Abstract
In patients with end-stage renal disease (ESRD), hypertension is common, difficult to diagnose, and often poorly controlled. Blood pressure (BP) recordings obtained before or after hemodialysis display a flat or even U-shaped association with cardiovascular events or survival, but this may reflect their poor accuracy, since elevated BP recorded with home or ambulatory BP monitoring is directly associated with shorter survival. Sodium and volume excess is the prominent pathogenic mechanism of hypertension in dialysis patients, but non-volume-mediated pathways, such as activation of the renin-angiotensin-aldosterone and sympathetic nervous systems, structural arterial wall alterations, endothelial dysfunction, sleep apnea, and the use of particular medications like erythropoietin-stimulating agents (ESAs), are also involved in the complex mechanistic background of hypertension in these individuals. Since sodium and volume excess is the most important cause, non-pharmacologic strategies such as dietary sodium restriction, individualized dialysate sodium prescription, and gradual dry-weight reduction should be the initial therapeutic approaches to achieve BP control. If hypertension remains poorly controlled, pharmacologic therapy should be commenced, taking into consideration the particular characteristics of antihypertensive agents. In this chapter, we discuss the epidemiology, pathogenesis, diagnosis, and treatment of hypertension among patients on dialysis in the light of currently available evidence.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Among end-stage renal disease (ESRD) patients receiving maintenance hemodialysis or peritoneal dialysis, hypertension is very common, difficult to diagnose and often poorly controlled [1]. Elevated blood pressure (BP), especially recorded outside of the hemodialysis unit with home or ambulatory BP monitoring, is associated with shorter survival [2,3,4]. Sodium and volume overload is the most important cause of hypertension in dialysis patients; accordingly, non-pharmacologic strategies such as dietary sodium restriction, individualized dialysate sodium prescription, and gradual dry-weight reduction should be the initial therapeutic approaches to achieve BP control [1, 5]. However, this approach still remains inadequately implemented [6, 7]. Even following proper management of sodium and volume excess, hypertension remains poorly controlled in a substantial proportion of dialysis patients; in these patients, pharmacologic therapy is obviously necessary to control BP [1, 5].
In this chapter, we discuss the epidemiology, pathogenesis, diagnosis, and treatment of hypertension among patients on dialysis in the light of currently available evidence derived from observational and randomized controlled studies; non-pharmacological and pharmacological strategies to manage hypertension in dialysis are both included in our discussion. We discuss data from the fewer relevant studies in peritoneal dialysis patients, summarizing clinical evidence that may be useful for the management of hypertension in these individuals.
2 Diagnosis
In the 2004 National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guideline document [8], the diagnosis of hypertension among patients on hemodialysis was based on BP measurements obtained shortly before or after dialysis, i.e., when predialysis BP is >140/90 mmHg or when postdialysis BP is >130/80 mmHg, respectively [8]. Whether using conventional peridialytic BP recordings is efficient to diagnose and guide the management of hypertension in the hemodialysis population is a matter of debate for several reasons. Pre- and postdialysis BP is typically recorded by the dialysis unit staff and without the necessary attention to the technique of BP measurement and the prerequisites for objective office BP recordings [9]. The high variability of BP from pre- to postdialysis and from one day to the next in response to the shifts and fluctuations in volume status and other parameters during the intra- and interdialytic period is another important issue that imposes particular difficulties in the accurate detection of hypertension [10]. The typical pattern of hemodynamic response to ultrafiltration is a BP decrease from pre- to postdialysis; the magnitude of intradialytic BP reduction is at least partially related to the magnitude and the rate of volume withdrawal during dialysis. The exact opposite phenomenon occurs during the out-of-dialysis interval [11], with several studies showing that interdialytic weight gain is closely associated with higher predialysis BP [12]. It is therefore not uncommon that predialysis BP levels are within the hypertensive range, whereas postdialysis BP measurements in the same patient are in the normotensive range. The poor diagnostic accuracy of peridialytic BP recordings is supported by a meta-analysis of clinical studies showing that both pre- and postdialysis BP provide imprecise estimates of the mean interdialytic BP recorded with 44-h ambulatory BP monitoring [13]. Furthermore, peridialytic BP recordings have little or no prognostic relations with mortality in hemodialysis patients [2, 3].
The rate of hypertension misdiagnosis when using peridialytic BP measurements is unacceptably high [14]. Using BP measurements obtained during the dialysis session in combination with the pre- and postdialysis BP may be an alternative approach to improve the reproducibility, precision, and accuracy of hypertension diagnosis among hemodialysis patients [15]. Intradialytic BP is usually recorded every 30 min with the use of an oscillometric devices, sometimes attached to the dialysis machine. In a diagnostic test study using 44-h interdialytic ambulatory BP as the reference standard, the average intradialytic BP in combination with peridialytic BP was shown to have greater diagnostic value compared with peridialytic BP recordings alone [16]. A median intradialytic cutoff BP of 140/90 mmHg during a midweek dialysis session provided greater sensitivity and specificity in detecting interdialytic hypertension as compared with pre- and postdialysis BP measurements [16]. Despite the fact that the diagnostic accuracy is improved when peridialytic BP recordings are considered together with intradialytic BP, this approach should remain a method of last resort, as BP measurements obtained outside of the dialysis unit appear better methods for the diagnosis of hypertension in these patients [14].
Home BP monitoring is a widely applied and recommended international guideline method to diagnose and manage hypertension in the general population [17, 18]. Among patients on dialysis, home BP monitoring is reported to have several advantages over conventional peridialytic BP recordings [19]. Compared with BP recordings obtained pre- or postdialysis, home BP exhibits stronger associations with mean 44-h ambulatory BP [20, 21]. In the Dry-Weight Reduction in Hemodialysis Patients (DRIP) trial, changes in home BP after 4 and 8 weeks of dry-weight probing were closely associated with the relevant changes in 44-h ambulatory BP; in contrast, predialysis and postdialysis BP recordings were unable to detect the changes in ambulatory BP caused in response to dry-weight reduction [22]. Contrary to the high variability and poor reproducibility of conventional peridialytic BP recordings, home BP was shown to have high short-term reproducibility from 1 week to the next [21]. Compared with the BP measurements obtained within the dialysis unit, home BP exhibits stronger associations with indices of target-organ damage [23,24,25] and represents a more powerful predictor of future cardiovascular events or all-cause mortality [2, 3].
The notion that home BP may be a useful tool to guide the management of hypertension among dialysis patients is supported by a pilot study which randomized 65 hypertensive hemodialysis patients to have their antihypertensive drug therapy adjusted either on the basis of routine predialysis BP recording or with the use of home BP monitoring [26]. Over a mean follow-up period of 6 months, a significant reduction in interdialytic ambulatory BP of 9/7 mmHg was noted in the home BP-guided group, but not in the predialysis BP-guided group [26]. Another study randomized 34 hemodialysis patients to home BP-guided management plus usual care or usual care alone for management of hypertension. After 12 weeks of follow-up, the use of home BP recordings in decision making resulted also in significant reduction of the average weekly systolic BP as compared with the usual care alone [27].
Ambulatory BP monitoring is considered the “gold standard” method for diagnosing hypertension among patients receiving dialysis [1, 18, 28]. The superiority of this technique over the conventional peridialytic BP measurements is strongly supported by comparative studies showing that mean 44-h interdialytic BP can better predict the presence of target-organ damage (such as echocardiographic LV hypertrophy) [23] and is more closely associated with all-cause and cardiovascular mortality [2, 4]. The use of ambulatory BP monitoring has also the advantage of recording BP during nighttime, providing additional information with respect to the circadian variation of BP; the presence of a non-dipping nocturnal BP pattern is very common among dialysis patients and has been associated with LVH [29] and increased risk of all-cause and cardiovascular mortality [30]. It is important noting that the superiority of ambulatory BP monitoring over peridialytic BP recordings can only partially be explained by the higher number of BP measurements, as interdialytic BP recordings retain their strong prognostic association with cardiovascular outcomes even when a small number of randomly selected measurements are used to assess the interdialytic BP burden [31]; the latter suggests that the location and time frame covered and not the quantity of BP recordings are the major factor determining the strong prognostic significance of interdialytic ambulatory BP. Despite the above advantages, ambulatory BP monitoring is still perceived as a technique with limited applicability in dialysis patients in a reservation arising partly from the fact that many studies on ambulatory BP monitoring in this population dialysis patients were performed in a single American academic hemodialysis unit [2, 11, 23]. The high prevalence of non-dipping and nocturnal hypertension among dialysis patients [32] suggests that the application of ABPM for the diagnosis and the treatment of hypertension is more compelling than in the general population, where ABPM has already been firmly recommended by different guidelines [33, 34]. Additional research efforts are needed in order to fully elucidate the particular indications, tolerability, and cost-effectiveness of ABPM. Until such studies are completed, the wide application of home BP monitoring should be encouraged as a simple and efficient approach to measure BP and make therapeutic decisions among patients on dialysis [14]. Figure 24.1 summarizes the thresholds to define hypertension using home and ambulatory BP monitoring proposed in a recent document of the EURECA-m working group of ERA-EDTA [18].
Definition of hypertension in CKD and in ESRD patients (reprinted with permission from Parati et al. [18])
Contrary to the typical decline in BP during dialysis, in approximately 10–15% of dialysis patients, BP exhibits a “paradoxical” intradialytic elevation [35, 36]. Despite the fact that this abnormal pattern of intradialytic hemodynamic response has been for long recognized, there is no universally agreed definition of intradialysis hypertension. For example, in some studies, intradialysis hypertension was defined as a rise of at least 10 mmHg in systolic BP during dialysis or immediately postdialysis in a certain number of dialysis treatments [35, 36]. In other studies, patients were considered as suffering from intradialysis hypertension when their BP showed a change of >0 mmHg from pre- to postdialysis; another definition was the regression of all intradialytic BP measurements over time with a slope greater than zero [37]. Of note, intradialysis hypertension is not solely related to mechanistic changes exerted during the dialysis session but also related to the BP burden during the interdialytic period. In a case-control study comparing the interdialytic BP profile of 25 patients with intradialysis hypertension (increase in systolic BP >10 mmHg from pre- to postdialysis in four out of six consecutive dialysis treatments) with that of 25 age- and sex-matched controls with normal intradialytic hemodynamic response, Van Buren et al. [38] made the important observation that intradialysis hypertension is a phenomenon superimposed to systemic background hypertension. Patients with intradialysis hypertension had higher 44-h interdialytic BP than controls, as well as a gradual BP decline during the first 24 h after dialysis, which contrasted with the (typical) gradual increase from postdialysis onward in patients without intradialytic hypertension [38].
3 Epidemiology
The estimates of the prevalence, treatment, and control of hypertension among patients on chronic dialysis are highly variable, depending on the definitions used to diagnose hypertension as well as on the setting of BP measurement (i.e., routine peridialytic BP recordings or interdialytic ambulatory BP monitoring) [39,40,41,42,43].
3.1 Epidemiology Based on Peridialytic BP Recordings
Hypertension is highly prevalent among patients with chronic kidney disease (CKD) not yet on dialysis. In a cross-sectional analysis of 10,813 CKD patients participating in the Kidney Early Evaluation Program (KEEP) in the USA, hypertension (defined as BP >130/80 mmHg or use of antihypertensives) was detected in 86.2% of the overall study cohort; prevalence of hypertension exhibited a stepwise increase with advancing stage of CKD, increasing from 79.1% in participants with stage 1 CKD to approximately 95%% (or 91% with the use of 140/90 threshold) in participants with stage 4 and 5 CKD [44]. An analysis of 238 patients with predialysis CKD followed in a low clearance clinic in the UK confirmed that the prevalence of hypertension is at 95% (Fig. 24.2) [45]; the mean estimated glomerular filtration rate (eGFR) in this cohort was 14.5 mL/min/1.73 m2, suggesting that nearly all CKD patients just before the initiation of renal replacement therapy are already hypertensives.
Prevalence (at the 130/80 threshold for office BP), treatment, and control of hypertension in predialysis patients followed in a low clearance clinic with average eGFR 14.5 mL/min/1.73 m2 (reprinted with permission from Sarafidis et al. [45])
Initiation of dialysis per se may have a substantial impact on management of hypertension, given the severely impaired ability of patients with advanced CKD for sodium excretion and the fact that dialysis represents a potent therapeutic tool to remove the sodium excess [1]. Achievement of sodium and volume control via dialysis often decreases the need for antihypertensive drug therapy in incident dialysis patients. It is therefore unsurprising that the rates of hypertension prevalence may be higher among predialysis CKD patients than among ESRD patients receiving renal replacement therapy, as discussed below. Moreover, hypertension prevalence after initiation of dialysis depends on the clinical policies adopted in the renal units where the patients are being treated. In some renal units which apply long dialysis and strict control of salt intake, hypertension has a lower prevalence than in those which don’t apply such a clinical policy [46].
Using the definition of predialysis mean arterial pressure ≥114 mmHg, Salem et al. [42] reported that the prevalence of hypertension among 649 hemodialysis patients from ten dialysis units in Mississippi was 72%. Eighty percent of hypertensive patients had combined systolic and diastolic hypertension and 20% isolated systolic hypertension. Race, dialysis vintage, primary cause of ESRD, or adequacy of dialysis had no association with the hypertension status in this study [42]. In 5369 patients participating in the Dialysis Morbidity and Mortality Study Wave 1 [40], the prevalence of hypertension was 63% using the JNC 6 classification to define hypertension. A hypertension prevalence rate of 70% was reported in a cross-sectional analysis of the baseline characteristics of 1238 chronic hemodialysis patients enrolled in the HEMO study [41]. A more detailed evaluation of prevalence, treatment, and control of hypertension was provided by a cross-sectional analysis of 2535 clinically stable, hemodialysis patients participating in a multicenter trial of the safety and tolerability of an intravenous iron preparation [39]. In this survey, hypertension was defined as a 1-week average predialysis systolic BP >150 mmHg or diastolic BP >85 mmHg or the use of antihypertensive drugs with prevalence at 86%, and despite the fact that 88% of hypertensives were treated, only 30% of them had their BP adequately controlled [39]. Information on hypertension prevalence in countries other than the USA is limited. In surveys made within the frame of the DOPPS [47], the prevalence of hypertension was very high and rising over time in all countries. In the last of these surveys [48], hypertension prevalence ranged from 78% in Japan to 95.9% in Germany. All the above estimates should be interpreted within the context of the unavoidable limitation of the use of routine peridialytic BP recordings to assess the hypertension status of study participants.
3.2 Epidemiology Based on Interdialytic Ambulatory BP Monitoring
A more valid estimation of hypertension prevalence and control among dialysis patients was provided by a recent study using the “gold standard” method of 44-h interdialytic ambulatory BP monitoring and defining hypertension as average systolic BP values ≥135 mmHg and/or diastolic BP ≥85 mmHg or the use of antihypertensive medications in a population of 369 predominantly African-American patients who received hemodialysis treatment in units affiliated with the Indiana University in Indianapolis. The prevalence of hypertension was 82% [43], and although 89% of hypertensives were treated with antihypertensive drugs, the rate of adequate 44-h BP control was as low as 38% [43]. Poor hypertension control in this study was associated with excessive antihypertensive drug use and volume expansion as measured by the inferior vena cava diameter in expiration [49]. Of note, other studies suggest that the higher the number of antihypertensive agents prescribed, the greater the likelihood a dialysis patient to be on a volume-expanded state [43]. Apart from this study in African-Americans, no large surveys reporting hypertension prevalence based on ABPM have been made in other ethnicities and in other countries.
3.3 The Association of BP with All-Cause and Cardiovascular Mortality
The relationship of BP with all-cause and cause-specific mortality among patients on dialysis is an issue surrounded by substantial controversy, due to the diverse patterns of association between BP and mortality according to timing (i.e., predialysis, postdialysis, or intradialysis) or the technique of BP measurement (i.e., peridialytic BP recordings vs. interdialytic BP recording either with home or ambulatory BP monitoring). Several studies have shown a U-shaped association of the BP recorded either predialysis or postdialysis with all-cause and cardiovascular mortality [50,51,52], a phenomenon described as “reverse epidemiology of hypertension” in the dialysis population. This observation has raised substantial concerns on whether BP lowering is a strategy associated with benefits for ESRD patients receiving hemodialysis [53]. However, this U-shaped association seems to be due to the incapacity of peridialytic BP recordings per se to describe the true BP load, rather than reflect a true U-shaped relation of BP with cardiovascular morbidity and mortality.
Contrary to the unclear association of peridialytic BP recordings with all-cause and cardiovascular mortality, prospective cohort studies have shown that interdialytic BP recorded either with home or with ambulatory BP monitoring associates directly with mortality and cardiovascular events relevant to what happens in non-dialysis populations. In a cohort of 57 treated hypertensive hemodialysis patients prospectively followed for a mean period of 34.4±20.4 months, Amar et al. [4] showed elevated 24-h ambulatory pulse pressure (PP) [relative risk (RR), 1.85 for each 10 mmHg increase in PP; 95% confidence intervals (CIs), 1.28–2.65] as well as elevated nocturnal systolic BP (RR, 1.41 for each 10 mmHg increase in nocturnal systolic BP; 95% CIs, 1.08–1.84) to be independently associated with increased risk of cardiovascular mortality [4]. In larger study by Tripepi et al., in 168 nondiabetic hemodialysis patients, nocturnal BP burden (as estimated by the night/day ratio) was a direct predictor of death and cardiovascular events as well as of LVH [30]. In a subsequent cohort study of 150 hemodialysis patients, Alborzi et al. [3] showed that increasing interdialytic BP measured with home and ambulatory BP monitoring was directly associated with heightened risk of mortality over a mean follow-up period of 24 months. No such relationship was detectable using BP measurements obtained before or after dialysis (Fig. 24.3) [3]. In a larger cohort of hemodialysis patients followed for 32 months, Agarwal et al. confirmed that the higher quartiles of home and 44-h ambulatory systolic BP were independently associated with increased risk of mortality [2]. Once again, BP recorded outside of the dialysis unit was of stronger prognostic significance as compared with BP recorded before or after dialysis.
Hazard ratios for all-cause mortality for quartiles of predialysis, postdialysis, and home and ambulatory systolic blood pressure (BP). Higher levels of home BP and ambulatory BP were significantly associated with mortality, whereas pre- and postdialysis BP was not. P values are those reported for linear trend. HD indicates hemodialysis and Q quartile (reproduced with permission from Alborzi et al. [3])
Additional support to the notion that out-of-dialysis BP recordings have closer association with outcomes is provided by a recent prospective analysis of 326 patients participating in the Chronic Renal Insufficiency Cohort (CRIC) study [54]. The prognostic association of systolic BP with all-cause mortality was assessed in three different time points of this prospective cohort: (1) when participants had stage 4 CKD (eGFR <30 mL/min/1.73 m2), (2) when participants initiated hemodialysis and dialysis unit BP measurements were available, and (3) when incident hemodialysis patients had an out-of-dialysis BP measurement obtained during a prespecified follow-up visit at home [54]. Systolic BP had no association with mortality among participants not yet on dialysis. In accordance with earlier reports from other cohorts of hemodialysis patients, dialysis unit systolic BP provided a U-shaped association with mortality. In contrast, a direct linear association between systolic BP and all-cause mortality was evident when BP measurements were obtained outside of the unit (HR, 1.26 for each 10 mmHg higher systolic BP; 95% CIs, 1.14–1.40) [54].
The pattern of intradialytic hemodynamic response (i.e., the change in BP from pre- to postdialysis) has been also associated with increased risk of all-cause and cardiovascular mortality [54, 55]. In this regard, Park et al. [56] revealed a U-shaped association between intradialytic change in systolic BP and mortality. In a huge cohort study of 113,215 US hemodialysis patients retrospectively followed over a median period of 5 years, it was shown that drops in systolic BP from pre- to postdialysis between 30 and 0 mmHg were associated with better survival, but large declines in systolic BP (>30 mmHg) and intradialytic rise in systolic BP of any degree were both linked with increased risk of mortality [56].
3.4 Epidemiology of Hypertension Among Patients Receiving Peritoneal Dialysis
The prevalence of hypertension among patients on peritoneal dialysis was evaluated in a cross-sectional study conducted in 504 patients in 27 peritoneal dialysis centers belonging to the Italian Co-operative Peritoneal Dialysis Study Group [57]. Valid ambulatory BP measurements were obtained in 414 patients (82%) using the WHO/ISH and the JNC 7 report criteria; Cocchi et al. reported that the prevalence of hypertension was 88.1%. Applying the definition of a BP load >30% over a 24-h ambulatory BP monitoring, the estimated prevalence of hypertension was lower (69%). The average 24-h blood pressure in this study was 139±19/81±11 mmHg, clearly indicating that the prevalence of hypertension as defined by the joint document of the American Society of Nephrology and the American Society of Hypertension (SBP >135 and/or DBP >85 mmHg) [1] exceeds 50–60% in the peritoneal dialysis population [57]. Of note, as much as 53% of patients in this study were non-dippers and an additional 9% had an inverted day/night BP profile. Small studies comparing the ambulatory BP profile between patients treated with automated peritoneal dialysis vs. continuous ambulatory peritoneal dialysis showed that the average 24-h BP, diurnal BP variation, and BP control rates were no different between these two modalities [58, 59]. Other studies have described an association between BP and peritoneal transport status. Patients with high peritoneal transport (reflecting poor peritoneal ultrafiltration) have higher BP levels during both daytime and nighttime periods as well as higher LVMI as compared to “low transporters,” and this difference most likely reflects volume overload triggered by high peritoneal transport in the first group. Volume expansion is more marked in peritoneal than in hemodialysis patients [60], and these patients more frequently require antihypertensive drugs (65%) than hemodialysis patients (38%, P<0.001). The detrimental role of volume expansion in patients maintained on peritoneal dialysis is notorious [61].
Given the more continuous nature of renal replacement therapy and the absence of cyclic variations in volume status and in several other metabolic parameters in patients receiving peritoneal dialysis, it is long hypothesized that BP control and diurnal variation of BP may be substantially different between patients treated with peritoneal dialysis and those receiving thrice-weekly hemodialysis. However, only two small studies have so far tested this hypothesis. Tonbul et al. [62] compared the 44-h ambulatory BP profile of 22 hemodialysis patients with that of 24 patients treated with continuous ambulatory peritoneal dialysis. Mean 44-h systolic and diastolic BP was no different between the two dialytic modalities; however, in hemodialysis nighttime BP recorded on the dialysis-off day was significantly higher, and daytime BP recorded on the dialysis-on day was significantly lower than the relevant BP recordings obtained in the same time periods in patients treated with continuous ambulatory peritoneal dialysis [62]. Another comparative study including 33 hemodialysis and 27 peritoneal dialysis patients showed that diurnal BP pattern (i.e., dipping status) did not differ between the two dialytic modalities over a 48-h ambulatory BP recording, but average ambulatory systolic BP (142.1±16.3 vs. 130.4±17.1 mmHg, P<0.01) and systolic loads (54±29% vs. 30±31%, P<0.01) were higher in those receiving hemodialysis [63]. It has to be noted, however, that methodologically rigorous randomized comparisons between hemodialysis and peritoneal dialysis are missing, and the studies performed so far are small and largely inconclusive.
4 Pathogenesis
Increase in cardiac output or in peripheral vascular resistance or in both these hemodynamic parameters may result in sustained BP elevation among patients on dialysis. Undoubtedly, sodium and volume expansions are considered the prominent pathogenic mechanisms of hypertension in these individuals. A number of non-volume-mediated pathways, such as activation of the renin-angiotensin-aldosterone and sympathetic nervous systems, structural arterial wall alterations related to the long-term arteriosclerotic process, endothelial dysfunction, sleep apnea, and the use of particular medications like erythropoietin-stimulating-agents (ESAs), are also reported to play an important role in the complex mechanistic background of hypertension in dialysis patients.
4.1 Volume Overload
In patients with ESRD, even when residual renal function is preserved, the sodium and fluid excretory capacity is substantially impaired; subsequently, the presence of sodium and volume expansion is very common and often not easily identifiable in dialysis patients. Moreover, patients with ESRD are those with the highest sodium sensitivity of BP [64, 65]. In addition, it is now well documented that in addition to classical osmotic volume expansion, sodium retention may occur in the form of osmotically inactive sodium in the connective tissue and the skin where sodium accumulates linked to glycosaminoglycans [66]. Such a non-osmotic sodium retention triggers local macrophage recruitment, lympho-angiogenesis, and hypertensive mechanisms independent of those traditionally ascribed to isoosmotic volume retention. In hemodialysis patients, sodium and water in skin and muscle are increased and vascular endothelial growth factor is reduced as compared to age-matched healthy individuals, and these phenomena may also contribute to hypertension [67]. Fluid and sodium accumulation between subsequent dialysis treatments exerts a substantial impact on the patterns and rhythms of interdialytic BP, which is superimposed on the circadian variation of BP. Among hemodialysis patients, BP steadily increases during the interdialytic interval and the rate of BP increment is directly proportional to the interdialytic weight gain [68]. Studies including 48-h ambulatory monitoring of central hemodynamic indices in hemodialysis patients showed a gradual increase in peripheral and central aortic BP between the intra- and interdialytic periods [69]. Excess volume accumulation over the long interdialytic interval in patients receiving thrice-weekly hemodialysis imposes an additional BP load during the third interdialytic day (Fig. 24.4). In a study of 55 hemodialysis patients having a 72-h ambulatory aortic BP monitoring, a significant increase of 5/3.5 mmHg in aortic BP was noted between the third and the second day of the long interdialytic intervals; nighttime BP and the proportion of patients with a non-dipping circadian BP pattern were also higher during the third interdialytic day [70]. Unless extracellular fluid and sodium overload is removed with ultrafiltration, a rise in vascular resistance would sustain hypertension in these individuals. In this context, strict volume and sodium control emerges as the principal target of therapy in hypertensive patients with ESRD.
Changes in aortic blood pressures, wave reflections, and arterial stiffness parameters between the first and the second interdialytic day Δ[day(2)–day(1)], in comparison with relevant changes between the second and the third interdialytic day Δ[day(3)–day(2)] (reprinted with permission from Koutroumpas et al. [70])
4.2 Renin-Angiotensin-Aldosterone System
Activation of the renin-angiotensin-aldosterone system (RAAS) even in patients with ESRD under renal replacement therapy is long known [71, 72]. Plasma renin activity (PRA) is maintained within the normal range in the majority of dialysis patients; however, PRA may be inappropriately elevated in relation to the total exchangeable sodium and may contribute to the sustained BP elevation [73]. This notion is supported by clinical studies showing a significant increase in PRA and plasma aldosterone levels from pre- to postdialysis, suggesting that residual functioning nephrons in the failing kidneys of ESRD patients retain their ability to sense acute changes in sodium and intravascular volume status that occur in response to ultrafiltration [71, 73]. Additional support to the fact that BP elevation in a subset of dialysis patients may be in part renin mediated is provided by earlier studies showing a sustained BP reduction in hypertensive dialysis patients after the administration of the angiotensin II antagonist saralasin; removal of the native kidneys from the BP responders was associated with long-term normalization of their BP levels [74]. More recent studies have shown a dose-dependent elevation in pre- and postdialysis PRA levels along with a parallel fall in 44-h [75] interdialytic ambulatory BP in response to the supervised administration of the angiotensin-converting enzyme inhibitor (ACEI) lisinopril [75]. In addition to the above, the relationship between PRA, aldosterone, and major clinical outcomes in dialysis patients is complex and much influenced by malnutrition and inflammation. Indeed, independently of predialysis BP, aldosterone is an inverse predictor of mortality and CV events in this population, and this seemingly paradoxical relationship is abolished by adjustment for inflammation, protein energy malnutrition, and volume expansion biomarkers indicating that it is the mere expression of the confounding effect of these factors [76].
4.3 Sympathetic Nervous System
Seminal microneurography studies assessing efferent sympathetic nerve activity have provided evidence that sympathetic overactivity is an important cause of hypertension among patients on dialysis. These clinical studies showed a doubling in the rate of sympathetic discharge in hemodialysis patients with intact native kidneys; in contrast, sympathetic nerve activity in bilaterally nephrectomized hemodialysis patients was similar to that of healthy individuals [77]. Bilateral nephrectomy of native failing kidneys was shown to be associated with sustained reduction in peripheral vascular resistance as well as with dramatic drop in BP levels [78]. The notion that sympathetic overactivity is implicated in the causal pathway of hypertension in dialysis patients is also supported by recent reports in small groups of patients suggesting that renal denervation exerts a significant BP-lowering effect and improves sympathetic nerve discharge among dialysis patients with hypertension that remains unresponsive to multidrug antihypertensive therapy and ultrafiltration intensification [79, 80]. In a proof-of-concept study, Schlaich et al. [81] performed renal nerve ablation in 12 hemodialysis patients with uncontrolled hypertension (office BP>140/90 mmHg) despite the current use of ≥3 antihypertensive drugs. The procedure of renal denervation was feasible in nine out of 12 study participants; among these patients, a significant drop of 28/10 mmHg in office BP was noted over a mean 12-month-long follow-up period [81].
Renalase, an enzyme that metabolizes catecholamines and catecholamine-like substances, may contribute to the excessive sympathetic overactivity and hypertension in CKD [82]. Renalase is a flavin adenine dinucleotide-dependent amine oxidase which is secreted in the blood by the kidney [82]. Infusion of recombinant renalase in rats produces a significant reduction in BP and heart rate, an effect predominantly mediated through reduced peripheral vascular tone and cardiac output [83]. The plasma concentration of renalase was shown to be markedly decreased in hemodialysis patients as compared to age- and sex-matched controls with normal renal function [84].
4.4 Arterial Stiffness
Patients with ESRD display a distinct form of early increase in arterial stiffness, due to a combination of factors, mostly relevant to inappropriate calcium-phosphate homeostasis [85]. Among dialysis patients, arterial stiffness, as assessed by aortic pulse wave velocity (PWV), is a relevant determinant of the patterns and rhythms of BP recorded over the entire interdialytic period [85,86,87]. Analyzing 11,833 interdialytic BP measurements obtained from 125 hemodialysis patients with the use of a generalized cosinor model, Agarwal et al. [86] showed that each one log increase in aortic PWV was associated with a rise of 18.8/7.08 mmHg in the intercept of systolic/diastolic BP and with elevation of 11.7 mmHg in the intercept of PP. Increasing aortic PWV tended also to blunt the circadian amplitude of systolic BP and PP [86]. Subsequently, in a post hoc analysis of the HDPAL trial, it was shown that increasing aortic PWV at baseline was an independent determinant of 44-h ambulatory systolic BP and PP. After adjustment for several confounding factors, each 1-m/s higher baseline aortic PWV was associated with 1.34-mmHg higher baseline systolic BP and 1.02-mmHg higher PP [87]. However, aortic PWV at baseline was unable to predict the treatment-induced reduction in 44-h ambulatory systolic and diastolic BP at 3, 6, and 12 months of follow-up [87]; the latter suggests that among dialysis patients, arterial stiffness does not make hypertension more resistant to the BP-lowering therapy. Studies evaluating acute changes in arterial stiffness indexes during the interdialytic periods showed that augmentation index (AIx) and central aortic PP are increased during both 3-day and 2-day interdialytic intervals; aortic and brachial PWV was unchanged in this short time frame [88]. This increase in wave reflection indices was by 30% higher during the 3-day as compared to the 2-day interdialytic interval and was linearly associated with interdialytic weight gain [88]. This observation was confirmed in subsequent studies showing a gradual interdialytic increase in wave reflection indices and central aortic BP with the use of ambulatory BP monitoring [69, 70].
4.5 Endothelial Dysfunction
An imbalance between endothelium-derived vasoconstrictors and vasodilators in favor of the former may be another mechanistic pathway of hypertension among patients on dialysis [89]. This is supported by animal studies showing downregulation of the endothelial and inducible nitric oxide synthase activity in 5/6 nephrectomized rats, an alteration that resulted in sustained BP elevation [90]. Endothelial dysfunction results from several mechanisms including high circulating levels of asymmetric dimethylarginine (ADMA) [91, 92]; an endogenous nitric oxide synthase inhibitor and its accumulation result in reduced generation of nitric oxide [93]. The higher levels of ADMA in ESRD result from both a diminished intracellular degradation by desamino-d-argininehydrolase and diminished renal clearance of ADMA, since this molecule is mainly excreted by the kidney [93]. Among ESRD patients, ADMA is associated with increased LV relative wall thickness and reduced ejection fraction. Importantly, prospective cohort studies have associated increased ADMA levels with excessive risk of cardiovascular morbidity and mortality in hemodialysis patients [91, 93].
4.6 Sleep Apnea
Sleep apnea is highly prevalent among dialysis patients and volume expansion may be a major player in this alteration [94]. In the recumbent position, volume overload may promote sleep-disordered breathing and nocturnal hypoxemia through an overnight fluid shift from the legs to the neck soft tissues that increases peripharyngeal and upper airway resistance [95]. Nocturnal hypoxemia in sleep apnea has been associated with a reversed circadian BP pattern, triggering in this way nocturnal hypertension. This notion is supported by a study of 32 hemodialysis patients showing that those patients experiencing sleep apnea had higher nocturnal systolic BP and higher LV relative wall thickness than those without sleep apnea; an inverse relationship was noted between the average nocturnal arterial oxygen saturation and LV relative wall thickness [29]. In another study, Abdel-Kader et al. [96] showed that ESRD patients with sleep apnea had 7.1 times higher risk of developing resistant hypertension (defined as office BP >140/90 mmHg despite the use of >3 different antihypertensive agents); in contrast, no such association between sleep apnea and resistant hypertension was noted among patients with non-dialysis-requiring CKD [96]. Whether strict management of volume status improves sleep apnea symptoms and restores the blunted nocturnal BP fall in dialysis patients still remains elusive.
4.7 Erythropoietin-Stimulating Agents
Hypertension is a common but frequently overlooked complication of erythropoietin therapy [97]. New-onset hypertension or worsening of pre-existing hypertension can be easily missed due to the high variability of BP in dialysis patients [10] particularly in the absence of properly performed home or ambulatory BP measurements. Studies that did not detect BP elevation in response to erythropoietin therapy may have managed hypertension more aggressively through intensification of antihypertensive drug therapy and closer monitoring of volume status [97]. Existing studies have associated erythropoietin-induced hypertension with increased circulating endothelin-1 concentration or enhanced vasoconstrictive response to endothelin-1 [98, 99], increased sensitivity to the pressor effect of angiotensin II [100], and increased vascular reactivity to norepinephrine [101].
5 Treatment
5.1 Non-pharmacological Management of Hypertension
Once an accurate diagnosis of hypertension is made (see above), the management of hypertension in dialysis patients should start with non-pharmacological therapeutic measures aiming to control sodium and volume excess. This includes (1) dietary sodium restriction [102, 103], (2) individualized prescription of the sodium concentration in the dialysate to avoid intradialytic sodium loading, (3) proper adjustment of dry weight, and (4) avoiding shorter dialysis. Outside the realm of hypertensive urgencies and emergencies [6], and the fact that common antihypertensive agents may be needed for other indications (i.e., β-blockers for angina symptoms, heart failure, or rate control, RAS blockers for heart failure, etc.), administration of antihypertensive drug therapy in dialysis patients considered to be volume overloaded should follow the attainment of dry weight.
5.1.1 Restricting Dietary Sodium Intake
Among dialysis patients, restricting dietary sodium is proposed as a simple and effective maneuver to limit the sense of thirst, reduce interdialytic weight gain, and facilitate the achievement of dry weight [102]. Instead of dietary sodium restriction, patients on dialysis are often instructed to avoid excess fluid accumulation during the interdialytic interval. With the exception of treating hyponatremia, there is no specific indication to prescribe fluid-restrictive diets in chronic dialysis patients [104]. Currently available recommendations suggest that among dialysis patients, dietary sodium intake should not exceed 1.5 g (or approximately 65 mmol) sodium per day [103].
5.1.2 Individualizing the Dialysate Sodium Prescription
To ensure hemodynamic stability during dialysis and limit the risk of intradialytic symptoms (i.e., disequilibrium, nausea, vomiting, muscle cramps, etc.), prescription of a high dialysate sodium concentration was initially the most preferable therapeutic choice for patients receiving long-term dialysis [105, 106]. Earlier studies supported the notion that high dialysate sodium minimizes the intradialytic hypotensive episodes without worsening interdialytic hypertension [107, 108]. However, more recent works challenged the conclusions of those studies and emphasized that a high dialysate sodium concentration may increase thirst and, therefore, interdialytic weight gain leading to the need for higher ultrafiltration during the next dialysis session [105, 106]. Indeed, in a study in 1084 hemodialysis patients, Munoz Mendoza et al. [109] found that dialysate sodium prescriptions ranging from 136 to 149 (median, 140) mEq/L, with most patients being dialyzed against a positive sodium gradient, resulted in over 90% of patients having a rise in serum sodium across dialysis and thus higher postdialysis thirst and interdialysis weight gain. A consensus document by the chief medical officers of US dialysis providers warns against the use of dialysate with a sodium concentration exceeding predialysis serum sodium [105, 106]. This increase in interdialytic weight gain leads to the need for higher ultrafiltration during the next dialysis session, which may act as a triggering factor for more frequent episodes of intradialytic hypotension and prescription of even a higher dialysate sodium concentration, precipitating in this way a vicious cycle [105, 106].
A positive intradialytic sodium balance may also arise in patients receiving sodium-profiling dialysis. A randomized crossover study of 11 dialysis patients compared the effect of performing sodium-profiling dialysis with a time-averaged concentration (TAC) of dialysate sodium of 140 mmol/L [TAC(140)] vs. sodium-profiling dialysis with a TAC of 147 mmol/L [TAC(147)] vs. conventional dialysis with a dialysate sodium of 138 mmol/L [110]. An increase in mean 24-h interdialytic BP, in interdialytic weight gain, as well as in interdialytic discomfort symptoms was evident during the period of TAC(147) sodium-profiling dialysis as compared with the periods of TAC(140) and TAC(138). Increase in interdialytic weight gain and interdialytic systolic BP was directly proportional to the TAC of the dialysate sodium [110].
The vicious cycle of intradialytic sodium loading can be interrupted by individualizing the prescription of the sodium concentration in the dialysate. A single-blind, randomized, crossover study compared the effect of individualized prescription of the dialysate sodium concentration (the dialysate sodium set to match predialysis sodium during standard dialysis applying a 138 mEq/L sodium concentration multiplied by 0.95 to allow for the Gibbs-Donnan effect) with that of a standard dialysate sodium concentration set to 138 mEq/L in nondiabetic, non-hypotension-prone dialysis patients. Compared with the period of standard dialysate sodium, a significant reduction in interdialytic weight gain (2.91±0.87 vs. 2.29±0.65 kg, P<0.001), interdialytic thirst score, and episodes of intradialytic hypotension was evident during the period of individualized dialysate sodium prescription [111]. A pilot study using a biofeedback software system to progressively reduce postdialysis plasma conductivity from 14.0 to 13.5 mS/cm [112] showed that this maneuver resulted in significant reduction of postdialysis plasma sodium from 137.8 to 135.6 mmol/L. Diffusive sodium removal in addition to convective losses induced a nearly 100 mmol/L higher net intradialytic sodium loss resulting in reduction in the extracellular body water compartment, lower interdialytic weight gain, and drop in predialysis BP [112]. In a subsequent single-blind, crossover study of 15 patients receiving thrice-weekly in-center, nocturnal dialysis, lowering the dialysate sodium concentration from 140 to 136 or 134 mEq/L for a 12-week treatment period decreased interdialytic weight gain by 0.6±0.6 kg and predialysis systolic BP by 8.3±14.9 mmHg without increasing intradialytic hypotensive episodes [113]. In a 3-week, two-arm, randomized, crossover trial of 16 dialysis patients with intradialysis hypertension, Inrig et al. [114] compared the effect of a high (5 mEq/L above serum sodium) vs. a low dialysate sodium concentration (5 mEq/L below serum sodium) on intradialytic BP and endothelium-derived vasoregulators. The weekly averaged predialysis systolic BP was lower during the period of low dialysate sodium concentration compared with dialysis treatments with high dialysate sodium (parameter estimate, −9.9 mmHg; 95% CI, −13.3 to −6.4 mmHg; P<0.001) [114]. Overall these studies suggest that a single dialysate sodium prescription may not fit all patients. Individualizing the dialysate sodium prescription may facilitate the achievement of euvolemia without aggravating the risk of intradialytic hemodynamic instability.
Similarly to the low dialysate sodium in hemodialysis patients, increasing the diffusive component of sodium removal with the use of low-sodium peritoneal dialysis fluids is suggested to be an effective maneuver to improve BP control among patients receiving peritoneal dialysis. In a nonrandomized interventional study comparing a standard vs. a low-sodium peritoneal dialysis solution substituted for one 3- to 5-h exchange over a mean follow-up period of 2 months, low-sodium concentration in the dialysate resulted in a significant increase of 30–50 mmol/dwell diffusive peritoneal sodium removal [115]. Associated benefits of this intervention were significant reductions in the sense of thirst and total body water assessed by bioelectrical impendence analysis, together with a significant fall of 8 mmHg in nighttime systolic BP [115]. Prescribing low-sodium dialysate solutions and achieving adequate volume control through icodextrin solutions may have additive benefits in patients being on a volume-expanded state. A small, open-label randomized study lasting 12 months showed that compared with standard glucose peritoneal dialysis solutions, the use of icodextrin as an osmotic agent is associated with better extracellular volume control and greater reduction in systolic and diastolic 24-h ambulatory BP [116].
5.1.3 Probing of Dry Weight
The adequate management of dry weight among dialysis patients is challenging [117]. The most important issue is the absence of a widely accepted definition of dry weight. Sinha and Agarwal [118] defined dry weight as the lowest tolerated postdialysis weight achieved through gentle and gradual reduction in postdialysis weight at which patients experience minimal signs or symptoms of either hypovolemia or hypervolemia [118].
Another challenge in the management of volume status among dialysis patients is the absence of a single clinical test to reliably adjudicate whether a patient has reached the “ideal” dry weight or whether the patient remains volume overloaded. The presence of pedal edema is frequently used in daily clinical practice as a simple physical sign to assess dry-weight achievement. The reliability of pedal edema as a sign of volume excess was investigated in a cross-sectional analysis of 146 asymptomatic dialysis patients, in which echocardiographic parameters, blood volume monitoring, plasma volume markers, and inflammatory markers were measured as exposure variables, whereas pedal edema was assessed as an outcome variable [119]. This study showed that pedal edema exhibited significant associations with several cardiovascular risk factors such as age, body mass index, and LV mass index. However, indices reflecting intravascular volume, such as inferior vena cava diameter, blood volume monitoring, and plasma volume biomarkers, were not independent determinants of the presence of pedal edema [119].
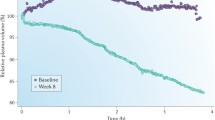
Achievement of dry weight is a long-term process, in which the interaction between the doctor and the patient plays a prominent role. Dry-weight reduction is often accompanied by uncomfortable intradialytic symptoms such as hypotension, dizziness, cramps, nausea, and vomiting. Physicians often respond falsely to these symptoms with therapeutic interventions such as cessation of ultrafiltration, intravenous saline infusion, premature termination of dialysis, increasing the dialysate sodium concentration or finally raising the dry weight, and subsequently increasing the number of prescribed antihypertensive medications, which all finally act as barriers to the dry-weight achievement [1, 106]. The strongest evidence that probing of dry weight is an effective intervention in order to improve BP control among patients on dialysis is provided by the DRIP trial [120]. In this trial, 100 long-term hypertensive dialysis patients were randomly assigned to an intensive ultrafiltration group, in which the dry weight was probed without increasing the frequency or duration of dialysis; another 50 patients were randomly assigned to a control group, in which patients had only physician visits without any modification in their volume status [120]. The primary trial end point was the difference between the ultrafiltration and control groups in the change of 44-h interdialytic ambulatory BP during follow-up. Postdialysis weight was reduced by 0.9 kg at 4 weeks and resulted in a significant reduction of 6.9 mmHg (95% CI, −12.4 to −1.3 mmHg) in systolic BP; diastolic BP exhibited also a significant drop of 3.1 mmHg (95% CI, −6.2 to −0.02 mmHg). The overall dry-weight reduction achieved at study completion (8 weeks) was 1 kg; the associated BP-lowering benefit was a reduction of 6.6/3.3 mmHg in 44-h interdialytic ambulatory BP at 8 weeks of dry-weight probing (Fig. 24.5) [120]. The DRIP trial provided the net BP-lowering efficacy of dry-weight reduction, since background antihypertensive treatment of study participants remained unchanged throughout the trial. Of importance, this benefit was seen without any deterioration in parameters of health-related quality of life [120] and with a reduction in LV chamber volume [121]. The findings of the DRIP trial are in general agreement with previous uncontrolled observations in small series of patients [122,123,124].
The effect of dry-weight reduction on changes in interdialytic (44-h) ambulatory systolic and diastolic BP over 4 and 8 weeks in hypertensive hemodialysis patients (reprinted with permission from Agarwal et al. [120])
In contrast to the above, benefits on BP control of intensification of ultrafiltration without prolonging dialysis time may be counterbalanced by a higher risk of hospitalizations for cardiovascular complications and arteriovenous fistula clotting [125]. High ultrafiltration rates increase the risk of dialysis hypotension, and in one observational study, ultrafiltration rates greater than 12.4 mL/kg per hour were associated with increased mortality [126]. Overall, dry-weight reduction may be more easily and safely achieved in multiple sessions or by prolonging the dialysis time to achieve a slower ultrafiltration rate, as discussed below.
5.1.4 Avoiding Shorter Delivered Dialysis
Current best practice guidelines recommend that patients with ESRD should receive renal replacement therapy with at least three dialysis sessions weekly, and the total duration of dialysis time should be at least 12 h per week [127]. Exception to this recommendation is proposed to be incident dialysis patients with substantial residual renal function or patients who started earlier dialysis; these specific subgroups of dialysis patients may be able to maintain the homeostasis of volume and metabolic parameters over a longer dialysis-free interval [127,128,129]. In contrast to guidelines, real-world data derived from the ESRD Clinical Performance Measures Project in the USA suggest that one quarter of the 32,065 patients participating in this program were receiving less than 3 h and 15 min of dialysis/session and only one quarter of patients were receiving an extended-time (>4 h/session) dialysis regimen [130].
Among several other potential hazards, shorter delivered dialysis is reported to be an important barrier to the achievement of adequate BP control. This notion is supported by a post hoc analysis of the DRIP trial [131], in which median intradialytic systolic BP at baseline and its change over time were modeled against the duration of delivered dialysis. At baseline, median intradialytic systolic BP was higher with fewer hours of delivered dialysis. Among patients who did not have their dry weight probed (control group), median intradialytic systolic BP followed an increasing trend over the course of the trial. Dry-weight reduction in the ultrafiltration group induced a significant drop in median intradialytic systolic BP regardless of the duration of delivered dialysis [131]. However, patients with longer delivered dialysis required fewer dialysis sessions in order to gain the BP-lowering benefit of dry-weight reduction. A similar relationship was evident between the duration of delivered dialysis and the magnitude of change in 44-h interdialytic ambulatory systolic BP over time [131].
Increasing the duration or the frequency of the delivered dialysis may represent an alternative approach to control BP among dialysis patients who either experience frequent episodes of intradialytic hemodynamic instability or remain hypertensive despite the intensification of volume withdrawal that can be achieved within the conventional thrice-weekly 12-h dialysis regimen [132]. For example, in a crossover study of 38 dialysis patients comparing the frequency of intradialytic symptoms during 5-h vs. 4-h duration dialysis sessions, the incidence of intradialytic hypotension and postdialysis orthostatic hypotension was shown to be less common during the period of extended-time dialysis [133]. This notion is also supported by several other randomized and nonrandomized observations showing that patients assigned to longer or more frequent dialysis regimens achieve adequate BP control with minimal requirements for antihypertensive medications, a benefit that is possibly mediated through better achievement of postdialysis dry weight [132, 134,135,136].
5.2 Pharmacological Management of Hypertension
Two meta-analyses of randomized controlled trials have provided evidence that BP lowering with the use of antihypertensive drugs is associated with reduced cardiovascular morbidity and mortality in dialysis patients [137, 138]. The first meta-analysis included eight randomized controlled trials incorporating data from 1697 ESRD patients and 495 cardiovascular events [138]. The weighted mean difference in the change of BP between the active treatment and control groups was −4.5 mmHg for systolic and −2.3 mmHg for diastolic BP. This BP-lowering effect of antihypertensive drug treatment was associated with 29% reduction in the risk of all-cause mortality (pooled RR, 0.71; 95% CIs, 0.55–0.92) and 29% reduction in the risk of cardiovascular mortality (pooled RR, 0.71; 95% CIs, 0.50–0.99) [138]. The second meta-analysis [137] included five randomized trials with 1202 study participants. Compared with placebo or control therapy, the overall cardiovascular benefit of BP lowering with antihypertensive therapy was a 31% reduction in the risk of future cardiovascular events (pooled HR, 0.69; 95% CIs, 0.56–0.84) [137]. In a sub-analysis according to the hypertension status of patients participating in the individual studies, it was shown that cardiovascular protection provided by BP lowering was lesser when normotensive patients were included in the analysis (pooled HR, 0.86; 95% CIs, 0.67–1.12) [137]. These meta-analyses indicate that the use of antihypertensive drugs in dialysis patients may afford cardiovascular protection both in hypertensive patients and in normotensive patients with LV systolic dysfunction [137]; the cardiovascular benefit seems to be greater for hypertensives [137].
All major antihypertensive drug classes are useful for pharmacological treatment of hypertension [1, 139, 140]. Exception may be diuretic compounds, which are generally ineffective for BP control in patients with ESRD [1, 139, 140]. Echocardiographic studies conducted in anuric hemodialysis patients showed that intravenous administration of loop diuretics, even at high doses, exerts only minimal alterations in central hemodynamic indices [141]. Given the high risk of ototoxicity, the use of loop diuretics in anuric dialysis patients should be avoided. It remains to be elucidated whether these compounds have a beneficial role in those patients with preserved residual diuresis as a therapeutic intervention targeting to enhance urine output and limit fluid accumulation between subsequent dialysis treatments [142].
5.2.1 Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
Inhibition of the RAAS is often recommended as first-line BP-lowering therapy for dialysis patients, by extrapolation of the cardiovascular benefits of RAAS-blockers in the general population. However, whether RAAS-blockade affords the same benefits in hypertensive dialysis patients with hypertensive patients in the general population still remains unclear. In the Fosinopril in Dialysis (FOSIDIAL) trial [143] (Table 24.1), 397 hemodialysis patients were randomized to receive the ACEI fosinopril (titrated up to 20 mg/day) or placebo for a mean follow-up period of 48 months. Patients participating in the FOSIDIAL trial had by protocol LV hypertrophy, but were not necessarily hypertensives. Although therapy with fosinopril resulted in a significant reduction of predialysis BP vs. placebo in the subgroup of hypertensive participants, occurrence of fatal and nonfatal cardiovascular events during the follow-up did not significantly differ between the active treatment and placebo arms (RR, 0.93; 95% CIs, 0.68–1.26) [143].
Three trials (Table 24.1) [144,145,146] all performed in Japan compared angiotensin II receptor blockers (ARBs) to placebo or active therapy. The first enrolled 80 hemodialysis patients without overt cardiovascular disease and showed candesartan was superior to placebo in improving cardiovascular event-free survival [144]. In the second, 360 hypertensive hemodialysis patients were randomly assigned to receive ARB therapy (valsartan, candesartan, or losartan) or control therapy not including ACEIs or ARBs [145]. Over a mean follow-up period of 36 months, ARB therapy was associated with a 49% reduction in the risk of cardiovascular death, nonfatal myocardial infarction (MI), stroke, coronary revascularization, and hospitalized congestive heart failure (CHF) as compared with control therapy not including RAAS inhibitors (HR, 0.51; 95% CI, 0.33–0.79) [145]. In the subsequent Olmesartan Clinical Trial in Okinawan Patients Under Okinawa Dialysis Study (OCTOPUS) trial [146], 469 hypertensive hemodialysis patients were randomized to the ARB olmesartan (10–40 mg/day) or control therapy not including ACEIs or ARBs. Over a mean follow-up of 3.5 years, incidence of all-cause death, nonfatal stroke, MI, and coronary revascularization was similar in the olmesartan and control groups (HR, 1.00; 95% CI, 0.71–1.40); mortality was also not different (Fig. 24.6) [146]. A meta-analytical estimate of the risk reduction by ARBs in these trials (which included around 900 patients and 175 deaths) showed a nonsignificant (P = 0.10) 42% risk reduction [147]. Overall, a superiority of ACEIs and ARBs over other antihypertensive drugs seems unlikely in dialysis patients, and antihypertensive treatment per se and not the use of a RAAS blocker is rather the factor reducing cardiovascular risk. It should be also noted that there are important differences between ACEIs and ARBs in renal clearance and removal during dialysis [5]; most ARBs are not dialyzed during conventional dialysis and may be therefore preferred in these patients for BP reduction.
Effects of olmesartan vs. other antihypertensive treatments on primary outcome (death, nonfatal stroke, myocardial infarction, and coronary revascularization) and all-cause mortality in hemodialysis patients in the OCTOPUS trial (reprinted with permission from Iseki et al. [146])
5.2.2 β-Blockers
Sympathetic overactivity as measured by plasma norepinephrine is a powerful predictor of death and cardiovascular events in dialysis patients [148]. Susceptibility of dialysis patients to serious arrhythmias and sudden death along with the excessive activation of the sympathetic nervous system makes β-blockers an attractive therapeutic option toward cardiovascular protection in this population [139]. In the first clinical trial with hard cardiovascular outcomes using a β-blocker in hemodialysis, 114 patients with dilated cardiomyopathy were randomly assigned to carvedilol (titrated up to 25 mg twice daily) or placebo. Over a follow-up of 2 years, carvedilol treatment improved LV systolic function and lowered by 56% the risk of all-cause hospitalization (HR, 0.44; 95% CI, 0.25–0.77) and by 49% the risk of all-cause death (HR, 0.51; 95% CI, 0.32–0.82) compared to placebo [149].
Additional support to the cardioprotective properties of β-blockade is provided by the Hypertension in Hemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) trial [150], which performed a head-to-head comparison between the β-blocker atenolol and the ACEI lisinopril (both administered in a thrice-weekly regimen immediately postdialysis) in 200 hypertensive hemodialysis patients with echocardiographically documented LV hypertrophy (Table 24.1). This study was prematurely terminated for safety reasons due to significantly higher risk of cardiovascular events in the lisinopril group, although the number of events was generally not different from that recorded in registries of hemodialysis patients. The incidence of the combined outcome of MI, stroke, hospitalized CHF, and cardiovascular death was 2.29 times higher in lisinopril than in atenolol group [incidence rate ratio (IRR), 2.29; 95% CI, 1.07–5.21] [129]. LV mass index (the primary outcome) improved to a similar extent in the atenolol and lisinopril groups [150]. However, atenolol was shown to be superior to lisinopril in terms of its BP-lowering efficacy; although no significant differences in BP were noted between groups, lisinopril-treated patients had always numerically higher BP levels and required more aggressive volume management during dialysis and administration of higher number of antihypertensive drugs as add-on therapy to achieve the prespecified home BP target of 140/90 mmHg. In a secondary analysis of the HDPAL trial, atenolol was shown to be superior to lisinopril in improving aortic pulse wave velocity [87], which is a strong and independent cardiovascular risk predictor among dialysis patients [85]. This beneficial effect of atenolol on aortic stiffness was predominantly mediated through its potent BP-lowering efficacy.
The Beta-blocker to LOwer CArdiovascular Dialysis Events (BLOCADE) trial failed to advance our understanding on the cardioprotective role of β-blockade due to the low recruitment rate in the feasibility study that resulted in a small sample size [151]. The study aimed to enroll 150 patients; among 1443 patients screened, including 176 who were already on treatment with beta-blockers, only 354 were eligible, 91 consented, and 72 entered the 6-week active treatment run-in period. Of these, only 49 participants (68%; 95% CI, 57–79%) tolerated carvedilol therapy (6.25 mg twice daily) during the run-in and progressed to randomization [151]. Narrow inclusion criteria led to exclusion of high-risk patients, who were more likely to benefit from the cardioprotective actions of carvedilol.
Although actual data are scarce, some suggest vasodilating β-blockers (i.e., carvedilol) to be particularly useful in the setting of intradialysis hypertension, as they may favorably affect endothelial dysfunction, which is suggested as a major mechanistic pathway of intradialysis hypertension [152,153,154]. In an uncontrolled interventional study of 25 patients with intradialysis hypertension, Inrig et al. [155] showed that carvedilol treatment was associated with an improvement in endothelium-dependent flow-mediated vasodilatation; this effect was accompanied by reduced occurrence of intradialytic hypertensive episodes during follow-up and with a significant drop of 7 mmHg in 44-h interdialytic ambulatory systolic BP. Again, it must be noted that there are differences in renal clearance and dialyzability between different β-blockers that need to be taken into account when prescribing these agents in hemodialysis patients [5].
5.2.3 Calcium Channel Blockers
Calcium channel blockers (CCBs) can effectively lower BP, even in the volume-expanded state [156], and are often used as combination therapy for management of hypertension in dialysis patients. Tepel et al. [157] randomized 251 hypertensive hemodialysis patients to receive amlodipine (5–10 mg/day) or placebo for 30 months (Table 24.1). Amlodipine insignificantly improved survival as compared with placebo, but reduced by 47% the composite secondary end point of all-cause death, nonfatal stroke, MI, coronary revascularization, and angioplasty for peripheral vascular disease (HR, 0.53; 95% CI, 0.31–0.93) [157]. Other small studies suggested that dihydropyridine CCBs are equally effective with ACEIs or ARBs in reducing oxidative stress and regressing LV hypertrophy and carotid intima-media thickness [158]. Data on non-dihydropyridine CCB use in hemodialysis patients are scarce; using these agents should at least follow the recommendations for the general population. An important benefit of all CCBs is that they are practically not removed during standard hemodialysis and, thus, can be dosed once daily in these patients [5].
5.2.4 Mineralocorticoid Receptor Antagonists
A cardioprotective action of mineralocorticoid receptor antagonist (MRA) therapy among dialysis patients is strongly supported by background evidence [159] and two recent trials (Table 24.1) [160, 161]. In the Dialysis Outcomes Heart Failure Aldactone Study (DOHAS), 309 oligoanuric hemodialysis patients were randomized to spironolactone (25 mg/day) or no add-on therapy for 3 years. Spironolactone reduced by 62% the risk of cardiovascular mortality or cardiovascular-related hospitalization (HR, 0.38; 95% CI, 0.17–0.83), with incidence of drug discontinuation due to serious hyperkalemia being 1.9% [160]. Another study randomized 253 patients without heart failure receiving hemodialysis or peritoneal dialysis to 2-year-long add-on therapy with spironolactone (25 mg/day) or placebo. Add-on MRA therapy reduced by 58% the occurrence of the composite primary end point of cardio-cerebrovascular mortality, aborted cardiac arrest, and sudden death (HR, 0.42; 95% CI, 0.26–0.78) [161]. The reduction in the risk of adverse clinical outcomes in these trials exceeded 50%, i.e., it was apparently superior to the effect of frequent in-center hemodialysis on the combined end point death and LVH progression [134] and largely unexpected in a population like the hemodialysis population that is notoriously less sensitive to interventions aimed at reducing death and cardiovascular events than other patient populations [162]. The safety profile of MRAs in the dialysis population was investigated in a recent study, in which 146 hemodialysis patients were randomly assigned to eplerenone (25–50 mg daily) or matching placebo for 13 weeks [163]. Eplerenone treatment significantly increased the incidence of hyperkalemia (defined as predialysis serum potassium >6.5 mmol/L) as compared with placebo (RR, 4.50; 95% CI, 1.0–20.2) [163], but permanent drug discontinuation due to hyperkalemia or hypotension, which was the primary study end point, was no different between eplerenone and placebo groups [163]. Large, properly designed studies, like the ongoing ALCHEMIST [164] (ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial; NCT01848639), are needed to assess the safety and the effectiveness of mineralocorticoid receptor blockade in ESRD.
Conclusion
Hypertension in patients undergoing hemodialysis and peritoneal dialysis patients poses almost unique diagnostic, prognostic, and therapeutic problems. Evolution of studies using home or ambulatory BP monitoring is currently needed in order to better define the true burden of hypertension, to provide solid data on hypertension prevalence and prognostic associations, and to enable international organizations to propose objective thresholds for diagnosis and targets for treatment for these patients. As sodium and volume excess is the most important contributor to BP increase in the dialysis population, non-pharmacologic interventions targeting these factors are fundamental in this population and should precede pharmacological treatment. In patients whose BP remains unresponsive to the volume management strategies, the use of antihypertensive drugs is necessary. Among dialysis patents, BP lowering with the use of antihypertensive agents is associated with improvement in cardiovascular outcomes; the use of β-blockers followed by ACEIs and ARBs should be strongly considered, on the basis of evidence suggesting that these agents likely offer cardioprotection. Additional research efforts, mainly properly designed clinical trials, are warranted to identify the optimal non-pharmacologic and pharmacologic measures to treat hypertension and reduce cardiovascular disease in dialysis patients.
References
Agarwal R, Flynn J, Pogue V et al (2014) Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol 25(8):1630–1646
Agarwal R (2010) Blood pressure and mortality among hemodialysis patients. Hypertension 55(3):762–768
Alborzi P, Patel N, Agarwal R (2007) Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2(6):1228–1234
Amar J, Vernier I, Rossignol E et al (2000) Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int 57(6):2485–2491
Levin NW, Kotanko P, Eckardt KU et al (2010) Blood pressure in chronic kidney disease stage 5D-report from a kidney disease: improving global outcomes controversies conference. Kidney Int 77(4):273–284
Zoccali C, Mallamaci F (2015) Hypertension. In: Daugirdas JT, Blake PG, Ing TS (eds) Handbook of dialysis, 5th edn. Wolters Kluwer, Philadelphia, pp 578–591
Agarwal R, Martinez-Castelao A, Wiecek A et al (2011) The lingering dilemma of arterial pressure in CKD: what do we know, where do we go? Kidney Int Suppl 1(1):17–20
Kidney Disease Outcomes Quality Initiative (K/DOQI) (2004) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43(5 Suppl 1):S1–290
Rahman M, Griffin V, Kumar A et al (2002) A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis 39(6):1226–1230
Rohrscheib MR, Myers OB, Servilla KS et al (2008) Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol 3(5):1407–1414
Agarwal R (2009) Volume-associated ambulatory blood pressure patterns in hemodialysis patients. Hypertension 54(2):241–247
Inrig JK, Patel UD, Gillespie BS et al (2007) Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis 50(1):108–118.e4
Agarwal R, Peixoto AJ, Santos SF, Zoccali C (2006) Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 1(3):389–398
Zoccali C, Tripepi R, Torino C et al (2015) Moderator’s view: ambulatory blood pressure monitoring and home blood pressure for the prognosis, diagnosis and treatment of hypertension in dialysis patients. Nephrol Dial Transplant 30(9):1443–1448
Sinha AD, Agarwal R (2009) Peridialytic, intradialytic, and interdialytic blood pressure measurement in hemodialysis patients. Am J Kidney Dis 54(5):788–791
Agarwal R, Metiku T, Tegegne GG et al (2008) Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol 3(5):1364–1372
Stergiou GS, Bliziotis IA (2011) Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens 24(2):123–134
Parati G, Ochoa JE, Bilo G et al (2016) Hypertension in chronic kidney disease part 1: out-of-office blood pressure monitoring: methods, thresholds, and patterns. Hypertension 67(6):1093–1101
Agarwal R (2010) Managing hypertension using home blood pressure monitoring among haemodialysis patients—a call to action. Nephrol Dial Transplant 25(6):1766–1771
Agarwal R, Andersen MJ, Bishu K, Saha C (2006) Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int 69(5):900–906
Agarwal R, Satyan S, Alborzi P et al (2009) Home blood pressure measurements for managing hypertension in hemodialysis patients. Am J Nephrol 30(2):126–134
Agarwal R, Light RP (2010) Median intradialytic blood pressure can track changes evoked by probing dry-weight. Clin J Am Soc Nephrol 5(5):897–904
Agarwal R, Brim NJ, Mahenthiran J et al (2006) Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 47(1):62–68
Moriya H, Ohtake T, Kobayashi S (2007) Aortic stiffness, left ventricular hypertrophy and weekly averaged blood pressure (WAB) in patients on haemodialysis. Nephrol Dial Transplant 22(4):1198–1204
Moriya H, Oka M, Maesato K, Mano T, Ikee R, Ohtake T, Kobayashi S (2008) Weekly averaged blood pressure is more important than a single-point blood pressure measurement in the risk stratification of dialysis patients. Clin J Am Soc Nephrol 3(2):416–422
da Silva GV, de Barros S, Abensur H et al (2009) Home blood pressure monitoring in blood pressure control among haemodialysis patients: an open randomized clinical trial. Nephrol Dial Transplant 24(12):3805–3811
Kauric-Klein Z, Artinian N (2007) Improving blood pressure control in hypertensive hemodialysis patients. CANNT J 17(4):24–28
Agarwal R (2015) Pro: ambulatory blood pressure should be used in all patients on hemodialysis. Nephrol Dial Transplant 30(9):1432–1437
Zoccali C, Benedetto FA, Tripepi G et al (1998) Nocturnal hypoxemia, night-day arterial pressure changes and left ventricular geometry in dialysis patients. Kidney Int 53(4):1078–1084
Tripepi G, Fagugli RM, Dattolo P et al (2005) Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int 68(3):1294–1302
Agarwal R, Andersen MJ, Light RP (2008) Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. Am J Nephrol 28(2):210–217
Parati G, Ochoa JE, Bilo G et al (2016) Hypertension in chronic kidney disease part 2: role of ambulatory and home blood pressure monitoring for assessing alterations in blood pressure variability and blood pressure profiles. Hypertension 67(6):1102–1110
McManus RJ, Caulfield M, Williams B (2012) NICE hypertension guideline 2011: evidence based evolution. BMJ 344:e181
Piper MA, Evans CV, Burda BU et al (2015) Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force blood pressure screening methods and consideration of rescreening intervals. Ann Intern Med 162(3):192–204
Georgianos PI, Sarafidis PA, Zoccali C (2015) Intradialysis hypertension in end-stage renal disease patients: clinical epidemiology, pathogenesis, and treatment. Hypertension 66(3):456–463
Inrig JK (2010) Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis 55(3):580–589
Agarwal R, Light RP (2010) Intradialytic hypertension is a marker of volume excess. Nephrol Dial Transplant 25(10):3355–3361
Van Buren PN, Kim C, Toto R, Inrig JK (2011) Intradialytic hypertension and the association with interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 6(7):1684–1691
Agarwal R, Nissenson AR, Batlle D et al (2003) Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115(4):291–297
Rahman M, Fu P, Sehgal AR, Smith MC (2000) Interdialytic weight gain, compliance with dialysis regimen, and age are independent predictors of blood pressure in hemodialysis patients. Am J Kidney Dis 35(2):257–265
Rocco MV, Yan G, Heyka RJ et al (2001) Risk factors for hypertension in chronic hemodialysis patients: baseline data from the HEMO study. Am J Nephrol 21(4):280–288
Salem MM (1995) Hypertension in the hemodialysis population: a survey of 649 patients. Am J Kidney Dis 26(3):461–468
Agarwal R (2011) Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. Am J Nephrol 34(4):381–390
Sarafidis PA, Li S, Chen SC et al (2008) Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 121(4):332–340
Sarafidis PA, Sharpe CC, Wood E et al (2012) Prevalence, patterns of treatment, and control of hypertension in predialysis patients with chronic kidney disease. Nephron Clin Pract 120(3):c147–c155
Katzarski KS, Charra B, Luik AJ et al (1999) Fluid state and blood pressure control in patients treated with long and short haemodialysis. Nephrol Dial Transplant 14(2):369–375
Hypertension, by DOPPS country and cross-section. Available at: http://www.dopps.org/annualreport/html/MCOMHTN. Accessed 16 Jul 2016
Robinson B, Fuller D, Zinsser D et al (2011) The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: rationale and methods for an initiative to monitor the new US bundled dialysis payment system. Am J Kidney Dis 57(6):822–831
Agarwal R, Bouldin JM, Light RP, Garg A (2011) Inferior vena cava diameter and left atrial diameter measure volume but not dry weight. Clin J Am Soc Nephrol 6(5):1066–1072
Port FK, Hulbert-Shearon TE, Wolfe RA et al (1999) Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33(3):507–517
Salem MM (1999) Hypertension in the haemodialysis population: any relationship to 2-years survival? Nephrol Dial Transplant 14(1):125–128
Zager PG, Nikolic J, Brown RH et al (1998) “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic Inc. Kidney Int 54(2):561–569
Lacson E Jr, Lazarus JM (2007) The association between blood pressure and mortality in ESRD-not different from the general population? Semin Dial 20(6):510–517
Inrig JK, Patel UD, Toto RD, Szczech LA (2009) Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis 54(5):881–890
Inrig JK, Patel UD, Toto RD, Reddan DN, Himmelfarb J, Lindsay RM, Stivelman J, Winchester JF, Szczech LA (2009) Decreased pulse pressure during hemodialysis is associated with improved 6-month outcomes. Kidney Int 76(10):1098–1107
Park J, Rhee CM, Sim JJ, Kim YL, Ricks J, Streja E, Vashistha T, Tolouian R, Kovesdy CP, Kalantar-Zadeh K (2013) A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int 84(4):795–802
Cocchi R, Degli EE, Fabbri A et al (1999) Prevalence of hypertension in patients on peritoneal dialysis: results of an Italian multicentre study. Nephrol Dial Transplant 14(6):1536–1540
Atas N, Erten Y, Okyay GU et al (2014) Left ventricular hypertrophy and blood pressure control in automated and continuous ambulatory peritoneal dialysis patients. Ther Apher Dial 18(3):297–304
Cnossen TT, Konings CJ, Fagel WJ et al (2012) Fluid state and blood pressure control: no differences between APD and CAPD. ASAIO J 58(2):132–136
Enia G, Mallamaci F, Benedetto FA et al (2001) Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant 16(7):1459–1464
Davies SJ (2015) What are the consequences of volume expansion in chronic dialysis patients? volume expansion in peritoneal dialysis patients. Semin Dial 28(3):239–242
Tonbul Z, Altintepe L, Sozlu C et al (2002) Ambulatory blood pressure monitoring in haemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients. J Hum Hypertens 16(8):585–589
Rodby RA, Vonesh EF, Korbet SM (1994) Blood pressures in hemodialysis and peritoneal dialysis using ambulatory blood pressure monitoring. Am J Kidney Dis 23(3):401–411
Hall JE (1991) The renin-angiotensin system: renal actions and blood pressure regulation. Compr Ther 17(5):8–17
Ritz E, Fliser D (1991) The kidney in congestive heart failure. Eur Heart J 12(Suppl C):14–20
Machnik A, Neuhofer W, Jantsch J et al (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15(5):545–552
Dahlmann A, Dorfelt K, Eicher F et al (2015) Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int 87(2):434–441
Kelley K, Light RP, Agarwal R (2007) Trended cosinor change model for analyzing hemodynamic rhythm patterns in hemodialysis patients. Hypertension 50(1):143–150
Karpetas A, Sarafidis PA, Georgianos PI et al (2015) Ambulatory recording of wave reflections and arterial stiffness during intra- and interdialytic periods in patients treated with dialysis. Clin J Am Soc Nephrol 10(4):630–638
Koutroumbas G, Georgianos PI, Sarafidis PA et al (2015) Ambulatory aortic blood pressure, wave reflections and pulse wave velocity are elevated during the third in comparison to the second interdialytic day of the long interval in chronic haemodialysis patients. Nephrol Dial Transplant 30(12):2046–2053
Bazzato G, Coli U, Landini S et al (1984) Prevention of intra- and postdialytic hypertensive crises by captopril. Contrib Nephrol 41:292–298
Kornerup HJ, Schmitz O, Danielsen H et al (1984) Significance of the renin-angiotensin system for blood pressure regulation in end-stage renal disease. Contrib Nephrol 41:123–127
Henrich WL, Katz FH, Molinoff PB, Schrier RW (1977) Competitive effects of hypokalemia and volume depletion on plasma renin activity, aldosterone and catecholamine concentrations in hemodialysis patients. Kidney Int 12(4):279–284
Lifschitz MD, Kirschenbaum MA, Rosenblatt SG, Gibney R (1978) Effect of saralasin in hypertensive patients on chronic hemodialysis. Ann Intern Med 88(1):23–27
Agarwal R, Lewis R, Davis JL, Becker B (2001) Lisinopril therapy for hemodialysis hypertension: hemodynamic and endocrine responses. Am J Kidney Dis 38(6):1245–1250
Abd ElHafeez S, Tripepi G, Mallamaci F, Zoccali C (2015) Aldosterone, mortality, cardiovascular events and reverse epidemiology in end stage renal disease. Eur J Clin Invest 45(10):1077–1086
Converse RL Jr, Jacobsen TN, Toto RD et al (1992) Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327(27):1912–1918
Hausberg M, Kosch M, Harmelink P et al (2002) Sympathetic nerve activity in end-stage renal disease. Circulation 106(15):1974–1979
Ott C, Schmid A, Ditting T et al (2012) Renal denervation in a hypertensive patient with end-stage renal disease and small arteries: a direction for future research. J Clin Hypertens (Greenwich) 14(11):799–801
Papademetriou V, Doumas M, Anyfanti P et al (2014) Renal nerve ablation for hypertensive patients with chronic kidney disease. Curr Vasc Pharmacol 12(1):47–54
Schlaich MP, Bart B, Hering D et al (2013) Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol 168(3):2214–2220
Desir GV, Wang L, Peixoto AJ (2012) Human renalase: a review of its biology, function, and implications for hypertension. J Am Soc Hypertens 6(6):417–426
Desir GV (2009) Regulation of blood pressure and cardiovascular function by renalase. Kidney Int 76(4):366–370
Malyszko J, Koc-Zorawska E, Malyszko JS et al (2012) Renalase, stroke, and hypertension in hemodialyzed patients. Ren Fail 34(6):727–731
Georgianos PI, Sarafidis PA, Lasaridis AN (2015) Arterial stiffness: a novel cardiovascular risk factor in kidney disease patients. Curr Vasc Pharmacol 13(2):229–238
Agarwal R, Light RP (2008) Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. Am J Physiol Renal Physiol 294(2):F303–F308
Georgianos PI, Agarwal R (2015) Aortic stiffness, ambulatory blood pressure, and predictors of response to antihypertensive therapy in hemodialysis. Am J Kidney Dis 66(2):305–312
Georgianos PI, Sarafidis PA, Haidich AB et al (2013) Diverse effects of interdialytic intervals on central wave augmentation in haemodialysis patients. Nephrol Dial Transplant 28(8):2160–2169
Townsend RR (2014) Pathogenesis of drug-resistant hypertension. Semin Nephrol 34(5):506–513
Vaziri ND, Ni Z, Wang XQ et al (1998) Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH. Am J Physiol 274(4 Pt 2):F642–F649
Mallamaci F, Tripepi G, Maas R et al (2004) Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J Am Soc Nephrol 15(2):435–441
Vallance P, Leone A, Calver A et al (1992) Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339(8793):572–575
Raptis V, Kapoulas S, Grekas D (2013) Role of asymmetrical dimethylarginine in the progression of renal disease. Nephrology(Carlton) 18(1):11–21
Tada T, Kusano KF, Ogawa A et al (2007) The predictors of central and obstructive sleep apnoea in haemodialysis patients. Nephrol Dial Transplant 22(4):1190–1197
Ogna A, Forni OV, Mihalache A et al (2015) Obstructive sleep apnea severity and overnight body fluid shift before and after hemodialysis. Clin J Am Soc Nephrol 10(6):1002–1010
Abdel-Kader K, Dohar S, Shah N et al (2012) Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. J Hypertens 30(5):960–966
Boyle SM, Berns JS (2014) Erythropoietin and resistant hypertension in CKD. Semin Nephrol 34(5):540–549
Carlini RG, Dusso AS, Obialo CI, Alvarez UM, Rothstein M (1993) Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int 43(5):1010–1014
Kang DH, Yoon KI, Han DS (1998) Acute effects of recombinant human erythropoietin on plasma levels of proendothelin-1 and endothelin-1 in haemodialysis patients. Nephrol Dial Transplant 13(11):2877–2883
Eggena P, Willsey P, Jamgotchian N et al (1991) Influence of recombinant human erythropoietin on blood pressure and tissue renin-angiotensin systems. Am J Physiol 261(5 Pt 1):E642–E646
Hand MF, Haynes WG, Johnstone HA et al (1995) Erythropoietin enhances vascular responsiveness to norepinephrine in renal failure. Kidney Int 48(3):806–813
Kooman JP, van der Sande F, Leunissen K, Locatelli F (2003) Sodium balance in hemodialysis therapy. Semin Dial 16(5):351–355
Bibbins-Domingo K, Chertow GM, Coxson PG et al (2010) Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362(7):590–599
Tomson CR (2001) Advising dialysis patients to restrict fluid intake without restricting sodium intake is not based on evidence and is a waste of time. Nephrol Dial Transplant 16(8):1538–1542
Santos SF, Peixoto AJ (2008) Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol 3(2):522–530
Weiner DE, Brunelli SM, Hunt A et al (2014) Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis 64(5):685–695
Cybulsky AV, Matni A, Hollomby DJ (1985) Effects of high sodium dialysate during maintenance hemodialysis. Nephron 41(1):57–61
Ogden DA (1978) A double blind crossover comparison of high and low sodium dialysis. Proc Clin Dial Transplant Forum 8:157–165
Munoz Mendoza J, Sun S, Chertow GM et al (2011) Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant 26(4):1281–1287
Song JH, Lee SW, Suh CK, Kim MJ (2002) Time-averaged concentration of dialysate sodium relates with sodium load and interdialytic weight gain during sodium-profiling hemodialysis. Am J Kidney Dis 40(2):291–301
de Paula FM, Peixoto AJ, Pinto LV et al (2004) Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int 66(3):1232–1238
Manlucu J, Gallo K, Heidenheim PA, Lindsay RM (2010) Lowering postdialysis plasma sodium (conductivity) to increase sodium removal in volume-expanded hemodialysis patients: a pilot study using a biofeedback software system. Am J Kidney Dis 56(1):69–76
Munoz MJ, Bayes LY, Sun S et al (2011) Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice-weekly in-center nocturnal hemodialysis: a quality improvement study. Am J Kidney Dis 58(6):956–963
Inrig JK, Molina C, D'Silva K et al (2015) Effect of low versus high dialysate sodium concentration on blood pressure and endothelial-derived vasoregulators during hemodialysis: a randomized crossover study. Am J Kidney Dis 65(3):464–473
Davies S, Carlsson O, Simonsen O et al (2009) The effects of low-sodium peritoneal dialysis fluids on blood pressure, thirst and volume status. Nephrol Dial Transplant 24(5):1609–1617
Paniagua R, Orihuela O, Ventura MD et al (2008) Echocardiographic, electrocardiographic and blood pressure changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney Int Suppl 108:S125–S130
Agarwal R, Weir MR (2010) Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol 5(7):1255–1260
Sinha AD, Agarwal R (2009) Can chronic volume overload be recognized and prevented in hemodialysis patients? The pitfalls of the clinical examination in assessing volume status. Semin Dial 22(5):480–482
Agarwal R, Andersen MJ, Pratt JH (2008) On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3(1):153–158
Agarwal R, Alborzi P, Satyan S, Light RP (2009) Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension 53(3):500–507
Agarwal R, Bouldin JM, Light RP, Garg A (2011) Probing dry-weight improves left ventricular mass index. Am J Nephrol 33(4):373–380
Ozkahya M, Toz H, Qzerkan F et al (2002) Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol 15(6):655–660
Kayikcioglu M, Tumuklu M, Ozkahya M et al (2009) The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant 24(3):956–962
Cirit M, Akcicek F, Terzioglu E et al (1995) ‘Paradoxical’ rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant 10(8):1417–1420
Curatola G, Bolignano D, Rastelli S et al (2011) Ultrafiltration intensification in hemodialysis patients improves hypertension but increases AV fistula complications and cardiovascular events. J Nephrol 24(4):465–473
Movilli E, Gaggia P, Zubani R et al (2007) Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 22(12):3547–3552
Tattersall J, Martin-Malo A, Pedrini L et al (2007) EBPG guideline on dialysis strategies. Nephrol Dial Transplant 22(Suppl 2):ii5–21
Kalantar-Zadeh K, Unruh M, Zager PG et al (2014) Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis 64(2):181–186
Kalantar-Zadeh K, Casino FG (2014) Let us give twice-weekly hemodialysis a chance: revisiting the taboo. Nephrol Dial Transplant 29(9):1618–1620
Foley RN, Gilbertson DT, Murray T, Collins AJ (2011) Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365(12):1099–1107
Tandon T, Sinha AD, Agarwal R (2013) Shorter delivered dialysis times associate with a higher and more difficult to treat blood pressure. Nephrol Dial Transplant 28(6):1562–1568
Georgianos PI, Sarafidis PA, Sinha AD, Agarwal R (2015) Adverse effects of conventional thrice-weekly hemodialysis: is it time to avoid 3-day interdialytic intervals? Am J Nephrol 41(4-5):400–408
Brunet P, Saingra Y, Leonetti F et al (1996) Tolerance of haemodialysis: a randomized cross-over trial of 5-h versus 4-h treatment time. Nephrol Dial Transplant 11(Suppl 8):46–51
Chertow GM, Levin NW, Beck GJ et al (2010) In-center hemodialysis six times per week versus three times per week. N Engl J Med 363(24):2287–2300
Ok E, Duman S, Asci G et al (2011) Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dial Transplant 26(4):1287–1296
Chertow GM, Levin NW, Beck GJ et al (2016) Long-term effects of frequent in-center hemodialysis. J Am Soc Nephrol 27(6):1830–1836
Agarwal R, Sinha AD (2009) Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension 53(5):860–866
Heerspink HJ, Ninomiya T, Zoungas S et al (2009) Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 373(9668):1009–1015
Denker MG, Cohen DL (2015) Antihypertensive medications in end-stage renal disease. Semin Dial 28(4):330–336
Inrig JK (2010) Antihypertensive agents in hemodialysis patients: a current perspective. Semin Dial 23(3):290–297
Hayashi SY, Seeberger A, Lind B et al (2008) Acute effects of low and high intravenous doses of furosemide on myocardial function in anuric haemodialysis patients: a tissue Doppler study. Nephrol Dial Transplant 23(4):1355–1361
Lemes HP, Araujo S, Nascimento D et al (2011) Use of small doses of furosemide in chronic kidney disease patients with residual renal function undergoing hemodialysis. Clin Exp Nephrol 15(4):554–559
Zannad F, Kessler M, Lehert P et al (2006) Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int 70(7):1318–1324
Takahashi A, Takase H, Toriyama T et al (2006) Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis—a randomized study. Nephrol Dial Transplant 21(9):2507–2512
Suzuki H, Kanno Y, Sugahara S et al (2008) Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis 52(3):501–506
Iseki K, Arima H, Kohagura K et al (2013) Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant 28(6):1579–1589
Zoccali C, Mallamaci F (2014) Pleiotropic effects of angiotensin II blockers in hemodialysis patients: myth or reality? Kidney Int 86(3):469–471
Zoccali C, Mallamaci F, Parlongo S et al (2002) Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105(11):1354–1359
Cice G, Ferrara L, D'Andrea A et al (2003) Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol 41(9):1438–1444
Agarwal R, Sinha AD, Pappas MK et al (2014) Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant 29(3):672–681
Roberts MA, Pilmore HL, Ierino FL et al (2016) The beta-blocker to lower cardiovascular dialysis events (BLOCADE) feasibility study: a randomized controlled trial. Am J Kidney Dis 67(6):902–911
El-Shafey EM, El-Nagar GF, Selim MF et al (2008) Is there a role for endothelin-1 in the hemodynamic changes during hemodialysis? Clin Exp Nephrol 12(5):370–375
Inrig JK, Van BP, Kim C et al (2011) Intradialytic hypertension and its association with endothelial cell dysfunction. Clin J Am Soc Nephrol 6(8):2016–2024
Raj DS, Vincent B, Simpson K et al (2002) Hemodynamic changes during hemodialysis: role of nitric oxide and endothelin. Kidney Int 61(2):697–704
Inrig JK, Van BP, Kim C et al (2012) Probing the mechanisms of intradialytic hypertension: a pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol 7(8):1300–1309
London GM, Marchais SJ, Guerin AP et al (1990) Salt and water retention and calcium blockade in uremia. Circulation 82(1):105–113
Tepel M, Hopfenmueller W, Scholze A et al (2008) Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant 23(11):3605–3612
Aslam S, Santha T, Leone A, Wilcox C (2006) Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int 70(12):2109–2115
Pitt B, Rossignol P (2014) Mineralocorticoid receptor antagonists in patients with end-stage renal disease on chronic hemodialysis. J Am Coll Cardiol 63(6):537–538
Matsumoto Y, Mori Y, Kageyama S et al (2014) Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol 63(6):528–536
Lin C, Zhang Q, Zhang H, Lin A (2016) Long-term effects of low-dose spironolactone on chronic dialysis patients: a randomized placebo-controlled study. J Clin Hypertens (Greenwich) 18(2):121–128
Kramann R, Floege J, Ketteler M et al (2012) Medical options to fight mortality in end-stage renal disease: a review of the literature. Nephrol Dial Transplant 27(12):4298–4307
Walsh M, Manns B, Garg AX et al (2015) The safety of eplerenone in hemodialysis patients: a noninferiority randomized controlled trial. Clin J Am Soc Nephrol 10(9):1602–1608
Brest UH (2000–2016) ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial (ALCHEMIST). In: ClinicalTrials.gov. National Library of Medicine (US), Bethesda, MD. Available from https://clinicaltrials.gov/show/NCT01848639: NCT01848639
Conflicts of Interest
None relevant to this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Sarafidis, P.A., Georgianos, P., Zoccali, C. (2018). Hypertension in Dialysis Patients: Clinical Epidemiology, Pathogenesis, Diagnosis, and Treatment. In: Berbari, A., Mancia, G. (eds) Disorders of Blood Pressure Regulation. Updates in Hypertension and Cardiovascular Protection. Springer, Cham. https://doi.org/10.1007/978-3-319-59918-2_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-59918-2_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59917-5
Online ISBN: 978-3-319-59918-2
eBook Packages: MedicineMedicine (R0)