence of POFs exclusively to post-spawning common octopus. However, POFs should occur as soon as mature females ovulate and spawn. In fact, in loliginids and other octopods, POFs were found from partially spent females (Melo and Sauer 1999, 2007; Macewicz et al. 2004; Olivares-Paz et al. 2001; Zamora and Olivares 2004; Arizmendi-Rodríguez et al. 2012). We were, however, not able to identify POFs probably due to the low sample size of spawning females, and their advanced stage of spawning that hampered POF recognition due to their rapid resorption and probable misidentification with atretic oocytes. In fact, POFs were only reliably identifiable within 14 h after spawning in Loligo reynaudii, being old postovulatory follicles difficult to distinguish from atretic oocytes afterwards (Melo and Sauer 2007).
Atresia occurrence and reproductive strategy
Cuccu et al. (2013) found atretic oocytes restricted to the post-spawning stage in female common octopus. By contrast, we found ovarian atresia throughout the whole ovary development and affecting all common octopus females when spawning begins. In fact, the peak of atretic females was fairly coincident with the maximum of maturation in spring before spawning (Sieiro et al. 2014). Thus, the number of atretic females increased from immature to mature individuals. Moreover, atresia was predominately previtellogenic throughout sexual maturation with the exception of spawning when all females have atretic ovaries of vitellogenic type. Therefore, atresia in vitellogenic oocytes before spawning seems to be a rare event during the normal ovary development. This is fairly similar to Loligo reynaudii, which has ovarian atresia throughout the year with the vitellogenic type being particularly prevalent in fully spent females (Melo and Sauer 1998). In other octopods, apart from the post-spawning phase, atretic oocytes were also found in earlier maturity stages such as the cases of Eledone cirrhosa (Boyle and Chevis 1992), Octopus mimus (Olivares-Paz et al. 2001) and Graneledone macrotyla (Guerra et al. 2013).
Therefore, the occurrence of ovarian atresia in O. vulgaris is a common and regulative process inherent to the normal ovary development as observed in other cephalopods such as Dosidicus gigas (Hernández-Muñoz et al. 2016), Doroteuthis opalescens (Macewicz et al. 2004) and Loligo reynaudii (Melo and Sauer 1998). However, ovarian atresia in common octopus has not yet been considered as an important physiological process for the species, which would have further implications for the reproductive strategy and the potential fecundity. O. vulgaris has an asynchronic ovary development since all microstages were identified over the whole ovary maturation (Gonçalves et al. 2002; Cuccu et al. 2013; Sieiro et al. 2014). Additionally, all previtellogenic oocytes end up being reabsorbed before spawning begins, particularly in LV ovaries. Moreover, FV microstage starts to degenerate at the spawning onset contrary to LV and OV oocytes. Once egg laying ends, atresia continues in those LV and OV oocytes that will remain within the spawned female. This is in contrast to Mangold-Wirz (1963), who stated that a total egg laying occur in this species. Given these elements, we can confirm histologically that ovulation follows a synchronic pattern unlike ovary maturation. All these facts would explain why spawning duration that has been recently estimated at around 35 days into the wild (Garci et al. 2016) is shorter than ovary maturation, and why common octopus would differ from other cephalopods such as the intermittent terminal spawner Loligo reynaudii (Melo and Sauer 1999), or the multiple spawner jumbo squid, Dosidicus gigas (Hernández-Muñoz et al. 2016).
Our findings also have implications concerning potential fecundity, that is, only LV oocytes in pre-spawning ovaries of common octopus should be considered in fecundity estimations since this microstage does not experience atresia until spawning ends. Another plausible method would be to apply a correction factor if percentages of atretic oocytes could be calculated for each type of ovary. In this regard, we propose for future works to calculate the intensity of atresia for merely the FV microstage during ovary development of common octopus since atresia is predominately previtellogenic during maturation in this species. This would allow shortening the time required when an appropriate stereological method would be applied for this purpose.
References
Alejo-Plata MC, Gómez-Márquez JL (2015) Reproductive biology of Octopus hubbsorum (Cephalopoda: Octopodidae) from the coast of Oaxaca, Mexico. Am Malacol Bull 33:1–12
Arizmendi-Rodríguez DI, Rodríguez-Jaramillo C, Quiñonez-Velázquez C, Salinas-Zavala CA (2012) Reproductive indicators and gonad development of the Panama brief squid Lolliguncula panamensis (Berry 1911) in the Gulf of California, Mexico. J Shellfish Res 31:817–826
Besseau L, Faliex E (1994) Resorption of unemitted gametes in Lithognathus mormyrus (Sparidae, Teleostei): a possible synergic action of somatic and immune cells. Cell Tissue Res 276:123–132
Blazer VS (2002) Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol Biochem 26:85–101
Boyle PR, Chevis D (1992) Egg development in the octopus Eledone cirrhosa. J Zool 227:623–638
Bruslé-Sicard S, Debas L, Fourcault B, Fuchs J (1992) Ultrastructural study of sex inversion in a protogynous hermaphrodite, Epinephelus microdon (Teleostei, Serranidae). Reprod Nutr Dev 32:393–406
Buckley SKL (1977) Oogenesis and its hormonal control in Octopus vulgaris. Ph.D. Thesis, University of Cambridge, Cambridge
Cabrera-Páez Y, Aguilar-Betancourt C, González-Sansón G, Antonelli F (2009) La atresia en Stegastes partitus (Poey, 1868) (Actinopterygii: Pomacentridae) como indicador de impacto ambiental. Rev Investig Mar 30:107–115
Camacho-Mondragón MA, Arellano-Martínez M, Ceballos-Vázquez BP (2012) Particular features of gonadal maturation and size at first maturity in Atrina maura (Bivalvia: Pinnidae). Sci Mar 76:539–548
Castellanos-Martínez S, Prado-Álvarez M, Lobo-da-Cunha A, Azevedo C, Gestal C (2014) Morphologic, cytometric and functional characterization of the common octopus (Octopus vulgaris) hemocytes. Dev Comp Immunol 44:50–58
Chapela A, González AF, Dawe EG, Rocha F, Guerra A (2006) Growth of common octopus (Octopus vulgaris) in cages suspended from rafts. Sci Mar 70:121–129
Cheng TC (1975) Functional morphology and biochemistry of molluscan phagocytes. Ann N Y Acad Sci 266:343–379
Cuccu D, Mereu M, Porcu C, Follesa MC, Cau AL, Cau A (2013) Development of sexual organs and fecundity in Octopus vulgaris Cuvier, 1797 from the Sardinian waters (Mediterranean Sea). Mediterr Mar Sci 14:270–277
Cuevas N, Zorita I, Costa PM, Franco J, Larreta J (2015) Development of histopathological indices in the digestive gland and gonad of mussels: integration with contamination levels and effects of confounding factors. Aquat Toxicol 162:152–164
Di Cosmo A, Di Cristo C, Paolucci M (2001) Sex steroid hormone fluctuations and morphological changes of the reproductive system of the female of Octopus vulgaris throughout the annual cycle. J Exp Zool 289:33–47
Drummond CD, Bazzoli N, Rizzo E, Sato Y (2000) Postovulatory follicle: a model for experimental studies of programmed cell death or apoptosis in teleosts. J Exp Zool 287:176–182
Flores-Quintana C, Blanco-Cohene T, Arbúes R, Domitrovic H, González J (2012) Follicular atresia in ovaries of Prochilodus lineatus. Int J Morphol 30:1301–1308
Ganias K (2012) Thirty years of using the postovulatory follicles method: overview, problems and alternatives. Fish Res 117–118:63–74
Ganias K, Somarakis S, Koutsikopoulos C, Machias A, Theodorou A (2003) Ovarian atresia in the Mediterranean sardine, Sardina pilchardus sardina. J Mar Biol Assoc UK 83:1327–1332
Ganias K, Nunes G, Stratoudakis Y (2007) Degeneration of postovulatory follicles in the Iberian sardine Sardina pilchardus: structural changes and factors affecting resorption. Fish Bull 105:131–139
Garci ME, Hernández-Urcera J, Gil-Coto M, Fernández-Gago R, González AF, Guerra A (2016) From brooding to hatching: new insights from a female Octopus vulgaris in the wild. J Mar Biol Assoc UK 96:1341–1346
Gonçalves I, Sendão J, Borges TC (2002) Octopus vulgaris (Cephalopoda: Octopodidae) gametogenesis: a histological approach to the verification of the macroscopic maturity scales. Abh Geol Bundesanst A 57:79–88
Guerra A (2006) Estrategias evolutivas de los cefalópodos. Investig Cienc 355:50–59
Guerra A, Sieiro MP, Roura A, Portela JM, del Río JL (2013) On gonadic maturation and reproductive strategy in deep-sea benthic octopus Graneledone macrotyla. Helgol Mar Res 67:545–554
Guraya SS (1986) The cell and molecular biology of fish oogenesis. Monogr Dev Biol 18:1–223
Guraya SS (1998) The comparative cell biology of accessory somatic (follicle or granulosa) cells in the animal ovary. Proc Indian Natl Sci Acad Part B Biol Sci 64:161–195
Hernández-Muñoz AT, Rodríguez-Jaramillo C, Mejía-Rebollo A, Salinas-Zavala CA (2016) Reproductive strategy in jumbo squid Dosidicus gigas (D’Orbigny 1835: a new perspective. Fish Res 173:145–150
Hoving HJT, Laptikhovsky VV, Lipinski MR, Jürgens E (2014) Fecundity, oogenesis, and ovulation pattern of southern African Lycoteuthis lorigera (Steenstrup, 1875). Hydrobiologia 725:23–32
Hunter JR, Lo NCH (1997) The daily egg production method of biomass estimation: some problems and potential improvements. Ozeanografika 2:41–69
Hunter JR, Macewicz BJ (1985) Rates of atresia in the ovary of captive and wild northern anchovy, Engraulis mordax. Fish Bull 83:119–136
Inejih CAO (2000) Dynamique spatio-temporelle et biologie du poulpe (Octopus vulgaris) dans les eaux Mauritaniennes: modélisation de l’abondance et aménagement des pêcheries. Ph.D. Thesis, Université de Bretagne Occidentale, France
Kurita Y, Meier S, Kjesbu OS (2003) Oocyte growth and fecundity regulation by atresia of Atlantic herring (Clupea harengus) in relation to body condition throughout the maturation cycle. J Sea Res 49:203–219
Laptikhovsky V (2013) Reproductive strategy of deep-sea and Antarctic octopods of the genera Graneledone, Adelieledone and Muusoctopus (Mollusca: Cephalopoda). Aquat Biol 18:21–29
Laptikhovsky VV, Arkhipkin AI (2001) Oogenesis and gonad development in the cold water loliginid squid Loligo gahi (Cephalopoda: Myopsida) on the Falkland shelf. J Molluscan Stud 67:475–482
Le Pabic C, Goux D, Guillamin M, Safi G, Lebel JM, Koueta N, Serpentini A (2014) Hemocyte morphology and phagocytic activity in the common cuttlefish (Sepia officinalis). Fish Shellfish Immunol 40:362–373
Le Pennec M, Beninger PG, Dorange G, Paulet YM (1991) Trophic sources and pathways to the developing gametes of Pecten maximus (Bivalvia: Pectinidae). J Mar Biol Assoc UK 71:451–463
Linares-Casenave J, Van Eenennaam JP, Doroshov SI (2002) Ultrastructural and histological observations on temperature-induced follicular ovarian atresia in the white sturgeon. J Appl Ichthyol 18:382–390
López-Peraza DJ, Hernández-Rodríguez M, Barón-Sevilla B, Bückle-Ramírez LF (2013) Histological analysis of the reproductive system and gonad maturity of Octopus rubescens. Int J Morphol 31:1459–1469
Macewicz BJ, Hunter JR, Lo NCH, LaCasella EL (2004) Fecundity, egg deposition, and mortality of market squid (Loligo opalescens). Fish Bull 102:306–327
Malham SK, Runham NW, Secombes CJ (1997) Phagocytosis by haemocytes from the lesser octopus Eledone cirrhosa. Iberus 15:1–11
Mangold-Wirz K (1963) Biologie des céphalopodes benthiques et nectoniques de la mer catalane. Vie et Milieu, Supl. 13. Hermann, Banyuls-sur-Mer, Paris
McMillan DB (2007) Fish histology female reproductive systems. Springer, Dordrecht
Melo YC, Sauer WHH (1998) Ovarian atresia in cephalopods. S Afr J Mar Sci 20:143–151
Melo YC, Sauer WHH (1999) Confirmation of serial spawning in the chokka squid Loligo vulgaris reynaudii off the coast of South Africa. Mar Biol 135:307–313
Melo YC, Sauer WHH (2007) Determining the daily spawning cycle of the chokka squid, Loligo reynaudii off the South African Coast. Rev Fish Biol Fish 17:247–257
Miranda ACL, Bazzoli N, Rizzo E, Sato Y (1999) Ovarian follicular atresia in two teleost species: a histological and ultrastructural study. Tissue Cell 31:480–488
Morais RD, Thomé RG, Lemos FS, Bazzoli N, Rizzo E (2012) Autophagy and apoptosis interplay during follicular atresia in fish ovary: a morphological and immunocytochemical study. Cell Tissue Res 347:467–478
Nesis KN, Nigmatullin ChM, Nikitina IV (1998) Spent females of deepwater squid Galiteuthis glacialis under the ice at the surface of the Weddell Sea (Antarctic). J Zool 244:185–200
Novoa B, Tafalla C, Guerra A, Figueras A (2002) Cellular immunological parameters of the octopus, Octopus vulgaris. J Shellfish Res 21:243–248
Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ (2009) Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ Microbiol 11:483–493
Olivares-Paz A, Zamora M, Portilla P, Zuñiga O (2001) Estudio histológico de la ovogénesis y maduración ovárica en Octopus mimus (Cephalopoda: Octopodidae) de la II Región de Chile. Estud Oceanol 20:13–22
Ortiz-Zarragoitia M, Garmendia L, Barbero MC, Serrano T, Marigómez I, Cajaraville MP (2011) Effects of the fuel oil spilled by the Prestige tanker on reproduction parameters of wild mussel populations. J Environ Monit 13:84–94
Otero J, González AF, Sieiro MP, Guerra A (2007) Reproductive cycle and energy allocation of Octopus vulgaris in Galician waters, NE Atlantic. Fish Res 85:122–129
Pierce GJ, Allcock L, Bruno I, Bustamante P, González A, Guerra A, Jereb P, Lefkaditou E, Malham S, Moreno A et al (2010) Cephalopod biology and fisheries in Europe. ICES Cooperative Research Report No. 303
Rocha F, Guerra A, González AF (2001) A review of reproductive strategies in cephalopods. Biol Rev 76:291–304
Rodríguez-Domínguez H, Soto-Búa M, Iglesias-Blanco R, Crespo-González C, Arias-FernándeZ C, García-Estévez J (2006) Preliminary study on the phagocytic ability of Octopus vulgaris Cuvier, 1797 (Mollusca: Cephalopoda) haemocytes in vitro. Aquaculture 254:563–570
Saidapur SK (1978) Follicular atresia in the ovaries of nonmammalian vertebrates. Int Rev Cytol 54:225–244
Santos HB, Rizzo E, Bazzoli N, Sato Y, Moro L (2005) Ovarian regression and apoptosis in the South American teleost Leporinus taeniatus Lütken (Characiformes, Anostomidae) from the São Francisco Basin. J Fish Biol 67:1446–1459
Santos HB, Thomé RG, Arantes FP, Sato Y, Bazzoli N, Rizzo E (2008) Ovarian follicular atresia is mediated by heterophagy, autophagy and apoptosis in Prochilodus argenteus and Leporinus taeniatus (Teleostei: Characiformes). Theriogenology 70:1449–1460
Schäfer S, Köhler A (2009) Gonadal lesions of female sea urchin (Psammechinus miliaris) after exposure to the polycyclic aromatic hydrocarbon phenanthrene. Mar Environ Res 68:128–136
Sharma RK, Bhat RA (2014) Histomorphology of atretic follicles in rainbow trout (Oncorhynchus mykiss) from Kashmir. J Entomol Zool Stud 2:21–26
Sieiro MP, Otero J, Guerra A (2014) Contrasting macroscopic maturity staging with histological characteristics of the gonads in female Octopus vulgaris. Hydrobiologia 730:113–125
Suárez-Alonso P, Álvarez-González C, Molist-García P, San Juan-Serrano F (2007) Atresia gonadal durante el ciclo gametogénico de Mytilus galloprovincialis Lamarck, 1819 cultivado en la ría de Vigo (Noroeste de la Península Ibérica). Bol Inst Esp Oceanogr 23:3–10
Troncone L, De Lisa E, Bertapelle C, Procellini A, Laccetti P, Polese G, Di Cosmo A (2015) Morphofunctional characterization and antibacterial activity of hemocytes from Octopus vulgaris. J Nat Hist 49:21–24
Üçüncü SI, Çakici Ö (2009) Atresia and apoptosis in preovulatory follicles in the ovary of Danio rerio (zebrafish). Turk J Fish Aquat Sci 9:215–221
Valdebenito I, Paiva L, Berland M (2011) Follicular atresia in teleost fish: a review. Arch Med Vet 43:11–25
Wang W, Dong G, Yang J, Zheng X, Wei X, Sun G (2015) The development process and seasonal changes of the gonad in Octopus ocellatus Gray off the coast of Qingdao, Northeast China. Fish Sci 81:309–319
Wood AW, Van Der Kraak GJ (2001) Apoptosis and ovarian function: novel perspectives from the teleosts. Biol Reprod 64:264–271
Yamamoto Y, Luckenbach JA, Goetz FW, Young G, Swanson P (2011) Disruption of the salmon reproductive endocrine axis through prolonged nutritional stress: changes in circulating hormone levels and transcripts for ovarian genes involved in steroidogenesis and apoptosis. Gen Comp Endocrinol 172:331–343
Zamora CM, Olivares PA (2004) Variaciones bioquímicas e histológicas asociadas al evento reproductivo de la hembra de Octopus mimus (Mollusca: Cephalopoda). Int J Morphol 22:207–216
Zara FJ, Gaeta HH, Costa TM, Toyama MH, Caetano FH (2013) The ovarian cycle histochemistry and its relationship with hepatopancreas weight in the blue crab Callinectes danae (Crustacea: Portunidae). Acta Zool 94:134–146
Acknowledgments
We thank the “Asociación de Naseiros de Meira” for providing the majority of the octopus individuals, and Jose Manuel Antonio, Manuel. E. Garci, and María Teresa Fernández for their help in processing the samples. The Fisheries Group (CSIC) allowed us to use their image analyser, and Alex Alonso in particular provided valuable support and helpful suggestions during the manuscript preparation. We also acknowledge Dra. Sheila Castellanos-Martínez for her help regarding the haemocyte identification. This work was funded by the Comisión Interministerial de Ciencia y Tecnología (CICYT, Spain) (Project VEM 2003–20010) and by Xunta de Galicia (PGIDIT04PXIC40207PN). P. Sieiro was supported by a fellowship of Departamento de Postgrado y Especialización (CSIC), and J. Otero was supported by a “Junta para la Ampliación de Estudios” Fellowship (JAE Doc programme 2011) from the CSIC and ESF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We have worked with individuals obtained from the commercial fishery that were captured according to the legal standards established in our region.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00435-017-0355-x.
Rights and permissions
About this article
Cite this article
Sieiro, P., Otero, J. & Guerra, Á. Histomorphological study of ovarian atresia over the reproductive cycle of Octopus vulgaris from Galician waters (NW Spain). Zoomorphology 135, 419–431 (2016). https://doi.org/10.1007/s00435-016-0322-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0322-y