Abstract
Biological sulphate-reduction is a microbial-mediated process where sulphate is reduced to sulphide, which can be used to recover metals as sulphidic precipitates. To date, this technology has been assessed at full scale to recover valuable metals such as Cu, Ni and Zn. Despite this, research gaps are still encountered in this technology for improving and expanding its scope. Accordingly, the present review discusses: (1) the state of the art of the sulphate-reduction process, (2) the substrate options available that can meet the needs of the process, (3) the bioreactor configurations and their suitability for metal recovery, (4) the principles and factors affecting metal sulphide-precipitation and (5) the basis and advances on modelling and control of the process. The high diversity and versatility of sulphate-reducing bacteria allows exploring the use of substrates and operational conditions that facilitate the recovery of metals in bioreactors. Due to the lack of organics on industrial and mining waste streams that can sustain sulphate-reducing bacteria, the selection of a degradable, cost-effective, available, and non-pollutant substrate becomes crucial for the process. Different bioreactor configurations have been tested for the removal of metals from waste streams upon variations of the several operational conditions, concentration and type of metals tested, but metal recovery is hardly reported. Sulphate-reduction modelling has been developed to predict sulphide-inhibition/toxicity, microbial competition, kinetic parameters, biofilm and granulation development, sulphide-equilibrium and for scale-up design. Physicochemical reactions such as sorption/desorption and precipitation/solubilisation are not included in sulphate-reduction models despite that they are highly important for metal recovery in these systems. Sulphide and pH control in sulphate-reducing bioreactors is inherently essential to achieve metal recovery and to avoid unnecessary electron donor addition and over production of sulphide.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Global population growth, including urbanisation and industrial prosperity, has led to a strong increase in commodities coming from mining and metal industries. Additionally, mining activities have caused pollution problems all over the world (Pokhrel and Dubey 2013; Haslehner Roland and Stelter Benjamin 2015). Acid-mine drainage is infamous as one of the most widespread causes of pollution in the world. Acid-mine drainage is formed when pyrite and other sulphide minerals are oxidised by bacteria to produce a leachate containing dissolved metals, sulphate and acidity (Pokhrel and Dubey 2013). These issues have triggered the development of sustainable technologies to obtain metals from wastes produced from existing and past mining operations.
Metal precipitation with biogenic sulphide can be considered a sustainable technology for treating acid-mine drainage for two main reasons: (1) it allows the recovery and reuse of metals, as many metal-refining operations process sulphidic ores (Brooks 1991), and (2) biogenic sulphide, produced by sulphate-reducing bacteria, may be produced from the sulphate already present in the wastewaters coming from metal refineries and acid-mine drainage (Boonstra et al. 1999). This technology has been already assessed at full scale to recover valuable metals such as Cu, Ni and Zn from wastewaters coming from metal associated processes (Paques 2016). Despite this, the sulphate-reduction process for recovering metals presents significant challenges with respect to cost effective and reclaimable substrates, metal recovery and bioreactor configurations that facilitate the recovery of metals as well as process control and automation.
3.2 The Sulphate-Reduction Process
3.2.1 Microbial Sulphate-Reduction
Sulphate-reducing bacteria are anaerobic micro-organisms found in a wide range of different environmental conditions that are capable of reducing sulphate by using as electron donor and carbon source, hydrogen and CO2/CO (autotrophs) or organic compounds (heterotrophs) (Parshina et al. 2010; Hao et al. 2014). The outcome of this metabolism is hydrogen sulphide and bicarbonate ions in the case of complete oxidation, and acetate when incomplete oxidation occurs (Hao et al. 2014).
Figure 3.1 shows the anaerobic degradation process and some examples of the complete oxidation of the substrates formed in each stage by sulphate-reduction. The biological reactions shown are those that have been more commonly reported in the literature. Although there are some other reports of fermentative and sulphidogenic growth on complex organic material, sugars and amino-acids (Muyzer and Stams 2008), these reaction pathways are not directly dissimilatory sulphate-reduction (Neculita et al. 2007) nor assessed in bioreactors. The syntrophic relationship between anaerobic micro-organisms promotes competition for substrates, i.e. methanogens can compete for hydrogen and acetate with sulphate-reducing bacteria, as both use these substrates as electron donor (Muyzer and Stams 2008). In recent years, sulphate-reduction with CH4 as electron donor has been studied in bioreactors (Meulepas et al. 2010). However, the extremely low biomass growth rates (doubling time between 1.1 and 7.5 months) is limiting for biotechnological application (Meulepas et al. 2010). The ΔG0 values represent the amount of energy obtained from the reaction of sulphate (electron acceptor) with each substrate (electron donor) mentioned (Rittmann and McCarty 2001). Figure 3.1 shows that there is a trend in the ΔG0 values that correlates with the location of the substrate in the anaerobic degradation stage. This explains the faster growth of sulphate-reducing bacteria on lactate (ΔG0= −492 kJ/mol) over CH4 (ΔG0= −21.4 kJ/mol) as electron donor, as microorganisms obtain more energy from this reaction.
Anaerobic degradation process of complex organic material (left), examples of the sulphate-reduction reactions generated from the substrates formed in each stage (middle) and the free Gibbs energy of the sulphate-reduction reactions with each substrate at standard conditions and pH 7 (ΔG0) (left). Reactions and ΔG0 calculated from Rittmann and McCarty (2001), half reactions for sulphate as electron acceptor and the substrates mentioned as electron donor under complete oxidation
3.2.2 Sulphate-Reducing Bacteria Diversity
The study of sulphate-reducing bacteria diversity has been facilitated due to the increase in molecular tools available such as the use of marker genes like the 16S ribosomal RNA (rRNA) (Vanwonterghem et al. 2014; Cabezas et al. 2015). Based on this tool, sulphate-reducing bacteria can be grouped into seven phylogenetic lineages, five within the bacteria (Deltaproteobacteria, Nitrospirae, Clostridia, Thermodesulfobacteria and Thermodesulfobiaceae) and two within the archaea (Euryarchaeota, and Crenarchaeota) (Muyzer and Stams 2008).
In bioreactor systems, the diversity of sulphate-reducing bacteria highly depends on the operational conditions such as hydraulic retention time, sludge retention time, temperature, pH and salinity (Hao et al. 2014; Ňancucheo and Johnson 2012). Higher diversity is encountered in bioreactors fed with easily biodegradable compounds (Sánchez-Andrea et al. 2014; Hiibel et al. 2011) at higher COD/sulphate ratios (Deng et al. 2016) and low sulphide concentrations (Dar et al. 2009). The 16S rRNA sequences of sulphate-reducing bioreactors operating at COD/sulphate ratios of 0.2, 1 and 2 with municipal wastewater as electron donor for the treatment of acid-mine drainage, showed that the most diverse and evenly distributed microbial community was found at a COD/sulphate ratio of 2, where clones were closely related to dehalogenating and fermentative Clostridium sp., anaerobic sugar fermenting psychrotolerant nitrate-reducing P. bellariivorans, dechlorinating associated Sedimentibacter sp., and neutrophilic and acidophilic Desulfovibrio sp. and Desulfomicrobium spp. (Deng et al. 2016). Interestingly, literature on sulphate-reducing bioreactors operated at low pH, mimicking the pH conditions of mining waste streams (Johnson and Hallberg 2005), do not present an important decrease in sulphate-reducing bacteria diversity (Koschorreck et al. 2010; Sánchez-Andrea et al. 2014; Ňancucheo and Johnson 2012), but in the predominant species, which are Desulfosporosinus and Desulfitobacterium regardless the inoculum source (Sánchez-Andrea et al. 2014).
3.2.3 Substrates Used in Sulphate-Reduction as Electron Donor and Carbon Source
Industrial and mining waste streams usually contain low concentrations of electron donor and carbon source that can sustain sulphate-reducing activity, therefore, the selection of these, becomes of great importance. Several aspects must be considered in choosing an electron donor, including: (1) the degradation feasibility of the organic compound and biomass yield, (2) the cost of the electron donor per unit of sulphide produced, (3) the local availability of the electron donor and (4) the production of by-products that can cause pollution or toxicity problems (Dijkman et al. 1999; Kaksonen and Puhakka 2007; Liamleam and Annachhatre 2007; Papirio et al. 2012; Bijmans et al. 2011).
In the last years, a considerable amount of electron donors have been used in sulphate-reducing bioreactors. These can be grouped in: (a) easily biodegradable, (b) complex and (c) gaseous substrates. Easily biodegradable compounds have been largely studied and include: volatile fatty acids (acetate, propionate, pyruvate, butyrate), lactate, alcohols (methanol, ethanol), and sugars (molasses, glucose and sucrose) (Papirio et al. 2012; Liamleam and Annachhatre 2007; Kaksonen and Puhakka 2007). Most commonly, easily biodegradable compounds present more advantages over complex substrates in terms of degradation feasibility and biomass yield. Nevertheless, in many cases easily biodegradable compounds present important drawbacks regarding costs and competition with methanogens.
Complex substrates such as organic waste materials from pruning (grass clippings, leaf compost, maple wood chips and sawdust) and agriculture (straw and hay, oak chips and spent mushroom) and from cattle (manure, whey and slurry) have been studied in sulphate-reducing bioreactors for the treatment of acid-mine drainage, either as raw, composted or after silage (Lefticariu et al. 2015; Chang et al. 2000; Gibert et al. 2004; Wakeman et al. 2010; Song et al. 2012). In some cases, solid substrates from pruning and agriculture not only participate as slow releasing electron donor but also as packing material, functioning as bacterial support in bioreactors. These substrates can also retain metals via sorption mechanisms thus contributing to metal removal from the waste streams (Lefticariu et al. 2015; Neculita et al. 2007). An important drawback of these substrates is their lignocellulosic structure that prevents access to the electron donors (Wakeman et al. 2010) and the potential inhibition of the system due to volatile fatty acid accumulation (Wakeman et al. 2010; Lakaniemi et al. 2010).
Complex substrates also come from waste streams of the food, beverage and paper industry, as well as municipal wastewater (Mes et al. 2003; Deng et al. 2016; Deng and Lin 2013; Sanchez-Andrea et al. 2012; Costa et al. 2009). The most important advantages of these substrates are that they are, in many cases, cost effective (Liamleam and Annachhatre 2007), locally available (reducing transportation costs), and part of an alkaline waste stream, thus raising the pH of the metal-containing waste streams upon mixing (Deng et al. 2016). However, these substrates may not be easily biodegradable and may contain some inert material, which need to be removed by pre or post treatment (Meulepas et al. 2010; Bijmans et al. 2010; Neculita et al. 2007).
Gaseous substrates have the advantage of leaving no residual electron donor in the effluent, but they are voluminous and therefore need to be compressed during transportation (Meulepas et al. 2010). Gaseous substrates are preferable when the bioreactor operating pH is suboptimal for sulphate-reducing bacteria, as these substrates do not form toxic species upon pH variations as compared with organic substrates (Bijmans et al. 2010; Bijmans et al. 2008). Gaseous substrates used as electron donors to sustain sulphate-reduction include H2 (coupled with CO2 or CO as carbon source) (Parshina et al. 2010), synthesis gas (H2 + CO2 + CO) (Muyzer and Stams 2008), CO (Parshina et al. 2010) and CH4 (Meulepas et al. 2010). Co-utilization of H2 with CO2 gives high sulphate-reduction rates (up to 30 g/L·d) at both mesophilic and thermophilic conditions and at laboratory and full scale (Meulepas et al. 2010; Muyzer and Stams 2008; Hao et al. 2014). The use of H2/CO for sulphate-reduction has attracted much interest despite the low sulphate-reduction rates (up to 1.9 g/L·d) (Hao et al. 2014). This is because it may allow using cheap CO-rich synthesis gas for sulphate-reduction (Sipma et al. 2006, 2007), without the need for prior elimination of CO to prevent sulphate-reducing bacteria toxicity (Parshina et al. 2010). Progress has been made on this purpose with the recent discovery of CO tolerant sulphate-reducing bacteria species (Desulfotomaculum kuznetsovii and Desulfotomaculum thermobenzoicum subsp. Thermosyntrophicum and Desulfotomaculum carboxydivorans sp. nov.), which use CO in the presence of H2 (Parshina et al. 2005a) or exclusively CO (Parshina et al. 2005b). This may not only allow a direct application of CO-rich synthesis gas, but also the use of CO, as sole electron donor (Sipma et al. 2006; Parshina et al. 2010).
CH4 as electron donor for sulphate-reduction has been studied in natural environments and in bioreactors (Meulepas et al. 2010; Caldwell et al. 2008; Zhang et al. 2010). CH4 as electron donor presents the following advantages: (a) it opens the possibility of using natural gas in sulphate-reducing bioreactors, which is less expensive and more accessible in certain world regions over other gaseous substrates, (b) the solubility of CH4 is slightly higher compared with that of H2 and (c) a higher number of electrons are donated per mole of compound compared to H2 (Meulepas et al. 2010). Still, bottlenecks such as the sub-optimal conditions such as low temperatures (5–25 °C), pH above 7.5 and high salinity (30%) required to carry out the process hamper its biotechnological application.
3.2.4 Sulphate-Reducing Bioreactors and Process Configurations
Over the last years, different bioreactors have been studied for sulphate-reduction and treatment of metal containing waste streams in a single stage or in multistage process (Table 3.1). These bioreactors include: a) completely stirred tank reactor (CSTR), b) up-flow anaerobic packed-bed reactor (UAPBR), c) gas-lift reactor (GLR), d) up-flow anaerobic sludge blanket (UASB) reactor, e) up-flow fluidized bed reactor (UFBR), f) down-flow fluidized bed reactor (DFBR), g) anaerobic baffled reactor (ABR), h) membrane (side-stream membrane or immerse) bioreactor (MBR) (Kaksonen and Puhakka 2007; Papirio et al. 2012). The classifications of these systems can be based on: (a) the flow mode (batch, continuous or semi continuous), (b) biomass retention (suspended or attached), (c) state of the substrate used (liquid, gas or solid) and (d) whether is possible or not to recover the metals in a single stage, or if it requires additional stages (multistage process).
A single stage process is attractive because it reduces the number of process units and thus construction costs. Over the last years, the process feasibility has been demonstrated in the bioreactor configurations mentioned above over a wide range of operational conditions including low pH values and high metal loads. Table 3.1 shows the configurations, operational conditions and results obtained in several sulphate-reducing bioreactors used for the treatment of metal containing-waste streams. The performance of these bioreactors has been evaluated mainly based on the COD and sulphate-removal efficiencies (%) as well as on the sulphate-reduction rates (g SO4 2− reduced/L·d) upon variations of substrate, hydraulic retention time, temperature, influent pH, COD/SO4 2− ratio and concentration and type of metals tested. It can be noticed that the substrate has a substantial impact on the sulphate and COD-removal efficiencies and on the sulphate-reduction rates. For instance, studies in an UAPBR using complex organic substrates have the lowest sulphate-reduction rates (0.06–0.47 g SO4 2− reduced/L·d), while an UFBR and an DFBR, using lactate and ethanol as substrate, present the highest sulphate-reduction rates (2.6–4.6 g SO4 2− reduced/L·d). In general, the studies presented in Table 3.1 report a fair performance despite the low pH and high removal efficiencies of the metals tested, which is higher than 80% in most cases (data not shown). This is partly due to the production of bicarbonate ions by the sulphate-reducing process and the sulphide present, which allow rising the pH and precipitate any metals upon introduction to the bioreactor.
A missing information in most of these studies is encountered in whether the metals could leave the system and be recovered, or if they accumulate within the bioreactor hampering long-term operation. Although the metals can be recovered from the metal sulphide-containing sludge (Tabak et al. 2003), this might imply biomass loss in the process leading to the reduction of the system performance.
Sulphate-reduction and metal recovery can be also performed in a multistage process consisting of: (1) a biological stage, separated from the precipitation stage (Hao 2000; Kaksonen and Puhakka 2007), (2) a biological stage and precipitation stage, separated from the settling stage (Hao 2000; Muyzer and Stams 2008) and (3) a biological stage separated from several precipitation settlers operating at different pH or sulphide concentration in order to achieve selective metal recovery (Veeken et al. 2003b; Esposito et al. 2006; König et al. 2006; Sampaio et al. 2009). In the last decade, these process configurations have been demonstrated for Cu and Zn at lab-scale (Foucher et al. 2001; Al-Tarazi et al. 2005a; Gramp et al. 2006; Esposito et al. 2006) and for Cu, Zn, As, Fe and Ni at full scale (Muyzer and Stams 2008). At full scale, the Nyrstar plant in The Netherlands treats a process water containing ZnSO4, where sulphate-reduction takes place in a full-scale (500 m3) gas lift reactor with hydrogen as electron donor (Muyzer and Stams 2008). Then the ZnS produced is collected in a settler and the excess sulphide is oxidized in an aerobic bioreactor. The process has also been assessed in a sulphate and metal-rich effluent coming from a coal process in South Africa and for the treatment of acid-mine drainage at the former Wheal Jane mine in Cornwall, UK (Paques 2016).
3.2.5 Operational Conditions Affecting Sulphate-Reduction in Bioreactors
3.2.5.1 Effect of pH
Although sulphate-reducing bacteria are naturally present in extreme pH environments (Muyzer and Stams 2008), optimal growth conditions in lab-scale are reported at pH values between 5.5 and 10 (Hao et al. 2014). Many studies have successfully demonstrated the application of the sulphate-reduction process for acid waste streams (2.5–3), since the process itself generates bicarbonate ions, which increase the waste stream pH up to 7.5–8.5 (Kaksonen and Puhakka 2007; Bekmezci et al. 2011). However, research on sulphate-reduction at acidophilic (pH below 7) (Ňancucheo and Johnson 2014) and alkaline (pH above 7) conditions (Sousa et al. 2015; Zhou and Xing 2015; Zhou et al. 2015) is still attractive for metal recovery purposes. This is because the manipulation of the pH in a wider range than neutral in bioreactors can allow selective precipitation, and thus selective recovery, of metals from multi metal streams (Huisman et al. 2006; Tabak et al. 2003) as metal sulphide solubility is pH dependent (Lewis 2010).
The effect of alkaline pH, accompanied with high salinity, on the sulphate-reduction process decreases the microbial growth rate and aggregation of biomass (Sousa et al. 2015). The effect of acidic pH on the sulphate-reduction process has been widely studied so it is known that the inhibition is caused by: (a) the increase in protons (H+) (Sánchez-Andrea et al. 2014), (b) the formation of H2S, which is the unionized sulphide-species (Reis et al. 1992; Hulshoff Pol et al. 1998; Hulshoff Pol et al. 2001; Willow and Cohen 2003; Lopes et al. 2007; Bijmans et al. 2008) and c) the formation of unionized organic acids (Kimura et al. 2006) either from organics added as electron donor, or those formed during the anaerobic degradation (e.g. acetate). The concentration of sulphide and acetate is a function of the pH due to chemical equilibrium (Fig. 3.2). Therefore, unionized acetate and sulphide concentration is higher at low pH values.
The inhibitory effect of H2S and undissociated organic compounds relies on the ability to penetrate the cell membrane thus affecting the functioning of metabolic coenzymes and, denaturizing proteins (Kaksonen and Puhakka 2007). To avoid this, several operational strategies have been developed:
-
(a)
The use of electron donors that do not form unionized organic acids, for instance, glycerol (Kimura et al. 2006; Ňancucheo and Johnson 2012), formate and hydrogen (Bijmans et al. 2010).
-
(b)
Continuous sulphide-removal through N2 stripping of the liquid media (Lopes et al. 2007; Bijmans et al. 2008).
-
(c)
Recirculation of the effluent in order to dilute the sulphide concentration (Celis-García et al. 2007; Kaksonen et al. 2004).
-
(d)
Sulphide-precipitation with metals (Hulshoff Pol et al. 2001; Kaksonen and Puhakka 2007), for example by Fe (Vakili et al. 2012).
3.2.5.2 Effect of Hydraulic Retention Time
Several studies have assessed the effect of the hydraulic retention time on the sulphate-reducing process with or without the presence of metals (Qinglin et al. 2012; Kaksonen et al. 2004; Dries et al. 1998; Mizuno et al. 1998; Nagpal et al. 2000; Alphenaar et al. 1993; Celis-García et al. 2007; Sahinkaya et al. 2009; Villa-Gómez et al. 2011; Villa Gómez et al. 2015). These experiments have been carried out by sudden or stepwise reduction of the hydraulic retention time. In every study, the term defined as “long” and “short” hydraulic retention time depends on the range used, type of bioreactor and operating conditions used. Regardless this, some generalizations on the effect of the hydraulic retention time in sulphate-reducing bioreactors can be made:
-
A long hydraulic retention time (48 h) is applied at bioreactor start up to enhance biomass retention or immobilization within the bioreactor (Celis et al. 2009; Villa-Gómez et al. 2011).
-
Depending on the electron donor used, methanogens or sulphate-reducing bacteria outcompete upon variations of the hydraulic retention time. For instance, acetate was used by sulphate-reducing bacteria at higher hydraulic retention times (40.2 h) when acetic acid (50%), propionic acid (40%) and sucrose (10%) was fed in a UASB/CSTR bioreactor, thus outcompeting methanogens (Alphenaar et al. 1993), while methanogens outcompeted sulphate-reducing bacteria in a GLR (55 °C) fed with CO when this bioreactor was operated at hydraulic retention times higher than 9 h (Sipma et al. 2007).
-
Higher sulphate and COD removal efficiencies are achieved at long hydraulic retention times (Mizuno et al. 1998; Nagpal et al. 2000; Kaksonen et al. 2004; Celis-García et al. 2007; Sahinkaya et al. 2009; Sipma et al. 2007). However, substrate limitation can also occur (Nagpal et al. 2000), as biomass growth rate and electron donor/acceptor conversion rates overpass the electron donor/acceptor supply rate.
-
Loss of biomass due to wash out is reported at short hydraulic retention times resulting in lower conversion rates due to low biomass concentration in the biroeactor (Dries et al. 1998; Kaksonen et al. 2004; Sipma et al. 2007; Villa Gómez et al. 2015; Alphenaar et al. 1993).
-
Short hydraulic retention times can lead to low sulphide-production impeding total metal precipitation of metal-containing waste streams, thus allowing free metal toxicity (Villa Gómez et al. 2015).
-
Due to faster growth rates of incomplete oxidizers as compared to complete oxidizers and acetotrophic sulphate-reducing bacteria, acetate accumulation has been reported at short hydraulic retention times (Nagpal et al. 2000; Kaksonen et al. 2004).
3.2.5.3 Effect of Metal Concentration
Metals can stimulate or inhibit sulphate-reducing bacteria depending on process-related factors such as pH, redox potential (Chen et al. 2008) and the reactive species in the mixed liquor (Labrenz et al. 2000; Gonzalez-Silva et al. 2009). These parameters drive the dissolved metal species concurrent with concentration. Dissolved metals can affect the sulphate-reducing bacteria metabolism by deactivating the enzymes and denaturing the proteins (Cabrera et al. 2006).
In sulphate-reducing bioreactors treating metal containing wastewaters, the sulphide reacts with metals forming insoluble metal sulphide particles, which reduces the metal toxicity and bioavailability (Kaksonen and Puhakka 2007). Despite this, inhibition of sulphate-reduction by insoluble metal sulphides can still occur (Gonzalez-Silva et al. 2009; Utgikar et al. 2004; Utgikar et al. 2002), particularly at pH values below neutral (Moosa and Harrison 2006; Reis et al. 1992) or if the metal sulphide-precipitation occurs onto the sulphate-reducing bacterial cells (Villa Gómez et al. 2015). Gonzalez-Silva et al. (2009) found that Cd (3 mM) precipitation with sulphide did not decrease the inhibition of cadmium on the sulphate-reduction process in a study investigating the inhibition effect of Fe, Cd and sulphide on the substrate utilization rate of sulphate-reducing granular sludge in a UASB bioreactor.
3.3 Metal Sulphide-Precipitation Process
3.3.1 Formation of Metal Sulphide Precipitates
Besides the bioreactor configuration and the number of stages in the treatment process, metal sulphide-precipitation itself is a complex process that needs to be understood for optimal metal recovery. The kinetic phenomena associated with metal sulphide-precipitation are nucleation, and crystal growth (Fig. 3.3). The driving force of both phenomena is the supersaturation. The supersaturation level is the amount by which the solute concentration exceeds the saturation concentration. Crystallization only occurs if the system is supersaturated (Larsen et al. 2006). In general, high supersaturation levels favour nucleation, thus to produce large particles the supersaturation should be minimized. The supersaturation level σ for metal sulphides can be expressed in terms of the solubility product (Veeken et al. 2003a):
Where σ is the supersaturation level, (Me2+) is metal activity (mol/L), (S2−) is sulphide activity (mol/L). Later agglomeration and break up of crystals also occur.
At high levels of supersaturation both phenomena compete for the available solute (metal and sulphide). Depending on the conditions, either nucleation or crystal growth may be predominant over the other, and as a result, crystals with different sizes and shapes are obtained (Mersmann 1999).
Metal sulphide precipitates have a low solubility (Fig. 3.4), as a consequence, the supersaturation is high (Hammack et al. 1994) and difficult to control, especially at the feeding points (Lewis and van Hille 2006) due to micro mixing limitations (Tabak and Govind 2003). This results in the formation of small particles, called fines, which are difficult to recover (Mokone et al. 2010; Villa-Gómez et al. 2011). This scenario has been observed in bioreactors as well (Villa-Gómez et al. 2011).
Solubility product constants (Ksp) of metal (Mex+) sulphides (S2−) at standard conditions (25 °C, 1 atm) (Data from Sampaio et al. 2009)
3.3.1.1 Solubility Product
The solubility product determines whether a metal sulphide will stay dissolved or will precipitate. For a solid precipitate of metal sulphide MxSy(s), the following general solubility expression can be written:
The solubility product (K sp) of the metal sulphide is defined as:
Where Ksp is in mol2/L2 when x = y = 1, [M2+] is the equilibrium activity of metal ion M2+ (mol/L) and [S2−] is the equilibrium activity of S2− (mol/L) (Sampaio et al. 2009).
Several authors have found that the metal sulphide-precipitation rate is ruled by the sulphide concentration by means of the solubility product (Bryson and Bijsterveld 1991; Mishra and Das 1992; Lewis and Swartbooi 2006; Veeken et al. 2003b). Veeken et al. (2003b) showed the different precipitation rates of Cd, Cu, Ni, Pb and Zn upon the variations of the sulphide concentration expressed in the logarithm of the S2− species (pS) at a fixed pH of 6. Based on this principle, selective precipitation of individual metals has been demonstrated in additional stages by controlling the pH and pS (Veeken et al. 2003a; Esposito et al. 2006; König et al. 2006; Sampaio et al. 2009).
Many studies on metal sulphide precipitation have focused on studying the way to reduce the high level of supersaturation on the metal sulphide precipitation in order to increase the size of the precipitates for better solid-liquid separation. Van Hille et al. (2005) studied the influence of the sulphide to Cu molar ratio, recycle flow rate, inlet Cu flow rate and the inlet Cu concentration on the Cu conversion and removal efficiency. They found that the sulphide to Cu molar ratio and the bisulfide ion formation were the most important factors determining local supersaturation. Al-Tarazi (2005b) used gaseous H2S as precipitating reagent for Cu and Zn to reduce the high level of supersaturation in a bubble column and concluded that the morphology of the metal sulphide precipitates was more favourable than that of the precipitates produced using an aqueous sulphide source.
Some studies have shown the influence of the geometry and operating conditions of the precipitator reactor on the crystallization process. For instance, Al-Tarazi et al. (2005b) studied the effects of the configuration on three different types of reactors for the precipitation of Zn and Cu: laminar jet, bubble column and a Mixed Solution Mixed Product Removal (MSMPR) reactor, studying the effects of mass transfer and process conditions on the morphology of the produced crystals. They found that the largest crystals of metal sulphides were obtained at high supersaturation conditions, moderate stirrer speeds, short residence times, a pH value of around 5 and high Cu2+ to sulphide ratios. Sampaio et al. (2009) observed that the particle size of CuS in a continuously stirred tank reactor increased if allowed to settle (from 36 to 180 μm), whereas upon vigorously stirring, the particles decreased to below 3 μm.
In addition to the influence of geometry and operating conditions, foreign particles determine the particle size of the metal sulphides by affecting the relative rates of nucleation and crystal growth (Mersmann 1999). Gramp et al. (2006) showed the differences between biogenic and abiotic sulphide used to precipitate copper in cultures of sulphate-reducing bacteria and Na2S solutions. They found that bacterial cells alter crystal formation by inhibiting particle nucleation and as a consequence the chemically produced covellite (CuS) should be more resistant to biogeochemical oxidation as compared to poorly crystalline biogenic Cu-sulphide. Contrary to the previous authors, Bijmans et al. (2009c) suggested that the biomass functioned as nucleation seeds, enhancing crystal growth, reporting NiS precipitates formed with biogenic sulphide ranging from 13 to 73 μm.
3.3.2 Factors Affecting Metal Sulphide Precipitation
3.3.2.1 pH
The pH has an influence on the speciation of the components present in the liquid phase of bioreactors, which affects metal sulphide precipitation. Villa-Gómez et al. (2014b) studied the morphology, mineralogy, and solid-liquid phase separation of the Cu and Zn precipitates formed with biogenic sulphide at pH 3, 5, and 7. They found that at pH 5, the dissolved organic matter present in the bioreactor liquor induced crystallization and hampered agglomeration of the metal sulphides, while at pH 7 and 3, the agglomeration phenomena were clearly predominant (Fig. 3.5).
Scanning electron microscopy images of the precipitates formed at pH 7 (a), pH 5 (b) and pH 3 (c) with biogenic sulphide and metals (Zn, Cu) at 10 μm magnification (zoomed picture b at 1 μm magnification) (Source: Villa-Gómez et al. 2014b)
The pH-dependent solubility of the metal sulphides has been used to selectively recover metals from complex and simple mixed metal systems (Tokuda et al. 2008; Bijmans et al. 2009c; Sahinkaya et al. 2009; Tabak et al. 2003). Foucher et al. (2001) found that Cu and Zn sulphides could be selectively recovered at pH 2.8 and 3.5, while Ni and Fe sulphides could only be removed (not recovered) at pH 6. Similar results were found by Sampaio et al. (2009), who selectively precipitated CuS (covellite) at pH 2 and 3 and ZnS (sphalerite) at pH 3 and 4. Sampaio et al. (2010) additionally demonstrated that Ni can be selectively recovered from a Ni-Fe solution at pH 5 using a single stage bioreactor operating at low pH. The results also suggested that the pH should be lower than 4.8 for complete Ni-Fe separation.
3.3.3 Competing Metal Removal Mechanisms
Although the low solubility of metal sulphides favour metal removal mechanism (Fig. 3.4), other precipitation reactions can also occur in sulphate-reducing bioreactors. Several authors have confirmed alternative precipitates are formed at low sulphide concentrations, particularly for metals with higher solubility such as Zn (Mokone et al. 2010; Neculita et al. 2007; Villa-Gómez et al. 2012). Although the decrease of supersaturation is preferable for crystal growth (Fig. 3.3), it also allows the formation of alternative precipitates such as brochantite (Cu4(OH)6SO4) (Mokone et al. 2010), Zn-phosphate (Villa-Gómez et al. 2012) and hydroxides (Samaranayake et al. 2002; Neculita et al. 2008). Other metal removal mechanisms that can occur in bioreactors include: biosorption as well as sorption onto previously formed metal sulphide precipitates (Neculita et al. 2007; Villa-Gómez et al. 2012; Villa-Gómez et al. 2014b; van Hullebusch et al. 2003).
Elucidation of the metal removal mechanisms that can occur apart from metal sulphide precipitation in bioreactors is difficult, as the solid phase techniques used for the identification of the chemical species are limited to scanning electron microscopy and X-ray diffraction, which display low resolution on poorly crystallized samples immersed in biological tissues (Neculita et al. 2007). Various studies have addressed different metal removal mechanisms based on chemical equilibrium calculations to predict the species formed in bioreactors (Bartacek et al. 2008; Villa-Gómez et al. 2012, 2015). Despite being a great help to understand the chemical speciation, these are based on the formation of species at thermodynamic equilibrium, which is not always reached in bioreactors.
X-ray absorption spectroscopy, which consists of two complementary techniques, X-ray absorption near edge spectroscopy (XANES) and extended X-ray absorption fine structure (EXAFS), is an accurate technique for the analysis of metals in biological samples (Prange and Modrow 2002; van Hullebusch et al. 2009; Lenz et al. 2011; Shakeri Yekta et al. 2012; Villa-Gómez et al. 2012, 2014b). This technique was applied by Villa-Gómez et al. (2014b) to identify the Cu and Zn removal mechanisms in sulphate-reducing bioreactors. Figure 3.6 shows Zn K-edge XANES spectra of the Zn precipitates obtained when the metals were put in contact with biogenic sulphide at pH 3, 5 and 7. The spectroscopic similarities with sphalerite were the highest at pH 5, where XANES features A to E were almost identical, while at pH 3, these features were in a lesser extent similar. In contrast, the features A and E at pH 7 indicated a contribution from another Zn environment, where the feature E was slightly shifted towards lower energies, thus suggesting the presence of minor amounts of Zn-O, as in Zn-sorbed hydroxyapatite, Zn:Ca5(PO4)3•(OH).
Zn K-edge XANES spectra for selected model compounds (c-ZnS and Zn sorbed on apatite) as compared to the sulphide precipitates formed in three batch samples of the study of Villa-Gómez et al. (2014b)
3.4 Modelling and Control of the Sulphate-Reduction Process for Metal Recovery
3.4.1 Modelling
Mathematical modelling is a powerful tool for process analysis and design. It also forms the basis for monitoring and control schemes of bioreactors. The baseline for modelling anaerobic digestion systems, including sulphate-reduction, is the anaerobic digestion model no. 1 (ADM1), developed by the IWA task group (Batstone et al. 2002). It comprehends mass balances, kinetics, biochemical and physicochemical components (Batstone 2006). Further extensions of sulphate-reduction modelling include additions in kinetics, biochemical and physicochemical components (Cassidy et al. 2015; Barrera et al. 2015). Sulphate-reduction modelling has been developed in several bioreactor configurations to predict sulphide inhibition/toxicity, microbial competition, kinetic parameters, biofilm or granulation development (Cassidy et al. 2015), sulphide equilibrium (Barrera et al. 2015) and for scale-up design (Tabak and Govind 2003).
3.4.1.1 Model Components
Figure 3.7 shows the basic components of sulphate-reduction models. These components are interrelated and depend on each other. Thus, mass balances, which describe accumulation and reaction within a system in relation to flow across the system boundaries for each component involved in the system (Batstone 2006), require the rate of the biological and physicochemical reactions that can occur in the system to predict the final concentrations of each component.
3.4.1.1.1 Kinetics
Biological reactions include growth, uptake, decay and inhibition of microorganisms. The growth rate and uptake rate of electron donor/acceptor are most commonly described by Monod (or Michaelis-Menten) kinetics, while decay rates are better expressed by first order kinetics (Batstone 2006). Inhibition is also included within the biological reactions as it may have a strong effect on biochemical processes by decreasing the conversion or growth rate, thus affecting the overall performance. In sulphate-reducing bioreactors, pH, metals, substrate, ammonia, and sulphide mainly induce inhibition. Commonly, inhibition increases with an increase in the inhibitor concentration, leading to a gradual decrease in the specific substrate utilization rate (Cassidy et al. 2015). Inhibition by pH is implemented through empirical equations when both high and low pH inhibition occur, or when only low pH inhibition occurs (Batstone et al. 2002). Inhibition due to metals substrate, ammonia and sulphide are most commonly represented by non-competitive functions (Batstone et al. 2002; Cassidy et al. 2015). This function has been used to study the inhibition by Fe, Cd and sulphide in a UASB bioreactor (Gonzalez-Silva et al. 2009) and by sulphide in an up-flow fluidized bed bioreactor (Kaksonen et al. 2004).
3.4.1.1.2 Physicochemical Components
Physicochemical reactions are defined as those not mediated by microorganisms, and include (Fig. 3.5): (a) liquid-liquid reactions, (b) gas-liquid exchanges, (c) liquid-solid transformations. Liquid-liquid reactions, comprehending ion association/dissociation, are acknowledge in anaerobic digestion models but, since they occur at a relatively rapid scale (Batstone et al. 2002), the reaction rates are omitted. Gas-liquid transformations have been mainly described for CH4, CO2 and H2 and less often for sulphide. Barrera et al. (2015) presented an extension of the ADM1 with sulphate- reduction for a very high strength and sulphate-rich wastewater where the concentrations of total aqueous, free and gas phase sulphides were accurately predicted.
Physicochemical components include chemical equilibrium, pH, temperature and gas-liquid partitioning. Chemical equilibrium such as acid-base equilibrium for inorganic carbon and nitrogen, acetate, propionate, valerate, butyrate and hydrogen is either by formulation of the base or acid concentration or by calculation of the equilibrium in algebraic equations (Lauwers et al. 2013). pH calculation involves solving a set of algebraic or differential equations to calculate the concentrations of ionic acids and bases related with ionic, active concentration state variables (Batstone 2006). Changes in temperature have a fundamental influence on the physicochemical system, mainly because of changes in equilibrium constants, for this, the most widely used is the Van’t Hoff equation (IWA 2002). The main components considered in gas-liquid partitioning are CO2, CH4, H2 and H2S. When the liquid phase is relatively dilute, Henry’s law can be used to describe the equilibrium relationship (IWA 2002).
Liquid-solid reactions include sorption/desorption and precipitation/solubilisation mechanisms. These have been less studied and are not included in the ADM1 (IWA 2002). Sorption/desorption mechanisms are highly relevant when metals are present in bioreactors. These are mainly attributed to an ion exchange mechanism on the surface of the biomass (van Hullebusch et al., 2003) or by extracellular polymers (van Hullebusch et al. 2005; Liu et al. 2015). Metal sorption mechanisms in the anaerobic granular sludge have been described, aside of the overall bioreactor system, with Langmuir, Freundlich and Redlich-Peterson equations (van Hullebusch et al. 2004, 2005, 2006; Pat-Espadas et al. 2016).
Precipitation/solubilisation mechanisms are mainly important in bioreactors where sulphate-reduction occurs for metal sulphide precipitation. The simple method to include them is an equilibrium reaction or simple first order kinetics (Parker and Wu 2006; IWA 2002). This can be fairly valid as the solubility product coefficients for metal sulphide precipitates are extremely small, thus reacting fast. Parker and Wu (2006) introduced a first-order metal sulphide rate coefficient (arbitrarily) of 106 M/d in a modified ADM1 to describe the effect of metal sulphide precipitation in the formation and emission of odorous compounds in anaerobic sludge digestion. The model was capable to predict the changes in emissions of H2S upon variations of metal concentrations due to metal sulphide precipitation.
It is important to stress that the precipitation process comprises: nucleation, and crystallization, and the predominance of one of these defines the size of the metal sulphide precipitates and thus the suitability of metal recovery in bioreactors. As the predominance of one mechanism over another is influenced by the sulphide concentration and pH, these mechanisms should, therefore, be considered in the modelling of sulphate-reducing bioreactors. The metal precipitation process has been modelled separately from the sulphate-reducing process in completely stirred tank reactors to obtain crystallization kinetics (Al-Tarazi et al. 2004) to predict the effects of organic substances and sulphide concentration (König et al. 2006), pH variation (Luptakova and Kusnierova 2005; König et al. 2006; Sampaio et al. 2009) and metal concentration (König et al. 2006), and to design an adequate control strategy to estimate the effluent metal concentration (Sampaio et al. 2009). This knowledge could be the start point to incorporate the precipitation mechanisms in sulphate-reduction models.
3.4.1.1.3 Biochemical Components
Biochemical components include the stoichiometric microbial degradation pathways hydrolysis, acidogenesis, acetogenesis and methanogenesis (Fig. 3.1) and sulphate-reduction as well as biomass-associated products and substrate-utilization-associated products (Batstone et al. 2002). Sulphate-reduction, regardless the electron donor used, has been generalized in one component, as some models consider only the oxidation of the available hydrogen (Batstone, 2006) or from other substrates such as volatile fatty acids directly by sulphate-reducing bacteria (Barrera et al. 2015; Fedorovich et al. 2003). The biomass-associated products and substrate-utilization-associated products have been recently introduced in sulphate-reduction models to understand the onset of sulphate-reduction in denitrifying membrane biofilm reactors (Tang et al. 2013) and to determine the impact of sulphate and polyhydroxybutyrate-accumulation on process control of sulphate-reducing bioreactors (Cassidy et al. 2017).
3.4.2 Control and Automation
As mentioned before, metal precipitation/recovery is highly dependent on the sulphide concentration and pH. Therefore, control and automation of sulphate-reducing bioreactors are inherently essential to achieve metal recovery in bioreactors. Additionally, industrial and mining wastewaters are deficient in organic compounds as electron donor source for sulphate-reduction. Thus, for practical implementation, steering the sulphide-production towards its required stoichiometric amount in bioreactors is highly relevant to avoid unnecessary electron donor addition and over production of sulphide (Villa-Gómez et al. 2014a).
The basic components of a control system are: (i) the process (sulphate-reducing bioreactor), (ii) the measurement device (sensors) for process monitoring and iii) the controller (Fig. 3.8). A control system requires monitoring of the process to increase knowledge on the process, and thus, to design an adequate control strategy (Cassidy et al. 2015). Advances in instrumentation have enabled the on-line monitoring of critical parameters for early detection of process disturbances (Nguyen et al. 2015). Online sensors such as pH, oxygen redox potential and ion selective electrodes have been used for the measurement of crucial variables in the sulphate-reducing process (Villa-Gómez et al. 2014a; Torner-Morales and Buitrón 2010). An oxygen, redox potential sensor and pH electrode were used to maintain sulphate-reduction/sulphide oxidation in a single sequencing batch reactor with a significant yield of 64% of elemental sulphur (Torner-Morales and Buitrón 2010). A S2− selective electrode was used for sulphide monitoring in a down-flow fluidized bed bioreactor for the design of a control strategy to control the sulphide production in sulphate-reducing bioreactors (Villa-Gómez et al. 2014a, b). Other sensors such as SO4 2− selective electrodes, not yet tested in the sulphate-reduction process, could be potentially useful for process control of these systems (Cassidy et al. 2015).
Once the sensor has sent the data, the controller, can decide the output applied to the manipulated variable. The controllers used in anaerobic digestion systems, with scarce contribution to sulphate-reducing systems, include proportional integral (PI) control, PI derivative (PID) control, adaptive control, robust adaptive, fuzzy logic, neural network, and neural fuzzy (Nguyen et al. 2015). In the anaerobic digestion process, these controllers have been used to steer the feeding rate, volatile fatty acid concentration, pH, bicarbonate alkalinity, biogas and methane production rate (Pind et al. 2003; Nguyen et al. 2015), while for sulphate-reduction, only the pH has successfully been controlled with commercially available pH controllers especially designed for bioreactors (Bijmans et al. 2009a; Bijmans et al. 2010).
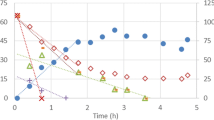
A first approach towards the control of the sulphide concentration in a sulphate-reducing bioreactor for metal precipitation/recovery was studied by Villa-Gómez et al. (2014a). Step changes in the organic loading rate were applied by changing the lactate concentration or the hydraulic retention time, and the sulphide concentration and pH were measured using pS and pH electrodes connected to the LabView software version 2009®. The pS output values resulting from both control strategies were used to determine the PID parameters. Despite that the controller was not tested, the knowledge gained on the critical factors affecting sulphide control in bioreactors put the automation of these systems one step further, not only for metal recovery but also for other biotechnological applications where biological sulphide production control is required.
3.5 Conclusion
This chapter overviewed the factors affecting metal recovery in sulphate-reducing bioreactors. Different bioreactor configurations have been applied for sulphate-reduction and metal precipitation. However, metal recovery cannot always be achieved in these bioreactors, since metals precipitate partly in the biomass, which hampers metal recovery. Many mining and metallurgical waste streams are deficient in organic compounds as electron donor for sulphate-reduction, thus, cheap electron donors and steering the sulphide production towards its required stoichiometric amount in bioreactors is highly relevant to avoid unnecessary electron donor addition and overproduction of sulphide. Process control in sulphate-reducing bioreactors is essential to align the sulphide production to the amount of metals desired to precipitate.
Abbreviations
- COD:
-
Chemical oxygen demand
- CSTR:
-
Completely stirred tank reactor
- UAPBR:
-
Up-flow anaerobic packed-bed reactor
- GLR:
-
Gas-lift reactor
- UASB:
-
Up-flow anaerobic sludge blanket
- UFBR:
-
Up-flow fluidized bed reactor
- DFBR:
-
Down-flow fluidized bed reactor
- ABR:
-
Anaerobic baffled reactor
- MBR:
-
Membrane (side-stream membrane or immerse) bioreactor
References
Alphenaar AP, Visser A, Lettinga G (1993) The effect of liquid upward velocity and hydraulic retention time on granulation in UASB reactors treating wastewater with a high sulphate content. Bioresour Technol 43(3):249–258. doi:10.1016/0960-8524(93)90038-D
Al-Tarazi M, Heesink ABM, Azzam MOJ, Yahya SA, Versteeg GF (2004) Crystallization kinetics of ZnS precipitation; an experimental study using the mixed-suspension-mixed-product-removal (MSMPR) method. Cryst Res Technol 39(8):675–685. doi:10.1002/crat.200310238
Al-Tarazi M, Heesink ABM, Versteeg GF, Azzam MOJ, Azzam K (2005) Precipitation of CuS and ZnS in a bubble column reactor. AICHE J 51(1):235–246. doi:10.1002/aic.10310
Al-Tarazi M, Heesink ABM, Versteeg GF (2005) Effects of reactor type and mass transfer on the morphology of CuS and ZnS crystals. Cryst Res Technol 40(8):735–740. doi:10.1002/crat.200410421
Barrera EL, Spanjers H, Solon K, Amerlinck Y, Nopens I, Dewulf J (2015) Modeling the anaerobic digestion of cane-molasses vinasse: extension of the anaerobic digestion model no. 1 (ADM1) with sulfate reduction for a very high strength and sulfate rich wastewater. Water Res 71:42–54. doi:10.1016/j.watres.2014.12.026
Bartacek J, Fermoso F, Baldó-Urrutia A, van Hullebusch E, Lens P (2008) Cobalt toxicity in anaerobic granular sludge: influence of chemical speciation. J Ind Microbiol Biotechnol 35(11):1465–1474. doi:10.1007/s10295-008-0448-0
Batstone DJ (2006) Mathematical modelling of anaerobic reactors treating domestic wastewater: rational criteria for model use. Rev Environ Sci Biotechnol 5(1):57–71. doi:10.1007/s11157-005-7191-z
Batstone DJ, Keller J, Angelidaki I, Kalyuzhnyi SV, Pavlostathis SG, Rozzi A, Sanders WT, Siegrist H, Vavilin VA (2002) The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci Technol 45(10):65–73
Bekmezci OK, Ucar D, Kaksonen AH, Sahinkaya E (2011) Sulfidogenic biotreatment of synthetic acid mine drainage and sulfide oxidation in anaerobic baffled reactor. J Hazard Mater 189(3):670–676. doi:10.1016/j.jhazmat.2011.01.087
Bijmans MFM, Dopson M, Ennin F, Lens PNL, Buisman CJN (2008) Effect of sulfide removal on sulfate reduction at pH 5 in a hydrogen fed gas-lift bioreactor. J Microbiol Biotechnol 18(11):1809–1818. doi:10.4014/jmb.0800.109
Bijmans MF, Dopson M, Peeters TW, Lens PN, Buisman CJ (2009a) Sulfate reduction at pH 5 in a high-rate membrane bioreactor: reactor performance and microbial community analyses. J Microbiol Biotechnol 19(7):698–708
Bijmans MFM, van Helvoort P-J, Buisman CJN, Lens PNL (2009b) Effect of the sulfide concentration on zinc bio-precipitation in a single stage sulfidogenic bioreactor at pH 5.5. Sep Purif Technol 69(3):243–248
Bijmans MFM, van Helvoort P-J, Dar SA, Dopson M, Lens PNL, Buisman CJN (2009c) Selective recovery of nickel over iron from a nickel-iron solution using microbial sulfate reduction in a gas-lift bioreactor. Water Res 43(3):853–861. doi:10.1016/j.watres.2008.11.023
Bijmans MFM, de Vries E, Yang C-H, N Buisman CJ, Lens PNL, Dopson M (2010) Sulfate reduction at pH 4.0 for treatment of process and wastewaters. Biotechnol Prog 26(4):1029–1037. doi:10.1002/btpr.400
Bijmans MFM, Buisman CJN, Meulepas RJW, Lens PNL (2011) 6.34 – Sulfate reduction for inorganic waste and process water treatment. In: Murray M-Y (ed) Comprehensive biotechnology, 2nd edn. Academic Press, Burlington, pp 435–446. doi:10.1016/B978-0-08-088504-9.00471-2
Boonstra J, van Lier R, Janssen G, Dijkman H, Buisman CJN (1999) Biological treatment of acid mine drainage. Proc Metal 9B:559–567. doi:10.1016/S1572-4409(99)80145-X
Borja PR, Colmenarejo Morcillo MF, Durán-Barrantes MM, Jiménez-Rodríguez AM, Raposo Bejines F, Sánchez E (2010) Biological sulphate removal in acid mine drainage using anaerobic fixed bed reactors with cheese whey as a carbon source. Latin American Appl Res 335:329–335
Brooks CS (1991) Metal recovery from industrial wastes. Lewis Publishers Inc., Chelsea. doi:10.1007/BF03258716
Bryson AW, Bijsterveld CH (1991) Kinetics of the precipitation of manganese and cobalt sulphides in the purification of a manganese sulphate electrolyte. Hydrometallurgy 27(1):75–84. doi:10.1016/0304-386X(91)90079-2
Cabezas A, de Araujo J, Callejas C, Galès A, Hamelin J, Marone A, Sousa D, Trably E, Etchebehere C (2015) How to use molecular biology tools for the study of the anaerobic digestion process? Rev Environ Sci Bio/Technol 14(4):555–593. doi:10.1007/s11157-015-9380-8
Cabrera G, Pérez R, Gómez JM, Ábalos A, Cantero D (2006) Toxic effects of dissolved heavy metals on Desulfovibrio vulgaris and Desulfovibrio sp. strains. J Hazard Mater 135(1–3):40–46. doi:10.1016/j.jhazmat.2005.11.058
Caldwell SL, Laidler JR, Brewer EA, Eberly JO, Sandborgh SC, Colwell FS (2008) Anaerobic oxidation of methane: mechanisms, bioenergetics, and the ecology of associated microorganisms. Environ Sci Technol 42(18):6791–6799. doi:10.1021/es800120b
Cassidy J, Lubberding HJ, Esposito G, Keesman KJ, Lens PN (2015) Automated biological sulphate reduction: a review on mathematical models, monitoring and bioprocess control. FEMS Microbiol Rev 39(6):823–853. doi:10.1093/femsre/fuv033
Cassidy J, Frunzo L, Lubberding HJ, Villa-Gómez DK, Esposito G, Keesman KJ, Lens PNL (2017) Role of microbial accumulation in biological sulphate reduction using lactate as electron donor in an inversed fluidized bed bioreactor: operation and dynamic mathematical modelling. Int Biodeterior Biodegrad 121:1–10. doi:http://dx.doi.org/10.1016/j.ibiod.2017.03.006
Celis L, Villa-Gómez D, Alpuche-Solís A, Ortega-Morales B, Razo-Flores E (2009) Characterization of sulfate-reducing bacteria dominated surface communities during start-up of a down-flow fluidized bed reactor. J Ind Microbiol Biotechnol 36(1):111–121. doi:10.1007/s10295-008-0478-7
Celis-García LB, Razo-Flores E, Monroy O (2007) Performance of a down-flow fluidized bed reactor under sulfate reduction conditions using volatile fatty acids as electron donors. Biotechnol Bioeng 97(4):771–779. doi:10.1002/bit.21288
Chang IS, Shin PK, Kim BH (2000) Biological treatment of acid mine drainage under sulphate-reducing conditions with solid waste materials as substrate. Water Res 34(4):1269–1277. doi:10.1016/S0043-1354(99)00268-7
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064. doi:10.1016/j.biortech.2007.01.057
Costa MC, Santos ES, Barros RJ, Pires C, Martins M (2009) Wine wastes as carbon source for biological treatment of acid mine drainage. Chemosphere 75(6):831–836. doi:10.1016/j.chemosphere.2008.12.062
Dar S, Bijmans M, Dinkla I, Geurkink B, Lens P, Dopson M (2009) Population dynamics of a single-stage sulfidogenic bioreactor treating synthetic zinc-containing waste streams. Microb Ecol 58(3):529–537. doi:10.1007/s00248-009-9509-9
Deng D, Lin L-S (2013) Two-stage combined treatment of acid mine drainage and municipal wastewater. Water Sci Technol 67(5):1000–1007. doi:10.2166/wst.2013.653
Deng D, Weidhaas JL, Lin L-S (2016) Kinetics and microbial ecology of batch sulfidogenic bioreactors for co-treatment of municipal wastewater and acid mine drainage. J Hazard Mater 305:200–208. doi:10.1016/j.jhazmat.2015.11.041
Dijkman H, Buisman CJN, Bayer HG (1999) Biotechnology in the mining and metallurgical industries: cost savings through selective precipitation of metal sulfides. In: Young DBD SK, Hackl RP, Dixon DG (ed) Copper 99 – Cobre 99 International Conference, Vol. IV: Hydrometallurgy of Copper 1999 phoenix, Arizona, USA. The minerals, metals & materials society, Warrendale, Pennsylvania, USA, 10–13 Oct 1999, pp 113–126
Dries J, De Smul A, Goethals L, Grootaerd H, Verstraete W (1998) High rate biological treatment of sulfate-rich wastewater in an acetate-fed EGSB reactor. Biodegradation 9(2):103–111. doi:10.1023/a:1008334219332
Esposito G, Veeken A, Weijma J, Lens PNL (2006) Use of biogenic sulfide for ZnS precipitation. Sep Purif Technol 51(1):31–39. doi:10.1016/j.seppur.2005.12.021
Fedorovich V, Lens P, Kalyuzhnyi S (2003) Extension of Anaerobic digestion model no. 1 with processes of sulfate reduction. Appl Biochem Biotechnol 109(1–3):33–45. doi:10.1385/ABAB:109:1-3:33
Foucher S, Battaglia-Brunet F, Ignatiadis I, Morin D (2001) Treatment by sulfate-reducing bacteria of Chessy acid-mine drainage and metals recovery. Chem Eng Sci 56(4):1639–1645. doi:10.1016/S0009-2509(00)00392-4
Gallegos-Garcia M, Celis LB, Rangel-Méndez R, Razo-Flores E (2009) Precipitation and recovery of metal sulfides from metal containing acidic wastewater in a sulfidogenic down-flow fluidized bed reactor. Biotechnol Bioeng 102(1):91–99. doi:10.1002/bit.22049
Gibert O, de Pablo J, Luis Cortina J, Ayora C (2004) Chemical characterisation of natural organic substrates for biological mitigation of acid mine drainage. Water Res 38(19):4186–4196. doi:10.1016/j.watres.2004.06.023
Gonzalez-Silva BM, Briones-Gallardo R, Razo-Flores E, Celis LB (2009) Inhibition of sulfate reduction by iron, cadmium and sulfide in granular sludge. J Hazard Mater 172(1):400–407. doi:10.1016/j.jhazmat.2009.07.022
Gramp JP, Sasaki K, Bigham JM, Karnachuk OV, Tuovinen OH (2006) Formation of covellite (CuS) under biological sulfate-reducing conditions. Geomicrobiol J 23(8):613–619. doi:10.1080/01490450600964383
Hammack RW, Edenborn HM (1992) The removal of nickel from mine waters using bacterial sulfate reduction. Appl Microbiol Biotechnol 37(5):674–678. doi:10.1007/bf00240748
Hammack RW, Edenborn HM, Dvorak DH (1994) Treatment of water from an open-pit copper mine using biogenic sulfide and limestone: a feasibility study. Water Res 28(11):2321–2329. doi:10.1016/0043-1354(94)90047-7
Hao OJ (2000) Metal effects on sulfur cycle bacteria and metal removal by sulfate reducing bacteria. In: PNL L, Hulshoff Pol L (eds) Environmental technologies to treat sulfur pollution: principles and engineering. IWA Publishing, London, pp 393–409
Hao T-W, Xiang P-Y, Mackey HR, Chi K, Lu H, Chui H-K, van Loosdrecht MCM, Chen G-H (2014) A review of biological sulfate conversions in wastewater treatment. Water Res 65:1–21. doi:10.1016/j.watres.2014.06.043
Hiibel SR, Pereyra LP, Breazeal MVR, Reisman DJ, Reardon KF, Pruden A (2011) Effect of organic substrate on the microbial community structure in pilot-scale sulfate-reducing biochemical reactors treating mine drainage. Environ Eng Sci 28(8):563–572. doi:10.1089/ees.2010.0237
Huisman JL, Schouten G, Schultz C (2006) Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 83(1–4):106–113. doi:10.1016/j.hydromet.2006.03.017
Hulshoff Pol LW, Lens PNL, Stams AJM, Lettinga G (1998) Anaerobic treatment of sulphate-rich wastewaters. Biodegradation 9(3):213–224. doi:10.1023/A:1008307929134
Hulshoff Pol LW, Lens PNL, Weijma J, Stams AJM (2001) New developments in reactor and process technology for sulfate reduction. Water Sci Technol 44(8):67–76
IWA TGfMMoADP (2002) Anaerobic digestion Model No. 1 (ADM1), IWA.
Johnson DB, Hallberg KB (2005) Biogeochemistry of the compost bioreactor components of a composite acid mine drainage passive remediation system. Sci Total Environ 338(1–2):81–93. doi:10.1016/j.scitotenv.2004.09.008
Jong T, Parry DL (2003) Removal of sulfate and heavy metals by sulfate reducing bacteria in short-term bench scale upflow anaerobic packed bed reactor runs. Water Res 37(14):3379–3389
Kaksonen AH, Puhakka JA (2007) Sulfate reduction based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng Life Sci 7(6):541–564. doi:10.1002/elsc.200720216
Kaksonen AH, Riekkola-Vanhanen ML, Puhakka JA (2003) Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater. Water Res 37(2):255–266. doi:10.1016/S0043-1354(02)00267-1
Kaksonen AH, Franzmann PD, Puhakka JA (2004) Effects of hydraulic retention time and sulfide toxicity on ethanol and acetate oxidation in sulfate-reducing metal-precipitating fluidized-bed reactor. Biotechnol Bioeng 86(3):332–343. doi:10.1002/bit.20061
Kimura S, Hallberg K, Johnson D (2006) Sulfidogenesis in low pH (3.8–4.2) media by a mixed population of acidophilic bacteria. Biodegradation 17(2):57–65. doi:10.1007/s10532-005-3050-4
König J, Keesman KJ, Veeken A, Lens PNL (2006) Dynamic modelling and process control of ZnS precipitation. Sep Sci Technol 41(6):1025–1042. doi:10.1080/01496390600641546
Koschorreck M, Geller W, Neu T, Kleinsteuber S, Kunze T, Trosiener A, Wendt-Potthoff K (2010) Structure and function of the microbial community in an in situ reactor to treat an acidic mine pit lake. FEMS Microbiol Ecol 73(2):385–395. doi:10.1111/j.1574-6941.2010.00886.x
La H-J, Kim K-H, Quan Z-X, Cho Y-G, Lee S-T (2003) Enhancement of sulfate reduction activity using granular sludge in anaerobic treatment of acid mine drainage. Biotechnol Lett 25(6):503–508. doi:10.1023/A:1022666310393
Labrenz M, Druschel GK, Thomsen-Ebert T, Gilbert B, Welch SA, Kemner KM, Logan GA, Summons RE, Stasio GD, Bond PL, Lai B, Kelly SD, Banfield JF (2000) Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 290(5497):1744–1747. doi:10.1126/science.290.5497.1744
Lakaniemi A-M, Nevatalo LM, Kaksonen AH, Puhakka JA (2010) Mine wastewater treatment using Phalaris arundinacea plant material hydrolyzate as substrate for sulfate-reducing bioreactor. Bioresour Technol 101(11):3931–3939. doi:10.1016/j.biortech.2010.01.020
Larsen PA, Patience DB, Rawlings JB (2006) Industrial crystallization process control. IEEE Ctl Syst Mag 26(4):70–80. doi:10.1109/MCS.2006.1657878
Lauwers J, Appels L, Thompson IP, Degrève J, Impe JFV, Dewil R (2013) Mathematical modelling of anaerobic digestion of biomass and waste: power and limitations. Prog Energy Combust Sci 39(4):383–402. doi:10.1016/j.pecs.2013.03.003
Lefticariu L, Walters ER, Pugh CW, Bender KS (2015). Sulfate reducing bioreactor dependence on organic substrates for remediation of coal-generated acid mine drainage: field experiments. Appl Geochem 63:70–82. doi: 10.1016/j.apgeochem.2015.08.002
Lenz M, van Hullebusch ED, Farges F, Nikitenko S, Corvini PFX, Lens PNL (2011) Combined speciation analysis by X-ray absorption near-edge structure spectroscopy, ion chromatography, and solid-phase microextraction gas chromatography-mass spectrometry to evaluate biotreatment of concentrated selenium wastewaters. Environ Sci Technol 45(3):1067–1073. doi:10.1021/es1022619
Lewis AE (2010) Review of metal sulphide precipitation. Hydrometallurgy 104(2):222–234. doi:10.1016/j.hydromet.2010.06.010
Lewis A, Swartbooi A (2006) Factors affecting metal removal in mixed sulfide precipitation. Chem Eng Technol 29(2):277–280. doi:10.1002/ceat.200500365
Lewis A, van Hille R (2006) An exploration into the sulphide precipitation method and its effect on metal sulphide removal. Hydrometallurgy 81(3–4):197–204. doi:10.1016/j.hydromet.2005.12.009
Liamleam W, Annachhatre AP (2007) Electron donors for biological sulfate reduction. Biotechnol Adv 25(5):452–463. doi:10.1016/j.biotechadv.2007.05.002
Liu W, Zhang J, Jin Y, Zhao X, Cai Z (2015) Adsorption of Pb(II), Cd(II) and Zn(II) by extracellular polymeric substances extracted from aerobic granular sludge: efficiency of protein. J Environ Chem Eng 3(2):1223–1232. doi:10.1016/j.jece.2015.04.009
Lopes SIC, Sulistyawati I, Capela MI, Lens PNL (2007) Low pH (6, 5 and 4) sulfate reduction during the acidification of sucrose under thermophilic (55°C) conditions. Process Biochem 42(4):580–591. doi:10.1016/j.procbio.2006.11.004
Luptakova A, Kusnierova M (2005) Bioremediation of acid mine drainage contaminated by SRB. Hydrometallurgy 77(1–2):97–102. doi:10.1016/j.hydromet.2004.10.019
Mersmann A (1999) Crystallization and precipitation. Chem Eng Process 38(4–6):345–353. doi:10.1016/S0255-2701(99)00025-2
Mes TZDD, Stams AJM, Reith JH, Zeeman G (2003) Methane production by anaerobic digestion of wastewater and solid wastes. In: Reith JH, Wijffels RH, Barten H (eds) Bio-methane & bio-hydrogen: status and perspectives of biological methane and hydrogen production. The Hague, Dutch Biological Hydrogen Foundation – NOVEM, pp 58–102
Meulepas RJW, Stams AM, Lens PL (2010) Biotechnological aspects of sulfate reduction with methane as electron donor. Rev Environ Sci Biotechnol 9(1):59–78. doi:10.1007/s11157-010-9193-8
Mishra PK, Das RP (1992) Kinetics of zinc and cobalt sulphide precipitation and its application in hydrometallurgical separation. Hydrometallurgy 28(3):373–379. doi:10.1016/0304-386X(92)90042-X
Mizuno O, Li YY, Noike T (1998) The behavior of sulfate-reducing bacteria in acidogenic phase of anaerobic digestion. Water Res 32(5):1626–1634. doi:10.1016/s0043-1354(97)00372-2
Mokone TP, van Hille RP, Lewis AE (2010) Effect of solution chemistry on particle characteristics during metal sulfide precipitation. J Colloid Interface Sci 351(1):10–18. doi:10.1016/j.jcis.2010.06.027
Moosa S, Harrison STL (2006) Product inhibition by sulphide species on biological sulphate reduction for the treatment of acid mine drainage. Hydrometallurgy 83(1–4):214–222. doi:10.1016/j.hydromet.2006.03.026
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Micro 6(6):441–454. doi:10.1038/nrmicro1892
Nagpal S, Chuichulcherm S, Peeva L, Livingston A (2000) Microbial sulfate reduction in a liquid-solid fluidized bed reactor. Biotechnol Bioeng 70(4):370–380. doi:10.1002/1097-0290(20001120)70:4<370::aid-bit2>3.0.co;2-7
Ňancucheo I, Johnson DB (2012) Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb Biotechnol 5(1):34–44. doi:10.1111/j.1751-7915.2011.00285.x
Ňancucheo I, Johnson DB (2014) Removal of sulfate from extremely acidic mine waters using low pH sulfidogenic bioreactors. Hydrometallurgy 150:222–226. doi:10.1016/j.hydromet.2014.04.025
Neculita C-M, Zagury GJ, Bussiere B (2007) Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: critical review and research needs. J Environ Qual 36(1):1–16. doi:10.2134/jeq2006.0066
Neculita C-M, Zagury GJ, Bussière B (2008) Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: II. Metal removal mechanisms and potential mobility. Appl Geochem 23(12):3545–3560. doi:10.1016/j.apgeochem.2008.08.014
Nguyen D, Gadhamshetty V, Nitayavardhana S, Khanal SK (2015) Automatic process control in anaerobic digestion technology: a critical review. Bioresour Technol 193:513–522. doi:10.1016/j.biortech.2015.06.080
Papirio S, Villa-Gómez DK, Esposito G, Pirozzi F, Lens PNL (2012) Acid mine drainage treatment in fluidized-bed bioreactors by sulfate-reducing bacteria: a critical review. Crit Rev Environ Sci Technol 43(23):2545–2580. doi:10.1080/10643389.2012.694328
Paques BV (2016) Paques, leading in biological wastewater and gas treatment [Online]. Available: www.paques.nl. Accessed 10 Jan 2016
Parker WJ, Wu GH (2006) Modifying ADM1 to include formation and emission of odourants. Water Sci Technol 54(4):111–117. doi:10.2166/wst.2006.532
Parshina SN, Kijlstra S, Henstra AM, Sipma J, Plugge CM, Stams AJM (2005a) Carbon monoxide conversion by thermophilic sulfate-reducing bacteria in pure culture and in co-culture with Carboxydothermus hydrogenoformans. Appl Microbiol Biotechnol 68(3):390–396
Parshina SN, Sipma J, Nakashimada Y, Henstra AM, Smidt H, Lysenko AM, Lens PNL, Lettinga G, Stams AJM (2005b) Desulfotomaculum carboxydivorans sp. nov., a novel sulfate-reducing bacterium capable of growth at 100% CO. Int J Syst Evol Microbiol 55(5):2159–2165. doi:10.1099/ijs.0.63780-0
Parshina SN, Sipma J, Henstra AM, Stams AJM (2010) Carbon monoxide as an electron donor for the biological reduction of sulphate Int J Microbiol 2010:9. doi: 10.1155/2010/319527
Pat-Espadas AM, Field JA, Otero-Gonzalez L, Razo-Flores E, Cervantes FJ, Sierra-Alvarez R (2016) Recovery of palladium(II) by methanogenic granular sludge. Chemosphere 144:745–753. doi:10.1016/j.chemosphere.2015.09.035
Pind P, Angelidaki I, Ahring B, Stamatelatou K, Lyberatos G (2003) Monitoring and control of anaerobic reactors. In: Biomethanation II, vol 82, Series on Advances in Biochemical Engineering/biotechnology. Springer, Berlin/Heidelberg, pp 135–182. doi:10.1007/3-540-45838-7_4
Pokhrel LR, Dubey B (2013) Global scenarios of metal mining, environmental repercussions, public policies, and sustainability: a review. Crit Rev Environ Sci Technol 43(21):2352–2388. doi:10.1080/10643389.2012.672086
Prange A, Modrow H (2002) X-ray absorption spectroscopy and its application in biological, agricultural and environmental research. Rev Environ Sci Biotechnol 1(4):259–276. doi:10.1023/a:1023281303220
Puigdomènech I (2010) Make equilibrium diagrams using sophisticated algorithms (MEDUSA), software version 18 February 2004. Inorganic Chemistry Department, Royal Institute of Technology, Stockholm
Qinglin X, Yanhong L, Shaoyuan B, Hongda J (2012) Effects of ORP, recycling rate, and HRT on simultaneous sulfate reduction and sulfur production in expanded granular sludge bed (EGSB) reactors under micro-aerobic conditions for treating molasses distillery wastewater. Water Sci Technol 66(6):1253–1262. doi:10.2166/wst.2012.311
Reis MAM, Almeida JS, Lemos PC, Carrondo MJT (1992) Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol Bioeng 40(5):593–600. doi:10.1002/bit.260400506
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill, New Delhi
Roland H, Benjamin S (2015) Mining & metals in a sustainable world 2050. Switzerland
Sahinkaya E, Gungor M (2010) Comparison of sulfidogenic up-flow and down-flow fluidized-bed reactors for the biotreatment of acidic metal-containing wastewater. Bioresour Technol 101(24):9508–9514. doi:10.1016/j.biortech.2010.07.113
Sahinkaya E, Yucesoy Z (2010) Biotreatment of acidic zinc- and copper-containing wastewater using ethanol-fed sulfidogenic anaerobic baffled reactor. Bioprocess Biosyst Eng 33(8):989–997. doi:10.1007/s00449-010-0423-9
Sahinkaya E, Gungor M, Bayrakdar A, Yucesoy Z, Uyanik S (2009) Separate recovery of copper and zinc from acid mine drainage using biogenic sulfide. J Hazard Mater 171(1–3):901–906. doi:10.1016/j.jhazmat.2009.06.089
Sahinkaya E, Gunes FM, Ucar D, Kaksonen AH (2011) Sulfidogenic fluidized bed treatment of real acid mine drainage water. Bioresour Technol 102(2):683–689. doi:10.1016/j.biortech.2010.08.042
Samaranayake R, Singhal N, Lewis G, Hyland M (2002) Kinetics of biochemically driven metal precipitation in synthetic landfill leachate. Remed J 13(1):137–150. doi:10.1002/rem.10060
Sampaio RMM, Timmers RA, Xu Y, Keesman KJ, Lens PNL (2009) Selective precipitation of Cu from Zn in a pS controlled continuously stirred tank reactor. J Hazard Mater 165(1–3):256–265. doi:10.1016/j.jhazmat.2008.09.117
Sampaio RMM, Timmers RA, Kocks N, André V, Duarte MT, van Hullebusch ED, Farges F, Lens PNL (2010) Zn-Ni sulfide selective precipitation: the role of supersaturation. Sep Purif Technol 74(1):108–118. doi:10.1016/j.seppur.2010.05.013
Sanchez-Andrea I, Triana D, Sanz JL (2012) Bioremediation of acid mine drainage coupled with domestic wastewater treatment. Water Sci Technol 66(11):2425–2431. doi:10.2166/wst.2012.477
Sánchez-Andrea I, Sanz JL, Bijmans MFM, Stams AJM (2014) Sulfate reduction at low pH to remediate acid mine drainage. J Hazard Mater 269:98–109. doi:10.1016/j.jhazmat.2013.12.032
Shakeri Yekta S, Gustavsson J, Svensson BH, Skyllberg U (2012) Sulfur K-edge XANES and acid volatile sulfide analyses of changes in chemical speciation of S and Fe during sequential extraction of trace metals in anoxic sludge from biogas reactors. Talanta 89:470–477. doi:10.1016/j.talanta.2011.12.065
Sierra-Alvarez R, Karri S, Freeman S, Field JA (2006) Biological treatment of heavy metals in acid mine drainage using sulfate reducing bioreactors. Water Sci Technol 54(2):179–185
Sipma J, Henstra AM, Parshina SN, Lens PNL, Lettinga G, Stams AJM (2006) Microbial CO conversions with applications in synthesis gas purification and bio-desulfurization. Crit Rev Biotechnol 26(1):41–65. doi:10.1080/07388550500513974
Sipma J, Begoña Osuna M, Lettinga G, Stams AJM, Lens PNL (2007) Effect of hydraulic retention time on sulfate reduction in a carbon monoxide fed thermophilic gas lift reactor. Water Res 41(9):1995–2003. doi:10.1016/j.watres.2007.01.030
Song H, Yim G, Ji S, Nam I, Neculita C, Lee G (2012) Performance of mixed organic substrates during treatment of acidic and moderate mine drainage in column bioreactors. J Environ Eng 138(10):1077–1084. doi:10.1061/(ASCE)EE.1943-7870.0000567
Sousa JAB, Plugge CM, Stams AJM, Bijmans MFM (2015) Sulfate reduction in a hydrogen fed bioreactor operated at haloalkaline conditions. Water Res 68:67–76. doi:10.1016/j.watres.2014.09.035
Tabak HH, Govind R (2003) Advances in biotreatment of acid mine drainage and biorecovery of metals: 2. Membrane bioreactor system for sulfate reduction. Biodegradation 14(6):437–452. doi:10.1023/A:1027332918844
Tabak HH, Scharp R, Burckle J, Kawahara FK, Govind R (2003) Advances in biotreatment of acid mine drainage and biorecovery of metals: 1. Metal precipitation for recovery and recycle. Biodegradation 14(6):423–436. doi:10.1023/A:1027332902740
Tang Y, Ontiveros-Valencia A, Feng L, Zhou C, Krajmalnik-Brown R, Rittmann BE (2013) A biofilm model to understand the onset of sulfate reduction in denitrifying membrane biofilm reactors. Biotechnol Bioeng 110(3):763–772. doi:10.1002/bit.24755
Tokuda H, Kuchar D, Mihara N, Kubota M, Matsuda H, Fukuta T (2008) Study on reaction kinetics and selective precipitation of Cu, Zn, Ni and Sn with H2S in single-metal and multi-metal systems. Chemosphere 73(9):1448–1452. doi:10.1016/j.chemosphere.2008.07.073
Torner-Morales FJ, Buitrón G (2010) The redox potential as the limiting factor to carry out a combined sulfate-reducing/sulfide-oxidizing process in a single SBR. In: Proceedings of the 12th world congress on anaerobic digestion, Guadalajara, Mexico, 31 October–4 November
Utgikar VP, Harmon SM, Chaudhary N, Tabak HH, Govind R, Haines JR (2002) Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage. Environ Toxicol 17(1):40–48. doi:10.1002/tox.10031/abstract
Utgikar VP, Chaudhary N, Koeniger A, Tabak HH, Haines JR, Govind R (2004) Toxicity of metals and metal mixtures: analysis of concentration and time dependence for zinc and copper. Water Res 38(17):3651–3658. doi:10.1016/j.watres.2004.05.022
Vakili M, Gholami Z, Gholami F (2012) Removal of hydrogen sulfide from gaseous streams by a chemical method using ferric sulfate solution. World Appl Sci J 19(2):241–245. doi:10.5829/idosi.wasj.2012.19.02.1541
van Hille RP, Peterson KA, Lewis AE (2005) Copper sulphide precipitation in a fluidised bed reactor. Chem Eng Sci 60(10):2571–2578. doi:10.1016/j.ces.2004.11.052
van Hullebusch E, Zandvoort M, Lens P (2003) Metal immobilisation by biofilms: mechanisms and analytical tools. Rev Environ Sci Biotechnol 2(1):9–33. doi:10.1023/B:RESB.0000022995.48330.55
van Hullebusch ED, Zandvoort MH, Lens PNL (2004) Nickel and cobalt sorption on anaerobic granular sludges: kinetic and equilibrium studies. J Chem Technol Biotechnol 79(11):1219–1227. doi:10.1002/jctb.1116
van Hullebusch ED, Peerbolte A, Zandvoort MH, Lens PNL (2005) Sorption of cobalt and nickel on anaerobic granular sludges: isotherms and sequential extraction. Chemosphere 58(4):493–505. doi:10.1016/j.chemosphere.2004.09.017
van Hullebusch ED, Gieteling J, Zhang M, Zandvoort MH, Daele WV, Defrancq J, Lens PNL (2006) Cobalt sorption onto anaerobic granular sludge: isotherm and spatial localization analysis. J Biotechnol 121(2):227–240. doi:10.1016/j.jbiotec.2005.07.011
van Hullebusch ED, Rossano S, Farges F, Lenz M, Labanowski J, Lagarde P, Flank AM, Lens PNL (2009) Sulfur K-edge XANES spectroscopy as a tool for understanding sulfur chemical state in anaerobic granular sludge. J Phys Conf Ser 190:012184. doi:10.1088/1742-6596/190/1/012184
Vanwonterghem I, Jensen PD, Ho DP, Batstone DJ, Tyson GW (2014) Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr Opin Biotechnol 27:55–64. doi:10.1016/j.copbio.2013.11.004
Veeken AHM, Akoto L, Hulshoff Pol LW, Weijma J (2003a) Control of the sulfide (S) concentration for optimal zinc removal by sulfide precipitation in a continuously stirred tank reactor. Water Res 37(15):3709–3717. doi:10.1016/S0043-1354(03)00262-8
Veeken AHM, de Vries S, van der Mark A, Rulkens WH (2003b) Selective precipitation of heavy metals as controlled by a sulfide-selective electrode. Sep Sci Technol 38(1):1–19. doi:10.1081/SS-120016695
Villa-Gómez DK, Enright AM, Rini EL, Buttice A, Kramer H, Lens P (2015) Effect of hydraulic retention time on metal precipitation in sulfate reducing inverse fluidized bed reactors. J Chem Technol Biotechnol 90(1):120–129. doi:10.1002/jctb.4296
Villa-Gómez D, Ababneh H, Papirio S, Rousseau DPL, Lens PNL (2011) Effect of sulfide concentration on the location of the metal precipitates in inversed fluidized bed reactors. J Hazard Mater 192(1):200–207. doi:10.1016/j.jhazmat.2011.05.002
Villa-Gómez DK, Papirio S, van Hullebusch ED, Farges F, Nikitenko S, Kramer H, Lens PNL (2012) Influence of sulfide concentration and macronutrients on the characteristics of metal precipitates relevant to metal recovery in bioreactors. Bioresour Technol 110(0):26–34. doi:10.1016/j.biortech.2012.01.041
Villa-Gómez DK, Cassidy J, Keesman KJ, Sampaio R, Lens PNL (2014a) Sulfide response analysis for sulfide control using a pS electrode in sulfate reducing bioreactors. Water Res 50(0):48–58. doi:10.1016/j.watres.2013.10.006
Villa-Gómez DK, van Hullebusch ED, Maestro R, Farges F, Nikitenko S, Kramer H, Gonzalez-Gil G, Lens PNL (2014b) Morphology, mineralogy, and solid-liquid phase separation characteristics of Cu and Zn precipitates produced with biogenic sulfide. Environ Sci Technol 48(1):664–673. doi:10.1021/es402795x
Wakeman KD, Erving L, Riekkola-Vanhanen ML, Puhakka JA (2010) Silage supports sulfate reduction in the treatment of metals- and sulfate-containing waste waters. Water Res 44(17):4932–4939. doi:10.1016/j.watres.2010.07.025
Willow MA, Cohen RRH (2003) pH, dissolved oxygen, and adsorption effects on metal removal in anaerobic bioreactors. J Environ Qual 32(4):1212–1221. doi:10.2134/jeq2003.1212
Zhang Y, Henriet JP, Bursens J, Boon N (2010) Stimulation of in vitro anaerobic oxidation of methane rate in a continuous high-pressure bioreactor. Bioresour Technol 101(9):3132–3138. doi:10.1016/j.biortech.2009.11.103
Zhou J, Xing J (2015) Effect of electron donors on the performance of haloalkaliphilic sulfate-reducing bioreactors for flue gas treatment and microbial degradation patterns related to sulfate reduction of different electron donors. Biochem Eng J 96:14–22. doi:10.1016/j.bej.2014.12.015
Zhou J, Zhou X, Li Y, Xing J (2015) Bacterial communities in haloalkaliphilic sulfate-reducing bioreactors under different electron donors revealed by 16S rRNA MiSeq sequencing. J Hazard Mater 295:176–184. doi:10.1016/j.jhazmat.2015.04.010
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Gómez, D.K.V., Lens, P.N.L. (2017). Metal Recovery from Industrial and Mining Wastewaters. In: Rene, E., Sahinkaya, E., Lewis, A., Lens, P. (eds) Sustainable Heavy Metal Remediation. Environmental Chemistry for a Sustainable World. Springer, Cham. https://doi.org/10.1007/978-3-319-61146-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-61146-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61145-7
Online ISBN: 978-3-319-61146-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)