Abstract
The treatment of acidic (pH 6.5–3), sulfate- (2–3 g/L), Zn- and Cu- (total metal 0–500 mg/L) containing wastewater was studied in a four-stage anaerobic baffled reactor (ABR) at 35 °C for 250 days. Ethanol was supplemented (COD/SO4 2− = 0.67) as carbon and electron source for sulfate reducing bacteria. Sulfate reduction, COD oxidation and metal precipitation efficiencies were 70–92, 80–94 and >99%, respectively. The alkalinity produced from sulfidogenic ethanol oxidation increased the wastewater pH from 3.0 to 7.0–8.0. The electron flow from organic oxidation to sulfate averaged 87%. Decreasing feed pH to 3 and increasing total metal concentrations to 500 mg/L did not adversely affect the performance of ABR and sufficient alkalinity was produced to increase the effluent pH to neutral values. More than 99% of metals were precipitated in the form of metal-sulfides. Accumulation of precipitated metals in the first compartment allowed metal recovery without disturbing reactor performance seriously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mining of sulfide ores or coal generates acid mine drainage (AMD) upon interaction of air and water with sulfides, mainly pyrite (FeS2) [1–3]. The formation of acidic wastewaters can continue even tens or hundreds of years after mine closure if the conditions remain favorable [4, 5].
Sulfate reducing bioprocesses have become an alternative to conventional chemical treatment of AMD due to low cost and high efficiency [6]. In the presence of organic compounds, sulfate is microbially reduced to H2S under anaerobic conditions and heavy metals (especially, Cu, Pb, Zn, Cd, Ni, Fe) form stable precipitates with the produced H2S. Moreover, produced bicarbonate increases the pH of the wastewater. This way, metals and sulfate are concomitantly removed and pH can be increased to neutral values in a single reactor [2, 3, 7, 8].
The dissolved organic carbon content of metal-containing wastewater is very low and usually <10 mg/L [9]. Therefore, addition of a suitable carbon source and electron donor for sulfate reduction is necessary to promote biogenic H2S production. Sulfate reducing bacteria (SRB) utilize several low molecular weight substrates, such as lactate, formate, acetate, ethanol and hydrogen. Ethanol is a good substrate for SRB and suitable for large-scale applications [2, 7]. Under sulfidogenic conditions, ethanol is first converted to acetate and in the second step acetate may further be oxidized to CO2. In this case, acetate oxidation is obligatory to produce alkalinity (Eqs. 1, 2) as the first step (conversion of ethanol to acetate) does not produce alkalinity [6]. Some SRB oxidize organic substrates completely to CO2, while others incompletely to acetate [10]. It is well known that acetate may accumulate in the reactor at high loadings, which may decrease the sulfide and alkalinity productions [6–8, 11]. Hence, in the case of ethanol supplementation, the loading rate should be carefully adjusted not to accumulate acetate and cease alkalinity production:
In the literature, several studies have been conducted on the treatment of AMD using different attached growth reactor configurations (see Kaksonen et al. [12] for review). When attached growth reactors are used, the metal-sulfide precipitates may accumulate within the reactor and metal recovery becomes difficult. When metal precipitates were drawn from the attached growth reactors, active biomass may also be lost and the reactor performance may be adversely affected. Generally, metal-sulfide precipitates and biomass are recovered in separate units, which may increase the process cost. Hence, there is a great interest to develop a technology for effective biotreatment of AMD and metal recovery in one reactor [13]. For this purpose, sulfidogenic down-flow fluidized-bed reactor was used by Gallegos-Garcia et al. [13]. In this study, however, accumulation of acetate led to relatively low sulfate and COD removal efficiencies as well as low alkalinity production.
Another alternative for the treatment of AMD and recovering metals is the use of anaerobic baffled reactor (ABR). The main advantage of ABR for AMD treatment is the accumulation of precipitated metals in the first compartment, which allows metal-sulfide recovery without distributing reactor performance seriously. The reactor has several compartments and drawing sludge from the first compartment during metal recovery may not adversely affect the system performance. Metal-sulfide precipitates can be recovered from the first compartment, which can be reseeded by sulfate reducers drawn from other compartments. Hence, ABR is an attractive reactor configuration for metal-containing wastewater treatment and metal recovery. Although several studies have shown that ABR is very effective in anaerobic wastewater treatment and biomass granulation [14–16], limited information is available on the potential of ABR for the treatment of AMD and metal recovery [11]. In this context, the present study aims at evaluating the potential of ethanol-fed sulfidogenic ABR in the treatment of Zn- and Cu-containing AMD and metal recovery.
Materials and methods
Bioreactor
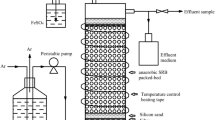
A laboratory scale ABR (Fig. 1) previously fed with lactate and synthetic AMD for around 300 days [11] was used in the present study. Reactor was divided into four equal 5-L compartments by vertical baffles, each compartment having down-comer and riser regions created by further vertical baffle. The reactor was maintained at 35 °C in a temperature controlled room. The hydraulic retention time (HRT) of the reactor was kept constant at 2 days throughout the study. Hence, the HRT in each compartment was equal to 0.5 days as the reactor had four equal compartments. The synthetic wastewater (pH 3–7.0) was fed to the reactor at a rate of 10 L/day. Sulfate concentration in the synthetic wastewater containing micro- and macro-nutrients (56 mg/L KH2PO4; 110 mg/L NH4Cl; 11 mg/L ascorbic acid and 50 mg/L yeast extract) was 2,000 or 3,000 mg/L (Table 1). Ethanol (1,340 or 2,010 mg COD/L) was used as a carbon and electron source stoichiometrically to reduce sulfate to hydrogen sulfide and oxidize ethanol completely to CO2 and H2O. Hence, COD/SO4 2− ratio was kept constant at 0.67 on mass ratio throughout the study (Eqs. 1, 2). The feed solution was prepared daily. COD removal, Cu and Zn precipitation, and sulfate reduction were not observed in the feed container.
Experimental procedure
The performance of ethanol-fed ABR reactor was evaluated at different feed organic, sulfate and metal loadings for around 250 days (Table 1). Firstly, the reactor was fed with an alkaline solution containing 2,000 mg SO4 2−/L without metal supplementation (Period I, days 0–42) to enrich ethanol oxidizing SRB. Then, the reactor performance was evaluated at increasing metal (Cu and Zn at equal concentrations), sulfate and organic loadings (Table 1) with decreasing pH.
The reactor influent, each compartment, and the effluent were sampled 3–4 times in a week for the measurement of pH, alkalinity, total volatile fatty acids (VFAs), acetate, COD, sulfate, dissolved sulfide, and soluble and total metals (Zn and Cu).
Electron flow from carbon oxidation to sulfate reduction was calculated assuming 0.67 mg COD is required to reduce per mg sulfate based on Eqs. 1 and 2. The following equation was used in the electron flow calculations:
where SO4,0 and SO4,e are influent and effluent sulfate concentrations (mg/L), and COD0 and CODe are influent and effluent COD concentrations (mg/L), respectively.
Analytical techniques
Samples were centrifuged using Hettich Rotofix 32 centrifuge at 4,000 rpm for 10 min, before the measurement of sulfate, dissolved sulfide, soluble metals (Zn and Cu), acetate and COD from the supernatant. Before centrifugation for sulfide measurement, the pH was increased to around 10 with 1 M NaOH and glass sampling tube was sealed using a Teflon crimp cap so that not to cause any loss of sulfide. Total sulfide was analyzed spectrometrically using a Shimadzu UV-1601 Spectrophotometer following the method described by Cord-Ruwisch [17]. A turbidimetric method was used to measure sulfate concentrations [18]. COD and alkalinity were also measured according to Standard Methods [18]. Before COD measurements, sample pH was decreased below 2 with concentrated H2SO4 and the sample was purged with N2 gas around 5 min to remove H2S from the sample. For the alkalinity measurements, unfiltered samples were titrated by 0.1 N HCl to pH 4.5. Total VFA concentration was measured following the procedure described by Alvarez et al. [19]. A gas chromatograph (Varian 3900 GC) equipped with a 30 m × 0.25 mm (i.d.) × 0.50 μm (film) FFAP capillary column and flame ionization detector was used for acetate measurements after acidification of samples with HCl. For soluble Zn and Cu measurements, sample was first filtered through 0.45-μm polyethersulfone membrane syringe filters and then acidified with concentrated HCl to pH below 2. For total Zn and Cu concentration measurements, samples were first acidified to pH around 1.0 with concentrated HCl to solubilize Zn and Cu particles. Then, samples were filtered through 0.45-μm syringe filters to remove biomass and other particles. Metal concentrations were measured with an atomic absorption spectrophotometer (Varian AA 140). In order to determine the mechanism of metal removal, Panalytical Axios-Advanced wavelength dispersive X-ray fluorescence spectrometer (XRF) was employed to analyze the elemental composition of sludge samples drawn from the Compartment 1 at the end of reactor operation (day 245). All measurements were done at least in duplicate. Mean values and standard deviations were presented.
Results and discussion
Sulfate reduction and COD oxidation
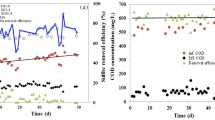
The performance of ABR in terms of sulfate reduction and COD removal is presented in Figs. 2 and 3, respectively. The reactor started with 65% sulfate reduction and 80% COD removal without any lag period due to the fact that the reactor was fed with a lactate containing synthetic wastewater for more than 300 days in a previous study [11]. During the electron donor change from lactate to ethanol, high sulfate reduction and COD removal efficiencies were observed and hence the electron donor change did not adversely affect the system performance, similar to the study of Kaksonen et al. [7]. In the first period of the reactor operation, sulfate reduction and COD oxidation averaged 60 and 80%, respectively. The reactor performance improved until Period IV at which sulfate reduction and COD oxidation efficiencies reached to around 92 and 95%, respectively (Figs. 2, 3, 4). Sulfate and COD concentration profiles were generally steady and showed similar pattern over the entire course of the experiments. Sulfate concentration in the Compartment 4 reached 1,000 mg/L during the start-up period (Period 1) and decreased continuously to approximately 200 mg/L at the end of the Period IV (Figs. 2, 3, 4). Similarly, after start-up period, COD concentration in the Compartment 4 remained around 100 mg/L until the end of Period IV (Figs. 2, 3, 4). Hence, decreasing pH and increasing feed metal concentration (between Periods 1 and 4) did not adversely affect the reactor performance. The treatment efficiency of the reactor showed an increasing trend in terms of sulfate reduction and COD oxidation efficiencies (Figs. 2, 3, 4). There may be two possible reasons for this increase. The first is that sulfide is toxic to SRB and addition of metal caused removal of sulfide and increasing sulfate reduction performance. The second is the adaptation of SRB to the operating conditions.
Steady-state performance of cumulative sulfate reductions (a) and cumulative COD removals (b) for each ABR compartment at different operational periods. See Table 1 for operational periods
The sulfate reduction and COD oxidation efficiencies started to decrease at Period V, at which the influent sulfate concentration was increased from 2,000 to 3,000 mg/L. A transient increase was observed in COD concentration of Compartment 1 in response to step increase in sulfate and COD loadings at Period V, but it dropped further to around 750 mg/L when the reactor had acclimatized to the new feed regime. Although sulfate reduction and COD oxidation efficiencies decreased at Period V (Figs. 2, 3, 4), the sulfate reduction and COD removal rates dramatically increased from 1 and 0.63 g/(L day) at Period IV to 1.3 and 0.90 g/(L day) at Period V, respectively. The maximum sulfate reduction rate in an ethanol-fed fluidized-bed reactor was reported as around 4 g/(L day) [7, 20]. The reason for observing low removal rates in our study was the long HRT of the ABR over the entire course of the experiment. If we only consider first compartment (HRT was 0.5 day), the sulfate reduction rate can be calculated as approximately 3 g/(L day), pretty similar to the previously reported values [7, 20]. Operating the reactor at long HRT prevented the accumulation of acetate in the reactor as it is well known that short HRTs may cause build up of acetate in the reactor, which in turn reduces alkalinity production and eventually results in reactor failure for acidic influents [20].
The operational conditions of the reactor were not changed until steady-state conditions were reached. The reactor was assumed to be operating at steady state when the measured effluent sulfate, alkalinity, sulfide and COD concentrations did not show variations higher than 10% at least for the last three measurements. The steady-state performances of sulfate reduction and COD oxidation were provided in Fig. 4. Total VFA and acetate concentrations were almost similar for all compartments. This may indicate that ethanol is converted to acetate, which may be further oxidized to CO2 and H2O as previously reported in other studies [2, 3, 7, 21]. The COD (Fig. 3) and acetate (Fig. 5) measurements showed that the remaining COD in the effluent of the reactor was due to acetate. Although serious acetate accumulation was not observed in the present study, acetate accumulation may take place at higher loadings as previously reported [2, 3, 7, 21–23]. Moreover, Sahinkaya et al. [22] reported acetate oxidation is the limiting step in sulfate reducing bioprocesses.
Alkalinity production and metal removal
According to Eqs. 1 and 2, sulfidogenic oxidation of ethanol to acetate does not produce alkalinity and acetate oxidation is necessary to produce sufficient alkalinity to increase the pH of AMD to neutral values. Alkalinity and pH variations for each compartment were illustrated in Fig. 5a and c, respectively. The pH increased from Compartment 1 to 4 due to alkalinity production (Fig. 5c) during sulfidogenic acetate oxidation (Eq. 2). The pH at the effluent of Compartment 4 was close to 7.0 although the feed pH was decreased to around 3 at Period VII (Fig. 5c). When the feed sulfate concentration was 2,000 mg/L (up to Period V), the effluent alkalinity averaged 1,500 mg CaCO3/L and it increased to approximately 2,000 mg CaCO3/L with increasing feed sulfate concentration to 3,000 mg/L. At the beginning of Period VI, the alkalinity and pH in the Compartment 1 transiently dropped to 0 and 4, respectively, and then fluctuated around 500 mg/L CaCO3 and 6.0, respectively, for the rest of the experiment. Although alkalinity values in Compartment 1 fluctuated transiently following each step increase in metal loads and pH decrease between Periods VI and VII, the alkalinity and pH remained stable and averaging 1,750 mg CaCO3/L and 7.2, respectively, in the Compartment 4. This shows that ABR has quite high application potential for the treatment of acidic metal- and sulfate-containing wastewater.
The alkalinity and sulfide profiles showed almost the same pattern over the entire course of the experiment (Fig. 5a, b). Sulfide concentration increased from Compartment 1 to 4 and effluent sulfide concentration averaged 380 mg/L when feed sulfate concentration was 2,000 mg/L (Fig. 5b). The average sulfide concentration increased to around 600 mg/L with increasing feed sulfate concentration to 3,000 mg/L (Period V) and it decreased to around 400 mg/L for the rest of reactor operation due to increase in feed metal concentrations. Sulfide concentration in Compartment 1 fluctuated after Period IV and showed a decreasing trend toward the end of the experiment. However, sulfide concentration remained stable in Compartment 4, similar to the alkalinity and pH trends (Fig. 5a, b).
Over 99% removal efficiencies were obtained for both Zn and Cu (Fig. 6). The soluble metal concentrations decreased from Compartment 1 to 4 throughout the study. The effluent Zn and Cu concentrations averaged 0.47 and 0.14 mg/L, respectively (Fig. 6). Although the feed Zn and Cu concentrations were the same as mg/L, the effluent Zn concentration was much higher than the effluent Cu concentration. The reason of this observation is the much lower log Ksp value of CuS (−35.1) compared to that of ZnS (−23.8). Therefore, it is much easier to precipitate Cu compared to Zn using biogenically produced sulfide [24].
Similar to previous studies [11, 24], although sulfide concentration in the reactor was very high, Zn and Cu concentrations were still in detectable levels (Fig. 6). The reason for this observation may be the formation of small metal-sulfide particles at high sulfide concentrations that can pass through the 0.45-μm membrane filter [25].
In addition to soluble metal, total metal concentrations were followed at each compartment of the reactor. Total Cu concentrations were similar to soluble Cu concentrations, which showed that CuS particles settled in the reactor efficiently. Slightly high total Zn concentrations (between 5 and 10 mg/L) were observed in Compartment 1, whereas the concentrations decreased as water passed through the compartments and effluent total Zn concentration was always lower than 2 mg/L. Hence, CuS particles were much easily settled compared to ZnS particles. Similarly, Sahinkaya et al. [22] reported that CuS particles settled much better than the ZnS particles as the mode of particle size distribution of ZnS and CuS precipitates was around 17 and 46 μm, respectively.
Another important point is that more than 99% of the precipitated metals were accumulated in the first compartment. Hence, precipitated metals can directly be recovered from Compartment 1 without losing high amount of SRB. After drawing sludge from Compartment 1 for metal recovery, the compartment can be reseeded from other compartments, without adversely affecting the reactor operation. For other types of reactors, such as fluidized-bed reactor, recovering metal-sulfide precipitate may cause losing high amount of SRB, which may adversely affect the reactor operation [13]. Hence, ABR has a high application potential for the sulfidogenic biotreatment of AMD and metal recovery.
In order to verify that the main metal removal mechanism in the reactor was precipitating them with biogenically produced sulfide, sludge samples were collected from Compartment 1 for elemental analysis of precipitate (day 245). XRF analyses showed that the sludge samples contained high amount of Zn, Cu and S, showing that sulfide was responsible for metal precipitation.
Electron flow to sulfate reduction
Methane production was not observed throughout the study as the reactor was previously fed with lactate containing synthetic AMD at COD/SO4 2− mass ratio of 0.67 for more than 300 days [11]. High sulfide concentrations are more toxic to methanogenes compared to SRB [26]. The electron flow from organic oxidation to sulfate was on average 87.3 ± 14% (Fig. 7a). The rest of the electrons were most probably used for biomass growth as previous studies [6, 20] showed that 0.05–0.15 mg VSS produced per mg sulfate reduced depending on the reactor configuration and operational conditions. Similarly, Bayrakdar et al. [11] reported that the percent electron flow to sulfate reduction was 86 ± 11% in a lactate-fed sulfidogenic ABR. Sahinkaya [6] reported this value as 83% in a sulfidogenic CSTR treating acidic and Zn-containing wastewater. Kaksonen et al. [20] reported the average electron donor utilized for sulfate reduction as 76 ± 10% in a mesophilic ethanol-fed FBR.
Sulfide balance
The sulfate reduction rate, total sulfide production rate and sulfide recovery over the entire course of the experiment were illustrated in Fig. 7b. Produced total sulfide (soluble sulfide + precipitated sulfide as ZnS and CuS) was calculated assuming 1 mmol sulfide was used to precipitate 1 mmol metal according to the following reaction [11]:
The recovery of sulfide was calculated using the following equation:
The sulfate reduction rate was slightly higher than total sulfide production rate and the sulfide recovery averaged 83 ± 10% (Fig. 7b). In a previous study, Bayrakdar et al. [11] reported the sulfide recovery as 70%. In the present study, there was around 17% uncoupling between sulfate reduction and total sulfide production, which was most probably due to the chemical oxidation of sulfide to elemental sulfur within the reactor, volatilization of sulfide to the gas phase or partial loss of sulfide during the sampling and measurement.
Results of this study showed that sulfidogenic ABR is a powerful alternative for AMD treatment and metal recovery. However, the effluent may not be suitable for the discharge due to high sulfide concentration. Direct and indirect biological methods can be used for the removal of sulfide from liquid or gas streams of the sulfidogenic reactor. In the direct approach, sulfide oxidizing bacteria use sulfide as electron donor and convert it to sulfur. Oxygen, nitrate or nitrite can be used as terminal electron acceptors for sulfide oxidation. In the indirect method, ferric iron is used to oxidize sulfide to sulfur, and iron oxidizing bacteria are used to regenerate ferric iron [27].
Conclusions
Ethanol-supplemented ABR has a high application potential for the treatment of acidic and metal-containing AMD. The sulfate reduction and COD oxidation efficiencies were between 60–85 and 80–95%, respectively. Decreasing feed pH to 3.0 and increasing total metal concentrations to 500 mg/L did not adversely affect the reactor performance and sufficient alkalinity was produced to increase the effluent pH to neutral values. Throughout the study, more than 99% metal precipitation was observed. The accumulation of precipitated metals in the first compartment allows metal recovery without disturbing reactor operation seriously. Approximately 87% of the electrons were channeled to sulfate reduction.
References
Foucher S, Battaglia-Brunet F, Ignatiadis I, Morin D (2001) Treatment by sulfate reducing bacteria of Chessy acid mine drainage and metal recovery. Chem Eng Sci 56:1639–1645
Nagpal S, Chuichulcherm S, Livingston A, Peeva L (2000) Ethanol utilization by sulfate reducing bacteria: an experimental and modelling study. Biotechnol Bioeng 70:533–543
Nagpal S, Chuichulcherm S, Peeva L, Livingston A (2000) Microbial sulfate-reduction in a liquid-solid fluidized bed reactor. Biotechnol Bioeng 70:370–380
Bechard G, Yamazaki H, Gould WD, Bedard P (1994) Use of cellulosic substances for the microbial treatment of acid mine drainage. J Environ Qual 23:111–116
Szczepanska J, Twardowska I (1999) Distribution and environmental impact of coal-mining wastes in Upper Silesia, Poland. Environ Geol 38:249–258
Sahinkaya E (2009) Biotreatment of zinc-containing wastewater in a sulfidogenic CSTR: performance and artificial neural network (ANN) modelling studies. J Hazard Mater 164:105–113
Kaksonen AH, Franzmann PD, Puhakka JA (2003) Performance and ethanol oxidation kinetics of a sulfate-reducing fluidized-bed reactor treating acidic metal-containing wastewater. Biodegradation 14:207–217
Kaksonen AH, Riekkola-Vanhanen ML, Puhakka JA (2003) Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater. Water Res 37:255–266
Johnson DB (2000) Biological removal of sulphurous compounds from inorganic wastewaters. In: Lens PNL, Hulshoff Pol L (eds) Environmental technologies to treat sulphur pollution, principles and engineering. IWA Publishing, London, pp 175–205
Widdel F (1988) Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 469–585
Bayrakdar A, Sahinkaya E, Gungor M, Uyanik S, Atasoy AD (2009) Performance of sulfidogenic anaerobic baffled reactor (ABR) treating acidic and zinc-containing wastewater. Bioresour Technol 100:4354–4360
Kaksonen AH, Puhakka JA (2007) Sulfate reduction based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng Life Sci 7:541–564
Gallegos-Garcia M, Celis LB, Rangel-Mendez R, Razo-Flores E (2009) Precipitation and recovery of metal sulfides from metal containing acidic wastewater in a sulfidogenic down-flow fluidized bed reactor. Biotechnol Bioeng 102:91–99
Uyanik S, Sallis PJ, Anderson GK (2002) The effect of polymer addition on granulation in an anaerobic baffled reactor (ABR). Part I. Process performance. Water Res 36:933–943
Uyanik S, Sallis PJ, Anderson GK (2002) The effect of polymer addition on granulation in an anaerobic baffled reactor (ABR). Part II. Compartmentalization of bacterial populations. Water Res 36:944–955
She Z, Zheng X, Yang B, Jin C, Gao M (2006) Granule development and performance in sucrose fed anaerobic baffled reactors. J Biotechnol 122:198–208
Cord-Ruwisch R (1985) A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate reducing bacteria. J Microbiol Meth 4:33–36
APHA (1999) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC
Alvarez MT, Crespo C, Mattiasson B (2007) Precipitation of Zn(II), Cu(II) and Pb(II) at bench-scale using biogenic hydrogen sulphide from the utilization of volatile fatty acids. Chemosphere 66:1677–1683
Kaksonen H, Franzmann PD, Puhakka JA (2004) Effects of hydraulic retention time and sulfide toxicity on ethanol and acetate oxidation in sulfate-reducing metal-precipitating fluidized-bed reactor. Biotechnol Bioeng 86:332–343
Omil F, Oude Elferink SJWH, Lens P, Hulshoff Pol L, Lettinga G (1997) Effect of the inoculation with Desulforhabdus amnigenus and pH or O2 shocks on the competition between sulphate reducing and methanogenic bacteria in an acetate fed UASB reactor. Bioresour Technol 60:113–122
Sahinkaya E, Özkaya B, Kaksonen AH, Puhakka JA (2007) Sulfidogenic fluidized-bed treatment of metal-containing wastewater at low and high temperatures. Biotechnol Bioeng 96:1064–1072
Vallero MVG, Sipma J, Lettinga G, Lens PNL (2004) High-rate sulfate reduction at high salinity (up to 90 mS/cm) in mesophilic UASB reactors. Biotechnol Bioeng 86:226–236
Sahinkaya E (2009) Biotreatment of zinc-containing wastewater in a sulfidogenic CSTR: performance and artificial neural network (ANN) modelling studies. J Hazard Mater 164:105–113
Veeken AHM, Akoto L, Hulshoff Pol LW, Weijma J (2003) Control of the sulphide concentration for optimal zinc removal by sulphide precipitation in a continuously stirred tank reactor. Water Res 37:3709–3717
Hulshoff Pol LW, Lens PNL, Stams AJM, Lettinga G (1998) Anaerobic treatment of sulfate-rich wastewaters. Biodegradation 9:213–224
Tang K, Baskaran V, Nemati M (2009) Bacteria of the sulphur cycle: an overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem Eng J 44:73–94
Acknowledgments
This study was funded by the Scientific & Technological Research Council of Turkey (TÜBİTAK project no. 108Y036).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahinkaya, E., Yucesoy, Z. Biotreatment of acidic zinc- and copper-containing wastewater using ethanol-fed sulfidogenic anaerobic baffled reactor. Bioprocess Biosyst Eng 33, 989–997 (2010). https://doi.org/10.1007/s00449-010-0423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-010-0423-9