Abstract
Globally, there is a widespread interest in the use of legumes due to their multifaceted functions. Also, legumes (Fabaceae, Syn. Leguminosae) are essential components in natural and managed terrestrial ecosystems due to their ability to intimately interact with different rhizosphere microorganisms. Among soil microbiota, the arbuscular mycorrhizal fungi (AMF) are universal and ubiquitous rhizosphere microflora forging symbiosis with plethora of plant species roots and acting as biofertilizers, bioprotectants, mycoremediators, and biodegraders. The arbuscular mycorrhizal-legume (herb or tree) symbiosis is viewed as a better alternative for enhancing soil fertility and the rehabilitation of arid lands and, therefore, provides an important direction for future agricultural research. The sole application of AMF has been found to improve the overall performance of leguminous plants growing under diverse farming practices. In addition, the interaction of AM fungi with other plant growth-promoting rhizobacteria has shown considerable increase in growth and yield of legumes. Here, legume growth responses to single or composite inoculation of AMF for sustainable production of legumes cultivated in different agroecological niches are highlighted. Furthermore, mycorrhizal dependency of legumes and effects of arbuscular mycorrhizal fungi on productivity of legumes grown under stressed environment are described.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
The constantly declining cultivable lands and consistently rising human populations require that the production of crops be increased substantially at global level. Due to changes in abiotic and biotic soil properties, reestablishment of proper vegetation cover has been adversely affected (Miller 1987). Due to these factors and increased pressure for food production, there is urgent need to upgrade farming practices so that food demands are fulfilled. In this regard, farming communities are adopting intensive agricultural systems that involve the use of significant quantities of agrochemicals to optimize crop production (Hooker and Black 1995). However, the cost and environmental threats associated with the use of high-input strategy demands that agricultural systems should be modified in order to make them more productive and sustainable (van der Vossen 2005). To circumvent such problems, the use of microorganisms especially symbiotic fungi opens up the new possibility of a more sustainable and low-cost agricultural practices.
Legumes form symbioses with both rhizobia (Spaink 1996) and mycorrhizal fungi (Harrison 1999; Lodwig et al. 2003). Legumes have been grown for food, as fodder, fiber, industrial and medicinal compounds, flowers, and other end uses. Leguminous plants are also highly suitable for agroforestry system, the area that receives due attention for sustainable agriculture. Nutrient-acquisition symbioses between plants and soil microbes are important to plant evolution and ecosystem function (Simms and Taylor 2002). A complex yet positive interactions between plants and soil microbes determine the soil fertility and consequently the plant health (Jeffries et al. 2003). Among numerous useful soil microflora, arbuscular mycorrhizas are the most important organisms that form symbioses with majority of plants including legumes (Barea and Azcon-Aguilar 1983) grown under P-deficient soils and influence plant community development, nutrient uptake, water relations, and aboveground productivity (van der Heijden et al. 2008). Arbuscular mycorrhizas also act as bioprotectants and protect plants from pathogens and toxic stresses (van der Heijden et al. 2008). However, in order to optimize their beneficial impacts, it is important to ensure that management practices such as minimum tillage, reduced use of inappropriate fertilizer, appropriate crop rotations with minimal fallow, and rationalized pesticide use be adopted regularly.
Arbuscular mycorrhizal fungi and rhizobia play a key role in enhancing plant productivity, plant nutrition, and plant resistance (Demir and Akköprü 2007). The activities of nitrogen-fixing bacteria are enhanced in the rhizosphere of mycorrhizal plants where synergistic interactions of such microorganisms with mycorrhizal fungi have been demonstrated (Barea et al. 2002), and hence, the symbiosis of AMF with rhizobia is considered crucial for legumes (van der Heijden et al. 2006). Realizing the importance of rhizobia and AMF symbiosis, pot and field experiments were conducted where both symbionts showed higher plant biomass and better N and P acquisition, although these effects were also dependent on the specific symbiont combination (Azcón et al. 1991; Requena et al. 2001; Xavier and Germida 2002). Similarly, the tripartite symbiosis of legume-mycorrhiza-rhizobium has conclusively shown improvements in overall growth of leguminous plants (Babajide et al. 2008; Wu et al. 2009). For some plant species, the association with mycorrhizal fungi is indispensable. The degree of dependence however varies with plant species, particularly the root morphology, and conditions of soil and climate. Mycorrhizal dependencies of leguminous plants such as Acacia and Albizia have been well demonstrated (Plenchette et al. 2005; Ghosh and Verma 2006). Several research findings have also indicated the remarkable roles of AM fungi in amelioration of various types of stresses (abiotic/biotic) in leguminous plants (Rabie and Almadini 2005; Khan 2006; Aysan and Demir 2009). Mycorrhizal legumes are also well known for rehabilitation of badly degraded lands and/or desertified habitats emphasizing the ecological significance of this special association (Requena et al. 2001; Quatrini et al. 2003). Under conditions of low N and P availability which occur in many tropical soils, the possible transfer of nutrients from the host plant to another plant by AMF active hyphae may take place. Hyphae of mycorrhizas may spread from one infected plant and enter the roots of one or more other plants (Heap and Newman 1980). It has been shown that plant assimilates may be transported from one plant to another through AM hyphal connections. In a study, the transfer of 14C photosynthate from one plant to another was found primarily through AM hyphae rather than leakage from the roots of the donor plants (Francis and Read 1984). More specifically, different experimental results (Snoeck et al. 2000; Li et al. 2009) have verified the transfer of fixed N from legume mycorrhizal plants to nearby/adjacent nonleguminous plants via active hyphal connections. Diverse experimental results show that AMF differ in their capacity to supply plant nutrients such as P (van der Heijden et al. 2003; Ghosh and Verma 2006) suggesting mass production of suitable strains for sustainable inoculum development. Although the technology for the production of rhizobial and free-living PGPR inoculants are commercially available, the production of AM fungi inocula and the development of inoculation techniques have restricted the manipulation of AM fungi. An appropriate management of selected AM fungi is now available for exploiting the benefits of these microorganisms in agriculture, horticulture, and revegetation of degraded ecosystems (Barea et al., 2005). And large quantities of AMF inoculum can be produced by pot culture technique (Nopamornbodi et al. 1988). The traditional and most widely used approach has been to grow the fungus with suitable host plants in solid growth medium individually or in combination on the solid growth media (Tiwari and Adholeya 2002). However, the current biotechnology practices now allow the production of efficient AM fungal inoculants to mass propagate them for large-scale production systems (Gianinazzi and Vosátka 2004).
10.2 Mycorrhizal Association with Legumes
Legumes are an important plant group which can form symbiosis with P-acquiring arbuscular mycorrhizal fungi (AMF) (Pagano et al. 2007; Valsalakumar et al. 2007; Molla and Solaiman 2009). Scheublin et al. (2004) have analyzed the AMF community composition in the roots of three nonlegumes and in the roots and root nodules of three legumes growing in a natural dune grassland and found differences in AMF communities between legumes and nonlegumes and between legume roots and root nodules. One AMF sequence type was much more abundant in legumes than in nonlegumes (39% and 13%, respectively). Root nodules contained characteristic AMF communities that were different from those in legume roots, even though the communities were similar in nodules from different legume species. Legumes and root nodules have relatively high N concentrations and high P demands. Accordingly, the presence of legume- and nodule-related AMF can be explained by the specific nutritional requirements of legumes or by host-specific interactions among legumes, root nodules, and AMF. In other experiments, Muleta et al. (2007, 2008) have reported more AMF spore counts under Acacia abyssinica, Albizia gummifera, and Millettia ferruginea shade trees than under nonleguminous shade trees in both natural coffee forest and in soils of smallholder agroforestry coffee system in southwestern Ethiopia. Similar observations have also been reported elsewhere under canopies of legume plants (He et al. 2004). Colozzi and Cardoso (2000) have also demonstrated that legume intercropping cultivation increased spores concentration of AMF in the soil.
Valsalakumar et al. (2007) in a field study identified the AM fungi associated with greengram [Phaseolus aureus Roxb. (=Vigna radiata var. radiata)]. The findings show that Glomus mosseae, G. microcarpum, Gigaspora margarita, and Scutellospora sp. colonized the greengram. Glomus mosseae was the most abundant AM fungal associate (81%) followed by G. microcarpum (24%) and G. margarita (24%) and Scutellospora sp. (5%) identified in soils studied. The range of distribution varied from a single species of AM fungus to three species belonging to two genera in one sample. Similarly, Bakarr and Janos (1996) examined the fine roots of 27 forest tree species for mycorrhizal colonization, a forestry plantation and a reforestation site in Sierra Leone, West Africa. Twenty tree species had arbuscular mycorrhizas, of which seven species were ectomycorrhiza colonizing six legume species belonging to Caesalpinioideae. Three species of Australian Acacia used widely in reforestation in Sierra Leone had arbuscular mycorrhizas. The effects of AMF, P addition, and their interaction on the growth and P uptake of three facultative mycotrophic legume trees (Anadenanthera peregrina, Enterolobium contortisiliquum, and Plathymenia reticulata) were investigated (Pagano et al. 2007). Phosphorus fertilization improved the growth of all the legume tree species. In turn, P enhanced the positive effects of AMF on the three studied species. Tissue nutrient concentrations showed slight variation among species and were influenced by both AMF inoculation and P. Plants inoculated with higher doses of KH2PO4 showed more vigorous seedlings. Results suggest that in low fertility soils, A. peregrina, E. contortisiliquum, and P. reticulata seedlings should be inoculated with AMF to enhance plant growth.
The application of AMF in soils has shown a tremendous improvement in growth and yields of diverse leguminous plants raised under both greenhouse and field conditions. For instance, inoculation with AMF improved growth of chickpea and doubled P uptake at low and intermediate levels of P fertilization in a pot experiment on sterilized low P calcareous soil (Weber et al. 1992). In a follow-up study, Ndiaye et al. (2009) evaluated the effects of different indigenous AM fungi on the mobilization of P from Senegalese natural rock phosphate (NRP) for growth of Gliricidia sepium and Sesbania sesban seedlings. In this study, the levels of NRP were found compatible with high AM fungal proliferation but changed the pattern of root colonization which varied with plant cultivars and fungal species. The mixed applications of NRP and AM fungi facilitated the measured growth parameters of G. sepium and S. sesban after 4 months cultivation. AM fungi in the presence of 600 or 800 mg NRP enhanced the weight of S. sesban by 200%. For Gliricidia, only G. aggregatum in the presence of high NRP levels showed similar growth promotory effects. On the other hand, G. fasciculatum enhanced the height of Sesbania by twofolds when grown in the presence of 400, 600, and 800 mg NRP. Generally, the impact of composite application of AM fungi and NRP on nutritional content was more obvious for Sesbania than for Gliricidia seedlings.
10.3 Mycorrhizal Status of Legumes That Do Not Form Nodules
It is interesting that certain leguminous tribes that cannot form nodules may be colonized by AM fungi. Cárdenas et al. (2006) investigated early responses to Nod factors and mycorrhizal colonization in a non-nodulating Phaseolus vulgaris mutant. The results indicate that even though P. vulgaris non-nodulating mutant (NN-mutant) is deficient in early nodulin gene expression when inoculated with Rhizobium etli, it can be effectively colonized by AM fungus, G. intraradices. Sometimes Nod mutants of other legumes fail to establish a mycorrhizal symbiosis (Bradbury et al. 1991) indicating that common elements of the infection process may exist in both associations.
10.4 Dual Inoculation of Legume Plants with Mycorrhizal Fungi and Rhizobia
The majority of legumes have the capacity to form a dual symbiotic interaction with N2-fixing rhizobia and P-acquiring AM fungi (Lodwig et al. 2003; Navazio et al. 2007). Arbuscular mycorrhizal fungi and rhizobia together play a key role in natural ecosystems and influence plant productivity, nutrition, resistance, and plant community structure (van der Heijden et al. 2006; Demir and Akköprü 2007). The bioavailability of N and P is enhanced in the rhizosphere of mycorrhizal plants following synergistic interactions between the two groups of microorganisms (Barea et al. 2002). The authors further suggested that the inoculation of such phytobeneficial microbes has been shown to improve the overall performance of legumes indicating the importance of the tripartite symbiosis between legume-mycorrhiza and rhizobia in a given ecosystem. Studies have demonstrated that the two symbioses share some components of their developmental programs (Harrison 2005; Navazio et al. 2007). Synergistic effect of dual colonization of roots with AMF and Rhizobium on growth, nutrient uptake, and N2 fixation in many legume plants has been reported (Xavier and Germida 2002; Stancheva et al. 2008) and discussed in the following section.
10.4.1 Dual Inoculation of AM Fungi and Rhizobia Under Greenhouse Conditions
Response of Leucaena leucocephala to inoculation with Glomus fasciculatum and/or Rhizobium was studied in a P-deficient unsterile soil (Manjunath et al. 1984). The findings show that G. fasciculatum inoculation alone improved nodulation by native rhizobia and Rhizobium only treatment increased colonization of roots by native mycorrhizal fungi. However, when AM fungi and Rhizobium were used together, it improved nodulation, mycorrhizal colonization, dry weight, and N and P contents of the plants compared to single inoculation of each organism in a similar study. Eom et al. (1994) evaluated two wild legume plants, Glycine soja and Cassia mimosoides var. nomame, and a cultivated plant, soybean, inoculated with Scutellospora heterogama, isolated from natural soils and rhizobial cells. The AMF-colonized wild legume plants showed greater growth compared to soybean, whereas the soybean showed more nodulation than AM-colonized Cassia mimosoides plants. Moreover, S. heterogama appeared to stimulate the triple symbiosis of the wild legume plants. In addition, Babajide et al. (2008) in a greenhouse experiments determined the effect of different rhizobial and mycorrhizal species (G. clarum) on growth, nodulation, and biomass yield of soybean grown under low fertile eroded soil condition. Plant growth and biomass yield were significantly enhanced by AM fungus in both sterile and non-sterile soils compared to the control. However, combined inoculation of mycorrhiza with any of the rhizobial strains further improved plant growth and biomass production. The effect of composite inoculation of mycorrhiza + R25B Rhizobium was more pronounced, which substantially increased the plant height (68.8 cm), stem circumference (2.94 cm), number of leaves (39.0), shoot dry weight (16.1 g), and root dry weight (4.6 g), relative to control values of 33.2, 0.60 cm, 15, 4.4, and 1.6 g, respectively. Nodulation was equally enhanced by mycorrhizal and rhizobial inoculations under sterile and non-sterile soils. The percentage of mycorrhizal root colonization ranged from 4 to 42%, and root colonization was highest for mycorrhizal inoculated plants grown in sterile soil. From these findings authors concluded that dual inoculation of mycorrhiza and Rhizobium may be beneficial to soybean production in the tropics, where nutrients particularly available P and total N are very low. Ahmad (1995) studied the effect of dual inoculation on three local cultivars (Miss Kelly, Portland Red, Round Red) of red kidney bean with four strains of R. phaseoli (B36, B17, T2, and CIAT652) and three species of AM fungi (G. pallidum, G. aggregatum, and Sclerocystis microcarpa) in sterilized and non-sterilized soil. Symbiotic efficiency including improved plant growth and enhanced N and P was dependent on the specific combinations of Rhizobium strain, AM fungus, and cultivars of kidney bean. The rhizobial strains B36 and B17 co-inoculated with G. pallidum or G. aggregatum increased the growth of Miss Kelly and Portland Red, while rhizobial strain T2 paired with any of the three AM fungi was found as the best compatible pairing for the Round Red kidney beans. From these results, the author suggested that even though dual inoculation significantly improved the growth of the bean plants, the best performing combination of AM fungus and rhizobia requires further trials so that it is recommended for legume promotion in different geographical regions. Tajini et al. (2012) have also investigated the effect of dual inoculation of common bean with G. intraradices and Rhizobium tropici CIAT899 under glasshouse conditions. Two common bean genotypes (i.e., CocoT and Flamingo) varying in their effectiveness for nitrogen fixation were inoculated with G. intraradices and R. tropici CIAT899 and grown for 50 days in soil–sand substrate. Inoculation of common bean plants with the AM fungi resulted in a significant increase in nodulation compared to plants without inoculation. The combined inoculation of AM fungi and rhizobia significantly increased various plant growth parameters compared to simple inoculated plants. In addition, the combined inoculation of AM fungi and rhizobia resulted in significantly higher nitrogen and phosphorus accumulation in the shoots of common bean plants and improved phosphorus use efficiency compared to their controls, which were not dually inoculated. It is concluded that inoculation with rhizobia and AM fungi could improve the efficiency in P use for symbiotic nitrogen fixation especially under phosphorus deficiency. Combined inoculation with G. intraradices and R. tropici CIAT899 increases P use efficiency for symbiotic nitrogen fixation in common bean. Similarly, potted bean plants were grown in a glasshouse with and without organic and chemical fertilizers, uninoculated or inoculated with rhizobia (a mixed culture of R. leguminosarum bv. phaseoli and R. tropici) and AMF (Glomus spp.), singly or in combination (Aryal et al. 2003). Treatment effects on growth, nodulation, AMF colonization, and nutrient uptake of plants were evaluated. Rhizobial inoculation positively influenced root dry weight and nodulation of plants. Shoot and root dry weights and nodulation were again higher in dually inoculated plants compared to singly inoculated plants. Compared to control, single inoculation either with rhizobia or AMF did not increase pod yield. But, dual inoculation significantly increased pod yield compared to control or singly inoculated plants. Inoculation also significantly increased pod yield in organic fertilization treatment, but not in chemical fertilization treatment. AMF colonization, spore population, and shoot N and P were also significantly higher in dually inoculated plants. Under fertilized conditions, nodulation, AMF colonization, and spore population were generally more pronounced in dually inoculated organic plants than in chemical plants. Shoot Ca and K remained unaffected by inoculation either in fertilized or unfertilized conditions. Dual inoculation significantly increased the concentration of shoot Mg in organic plants, but not in chemical. In general, better positive effects of inoculations were observed in organic plants than in chemical suggesting higher dependency of organic plants on these symbionts for better growth and development. A similar study was conducted by Jia et al. (2004) to investigate the effects of the interactions between Rhizobium and AMF on N and P accumulation by broad bean and how increased N and P content influence biomass production, leaf area, and net photosynthetic rate. The AM fungus increased biomass production and photosynthetic rates by stimulating the ratio of P to N accumulation, and an increase in P was consistently correlated with an increase in N accumulation and N productivity, expressed in terms of biomass and leaf area. Photosynthetic N use efficiency, irrespective of the inorganic source of N (e.g., NO3− or N2) was enhanced by increased P supply due to AMF colonization. However, Rhizobium significantly declined AMF colonization irrespective of N supply and without Rhizobium; AMF colonization was higher in low N treatments. Presence or absence of AMF did not have a significant effect on nodule mass but high N with or without AMF led to a significant decline in nodule biomass. Furthermore, plants with the Rhizobium and AMF had higher photosynthetic rates per unit leaf area. Geneva et al. (2006) reported that the dual inoculation of pea plants with G. mosseae or G. intraradices and R. leguminosarum bv. viciae, strain D 293, significantly increased the plant biomass, photosynthetic rate, nodulation, and N2 fixing activity in comparison to single inoculation of R. leguminosarum bv. viciae strain D 293. In addition, the co-inoculation significantly increased the total P content in plant tissues, acid phosphatase activity, and percentage of root colonization. Among all the microbial pairings, the co-inoculation of R. leguminosarum with G. mosseae was most effective at low P level, while G. intraradices inoculated with R. leguminosarum was most effective at higher P level. Xavier and Germida (2002) investigated also the effect of synergism between AMF and R. leguminosarum bv. viciae strains on lentil (Lens culinaris cv. Laird). Plants were inoculated with the AMF species G. clarum NT4 or G. mosseae NT6 and/or nine Rhizobium strains varying in efficacy and grown for 110 days in soil containing indigenous AMF and rhizobia. The results suggest that synergistic interactions between AMF and Rhizobium strains can enhance lentil productivity. In another study, Wu et al. (2009) have determined the single and combined effects of G. mosseae and Rhizobium on Medicago sativa grown on three types of coal mine substrates, namely, a mixture of coal wastes and sands (CS), coal wastes and fly ash (CF), and fly ash (FA) in pot experiment. When Rhizobium was used alone, it did not result in any growth response but sole application of G. mosseae had a significant effect on plant growth. Inoculation of G. mosseae also increased the survival rate of M. sativa in CS substrate. When G. mosseae inoculated M. sativa plant was grown with CF and FA substrates, the dry matter accumulation in the test plants was 1.8 and 5.1 times higher than those without inoculation. However, when M. sativa was inoculated with G. mosseae and Rhizobium together and grown in CS and CF substrates, the N, P, and K uptake by the test plant increased substantially suggesting a synergistic effect of the two phylogenetically distinct organisms which could be exploited for revegetation of coal mine substrates. In another greenhouse trial, Mehdi et al. (2006) reported that the effects of AM fungi (G. mosseae and G. intraradices), rhizobial (R. leguminosarum bv. viciae) strains, and P (superphosphate and phosphate rock) fertilizers significantly increased the dry biomass of shoots and seeds, P and N contents (shoots and seeds) of lentil plants, and percent of root colonized by AM fungus. The rhizobial strain possessing P-solubilizing ability showed a more beneficial effect on plant growth and nutrient uptake than the strain without this activity, although both strains had similar N2-fixing efficiency. Synergistic relationships were observed between AM fungi and some rhizobial strains that related to the compatible pairing of these two microsymbionts. Moreover, the P uptake efficiency was increased when P fertilizers were applied along with AM fungi and/or P-solubilizing rhizobial strains emphasizing the remarkable importance of dual inoculation in the improvement of plant growth responses. Likewise, Meghvansi et al. (2008) observed the comparative efficacy of three AMF combined with cultivar-specific B. japonicum (CSBJ) in soybean under greenhouse conditions. Soybean seeds of four cultivars, namely, JS 335, JS 71-05, NRC 2, and NRC 7, were inoculated with three AMF (G. intraradices, Acaulospora tuberculata, and Gigaspora gigantea) and CSBJ isolates, individually or in combination, and were grown in pots using autoclaved alluvial soil of a nonlegume cultivated field of Ajmer (Rajasthan). Their findings indicate that among the single inoculations of three AMF, G. intraradices produced the largest increases in the parameters (nodulation, plant growth, and seed yield) studied followed by A. tuberculata and G. gigantea indicating that plant acted selectively on AMF symbiosis. The dual inoculation with AMF + B. japonicum CSBJ further improved these parameters demonstrating synergism between the two microsymbionts. Among all the dual treatments, G. intraradices + B. japonicum showed the greatest increase (115.19%), in seed weight per plant suggesting a strong selective synergistic relationship between AMF and B. japonicum. The cv. JS 335 exhibited maximum positive response toward inoculation. The variations in efficacy of different treatments with soybean cultivars, however, indicated the specificity of the inoculants. These results provide a basis for selection of an appropriate combination of specific AMF and Bradyrhizobium which could further be utilized for identifying the symbiotic effectiveness and competitive ability of microsymbionts under field conditions. Likewise, a pot trial was set up (Stancheva et al. 2008) to evaluate the response of alfalfa (Medicago sativa L.) to AMF species G. intraradices and S. meliloti, strain 1021, regarding the dry biomass accumulation, mycorrhizal fungi colonization, nodulation, and nitrogen fixation activity. Alfalfa plants were grown in a glasshouse until the flowering stage (58 days), in 4 kg plastic pots using leached cinnamon forest soil (Chromic luvisols—FAO) at P levels 42 mg P2O5 kg−1 soil (applied as 133 mg kg soil−1 tunisian phosphorite). The results demonstrated that the dual inoculation of alfalfa plants with G. intraradices and S. meliloti, strain 1021, significantly increased the percent of root colonization and acid phosphatase activities in the root tissue and in soil in comparison to a single inoculation with G. intraradices. Co-inoculation also significantly increased the plant biomass, total P and N content in plant tissues. Under conditions of dual inoculation, high nitrogenase activity was established, especially at the floral budding stage compared to the single inoculation of S. meliloti strain 1021. In addition, the interaction between AMF, S. meliloti, and Medicago truncatula Gaertn was investigated (de Varennes and Goss 2007). To generate a differential inoculum potential of indigenous AMF, five cycles of wheat, each of 1 month, were grown in sieved or undisturbed soil before M. truncatula was sown. The early colonization of M. truncatula roots by indigenous AMF was faster in undisturbed soil compared to sieved soil. M. truncatula grown in undisturbed soil had accumulated a greater biomass in aboveground tissues, had a greater P concentration, and derived more N from the atmosphere than plants grown in disturbed soil, although soil compaction resulted in plants having a smaller root system than those from disturbed soil. The difference in plant P content could not be explained by modifications in hydrolytic soil enzymes related to the P cycle as the activity of acid phosphatase was greater in sieved than in undisturbed soil, and the activity of alkaline phosphatase was unaffected by the treatment. Thus, the results observed were a consequence of the different rates of AMF colonization caused by soil disturbance. This study confirms that soil disturbance modifies the interaction between indigenous AMF, rhizobia, and legumes leading to a reduced efficacy of the bacterial symbiont.
Chickpea plants were also inoculated with six strains of M. ciceri and three AMF species, G. intraradices (GI), G. mosseae (GM), and G. etunicatum (GE), under pot experiments (Tavasolee et al. 2011). The plants inoculated with a number of AMF species, and bacterial strains increased overall plant dry mass compared to non-inoculated plants. GE was the most efficient in increasing plant dry matter. Individual AMF species were more effective than when mixed (GI + GM + GE). Bacterial treatments had increasing effect on root colonization by GI, GM, and GI + GM + GE. The results revealed that dual inoculation with AMF and rhizobia enhanced N, P, Zn, Fe, and Cu content in plants, but these increasing effects were different between fungal and bacterial treatments. Chaitra and Lakshman (2016) have also investigated the interaction between AM fungus, Rhizobium and Azospirillum on three leguminous crop plants (Cicer arietinum L., Vigna unguiculata L., and Vigna radiata L.) under greenhouse condition. Results revealed that triple inoculation of Rhizobium, AM fungus (G. geosporum) with Azospirillum, showed a significant plant growth biomass yield, percent root colonization, spore number, nodule number, nitrogen, and phosphorus content in shoots of C. arietinum L. and V. unguiculata L. compared to dual inoculation or single inoculation. However no improvement was observed in control/non-inoculated plants. The V. radiata responded positively with dual inoculation of Rhizobiuam with AM fungus (G. geosporum). This change has not been recorded in control plants compared to single/triple inoculation. Response to mineral fertilization and inoculation with rhizobia and/or arbuscular mycorrhiza fungi (AMF) of the Anadenanthera colubrina, Mimosa bimucronata, and Parapiptadenia rigida (Leguminosae–Mimosoideae) native trees from Brazilian riparian forests were studied in nursery conditions (Patreze and Cordeiro 2004). There were seven treatments varying in N, P fertilization, and inoculation with rhizobia (r), mycorrhiza (m), or both (rm): NP, P, P + r, P + rm, N, N + m, and N + rm. Results showed that AMF inoculations did not enhance the mycorrhizal colonization, and P uptake was not sufficient to sustain good growth of plants. The level of P mineral added affected negatively the AMF colonization in A. colubrina and M. bimucronata, but not in P. rigida. Native fungi infected the three legume hosts. The absence of mineral N limited growth of A. colubrina and P. rigida, but in M. bimucronata the lack of N was corrected by biological nitrogen fixation. N mineral added inhibited the nodulation, although spontaneous nodulation had occurred in A. colubrina and M. bimucronata. Rhizobia inoculation enhanced the number of nodules, nitrogenase activity, and leghemoglobin content of these two species. Thus, the extent of rhizobial and mycorrhizal symbiosis in these species under nursery conditions can affect growth and consequently the post-planting success.
10.4.2 Dual Inoculation with AMF and Rhizobia Under Field Conditions
Field investigations were conducted to study the effects of AM inoculation and triple superphosphate fertilization on nodulation, dry matter yield, and tissue N and P contents of Bradyrhizobium-inoculated soybean and lablab bean (Mahdi and Atabani 1992). Inoculation of both legumes with any of four AM fungi enhanced nodulation, dry matter yield, and plant N and P contents more than did triple superphosphate. Gigaspora margarita and G. mosseae were superior to G. calospora and Acaulospora species and resulted in more extensive root infection, especially in soyabean. The integration of N2 fixing trees into stable agroforestry systems in the tropics is being tested due to their ability to produce high biomass N and P yields, when symbiotically associated with rhizobia and mycorrhizal fungi (Marques et al. 2001; Kayode and Franco 2002). Accordingly, in a field trial, Marques et al. (2001) evaluated the effect of dual inoculation of Rhizobium spp. and mycorrhizal fungi on the growth of Centrolobium tomentosum Guill. ex Benth, a native leguminous tree of the Brazilian Atlantic Forest. Complete fertilization was compared to inoculation treatments of selected rhizobia strains BHICB-Ab1 or BHICB-Ab3, associated or not to AM fungi. Plants inoculated with strain BHICB-Ab1 and AMF increased the dry matter by 56% over uninoculated control, and N accumulation was greater than those observed for BHICB-Ab3 inoculated plants. Strain BHICB-Ab1 formed a synergetic relationship with mycorrhizal fungi as the combined inoculation enhanced plant height and dry weight more than single inoculation, while the growth of BHICB-Ab3 plants was not modified by AMF inoculation. Arbuscular mycorrhizal fungi also improved plants survival and possibly favored the nodule occupation by rhizobial strains as compared to the non-mycorrhizal plants. Similarly, Acacia mangium inoculated with rhizobial strains (BR 3609 and BR 3617) and three AM fungi, G. clarium, Gigaspora margarita, and Scutellospora heterogama, grew better than seeds planted without rhizobia and AMF inoculants (Kayode and Franco 2002). The authors observed that S. heterogama facilitated the growth better in both fallow and degraded soils. Seeds inoculated with rhizobia strains and AMF, however, produced more nodules and had higher AMF infection rates than seeds inoculated with rhizobia or AMF inoculants alone (Marques et al. 2001; Kayode and Franco 2002). Singh et al. (1991) evaluated the effect of live yeast cells (Saccharomyces cerevisiae) on nodulation and dry biomass of shoot and roots of legumes like Leucaena leucocephala, Glycine max, Cajanus cajan, Phaseolus mungo, Phaseolus aureus, and Vigna unguiculata in the presence of both AMF and Rhizobium strains. The results indicate that inoculation with live yeast cells remarkably enhanced the measured plant parameters. Root infection (native AMF) and the formation of vesicles, arbuscules, and spores were also increased with yeast inoculation. The increase in the parameters, however, varies with legumes and the type of yeast culture. On the other hand, the effect of whey application, the inoculation of Glomus intraradices Schenck & Smith and Mesorhizobium ciceri on root colonization, nodulation, yield, and the components of yield in chickpea (cv. Aziziye-94) were studied under rain-fed and irrigation management (Erman et al. 2011). Experiments were carried out in a split plot design with four replications in 2003 and 2004. The abovementioned factors were all applied to plants in single, double, and triple combinations. Arbuscular mycorrhizal fungus (AMF) inoculation, alone or in combination with other treatments, was very effective under rain-fed conditions, resulting in large increases in yield, root colonization, and phosphorus content of the seed and shoot. On the other hand, rhizobial inoculation increased significantly all traits examined, particularly root nodulation and the nitrogen content of seeds and shoots under irrigated conditions. Whey combined with AMF significantly increased root colonization, while its combination with Rhizobium increased the number of nodules. Combinations of two or three treatments were more effective than individual applications. The greatest yield, root colonization, and nodulation were obtained from the combination of all three treatments under irrigation. Although voluminous literature reports show superiority of plant performances under dual inoculation, sometimes the usual synergism was found to be less effective. For example, Nambiar and Anjaiah (1989), in a field experiment, reported that the effects of AMF on competition among inoculated bradyrhizobia were less evident, but inoculation with Bradyrhizobium strains increased root colonization by AMF and certain AMF/Bradyrhizobium inoculum strain combinations produced higher nodule numbers. Plants grown without Bradyrhizobium and AMF, but supplied with ammonium nitrate (300 g mL−1) and potassium phosphate (16 g mL−1), produced higher dry matter yields than those inoculated with both symbionts in the pot experiment. Inoculation with either symbiont in the field, however, did not result in higher pod yields at harvest. In a similar trial, Camila and Lazara (2004) have tested response to mineral fertilization and inoculation with rhizobia and/or AMF of the Anadenanthera colubrina, Mimosa bimucronata, and Parapiptadenia rigida (Leguminosae–Mimosoideae) native trees from Brazilian riparian forests, in nursery conditions. The findings showed that AMF inoculations did not enhance the mycorrhizal colonization, and P uptake was not sufficient to sustain good growth of plants. The level of P mineral added affected negatively the AMF colonization in A. colubrina and M. bimucronata, but not in P. rigida. Native fungi infected the three legume hosts. The absence of mineral N limited the growth of A. colubrina and P. rigida, but in M. bimucronata the lack of N was corrected by BNF. The applied N mineral, however, inhibited nodulation, although spontaneous nodulation occurred in A. colubrina and M. bimucronata. Rhizobia inoculation enhanced the number of nodules, nitrogenase activity, and leghemoglobin content of these two species. Thus, the extent of rhizobial and mycorrhizal symbiosis in these species under nursery conditions affected growth and consequently the post-planting success. Evidence is also available that improved formation of AM can inhibit nodulation, possibly due to inter-endophyte incompatibility of competition (Behlenfalvay et al. 1985). On the contrary, (Pacovsky et al. 1986) revealed that even though nodule numbers may not significantly be increased by AM colonization, yet the size and nitrogen-fixing activity may be increased. However, there is a report that suggests that symbiotic N2 fixation is clearly accelerated in legume following AMF inoculation, but the response of Rhizobium symbiosis may vary according to the strains of the AM fungus involved (Linderman and Paulitz 1990). These and other associated data thus indicate that the Rhizobium–AMF partnership nearly always exists but may not necessarily be optimal with the best combination of symbionts for the host legumes.
10.5 Mycorrhizal Dependency of Legumes
For some plant species, the association with mycorrhizal fungi is indispensable. The degree of dependence, however, varies with plant species, particularly the root morphology and conditions of soil and climate (Hayman 1986). Plants with thick roots poorly branched and with few root hairs are usually more dependent on mycorrhizas for normal growth and development. These species include onions, grapes, citrus, cassava, coffee, and tropical legumes. When the level of soil fertility and humidity are increased, the dependence on the mycorrhizal condition decreases to a point where the plant becomes immune to colonization (Khaliel et al. 1999). Furthermore, mycorrhizal dependencies of leguminous plants grown in stressed situations have also been well documented (Plenchette et al. 2005; Ghosh and Verma 2006). Growth and mineral uptake of 24 tropical forage legumes and grasses were compared under glasshouse conditions in a sterile low P oxisol, one part inoculated and the other not inoculated with mycorrhizal fungi (Duponnois et al. 2001). Shoot and root dry weights and total uptake of P, N, K, Ca, and Mg of the entire test plants were significantly increased by mycorrhizal inoculation. Mycorrhizal inoculation, with few exceptions, decreased the root/shoot ratio. Non-mycorrhizal plants, on the other hand, had lower quantities of mineral elements than mycorrhizal plants. Plant species, however, did not show any correlation between percentage mycorrhizal infection and growth. A great variation in dependence on mycorrhiza was observed among forage species. Total uptake of all elements by non-mycorrhizal legumes and uptake of P, N, and K by non-mycorrhizal grasses correlated inversely with mycorrhizal dependency. Mycorrhizal plants of all species used significantly greater quantities of soil P than the non-mycorrhizal plants, and utilization of soil P by non-mycorrhizal plants was correlated inversely with mycorrhizal dependency. As the production of grain and herbaceous legumes is often limited by low levels of available P in most savanna soils, the potential for managing AMF by selecting lines or accessions dependent on AMF as a strategy to improve plant P nutrition and productivity is required (Plenchette et al. 2005; Ghosh and Verma 2006). Accordingly, Nwoko and Sanginga (1999) evaluated the interactions between AMF and Bradyrhizobium species and their effects on growth and mycorrhizal colonization of ten recent selections of promiscuous soybean breeding lines and two herbaceous legumes (Lablab purpureus and Mucuna pruriens). Mycorrhizal colonization differed among promiscuous soybean lines (ranging from 16 to 33%) and was on average 20% for mucuna and lablab. Three groups of plants were identified according to mycorrhizal dependency (MD): (1) the highly dependent plants with MD >30% (e.g., soybean line 1039 and mucuna), (2) the intermediate group, with MD between 10 and 30% (e.g., soybean line 1576 and lablab), and (3) the majority of soybean lines (five lines out of ten) that were not mycorrhizal dependent. This great variability in MD and response to P application among promiscuous soybean and herbaceous legumes offers a potential for the selection of plant germplasm able to grow in P-deficient soil. Similar results have also been reported for different species of woody leguminous trees. For instance, Ghosh and Verma (2006) evaluated the effects of three AMF species (G. occultum, G. aggregatum, and G. mosseae) inoculations on growth responses of Acacia mangium in lateritic soil. All inoculations significantly enhanced growth with respect to shoot height, root diameter, leaf area, chlorophyll content, and biomass of A. mangium compared to uninoculated control seedlings. The mycorrhizal dependency factor indicated that the growth of A. mangium was 57% dependent on G. occultum, 47% on G. mosseae, and 46% on G. aggregatum. The findings indicate the presence of disparity among AMF species with regard to their growth enhancement in a particular mycorrhizal legume. It has also been demonstrated that mycorrhizal dependence and responsiveness of legumes declines with an increase in P added to the soil (Khaliel et al. 1999).
10.6 How Arbuscular Mycorrhizal Fungi Enhance Legumes’ Performance
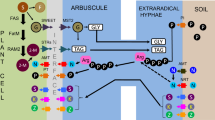
The AM fungi affect the growth and development of plants both directly and indirectly (Table 10.1). However, broadly, the principal contribution of AM fungi to plant growth is due to uptake of nutrients by extraradical mycorrhizal hyphae (Marschner 1998; Hodge and Campbell 2001; van der Heijden et al. 2006). The most prominent effect of AMF is to improve P nutrition of the host plant in soils with low P levels due to the large surface area of their hyphae and their high affinity P uptake mechanisms (Muchovej 2001). To substantiate this concept of plant growth promotion by AM fungi, several studies have shown that AM fungi contribute to up to 90% of plant P demand (Jakobsen et al. 1992; van der Heijden et al. 2006). For instance, the P depletion zone around non-mycorrhizal roots extends to only 1–2 mm, nearly the length of a root hair, whereas extraradical hyphae of AMF extend 8 cm or more beyond the root making the P in this greater volume of soil available to the host (Fig. 10.1). There are also reports of production of organic acids by AMF that could solubilize the insoluble mineral P (Lapeyrie 1988), an added advantage in terms of improvement of P uptake by host plants. In addition, AMF mycelia have also been shown to increase uptake of many other nutrients, including N, S, B, Cu, K, Zn, Ca, Mg, Na, Mn, Fe, Al, and Si (Clark and Zeto 2000). Apparently, besides providing P to their host plants, AM fungi can facilitate N2 fixation by providing legumes with P and other immobile nutrients such as Cu and Zn, essential for N2 fixation (Li et al. 1991; Kothari et al. 1991; Clark and Zeto 2000). There are reports that N fixation can be reduced or even completely inhibited in the absence of AMF at low nutrient availability (Azcón et al. 1991). The improvement in plant growth under both greenhouse and field conditions has also been suggested due to increased photosynthesis and improved carbon flow to the nodule and to AM sinks, giving rise to more and larger nodules that fix more nitrogen for the plant (Linderman and Paulitz 1990). In some cases, AMF may be responsible for acquiring 100% of host nutrients (Smith et al. 2004). Thus, Marschner (1998) and Hodge and Campbell (2001) have suggested that the overall improvement in plant nutrition following AM inoculation is due to (1) increased root surface through extraradical hyphae, which can extend beyond root depletion zone, (2) degradation of organic material, and (3) alteration of the microbial composition in the rhizosphere. More specifically, mechanisms as to how AMF contribute to plant health have been extensively studied leading to development of several hypotheses (Linderman 1994). The most important are (a) increased nutrient uptake that results in higher resistance of the plant to pathogen invasion or a compensation of the symptoms, (b) competition for photosynthates or space, (c) plant morphological changes and barrier formation, (d) changes in biochemical compounds related with plant defense, and (e) increased percentage of microbial antagonists in the rhizosphere. Under conditions of low N and P availability which exist in many tropical soils, the possible transfer of nutrients from the mycorrhizal plant to another plant via AMF hyphal network may occur. Underground hyphal links can be formed when hyphae of mycorrhizal fungi spread from one infected plant and enter the roots of one or more other plants (Heap and Newman 1980). Studies have ascertained that AM fungi did enhance N transfer from mycorrhizal legumes to another nonleguminous plant (Vankessel et al. 1985). Similarly, Snoeck et al. (2000) demonstrated that nearly 30% of the nitrogen fixed by legumes like Desmodium and Leucaena was transferred to associated coffee trees.
Root colonized by endomycorrhizal fungus . Zone of P (or other nutrient) absorption by a non-mycorrhizal root (a) and by a mycorrhizal root (b) P phosphate ion (adapted from Muchovej (2001))
10.6.1 Alleviation of Environmental Stresses in Mycorrhizal Legumes
Currently, wide arrays of environmental stresses (abiotic/biotic) are increasing worldwide due to various types of anthropological activities that have seriously threatened plant distribution and function in a given ecosystem. Although plants have evolved mechanisms to cope such unfavorable factors, but they can perform better if grown with beneficial rhizosphere microbes (Aroca and Ruiz-Lozano 2009). Generally, phytobeneficial microbes greatly enhance tolerances of plants to a wide array of stresses (Fig. 10.2). The role of AM fungi and other phytobeneficial microbes in the promotion of biological and chemical properties of legumes under stressed environment is briefly discussed in the following section.
10.6.1.1 Tolerance to Salt/Alkaline and Acidity
Salinity is one of the most important abiotic stresses that limit crop growth and productivity across the globe. Soil salinity also decreases nodulation and N2 fixation and nitrogenase activity of nodulated legumes (Karmakar et al. 2015). Thus, the development of salt-tolerant symbioses is an absolute necessity to enable cultivation of leguminous crops in salt-affected soils. For example, Rabie and Almadini (2005) while investigating the effects of dual inoculation of Azospirillum brasilense nitrogen fixing bacterium (NFB) and AMF (G. clarum) on Vicia faba grown with five levels of NaCl (0.0–6.0 dS m−1) observed that AM-inoculated faba plants showed decreases in salinity tolerance, % of mycorrhizal infection, and higher accumulation of proline with increasing levels of salinity. In addition, AMF infection significantly increased mycorrhizal dependency, N and P level, phosphatase enzymes, nodule numbers, protein content, and nitrogenase enzymes of all salinized faba plants compared to control and non-AM plants either in the absence or presence of NFB. In shoots of non-AM plants, Na+ concentration was increased, while the concentrations of K+, Mg2+, and Ca2+ were decreased with increasing salinity. AM-colonized plants, on the other hand, had greater K+/Na+, Mg2+/Na+, and Ca2+/Na+ ratios relative to non-AM plants at all salinity levels. The Na+ level in shoots of AM plants showed slight increase with gradual increase in salinity, while a noticeable increase was observed in K+ and Ca2+ concentrations especially at higher salinity levels. The results clearly showed that the inoculation of NFB along with AM plants synergistically increased the performance of the test legume under salinity stress providing evidence for reducing the salt affected negative impact on legumes as also reported for Trifolium alexandrinum plants grown under different salinity levels (2.2, 5, and 10 dS m−1) in a pot experiment under glasshouse conditions (Shokri and Maadi 2009). Another study in Egypt (Abd-Alla et al. 2014) was devoted to investigating the synergistic interaction of Rhizobium and arbuscular mycorrhizal fungi for improving growth of faba bean grown in alkaline soil. Out of 20 isolates 3 of them were selected as tolerant isolates and named as Rhizobium sp. Egypt 16 (HM622137), Rhizobium sp. Egypt27 (HM622138), and R. leguminosarum bv. viciae STDF-Egypt 19 (HM587713). The best alkaline tolerant was R. leguminosarum bv. viciae STDF-Egypt 19 (HM587713). The effect of R. leguminosarum bv. viciae STDF-Egypt 19 and mixture of AMF (Acaulospora laevis, G. geosporum, G. mosseae, and Scutellospora armeniaca) both individually and in combination on nodulation, nitrogen fixation, and growth of Vicia faba under alkalinity stress were assessed. A significant increase over control in number and mass of nodules, nitrogenase activity, leghemoglobin content of nodule, mycorrhizal colonization, and more dry mass of root and shoot was recorded in dual inoculated plants than plants with individual inoculation. The enhancement of nitrogen fixation of faba bean could be attributed to AMF facilitating the mobilization of certain elements such as P, Fe, K, and other minerals that involve in synthesis of nitrogenase and leghemoglobin. Thus it is clear that the dual inoculation with Rhizobium and AMF biofertilizer is more effective for promoting growth of faba bean grown in alkaline soils than the individual treatment, reflecting the existence of synergistic relationships among the inoculants.
The ability of crop plants to tolerate low soil pH has become extremely important in the agricultural production systems of the humid tropics with soils of low pH (Kamprath and Foy 1985). Studies by Dodd et al. (1990) and Sieverding (1991) show that over 50 field trials with effective AMF in acid soils of varying fertility resulted in an average increase of 20–25% in yields (3 tons ha−1) and a greater stability in production year after year. Later on, the influence of soil acidity on the levels of colonization by the microsymbionts and the dependency of pioneer plants on the microsymbionts was investigated in an abandoned quarry of acid sulfate soil (Maki et al. 2008). The levels of AM colonization in pioneer grass, forbs, and legume shrubs grown in the field were assessed, and no significant decline in the levels with an increase in soil acidity was observed. Most of the legume shrubs formed root nodules. Several AM fungi and bradyrhizobia were cultured from the rhizosphere soils of pioneer plants grown in the quarry. Pot experiments revealed that the microsymbionts isolated from the field significantly promoted the growths of pioneer grasses and legume shrubs in acid sulfate soil at pH 3.4. On the other hand, Dodd et al. (1990) supported the idea that increasing the AMF inoculum potential of acid-infertile soils by inoculation or pre-crops can greatly increase the rate of establishment of mycorrhiza-dependent host plants. Thus, from these and other studies, it was suggested that bacterial-AM-legume tripartite symbioses could be a new approach to increase the tolerance of legume plants under stressed environment. Integration of microbial inoculants with NPK application in acidic soils has showed promising results. For example, Bai et al. (2016) in a recent experiment quantified the influence of integrated use of AMF, Rhizobium, and N and P on growth, productivity, profitability, and nutrient use efficiencies of garden pea grown under acid Alfisol field. The experiment was laid out in randomized block design (RBD) replicated thrice comprising 13 treatments involving AMF (G. mosseae), Rhizobium (R. leguminosarum), and inorganic N and P fertilizers. The results revealed that dual inoculation of pea seed with AMF and Rhizobium enhanced the plant height, leaf area index, and dry matter accumulation significantly by 19.4 and 13.1, 10.7 and 10.7, and 16.6 and 16.7%, respectively, at 60 and 120 days after sowing (DAS). Similarly, dual inoculation exhibited significant respective increases of 9.5 and 14.6% in absolute and crop growth rates over generalized recommended NPK dose (GRD) during 60–120 DAS. The dual inoculation led to significant respective increases of 1- and 2.2-, 1.06- and 1.74-, 0.21- and 1.5-, and 1.05- and 1.60-folds in partial factor productivity, crop recovery efficiency, physiological efficiency, and % recovery of applied N and P, respectively, over GRD. The magnitude of increase in pea productivity, net returns, and boron to carbon (B/C) ratio following dual inoculation was to the tune of 20, 54.4, and 104.1%, respectively, over GRD. Dual inoculation also exhibited significant increases of 19.4 and 53% in production and monetary efficiencies of pea over GRD. Overall, dual inoculation of AMF and Rhizobium with 75% soil-test-based N and P dose in pea has great potential in enhancing pea productivity, profitability, and nutrient use efficiency besides saving about 25% fertilizer N and P without impairing pea productivity in Himalayan acid Alfisol.
10.6.1.2 Heavy Metals and Drought Tolerance
Working with Trifolium repens, Vivas et al. (2003) studied the effect of inoculation with naturally occurring microorganisms (an AM fungus and rhizosphere bacteria) isolated from a Cd-polluted soil. One of the bacterial isolate identified as a Brevibacillus sp. showed a marked PGPR activity. Mycorrhizal colonization also enhanced Trifolium growth and N, P, Zn, and Ni content, and the dual inoculation of AM fungus and Brevibacillus sp. further enhanced growth and nutrition and reduced Cd concentration, particularly at the highest Cd level. Interestingly, increasing Cd level in soil decreased Zn and Pb accumulation in shoot. Co-inoculation of Brevibacillus sp. and AM fungus increased shoot biomass over single mycorrhizal plants by 18% (at 13.6 mg Cd kg−1), 26% (at 33.0 mg Cd kg−1), and 35% (at 85.1 mg Cd kg−1). In contrast, Cd transport from soil to plants was substantially reduced and at the highest Cd level; Brevibacillus sp. lowered this value by 37.5% in AM-colonized plants. However, the increase in Cd level highly reduced plant mycorrhization and nodulation. On the contrary, strong positive effect of this bacterium was observed for nodule formation in all treatments. In a similar study conducted by Al-Garni (2006), the composite inoculation of AM fungus and Rhizobium significantly increased dry weight, root/shoot ratios, leaf number and area, plant length, leaf pigments, total carbohydrates, and N and P content of cowpea plants grown in pots treated with 6 concentrations of Zn (0–1000 mg/kg dry soil) and Cd (0–100 mg/kg dry soil) compared to non-inoculated controls. Moreover, tolerance index of inoculated cowpea plants was greater than uninoculated plants. And microsymbionts dependencies of test plants increased at higher levels of Zn and Cd in polluted soil. Metals accumulated by microsymbionts-infected cowpea plant were mostly distributed in root tissues, suggesting that an exclusion strategy for metal tolerance exists in such organisms. Yet in another study, the influence of AM fungus G. macrocarpum Tul. and Tul on growth, nutrients, and Pb uptake by Bradyrhizobium-inoculated soybean (var. IAC-14) was assessed in soils treated with different levels of Pb (Andrade et al. 2004). The results revealed that soybean shoot dry biomass was not affected by increasing doses of Pb, but the number of pods decreased significantly. Nodule dry weights of mycorrhizal roots were reduced by soil Pb additions, although the mycorrhizas stimulated plant nodulation significantly. The inoculation of AMF in soybeans provided higher rates of nutrients uptake, mainly P, inducing greater mycorrhizal-soybean growth. Thus, mycorrhizas improved Pb uptake and produced shoots with Pb concentrations 30% lower than those of non-mycorrhizal plants, at the highest Pb concentration added to the soil. AM fungus was, however, more susceptible to the higher Pb rates added to the soil than the soybean plants, decreasing both root AM colonization and spore production. This work indicated that a concentration of 600 mg dm−3 of Pb in the soil interfered with the establishment of double symbioses between AMF and Bradyrhizobium and with the fungus perpetuation in soil. Recent surveys indicate that ecosystem restoration of heavy metal contaminated soils practices need to incorporate microbial biotechnology research and development in order to harness the optimum benefits of bacterial-AM-legume tripartite symbiosis under heavy metal contaminated soils (Al-Garni 2006; Khan 2006).
Water deficit is considered one of the most important abiotic factors limiting plant growth and yields. Several eco-physiological investigations have shown that the AM symbiosis often alters the rates of water movement into, through, and out of the host plants, with consequent effects on tissue hydration and plant physiology (Ruiz-Lazano 2003) and consequently improve water uptake by plants (Aliasgharzad et al. 2006). AM fungi in combination with rhizobia or PGPR usually have an accumulative beneficial effect on plant drought tolerance (Aroca and Ruiz-Lozano 2009). For instance, in a controlled pot culture experiment performed by Aliasgharzad et al. (2006), soybean plants were inoculated with two species of AM fungi, G. mosseae (Gm) or G. etunicatum (Ge), or left non-inoculated (NM) as control in a sterile soil. Four levels of soil moisture (field capacity, 0.85 FC, 0.7 FC, 0.6 FC) in the presence or absence of B. japonicum were applied to the pots. Relative water content (RWC) of leaf at both plant growth stages (flowering and seed maturation) decreased with the dryness of soil; RWC was higher in all mycorrhizal than non-mycorrhizal plants irrespective of soil moisture level. At the lowest moisture level (0.6 FC), Ge was more efficient than Gm in maintaining high leaf RWC. Leaf water potential (LWP) had the same trend as RWC at flowering stage, but it was not significantly influenced by decrease in soil moisture to 0.7 FC during seed maturation stage. Seed and shoot dry weights were affected negatively by drought stress. Mycorrhizal plants, however, had significantly higher seed and shoot dry weights than non-mycorrhizal plants at all moisture levels except for seed weight at 0.6 FC. Root mycorrhizal colonization was positively correlated with RWC, LWP, shoot N and K, and seed weight, implying improvement of plant water and nutritional status as a result of colonization. Shoot K was enhanced considerably by both bacterial and fungal inoculations, particularly in plants with dual inoculations where the highest shoot K levels were found. The relatively higher shoot and seed dry weights in plants inoculated with both G. etunicatum and B. japonicum could be ascribed to their higher RWC and LWP, suggesting that drought avoidance is main mechanism of this plant-microbe association in alleviation of water stress in soybean. Aroca and Ruiz-Lozano (2009) also emphasized that phytobeneficial soil microorganisms enhance plant drought tolerance by different mechanisms including decreased oxidative stress, improved water status, or regulation of aquaporins. In addition, the authors further suggested that AM symbiosis improves almost every physiological parameter, like water status, leaf transpiration, photosynthesis, or root water uptake of the host plant under drought stress. At the same time, AMF in combination with rhizobia or other PGPR results in additive or synergistive effect on plant drought tolerance, although this depends on the compatibility of strains used for inoculation. Therefore, although there is evidence which help to understand as to soil microorganisms induce plant drought tolerance at physiological level, the mechanistic basis of drought tolerance at molecular level is inadequate. Currently, it is well documented that desertification is a complex and dynamic process which obviously has a negative environmental impact, particularly in arid, semiarid, and subhumid areas of the world, where the process is claiming several million hectares per annum (Herrera et al. 1993; Aroca and Ruiz-Lozano 2009). Consequently, the proportion of plants living under water shortage conditions is increasing. Thus, management of indigenous plant-microbes symbioses assists in restoration of desertified ecosystems (Requena et al. 2001). Legumes are the most appropriate candidates for revegetation of water-deficient, low-nutrient environments/disturbed ecosystems because of their ability to establish tripartite symbiotic associations with nitrogen-fixing rhizobia and AMF which improve nutrient acquisition and help plants to become established and cope with stress situations (Herrera et al. 1993). Studies show that useful legume tree species may contribute around 12 tons of dry litter and 190 kg of N ha−1 y−1 to renovate degraded soil (Franco and De Faria 1997). Sometimes, the combined effect of microsymbionts may, however, cause deleterious effect to legume host under moderate water stress condition. For instance, four Phaseolus vulgaris varieties were single or dual inoculated with two different AM fungus and/or two different Rhizobium strains (Franzini et al. 2010). All plants were grown under moderate drought conditions. Surprisingly, most of the biological treatments involving one fungus and one Rhizobium together caused a deleterious effect on plant growth. However, these negative effects were dependent on the P. vulgaris variety used as well as on the symbionts implicated. The results showed that AM symbiosis inhibited nodule development and N2 fixation, causing diminution of plant growth. Therefore, under moderate drought conditions, the dual symbiosis formed by AM fungi and Rhizobium can be deleterious to P. vulgaris growth depending on the plant variety and the symbionts involved. Thus, under these common stress conditions, selection for the appropriate symbionts to each P. vulgaris variety is needed.
10.6.1.3 Tolerance of Soilborne Pathogens
The effects of AM fungi G. mosseae (Gm) and G. fasciculatum (Gf) and R. leguminosarum biovar phaseoli (Rlp) were examined on the patho-system of Sclerotinia sclerotiorum (Lib) de Bary (Ss) and common bean (Aysan and Demir 2009). The colonization and nodulation of two biological control agents exhibited differences as a result of reciprocal interactions of these items as well as the effect of Ss. Nodulation of Rlp decreased in triple inoculation. In addition, colonization of AMF significantly decreased in treatment of Ss + AMF than control AMF. Treatments of single inoculation of AMF and Rlp isolates reduced disease severity by 10.3–24.1%. It was found that single biological control agent’s inoculations were more effective than dual inoculations (AMF + Rlp). While comparing the morphological parameters of common beans, all measured morphological parameters were decreased in treatments having pathogen isolate. Besides this, all biological control agents increased total content of P and N in treated plants compared to the controls. Root colonization by AMF can improve plant resistance/tolerance to biotic stresses. Studies indicate a range of mechanisms are involved in controlling the pathogen by mycorrhizal roots such as exclusion of pathogen, lignifications of cell wall, changed P nutrition, exudation of low molecular weight compounds, and others (Sharma et al. 2004). Sundaredan et al. (1993) investigated the interaction of G. fasciculatum with a wilt-causing soilborne pathogen, Fusarium oxysporum, against cowpea plants. It was found that pre-establishment by AM fungus reduced the colonization of the pathogen and the severity of the disease, as determined by reduction in vascular discoloration index. In mycorrhizal plants, the production of phytoalexin compounds was always higher than in the non-mycorrhizal plants, and a direct correlation between the concentration of the phytoalexins and the degree of mycorrhizal association was found. It is argued that the production of phytoalexin compounds in mycorrhizal plant could be one of the mechanisms imparting tolerance to the plants against wilt disease. Moreover, multiple lines of evidence reveal that AM fungi significantly reduced disease symptoms caused by fungal pathogens such as Phytophthora, Gaeumannomyces, Fusarium, Pythium, Rhizoctonia, Verticillium, and Aphanomyces (Demir and Akköprü 2007). In another study Gao et al. (2012) have investigated the disease incidence and index of soybean red crown rot under different P regimes in field and found that the natural inoculation of rhizobia and AMF could affect soybean red crown rot, particularly without P addition. Further studies in sand culture experiments showed that inoculation with rhizobia or AMF significantly decreased severity and incidence of soybean red crown rot, especially for co-inoculation with rhizobia and AMF at low P. The root colony forming unit (CFU) decreased over 50% when inoculated with rhizobia and/or AMF at low P. However, P addition only enhanced CFU when inoculated with AMF. Furthermore, root exudates of soybean inoculated with rhizobia and/or AMF significantly inhibited pathogen growth and reproduction. Quantitative RT-PCR results indicated that the transcripts of the most tested pathogen defense-related (PR) genes in roots were significantly increased by rhizobium and/or AMF inoculation. Among them, PR2, PR3, PR4, and PR10 reached the highest level with co-inoculation of rhizobium and AMF. The results indicated that inoculation with rhizobia and AMF could directly inhibit pathogen growth and reproduction and activate the plant overall defense system through increasing PR gene expressions. Combined with optimal P fertilization, inoculation with rhizobia and AMF could be considered as an efficient method to control soybean red crown rot in acid soils.
10.7 Inoculum Development and Formulations
Since AM fungi are an obligate biotrophs, the AM inoculum production on a commercial scale via a host plant is still an obstacle and hence limits its utility as inoculants in sustainable agricultural production systems. Despite the limitation in inoculums development, certain progress has been made in this direction, and some commercial inoculum is currently marketed in some countries in the world (Gianinazzi and Vosátka 2004). Currently, there has been a remarkable boom in enterprises producing mycorrhizal fungi inocula and related services for the retail sector, commercial plantations, horticulture, and, more recently, the developing agricultural market. There are number of reasons for increasing interest in developing mycorrhizal inocula by the mycorrhizal industry. Firstly, the positive effects of mycorrhizal fungi on plant health, growth, and yield have generated a greater interest among end users of mycorrhizal technology (Gianinazzi and Vosátka 2004). Secondly, it offers an environmentally friendly and economically attractive option in commercial cultivation (Toro et al. 1997). Therefore, AM fungi are gaining popularity as “biofertilizers”/efficient scavengers of nutrients, “bioprotectors,” and “biocontrol” agents (Sylvia 1999), and hence, the industry of mycorrhizal inoculum production is expanding around the world (Todd 2004). However, extensive field trials are required to prove that bioagent indeed is effective and, hence, can be recommended for inoculant development and its consequent application over a wide range of soil, environmental conditions, and crop types (Leggett et al. 2007). The first consideration in inoculum production involves the selection of fungal isolates endowed with growth-promoting activity (Ryan and Graham 2002). Other factors to be considered in the production of inoculum include soil conditions, the host plant used to grow fungus (Sieverding 1991; Ryan and Graham 2002). Several host plants including Sudan grass (Sorghum bicolor var. Sudanese), bahia grass (Paspalum notatum), guinea grass (Panicum maximum), cenchrus grass (Cenchrus ciliaris), clover (Trifolium subterraneum), strawberry (Fragaria sp.), sorghum (Sorghum vulgare), maize (Zea mays), barley (Hordeum vulgare), and onion (Allium cepa) have been attempted to produce AM inoculum. However, mass propagation of AM fungi varies greatly with root structure and habitat of host plant (Bever et al. 1996). Furthermore, since there are greater variations in soils and climates around the world, the locally available materials for inoculum production should be tested (Sieverding 1991). The traditional and most widely used approach has been to grow the fungus with the host plant in solid growth medium individually or a combination of the solid growth media such as soil, sand, peat, vermiculite, perlite, clay, or various types of composted barks (Sylvia and Jarstfer 1992). For the commercial development of AM inoculants, numerous strategies have been adopted time to time with their own merits and demerits. Some of the recently followed techniques for the production of mycorrhizal inoculum including soil or soilless technologies, like nutrient film technique (NFT) (Mosse and Thompson 1984), circulation hydroponic culture system, aeroponic culture system (Sylvia and Hubbell 1986), root organ culture, and tissue culture (Nopamornbodi et al. 1988), are briefly discussed in the following section.
10.7.1 Inoculum Production Strategies
10.7.1.1 Soil-Based Pot Culture Method
Soil-based pot culture is a common method for production of AM fungal inoculum (Menge 1984). Soil inoculum contains all AM fungal structures; this inoculum source is highly infective (Sieverding 1991). The author further suggested that the success for good soil inoculum production depends on the selection of the host plant and the ambient conditions. The soil inoculum (containing AM-infected roots, AM spores, and mycelium) is chopped and homogenized before use. Soil may contain abiotic and biotic components which make it undesirable substrate in which to grow and subsequently to distribute the AM fungal inoculum. Soil inocula are considered impractical because of their bulk and the risk of contamination by insects, nematodes, and plant pathogens (Sylvia and Jarstfer 1992). However, chopped roots in peat blocks (Warner 1985) and spores within a clay matrix (Dehne and Backhaus 1986) have been proposed for field application. Soil-based pot culture method is cost-effective with low inputs, and thousands of infectious propagules can be extracted per gram of soil. However, the major disadvantage associated with this technique includes bulk amount, vulnerability of pest to infestation, and nutrient management (Sharma et al. 2000). To overcome these problems, soilless technologies were discovered and are discussed.
10.7.1.2 Nutrient Film Technique (NFT)
In this method, large volume of nutrient liquid in a film is recycled which flows over the roots of plants. However, any host in the NFT should be grown first in the soil substrate with AM inoculums in order to infect the roots. This technique eliminates the possibility of contamination and helps to produce large quantities of AM-infected roots. However, higher sporulation compared to soil system is not achieved. Yun-Jeong and Eckhard (2005) used NFT culture system for nursery production of arbuscular mycorrhizal horticultural crops. In the NFT system, a thin layer of glass beads was used to provide solid support for plant and fungus growth, and nutrient solution was supplied intermittently (15 min, six times per day). A modified nutrient solution (80 μM P) was used and was changed with fresh solution at 3-day intervals. The dry matter accumulation in Glomus mosseae (BEG 107)-colonized lettuce (Lactuca sativa var. capitata) was significantly higher than non-mycorrhizal lettuce in perlite during the precolonization period. The root colonization rate was also high at rates up to 80 μM P supply. On the NFT system, growth differences between mycorrhizal and non-mycorrhizal plants were less than in perlite. However, root colonization rate was not reduced during the NFT culture period. In this system, high amounts of fungal biomass were produced. The authors suggested that using this technique, metal and other nutrient concentrations in fungal hyphae can be determined. Furthermore, this modified NFT culture system would also be suitable for fungal biomass production on a large scale with a view to additional aeration by intermittent nutrient supply, optimum P supply, and a use of glass beads as support materials. Furthermore, bulk inoculum composition with a mixture of spores, colonized roots, and hyphae grown in soilless media by the modified NFT system might be a useful way to mass-produce mycorrhizal crops and inoculum for commercial horticultural purposes.
10.7.1.3 Aeroponic Method
A culture system which applies a fine mist of defined nutrient solution to the roots of trap plant is termed as aeroponic culture (Zobel et al. 1976). For this, plants are generally inoculated in sand or vermiculite before they are transferred to these systems. Plants have also been inoculated directly in the aeroponic system (Hung et al. 1991). Applying aeroponics higher number spores have been produced compared to soil-based pot cultures. Since no substrate is present with the inoculum with aeroponic culture of roots, it is possible to produce inoculum with hundreds of thousands of propagules per dry gram of roots (Sylvia and Jarstfer 1992). The aeroponics has distinct advantage over other AM-producing techniques, like the highly aerated rooting environment of aeroponics stimulates rapid and abundant sporulation of the AM fungi and this system reduces the risk of contamination but this technique is a costly affair. For example, in an aeroponic culture, root colonization and sporulation of G. mosseae (Nicol. & Gerd.) Gerd. & Trappe and G. intraradices Schenck & Smith with bahia grass was found superior relative to a soil-based pot culture (Sylvia and Hubbell 1986). Similarly, Martin-Laurent et al. (1999) designed an experiment to produce Acacia mangium saplings associated with AM fungi using aeroponics (a soilless plant culture method). A. mangium seedlings were first grown in multipots and inoculated with Endorize (a commercial AM fungal inoculums) followed by transferring to aeroponic systems or to soil. Aeroponics was found as a better system than soil and doubled the production of tree saplings compared to soil. Moreover, compared to plants grown in soil, aeroponically grown saplings inoculated with AM fungal inoculum exhibited significantly different rates of mycorrhization, leading to an increase in chlorophyll contents in plant tissues. The authors suggested that the aeroponic system is an innovative and appropriate technology which could be used to produce large quantities of tree saplings associated with soil microorganisms, such as AM fungi, for reforestation of degraded land in the humid tropics. Aeroponically produced G. deserticola and G. etunicatum inocula retained their infectivity after cold storage (4°C) in either sterile water or moist vermiculite for at least 4 and 9 months, respectively (Hung and Sylvia 1988).
10.7.1.4 Root Organ Culture System
The root organ culture system is the most attractive and advanced cultivation method for AMF development. This technique uses root-inducing transfer-DNA-transformed roots of a host plant to develop the symbiosis on a specific medium in vitro which provides pure, viable, contamination-free inoculum using less space. Systems utilizing excised roots of various host plants and different media formulations have been developed to culture glomalean fungi monoxenically (Mugnier and Mosse 1987). Less than 5% of currently known AM species have, however, been successfully cultivated using dual culture approach. Gigaspora margarita (Miller-Wideman and Watrud 1984); G. fasciculatum, G. intraradices, and G. macrocarpum (Declerck et al. 1998); and G. versiforme (Diop et al. 1994) have been maintained and sporulated in association with excised tomato roots or roots of carrot transformed by “hairy root” inducing T-DNA from Agrobacterium rhizogenes. Evidently, the rate of in vitro spore formation of the AM fungus G. versiforme was followed in Petri dishes, using mycorrhizal root-segment inoculum associated with Ri T-DNA transformed carrot roots (Declerck et al. 1996). Three phases of sporulation were observed: a lag phase, a period of intensive spore production, and a plateau phase. An average of 9500 spores/Petri dish was produced after 5 months of dual culture. The root organ culture system supported extensive root colonization with many arbuscules and vesicles being formed. The fungus, both within root segments and as spores produced, was viable and able to complete its life cycle in vitro. The mycorrhizal root segments, however, exhibited higher inoculum potential due to numerous vesicles and extensive intraradical mycelium. The in vitro propagation on root organ culture, however, may not change drastically the traditional process but will improve the quality of strain and the supply of spores (Dalpé and Monreal 2004).
10.7.2 Formulations
Different formulated products are available in the market, which creates the need for the establishment of standards for widely accepted quality control. In most cases, fresh AMF inoculum is applied (Fig. 10.3). In preparation and formulation of mycorrhizal inoculum, the most widely used methods are based on the entrapment of fungal materials in natural polysaccharide gels (Sieverding 1991; Vassilev et al. 2005). The potential of such inoculant preparations is illustrated by various studies which include immobilization of mycorrhized root pieces, vesicles, and spores, in some cases co-entrapped with other plant beneficial microorganisms (Vassilev et al. 2001).
Various stages involved in production and inoculation of AMF: astrains to be selected must be the best performer, bcultivation using suitable host by employing either conventional soil-based or soilless techniques, cinclude immobilization of mycorrhized root pieces, vesicles, and spores, in some cases co-entrapped with other plant beneficial microorganisms, dsupplying propagules near seedlings in soil at appropriate rate (modified from Sieverding (1991))
In a study, Vassilev et al. (2001) assessed the applicability of microbial inoculants entrapped in alginate gel. For this, AM fungus G. deserticola enriched with rock phosphate, either in free form or entrapped in calcium alginate alone or in combination with P-solubilizing yeast (Yarrowia lipolytica), was inoculated into soil microcosms. Plant dry weight, soluble P acquisition, and mycorrhizal index were equal in treatments inoculated with free and alginate-entrapped AM fungus. Dual inoculation with entrapped G. deserticola and free cells of Y. lipolytica significantly increased all measured variables. The highest rates of the latter were obtained when both fungal microorganisms were applied co-entrapped in the carrier. The yeast culture behaved as a “mycorrhiza helper microorganism” enhancing mycorrhization of plant roots. These results indicate that dual inoculation with an AM fungus and a P-solubilizing microorganism co-entrapped in alginate can be an efficient technique for plant establishment and growth in nutrient-deficient soils. Likewise, Weber et al. (2005) studied dual inoculation of Acacia mangium grown in aeroponic culture using selected strains of Bradyrhizobium sp. and G. intraradices. A single-step technique with alginate as an embedding and sticking agent for an inoculum composed of AM-infected sheared roots was used to infect plants. This method resulted in the successful establishment of AM in 100% of the inoculated plants after 7 weeks. The results indicated that dual microbial inoculation with G. intraradices strain S-043 and Bradyrhizobium strain AUST 13C stimulated the growth of A. mangium in aeroponic culture. The effects of single and dual microbial inoculations were also evaluated at two levels of P in the nutrient medium. A concentration of 5 mg P kg−1 stimulated the development of AM without affecting plant development or establishment of Bradyrhizobium symbiosis. In contrast, saplings supplemented with a higher concentration of P (25 mg kg−1) alone or co-inoculated with Bradyrhizobium had lower AM frequencies.
Conclusion
The great agricultural and environmental importance of legumes together with its ability to harbor conventional symbionts and other PGPR make legumes a target crop in sustainable agriculture. Accordingly, beneficial soil microbes have become one of the established, promising, and sustainable low-input soil management options. Moreover, legumes, in general, have the potential to form mycorrhizal symbiosis. Mycorrhizal fungi affect the ecophysiology of nodulated legumes, the microbiota of soil, and associated nonlegume plants. Concomitantly, the inoculation of both rhizobia and AMF has increased growth and development of plants under varying conditions. Furthermore, co-inoculation with rhizobia and mycorrhizal fungi is currently being suggested as a possible solution to reforestation and amendment of soil fertility. Also, AMF alleviate various types of abiotic/biotic stresses and have been reported to increase tolerance of legumes to salt, heavy metals, acidic soils, drought, soilborne pathogens, etc. Due to these, use of AM inoculum may provide solutions to ever-increasing costs of agrochemicals and other health-problem causing factors. However, further research is needed to better understand the prospect of AM inoculum in legume production. Development of suitable technology for mass production of inoculants, simple application methods, and assessment of the mycorrhized fields are urgently required to harness the full potential of mycorrhizas. Apart from these, factors deleterious to mycorrhizal diversity and their associated activities, such as pesticides, fertilizers, and poor management practices, need to be carefully monitored.
References
Abd-Alla MH, El-Enany A-WE, Nafady NA, Khalaf DM, Morsy FM (2014) Synergistic interaction of Rhizobium leguminosarum bv. viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol Res 169:49–58
Ahmad MH (1995) Compatibility and co-selection of vesicular-arbuscular mycorrhizal fungi and rhizobia for tropical legumes. Crit Rev Biotechnol 15:229–239
Al-Garni SMS (2006) Increased heavy metal tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungi and nitrogen-fixer Rhizobium bacterium. Afr J Biotechnol 5:133–142
Aliasgharzad N, Neyshabouri MR, Salimi G (2006) Effects of arbuscular mycorrhizal fungi and Bradyrhizobium japonicum on drought stress of soybean. Biologia Bratislava 61(Suppl. 19):S324–S328
Andrade SAL, Abreu CA, de Abreu MF, Silveira APD (2004) Influence of lead additions on arbuscular mycorrhiza and Rhizobium symbioses under soybean plants. Appl Soil Ecol 26:123–131
Aroca R, Ruiz-Lozano JM (2009) Induction of plant tolerance to semi-arid environments by beneficial soil microorganisms–a review. In: Lichtfouse E (ed) Climate change, intercropping, pest control and beneficial microorganisms, sustainable agriculture reviews 2. Springer, Dordrecht, pp 121–135
Aryal UK, HL X, Fujita M (2003) Rhizobia and AM fungal inoculation improve growth and nutrient uptake of bean plants under organic fertilization. J Sustain Agric 21:27–39
Aysan E, Demir S (2009) Using arbuscular mycorrhizal fungi and Rhizobium leguminosarum biovar phaseoli against Sclerotinia sclerotiorum (Lib) de Bary in the common bean (Phaseolus vulgaris L.) Plant Pathol J 8:74–78
Azcón R, Rubio R, Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytol 117:399–404
Bai B, Suri VK, Kumar A, Choudhary KA (2016) Influence of dual inoculation of AM fungi and Rhizobium on growth indices, production economics, and nutrient use efficiencies in Garden Pea (Pisum sativum L.) Commun Soil Sci Plant Anal 47:941–954
Babajide PA, Akanbi WB, Alamu LO, Ewetola EA, Olatunji OO (2008) Growth, nodulation and biomass yield of soybean (Glycine max) as influenced by biofertilizers under simulated eroded soil condition. Res J Agron 2:96–100
Bakarr MI, Janos DP (1996) Mycorrhizal associations of tropical legume trees in Sierra Leone, West Africa. Forest Ecol Manag 89:89–92
Barea JM, Azcon-Aguilar C (1983) Mycorrhizas and their significance in nodulating nitrogen-fixing plants. In: Brady NC (ed) Advances in agronomy, vol 36. Academic, New York, pp 1–54
Barea JM, Werner D, Azcón-Guilar C, Azcón R (2005) Interactions of arbuscular mycorrhiza and nitrogen-fixing symbiosis in sustainable agriculture. In: Werner D, Newton WE (eds) Nitrogen fixation in agriculture, forestry, ecology and the environment. Springer, Dordrecht, pp 199–222
Barea JM, Azcon R, Azcon-Aguilar C (2002) Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek 81:343–351
Behlenfalvay GJ, Brown MS, Stafford AE (1985) Glycine-Rhizobium-symbiosis II. Antagonistic effects between mycorrhizal colonization and nodulation. Plant Physiol 79:1054–1058
Bever JD, Morton JB, Antonovics J, Schultz PA (1996) Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in mown grassland. J Ecol 84:71–82
Bradbury SM, Peterson RL, Bowley SR (1991) Interactions between three alfalfa nodulation genotypes and two Glomus species. New Phytol 119:115–120
Camila MP, Lazara C (2004) Nitrogen-fixing and vesicular-arbuscular mycorrhizal symbioses in some tropical legume trees of tribe mimoseae. Forest Ecol Manag 196:275–285
Cárdenas L, Alemán E, Nava N, Santana O, Sánchez F, Quinto C (2006) Early responses to Nod factors and mycorrhizal colonization in a non-nodulating Phaseolus vulgaris mutant. Planta 223:746–754
Chaitra B, Negalur Lakshman HC (2016) Interaction between AMF (Glomus geosporum) Rhizobium, Azospirillum and their effect on three leguminous plant to improve growth and N, P, K, uptake. Sci Res Rep 6:68–74
Clark RB, Zeto SK (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr 23:867–902
Colozzi A, Cardoso EJBN (2000) Detection of arbuscular mycorrhizal fungi in roots of coffee plants and Crotalaria cultivated between rows. Pesqui Agropecu Bras 35:2033–2042
Dalpé Y, Monreal M (2004) Arbuscular Mycorrhiza inoculum to support sustainable cropping systems. Proceedings of a symposium on the Great Plains Inoculant Forum, 27 and 28 March 2003, Saskatoon, Saskatchewan
Declerck S, Strullu DG, Plenchette C (1996) In vitro mass-production of the arbuscular mycorrhizal fungus, Glomus versiforme, associated with Ri T-DNA transformed carrot roots. Mycol Res 100:1237–1242
Declerck S, Strullu DG, Plenchette C (1998) Monoxenic culture of the intraradical forms of Glomus sp. isolated from a tropical ecosystem: a proposed methodology for germplasm collection. Mycologia 90:579–585
Dehne HW, Backhaus GF (1986) The use of vesicular-arbuscular mycorrhizal fungi in plant production. I Inoculum production. Z Pflanzenkr Pflanzenschutz 93:415–424
Demir S, Akköprü A (2007) Using of arbuscular mycorrhizal fungi (AMF) for biocontrol of soil borne fungal plant pathogens. In: Chincholkar SB, Mukerji KG (eds) Biological control of plant diseases. Haworth, Binghamton, NY, pp 17–37
de Varennes A, Goss MJ (2007) The tripartite symbiosis between legumes, rhizobia and indigenous mycorrhizal fungi is more efficient in undisturbed soil. Soil Biol Biochem 39:2603–2607
Diop TA, Plenchette C, Strullu DG (1994) Dual axenic culture of sheared-root inocula of vesicular-arbuscular mycorrhizal fungi associated with tomato roots. Mycorrhiza 5:17–22
Dodd JC, Arias I, Koomen I, Hayman DS (1990) The management of populations of vesicular-arbuscular mycorrhizal fungi in acid-infertile soils of a savanna ecosystem. I. The effect of pre-cropping and inoculation with VAM-fungi on plant growth and nutrition in the field. Plant Soil 122:229–240
Duponnois R, Plenchette C, Bâ AM (2001) Growth stimulation of seventeen fallow leguminous plants inoculated with Glomus aggregatum in Senegal. Eur J Soil Biol 37:181–186
Eom AH, Lee SS, Ahn TK, Lee MW (1994) Ecological roles of arbuscular mycorrhizal fungi in two wild legume plants. Mycoscience 35:69–75
Erman M, Demir S, Ocak E, Tufenkci S, Oguz F, Akkopru A (2011) Effects of Rhizobium, arbuscular mycorrhiza and whey applications on some properties in chickpea (Cicer arietinum L.) under irrigated and rain-fed conditions 1—yield, yield components, nodulation and AMF colonization. Field Crops Res 122:14–24
Francis R, Read DJ (1984) Direct transfer of carbon between plants connected by vesicular-arbuscular mycorrhizal mycelium. Nature 307:53–56
Franco AA, De Faria SM (1997) The contribution of N2-fixing tree legumes to land reclamation and sustainability in the tropics. Soil Biol Biochem 29:897–903
Franzini VI, Azco’n R, Mendes FL, Aroca R (2010) Interactionsbetween Glomus species and Rhizobium strains affect the nutritional physiology of drought-stressed legume hosts. J Plant Physiol 167:614–619
Gao X, Lu X, Wu M, Zhang H, Pan R, Tian J, Li S, Liao H (2012) Co-inoculation with Rhizobia and AMF inhibited soybean red crown rot: from field study to plant defense-related gene expression analysis. PLoS One 7(3):e33977. doi:10.1371/journal.pone.0033977
Geneva M, Zehirov G, Djonova E, Kaloyanova N, Georgiev G, Stancheva I (2006) The effect of inoculation of pea plants with mycorrhizal fungi and Rhizobium on nitrogen and phosphorus assimilation. Plant Soil Environ 52:435–440
Ghosh S, Verma NK (2006) Growth and mycorrhizal dependency of Acacia mangium Willd. Inoculated with three vesicular arbuscular mycorrhizal fungi in lateritic soil. New Forests 31:75–81
Gianinazzi S, Vosátka M (2004) Inoculum of arbuscular mycorrhizal fungi for production systems: science meets business. Can J Bot 82:1264–1271
Harrison MG (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59:19–42
Harrison MJ (1999) Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol 50:361–389
Hayman DS (1986) Mycorrhizae of nitrogen-fixing legumes. World J Microbiol Biotechnol 2:121–145
He X, Pen-Mouratov S, Steinberger Y (2004) Research note: spatial variation of AM fungal spore numbers under canopies of Acacia raddiana. Arid Land Res Manag 18:295–299
Heap AJ, Newman EL (1980) Links between roots by hyphae of vesiculararbuscular mycorrhizas. New Phytol 85:169–171
Herrera MA, Salamanca CP, Barea JM (1993) Inoculation of woody legumes with selected arbuscular mycorrhizal fungi and rhizobia to recover desertified mediterranean ecosystems. Appl Environ Microbiol 59:129–133
Hodge A, Campbell CDFAH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Hooker JE, Black KE (1995) Arbuscular mycorrhizal fungi as components of sustainable soil-plant systems. Crit Rev Biotechnol 15:201–212
Hung LL, O’Keefe DM, Sylvia DM (1991) Use of hydrogel as a sticking agent and carrier for vesicular–arbuscular mycorrhizal fungi. Mycol Res 95:427–429
Hung LLL, Sylvia DM (1988) Production of vesicular-arbuscular mycorrhizal fungus inoculum in aeroponic culturet. Appl Environ Microbiol 54:353–357
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. I: spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380
Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16
Jia Y, Gray VM, Straker CJ (2004) The influence of Rhizobium and arbuscular mycorrhizal fungi on nitrogen and phosphorus accumulation by Vicia faba. Ann Bot 94:251–258
Kamprath EJ, Foy CD (1985) Lime-fertilizer-plant interactions in acid soils. In: Englestad O (ed) Fertilizer technology and use, 3rd edn. Soil Science Society of America, Madison, WI
Karmakar K, Rana A, Rajwar A, Sahgal M, Johri BN (2015) Legume-rhizobia symbiosis under stress. In: Arora NK (ed) Plant microbes symbiosis: applied facets. Springer, India, pp 241–258
Kayode J, Franco AA (2002) Response of Acacia mangium to rhizobia and arbuscular mycorrhizal fungi. Trop Sci 42:116–119
Khaliel AS, Elkhider KA, Bahkali AH (1999) Response and dependence of haricot bean to inoculation with arbuscular mycorrhiza. Saudi J Biol Sci 6:126–132
Khan A (2006) Mycorrhizoremediation–an enhanced form of phytoremediation. J Zhejiang University Sci B7:503–514
Kothari SK, Marschner H, Römheld V (1991) Contribution of the VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant Soil 131:177–185
Lapeyrie F (1988) Oxalate synthesis from soil bicarbonate by fungus Paxillus involutus. Plant Soil 110:3–8
Leggett M, Cross J, Hnatowich G, Holloway G (2007) Challenges in commercializing a phosphate-solubilizing microorganism: Penicillium bilaiae, a case history. In: Velázquez E, Rodríguez-Barrueco C (eds) First international meeting on miccrobial phosphate solubilization. Springer, Dordrecht, pp 215–222
Li XL, Marschner H, George E (1991) Acquisition of phosphorus and copper by VA–mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 136:49–57
Li Y, Ran W, Zhang R, Sun S, Xu G (2009) Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil 315:285–296
Linderman RG (1994) Role of VAM fungi in biocontrol. In: Pfleger FL, Linderman RG (eds) Mycorrhizae and plant health. APS, St Paul, MN, pp 1–26
Linderman RG, Paulitz TC (1990) Mycorrhizal rhizobacterial interactions. In: Hornby D (ed) Biological control of soilborne plant pathogens. CABI International, Wallingford
Lodwig EM, Hosie AHF, Bourdès A, Findlay K, Allaway D, Karunakaran R, Downie JA, Poole PS (2003) Amino-acid cycling drives nitrogen fixation in the legume–Rhizobium symbiosis. Nature 422:722–726
Mahdi AA, Atabani IMA (1992) Response of Bradyrhizobium-inoculated soyabean and lablab bean to inoculation with vesicular-arbuscular mycorrhizae. Exp Agric 28:399–408
Maki T, Nomachi M, Yoshida S, Ezawa T (2008) Plant symbiotic microorganisms in acid sulfate soil: significance in the growth of pioneer plants. Plant Soil 310:55–65
Manjunath A, Bagyaraj DJ, Gowda HSG (1984) Dual inoculation with VA mycorrhiza and Rhizobium is beneficial to Leucaena. Plant Soil 78:445–448
Marques MS, Pagano M, Scotti M (2001) Dual inoculation of a woody legume (Centrolobium tomentosum) with rhizobia and mycorrhizal fungi in south-eastern Brazil. Agrofor Syst 50:107–117
Marschner H (1998) Mineral Nutrition of higher plants. Academic, London
Martin-Laurent F, Lee SK, Tham FY, Jie H, Diem HG (1999) Aeroponic production of Acacia mangium saplings inoculated with AM fungi for reforestation in the tropics. Forest Ecol Manag 122:199–207
Meghvansi MK, Prasad K, Harwani D, Mahna SK (2008) Response of soybean cultivars toward inoculation with three arbuscular mycorrhizal fungi and Bradyrhizobium japonicum in the alluvial soil. Eur J Soil Biol 44:316–323
Mehdi Z, Nahid S-R, Alikhani HA, Nasser A (2006) Responses of lentil to co-inoculation with phosphate-solubilizing rhizobial strains and arbuscular mycorrhizal fungi. J Plant Nutr 29:1509–1522
Menge JA (1984) Inoculum production. In: Powell CL, Bagyaraj DJ (eds) VA mycorrhiza. CRC, Boca Raton, FL, pp 187–203
Miller RM (1987) The ecology of vesicular-arbuscular mycorrhizae in grass and shrublands. In: Safir GR (ed) Ecophysiology of VA mycorrhizal plants. CRC, Boca Raton, FL, pp 135–170
Miller-Wideman MA, Watrud LS (1984) Sporulation of Gigaspora margarita on root cultures of tomato. Can J Microbiol 30:642–646
Molla MN, Solaiman ARM (2009) Association of arbuscular mycorrhizal fungi with leguminous crops grown in different agro-ecological zones of Bangladesh. Arch Agron Soil Sci 55:233–245
Mosse B, Thompson JP (1984) Vesicular-arbuscular endomycorrhizal inoculum production. I. Exploratory experiments with beans (Phaseolus vulgaris) in nutrient flow culture. Can J Bot 62:1523–1530
Muchovej RM (2001) Importance of mycorrhizae for agricultural crops. http://edis.ifas.ufl.edu/pdffiles/AG/AG11600.pdf. Accessed October 2009
Mugnier J, Mosse B (1987) Vesicular-arbuscular mycorrhizal infection in transformed root-inducing T-DNA roots grown axenically. Phytopathology 77:1045–1050
Muleta D, Assefa F, Nemomissa S, Granhall U (2007) Composition of coffee shade tree species and density of indigenous arbuscular mycorrhizal fungi (AMF) spores in Bonga natural coffee forest, southwestern Ethiopia. Forest Ecol Manag 241:145–154
Muleta D, Assefa F, Nemomissa S (2008) Granhall U (2008) Distribution of arbuscular mycorrhizal fungi spores in soils of smallholder agroforestry and monocultural coffee systems in southwestern Ethiopia. Biol Fertil Soils 44:653–659
Nambiar PTC, Anjaiah V (1989) Competition among strains of Bradyrhizobium and vesicular-arbuscular mycorrhizae for groundnut (Arachis hypogaea L.) root infection and their effect on plant growth and yield. Biol Fertil Soils 8:311–318
Navazio L, Moscatiello R, Genre A, Novero M, Baldan B, Bonfante P, Mariani P (2007) A diffusible signal from arbuscular mycorrhizal fungi elicits a transient cytosolic calcium elevation in host plant cells. Plant Physiol 144:673–681
Ndiaye F, Manga A, Diagne-Leye G, Samba SAN, Diop TA (2009) Effects of rock phosphate and arbuscular mycorrhizal fungi on growth and nutrition of Sesbania sesban and Gliricidia sepium. Afr J Microbiol Res 3:305–309
Nopamornbodi O, Rojanasiriwong W, Thomsurakul S (1988) Production of VAM fungi, Glomus intraradices and G. mosseae in tissue culture. In: Mahadevan A, Raman N, Natarajan K (eds) Mycorrhizae for green Asia. University of Madras, Madras, pp 315–316
Nwoko H, Sanginga N (1999) Dependence of promiscuous soybean and herbaceous legumes on arbuscular mycorrhizal fungi and their response to bradyrhizobial inoculation in low P soils. Appl Soil Ecol 13:251–258
Pacovsky RS, Fuller G, Stafford AE, Paul EA (1986) Nutrient and growth interactions in soybeans colonized with Glomus fasciculatum and Rhizobium japonicum. Plant Soil 92:37–45
Pagano MC, Cabello MN, Scotti MR (2007) Phosphorus response of three native Brazilian trees to inoculation with four arbuscular mycorrhizal fungi. J Agric Technol 3:231–240
Patreze CM, Cordeiro L (2004) Nitrogen-fixing and vesicular–arbuscular mycorrhizal symbioses in some tropical legume trees of tribe Mimoseae. Forest Ecol Manag 196:275–285
Plenchette C, Clermont-Dauphin C, Meynard JM, Fortin JA (2005) Managing arbuscular mycorrhizal fungi in cropping systems. Can J Plant Sci 85:31–40
Quatrini P, Scaglione G, Incannella G, Badalucco L, Puglia AM, Lamantia T (2003) Microbial inoculants on woody legumes to recover a municipal landfill site. Water Air Soil Pollut Focus 3:189–199
Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol 4:210–222
Requena N, Pérez-Solis E, Azcón-Aguilar C, Jeffries P, Barea JM (2001) Management of indigenous plant-microbe symbiosis aids restoration of desertified ecosystems. Appl Environ Microbiol 67:495–498
Ruiz-Lazano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317
Ryan MH, Graham JH (2002) Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant Soil 244:263–271
Scheublin TR, Ridgway KP, Young JPW, van der Heijden MGA (2004) Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 70:6240–6246
Sharma AK, Singh C, Akhauri P (2000) Mass culture of arbuscular mycorrhizal fungi and their role in biotechnology. Proc Indian Natl Sci Acad (PINSA) B66:223–238
Sharma MP, Tanu AG, Sharma OP (2004) Prospects of arbuscular mycorrhiza in sustainable management of root- and soil-borne diseases of vegetable crops. In: Mukerji KG (ed) Fruit and vegetable diseases. Kluwer Academic, Dordrecht, pp 501–539
Shokri S, Maadi B (2009) Effects of arbuscular mycorrhizal fungus on the mineral nutrition and yield of Trifolium alexandrinum plants under salinity stress. J Agron 8:79–83
Sieverding E (1991) Vesicular-arbuscular mycorrhizal management in tropical agrosystems. GTZ, Eschborn, Germany
Simms EL, Taylor DL (2002) Partner choice in nitrogen-fixation mutualisms of legumes and rhizobia. Integr Comp Biol 42:369–380
Singh CS, Kapoor A, Wange SS (1991) The enhancement of root colonisation of legumes by vesicular-arbuscular mycorrhizal (VAM) fungi through the inoculation of the legume seed with commercial yeast (Saccharomyces cerevisiae). Plant Soil 131:129–133
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524
Snoeck D, Zapata F, Domenach A (2000) Isotopic evidence of the transfer of nitrogen fixed by legumes to coffee trees. Biotechnol Agron Soc Environ 4:95–100
Spaink HP (1996) Regulation of plant morphogenesis by lipochin oligosaccharides. Crit Rev Plant Sci 15:559–582
Stancheva I, Geneva M, Djonova E, Kaloyanova N, Sichanova M, Boychinova M, Georgiev G (2008) Response of alfalfa (Medicago sativa L) growth at low accessible phosphorus source to the dual inoculation with mycorrhizal fungi and nitrogen fixing bacteria. Gen Appl Plant Physiol 34:319–326
Sundaredan Ρ, Raja ΝU, Gunasekaran Ρ (1993) Induction and accumulation of phytoalexins in cowpea roots infected with a mycorrhizal fungus Glomus fasciculatum and their resistance to Fusarium wilt disease. J Biosci 18:291–301
Sylvia DM (1999) Fundamentals and applications of arbuscular mycorrhizae: a ‘biofertilizer’ perspective. In: Siqueira JO (ed) Soil fertility, biology, and plant nutrition interrelationships. SBCS, Viçosa, pp 705–723
Sylvia DM, Hubbell DH (1986) Growth and sporulation of vesicular-arbuscular mycorrhizal fungi in aeroponic and membrane systems. Symbiosis 1:259–267
Sylvia DM, Jarstfer AG (1992) Sheared roots inocula of vesicular mycorrhizal fungi. Appl Environ Microbiol 58:229–232
Tajini F, Trabelsi M, Drevon J-J (2012) Combined inoculation with Glomus intraradices and Rhizobium tropici CIAT899 increases phosphorus use efficiency for symbiotic nitrogen fixation in common bean (Phaseolus vulgaris L.) Saudi J Biol Sci 19:157–163
Tavasolee A, Aliasgharzad N, Salehi Jouzani G, Mardi M, Asgharzadeh A (2011) Interactive effects of Arbuscular mycorrhizal fungi and rhizobial strains on chickpea growth and nutrient content in plant. Afr J Biotechnol 10:7585–7591
Tiwari P, Adholeya A (2002) In vitro co-culture of two AMF isolates Gigaspora margarita and Glomus intraradices on Ri T-DNA transformed roots. FEMS Microbiol Lett 206:39–43
Todd C (2004) Mycorrhizal fungi, nature’s key to plant survival and success. Pac Hort 65:8–12
Toro M, Azco’n R, Barea J (1997) Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate-solubilizing rhizobacteria to improve rock phosphate bioavailability [32P] and nutrient cycling. Appl Environ Microbiol 63:4408–4412
Valsalakumar N, Ray JG, Potty VP (2007) Arbuscular mycorrhizal fungi associated with green gram in South India. Agron J 99:1260–1264
van der Heijden MGA, Wiemken A, Sanders IR (2003) Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plant. New Phytol 157:569–578
van der Heijden MGA, Rinaudo V, Verbruggen E, Scherrer C, Bàrberi P, Giovannetti M (2008) The significance of mycorrhizal fungi for crop productivity and ecosystem sustainability in organic farming systems. 16th IFOAM Organic World Congress, Modena, Italy, 16–20 June 2008
van der Heijden MGA, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752
van der Vossen HAM (2005) A critical analysis of the agronomic and economic sustainability of organic coffee production. Exp Agric 41:449–473
Vankessel C, Singleton PW, Hoben HJ (1985) Enhanced N-transfer from a soybean to maize by vesicular arbuscular mycorrhizal (VAM) fungi. Plant Physiol 79:562–563
Vassilev N, Nikolaeva I, Vassileva M (2005) Polymer-based preparation of soil inoculants: applications to arbuscular mycorrhizal fungi. Rev Environ Sci Biotechnol 4:235–243
Vassilev N, Vassileva M, Azcon R, Medina A (2001) Preparation of gel-entrapped mycorrhizal inoculum in the presence or absence of Yarowia lipolytica. Biotechnol Lett 23:907–909
Vivas A, Vörös I, Biró B, Campos E, Barea JM, Azcón R (2003) Symbiotic efficiency of autochthonous arbuscular mycorrhizal fungus (G. mosseae) and Brevibacillus sp. isolated from cadmium polluted soil under increasing cadmium levels. Environ Pollut 126:179–189
Warner A (1985) US patent 4,551,165, November
Weber J, Ducousso M, Tham FY, Nourissier-Mountou S, Galiana A, Prin Y, Lee SK (2005) Co-inoculation of Acacia mangium with Glomus intraradices and Bradyrhizobium sp. in aeroponic culture. Biol Fertil Soils 41:233–239
Weber E, George E, Beck DP, Saxena MC, Marschner H (1992) Vesicular-arbuscular mycorrhiza and phosphorus uptake of chickpea grown in Northern Syria. Exp Agric 28:433–442
Wu FY, Bi YL, Wong MH (2009) Dual inoculation with an arbuscular mycorrhizal fungus and Rhizobium to facilitate the growth of Alfalfa on coal mine substrates. J Plant Nutr 32:755–771
Xavier LJC, Germida JJ (2002) Response of lentil under controlled conditions to co-inoculation with arbuscular mycorrhizal fungi and rhizobia varying in efficacy. Soil Biol Biochem 34:181–188
Yun-Jeong L, Eckhard G (2005) Development of a nutrient film technique culture system for arbuscular mycorrhizal plants. Hort Sci 40:378–380
Zobel RW, Dei Tredici P, Torren JG (1976) Method for growing plants aeroponically. Plant Physiol 57:344–346
Acknowledgments
The author would like to thank Prof. Md. Saghir Khan for his prompt and kind initiation to write this chapter and for his meticulous edition of the chapter. I would also like to express my earnest thanks to my wife Elfinesh Tolera for her unvarying encouragement and materials support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Muleta, D. (2017). Legume Response to Arbuscular Mycorrhizal Fungi Inoculation in Sustainable Agriculture. In: Zaidi, A., Khan, M., Musarrat, J. (eds) Microbes for Legume Improvement. Springer, Cham. https://doi.org/10.1007/978-3-319-59174-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-59174-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59173-5
Online ISBN: 978-3-319-59174-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)