Abstract
Cancer remains a leading cause of death in the United States and other developed countries. In nearly all cases, the cause of death is related to complications associated with tumor metastasis to distant sites such as the brain, lung, liver, and bone. A central feature of tumor progression is the acquisition of a blood supply, which provides nutrients for the growing tumor as well as conduits for transport of cancer cells. Our understanding of how a tumor acquires and manipulates a blood supply has been gleaned largely from animal models, but more recent advances in tissue engineering and microfabrication have led to clever 3D in vitro models of tumors that include blood vessels. This chapter will first briefly review the process of blood vessel growth including our knowledge of blood vessels within the cancer microenvironment, and discuss the most recent advances to mimic blood vessel growth in the tumor microenvironment using 3D in vitro culture methods. Finally, we discuss several important factors that control blood vessel growth including hypoxia, cellular metabolism, and tissue mechanics, which provide rich opportunities for future investigation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Angiogenesis, the process of vessels sprouting from existing blood vessels, is a critical event in numerous pathophysiological processes, including embryogenesis, wound healing, inflammation, diabetes, and cancer. Early growth of a neoplastic tissue engenders a metabolic deficit (e.g., glucose and oxygen) that limits growth. Compensatory mechanisms that permit additional growth include changes in metabolism and the acquisition of a blood supply. The access to vasculature allows tumors to disseminate cancer cells to distant organ sites, leading to the formation of metastatic lesions. The hypothesis that angiogenesis is required for tumor growth was first proposed by Folkman in 1971 [1]. Thereafter, there has been reaffirmation of this concept by independent groups [2,3,4,5]. As a result, anti-angiogenic therapy is part of the treatment regimen for some types of solid cancers including breast, lung, and renal cancers.
The biology of angiogenesis within the tumor microenvironment has been largely studied in either mouse models or simple 2D monolayer culture systems (Fig. 1). Although these classical model systems have provided a wealth of understanding, they have limitations and there remains a need for novel model systems that can further improve our understanding of angiogenesis. Animal models offer a complex tissue environment compared to 2D cell cultures, but the biology of these animal models is simply not human. A more recent model used to study cancer biology is the patient-derived xenograft (PDX) in which a human tumor is implanted either subcutaneously or orthotopically in an immunocompromised mouse. The major limitation in the subcutaneous model is that it lacks important features of the original tumor microenvironment. In contrast, orthotopic tumor xenografts are implanted at the original tumor site and are considered more reliable for studying the biology or predicting drug response in humans. Nonetheless, in both cases the tumor xenografts are surrounded by non-human tissue stroma, developed in the absence of a normal immune response, and take several months to grow [6, 7]. Furthermore, the animal models are expensive to maintain, are not high-throughput, provide barriers to some imaging technologies, and provide inherently limited spatial and temporal resolution. The 2D culture systems, on the other hand, are much easier to develop and utilize. However, these systems lack the fundamental complexity of the multicellular 3D tissue microenvironment. These limitations have led to the development of 3D culture systems, which are positioned between 2D and animal models in terms of advantages and limitations (Fig. 1).
Advantages and disadvantages of tumor model systems. Experimental model systems of the tumor microenvironment (TME) broadly include four categories: (a) traditional 2D monolayer cell culture, (b) 3D in vitro multicellular models, (c) animal models, and (d) human models. While simple and inexpensive, 2D monolayer culture cannot replicate the essential heterotypic cell-cell and cell-matrix interactions that have proven essential in the biology of the TME. Animal models can replicate the integrated response of the whole animal, but many times the immune system is compromised to allow the study of human cells and the temporal and spatial resolution of the TME is severely limited. While humans represent the “perfect” system, studies are limited to more advanced cancers, and most interventions, including genetic manipulation, are not possible. Advanced 3D in vitro multicellular models allow the step-by-step incorporation of key components in the TME, and high spatial and temporal resolution of dynamic events. While it may be difficult to fully recapitulate the TME in vitro, new advances in microfabrication and imaging provide opportunities to tease apart complex cell-cell and cell-matrix dynamics in the TME. The arrows linking the models are purposely two-way as observations made in one system can answer questions, but also generate new hypotheses that can be tested or confirmed in alternate systems. While all four approaches are important, a 3D in vitro model can provide unique opportunities to study angiogenesis in the tumor microenvironment
The idea of mimicking 3D tissue function in vitro is not new in cancer research; in fact, tumor spheroids were first presented nearly four decades ago [8]. Since then, the model systems have evolved from 3D tumor spheroids comprised solely of cancerous cells to tumor spheroids composed of a mixture of cancer and stromal cells, vascularized tumors, and more recently perfused vascularized tumors [8,9,10]. Despite the recent progress that has been made in the field using conventional biological models, we still do not have a complete picture of the impact of microenvironmental factors that dictate the formation and maintenance of cancer-associated blood vessels. Herein we provide a limited discussion of the biological pathways of angiogenesis; unique features of vessels within the cancer microenvironment; the important roles of hypoxia, cellular metabolism, and mechanics on tumor angiogenesis; and tumor metastasis as a backdrop to understand how the creation of in vitro 3D tumor organoids can be used to further augment our understanding of tumor angiogenesis and its role in tumor progression.
2 Cell Signaling Pathways in Tumor Angiogenesis
The growth of blood vessels from existing blood vessels to meet the metabolic needs of a tumor defines tumor angiogenesis (Fig. 2a) [11]. The important pro-angiogenic factors involved in cancer are vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and angiopoietin-1 (ANG1) [12]. These pro-angiogenic factors activate otherwise quiescent vessels and “turn on” the so-called angiogenic switch. VEGF has been extensively studied for how it activates the angiogenic program [12]. Vessel exposure to proangiogenic factors leads to vasodilation and increased vascular permeability. The endothelial cells lining the vessel degrade the basement membrane by proteolytic activity of matrix metalloproteinases (MMP). The endothelial cells detach and organize into a new branch in which the endothelial cell leading the branch is called a tip cell and the endothelial cells following are called stalk cells (Fig. 2b). The tip cells sense gradients of morphogens and guide the direction of the new sprout. The tip cell restricts the stalk cells from transforming into other tip cells by secreting delta-like ligand 4 and signaling through the NOTCH-mediated pathway. The stalk cells, on the other hand, are responsible for proliferating and forming continuous extension branching from the original vessel. Eventually vessel junctions are reestablished, proteolytic activity is neutralized, the basement membrane is synthesized, and pericytes are recruited to stabilize the nascent vessels. The processes that stabilize the vessels are conducted by numerous molecular pathways mediated by platelet-derived growth factor B (PDGF-B), ANG-1, transforming growth factor-β (TGF-β), ephrin-B2 and NOTCH. The WNT signaling pathway also plays a role in tumor angiogenesis [13, 14]. Endothelial cells express an array of WNT ligands and their frizzled (FZD) receptors, some of which are essential for stimulating endothelial cell proliferation [14]. One of the regulators of WNT signaling is β-catenin. Activation of WNT/β-catenin signaling can induce numerous tumor growth genes including Myc, Axin2, and Zeb1 in vivo. In contrast, abnormal β-catenin activation and signaling has been associated with solid stress from tumor masses [13, 14].
Angiogenesis in the tumor microenvironment. (a) Angiogenesis is required to promote tumor growth. The angiogenesis in tumors of mice was captured using optical frequency domain imaging (OFDI) technique. This high resolution microscopy shows tumor associated vasculature is dense and unorganized compared to surrounding non-tumor tissue. The red and yellow indicate the depth of the tissue and blue indicates lymphatics. Scale bar = 500 mm (reprinted with permission [11]). (b) Numerous signaling factors, including soluble growth factors and membrane bound receptors, integrins, and junction proteins, play a role in the development of vasculature during tumor progression. Such signaling is regulated both via tumor cells as well as vascular cells, including the tip cell of blood vessels undergoing angiogenesis
Another important cell signaling pathway that regulates vessel integrity is the angiopoietin (ANG) and TIE signaling system; this pathway stimulates basement membrane deposition to promote vessel tightness [15, 16]. However, dysregulation of ANG and TIE signaling in sprouting endothelial cells can lead to vascular permeability, inflammation, and defects that allow for tumor metastasis [17]. Finally, p21-activated kinases (PAKs) alter RhoGTPase signaling, causing irregular actomyosin contractility and actin dynamics, and altered cell motility and permeability in endothelial cells [18, 19]. Furthermore, work by Ghosh et al. has shown that endothelial cells in the tumor microenvironment demonstrate aberrant Rho activity and fail to respond to mechanical strain in the same manner as normal endothelial cells [20]. In addition to altering cell motility and permeability, disruption of actin dynamics also changes contractile forces and tension in cells, resulting in changes to the transcriptional regulators YAP and TAZ [21,22,23]. Studies are beginning to investigate the exact mechanisms that YAP and TAZ have on endothelial cells in cancer-associated blood vessels, but some preliminary work reveals that translocation of YAP/TAZ to the nucleus can upregulate the expression of target genes such as connective tissue growth factor (CTGF) and can lead to an increase in monocyte adhesion to endothelial cells [21].
3 Organoid Systems to Model Angiogenesis
The early 3D systems were mainly devoted to create vessels to study activation of angiogenic programs. In these systems, either a single suspension of endothelial cells or endothelial cell coated microbeads were embedded into extracellular matrix (ECM) gels, such as collagen, fibrin, or Matrigel [24, 25]. These gels were placed in micro-well plates or similar assemblies to generate a 3D culture system. These approaches have successfully yielded capillary microvessels with lumens, but these systems have several limitations. The vasculature was not designed for perfusion of fluid through the vascular lumen, the vasculature formed was not stable over time, and creating controllable temporal and spatial concentration gradients around the vasculature is difficult.
The rapidly emerging “organ-on-a-chip” field utilizes tissue engineering and microfabrication to create in vitro microtissues in platforms that are optically clear, cost-effective, have high spatial and temporal resolution, can capture events in real time, and have the potential to be high-throughput. These systems are proving critical for uncovering novel aspects of angiogenesis. The general method to fabricate the organ-on-a-chip platform begins with the computer-aided design of the device. A mask is printed from these designs, and used to create a master mold. The master mold is fabricated using soft lithography techniques, in which a silicon vapor coated with photoresist, such as SU8, is covered with the mask and exposed to UV. The UV light polymerizes the exposed area of photoresist, which is the area of device design on the mask. The non-polymerized photoresist is etched, and the master mold is used to create numerous replicas of the device design using polymers such as polydimethylsiloxane (PDMS). The devices made of PDMS are ideal for many cell culture applications, including tumor organoids, as they are permeable to oxygen, transparent, and have a similar refractive index to glass to facilitate optical imaging.
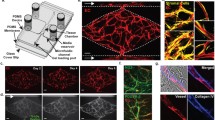
We and others have developed several microfluidic platforms that capture features of the human microcirculation (Fig. 3) [26,27,28,29,30,31,32]. A critical feature of the microcirculation is the hollow capillary structure through which blood or a blood substitute can flow. The studies use mainly two approaches to create these structures. The first approach is lining a conduit with endothelial cells. Some studies first create a perforated rectangular shaped conduit in PDMS, and coat one side or all sides of the conduit with endothelial cells [30]. Others have used a more sophisticated approach of creating a cylindrical conduit in an ECM gel (i.e., collagen or fibrin) and then coat the lumen of the cylinder with endothelial cells [27, 31]. Once the endothelial cells adhere to the wall of the conduit and spread they form tight junctions with physiological permeability coefficients [27]. This method generally creates large diameter (>100 μm) endothelial cell-lined tubes [27, 31, 32]. These endothelial-lined tubes can then be exposed to various levels of concentration gradients, which are created by using additional microfluidic lines. There are several creative designs to establish concentration gradients using microfluidics. These device designs initiate angiogenesis in response to physiological concentration levels of VEGF, bFGF, and several other proangiogenic factors or cocktails of factors [27, 31, 32]. As the endothelial cells are coated, a challenging task is to maintain the density of endothelial cells and shape of the tube to accurately mimic the in vivo vasculature.
In vitro blood vessels created by coating microfluidic channels with endothelial cells. (a1) Device design for coating endothelial cells on the surfaces of the PDMS and collagen gel. (a2) The endothelial cells (green) form monolayer in the device, and (a3) angiogenesis is observed from 2D coat into 3D collagen gel (reprinted with permission from [30]. (b1) Device design to create hollow tubes of circular cross section in collagen gel by using silicon master mold. The surface of the collagen tube are coated with endothelial cells to form a vessel. (b2) These vessels stained with endothelial specific CD31 (red) sprout and (b3) exhibit barrier function as they retain dextran in the lumen (green). Scale bar in b2 and b3 shows 100 μm (reprinted with permission from [27]). (c1) The channels in the hydrogel were developed by using viscous finger patterning and then (c2) endothelial cells (green) were coated on the hollow structures. (c3) These vessels show angiogenic response (red) to VEGF concentration gradients. Scale bar in c2 and c3 show 500 and 50 μm, respectively (reprinted with permission [32]). (d1) PDMS device in which a cylindrical tube is created in collagen gel using needle of 400 μm diameter. (d2) The gel cylinder was coated with endothelial cells (green). (d3) When the vessels exposed to angiogenic factors show angiogenesis. Scale bars in d2 and d3 are 100 μm. Scale bar in the insert of d3 is 50 μm (reprinted with permission from [31])
The second approach to generate microvessels follows the developmental process of vasculogenesis, in which the endothelial cells are encouraged to self-assemble into capillaries (Fig. 4). In this method, the microvessels are produced from endothelial cells and stromal cells that are initially randomly distributed in an ECM. The stromal cells are a necessary component as they secrete factors necessary to support vessel formation, in particular tube formation and stabilization [33,34,35]. Our lab has shown that cord blood-derived endothelial cells and human lung fibroblasts in a fibrin gel generate dynamic, interconnected, and perfusable networks of microvessels [29]. When implanted in the mouse, the microvessels anastomose to mouse vasculature and become functional [36, 37].This co-culture system provides a more physiologic alternative, compared to assays using only endothelial cells [27, 31, 32], to mimic in vivo angiogenesis. Moreover the physical dimensions, including diameter, of the microvasculature formed by our assay resembles that of the in vivo microvasculature (<50 μm).
Microfluidic systems to create in vitro vascular networks by vasculogenesis process. (a1) Device design for creating endothelial network from randomly distributed endothelial cells and normal lung fibroblasts in fibrin gel. (a2) The endothelial cells (green) form interconnected network of vessels (reprinted with permission from [29]). (b1) One approach to connect the vessels with the fluidic lines of PDMS device is to coat the fluidic lines with endothelial cells. (b2) The endothelial cells in the fluidic line connect with the vessel network in the gel forming a perfused network of vessels (reprinted with permission from [35]). (c1) Microfluidic platform design used to create vascular network from bone marrow derived mesenchymal stem cells and endothelial cells in fibrin gel. (c2) The micrograph shows endothelial network in this device (red). The scale bar is 200 μm (reprinted with permission [28]). (d1) Microfluidic platform used for co-culture of endothelial cells and pericytes in fibrin gel. (d2) The system supports formation of endothelial network (red) formation with pericyte coverage (green). The scale bar is 100 μm. The figure is reprinted from [26], and is covered under Creative Commons Attribution (CC BY) license
Vasculogenic vessel formation has also been shown to be facilitated by other types of stromal cells, such as bone marrow derived stromal cells [28]. In the context of tumor angiogenesis, such a system could be of interest, as bone marrow is one of the most frequent metastatic sites for multiple types of solid tumors, including breast, and colon cancers. Our lab has been working to develop microfluidic systems to place tumors in the immediate vicinity of perfused microvascular tissue developed by this vasculogenic process [38]. These systems are designed to recapitulate microenvironment of early and advanced tumors and study angiogenesis in response to the microenvironmental perturbations described in the following sections.
4 Hypoxia and Tumor Angiogenesis
4.1 Overview
The deficiency of oxygen, an essential nutrient for cell proliferation and survival, is a critical stimulus for acquiring new blood vessels. Hypoxic tumors activate molecular programs that lead to secretion of proangiogenic factors by the tumor as well as tumor-associated stromal cells. Tumor hypoxia has been associated with poor patient prognosis, with clinical studies showing that advanced breast cancers have a median oxygen tension of 10 mmHg, compared with 65 mmHg in normal breast tissue [39,40,41]. Hypoxic cores exist in advanced stage tumors [42], and can also exist in tumors as small as 400 μm in diameter [43]. In hypoxic conditions, angiogenesis is primarily regulated by hypoxia inducible factors (HIFs). Of the highly conserved HIF family of transcription factors, HIF-1 has been the best studied [44,45,46]. It is known to be a heterodimer of α-subunit (HIF-1α) and a β-subunit (HIF-1β), where subunits are members of the basic helix-loop-helix (bHLH)-containing PER-ARNT-SIM (PAS) domain family of transcription factors. In addition to HIF-1α and HIF-1β, there are two additional oxygen regulated α-subunits are (HIF-2α and HIF-3α) and two other constitutively expressed β-subunits (HIF-2β, and HIF-3β). Furthermore, the low oxygen environment stabilizes HIF-1α in endothelial cells as well [47].
The HIF-1 activation follows a series of molecular events. Starting at oxygen concentrations below 6%, HIF-1α stabilizes and translocates from the cytoplasm to the nucleus, where it dimerizes with HIF-1β [48]. HIF-1 then binds to hypoxia responsive elements (HREs) within the promoters of HIF target genes leading to the increased expression of proangiogenic factors such as vascular endothelial growth factor (VEGF), VEGF-R2, angiopoietin 1/2, fibroblast growth factor, platelet-derived growth factor, and the decreased expression of anti-angiogenic factors such as thrombospondin-1 and carbonic anhydrase-9 [49]. In addition to angiogenesis, HIF-1 can activate more than a hundred genes that control important cellular processes such as epithelial-mesenchymal transition, stem-cell maintenance, and metabolism that impact tumor cell invasion, metastasis, metabolic reprograming, and resistance to therapy [4].
4.2 Controlling and Measuring Oxygen In Vitro
The traditional method to create hypoxic conditions utilizes cell culture incubators, where blending excess nitrogen with air lowers oxygen concentration. Alternatively, the exchange of oxygen from air can be controlled by an air tight glove box equipment, or hypoxic conditions can simply be generated due to consumption of oxygen by cell culture. Additionally, chemicals that consume oxygen, such as sodium nitrate, can also be used to manipulate oxygen tension [50]. Alternatively, cobalt chloride can stabilize HIF-1α in the presence of normoxia, and allows for more flexible data collection. This “pseudo-hypoxic” condition can simulate the impact of HIF-1α, but cannot fully recapitulate all features that hypoxia has on cell function [51].
Microfluidic devices have become attractive systems to study hypoxia due to their inherently small size, and thus small diffusion distances. A common technique to reduce oxygen in microfluidic devices is to use separate channels containing an oxygen scavenger such as sodium nitrate (Fig. 5). These channels are separated from the tissue chambers by a semipermeable material, such as PDMS, that allows diffusion of oxygen but not water [52,53,54]. By altering the concentration and flow of the scavenger, the oxygen tension within the device can be controlled with high spatial and temporal resolution. PDMS is ideally suited as a material of construction for these device as it is a highly permeable material with respect to oxygen compared to relatively impermeable materials such as cyclic olefin copolymer, polystyrene, polypropylene, poly(methacrylic acid), polyurethane, and poly(methyl pentene) [55]. By choosing an appropriate coating and/or using an oxygen scavenger, a wide range of oxygen concentrations can be controlled to study tumor hypoxia and its effects.
Manipulating and measuring oxygen concentrations in vitro. (a) Oxygen scavenger lines can be designed into microfluidic platforms to generate hypoxic conditions inside tissue chambers. Typically these include materials such as sodium sulfite. (b) PhLM is a method used to measure oxygen concentrations in 3D culture systems. Using a pulsed laser to excite the oxygen sensitive dye and measuring the dye’s lifetime of decay, a longer phosphorescent lifetimes correspond to lower oxygen concentrations
A major advantage of using in vitro systems is that real time oxygen measurements can be performed in a live tissue culture with minimal disruption of biological processes. The gold standard for the oxygen sensors is Clark-type electrodes which measure oxygen by detecting a current flow caused by the reduction of oxygen [56]. However, the method is operationally complex and less sensitive for oxygen measurement relative to other methods. Recently, more sensitive techniques have been developed that employs an oxygen sensitive luminophore. The luminescence of oxygen sensitive dyes is inversely proportional to the concentration of oxygen. When the dyes are excited by a laser in the presence of oxygen, the excited state energy of the phosphorescent indicator molecule is absorbed by oxygen instead of being emitted as a luminescent photon. In other words, oxygen quenches the phosphorescence, and reduces the lifetime of the phosphorescence decay. Generally, a shorter luminescence lifetime indicates a higher oxygen concentration. The lifetime of the phosphorescence, as opposed to the intensity, is a more robust method as it is insensitive to photobleaching and independent of the concentration of the dye. Detecting the luminescence lifetime generally requires a more complicated experimental setup because a pulsed laser needs to be used [57].
While many research groups have focused on controlling the oxygen environment around tumor spheroids, some groups, including ours, have begun to control oxygen tension in vascularized tumors [52, 53, 58]. Due to the role that the vascular network has in oxygen regulation and the interaction between the tumor and the vasculature during hypoxia, the inclusion of these components in the next generation of tumor organoid models is critical for a complete understanding of angiogenesis in the tumor microenvironment.
5 Cellular Metabolism and Angiogenesis in the Tumor Microenvironment
5.1 Overview
Endothelial cells act as a semi-permeable barrier between the circulating blood and various tissues. Being in direct contact with the blood, endothelial cells have the most ready access to the nutrients needed for healthy cell growth, including glucose, glutamine, and oxygen but are also responsible for delivering these nutrients to the surrounding tissue. Endothelial cells are able to balance their own metabolic needs and transport duties by executing a specific metabolic program that shares many similarities with cancer cell metabolism.
Endothelial cells are highly glycolytic and consume glucose at a high rate. Even during quiescence, endothelial cells generate more than 80% of their ATP through glycolysis alone [59, 60]. Glycolysis in endothelial cells tends to favor lactate as its end product, as less than 1% of pyruvate generated by glycolysis is oxidized in the tricarboxylic acid (TCA) cycle. By reducing the utilization of oxidative phosphorylation (OxPhos) and thus reducing the amount of consumed oxygen, they are able to more effectively deliver oxygen to the tissues.
When appropriate signals are received to form tip cells and induce angiogenesis, phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFK2 or PFKFB3) expression is upregulated (Fig. 6). PFK2 converts fructose-6-phosphate into fructose-2,6-bisphosphate (F2,6BP), a potent regulator of phosphofructokinase-1 (PFK1) which converts F6P into fructose-1,6-bisphosphate (F1,6BP), considered the first committed step in glycolysis. VEGF and fibroblast growth factor 2 (FGF2) have been shown to increase PFK2 expression and glycolysis. Kruppel-Like Factor 2 (KLF2), a transcription factor which responds to hemodynamic-induced stress on the EC glycocalyx, has been shown to reduce PFK2 expression in quiescent endothelial cells (De Bock et al.; Doddaballapur et al.; De Bock, Georgiadou, and Carmeliet). By upregulating PFK2 and increasing levels of F2,6BP, PFK1 activity and flux through glycolysis are both greatly increased. In many other cells, such as immune cells, this activation would lead to a 20- to 30-fold increase in glycolytic flux but only a twofold increase occurs in endothelial cells. The end products, lactate, can later be used as a mitochondrial fuel by other stromal cells or regenerated through gluconeogenesis after reaching the liver.
Endothelial cell metabolism in cancer associated angiogenesis. (a) In normal tissues, ECs in blood vessels demonstrate a quiescent phenotype, with limited glycolysis for energy production due to hemodynamic stress-induced stimulation of Kruppel-Like Factor 2 (KLF2). In tip cells, glycolysis is upregulated by several growth factors and downregulation of KLF2 by reduced flow also serves to increase glycolytic flux as ECs become activated to proliferate and develop new vasculature via angiogenesis. (b) Nanostructure Imaging Mass Spectrometry of rat brain sections b. to resolve metabolic differences by brain region (reprinted with permission from [62]). An initiator is used, similar in function to the matrix from MALDI techniques, to desorb and ionize metabolites before analysis by mass spectrometry. Each metabolite can be visualized in its own ion image, as shown to the right, which allows for label-free spatial tracking of multiple metabolites in a tissue sample
Similar to endothelial cells, most tumor cells are highly glycolytic even in the presence of oxygen, known as Warburg Metabolism, and have reduced OxPhos [61]. In contrast to endothelial cells, tumor cells have a high rate of proliferation, and consume large quantities of glucose [61]. The rapid use of glucose and excretion of lactate in the tumor microenvironment stimulates angiogenesis. In low glucose conditions, endothelial cells can utilize Fatty Acid Oxidation (FAO) and amino acid metabolism, especially glutaminolysis, to supplement their energetic and macromolecular needs. FAO catabolizes circulating triglycerides to create acetyl-CoA, which can then be used for energy production in the TCA or for lipid synthesis and also yields NADPH as a byproduct. Glutaminolysis catabolizes glutamine, whose concentration in tissues is typically 5 μM, yielding glutamate, which is then converted to α-ketoglutarate and can enter the TCA cycle. Glutamate can also act as a nitrogen source for amino acid or nucleotide synthesis and an NADH source. These alternative energy sources facilitate angiogenesis into the tumor microenvironment, which is typically hypoxic and low in nutrients necessary for proliferation.
5.2 Methods to Characterize Cellular Metabolism In Vitro
There are several techniques that have been utilized to study metabolism in 2D culture and are being adapted to study 3D tissues in vitro. Three promising options include the Seahorse Extracellular Flux (XF) Assay, Fluorescence Lifetime Imaging Microscopy (FLIM), and Mass Spectrometry (MS) based metabolomics analysis. Direct in vitro studies of metabolism in complex tissues, including organoids composed of multiple cell types, create many challenges compared to standard 2D metabolic analyses due to the heterogeneity of the system and the need to analyze multiple focal planes.
The SeaHorse XF assays are useful to characterize a broad metabolic phenotype (i.e., primarily glycolytic or OxPhos) using a microwell plate format. They employ a probe containing an embedded oxygen-sensitive fluorophore and an embedded proton-sensitive fluorophore to monitor minute changes in acidity and oxygen concentration which can then inform the oxygen consumption rate and the extracellular acidification rate. The former is characteristic of OxPhos whereas the latter is indicative of glycolysis with lactate as the terminal end product (Fan et al.). The XF assays were originally developed for 2D cell culture, but have since been adapted to analyze spheroids as well, allowing for the rapid profiling of organoids grown in spheroid plates [62]. This system has the advantage of being label free, high-throughput, customizable for reagent studies, and fully adapted to 3D assays. However, it measures only two of the tens of thousands of possible metabolites, and contains no spatial resolution.
Fluorescence Lifetime Imaging Microscopy (FLIM) utilizes confocal or multiphoton microscopy with rapidly pulsed lasers to detect the lifetime of endogenous fluorophores. FLIM can offer incredible temporal resolution, on the order of nanoseconds, of the chemical state of a system. In vivo and in vitro, NADPH, NADH, and FAD are fluorescent molecules with a myriad of functions tied to the metabolic state of the cell and the ratios of protein bound to unbound forms is indicative of cellular metabolism [63, 64]. FLIM is incredibly sensitive due to the natural sensitivity of the fluorophores to their local chemical environment; bound forms of these molecules show a significant increase in lifetime (for NAD(P)H, 3.2 ns) over their unbound, free solution forms (for NAD(P)H, 0.8 ns) and can thus be used to metabolically profile cells [65, 66]. More recently FLIM has been used in 3D tumor organoids to assess cell proliferation [67]. Because this is a microscopic technique, it also offers high spatial resolution, permitting insight into the subcellular ratios of each of these fluorescent molecules. NADH and NADPH are nearly identical spectrally, but there have been some inroads into differentiating their FLIM signals, which is an important distinction due to the distinct roles of NADH in energy production and NADPH in biomolecule production and redox state maintenance [68, 69]. FLIM, however, is limited to those metabolites which are inherently fluorescent, and requires relatively expensive equipment.
By far the most robust technique for studying metabolism is Liquid Chromatography-Mass Spectrometry (LC-MS). LC-MS allows for the detection and quantification of nearly the entire metabolome of a sample. In 2D cell culture, cells are grown in culture medium, fixed (usually with ice cold methanol), and the metabolites are extracted using a mixture of organic and aqueous solvents before being passed through a chromatography column and analyzed by mass spectrometry. The combination of LC-MS allows for the separation of metabolites based on both the retention time (LC) and mass to charge ratio (MS) that allows for high resolution detection and identification of each metabolite. By comparing the metabolomes or specific metabolites of two nearly identical samples grown in different conditions, known as differential metabolomics, enriched pathways dependent on these differences can be elucidated. In addition, isotope labeling allows for the tracking of metabolites through different pathways through the detection of strong isotope peaks and the use of metabolic flux analysis using isotopically labeled metabolites yields a more complete picture of the metabolic network. The main shortcoming of this technique is that this represents an “average” metabolome for the sample, so extending this technique to 3D tissues results in a total loss of spatial resolution and a lack of cell specificity. To ameliorate the loss of specificity, cell sorting techniques can be used although this also presents its own set of challenges and disadvantages in sample handling.
Mass Spectrometry Imaging (MSI) is a Matrix Assisted Laser Desorption/Ionization (MALDI) variant that uses a laser and specific analyte preparation to desorb and ionize metabolites from finely sectioned tissues before running typical mass spectrometry and analysis. The details of MSI preparation and analysis are active areas of research and have been recently reviewed [70]. However, the result of an MSI experiment recreates the metabolome at each point with sub-micrometer resolution while retaining the spatial organization of the analyzed tissue [62]. By analyzing adjacent tissue sections histologically, metabolic differences between adjacent cells are resolvable as well. Very recently, MSI has been used to investigate tumor organoids in 3D to investigate topics such as drug delivery/penetration and the impact of hypoxia [71,72,73].
All three techniques outlined here have inherent strengths and weaknesses. Both XF and FLIM techniques can repeat measurements on the same sample and require little preparation, but offer somewhat limited information about the system, while LC-MS and MSI offer more complete information, but require more time, effort, and preparation to execute and analyze and are terminal experiments. As this is still a developing field of research, future advances may offer greater ease or scope for these techniques or new techniques altogether but metabolic studies of complex 3D tissues are finally possible. Being able to analyze cellular metabolism in tumor organoids will create a more complete picture of tumor angiogenesis.
6 Biomechanics in Tumor-Associated Angiogenesis
6.1 Overview
In addition to changes in metabolism and oxygen tension, biomechanical forces can regulate angiogenesis [74, 75]. Physical forces, including extracellular matrix (ECM) stiffness, compressive/contractile forces exerted due to cell proliferation and other cellular activities, and fluid forces exerted by blood and plasma flow have all been shown to be critical regulators of angiogenesis (Fig. 7) [76,77,78,79,80,81,82]. The composition, and therefore effective stiffness, as well as the organization of the ECM surrounding the tumor also plays a role in mechanically regulating angiogenesis [83,84,85,86,87,88]. Increased peritumoral ECM stiffness correlates with increased potential for angiogenesis and metastasis. Furthermore, both tumor cells and endothelial cells can actively remodel the ECM to facilitate enhanced angiogenesis as well as promote metastasis. Compressive forces in the tumor microenvironment are a result of the unchecked proliferation of tumor cells that are constrained by surrounding ECM and stromal cells [14, 89]. Finally, at the cellular level, contractile behaviors of cells in the tumor microenvironment can alter angiogenesis and tumor progression [90,91,92,93]. Enhanced mechanosignaling from cancer-associated fibroblasts (CAFs) and tumor cells alter ECM alignment and remodeling, promoting both enhanced angiogenesis and tumor cell metastasis [94,95,96,97,98,99]. Recent work has demonstrated that paracrine regulation to limit the number of tip cells occurs through the Notch pathway, which has been shown to be mechanosensitive [100, 101]. Furthermore, the shift from quiescent vascular endothelial cell to migrating tip cell mimics the phenotypic change seen in epithelial to mesenchymal transformation (EMT). In both cases cell-cell junction proteins are dramatically downregulated and cells become more migratory. Several groups have demonstrated that EMT is regulated through active biomechanical forces including cell-cell tension and contractile forces [92, 102,103,104].
Macromechanical forces in tumor progression. Typically, three different types of forces are considered important in tumor progression. Interstitial flow (red) generated by developing vasculature within the tumor promotes vessel growth at the periphery of the mass. Compressive forces and strains (purple) increase as tumor cells divide uncontrollably, putting pressure on the surrounding ECM. Finally, ECM surrounding the tumor can apply compressive forces (green) as more ECM is deposited by tumor and stromal cells, effectively creating a dense capsule of tissue containing the tumor mass
Endothelial cells are also exquisitely sensitive to fluid flow including intraluminal flow, interstitial flow over and around the basolateral surface, and intercellular (transmural) flow between cell junctions. While interstitial flow of plasma can enhance angiogenic signaling in endothelial cells as well as invasive pathways in tumor cells, luminal flow of blood through vessels limits the angiogenesis [30, 105, 106]. Importantly, the leaky and tortuous nature of blood vessels in the tumor microenvironment impacts the magnitude and variance of all of these flows.Luminal flow exerts shear stress that regulates nitric oxide production by endothelial cells, which in turn limits endothelial cell sprouting and angiogenesis [30]. The interstitial flow of plasma leaks across the capillary wall and is reabsorbed in post capillary venules. Thus, the interstitial flow exerts transmural shear forces on the endothelial junctions, pressure forces from apical to basal, and basal to apical sides of the endothelial tube. The transmural flow has been shown to facilitate angiogenesis in a shear stress dependent manner [107]. Transmural flow is characterized by Starling’s Law, in which the driving force is the arithmetic sum of hydrostatic and oncotic pressure differences across the wall of the vessels. The transmural flow exerts shear stress, the magnitude of which not only depends on the flow across the vessels but also on the size of vessel perforations. Interestingly, the vessel perforations in organs throughout the human body vary, indicating differential potential of organs for angiogenesis in response to transmural flow. The basal to apical interstitial flow, like that in post-capillary venules, has been shown to activate and directionally guide angiogenesis [108]. However, apical to basal flow, like that in capillaries, does not activate angiogenesis, indicating the direction of flow is important in activating the angiogenic program (Fig. 8).
Angiogenesis models for studying metastasis. (a) A microfluidic device that simulates features of intravasation includes a collagen filled central chamber with HT1080 fibrosarcoma cells (red) migrating towards an endothelial cell (green) lined fluidic line. Scale bar 30 μm (reprinted with permission from [155]). (b) An alternate 3D model of intravasation includes a spheroid of cells comprised of both endothelial cells (red) and SW620 colon carcinoma cells (green) embedded in a collagen gel that contains fibroblasts. Microvessels sprouts from the spheroid and cancer cells intravasate and migrate within the vessel lumen. Scale bar 100 μm (reprinted with permission from [9]). (c) A model of tumor cell extravasation using a similar microfluidic device as in (a). Here endothelial cells line a fluidic channel (gray) and a collagen gel (green) is placed adjacent to the abluminal surface at specified regions. Tumor cells (breast cancer, MDA-MB-231) are introduced through the microfluidic channel, and evidence of extravasation is demonstrated (white arrow). Red label is VE-cadherin, and blue is the DAPI stained nuclei of the endothelial cells (reprinted from [160] and is covered under Creative Commons Attribution (CC BY) license)
Finally, tumor cells themselves can sense shear stress, leading to changes in the production of soluble mediators that directly impact angiogenesis such as VEGF, HIF1, and matrix metalloproteinase 9 [109, 110]. The shear forces are transmitted in cells by several mechanisms, including integrin signaling pathways and surface glycocalyx signaling. Integrins on the surface of endothelial cells attach to the ECM surrounding the cells and begin the formation of focal adhesions (FAs) inside the cell. Over 200 proteins have found to be associated with FA formation and many of these or their downstream effectors are either mechanosensors or mechanoregulators. A full review of FA and glycocalyx signaling in tumor-associated angiogenesis is outside the scope of this book chapter but can be found in other excellent reviews [81, 111,112,113,114,115,116].
6.2 Angiogenesis Models to Elucidate the Impact of Mechanical Forces
Early understanding of biomechanical pathways involved in tumor-associated angiogenesis came exclusively from 2D monolayer cultures, either on uncoated or ECM-protein coated tissue culture plastic or glass. Another level of complexity in 2D monolayers is derived when polyacrylamide (PA) gels are functionalized with collagen or fibronectin before cells are seeded onto the substrate [117,118,119]. The advantage of this system is that the PA gels can be synthesized over a wide range of stiffness values with great control. ECM proteins, either collagen or fibronectin, can then be covalently bonded to the PA gels permitting cell culture. Numerous groups have used these gels to probe the mechanoregulation of cell behavior including FA formation and regulation of cell contractile behavior. The disadvantage of this widely used system is that it is limited to 2D studies. Recently, work from the Takyama lab has utilized this protocol in conjunction with PDMS-soft lithography and 3D collagen gels to generate hybrid platforms that allow for spatial control over cell seeding with the benefit 3D cell culture in ECM-based gels [120]. The 2D PA system has also been utilized to study the effects of matrix stiffness, specifically crosslinking of collagen by lysyl oxidase, on the upregulation of VEGF in hepatocellular carcinomas [119].
Additionally, there have been several studies that demonstrate remarkable differences in 2D and 3D signaling behavior, especially of endothelial cells [121]. A key demonstration of this occurs in integrin signaling regulation where FAs in 3D are formed and degraded much more quickly than in 2D. FAs are dynamic in 3D and the inhibition of specific integrins can either promote or inhibit faster migration [87, 113, 122, 123]. Results from studies such as these have prompted a new wave of tumor angiogenesis research incorporating the techniques used in tissue engineering to generate 3D organoids to replicate the native tumor microenvironment. These models have gone through several iterations, at first only including tumor cells and then adding more cell lines to recapitulate the complex multicellular environment. This includes stromal or support cells, immunological cells, and endothelial cells in the surrounding blood vessels. While numerous research groups have adopted 3D, multicellular approaches to study specific tumor types, metastatic potential, and cellular pathway regulators, there has been limited investigation of biomechanical regulation of tumor progression, specifically angiogenesis, in this type of model.
Tissue engineering protocols allow for several methods of spatial control over cellular seeding in either synthetic polymer or ECM-derived materials. One of the approaches is a “self-assembled” technique, in which cells are simply mixed together for co-culture before casting of the matrix in a premade mold or dish, and sometimes includes external mechanical stimulation from moving culture platforms including orbital shakers or rotating vessel wall bioreactors [85, 124,125,126,127]. Furthermore, many of these studies represent nascent research in developing novel platforms and are limited in their ability to interrogate the role of biomechanics in angiogenesis associated with tumor progression. Bates et al. demonstrated that blocking integrin function in such self-assembled organoid models of colorectal cancer blocked tumor progression [128]. Vascularized liver buds have been generated via these same techniques, with a possible dependence on stromal cell contractile behavior for tissue assembly [129,130,131]. The advantages of this protocol are that the cells naturally orient themselves in a manner replicating the native in vivo tumor microenvironment. However, there is incomplete spatial control of this process, limiting the type of results that can be gleaned from such studies. However, these models are still important tools to understand tumor angiogenesis, and have demonstrated the importance of CAFs in promotion of tumor associated blood vessel growth through factors including VEGF, HIF-1a, caveolin-1 [31, 97, 132].
Another approach to investigate the role of biomechanics on angiogenesis in 3D tumor organoids involves the use of animal models. A recent study demonstrated the mechanosensitive nature of angiogenesis using the avian choiroallantoic membrane (CAM) model in the developing avian embryo [133]. Rings containing a collagen gel were implanted on top of the membrane, with tension induced in only the outermost layer of tissue. Harvested gels showed invasion of blood vessels due to tensile forces generated by the implant. This same model was utilized by another group to explore how crosslinks affecting biomechanical properties of the collagen gels altered VEGF production in tumor spheroids seeded onto the CAM [134].
Microfluidic model systems, as described above, provide a novel technique to study the effects of luminal and interstitial flow in a 3D tumor microenvironment containing self-assembled vasculature network with the further advantage of high levels over spatiotemporal seeding, flow conditions, and ease of visualization in real time during experiments. By controlling device parameters and fluidic pressures in feeding chambers, the precise direction and magnitude of interstitial flow can be manipulated, allowing for creation and monitoring of soluble signaling factor gradients that alter angiogenesis in the tumor microenvironment. We have developed multiple microfluidic devices that controls interstitial flow and permits tumor growth in the presence of self-assembled vasculature [29, 38, 135,136,137]. Others have utilized similar techniques to study the effects of ECM composition and stiffness, effects of diffusion of growth factors, alterations in cell phenotypes in co-culture and nascent vasculature biomechanical properties such as permeability [138,139,140,141,142]. The continued advance of model systems will continue to enhance our understanding of the role of mechanical forces on angiogenesis in the tumor microenvironment.
7 Intravasation and Extravasation in Tumor-Associated Angiogenesis
7.1 Overview
Numerous outstanding recent reviews are available that cover the general metastatic process of cancer [143,144,145,146], as well as those focusing specifically on the role of the endothelium including intravasation (tumor cells entering the circulation) and extravasation (tumor cells exiting the circulation) [147,148,149,150]. In addition, a recent review describes in vitro and in vivo models that have been developed to probe the metastatic process including some of the very recent advances in microfluidic and “organ-on-a-chip” technologies [151]. Thus, this section will succinctly review the metastatic process, features of this process that involve the circulation which have been captured with in vitro models, and then focus on features of the metastatic cascade which specifically involve the circulation that have proven difficult to simulate including possible strategies moving forward.
Tumor metastasis is the process by which a primary tumor is able to successfully move, or metastasize, to another location in the body. While complex, many steps in the metastatic cascade have been described. For an epithelial-based tumor (which comprise approximately 80% of all tumors), the process can be summarized in five steps: (1) dedifferentiation from an epithelial phenotype to a migratory mesenchymal cell phenotype, usually termed epithelial-mesenchymal transition (EMT), (2) intravasation of the mesenchymal phenotype tumor cell, or clusters of tumor cells, from the primary tumor into the circulation, (3) survival within the circulation, (4) attachment to an endothelial cell at a distant site and extravasation from the circulation, and (5) survival and differentiation in a receptive stroma from the mesenchymal tumor cell phenotype back to an epithelial cell phenotype, termed mesenchymal-epithelial transition (MET). Engaging the circulation is a necessary step for successful metastasis. Intravasation, survival in the circulation, and extravasation (steps 2–4) all uniquely require or utilize the vascular network. Although specific features of these events have been demonstrated in vitro, several important features have not, and numerous important questions remain unanswered.
Intravasation occurs in what is generally referred as the metastatic niche, a tumor microenvironment that contains the necessary factors for successful migration and entry into the circulation. The development of the metastatic niche is complex but involves the release of growth factors and trophogens from the endothelium that encourage the clustering of tumor-associated myeloid cells, platelets, and tumor cells towards the vascular supply. For example, endothelial cells in cancer-associated blood vessels have differential expression of adhesion molecules, P- and E- selectin, that recruit attachment of leukocytes to the metastatic niche [74]. Also, increased release of stromal derived factor-1 (SDF1) from endothelial cells leads to recruitment of endothelial progenitor cells to the metastatic niche. The recruitment of additional cell types, along with altered expression on endothelial cells, leads to a cascade of cell secreted factors, such as VEGF, endostatin, and other pro-tumor growth factors that characterize the metastatic niche and contributes to metastasis [74, 152]. Using murine and zebrafish models, and 3D organotypic microvascular niches, Ghajar and co-workers demonstrated that endothelial tip cells of cancer-associated blood vessels have decreased expression of pro-dormancy factor, thrombospondin-1, and enhanced expression of pro-tumor factors, periostin and TGF-β1, that encourages tumor cell migration [153].
7.2 Methods to Investigate Intravasation and Extravasation of Tumor Cells
Intravasation from within the metastatic niche requires the tumor cell(s) to cross the basolateral side of the endothelial cell. This necessitates overcoming the endothelial basement membrane and intercellular functional proteins. There is general consensus that intravasation occurs in vessels that are part of the tumor microenvironment and thus have characteristic features which are different from vessels in non-cancerous tissue including increased permeability and less basement membrane. Several relatively sophisticated 3D in vitro models of intravasation have recently been presented. At least two groups have utilized soft lithography to create a microfluidic device to mimic intravasation. In both cases tumor cells could migrate across an ECM hydrogel (collagen or fibrin) and then engage the abluminal surface of an intact layer of endothelial cells [154, 155]. Zervantonakis et al. coated a microfluidic line on the other side of the ECM that was lined with a confluent layer of endothelial cells [155]. The tumor cells could then penetrate the abluminal surface of the endothelial cells, thus mimicking intravasation. Strengths of this approach include the 3D migration of tumor cells in response to controlled gradients, and the controlled migration of tumor cells across an endothelial monolayer. The models are also easily adaptable to include other cells such as macrophages [155], and stromal cells [154]. In either case, the endothelial cell phenotype was not conditioned by the tumor microenvironment and only immortalized cell lines were utilized.
Ehsan et al. presented an alternate strategy to create a 3D in vitro model of intravasation by co-culturing endothelial cells and tumors cells in a spheroid and placing this spheroid in a fibrin gel [9]. A spontaneous vessel network formed within the spheroid and also sprouted from the spheroid. A colon cancer cell line intravasated into the vessel network, and they showed this process was related to EMT and the expression of the transcription factor SNAIL. Strengths of this model include the creation of a vascular network in close proximity to the tumor, and a true 3D vascular network. The surrounding matrix did contain stromal cells, but their work was also limited to immortalized cancer cell linesand there was no flow within the vessel lumens.
Mimicking the step of tumor cell survival in the blood vessel is problematic due to the complexity of blood and its components (e.g., platelets, leukocytes, clotting factors) and the mechanical microenvironment. Most cancer cells in the circulation do not survive; those cancer cells that do survive are able to overcome the shear stress and immune system by aggregating together and/or interacting with platelets [149]. These events have generally been captured using in vivo mice models and post-sacrifice observations of metastasis [156, 157]. This approach has been useful to identify some of the key cells and proteins involved, but lack temporal and spatial resolution. No in vitro model to date has been able to capture these dynamic events.
Extravasation requires the tumor cell(s) to cross from the luminal side of the endothelial cell, and thus crossing the intercellular junctional proteins and basement membrane, in that order. In contrast to intravasation, extravasation occurs at sites distant from the primary tumor and thus the endothelium is generally considered to be normal, but specific to the organ. Many details of how a tumor cell attaches and transmigrates the endothelium have been worked out using Transwell© [158] chambers and 2D laminar flow chambers [159]. Some of the mechanisms parallel the steps of neutrophil adhesion and paracellular transmigration including the expression of PECAM1 and E-Selectin on the endothelial cells and αVβ3 integrin and CD44 on the cancer cell. A more advanced 3D microfluidic model was recently reported and demonstrated flow and extravasation of cancer cells through a microfluidic channel lined with endothelial cells [160]. The major weaknesses in the current in vitro models of extravasation are the lack of organ endothelial specificity. Most models have utilized human umbilical vein endothelial cells (HUVECs) [158, 161] cultured on a fibronectin coated membrane of collagen gel, and thus they do not contain organ specific features of the endothelial cell [162] or vascular architecture [163]. Overcoming these challenges in the mimicry of tumor cell extravasation represents a tremendous opportunity to enhance our understanding of tumor progression.
8 Summary and Future Directions
In this chapter, we have discussed the process of angiogenesis in tumor organoids, the development of novel model systems for its study, as well as numerous results garnered from such studies. Increasingly, researchers are trying to recapitulate the complex native in vivo tumor microenvironment to provide an enhanced understanding of tumor progression for the purpose of developing novel therapeutic strategies. Initial research strategies utilized simple co-culture systems, either 2D or 3D, or mouse models, both of which present limited spatial or temporal control in elucidating the cues that regulate angiogenesis and tumor progression. The recent trend is the generation of sophisticated organ-on-a-chip systems where researchers can control spatial and/or temporal patterning of cells in matrices that mimic the native tumor tissue. Furthermore, use of microfluidic systems based on optically transparent materials permits real-time analysis of angiogenesis in the tumor microenvironment. Modular control over factors including hypoxia, shear flow, biomechanical properties, and gradients of growth factors permit interrogation of tumor progression at previously unimaginable resolution and physiological relevance.
As this field develops further, we predict that we will generate models combining the numerous factors discussed in this chapter, to generate in vitro systems that fully recapitulate the complex, native tumor microenvironment. By enhancing our understanding of how these features alter not only tumor cell behavior, but also endothelial cells of tumor-associated vasculature and the processes accompanying tumor development including intravasation/extravasation, we increase the likelihood of a breakthrough scientific discovery that will allow for development of novel anti-cancer treatment strategies, targeting the processes we are only beginning to fully understand due to our refined models of tumor organoid angiogenesis.
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407(6801):249–257. doi:10.1038/35025220
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Semenza GL (2012) Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 33(4):207–214. doi:10.1016/j.tips.2012.01.005
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324(1):1–8. doi:10.1056/NEJM199101033240101
Morton CL, Houghton PJ (2007) Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2(2):247–250. doi:10.1038/nprot.2007.25
Richmond A, Su Y (2008) Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech 1(2–3):78–82. doi:10.1242/dmm.000976
Dalen H, Burki HJ (1971) Some observations on the three-dimensional growth of L5178Y cell colonies in soft agar culture. Exp Cell Res 65(2):433–438
Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CCW, George SC (2014) A three-dimensional in vitro model of tumor cell intravasation. Integr Biol (UK) 6(6):603–610. doi:10.1039/c3ib40170g
Carver K, Ming X, Juliano RL (2014) Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions. Mol Ther Nucl Acids 3. doi:ARTN e153 10.1038/mtna.2014.5
Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE (2009) Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med 15(10):1219–U1151. doi:10.1038/nm.1971
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3(6):401–410. doi:10.1038/nrc1093
Fernandez-Sanchez ME, Barbier S, Whitehead J, Bealle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brulle L, Girard E, Servant N, Rio-Frio T, Marie H, Lesieur S, Housset C, Gennisson JL, Tanter M, Menager C, Fre S, Robine S, Farge E (2015) Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature 523(7558):92–95. doi:10.1038/nature14329
Ou G, Weaver VM (2015) Tumor-induced solid stress activates beta-catenin signaling to drive malignant behavior in normal, tumor-adjacent cells. BioEssays 37(12):1293–1297. doi:10.1002/bies.201500090
Augustin HG, Koh GY, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10(3):165–177. doi:10.1038/nrm2639
Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K (2008) Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol 10(5):527–537. doi:10.1038/ncb1715
Cascone T, Heymach JV (2012) Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol (Official Journal of the American Society of Clinical Oncology) 30(4):441–444. doi:10.1200/jco.2011.38.7621
Radu M, Semenova G, Kosoff R, Chernoff J (2014) PAK signalling during the development and progression of cancer. Nat Rev Cancer 14(1):13–25
Fryer BH, Field J (2005) Rho, Rac, Pak and angiogenesis: old roles and newly identified responsibilities in endothelial cells. Cancer Lett 229(1):13–23. doi:http://dx.doi.org/10.1016/j.canlet.2004.12.009
Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE (2008) Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A 105(32):11305–11310. doi:10.1073/pnas.0800835105
Halder G, Dupont S, Piccolo S (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 13(9):591–600
Dupont S (2016) Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. doi:http://dx.doi.org/10.1016/j.yexcr.2015.10.034
Piccolo S, Cordenonsi M, Dupont S (2013) Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res (An Official Journal of the American Association for Cancer Research) 19(18):4925–4930. doi:10.1158/1078-0432.ccr-12-3172
Nakatsu MN, Hughes CCW (2008) An optimized three-dimensional in vitro model for the analysis of angiogenesis. Angiogenesis: in vitro systems. Methods Enzymol 443:65. doi:10.1016/S0076-6879(08)02004-1
Welch-Reardon KM, Ehsan SM, Wang KH, Wu N, Newman AC, Romero-Lopez M, Fong AH, George SC, Edwards RA, Hughes CCW (2014) Angiogenic sprouting is regulated by endothelial cell expression of Slug. J Cell Sci 127(9):2017–2028. doi:10.1242/jcs.143420
Kim J, Chung M, Kim S, Jo DH, Kim JH, Jeon NL (2015) Engineering of a biomimetic pericyte-covered 3D microvascular network. PLoS One 10(7):e0133880. doi:10.1371/journal.pone.0133880
Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA, Stroock AD (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 109(24):9342–9347. doi:10.1073/pnas.1201240109
Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M, Kamm RD (2014) Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol-Uk 6(5):555–563. doi:10.1039/c3ib40267c
Moya ML, Hsu YH, Lee AP, Hughes CC, George SC (2013) In vitro perfused human capillary networks. Tissue Eng Part C Methods 19(9):730–737. doi:10.1089/ten.TEC.2012.0430
Song JW, Munn LL (2011) Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A 108(37):15342–15347. doi:10.1073/pnas.1105316108
Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS (2013) Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A 110(17):6712–6717. doi:10.1073/pnas.1221526110
Bischel LL, Young EW, Mader BR, Beebe DJ (2013) Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34(5):1471–1477. doi:10.1016/j.biomaterials.2012.11.005
Newman AC, Chou W, Welch-Reardon KM, Fong AH, Popson SA, Phan DT, Sandoval DR, Nguyen DP, Gershon PD, Hughes CC (2013) Analysis of stromal cell secretomes reveals a critical role for stromal cell-derived hepatocyte growth factor and fibronectin in angiogenesis. Arterioscler Thromb Vasc Biol 33(3):513–522. doi:10.1161/ATVBAHA.112.300782
Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC (2011) The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell 22(20):3791–3800. doi:10.1091/mbc.E11-05-0393
Wang XL, Phan DTT, Sobrino A, George SC, Hughes CCW, Lee AP (2016) Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16(2):282–290. doi:10.1039/c5lc01050k
Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, George SC (2009) Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A 15(6):1363–1371. doi:10.1089/ten.tea.2008.0314
Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC (2010) Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A 16(2):585–594. doi:10.1089/ten.TEA.2009.0491
Alonzo LF, Moya ML, Shirure VS, George SC (2015) Microfluidic device to control interstitial flow-mediated homotypic and heterotypic cellular communication. Lab Chip 15(17):3521–3529. doi:10.1039/c5lc00507h
Gilkes DM, Semenza GL, Wirtz D (2014) Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 14(6):430–439. doi:10.1038/nrc3726
Harris AL (2002) Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer 2(1):38–47. doi:10.1038/nrc704
Vaupel P, Mayer A, Hockel M (2004) Tumor hypoxia and malignant progression. Methods Enzymol 381:335–354. doi:10.1016/S0076-6879(04)81023-1
Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN, Cleveland JL (2002) c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev 16(19):2530–2543. doi:10.1101/gad.1024602
Grimes DR, Kelly C, Bloch K, Partridge M (2014) A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J R Soc Interface 11(92):20131124. doi:10.1098/rsif.2013.1124
Adams JM, Difazio LT, Rolandelli RH, Lujan JJ, Hasko G, Csoka B, Selmeczy Z, Nemeth ZH (2009) HIF-1: a key mediator in hypoxia. Acta Physiol Hung 96(1):19–28. doi:10.1556/APhysiol.96.2009.1.2
Semenza GL (2007) Hypoxia-inducible factor 1 (HIF-1) pathway. Science’s STKE: signal transduction knowledge environment 2007 (407):cm8. doi:10.1126/stke.4072007cm8
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70(5):1469–1480. doi:10.1124/mol.106.027029
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9(6):653–660. doi:10.1038/nm0603-653
Zhou W, Dosey TL, Biechele T, Moon RT, Horwitz MS, Ruohola-Baker H (2011) Assessment of hypoxia inducible factor levels in cancer cell lines upon hypoxic induction using a novel reporter construct. PLoS One 6(11):e27460. doi:10.1371/journal.pone.0027460
Krock BL, Skuli N, Simon MC (2011) Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2(12):1117–1133. doi:10.1177/1947601911423654
Byrne MB, Leslie MT, Gaskins HR, Kenis PJ (2014) Methods to study the tumor microenvironment under controlled oxygen conditions. Trends Biotechnol 32(11):556–563. doi:10.1016/j.tibtech.2014.09.006
Piret JP, Mottet D, Raes M, Michiels C (2002) CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y Acad Sci 973:443–447
Brennan MD, Rexius-Hall ML, Elgass LJ, Eddington DT (2014) Oxygen control with microfluidics. Lab Chip 14(22):4305–4318. doi:10.1039/c4lc00853g
Funamoto K, Zervantonakis IK, Liu Y, Ochs CJ, Kim C, Kamm RD (2012) A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab Chip 12(22):4855–4863. doi:10.1039/c2lc40306d
Wang L, Liu W, Wang Y, Wang JC, Tu Q, Liu R, Wang J (2013) Construction of oxygen and chemical concentration gradients in a single microfluidic device for studying tumor cell-drug interactions in a dynamic hypoxia microenvironment. Lab Chip 13(4):695–705. doi:10.1039/c2lc40661f
Ochs CJ, Kasuya J, Pavesi A, Kamm RD (2014) Oxygen levels in thermoplastic microfluidic devices during cell culture. Lab Chip 14(3):459–462. doi:10.1039/c3lc51160j
Clark LC Jr, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 102:29–45
Esipova TV, Karagodov A, Miller J, Wilson DF, Busch TM, Vinogradov SA (2011) Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging. Anal Chem 83(22):8756–8765. doi:10.1021/ac2022234
Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, George SC (2005) Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng 11(1–2):257–266. doi:10.1089/ten.2005.11.257
De Bock K, Georgiadou M, Carmeliet P (2013) Role of endothelial cell metabolism in vessel sprouting. Cell Metab 18(5):634–647. doi:10.1016/j.cmet.2013.08.001
De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154(3):651–663. doi:10.1016/j.cell.2013.06.037
Verdegem D, Moens S, Stapor P, Carmeliet P (2014) Endothelial cell metabolism: parallels and divergences with cancer cell metabolism. Cancer Metab 2:19. doi:10.1186/2049-3002-2-19
Wenzel C, Riefke B, Grundemann S, Krebs A, Christian S, Prinz F, Osterland M, Golfier S, Rase S, Ansari N, Esner M, Bickle M, Pampaloni F, Mattheyer C, Stelzer EH, Parczyk K, Prechtl S, Steigemann P (2014) 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp Cell Res 323(1):131–143. doi:10.1016/j.yexcr.2014.01.017
Wright BK, Andrews LM, Jones MR, Stringari C, Digman MA, Gratton E (2012) Phasor-FLIM analysis of NADH distribution and localization in the nucleus of live progenitor myoblast cells. Microsc Res Tech 75(12):1717–1722. doi:10.1002/jemt.22121
Wright BK, Andrews LM, Markham J, Jones MR, Stringari C, Digman MA, Gratton E (2012) NADH distribution in live progenitor stem cells by phasor-fluorescence lifetime image microscopy. Biophys J 103(1):L7–L9. doi:10.1016/j.bpj.2012.05.038
Walsh AJ, Cook RS, Sanders ME, Aurisicchio L, Ciliberto G, Arteaga CL, Skala MC (2014) Quantitative optical imaging of primary tumor organoid metabolism predicts drug response in breast cancer. Cancer Res 74(18):5184–5194. doi:10.1158/0008-5472.CAN-14-0663
Walsh AJ, Castellanos JA, Nagathihalli NS, Merchant NB, Skala MC (2015) Optical imaging of drug-induced metabolism changes in murine and human pancreatic cancer organoids reveals heterogeneous drug response. Pancreas. doi:10.1097/MPA.0000000000000543
Okkelman IA, Dmitriev RI, Foley T, Papkovsky DB (2016) Use of Fluorescence Lifetime Imaging Microscopy (FLIM) as a timer of cell cycle S phase. PLoS One 11(12):e0167385. doi:10.1371/journal.pone.0167385
Blacker TS, Mann ZF, Gale JE, Ziegler M, Bain AJ, Szabadkai G, Duchen MR (2014) Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun 5:3936. doi:10.1038/ncomms4936
Stringari C, Cinquin A, Cinquin O, Digman MA, Donovan PJ, Gratton E (2011) Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc Natl Acad Sci U S A 108(33):13582–13587. doi:10.1073/pnas.1108161108
Jungmann JH, Heeren RM (2012) Emerging technologies in mass spectrometry imaging. J Proteome 75(16):5077–5092. doi:10.1016/j.jprot.2012.03.022
Feist PE, Sidoli S, Liu X, Schroll MM, Rahmy S, Fujiwara R, Garcia BA, Hummon AB (2017) Multicellular tumor spheroids combined with mass spectrometric histone analysis to evaluate epigenetic drugs. Anal Chem 89(5):2773–2781. doi:10.1021/acs.analchem.6b03602
Giordano S, Morosi L, Veglianese P, Licandro SA, Frapolli R, Zucchetti M, Cappelletti G, Falciola L, Pifferi V, Visentin S, D'Incalci M, Davoli E (2016) 3D mass spectrometry imaging reveals a very heterogeneous drug distribution in tumors. Sci Rep 6:37027. doi:10.1038/srep37027
Jiang L, Chughtai K, Purvine SO, Bhujwalla ZM, Raman V, Pasa-Tolic L, Heeren RM, Glunde K (2015) MALDI-mass spectrometric imaging revealing hypoxia-driven lipids and proteins in a breast tumor model. Anal Chem 87(12):5947–5956. doi:10.1021/ac504503x
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293. doi:10.1038/nrc2621
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307. doi:10.1038/nature10144
Shieh AC, Swartz MA (2011) Regulation of tumor invasion by interstitial fluid flow. Phys Biol 8(1):015012. doi:10.1088/1478-3975/8/1/015012
Jean C, Gravelle P, Fournie JJ, Laurent G (2011) Influence of stress on extracellular matrix and integrin biology. Oncogene 30(24):2697–2706. doi:10.1038/onc.2011.27
Csikasz-Nagy A, Escudero LM, Guillaud M, Sedwards S, Baum B, Cavaliere M (2013) Cooperation and competition in the dynamics of tissue architecture during homeostasis and tumorigenesis. Semin Cancer Biol 23(4):293–298. doi:10.1016/j.semcancer.2013.05.009
Butcher DT, Alliston T, Weaver VM (2009) A tense situation: forcing tumour progression. Nat Rev Cancer 9(2):108–122. doi:10.1038/nrc2544
DuFort CC, Paszek MJ, Weaver VM (2011) Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 12(5):308–319. doi:10.1038/nrm3112
Fu BM, Tarbell JM (2013) Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med 5(3):381–390. doi:10.1002/wsbm.1211
Sund M, Xie L, Kalluri R (2004) The contribution of vascular basement membranes and extracellular matrix to the mechanics of tumor angiogenesis. APMIS 112(7–8):450–462. doi:10.1111/j.1600-0463.2004.t01-1-apm11207-0806.x
Shen Y, Hou Y, Yao S, Huang P, Yobas L (2015) In vitro epithelial organoid generation induced by substrate nanotopography. Sci Rep 5:9293. doi:10.1038/srep09293
Bignon M, Pichol-Thievend C, Hardouin J, Malbouyres M, Brechot N, Nasciutti L, Barret A, Teillon J, Guillon E, Etienne E, Caron M, Joubert-Caron R, Monnot C, Ruggiero F, Muller L, Germain S (2011) Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood 118(14):3979–3989. doi:10.1182/blood-2010-10-313296
Yamamura N, Sudo R, Ikeda M, Tanishita K (2007) Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng 13(7):1443–1453. doi:10.1089/ten.2006.0333
Asparuhova MB, Secondini C, Ruegg C, Chiquet-Ehrismann R (2015) Mechanism of irradiation-induced mammary cancer metastasis: a role for SAP-dependent Mkl1 signaling. Mol Oncol 9(8):1510–1527. doi:10.1016/j.molonc.2015.04.003
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139(5):891–906. doi:10.1016/j.cell.2009.10.027
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196(4):395–406. doi:10.1083/jcb.201102147
Yu H, Mouw JK, Weaver VM (2011) Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol 21(1):47–56. doi:10.1016/j.tcb.2010.08.015
Matsumoto T, Yung YC, Fischbach C, Kong HJ, Nakaoka R, Mooney DJ (2007) Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng 13(1):207–217. doi:10.1089/ten.2006.0058
Hanna M, Liu H, Amir J, Sun Y, Morris SW, Siddiqui MA, Lau LF, Chaqour B (2009) Mechanical regulation of the proangiogenic factor CCN1/CYR61 gene requires the combined activities of MRTF-A and CREB-binding protein histone acetyltransferase. J Biol Chem 284(34):23125–23136. doi:10.1074/jbc.M109.019059
Gjorevski N, Piotrowski AS, Varner VD, Nelson CM (2015) Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Sci Rep 5:11458. doi:10.1038/srep11458
Mierke CT, Rosel D, Fabry B, Brabek J (2008) Contractile forces in tumor cell migration. Eur J Cell Biol 87(8–9):669–676. doi:10.1016/j.ejcb.2008.01.002
Augsten M (2014) Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol 4:62. doi:10.3389/fonc.2014.00062
Stanisavljevic J, Loubat-Casanovas J, Herrera M, Luque T, Pena R, Lluch A, Albanell J, Bonilla F, Rovira A, Pena C, Navajas D, Rojo F, Garcia de Herreros A, Baulida J (2015) Snail1-expressing fibroblasts in the tumor microenvironment display mechanical properties that support metastasis. Cancer Res 75(2):284–295. doi:10.1158/0008-5472.CAN-14-1903
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15(6):637–646. doi:10.1038/ncb2756
Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJ, Garcia R, Keely PJ, Sanchez-Mateos P, Cukierman E, Del Pozo MA (2011) Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146(1):148–163. doi:10.1016/j.cell.2011.05.040
Erez N, Truitt M, Olson P, Arron ST, Hanahan D (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147. doi:10.1016/j.ccr.2009.12.041
Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP (2012) Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res (MCR) 10(11):1403–1418. doi:10.1158/1541-7786.MCR-12-0307
Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445(7129):776–780. doi:10.1038/Nature05571
Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, Polverini PJ, Nor J, Kitajewski J, Wang CY (2005) Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell 8(1):13–23. doi:10.1016/j.ccr.2005.06.004
Gjorevski N, Boghaert E, Nelson CM (2012) Regulation of epithelial-mesenchymal transition by transmission of mechanical stress through epithelial tissues. Cancer Microenviron (Official Journal of the International Cancer Microenvironment Society) 5(1):29–38. doi:10.1007/s12307-011-0076-5
Gomez EW, Chen QK, Gjorevski N, Nelson CM (2010) Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. J Cell Biochem 110(1):44–51. doi:10.1002/jcb.22545
Sewell-Loftin MK, Delaughter DM, Peacock JR, Brown CB, Baldwin HS, Barnett JV, Merryman WD (2014) Myocardial contraction and hyaluronic acid mechanotransduction in epithelial-to-mesenchymal transformation of endocardial cells. Biomaterials. doi:S0142–9612(13)01535–4 [pii] 10.1016/j.biomaterials.2013.12.051
Chien S (2008) Role of shear stress direction in endothelial mechanotransduction. Mol Cell Biomech (MCB) 5(1):1–8
Li YS, Haga JH, Chien S (2005) Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38(10):1949–1971. doi:10.1016/j.jbiomech.2004.09.030
Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS (2014) Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A 111(22):7968–7973. doi:10.1073/pnas.1310842111
Vickerman V, Kamm RD (2012) Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Integr Biol-Uk 4(8):863–874. doi:10.1039/c2ib00184e
Buchanan CF, Verbridge SS, Vlachos PP, Rylander MN (2014) Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adhes Migr 8(5):517–524. doi:10.4161/19336918.2014.970001
Buchanan CF, Voigt EE, Szot CS, Freeman JW, Vlachos PP, Rylander MN (2014) Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng Part C Methods 20(1):64–75. doi:10.1089/ten.TEC.2012.0731
Ingber DE (2008) Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol 97(2–3):163–179. doi:S0079-6107(08)00015-1 [pii] 10.1016/j.pbiomolbio.2008.02.005
Lehoux S, Castier Y, Tedgui A (2006) Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 259(4):381–392. doi:10.1111/j.1365-2796.2006.01624.x
Ngu H, Feng Y, Lu L, Oswald SJ, Longmore GD, Yin FC (2010) Effect of focal adhesion proteins on endothelial cell adhesion, motility and orientation response to cyclic strain. Ann Biomed Eng 38(1):208–222. doi:10.1007/s10439-009-9826-7
Avraamides CJ, Garmy-Susini B, Varner JA (2008) Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 8(8):604–617. doi:10.1038/Nrc2353
Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC (2003) Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A 100(13):7988–7995. doi:10.1073/pnas.1332808100
Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454(3):345–359. doi:10.1007/s00424-007-0212-8
Pelham RJ Jr, Wang YL (1998) Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biol Bull 194(3):348–349. discussion 349–350
Pelham RJ Jr, Wang Y (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 94(25):13661–13665
Dong Y, Xie X, Wang Z, Hu C, Zheng Q, Wang Y, Chen R, Xue T, Chen J, Gao D, Wu W, Ren Z, Cui J (2014) Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin beta1. Biochem Biophys Res Commun 444(3):427–432. doi:10.1016/j.bbrc.2014.01.079
Kojima T, Moraes C, Cavnar SP, Luker GD, Takayama S (2015) Surface-templated hydrogel patterns prompt matrix-dependent migration of breast cancer cells towards chemokine-secreting cells. Acta Biomater 13:68–77. doi:10.1016/j.actbio.2014.11.033
Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D (2010) A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12(6):598–604. doi:10.1038/ncb2062
Zebda N, Dubrovskyi O, Birukov KG (2012) Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res 83(1):71–81. doi:10.1016/j.mvr.2011.06.007
Kim DH, Khatau SB, Feng Y, Walcott S, Sun SX, Longmore GD, Wirtz D (2012) Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci Rep 2:555. doi:10.1038/srep00555
Nagelkerke A, Bussink J, Sweep FC, Span PN (2013) Generation of multicellular tumor spheroids of breast cancer cells: how to go three-dimensional. Anal Biochem 437(1):17–19. doi:10.1016/j.ab.2013.02.004
Timmins NE, Nielsen LK (2007) Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med 140:141–151
Skardal A, Devarasetty M, Rodman C, Atala A, Soker S (2015) Liver-tumor hybrid organoids for modeling tumor growth and drug response in vitro. Ann Biomed Eng 43(10):2361–2373. doi:10.1007/s10439-015-1298-3
Fong EL, Wan X, Yang J, Morgado M, Mikos AG, Harrington DA, Navone NM, Farach-Carson MC (2015) A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 77:164–172. doi:10.1016/j.biomaterials.2015.10.059
Bates RC, Buret A, van Helden DF, Horton MA, Burns GF (1994) Apoptosis induced by inhibition of intercellular contact. J Cell Biol 125(2):403–415
Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, Nakauchi H, Yoshikawa HY, Taniguchi H (2015) Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16(5):556–565. doi:10.1016/j.stem.2015.03.004
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499(7459):481–484. doi:10.1038/nature12271
Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H (2014) Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc 9(2):396–409. doi:10.1038/nprot.2014.020
Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C (2010) Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng Part A 16(7):2133–2141. doi:10.1089/ten.TEA.2009.0670
Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P (2009) Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med 15(6):657–U145. doi:10.1038/Nm.1985
Liang Y, Jeong J, DeVolder RJ, Cha C, Wang F, Tong YW, Kong H (2011) A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials 32(35):9308–9315. doi:10.1016/j.biomaterials.2011.08.045
Hsu YH, Moya ML, Abiri P, Hughes CC, George SC, Lee AP (2013) Full range physiological mass transport control in 3D tissue cultures. Lab Chip 13(1):81–89. doi:10.1039/c2lc40787f
Hsu YH, Moya ML, Hughes CC, George SC, Lee AP (2013) A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip 13(15):2990–2998. doi:10.1039/c3lc50424g
Moya ML, Alonzo LF, George SC (2014) Microfluidic device to culture 3D in vitro human capillary networks. Methods Mol Biol 1202:21–27. doi:10.1007/7651_2013_36
Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD (2009) Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9(2):269–275. doi:10.1039/B807585a
Park YK, Tu TY, Lim SH, Clement IJM, Yang SY, Kamm RD (2014) In vitro microvessel growth and remodeling within a three-dimensional microfluidic environment. Cell Mol Bioeng 7(1):15–25. doi:10.1007/s12195-013-0315-6
Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, Kamm RD (2009) Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J 23(7):2155–2164. doi:10.1096/fj.08-122820
Cross MJ, Claesson-Welsh L (2001) FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 22(4):201–207. doi:10.1016/S0165-6147(00)01676-X
Lee H, Kim S, Chung M, Kim JH, Jeon NL (2014) A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc Res 91:90–98. doi:10.1016/j.mvr.2013.12.001
Alizadeh AM, Shiri S, Farsinejad S (2014) Metastasis review: from bench to bedside. Tumour Biol (The Journal of the International Society for Oncodevelopmental Biology and Medicine) 35(9):8483–8523. doi:10.1007/s13277-014-2421-z
Blazejczyk A, Papiernik D, Porshneva K, Sadowska J, Wietrzyk J (2015) Endothelium and cancer metastasis: perspectives for antimetastatic therapy. Pharmacol Rep (PR) 67(4):711–718. doi:10.1016/j.pharep.2015.05.014
Chang J, Erler J (2014) Hypoxia-mediated metastasis. Adv Exp Med Biol 772:55–81. doi:10.1007/978-1-4614-5915-6_3
Irmisch A, Huelsken J (2013) Metastasis: new insights into organ-specific extravasation and metastatic niches. Exp Cell Res 319(11):1604–1610. doi:10.1016/j.yexcr.2013.02.012
Garcia-Roman J, Zentella-Dehesa A (2013) Vascular permeability changes involved in tumor metastasis. Cancer Lett 335(2):259–269. doi:10.1016/j.canlet.2013.03.005
Miles FL, Pruitt FL, van Golen KL, Cooper CR (2008) Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis 25(4):305–324. doi:10.1007/s10585-007-9098-2
Reymond N, d’Agua BB, Ridley AJ (2013) Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13(12):858–870. doi:10.1038/nrc3628
van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 728(1–2):23–34. doi:10.1016/j.mrrev.2011.05.002
Bersini S, Jeon JS, Moretti M, Kamm RD (2014) In vitro models of the metastatic cascade: from local invasion to extravasation. Drug Discov Today 19(6):735–742. doi:10.1016/j.drudis.2013.12.006
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827. doi:10.1038/nature04186
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DYR, Chen EI, Lyden D, Bissell MJ (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15(7):807–817. doi:10.1038/ncb2767. http://www.nature.com/ncb/journal/v15/n7/abs/ncb2767.html#supplementary-information
Lee H, Park W, Ryu H, Jeon NL (2014) A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics 8(5):054102. doi:10.1063/1.4894595
Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD (2012) Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A 109(34):13515–13520. doi:10.1073/pnas.1210182109
Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR (2004) Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104(2):397–401. doi:10.1182/blood-2004-02-0434
Coupland LA, Chong BH, Parish CR (2012) Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res 72(18):4662–4671. doi:10.1158/0008-5472.CAN-11-4010
Reymond N, Im JH, Garg R, Vega FM, Borda d'Agua B, Riou P, Cox S, Valderrama F, Muschel RJ, Ridley AJ (2012) Cdc42 promotes transendothelial migration of cancer cells through beta1 integrin. J Cell Biol 199(4):653–668. doi:10.1083/jcb.201205169
Shirure VS, Liu T, Delgadillo LF, Cuckler CM, Tees DF, Benencia F, Goetz DJ, Burdick MM (2015) CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions. Am J Physiol Cell Physiol 308(1):C68–C78. doi:10.1152/ajpcell.00094.2014
Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL (2013) In vitro model of tumor cell extravasation. PLoS One 8(2):e56910. doi:10.1371/journal.pone.0056910
Heyder C, Gloria-Maercker E, Entschladen F, Hatzmann W, Niggemann B, Zanker KS, Dittmar T (2002) Realtime visualization of tumor cell/endothelial cell interactions during transmigration across the endothelial barrier. J Cancer Res Clin Oncol 128(10):533–538. doi:10.1007/s00432-002-0377-7
Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S (2013) Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26(2):204–219. doi:10.1016/j.devcel.2013.06.017
Moya ML, George SC (2014) Integrating organ-specific function with the microcirculation. Curr Opin Chem Eng 3:103–111. doi:10.1016/j.coche.2013.12.004
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Shirure, V.S., Sewell-Loftin, M.K., Lam, S.F., Todd, T.D., Hwang, P.Y., George, S.C. (2018). Building Better Tumor Models: Organoid Systems to Investigate Angiogenesis. In: Soker, S., Skardal, A. (eds) Tumor Organoids. Cancer Drug Discovery and Development. Humana Press, Cham. https://doi.org/10.1007/978-3-319-60511-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-60511-1_7
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-60509-8
Online ISBN: 978-3-319-60511-1
eBook Packages: MedicineMedicine (R0)