Abstract
Withania somnifera is a high value medicinal plant of the family Solanaceae. It is known for its anti-tumour, anti-stress, anti-aging, cardio-protective, neuro-protective and anti-inflammatory properties. Its pharmaceutical properties are attributed to a wide range of secondary metabolites, such as steroidal lactones, alkaloids, glycowithanolides, flavanol glycosides, phenolics and sterols. The traditional cultivation of W. somnifera is limited mainly by poor seed viability and germination, low yield and inconsistency in production of secondary metabolites. The infestation with various pests and pathogens also throw a major challenge in its cultivation. Biotechnological approaches involving organ, tissue and cell culture offer potential solution to the existing problems. In vitro propagation helps in rapid multiplication of elite cultivars and facilitate in raising quality planting materials. Genetic manipulation and secondary metabolite engineering hold great promise for enhancement of secondary metabolites and for overall crop improvement. The present chapter briefly discuss the challenges in W. somnifera and present a quick overview of biotechnological advances to address these challenges. It also highlights the futuristic approaches that would lay a foundation in the conglomeration of W. somnifera as an ideal model medicinal plant.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Withania somnifera
- In vitro propagation
- Secondary metabolites
- Stress
- Cell suspensions
- Genetic transformation
1 Introduction
Withania somnifera (L.) Dunal is a high value medicinal plant and is extensively used in Indian, African and Unani traditional system of medicine. It belongs to the family Solanaceae and is commonly referred to as Ashwagandha, Asgandh, Winter cherry or Indian ginseng. W. somnifera enjoys a position in the monographs on selected medicinal plants prepared by World Health Organization (WHO) (Mirjalili et al. 2009) and is among top 32 important medicinal plants chosen by National Medicinal Plant Board of India (www.nmpb.nic.in) due to its immense demand in the domestic and international markets (Prajapati et al. 2006). The genus Withania includes more than 23 species which are widely distributed in tropical and subtropical areas such as dry regions of Africa, South Asia and Central Asia, particularly in Bangladesh, Pakistan, Afghanistan, Sri Lanka, Egypt, Morocco, South Africa, India, Congo and Jordon (Kumar et al. 2007, 2011).
The plant is dicotyledonous, erect, woody evergreen, branched shrub that grows about 30–75 cm in height. The leaves are simple, petiolate, ovate, entire and arranged in alternate fashion (Mirjalili et al. 2009). Axil contains a cymose cluster of 5–25 inconspicuous pale green flowers. The roots are cylindrical and fleshy with light brown epidermis and white medulla. Flowers are 4–6 mm in diameter having short pedicel, gamosepalous, 5 sepals persistent and having acute linear lobes. Its corolla is gamopetalous, having 5-lobes that are recurved or spreading, acute pubescent and greenish yellow while stamens arise from the base of petals and are slender filaments. Fruit is enclosed in the green persistent calyx. It is green when unripe and turns orange-red when mature enclosing numerous small seeds (Singh and Kumar 1998). However, considerable morphogenetic variability exists in different accessions of W. somnifera (Kumar et al. 2007).
2 Economic Importance
W. somnifera is used in various forms (ointments, decoctions, powder, infusions and syrup) as herbal medicine (Kumar et al. 2007; Davis and Kuttan 2000). The extracts and different bioactive constituents are known to possess anticancer, anti-convulsant, anti-oxidative, anti-aging, anti-spasmodic, anti-inflammatory, cardio-protective, immunomodulatory, adaptogenic and neuroprotective properties (Singh et al. 2015b). The pharmacological traits of W. somnifera are due to the existence of a wide range of structurally diverse set of secondary metabolites in leaves and roots. Several bioactive alkaloids and phytochemicals such as tropine, psudotropine, hygrine, ashwagandhine, choline, cuscohygrine, flavanol glycosides, isopelletierine, anaferine, tropine, sitoindosides (saponins), anhygrine,withanolides, withanosomine, glycowithanolides, withanamides, sterols and phenolics are present in different parts of the plant (Misra et al. 2005, 2008; Matsuda et al. 2001; Chatterjee et al. 2010; Chaurasiya et al. 2012).
Conventionally, leaves and roots of W. somnifera are principally used in preparation of herbal medicines (Jayaprakasam et al. 2003; Kumar et al. 2011). Recently NMR and chromatographic techniques have been employed for metabolic profiling of leaf and root extract of W. somnifera. A total of 48 primary and secondary metabolites from roots and 62 from leaves have been reported. Among these, 29 metabolites are common to both the tissues. However, a noteworthy difference in the profile of withanolides has been reported in leaf and root tissues (Chatterjee et al. 2010). Withaferin A and Withanone are mainly found in leaves, whereas root is the major site of withanolide A (Singh et al. 2015b). Along with spatial variations, the distribution of secondary metabolites also shows temporal as well as quantitative and qualitative variations with respect to different chemotypes and growth conditions (Chatterjee et al. 2010; Dhar et al. 2013). Further, W. somnifera from different origins can be discriminated by the ratio of two groups of withanolides viz.4-OH and 5,6-epoxy withanolides and 5-OH and 6,7-epoxy withanolides in their leaves (Namdeo et al. 2011). Age of the plant has a significant bearing on the metabolic contents of the plant. The content of Withaferin A increases in leaves and roots with the growth of the plant from young (6 weeks old) to mature (12–18 weeks old) stage (Pal et al. 2011). However, accumulation of most of the withanolides declined at over-maturation stage (Dhar et al. 2013).
3 Major Challenges and Strategies for Crop Improvement in W. somnifera
Though W. somnifera is an important medicinal plant, it encounters several challenges in its mass propagation, improvement/production of secondary metabolites and protection from various biotic and abiotic stresses. The present chapter highlights some of the challenges and research endeavour to address these emerging issues.
3.1 Mass Propagation
W. somnifera is conventionally propagated via seeds. However, its traditional cultivation is restricted due to poor seed germination, low percentage of seed viability and seedling survival (Vakeswaran and Krishnasamy 2003), long gestation period between planting and harvesting (Rani et al. 2003) and susceptibility towards pathogens (Pati et al. 2008) and pests (Sharma and Pati 2011b). Further, effect of geographical and seasonal variations on the yield and composition of secondary metabolites in plants, low yield of withanolides under in vivo conditions, a narrow genetic base and presence of intraspecific chemotypic variations impose a challenge in its cultivation and improvement.
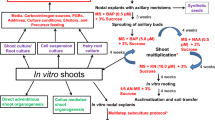
Biotechnological approach such as micropropagation provides a suitable option for rapid multiplication and raising uniform quality clones (Fig. 22.1). In W. somnifera, micropropagation has been performed using different explants viz. seeds (Supe et al. 2006; Sen and Sharma 1991), shoot tips (Furmanowa et al. 2001; Ray and Jha 2001; Sivanesan 2007; Ahmad Baba et al. 2013; Sen and Sharma 1991) and nodal segments (Sabir et al. 2008; Soni et al. 2011; Fatima et al. 2011; Sivanandhan et al. 2011). Most of these studies have documented the use of Murashige and Skoog’s (Murashige and Skoog 1962) medium for seed germination and micropropagation. Seed germination relies largely on the type of plant growth regulators (PGR), light and temperature. Maximum seed germination has been reported with GA at 25 °C (Khanna et al. 2013). Among different carbon sources, sucrose (2–3%) is mainly used for the proliferation of shoots and rooting (Sivanesan and Murugesan 2008).The growth and proliferation of microshoots and their rooting is significantly affected by the exogenous supply of PGRs. BAP is most effective for the formation of multiple shoots and its proliferation (Soni et al. 2011; Ray and Jha 2001; Nayak et al. 2013). However, BAP in combination with IAA (Sivanesan 2007; Sivanesan and Murugesan 2008) or IBA (Furmanowa et al. 2001; Sen and Sharma 1991) or KN (Sabir et al. 2008) have also been frequently used for multiple shoot formation and its proliferation. Plant growth medium supplemented with inorganic nutrients viz. ZnSO4, CuSO4 and polyamines showed enhanced shoot proliferation (Fatima et al. 2011; Sivanandhan et al. 2011). BAP was also found to be most suitable in liquid culture of W. somnifera (Sivanandhan et al. 2012). However, hyperhydricity of shoots limit the use of liquid culture system in W. somnifera. Microshoots have also been successfully rooted in both MS and half-strength MS medium. Among various auxins, IBA is most effective for in vitro rooting (Udayakumar et al. 2013; Ghimire et al. 2010). The rooted microshoots are hardened in a mixture of sand and soil (1:1) and the plantlets are established under field conditions (Kulkarni et al. 2000; Supe et al. 2006).
3.2 Withanolide Biosynthesis and Their Modulation in W. sominifera
W. somnifera has been extensively studied due to possession of a wide range of pharmaceutically important secondary metabolites. Among these, the major metabolites are withanolides that are a group of steroidal lactones (C-28) based on ergostane skeleton and constitute the prime metabolites of W. somnifera. Withanolides are synthesized from 24-methylene cholesterol, which itself originate from isoprenoid pathway (Sangwan et al. 2008). In plants, the biosynthesis of isoprenoid occurs via two independent pathways, Mevalonate (MVA) pathway and 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway (Fig. 22.2). The predominant MVA pathway operates in cytosol and accounts for 75% of carbon contribution for the synthesis of withanolides, whereas, non-MVA/MEP pathway operates in the plastids and contributes to 25% of carbon for the synthesis of withanolides (Chaurasiya et al. 2012). During MVA pathway, acetyl-CoA undergoes activation to form acetoacetyl-CoA. Acetoacetyl-CoA molecule condenses with acetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) in the presence of enzyme HMG-CoA synthase (HMGS). In an irreversible reaction catalysed by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), 3-hydroxy-3-methylglutaryl-CoA is converted into mevalonic acid. Next two steps are phosphorylation reactions that are catalysed by mevalonate kinase and phosphomevalonate kinase that results in the formation of 5-phosphomevalonate and 5-pyrophosphomevalonate, respectively. 5-pyrophosphomevalonate leads to the formation of 3-isopentenyl pyrophosphate (IPP) with the help of enzyme mevalonate-5-pyrophosphate decarboxylase. IPP condenses with its isomer 3,3-dimethyl allyl pyrophosphate (DMAPP) to yield geranyl pyrophosphate (GPP) in a reaction catalysed by farnesyl diphosphate synthase (FPPS). Further, trans-geranyl pyrophosphate condenses with another molecule of IPP to form farnesyl pyrophosphate (FPP). In the subsequent step, two molecules of farnesyl diphosphate condense by the enzyme squalene synthase (SQS) to yield squalene, a liner 30-carbon compound. Squalene epoxidase (SE) then convert squalene into 2,3-oxidosqualene, which after ring closure, yield various steroidal triterpenoidal skeletons (Singh et al. 2015b; Mirjalili et al. 2009).
Withanolide biosynthesis pathway and site of action of selected inhibitors. HMG-CoA 3-hydroxy-3-methylglutaryl-CoA, HMGS HMG-CoA synthase, HMGR 3-hydroxy-3-methylglutaryl-CoA reductase, IPP 3-isopentenyl pyrophosphate, DMAPP 3,3-dimethyl allyl pyrophosphate, IPPI isopentenyl pyrophosphate isomerase, GPP geranyl pyrophosphate, FPPS farnesyl diphosphate synthase, SQS squalene synthase, SE squalene epoxidase, CAS cycloartenol synthase, SMTI sterol methyltransferaseI, SMOI sterol-4ɑ-methyl oxidase 1, CECI cycloeucalenol cycloisomerase, OBT obtusifoliol 14-demethylase, FK delta 14-sterol reductase, HYDI C-7,8 sterol isomerase, DWFI delta-24 sterol reductase, SMO2 sterol-4a-methyl oxidase 2, STEI C-5 sterol desaturase, DWF5 sterol delta-7 reductase, BR6OX2 brassinosteroid-6-oxidase 2, GA-3P d-glyceraldehyde-3-phosphate, DXP 1-deoxy-d-xylulose-5-phosphate, DXS DXP synthase, DXR DXP reductoisomerase, MEP 2-methyl d-erythritol 4-phosphate, CMS 4-(cytidine-5-diphospho)-2-C-tmethyl-d-erythritol synthase, CDP-ME 4-diphospho-cytidyl-2-methyl-d-erythritol, CMK 4-(cytidine-5-diphospho)-2-C-methyl-d-erythritol kinase, CDP-MEP 2-C-methyl-d-erythritol-2-phosphate, MCS 2-C-methyl-d-erythritol-2,4-cyclodiphosphate synthase, ME-cPP – 2-C-methyl-D-erythritol-2,4-cyclodiphosphate, HDS hydroxyl methyl butenyl 4-diphosphate synthase, HMBPP hydroxyl methyl butenyl 4-diphosphate, HDR hydroxy methyl butenyl 4-diphosphate reductase, GPP geranyl diphosphate, GPS GPP synthase, GGPP geranylgeranyl diphosphate, GGPS geranylgeranyl diphosphate synthase, ABA abscisic acid
MEP pathway starts with the condensation of pyruvate with d-glyceraldehyde-3-phosphate (GA-3P) to yield 1-deoxy-d-xylulose-5-phosphate (DXP) and this reaction is catalysed by DXP synthase (DXS). DXP is further converted into 2-methyl d-erythritol 4-phosphate (MEP) by the enzyme DXP reductoisomerase (DXR). 4-(cytidine-5-diphospho)-2-C-tmethyl-d-erythritol synthase (CMS) in a CTP-dependent reaction catalyse the conversion of MEP into 4-diphospho-cytidyl-2-methyl-d-erythritol (CDP-ME). The later then undergoes phosphorylation to form 2-C-methyl-d-erythritol-2-phosphate (CDP-MEP) by 4-(cytidine-5-diphospho)-2-C-methyl-d-erythritol kinase (CMK). Further, in a reaction catalysed by 2-C-methyl-d-erythritol-2,4-cyclodiphosphate synthase (MCS), CDP-MEP leads to the formation of 2-C-methyl-d-erythritol-2,4-cyclodiphosphate (ME-cPP). In the subsequent steps, ME-cPP yield hydroxyl methyl butenyl 4-diphosphate (HMBPP) in a reaction catalysed by hydroxyl methyl butenyl 4-diphosphate synthase (HDS). The enzyme hydroxy methyl butenyl 4-diphosphate reductase (HDR), converts HMBPP into a mixture of IPP and DMAPP (Hunter 2007; Rodriguez-Concepcion and Boronat 2002).
There has been a huge interest in enhancement of withanolide production in W. somnifera. However, the major bottlenecks in this direction is the existence of chemotypic variations (Bhatia et al. 2013), unavailability of information for genome sequence and the cumbersome process of chemical synthesis of withanolides (Grover et al. 2013). In recent years various strategies have been adapted to modulate the withanolide production in W. somnifera.
3.3 In Vitro Culture System and Their Potential in Modulation of Withanolide Production
In vitro strategies viz. organ, tissue and cell culture and genetic manipulations can potentially overcome the challenges faced to increase the production of pharmaceutically important secondary metabolites under in vivo conditions. In W. somnifera, in vitro culture system is used as an alternative system for production of secondary metabolites. It has high metabolic rate and active growth in a short time period, leading to increased secondary metabolite production and its accumulation. A significant increase in withanolides production has been shown in in vitro shoot culture system as compared to field grown plants (Sharada et al. 2007; Sabir et al. 2008).
In in vitro culture system, the secondary metabolite production is governed by media formulations, type and concentration of carbon source and PGR. Half strength MS medium is most efficient for both the production of withanolide A and biomass accumulation in adventitious root culture (Praveen and Murthy 2012 & Praveen and Murthy 2013). In in vitro shoot culture, the concentration of sucrose is directly proportional to withaferin A and withanolide D production (Sivanandhan et al. 2015b). In most of the reports, BAP has been documented to be most suitable for withanolide production (Ray and Jha 2001; Mir et al. 2014). However, MS medium supplemented with a combination of BAP and KN resulted in higher accumulation of withanolide A (Sangwan et al. 2007). Differential accumulation of withanolides has been recorded in in vitro flowers and fruits. In vitro fruits have been shown to accumulate enhanced level of withanolide A and withanolide B, whereas, the level of withaferin A was more in in vitro flowers as compared to fruits. However, withanones did not show significant difference in in vitro fruits and flowers (Sivanandhan et al. 2015b). Further, exogenous application of elicitors such as MeJ and SA, and polyamines in the shoot culture medium led to marked improvement in secondary metabolite production (Sivanandhan et al. 2011, 2012).
Cell suspension culture holds the potential for enhancing secondary metabolites production (Mulabagal and Tsay 2004) and is also used to study cellular and molecular processes of secondary metabolite production and accumulation. The potential of cell suspension culture is affected by various factors, such as media composition, source of carbon and PGRs. MS medium is most effective for both the production of withanolide A and biomass accumulation (Nagella and Murthy 2010, 2011). As compared to glucose, fructose and maltose, sucrose was reported to be the best source of carbon for production of withanolide A (Sivanandhan et al. 2013b; Nagella and Murthy 2011). Manipulation of medium salt concentrations has important bearing in enhancing the production of withanolides. It has been observed that concentration of nitrogen and ammonia/nitrate ratio has great influence on the production of secondary metabolites. A high NO3 and low NH4 concentration supports both cell growth and production of withanolide (Nagella and Murthy 2011). PGRs also influence the potential of cell suspension culture for secondary metabolite production. MS medium having KN and 2,4-D led to higher accumulation of withanolide A (Nagella and Murthy 2011). In addition, elicitation acts as a promising strategy to raise the production of pharmaceutically important secondary metabolites in cell suspension cultures. Inorganic compounds viz. MeJ, SA and arachidonic acid, and biotic factors viz. components of plant cell wall and culture filtrates of Fusarium solani, Alternaria alternata and Verticilium dahilae act as potential elicitors (Sivanandhan et al. 2013b).
3.4 Enhancing Secondary Metabolite Production by Genetic Manipulation
Agrobacterium - mediated genetic transformation provides an excellent opportunity to study the function of various genes, understand their regulation and for increasing the productivity of novel secondary metabolites (Cucu et al. 2002). Several factors such as Agrobacterium strain, time of infection, method of co-cultivation, concentration of phytohormones, selection conditions, type and age of explant and germplasm of the plant have significant bearing on transformation efficiency (Herrera-Estrella et al. 2004). The earliest attempt to transform W. somnifera was made by infecting its leaves with wild type octopine and nopaline strains of A. tumefaciens, resulted to shooty teratomas only (Ray and Jha 1999). The fertile transgenic plants of W. somnifera were developed with very low transformation efficiency (1.67%) by infecting the leaf segments with A. tumefaciens (LBA4404) harbouring pIG121Hm vector (Pandey et al. 2010). The leaf segments were co-cultivated with A. tumefaciens in MS medium fortified with 8.9 μM BAP and 8.0 μM IAA. The growth of A. tumefaciens was suppressed by using augmentin and transformants were selected on 50 mg/l kanamycin. The transformation efficiency was improved up to 10% by employing the techniques of vacuum infiltration and sonication. Addition of thiol compounds, sodium thiosulphate (125 mg/l), L-cysteine (100 mg/l) and dithiothreitol (75 mg/l) in co-cultivation medium were also found to be beneficial in improving the transformation efficiency of W. somnifera (Sivanandhan et al. 2015a).
The development of genetic transformation system offers the advantage of exploring systematic metabolic engineering by modulating the expression of the key genes of withanolide biosynthetic pathway. Overexpression of squalene synthase, a rate limiting enzyme in the biosynthesis of withanolide, resulted in significant enhancement in Withanolide A content (2.5- folds) and squalene synthase activity (4-folds) in recombinant cell lines as compared to control. Withaferin A was additionally produced in transformed cell lines which was not produced in non-transformed cells (Grover et al. 2013). Transgenic plants overexpressing squalene synthase gene showed increase in the expression of squalene synthase and total withanolide content upto 2–5 folds and 1.5–2 folds, respectively (Patel et al. 2015). Due to significant enhancement in the production of withanolides in transformants, this strategy can be explored for effective metabolic engineering.
Transgenic hairy root cultures have revolutionized the secondary metabolites production due to their capacity to synthesis the metabolites at a much faster rate. Hairy roots results from integration of T-DNA of pRi plasmid (root inducing plasmid) from Agrobacterium rhizogenes to the susceptible explant and their induction frequency can be enhanced by subjecting the explants to sonication (15 s) followed by heat treatment for 5 min at 41 °C (Thilip et al. 2015). Hairy root cultures are genetically stable, produce biomass in large scale and are easily maintained. Various parameters viz. type and age of explant, culture medium, Agrobacterium stain, concentration of acetosyringone, and co-cultivation period are critical in determining transformation efficiency (Sivanandhan et al. 2014; Pawar and Maheshwari 2004). In W. somnifera, leaves and cotyledons show higher transformation potential compared to other explants (Murthy et al. 2008; Pawar and Maheshwari 2004). MS (Murashige and Skoog) medium is best suited for hairy roots growth and biomass accumulation (Murthy et al. 2008; Saravanakumar et al. 2012). Manipulation with source of carbon and its concentration results in differential accumulation of withanolides in hairy root cultures of W. somnifera. Low concentration of sucrose (3%) is effective for the growth of transformed hairy roots and production of withaferin A and withanolide A (Praveen and Murthy 2012). Whereas, high concentration of sucrose (4%) results in increased production of Withaferin A in hairy roots. Supplementing the medium with glucose (5%) leads to enhanced production of withanolide A as well as withaferin A (Doma et al. 2012). Withanolide production can also be increased in hairy root cultures by manipulating the concentration of macroelements and nitrogen source. Growth medium containing moderate concentration of NH4 (14.38 mM) with higher concentration of NO3 (37.60 mM) favours higher withanolide A production and biomass accumulation (Praveen and Murthy 2013).
The transformed hairy root cultures of W. somnifera were found to be distinct in their ability to produce a wide array of chemical compounds (Kim et al. 2002; Giri and Narasu 2000; Bandyopadhyay et al. 2007). Transformed root cultures were shown to accumulate enhanced amount of withanolide A, Withanolide D and Withaferin A (Ray et al. 1996; Ray and Jha 1999; Bandyopadhyay et al. 2007; Murthy et al. 2008). Hairy roots grown in liquid medium also showed significant enhancement in the antioxidant activity (Kumar et al. 2005). A positive correlation between elicitor treatment and withanolide production could be established in W. somnifera hairy roots. The optimum concentrations of MeJ and SA which leads to increased withanolide A, withaferin A and withanone production were reported to be 15 μM and 150 μM, respectively (Sivanandhan et al. 2013a). Constitutive expression of β-cryptogein (a fungal elicitor protein) in hairy root cultures of W. somnifera resulted in metabolic shift from withanolide biosynthesis to phenypropanoid biosynthetic pathway (Sil et al. 2015) resulting in higher amount of ferulic acid along with increase in activity of enzyme phenylalanine ammonia lyase (PAL) in cryptogein-cotransformed hairy roots.
3.5 Characterization of Withanolide Biosynthetic Pathway Genes
The major bottle neck in enhancement of pharamaceutically important secondary metabolites in W. somnifera has been the lack of proper information on their biosynthetic pathways. Recently, withanolide biosynthetic pathway genes such as HMGR (Akhtar et al. 2013), FPPS (Gupta et al. 2011), SQS (Bhat et al. 2012), SE (Razdan et al. 2013), DXS and DXR (Gupta et al. 2013), Cyt P450 (Rana et al. 2013), sterol glycosyltransferase (Sharma et al. 2007) and genes of oxido squalene cyclase (OSC) super-family: cycloartenol synthase (OSC/CAS), β-amyrin synthase (OSC/BS) and lupeol synthase (OSC/LS) (Dhar et al. 2014) have been identified, cloned and characterized. The expression analysis of HMGR, FPPS, SQS, DXS and DXR showed the differential expression of these genes in different chemotypes, tissue, mechanical injury and in response to elicitors (Gupta et al. 2011, 2013; Bhat et al. 2012; Akhtar et al. 2013). Also, the expression of HMGR, FPPS, SQS, DXS and DXR was enhanced in young leaves in comparison to mature leaves and roots. The high expression in leaves may attribute to higher rate of biosynthesis of withanolides in young leaves (Chaurasiya et al. 2007; Gupta et al. 2012 & Gupta et al. 2013). Further, low expression of DXS and DXR in roots suggests that these enzymes are localized in plastids. These studies indicate that although leaves are the major site for biosynthesis of withanolides, an independent system for the biosynthesis of withanolide may exist in roots also (Sangwan et al. 2008).
Comparative expression analysis of the genes combined with the overexpression and knockout studies have facilitated the characterization of withanolide biosynthetic pathway genes and also provide clue for enhancing the accumulation of withanolides. One of the most important aspects in the study of gene expression by quantitative real-time PCR (qRT-PCR) is the selection of suitable reference genes for data normalization. Author’s laboratory studied 11 candidate references genes viz. Actin (ACT), cyclophylin (CYP), 26S ribosomal RNA (26S), 18S ribosomal RNA (18S–rRNA), glyceral-dehyde-3-phosphate dehydrogenase (GAPDH), ubiquitin (UBQ), elongation factor-1 (EF-1), alpha-tubulin (TUA), beta-tubulin (TUB), Sand family protein (SAND) and ribosomal protein L2 (RPL2) to test the stability of their transcripts under various conditions. 26S, UBQ and TUB were found to be best reference genes for drought, cold and biotic stress respectively. T-SAND was most stably expressed gene in tissues treated with salt and salicylic acid (SA), whereas, 18S–rRNA was most stable in abscisic acid (ABA) treated samples (Singh et al. 2015c). This work has made significant contribution in improving the quality of q-PCR mediated gene expression data in W. somnifera.
Recently virus induced gene silencing (VIGS) approach has been exploited to analyse the function of the genes involved in withanolide biosynthetic pathway. VIGS of squalene synthase gene resulted in significant reduction in the level of sitosterol (15%), campesterol (16%), stigmasterol (26%) and total withanolides (54%) implicating a major role of squalene synthase in withanolide biosynthesis. Further, WsSQS-vigs causing down regulation of pathogenesis related (PR) proteins such as PR1 (46%), PR3 (50%), PR5 (76%) and nonexpressor of PR, NPR 1 (45%) and NPR3 (81%) resulted in reduced tolerance to biotic stress (Singh et al. 2015a). In another study, overexpression of WsHMGR2 in tobacco led to increase in the level of sitosterol (160%), stigmasterol (123%) and campesterol (546%). However, the increase in the level of these sterols was comparative low in WsDXR2 overexpressing tobacco, sitosterol (25%), stigmasterol (28%) and campesterol (66%). The enhanacement in cholesterol content was marginally higher in WsDXR2 (41%) than WsHMGR2 (38%). Further, RNAi mediated transient gene silencing approach was followed by using pART27:WsHMGR2i and pART27:WsDXR2i. A higher reduction in the level of sitosterol, stigmasterol and campesterol was observed in pART27:WsHMGR2i as compared to pART27:WsDXR2i. The reduction in the level of cholesterol was comparatively higher in pART27:WsDXR2i. The RNAi suppression data was further validated by using specific inhibitors such as mevinolin (HMGR inhibitor) and fosmidomycin (DXR inhibitor) and a similar trend of sterol reduction has been reported (Singh et al. 2014). These studies indicated that MVA pathway is predominant in contributing carbon flux for withanolide biosynthesis and thereby metabolic engineering involving manipulation of MVA pathway genes may prove to be more beneficial to enhance secondary metabolites production. A recent study has highlighted the importance of nitrogen in enhancing the content of sterols and withaferin A (Pal et al. 2016). Treatment with ammonium sulfate resulted in higher expression of key genes of withanolide biosynthetic pathway viz. FPPS, SMT1, SMT2, ODM, SMO1 and SMO2, leading to significant increase in the content of sterols. The improvement in the yield of secondary metabolites is probably linked to the expression of WRKY transcription factors. This study suggests that identification of transcription factors involved in withanolide biosynthetic pathway would be a promising approach for modulation of withanolide biosynthetic pathway.
4 Biotic and Abiotic Stress
For enhancing the commercial value of W. somnifera, studies are being conducted for the production of biotic and abiotic stress tolerant cultivar. W. somnifera is exposed to a plethora of various biotic (Sharma and Pati 2011a, b, 2012a, b) and abiotic (Rout and Sahoo 2012, 2013) stresses. At present, its agroecosystem is characterized by 26 major and minor insect pests (Chaudhary 2013) and various fungal pathogens (Pati et al. 2008; Maiti et al. 2007). In field conditions, this plant is frequently attacked by various pests viz. Henosepilachna vigintioctopunctata (Sharma and Pati 2011b), Oxyrachis tarandus (Sharma and Pati 2011a), Phenacoccus solenopsis (Sharma and Pati 2012a), Tetranychus utricae (Sharma and Pati 2012b), Meloidogyne incognita (Sharma and Pandey 2009), Helicoverpa armigera and Epilachna vigintioctopunctata (Kumar et al. 2009) and pathogens viz. Alternaria alternata (Pati et al. 2008), A. dianthicola (Maiti et al. 2007), Pithomyces chartarum (Verma et al. 2008) and phytoplasma (Khan et al. 2006). Among various diseases, leaf spot disease caused by A.alternata is one of the most prevalent diseases of W. somnifera (Sharma et al. 2014). This disease is characterized by brownish to black spots on the leaves (Fig. 22.3). During severe infection, 80–90% of leaves of a single plant may get infested with A. alternata, causing significant biodeterioration of pharmaceutical important secondary metabolites. A respective reduction of 15.4% and 76.3% in the content of withaferin A and total withanolides has been recorded in W. somnifera after infestation with A. alternata (Pati et al. 2008). Beside these, various post-infectional changes at morphological, biochemical and physiological level due to leaf spot disease have also been reported (Sharma et al. 2011; Sharma et al. 2014). Further, maintaining the quality of herbal products has been a critical issue for pharmaceutical industries. As leaf spot disease in W. somnifera is caused by A. alternata, the fungal infected herbal products might lead to serious health implications. Hence, consistent efforts in the development of sensitive detection system for fungal contamination in the leaves of W. somnifera are worth pursuing (Sharma 2013).
The research on stress tolerance in W. somnifera is at its nascent stage mainly due to limited information about different cultivars and their potential of resistance to pests and pathogens. A comprehensive study involving traditional and advanced biotechnological approaches need to be initiated. The traditional method of developing biotic resistance in plants involves conventional screening of plants resistant to major pests and pathogens for identification of resistant germplasms followed by breeding to inculcate resistant trait into sensitive cultivars. However, pests and pathogens frequently overcome single gene resistance in the host, marker-assisted breeding (MAS) would facilitate pyramiding of resistant genes providing broad spectrum resistant (Singh et al. 2001). Advanced biotechnological approaches involve overexpression of plant’s defence genes to confer biotic stress resistance in plants. Plants show a rapid change in its gene expression in response to attack of pathogen or abiotic stress, resulting in synthesis of specific proteins that help to combat stress. Many of these inducible proteins are pathogenesis-related (PR) proteins (Sarowar et al. 2005). To date, 17 distinct families of PR proteins (PR1 to PR17) are found in plants (Sels et al. 2008). Overexpression of some of these PR proteins in different plants has conferred resistance to a large number of pathogens (Liu et al. 1994; Datta et al. 1999; Fagoaga et al. 2001; Alexander et al. 1993; Niderman et al. 1995). Author’s laboratory is actively engaged in research on PR proteins, particularly PR1 and PR5. We are characterising WsPR1 and WsPR5 and also trying to elucidate their mechanism of action. One another approach that has gained considerable interest in recent years is to strengthen the plant resistance to stresses by enhancing the cell wall components such as lignin. Hence, modulation of phenylpropanoid pathway leading to the production of lignin holds great promise to combat biotic stress.
Global climate changes and unethical agricultural practices create stress conditions for plants and affect their growth and development. In this context, some preliminary studies on the influence of heavy metal stress on W. somnifera have been conducted. Copper toxicity induced reduction in fresh weight, chlorophyll and carotenoid content and the length of root and shoot has been observed. Further, increase in lipid peroxidation and level of O2 -· and H2O2 and a significant decline in the activity of antioxidant enzymes viz. catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), and superoxide dismutase (SOD) reflected the inability of W. somnifera to eliminate the reactive oxygen species (ROS) resulted from copper induced oxidative stress (Khatun et al. 2008). However, Copper treatment led to induction of new genes as revealed by the appearance of new proteins in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).These induced proteins are considered to be involved in the defence of Copper toxicity (Rout and Sahoo 2013). Similar morphological and physiological changes have also been reported in high concentrations of iron (Rout and Sahoo 2012).Various studies need to be conducted for producing salinity, drought, elevated temperature and heavy metal stress tolerant plants.
5 Conclusion
W. somnifera has generated considerable interest among researchers due to its possession of pharmaceutically important secondary metabolites. Efforts are being carried out to overcome the bottleneck in its cultivation and improvement. Various biotechnological approaches viz.in vitro propagation, organ and cell suspension culture and genetic manipulations are being explored to solve the challenges of W. somnifera. Overall a comprehensive approach involving genomics, transcriptomics, proteomics, interactomics and metabolomics along with metabolic engineering and identification of new transcription factors will pave the path to the development of an ideal model plant of W. somnifera, having high commercial value.
References
Ahmad Baba I, Alia A, Saxena RC, Itoo A, Kumar S, Ahmad M (2013) In vitro propagation of Withania Somnifera (L.) Dunal (Ashwagandha) an endangered medicinal plant. Intl J Pharm Sci Invention 2:6–11
Akhtar N, Gupta P, Sangwan NS, Sangwan RS, Trivedi PK (2013) Cloning and functional characterization of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Withania somnifera: an important medicinal plant. Protoplasma 250:613–622
Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E (1993) Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc Natl Acad Sci USA 90:7327–7331
Bandyopadhyay M, Jha S, Tepfer D (2007) Changes in morphological phenotypes and withanolide composition of Ri-transformed roots of Withania somnifera. Plant Cell Rep 26:599–609
Bhat WW, Lattoo SK, Razdan S, Dhar N, Rana S, Dhar RS, Khan S, Vishwakarma RA (2012) Molecular cloning, bacterial expression and promoter analysis of squalene synthase from Withania somnifera (L.) Dunal. Gene 499:25–36
Bhatia A, Bharti SK, Tewari SK, Sidhu OP, Roy R (2013) Metabolic profiling for studying chemotype variations in Withania somnifera (L.) Dunal fruits using GC-MS and NMR spectroscopy. Phytochemistry 93:105–115
Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS, Sidhu OP, Roy R, Khetrapal CL, Tuli R (2010) Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry 71:1085–1094
Chaudhary V (2013) Arthropods associated with ashwagandha, (Withania somnifera Dunal) in semi arid region of Gujarat. Karnataka J Agric Sci 26:433–435
Chaurasiya ND, Gupta VK, Sangwan RS (2007) Leaf ontogenic phase-related dynamics of withaferin a and withanone biogenesis in ashwagandha (Withania somnifera Dunal.)-an important medicinal herb. J Plant Biol 50:508–513
Chaurasiya ND, Sangwan NS, Sabir F, Misra L, Sangwan RS (2012) Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L.(Dunal). Plant Cell Rep 31:1889–1897
Cucu N, Negoianu-Tenea G, Gavrila L (2002) Genetically modified medicinal plants-II. Transfer and expression of a marker kanamycin resistance gene in Atropa belladonna plants. Roman Biotechnol Lett 7:869–874
Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK (1999) Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet 98:1138–1145
Davis L, Kuttan G (2000) Immunomodulatory activity of Withania somnifera. J Ethnopharmacol 71:193–200
Dhar N, Rana S, Bhat WW, Razdan S, Pandith SA, Khan S, Dutt P, Dhar RS, Vaishnavi S, Vishwakarma R (2013) Dynamics of withanolide biosynthesis in relation to temporal expression pattern of metabolic genes in Withania somnifera (L.) Dunal: a comparative study in two morpho-chemovariants. Mol Biol Rep 40:7007–7016
Dhar N, Rana S, Razdan S, Bhat WW, Hussain A, Dhar RS, Vaishnavi S, Hamid A, Vishwakarma R, Lattoo SK (2014) Cloning and functional characterization of three branch point oxidosqualene cyclases from Withania somnifera (L.) dunal. J Biol Chem 289:17249–17267
Doma M, Abhayankar G, Reddy VD, Kavi Kishor PB (2012) Carbohydrate and elicitor enhanced withanolide (withaferin A and withanolide A) accumulation in hairy root cultures of Withania somnifera (L.) Indian J Exp Biol 50:484–490
Fagoaga C, Rodrigo I, Conejero V, Hinarejos C, Tuset JJ, Jn A, Pina JA, Navarro L, Pena L (2001) Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Mol Breed 7:175–185
Fatima N, Ahmad N, Anis M (2011) Enhanced in vitro regeneration and change in photosynthetic pigments, biomass and proline content in Withania somnifera L.(Dunal) induced by copper and zinc ions. Plant Physiol Biochem 49:1465–1471
Ghimire BK, Seong ES, Kim EH, Lamsal K, Yu CY, Chung IM (2010) Direct shoot organogenesis from petiole and leaf discs of Withania somnifera (L.) Dunal. Afr J Biotechnol 9:7453–7461
Giri A, Narasu ML (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18:1–22
Grover A, Samuel G, Bisaria VS, Sundar D (2013) Enhanced withanolide production by overexpression of squalene synthase in Withania somnifera. J Biosci Bioeng 115:680–685
Gupta P, Akhtar N, Tewari SK, Sangwan RS, Trivedi PK (2011) Differential expression of farnesyl diphosphate synthase gene from Withania somnifera in different chemotypes and in response to elicitors. Plant Growth Regul 65:93–100
Gupta N, Sharma P, Kumar RJS, Vishwakarma RK, Khan BM (2012) Functional characterization and differential expression studies of squalene synthase from Withania somnifera. Mol Biol Rep 39:8803–8812
Gupta P, Goel R, Pathak S, Srivastava A, Singh SP, Sangwan RS, Asif MH, Trivedi PK (2013) De novo assembly, functional annotation and comparative analysis of Withania somnifera leaf and root transcriptomes to identify putative genes involved in the withanolides biosynthesis. PLoS One 8:e62714
Herrera-Estrella L, Simpson J, MartÃnez-Trujillo M (2004) Transgenic plants: an historical perspective. In: Transgenic plants: methods and protocols. Humana Press, Totowa, pp 3–31
Hunter WN (2007) The non-mevalonate pathway of isoprenoid precursor biosynthesis. J Biol Chem 282:21573–21577
Jayaprakasam B, Zhang Y, Seeram NP, Nair MG (2003) Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci 74:125–132
Khan JA, Srivastava P, Singh SK (2006) Sensitive detection of a phytoplasma associated with little leaf symptoms in Withania somnifera. Eur J Plant Pathol 115:401–408
Khanna PK, Kumar A, Chandra R, Verma V (2013) Germination behaviour of seeds of Withania somnifera (L.) Dunal: a high value medicinal plant. Physiol Mol Biol Plants 19:449–454
Khatun S, Ali MB, Hahn E-J, Paek K-Y (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot 64:279–285
Kim Y, Wyslouzil BE, Weathers PJ (2002) Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Devel Biol-Plant 38:1–10
Kulkarni AA, Thengane SR, Krishnamurthy KV (2000) Direct shoot regeneration from node, internode, hypocotyl and embryo explants of Withania somnifera. Plant Cell Tissue Organ Cult 62:203–209
Kumar V, Murthy KNC, Bhamid S, Sudha CG, Ravishankar GA (2005) Genetically modified hairy roots of Withania somnifera Dunal: a potent source of rejuvenating principles. Rejuvenation Res 8:37–45
Kumar A, Kaul MK, Bhan MK, Khanna PK, Suri KA (2007) Morphological and chemical variation in 25 collections of the Indian medicinal plant, Withania somnifera (L.) Dunal (Solanaceae). Genet Resour Crop Evol 54:655–660
Kumar A, Singh CP, Pandey R (2009) Influence of environmental factors on the population buildup of Helicoverpa armigeraHübner and Epilachna vigintioctopunctata (Febr.) on Aswagandha (Withania somnifera Linn.) J Entomol Res 33:123–127
Kumar A, Mir BA, Sehgal D, Dar TH, Koul S, Kaul MK, Raina SN, Qazi GN (2011) Utility of a multidisciplinary approach for genome diagnostics of cultivated and wild germplasm resources of medicinal Withania somnifera, and the status of new species, W. ashwagandha, in the cultivated taxon. Plant Syst Evol 291:141–151
Liu D, Raghothama KG, Hasegawa PM, Bressan RA (1994) Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci U S A 91:1888–1892
Furmanowa MA, Gajdzis-Kuls D, Ruszkowska J, Czarnocki Z, Gy O, A S, Rani R, Upadhyay SN (2001) In vitro propagation of Withania somnifera and isolation of withanolides with immunosuppressive activity. Planta Med 67:146–149
Maiti CK, Sen S, Paul AK, Acharya K (2007) First report of Alternaria dianthicola causing leaf blight on Withania somnifera from India. Plant Dis 91:467–467
Matsuda H, Murakami T, Kishi A, Yoshikawa M (2001) Structures of withanosides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withania somnifera DUNAL. And inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorg Med Chem 9:1499–1507
Mir BA, Khazir J, Hakeem KR, Koul S, Cowan DA (2014) Enhanced production of withaferin-A in shoot cultures of Withania somnifera (L) Dunal. J Plant Biochem Biotechnol 23:430–434
Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazón J (2009) Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 14:2373–2393
Misra L, Lal P, Sangwan RS, Sangwan NS, Uniyal GC, Tuli R (2005) Unusually sulfated and oxygenated steroids from Withania somnifera. Phytochemistry 66:2702–2707
Misra L, Mishra P, Pandey A, Sangwan RS, Sangwan NS, Tuli R (2008) Withanolides from Withania somnifera roots. Phytochemistry 69:1000–1004
Mulabagal V, Tsay H-S (2004) Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Dijkstra C, Anthony P, White DA, Davey MR, Power JB, Hahn EJ, Paek KY (2008) Establishment of Withania somnifera hairy root cultures for the production of withanolide A. J Integr Plant Biol 50:975–981
Nagella P, Murthy HN (2010) Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour Technol 101:6735–6739
Nagella P, Murthy HN (2011) Effects of macroelements and nitrogen source on biomass accumulation and withanolide-A production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tissue Organ Cult 104:119–124
Namdeo AG, Sharma A, Yadav KN, Gawande R, Mahadik KR, Lopez-Gresa MP, Kim HK, Choi YH, Verpoorte R (2011) Metabolic characterization of Withania somnifera from different regions of India using NMR spectroscopy. Planta Med 77:1958–1964
Nayak SA, Kumar S, Satapathy K, Moharana A, Behera B, Barik DP, Acharya L, Mohapatra PK, Jena PK, Naik SK (2013) In vitro plant regeneration from cotyledonary nodes of Withania somnifera (L.) Dunal and assessment of clonal fidelity using RAPD and ISSR markers. Acta Physiol Plant 35:195–203
Niderman T, Genetet I, Bruyere T, Gees R, Stintzi A, Legrand M, Fritig B, Mosinger E (1995) Pathogenesis-related PR-1 proteins are antifungal (isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans). Plant Physiol 108:17–27
Pal S, Singh S, Ashutosh KS, Madan MG, Suman PSK, Ajit KS (2011) Comparative withanolide profiles, gene isolation, and differential gene expression in the leaves and roots of Withania somnifera. J Hortic Sci Biotechnol 86:391–397
Pal S, Yadav AK, Singh AK, Rastogi S, Gupta MM, Verma RK, Nagegowda DA, Pal A, Shasany AK (2016) Nitrogen treatment enhances sterols and withaferin A through transcriptional activation of jasmonate pathway, WRKY transcription factors, and biosynthesis genes in Withania somnifera (L.) Dunal. Protoplasma: 1–11
Pandey V, Misra P, Chaturvedi P, Mishra MK, Trivedi PK, Tuli R (2010) Agrobacterium tumefaciens-mediated transformation of Withania somnifera (L.) Dunal: an important medicinal plant. Plant Cell Rep 29:133–141
Patel N, Patel P, Kendurkar SV, Thulasiram HV, Khan BM (2015) Overexpression of squalene synthase in Withania somnifera leads to enhanced withanolide biosynthesis. Plant Cell Tissue Organ Cult 122:409–420
Pati PK, Sharma M, Salar RK, Sharma A, Gupta AP, Singh B (2008) Studies on leaf spot disease of Withania somnifera and its impact on secondary metabolites. Indian J Microbiol 48:432–437
Pawar PK, Maheshwari VL (2004) Agrobacterium rhizogenes mediated hairy root induction in two medicinally important members of family Solanaceae. Indian J Biotechnol 3:414–417
Prajapati ND, Purohit SS, Sharma AK, Kumar T (2006) A handbook of medicinal plants a complete source book, reprint. Hindustan Printing Press, Agrobios, Jodhpur
Praveen N, Murthy HN (2012) Synthesis of withanolide a depends on carbon source and medium pH in hairy root cultures of Withania somnifera. Ind Crop Prod 35:241–243
Praveen N, Murthy HN (2013) Withanolide a production from Withania somnifera hairy root cultures with improved growth by altering the concentrations of macro elements and nitrogen source in the medium. Acta Physiol Plant 35:811–816
Rana S, Lattoo SK, Dhar N, Razdan S, Bhat WW, Dhar RS, Vishwakarma R (2013) NADPH-cytochrome P450 reductase: molecular cloning and functional characterization of two paralogs from Withania somnifera (L.) Dunal. PLoS One 8:e57068
Rani G, Virk GS, Nagpal A (2003) Callus induction and plantlet regeneration in Withania somnifera (L.) Dunal. In Vitro Cell Dev Biol Plant 39:468–474
Ray S, Jha S (1999) Withanolide synthesis in cultures of Withania somnifera transformed with Agrobacterium tumefaciens. Plant Sci 146:1–7
Ray S, Jha S (2001) Production of withaferin A in shoot cultures of Withania somnifera. Planta Med 67:432–436
Ray S, Ghosh B, Sen S, Jha S (1996) Withanolide production by root cultures of Withania somnifera transformed with Agrobacterium rhizogenes. Planta Med 62:571–573
Razdan S, Bhat WW, Rana S, Dhar N, Lattoo SK, Dhar RS, Vishwakarma RA (2013) Molecular characterization and promoter analysis of squalene epoxidase gene from Withania somnifera (L.) Dunal. Mol Biol Rep 40:905–916
Rodriguez-Concepcion M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130:1079–1089
Rout JR, Sahoo SL (2012) Morphological and protein profile alterations in Withania somnifera L. with response to iron stress. Indian J Life Sci 2:21–25
Rout JR, Sahoo SL (2013) Antioxidant enzyme gene expression in response to copper stress in Withania somnifera L. Plant Growth Regul 71:95–99
Sabir F, Sangwan NS, Chaurasiya ND, Misra LN, Tuli R, Sangwan RS (2008) Rapid micropropagation of Withania somnifera L. accessions from axillary meristems. Journal of Herbs, Spices & Medicinal Plants 13:123–133
Sangwan RS, Chaurasiya ND, Lal P, Misra L, Uniyal GC, Tuli R, Sangwan NS (2007) Withanolide a biogeneration in in vitro shoot cultures of Ashwagandha (Withania somnifera Dunal), a main medicinal plant in Ayurveda. Chem Pharm Bull 55:1371–1375
Sangwan RS, Chaurasiya ND, Lal P, Misra L, Tuli R, Sangwan NS (2008) Withanolide a is inherently de novo biosynthesized in roots of the medicinal plant Ashwagandha (Withania somnifera). Physiol Plant 133:278–287
Saravanakumar A, Aslam A, Shajahan A (2012) Development and optimization of hairy root culture systems in Withania somnifera (L.) Dunal for withaferin-A production. Afr J Biotechnol 11:16412
Sarowar S, Kim YJ, Kim EN, Kim KD, Hwang BK, Islam R, Shin JS (2005) Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep 24:216–224
Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC (2008) Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol Biochem 46:941–950
Sen J, Sharma AK (1991) Micropropagation of Withania somnifera from germinating seeds and shoot tips. Plant Cell Tissue Organ Cult 26:71–73
Sharada M, Ahuja A, Suri KA, Vij SP, Khajuria RK, Verma V, Kumar A (2007) Withanolide production by in vitro cultures of Withania somnifera and its association with differentiation. Biol Plant 51:161–164
Sharma A (2013) Study of leaf spot disease in WIthania somnifera (L.) Dunal employing morphological, biochemical and molecular approaches. PhD Thesis, Guru Nanak Dev University
Sharma P, Pandey R (2009) Biological control of root-knot nematode, Meloidogyne incognita in the medicinal plant, Withania somnifera and the effect of biocontrol agents on plant growth. Afr J Agric Res 4:564–567
Sharma A, Pati PK (2011a) First report of Withania somnifera (L.) Dunal, as a new host of cowbug (Oxyrachis tarandus, fab.) in plains of Punjab, northern India. World Appl Sci J 14:1344–1346
Sharma A, Pati PK (2011b) First record of 28-spotted ladybird beetle, Henosepilachna vigintioctopunctata (F.) infesting Withania somnifera (L.) Dunal in Punjab province of Northern India. Pest Technol 5:91–92
Sharma A, Pati PK (2012a) First record of Ashwagandha as a new host to the invasive mealybug (Phenacoccus solenopsis Tinsley) in India. Entomol News 123:59–62
Sharma A, Pati PK (2012b) First record of the carmine spider mite, Tetranychus urticae, infesting Withania somnifera in India. J Insect Sci 12:50
Sharma LK, Madina BR, Chaturvedi P, Sangwan RS, Tuli R (2007) Molecular cloning and characterization of one member of 3β-hydroxy sterol glucosyltransferase gene family in Withania somnifera. Arch Biochem Biophys 460:48–55
Sharma A, Sharma I, Pati PK (2011) Post-infectional changes associated with the progression of leaf spot disease in Withania somnifera. J Plant Pathol 93:397–405
Sharma A, Vats SK, Pati PK (2014) Post-infectional dynamics of leaf spot disease in Withania somnifera. Ann Appl Biol 165:429–440
Sil B, Mukherjee C, Jha S, Mitra A (2015) Metabolic shift from withasteroid formation to phenylpropanoid accumulation in cryptogein-cotransformed hairy roots of Withania somnifera (L.) Dunal. Protoplasma 252:1097–1110
Singh S, Kumar S (1998) Withania somnifera: the Indian ginseng ashwagandha. Central Institute of Medicinal and Aromatic Plants, Lucknow
Singh S, Sidhu JS, Huang N, Vikal Y, Li Z, Brar DS, Dhaliwal HS, Khush GS (2001) Pyramiding three bacterial blight resistance genes (xa5, xa13 and Xa21) using marker-assisted selection into indica rice cultivar PR106. Theor Appl Genet 102:1011–1015
Singh S, Pal S, Shanker K, Chanotiya CS, Gupta MM, Dwivedi UN, Shasany AK (2014) Sterol partitioning by HMGR and DXR for routing intermediates toward withanolide biosynthesis. Physiol Plant 152:617–633
Singh AK, Dwivedi V, Rai A, Pal S, Reddy SGE, Rao DKV, Shasany AK, Nagegowda DA (2015a) Virus-induced gene silencing of Withania somnifera squalene synthasenegatively regulates sterol and defence-related genes resulting in reduced withanolides and biotic stress tolerance. Plant Biotechnol J 13:1287–1299
Singh P, Guleri R, Singh V, Kaur G, Kataria H, Singh B, Kaur G, Kaul SC, Wadhwa R, Pati PK (2015b) Biotechnological interventions in Withania somnifera (L.) Dunal. Biotechnol Genet Eng Rev 31:1–20
Singh V, Kaul SC, Wadhwa R, Pati PK (2015c) Evaluation and selection of candidate reference genes for normalization of quantitative RT-PCR in Withania somnifera (L.) Dunal. PLoS One 10:e0118860
Sivanandhan G, Mariashibu TS, Arun M, Rajesh M, Kasthurirengan S, Selvaraj N, Ganapathi A (2011) The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiol Plant 33:2279–2288
Sivanandhan G, Rajesh M, Arun M, Jeyaraj M, Dev GK, Arjunan A, Manickavasagam M, Muthuselvam M, Selvaraj N, Ganapathi A (2012) Effect of culture conditions, cytokinins, methyl jasmonate and salicylic acid on the biomass accumulation and production of withanolides in multiple shoot culture of Withania somnifera (L.) Dunal using liquid culture. Acta Physiol Plant 35:715–728
Sivanandhan G, Dev GK, Jeyaraj M, Rajesh M, Arjunan A, Muthuselvam M, Manickavasagam M, Selvaraj N, Ganapathi A (2013a) Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult 114:121–129
Sivanandhan G, Dev GK, Jeyaraj M, Rajesh M, Muthuselvam M, Selvaraj N, Manickavasagam M, Ganapathi A (2013b) A promising approach on biomass accumulation and withanolides production in cell suspension culture of Withania somnifera (L.) Dunal. Protoplasma 250:885–898
Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M (2014) An efficient hairy root culture system for Withania somnifera (L.) Dunal. Afr J Biotechnol 13:4141–4147
Sivanandhan G, Dev GK, Theboral J, Selvaraj N, Ganapathi A, Manickavasagam M (2015a) Sonication, vacuum infiltration and thiol compounds enhance the Agrobacterium-mediated transformation frequency of Withania somnifera (L.) Dunal. PloS One 10:e0124693
Sivanandhan G, Theboral J, Dev GK, Selvaraj N, Manickavasagam M, Ganapathi A (2015b) Effect of carbon and nitrogen sources on in vitro flower and fruit formation and withanolides production in Withania somnifera (L.) Dunal. Indian J Exp Biol 53:177–183
Sivanesan I (2007) Direct regeneration from apical bud explants of Withania somnifera Dunal. Indian J Biotechnol 6:125
Sivanesan I, Murugesan K (2008) An efficient regeneration from nodal expiants of Withania somnifera Dunal. Asian J Plant Sci 7:551–556
Soni P, Bahadur AN, Tiwari U, Kanungo VK (2011) Micropropagation of a medicinal plant Withania somnifera (L.) Dunal by shoot bud culture. An International quarterly journal of Life Sci 6:135–137
Supe U, Dhote F, Roymon MG (2006) In vitro plant regeneration of Withania somnifera. Plant Tissue Cult Biotechnol 16:111–115
Thilip C, Raju CS, Varutharaju K, Aslam A, Shajahan A (2015) Improved Agrobacterium rhizogenes-mediated hairy root culture system of Withania somnifera (L.) Dunal using sonication and heat treatment. Biotech 5:949–956
Udayakumar R, Choi CW, Kim KT, Kim SC, Kasthurirengan S, Mariashibu TS, Sahaya Rayan JJ, Ganapathi A (2013) In vitro plant regeneration from epicotyl explant of Withania somnifera (L.) Dunal. J Med Plant Res 7:43–52
Vakeswaran V, Krishnasamy V (2003) Improvement in storability of Ashwagandha (Withania somnifera Dunal) seeds through pre-storage treatments by triggering their physiological and biochemical properties [abstract]. Seed Technol: 203–203
Verma OP, Gupta RBL, Shivpuri A (2008) A new host for Pithomyces chartarum, the cause of a leaf spot disease on Withania somnifera. Plant Pathol 57:385–385
Acknowledgements
We duly acknowledge the financial support received under University For Potential Excellence (UPE) program from UGC, New Delhi, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kaur, K. et al. (2017). Biotechnological Approaches in Propagation and Improvement of Withania somnifera (L.) Dunal. In: Kaul, S., Wadhwa, R. (eds) Science of Ashwagandha: Preventive and Therapeutic Potentials. Springer, Cham. https://doi.org/10.1007/978-3-319-59192-6_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-59192-6_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59191-9
Online ISBN: 978-3-319-59192-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)