Abstract
The main cause of cancer-related deaths is metastasis-spreading of cancer cells to different sites in the body. A critical step in metastasis formation is invasion of cells through the surrounding tissue. During invasion, cancer cells change their shape and apply forces. We have previously identified that about 30% of single, metastatic, breast cancer cells will indent impenetrable synthetic, non-degradable, polyacrylamide gels, when gel stiffness is in the range 1–10 kPa. By measuring the depth of indentation of an initially flat gel and monitoring time-dependent microscopic changes in cell morphology, we were able to distinguish between benign and metastatic cells, also identifying their metastatic potential (MP); benign cells do not indent the gels. Recent works have indicated that metastases from solid tumors occur predominantly by collective cell invasion. Hence, in the current study we evaluate the mechanical interactions of cell clusters with the impenetrable gel. We observe that indenting subpopulations of metastatic cells are doubled in clusters, and cells are also indent more deeply; this increases likelihood to successfully form metastasis in the body. Concurrently, double the fraction of high MP cells indent gels as compared to low MP cells, while benign cells do not indent even in clusters. We also show that the gel platform can be used to determine the time-dependent impact of chemotherapeutics on the cells’ ability to apply forces and indent gels. Our approach can provide a rapid, mechanical prediction of the likelihood for invasiveness of cancer cells and can further be applied in a patient-specific approach, thus providing a personalized prognosis that may improve treatment of cancer patients and increase their life expectancy.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Introduction
The main cause of cancer-associated mortality is metastasis-spreading of cancer cells to different sites in the body; this accounts for 90% of deaths. A critical step in metastasis formation is invasion of cells through the surrounding tissue, including cells and the extracellular matrix. During invasion, cancer cells change their shape and apply forces. We have previously identified that about 30% of single, high metastatic potential (MP), breast cancer cells will indent impenetrable synthetic, non-degradable, polyacrylamide gels, when gel stiffness is in the range 1–10 kPa (Kristal-Muscal et al. 2013, 2015). By measuring the depth of indentation of an initially flat gel and monitoring time-dependent microscopic changes in cell morphology, we were able to distinguish between benign and metastatic cells, also to identify their metastatic potential (Kristal-Muscal et al. 2013, 2015; Dvir et al. 2015); benign cells do not indent the gels.
Depending on the cell type and tissue environment, cells can migrate and invade in two main ways: individually or collectively, as multicellular groups. Most invasive solid tumors display predominantly collective invasion (Cheung et al. 2013), in which groups of cells invade their surroundings while cells remain either physically or chemically connected (Friedl et al. 2012). Hence, in the current study we evaluate the mechanical interactions of cell clusters with an impenetrable gel.
Materials and Methods
Cell Culture
We have used three commercially available human, epithelial breast cell lines: high MP cells-MDA-MB-231, low MP cells-MDA-MB-468, benign cells-MCF-10A (all cells from ATCC, Manassas, VA). Both metastatic cell lines were collected from lung metastases. The cells were cultured in 37 °C, 5% CO2, 90% humidity and in their appropriate media: Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen life technologies, CA) with supplementation of 10 vol.% fetal bovine serum (Hyclone, ThermoFisher Scientific, Waltham, MA), 1 vol.% each of L-glutamine, penicillin-streptomycin and sodium pyruvate (all from Biological Industries, Kibbutz Beit Haemek, Israel) for metastatic cells or with supplementation of 5 vol.% horse serum (Hyclone, ThermoFisher Scientific, Waltham, MA), 0.05 vol.% Hydrocortisone, 0.01 vol.% Cholera toxin, 0.1 vol.% Insulin (all from Sigma, St Louis, MO), 1 vol.% penicillin-streptomycin, 1 vol.% L-Glutamine (both from Biological Industries, Israel) and 0.01 vol.% Human EGF (Peprotech Asia, Israel) for benign cells.
Hydrogel Preparation
We have used polyacrylamide (PAM) hydrogel with fluorescent beads (200 nm) imbedded at its surface, prepared according to an established protocol (Kristal-Muscal et al. 2013, 2015). The gels were prepared at stiffness of 2400 Pa which is in a range of physiological stiffnesses of breast and lung (Levental et al. 2007).
Imaging and Indentation Depth Calculation
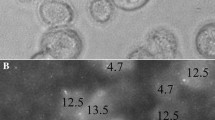
We seeded on separate gels 300,000 of each cell type, within their respective media. Approximately 45 min after seeding we began imaging the gels with the cells using an inverted, epifluorescence Olympus IX81 microscope, equipped with a 60x/0.7NA differential interference contrast (DIC, Nomarsky optics) air-immersion, long working-distance objective lens. At each randomly chosen field-of-view on the gel (typically 10 fields per gel), at least three images were taken: (1) a DIC image of the cells on the gel, (2) a fluorescence image of the particles embedded at the focal plane of the gel surface, and (3) a series of fluorescence images at the lowest focal depth where particles were in focus, identifying the indentation depth of each cell (Fig. 1). The indentation depth was calculated from difference in focal planes of fluorescent images. We also determined the number of indenting cells out of the total cells.
Indentation depth measurement. The highly metastatic potential cells seeded on 2.4 kPa PAM gel pushing the fluorescent beads imbedded at gel surface into the gel. The indentation depth is measured from difference in focal planes of beads at gel surface and beads pushed in-depth under the indenting cells. Scale bar is 20 µm
Statistical Analysis
Statistical analysis was performed to compare between the different cells types or to compare single and multiple cells of the same type. For comparison between the means of two groups, the general, linear mixed model, which is a multivariate regression method that helps to generalize the analysis of variance (ANOVA) was used (Edwards 2000). In all cases, a p value of less than 0.05 is considered statistically significant.
Results
We evaluated the number of indenting cells of each cell within the group, comparing high metastatic potential, low MP, and benign cells. We observe that the percentage of indenting subpopulations of metastatic cells are doubled in clusters, while benign cells almost did not indent the gels regardless of seeding density (Fig. 2). Concurrently, double the fraction of high MP cells indent gels as compared to low MP cells, while benign cells do not indent even in the 2-dimensional clusters. That could be explained by higher aggressiveness of cancer clusters compared to single cancer cell (Clark and Vignjevic 2015). Amplified invasiveness of cancer cell clusters was shown decades ago, when mice injected with 3-dimensional clumps of tumor cells had developed a higher number of metastases when compared to mice injected with an equal total number of single cancer cells (Fidler 1973).
The adjacent cells also indent more deeply. While single cells display Gaussian-like distribution of indentation depths (not shown) with no cells indenting deeper than 10 µm, the multicellular groups display a wide, bi-modal-like distribution with appearance of second indentation depth peak (Fig. 3). The indentation depths of single cells with high and low MP did not significantly differ. However, when cells are adjacent and in group organization, most of the high MP cells indented more deeply, in the second indentation peak. In contrast, most of the low MP cells still indented similarly to the single cells. Therefore we could distinguish between the cells with the high and low MP based on the number of deeply indenting cells. A similar phenomenon was obtained with intravital multiphoton microscopy for in vivo migration of cell clusters, while the velocity of single highly invasive (MDA-MB-231) and invasive (TN1) breast cancer cells was not significantly different, the multicellular groups of highly invasive cells enhance their velocity significantly (Patsialou et al. 2013).
The percentage (from total indenting cells) of high (green diamonds) and low (red circles) MP cancer cells indenting to average indentation depths (error bars are standard deviations). All single cells (empty markers) not indenting deeper than 10 µm. Most of adjacent cells with high MP indenting to the second indentation peak while most of the adjacent cells with low MP indenting to the first peak
Discussion
We found evidence that the groups of metastatic breast cancer cells from cell lines are able to indent in larger numbers and more deeply than single cells. That likely indicates that cell-groups are more aggressive/invasive than single cells and the proximity of metastatic cells increases likelihood to successfully form metastasis in the body. Using this approach we may be able to provide a rapid, mechanical prediction of the likelihood for invasiveness of cancer cells. Definitely, before our approach could be used as metastasis prediction platform a lot of future work with various cell lines and cells resected from primary tumors should be done. This approach can be potentially applied in a patient-specific way, thus providing a personalized prognosis that may improve treatment of cancer patients and increase their life expectancy.
References
Cheung KJ, Gabrielson E, Werb Z, Ewald AJ (2013) Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155(7):1639–1651
Clark AG, Vignjevic DM (2015) Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol 36:13–22
Dvir L, Nissim R, Alvarez-Elizondo MB, Weihs D (2015) Quantitative measures to reveal coordinated cytoskeleton-nucleus reorganization during in vitro invasion of cancer cells. New J Phys 17(4):43010
Edwards LJ (2000) Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol 30(4):330–344
Fidler IJ (1973) The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 9(3):223–227
Friedl P, Locker J, Sahai E, Segall JE (2012) Classifying collective cancer cell invasion. Nat Cell Biol 14(8):777–783
Kristal-Muscal R, Dvir L, Weihs D (2013) Metastatic cancer cells tenaciously indent impenetrable, soft substrates. New J Phys 15:35022
Kristal-Muscal R, Dvir L, Schvartzer M, Weihs D (2015) Mechanical interaction of metastatic cancer cells with a soft gel. Procedia IUTAM 12:211–219
Levental I, Georges PC, Janmey PA (2007) Soft biological materials and their impact on cell function. Soft Matter 3(3):299–306
Patsialou A, Bravo-Cordero JJ, Wang Y, Entenberg D, Liu H, Clarke M, Condeelis JS (2013) Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. Intravital 2(2):e25294
Acknowledgements
The work was partially supported by The Technion EVPR Funds-The Elias Fund for Medical Research and The Karbeling Fund for Bio-Medical Engineering Research, and also by a grant from the Ministry of Science, Technology and Space, Israel, and the National Science Council (NSC) of Taiwan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Merkher, Y., Weihs, D. (2018). Proximity of Metastatic Cells Strengthens the Mechanical Interaction with Their Environment. In: Gefen, A., Weihs, D. (eds) Computer Methods in Biomechanics and Biomedical Engineering. Lecture Notes in Bioengineering. Springer, Cham. https://doi.org/10.1007/978-3-319-59764-5_31
Download citation
DOI: https://doi.org/10.1007/978-3-319-59764-5_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59763-8
Online ISBN: 978-3-319-59764-5
eBook Packages: EngineeringEngineering (R0)