Abstract
Acid sulfate soil is generated by chemical and microbial oxidization of sulfide-rich minerals/sediments. Although revegetation of the soil is difficult due to low-pH and poor nutrient availability, pioneer plants may adapt to such an extreme environment via associating with mycorrhizal fungi and/or N-fixing bacteria for acquisition of mineral nutrients. In this study, an abandoned quarry in which acid sulfate soil was found was chosen to investigate the influence of soil acidity on the levels of colonization by the microsymbionts, the identities of the microsymbionts that associated with pioneer plants and the dependency of pioneer plants on the microsymbionts. The levels of arbuscular mycorrhizal (AM) colonization in pioneer grass, forbs and legume shrubs grown in the field were assessed, and no significant decline in the levels with an increase in soil acidity was observed. Most of the legume shrubs formed root nodules. Several AM fungi and bradyrhizobia were cultured from the rhizosphere soils of pioneer plants grown in the quarry and identified based on the sequences of the small subunit ribosomal RNA genes. Pot experiments revealed that the microsymbionts isolated from the field significantly promoted the growths of pioneer grasses and legume shrubs in acid sulfate soil at pH 3.4. These results suggest that plant–microbial symbiotic associations play significant roles in the growth of pioneer plants in acid sulfate soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid sulfate soil is widely distributed in volcanic and coastal areas in the world. The soil is formed by chemical and microbial oxidization of sulfide-rich minerals/sediments, in most cases pyrite (FeS2) that are exposed by large-scale land development, reclamation and quarrying. As a result of oxidation, a large amount of sulfuric acid is generated, thus the soil shows extremely low pH. Recently, the environmental impacts of acid sulfate soil have become a major concern. Drainage from acid sulfate soil causes serious damage to marine ecosystems via acidification, metal contamination, deoxygenation and iron precipitation (Powell and Martens 2005). Acidification and metal contamination of freshwater caused by the soil threaten not only terrestrial ecosystems but also human health (Appleyard et al. 2004). Revegetation of acid sulfate soil is one way of reducing the environmental impacts of the soil but is difficult due to strong acidity, high concentrations of toxic elements e.g. Al and Mn and deficiencies in the essential nutrients. In particular, P-deficiency often limits plant growth in the soil (Do et al. 2004).
It is considered that pioneer plants that are characterized as primary vegetation in disturbed areas may have developed diverse strategies to adapt to such extreme environments, although their adaptation mechanisms are largely unknown. Nutrient acquisition via symbiotic associations with soil microorganisms is likely to be one of the strategies of pioneer plants. Arbuscular mycorrhizal (AM) fungi form mutualistic associations with about 80% of land plants and improve phosphorus (P) nutrition of the host plants via enhanced P-uptake through extensive hyphal networks in soil (Smith and Read 1997). It is, therefore, expected that AM associations may play significant roles in the primary vegetation of acid sulfate soil. On the other hand, Allen (1991) suggested that the first plant colonizers after disturbance would be non-mycotrophic or at least facultative mycotrophic. This idea, however, contradicts the fact that ectomycorrhizal formation on Salix reinii, a pioneer tree species, was essential in facilitating seedling establishment of successional plant species in the early successional volcanic desert in Mt. Fuji (Nara and Hogetsu 2004). Given the fact that P-deficiency is a major limiting factor for plant growth in acid sulfate soil, it is worth evaluating the dependency of pioneer plants on AM fungi in the soil for further understanding of the roles of mycorrhiza in disturbed ecosystems.
Leguminous plants benefit not only from AM symbiosis but also from the symbiotic nitrogen (N)-fixing associations with rhizobia. Extensive studies have demonstrated that the efficiency of N-fixation in legume-rhizobial associations is promoted by AM fungal colonization (Barea et al. 1992), and this might be mainly due to the high-requirement of P in the N-fixing process (Bohlool et al. 1992). The effectiveness of plant-AM fungi-rhizobia tripartite associations has been demonstrated in the management of desertified ecosystems in the Mediterranean semi-arid region (Herrera et al. 1993; Requena et al. 1997, 2001). According to these observations, it is likely that the tripartite associations are of significance in the colonization of legume pioneers in acid sulfate soil.
It is likely that soil acidity has negative impacts on the formation and effectiveness of the symbiotic associations. Commercial Bradyrhizobium sp. that showed slower growth at lower pH in vitro could form nodules in acidic soil but did not fix appreciable N (Cline and Senwo 1993). The colonization, spore germination and the growth of germ tubes of an AM fungus that occurred in non-acidic soil were significantly reduced in acidic soil (Porter et al. 1987). The colonization levels of indigenous AM fungi on Plantago lanceolata in an acidic soil were significantly lower than those in limestone (non-acidic) soil, and the hyphal biomass of the fungi in the acidic soil was much smaller than that in the limestone soil (van Aarle et al. 2003). Based on these observations, it is hypothesized that the colonization of the symbiotic microorganisms on the host plants may be dramatically suppressed in extremely acidic soil. However, the ecology/physiology or even the presence of the symbiotic microorganisms in extremely acidic soil has not yet been reported.
In this study, an abandoned quarry located in the middle of Honshu Isl., Japan was chosen as a model field for studying plant-microbial associations in the pioneer vegetation of acid sulfate soil. The objectives of the present study were to investigate: (1) whether the colonization of the microsymbionts on pioneer plants is suppressed with an increase in soil acidity, (2) the origin and identity of the microorganisms that associate with pioneer plants under extremely acidic conditions and (3) whether the indigenous microsymbionts contribute to the growth of pioneer plants in acid sulfate soil. For these purposes, the levels of colonization in naturally occurring pioneer plants in the field were first assessed with respect to rhizosphere soil pH. Then AM fungi and rhizobia were isolated from the rhizosphere of the pioneer plants grown in the field and surrounding forest and identified, and the effectiveness on the growth of pioneer plants were evaluated by pot experiments.
Materials and methods
Sampling site
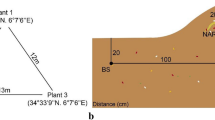
The experimental field is located in hilly area of Hazu-cho, Aichi prefecture, Japan (150–200 m altitude, 34°47′ N, 137°07′ E) where the annual mean temperature and rainfall are 16°C and 1,600 mm, respectively, and is surrounded by evergreen broad-leaved forest dominated by Quercus glauca Thunberg, Q. myrsinaefolia Blume and Castanopsis cuspidata Schottky. The understory of the forest is dominated by Sasa nipponica Makino et Shibata and Dryopteris hondoensis Koidz. The field had been quarried for a long time so that the bottom of the field (ca. 10 ha) is 20–100 m below the top of the surrounding hills and is flat except for the two ponds (2–4 m depth). The field has not been severely disturbed since 1999. A thin silt/sand layer (5–15 cm in depth) is formed on sulfur-rich gravel/base rock, and the most of plant roots grow within this layer. Alnus firma Sieb. et Zucc. (Betulaceae) (0.1 to 3 m height) is observed around the two ponds and along the border between the quarry and surrounding forest (Fig. 1). The rest of the area is not fully covered with vegetation, but scattered distributions of herbaceous plants, Miscunthus sinensis Andresson (Gramineae), Solidago altissima L. (Asteraceae) and Artemisia princeps L. (Asteraceae), and leguminous shrub, Lespedeza cyrtobotrya L. (Leguminosae) are observed. M. sinensis and L. cyrtobotrya dominate in the site, although the former is observed more frequently than the latter.
Sampling location of Miscanthus sinensis, Lespedeza cyrtobotrya, Solidago altissima and Artemisia princeps in the quarry in Hazu-cho, Aichi. The sample ID from which arbuscular mycorrhizal fungi were successfully cultured by the trap culture is indicated. Asterisk shows the sampling point of the soil for the pot experiments
Root and soil sampling
The roots and rhizosphere soils were collected both from the quarry and surrounding forest yearly from M. sinensis, L. cyrtobotrya, S. altissima and A. princeps in June from 2000 to 2002 as shown in Fig. 1. The roots were collected by two core samplers (100 ml in vol) from each plant, and then 2–3 kg rhizosphere soils were collected underneath the plants (top 5–10 cm depth, 30–40 cm in diam). Small parts of the soil samples were air-dried, crushed and passed through a 2 mm sieve for chemical analyses. The rest of the soils were stored at 7°C for the isolation of symbiotic microorganisms. Non-rhizospheric acid sulfate soil was collected as a culture medium for pot experiments from a sand pile in the quarry on which no vegetation was observed (Fig. 1).
The root samples collected by the two core samplers were combined, washed with tap water, cut into 1 cm pieces, cleared with 10% KOH (w/v) and stained with 0.05% Trypan blue in lactoglycerol. The percentage of mycorrhizal colonization was estimated by the grid-line intersect method (Giovannetti and Mosse 1980). Soil pH (H2O) was measured at 1:2.5 soil/water ratio, and pH (H2O2) was measured after oxidation by boiling in neutralized 30% H2O2. Total carbon (C) and nitrogen (N) contents were analyzed by a CHN analyzer (Perkin-Elmer, Yokohama, Japan). Available (Truog)-P was measured by the vanado-molybdate method after extraction with 0.001 M H2SO4 at ratio of 1:200 (w/v) (Truog 1930).
Isolation of microsymbionts
AM fungi in the rhizosphere of the pioneer plants were first cultured by the soil trap culture (Brundrett et al. 1999). A half liter of the rhizosphere soils collected in July 2000 and 2002 was layered between autoclaved river sand in a 4 l plastic pot, and M. sinensis, Arachis hypogaea L. and Sorghum bicolor L. were sown together in the same pot. The plants were fertilized with 0.25 g l−1 20–5–30 PETERS liquid fertilizer (W. R. Grace & Co., Forgelsville, PA) in sufficient amounts until the solution flowed out from the drain twice or three times a week and were grown in a greenhouse (20–35°C). After four months, the plants and media were dried in the greenhouse, and the roots and media were stored in plastic bags at room temperature. The cultures were maintained yearly by the sand culture system using the same host plants. The AM fungal spores were extracted by wet sieving, and the dominant spores were collected under a dissecting microscope. Ten to 20 spores were mounted on a glass slide to examine spore size and color under a light microscope.
Nodule bacteria were isolated from the rhizosphere soils or the root nodules of L. crytobotrya collected from the quarry and surrounding forest. The nodules were surface sterilized with 70% ethanol for 30 s and sodium hypochlorite (0.5% available chlorine) for 5 min, washed with sterilized deionized water and crushed in 100 μl sterilized deionized water. Then the suspension was spread on the yeast extract-mannitol agar (YMA) medium and cultured at 28°C. To isolate from the stored rhizosphere soils, 30 g of the soil was layered between autoclaved river sand 1 cm below surface in a 9 cm plastic pot (400 ml in vol), and L. bicolor L. was grown in the greenhouse. We used L. bicolor instead of L. crytobotrya as a host plant, because L. crytobotrya seed was not commercially available. The plants were watered by a capillary sheet through the drain hole and grown without fertilizer. The nodules formed on the roots were harvested after 2 months, and nodule bacteria were isolated and maintained on the YMA medium. Single strains isolated from each nodule or soil sample were randomly chosen and re-inoculated to L. bicolor grown on the non-rhizospheric acid sulfate soil under the same conditions to select acid-tolerant strains. The chemical properties of the soil are shown in Table 1, and the absence of AM fungi and rhizobia in the soil was confirmed by cultivation of L. bicolor in a greenhouse prior to the experiments. Acid-tolerant nodule bacteria were isolated from the nodules formed on the roots after 2 months and maintained on the YMA medium.
Molecular identification of microsymbionts
Ten to 50 spores of the AM fungi were crushed in 10 μL buffer of InstaGene matrix (BioRad Laboratories) under a microscope and mixed with 20–200 μl of the matrix in a 1.5 ml tube. DNA was purified according to the manufacturer’s instructions and stored at −30°C. A part (1.1 kbp) of the small subunit ribosomal RNA gene (18S rDNA) was amplified with the Expand High-Fidelity PCR System (Roche Diagnostics) with the NS1 and NS4 primer set (Table 2) using 1 μl of the template DNA. The PTC-150 MiniCycler or PTC-225 DNA Engine Tetrad (MJ Research) was employed for the amplification, and the program was as follows: initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 60 s, polymerization at 72°C for 80 s and final elongation at 72°C for 10 min.
DNA of the nodule bacteria was extracted from the colonies formed on the YMA medium by the InstaGene matrix according to the manufacturer’s instructions and stored at −30°C. A part (1.3 kbp) of the small subunit ribosomal RNA gene (16S rDNA) was amplified under the same conditions described for AM fungi except for the primer set and annealing temperature as follows. The 52f and 1320r primers were designed on the consensus regions of 16S rDNAs of the root nodule-forming bacteria across the four genera: Mesorhizobium loti (GenBank accession no. D14514), Rhizobium leguminosarum (D14513), Shinorhizobium meliloti (D14509), Bradyrhizobium japonicum (AF417550), B. elkanii (U35000) and B. yuanmingense (AF193818). The annealing temperature for this primer set was programmed at 60°C.
The PCR products were cloned into the pT7Blue T-vector (Novagen), and the nucleotide sequence of randomly chosen four clones (AM fungi) or a single clone (nodule bacteria) was determined by the dideoxy-sequencing method using the BigDye Terminator v3.0 or v3.1 Cycle Sequencing Kit with the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). The DNA sequences were subjected to phylogenetic analysis with published data by the ClustalW program.
Effects of microsymbionts on the growth of pioneer plants
Non-rhizospheric acid sulfate soil collected from the quarry (Table 1) was used as a culture medium for the experiments. A preliminary screening of effective nodule bacteria was carried out using L. bicolor as a host, and Bradyrhizobium sp. QC1 and F3b (described later) were chosen for this experiment. The bacteria were cultured in 10 ml yeast extract-mannitol broth for 4 days at 28°C, centrifuged at 1,500×g for 15 min, washed twice with deionized water then suspended in 50 ml deionized water. The mixed population of AM fungal inoculum was prepared by wet sieving from the soil trap culture of L. cyrtobotrya rhizosphere soil taken from the R2 plot (Fig. 1). L. bicolor or M. sinensis was sown on the acid sulfate soil in 400 ml plastic pots and inoculated with the AM fungi at 1,000 spores pot−1. In the case of L. bicolor, 2 ml of the bacterial suspension was applied to the medium in addition to the AM fungi. The non-mycorrhizal plants received the same amount of the filtrate of spore suspension. The seedlings were thinned to five (L. bicolor) or 10 (M. sinensis) plants pot−1 2 weeks after sowing and grown in the greenhouse without fertilization. After 3 months, the shoots were dried at 80°C and weighed. The roots were cut into 1 cm segments, randomized completely in water, cleared and stained with Trypan blue to assess the levels of AM colonization.
Statistical analysis
All statistical analyses were performed with the StatView software (SAS Institute Inc., Cary, NC). For calculating the average pH values of rhizosphere soils, the data were transformed to real numbers. For the analysis of the pH preference of pioneer plants, the t-test was employed. In this analysis, the transformed pH values (real numbers) were to be retransformed to logarithmic values for normalization. Analysis of variance (ANOVA) and the Fisher’s protected least significant difference test as a post hoc test (P < 0.05) were applied for the analyses of the effect of the microsymbionts on the growth of pioneer plants.
Results
Occurrence of pioneer plant–microbial associations with respect to soil acidity
The three herbaceous plants, M. sinensis, S. altissima and A. princeps, and the leguminous shrub, L. cyrtobotrya, in the quarry formed arbuscular mycorrhizas without exception, although the extent of colonization was highly variable among the individuals. The percentage of AM colonization of M. sinensis was 3–46% (n = 15), and those of L. cyrtobotrya and S. altissima were 3–40% (n = 13) and 2–57% (n = 7), respectively. A. princeps was found only in 2002, and the levels of AM colonization were 10–27% (n = 3). Most of the L. cyrtobotrya found in the quarry formed root nodules. The precise assessment of the presence of nodules, however, was difficult because the whole root system could not be collected from the gravel-rich soil. Soil pH (H2O) in the rhizosphere of the pioneer plants varied from 4.2 to 6.7, but most values were within a range of 4.2–5.5. Total C levels of the rhizosphere soils ranged from 2.1 to 20.4 g kg−1 with an average value of 6.2 g kg−1. Total N levels of the soils ranged from 0.38 to 1.23 g kg−1 with an average value of 0.49 g kg−1. Truog-P levels of the soils ranged from 20.5 to 71.3 mg kg−1 with an average value of 41.6 mg kg−1.
The levels of AM colonization and rhizosphere soil pH of the two dominant pioneer plants M. sinensis and L. cyrtobotrya were plotted, but the levels were not significantly decreased with an increase in soil acidity (Fig. 2a). The levels of AM colonization also did not correlate with the total C, N and Truog-P levels (data not shown). It is noteworthy that L. cyrtobotrya occurred preferentially in less acidic soils than for M. sinensis (Fig. 2b): the average soil pH of L. cyrtobotrya (4.96) was significantly higher than that of M. sinensis (4.64; P < 0.01). The average values of the other soil parameters did not differ significantly between the two pioneer species (data not shown).
Isolation and identification of microsymbionts
AM fungi were successfully cultured from the rhizosphere soils of R2 (L. cyrtobotrya), R5 (M. sinensis), R8 (S. altissima), R9 (L. cyrtobotrya), QR2 (M. sinensis) and QR3 (M. sinensis) plots (Fig. 1). HR1 of which spore diameter and wall thickness were 243.3 ± 46.5 μm and 16.0 ± 4.5 μm, respectively, dominated in the R2, R8, R9 and QR2 soils (Fig. 3a). The 1.1 kbp sequences of 18S rDNA of HR1 fell into the cluster of Gl. manihotis, Gl. clarum and Gl. intraradices to which HR1 sequences showed 97.8–99.1% similarity (Fig. 4), and thus HR1 was classified in the genus Glomus. HB1 was a dominant in the R5 soil, and its spore diameter and wall thickness were 135.5 ± 21.0 and 8.9 ± 3.0 μm, respectively (Fig. 3b). The spores occasionally retained a hyphal terminus that was a typical structure for the genus Entrophospora. The 18S rDNA sequences of HB1 were almost identical to that of Entrophospora colombiana. Based on the morphological and molecular data, HB1 was classified in the genus Entrophospora. WL1 was found in the QR2 and QR3 soils, and the spore diameter and wall thickness were 154.5 ± 30.4 and 8.9 ± 1.0 μm, respectively (Fig. 3c). The 18S rDNA sequences of WL1 showed highest similarity to that of Archaeospora leptoticha, and thus WL1 was classified in the genus Archaeospora. HRF1 was the only fungus that was cultured successfully from the surrounding forest (FR13 plot), and the spore morphology was similar to that of Glomus sp. HR1 (Fig. 3d). The 18S rDNA sequences of HRF1 and Glomus sp. HR1 showed highest similarity to each other (99–100% similarity), indicating that HRF1 was also classified in the genus Glomus.
Spore morphology of arbuscular mycorrhizal fungi cultured from the rhizosphere soils of pioneer plants. a HR1 isolated from the R2, R8, R9, R10 and QR2 soils from the quarry. b HB1 isolated from the R2 and R5 soils from the quarry. ht Hyphal terminus. c WL1 isolated from the QR3 soil from the quarry. d HRF1 isolated from the FR13 soil from the surrounding forest. Bars are 100 μm
Phylogenetic analysis of arbuscular mycorrhizal fungi isolated from the rhizosphere of pioneer plants grown in acid sulfate soil (HR1, HB1 and WL1) or in the surrounding forest (HRF1) based on the 1.1 kbp sequences of small subunit ribosomal RNA gene. Bootstrap values more than 70% (1,000 replications) are indicated. The numbers in the parentheses are GenBank accession numbers
All acid-tolerant nodule bacteria isolated from the quarry (QC1, QC2, QC3 and QC7) and the surrounding forest (F3b and F4b) were classified into the genus Bradyrhizobium (Fig. 5). The 1.3 kbp sequence of 16S rDNA of QC1 was identical to that of Bradyrhizobium sp. MAFF210318. The sequence of QC3 was identical to that of B. japonicum USDA62. All of the QC2, QC7, F3b and F4b sequences were identical and showed highest similarity to that of B. elkanii USDA76.
Phylogenetic analysis of nodule bacteria isolated from the rhizosphere soils or the nodules of Lespedeza cyrtobotrya grown in the quarry (QC1, QC2, QC3 and QC7) or in the surrounding forest (F3b and F4b) based on the 1.3 kbp sequences of small subunit ribosomal RNA gene. Bootstrap values more than 70% (1,000 replications) are indicated. The numbers in the parentheses are GenBank accession numbers
Effectiveness of indigenous microsymbionts in acid sulfate soil
The single inoculation of the AM fungi (Glomus sp. HR1 was a dominant) or the nodule bacterium, Bradyrhizobium sp. QC1 or F3b, did not improve the growth of L. bicolor significantly (Fig. 6a). Dual inoculation of the fungi and bacterium, however, promoted the growth of L. bicolor significantly compared with that of the non-mycorrhizal/non-bacterial control. The effectiveness of nodule bacterium on the growth did not differ between the two strains. The percentages of AM colonization of L. bicolor in the presence or absence of the nodule bacterium were 19.3 ± 3.5% (no bacterium), 11.6 ± 7.2% (QC1) and 24.5 ± 3.5% (F3b), respectively. No colonization was observed in the uninoculated plants. Non-mycorrhizal M. sinensis showed chlorosis and quite poor growth, whereas the growth of the mycorrhizal plants was more than 10-fold higher than that of the non-mycorrhizal plants (Fig. 6b). The levels of AM colonization were 39.0 ± 5.6% and 0% in the mycorrhizal and non-mycorrhizal plants, respectively.
Effectiveness of symbiotic microorganisms on the growth of pioneer plants in acid sulfate soil at pH 3.4. a The effect of dual inoculation of bradyrhizobium (QC1 or F3b) and arbuscular mycorrhizal fungi (AMF) on the growth of Lespedeza bicolor. b The effect of AMF on the growth of Miscanthus sinensis. Bradyrhizobium sp. QC1 and F3b were isolated from the quarry and the surrounding forest, respectively. NM Non-mycorrhizal control; AMF inoculated with the mixed population of AMF proliferated from the R2 plot soil (Glomus sp. HR1 was a dominant). Different letters show significant differences (Fisher’s LSD test, P < 0.05)
Discussion
Significance of pioneer plant–microbial associations in acid sulfate soil
The present study clearly demonstrated that the symbiotic associations play significant roles in the growth of pioneer plants in acid sulfate soil. Without exception, all of the pioneer herbs and shrubs formed AM associations, and the grass and legume pioneers showed significant growth responses to the indigenous microsymbionts. Pioneer grass species are generally regarded as facultative mycotrophic (Allen 1991; Oba et al. 2004). Wilson and Hartnett (1998) found, however, a strong relationship between the phenology of grass species and mycorrhizal responsiveness in a tallgrass prairie ecosystem: perennial warm-season C4 grasses were highly mycorrhiza-responsive but perennial cool-season C3 grasses were not. Given the fact that M. sinensis is a perennial C4 grass, our observations fit well with their results. Lespedeza spp. are known to be responsive to AM fungi (Wilson 1988; Wilson and Hartnett 1998), and Johnson et al. (2005) pointed out that the relatively coarse root system of Lespedeza spp. might be a key characteristic to receive benefit from AM fungi. On the other hand, it has been suggested that the establishment of Lespedeza spp. may be limited by the density of rhizobia in soil, based on the observations in N-poor grasslands (Larson and Siemann 1998). Considering that M. sinensis (Kayama 2001) and Lespdeza spp. (Cline and Silvernail 1997) are intrinsically acid-tolerant, it is likely that nutrient deficiency is a major limiting factor for these pioneers rather than soil acidity during early primary succession of acid sulfate soil. In addition, our observations also suggest that the establishment of symbiotic associations with the microorganisms is crucial for the initial growth and subsequent survival of the pioneer plants in this ecosystem. Further investigations, e.g. field inoculation experiments or pot experiments with a longer period are required to examine this hypothesis.
AM fungi in acid sulfate soil
The present study provides the first information about AM fungi in extremely acidic soil. It is considered that the fungi associated with the pioneer plants in the site are highly acid-tolerant due to the following reasons: (1) no significant decline in the levels of colonization with an increase in soil acidity was observed, and (2) the fungi isolated from the field promoted the growth of the pioneer plants significantly even at pH 3.4. Gl. clarum, E. colombiana and Gl. leptotichum (a synanamorph of Ar. leptoticha) as close relatives of Glomus sp. HR1, Entrophospora sp. HB1 and Archaeospora sp. WL1, respectively, have been isolated from acidic soils with pH less than 4 (http://invam.caf.wvu.edu/cultures/accessions.htm). Recently, the community compositions of AM fungi that associate with M. sinensis grown in acid sulfate soil have been investigated (An et al. 2008). Interestingly, Glomus sp. HR1 [corresponding to GLO3 phylotype in An et al. (2008)] was widely distributed in the rhizosphere from subarctic to subtropical Japan and occurred in a wide range of soil pH. Combining their observations with our finding that Glomus sp. HR1 was isolated from the rhizosphere of several pioneer species, it is suggested that this fungus is the ‘pioneer AM fungus’ that is widely distributed and plays major roles in pioneer vegetation of acid sulfate soil. In contrast to Glomus sp. HR1, Entrophospora sp. HB1 was cultured from only one soil sample in the present study. Oba et al. (2004) investigated the AM fungal communities in the pioneer vegetation of the lahar (volcanic mud flow) area of Mt. Pinatubo, Philippines and found a preferential association of E. colombiana with a leguminous plant Calpogonium mucunoides that was regarded as a member of secondary vegetation after the establishment of grass pioneers. According to An et al. (2008), Entrophospora spp. could not be detected from M. sinensis roots grown in acid sulfate soil even from Hazu site (the same field as the present study). These observations suggest that Entrophospora sp. HB1 may preferentially occur in a late phase of early succession. In the case of Archaeospora sp. WL1, information about ecosystems in which Ar. leptoticha occurs is much less available. Further investigations are required to evaluate the significance of this species in pioneer vegetation.
Rhizobia in acid sulfate soil
All of the nodule bacteria isolated in the present study were classified into the genus Bradyrhizobium. It has been reported that Lespedeza spp. associates with a wide range of nodule bacteria, e.g. Sinorhizobium spp., Rhizobium gelegae and B. elkanii formed nodules with L. bicolor (Yao et al. 2002). In acidic soil, however, it seems likely that Bradyrhizobium spp. are the major symbiotic partners (Cline and Senwo 1994). The acid-tolerant bradyrhizobia associated with Lespedeza spp. were able to grow on acidic agar media, but the lower limit of the in vitro growth of these strains was pH 4.5 (Cline and Senwo 1994), reflecting the acid-tolerance of the host plant that could not grow below pH 4.5 (Cline and Senwo 1993). Our observation that L. cyrtobotrya occurred in soils with pH more than 4.5 is in good agreement with that of Cline and Senwo (1993). Although the acid-tolerance of the bacterial symbionts is likely to be a significant factor for the effectiveness of N-fixation under acidic conditions, Richardson and Simpson (1989) found negative correlations between the acid tolerance of R. triforii isolates in vitro and their effectiveness in the field, indicating that the more acid-tolerant strains were less capable of infecting the host plants. In the present study, bradyrhizobia that promoted the growth of the host plants under extremely acidic conditions were successfully isolated by using acid sulfate soil as a selection medium, suggesting that choosing an appropriate medium/culture system for the screening is particularly important to obtain effective rhizobia. The P-status of the culture medium, in addition, should be taken into account for evaluating the effectiveness of selected bacteria under acidic conditions in which P-availability is generally low, because extremely low-P conditions suppress N-fixing activity. In this case, P-fertilization or the inoculation of suitable (acid-tolerant) AM fungi is to be considered.
Origin of microsymbionts in acid sulfate soil
It is likely that the symbiotic microorganisms observed in the quarry originated from the surrounding forest, because the phylogenetic analyses revealed that the AM fungi and nodule bacteria isolated from the quarry were closely related to those isolated from the surrounding forest. The idea that the forest soil is a potential habitat for acid-tolerant microsymbionts may be further supported by the fact that soil pH in the surrounding forest was 4.5–5.0 (data not shown), as is typical of temperate forest. It has been known that AM fungal propagules are dispersed into disturbed areas by wind, animal activities and erosion (Allen 1987; Warner et al. 1987). In the case of our field, soil erosion from the surrounding forest by rainfall might be a major vector of the fungal propagules and bradyrhizobia, because the ground level of the forest is 20–100 m higher than that of the quarry.
Conclusion
Isolation of acid-tolerant symbiotic microorganisms from pioneer plants will be a potential strategy for the application of the microorganisms to the revegetation program of acid sulfate soil. Further investigations are required, however, to clarify whether the inoculation of multiple stains is more effective than that of a single strain. For example, Jansa et al. (2008) demonstrated that the inoculation of multiple AM fungal species resulted in the greater P-uptake in medic and leek than that of the single species. Given the large heterogeneities in soil microenvironments and water regime, it is expected that the colonization with the multiple strains that differ in environmental preference may improve the adaptability of the host plants via maintaining the overall efficiency of acquisition of mineral nutrients under more diverse environments.
References
Allen MF (1987) Re-establishment of mycorrhizas on Mount St Helens Washington USA migration vectors. Trans Brit Mycol Soc 88:413–417
Allen MF (1991) The ecology of mycorrhizae. Cambridge University Press, Cambridge, NY, USA, pp 127–140
An G-H, Miyakawa S, Kawahara A, Osaki M, Ezawa T (2008) Community structures of arbuscular mycorrhizal fungi associated with pioneer grass species Miscanthus sinensis in acid sulfate soils: habitat segregation along pH gradients. Soil Sci Plant Nutri (in press) DOI 10.1111/j.1747-0765.2008.00267.x
Appleyard S, Wong S, Willis-Jones B, Angeloni J, Watkins R (2004) Groundwater acidification caused by urban development in Perth, Western Australia: source, distribution, and implications for management. Aust J Soil Res 42:579–585
Barea JM, Azcon R, Azconaguilar C (1992) Vesicular-arbuscular mycorrhizal fungi in nitrogen-fixing systems. Meth Microbiol 24:391–416
Bohlool BB, Ladha JK, Garrity DP, George T (1992) Biological nitrogen-fixation for sustainable agriculture – a perspective. Plant Soil 141:1–11
Brundrett MC, Abbott LK, Jasper DA (1999) Glomalean mycorrhizal fungi from tropical Australia I. Comparison of the effectiveness and specificity of different isolation procedures. Mycorrhiza 8:305–314
Cline GR, Senwo ZN (1993) Inhibitory effects of acidic minesoil on the Sericea lespedeza/Bradyrhizobium symbiotic relationship. J Plant Nutri 16:1867–1880
Cline GR, Senwo ZN (1994) Tolerance of lespedeza Bradyrhizobium to acidity, aluminum, and manganese in culture media containing glutamate or ammonium. Soil Biol Biochem 26:1067–1072
Cline GR, Silvernail AF (1997) Effects of soil acidity on the growth of sericea lespedeza. J Plant Nutri 20:1657–1666
Do TTR, Tinh TK, Nguyen TNM, Linh TB (2004) Applying mixed manure and inorganic phosphorus fertiliser to improve rice yield on acid sulfate soil (Hydraquentic Sulfaquept). Aust J Soil Res 42:693–698
Giovannetti M, Mosse B (1980) An evalu`tion of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Herrera MA, Salamanca CP, Barea JM (1993) Inoculation of woody legumes with selected arbuscular mycorrhizal fungi and rhizobia to recover desertified Mediterranean ecosystems. Appl Environ Microbiol 59:129–133
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789
Johnson NC, Wolf J, Reyes MA, Panter A, Koch GW, Redman A (2005) Species of plants and associated arbuscular mycorrhizal fungi mediate mycorrhizal responses to CO2 enrichment. Global Change Biol 11:1156–1166
Kayama M (2001) Comparison of the aluminum tolerance of Miscanthus sinensis Anderss. and Miscanthus sacchariflorus Bentham in hydroculture. Int J Plant Sci 162:1025–1031
Larson JL, Siemann E (1998) Legumes may be symbiont-limited during old-field succession. American Midland Naturalist 140:90–95
Nara K, Hogetsu T (2004) Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successional plant species. Ecology 85:1700–1707
Oba H, Shinozaki N, Oyaizu H, Tawaraya K, Wagatsuma T, Barraquio WL, Saito M (2004) Arbuscular mycorrhizal fungal communities associated with some pioneer plants in the Lahar area of Mt. Pinatubo, Philippines. Soil Sci Plant Nutri 50:1195–1203
Porter WM, Robson AD, Abbott LK (1987) Factors controlling the distribution of vesicular-arbuscular mycorrhizal fungi in relation to soil pH. J Appl Ecol 24:663–672
Powell B, Martens M (2005) A review of acid sulfate soil impacts, actions and policies that impact on water quality in Great Barrier Reef catchments, including a case study on remediation at East Trinity. Mar Poll Bull 51:149–164
Requena N, Jimenez I, Toro M, Barea JM (1997) Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in mediterranean semi-arid ecosystems. New Phytol 136:667–677
Requena N, Perez-Solis E, Azcon-Aguilar C, Jeffries P, Barea J-M (2001) Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl Environ Microbiol 67:495–498
Richardson AE, Simpson RJ (1989) Acid-Tolerance and symbiotic effectiveness of rhizobium-trifolii associated with a Trifolium-Subterraneum L-based pasture growing in an acid soil. Soil Biol Biochem 21:87–95
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, San Diego, CA, p 605
Truog E (1930) The determination of the readily available phosphorus of soils. J Am Soc Agron 22:874–882
van Aarle IM, Soderstrom B, Olsson PA (2003) Growth and interactions of arbuscular mycorrhizal fungi in soils from limestone and acid rock habitats. Soil Biol Biochem 35:1557–1564
Warner NJ, Allen MF, Macmahon JA (1987) Dispersal agents of vesicular-arbuscular mycorrhizal fungi in a disturbed arid ecosystem. Mycologia 79:721–730
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wilson D (1988) Differential plant response to inoculation with two VA mycorrhizal fungi isolated from a low-pH soil. Plant Soil 110:69–75
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738
Yao ZY, Kan FL, Wang ET, Wei GH, Chen WX (2002) Characterization of rhizobia that nodulate legume species of the genus Lespedeza and description of Bradyrhizobium yuanmingense sp. nov. Int J Syst Evol Microbiol 52:2219–2230
Acknowledgements
We are grateful to Drs. I. Nioh, K. Saito, M. Satio, M. Abe and Y. Hashimoto for invaluable suggestions, to M. Maesaka, S. Mizuno and Y. Tahara in Nagoya University for technical assistance and to Aichi prefecture for allowing us to collect the samples from the field. This study was supported by Tokai Gakujutsu Shoreikai and the Japan Society for the Promotion of Science (TE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: F. Andrew Smith.
Rights and permissions
About this article
Cite this article
Maki, T., Nomachi, M., Yoshida, S. et al. Plant symbiotic microorganisms in acid sulfate soil: significance in the growth of pioneer plants. Plant Soil 310, 55–65 (2008). https://doi.org/10.1007/s11104-008-9628-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9628-y