Abstract
This contribution highlights the role of stress and cortisol in tinnitus. Firstly, stressors such as loud noise, infection, and injury can cause hearing loss and tinnitus pathology. Secondly, stress-induced cortisol levels are decreased in individuals with tinnitus with consequences for functioning of the inner ear, the central auditory system, and its targets in the limbic brain circuit relevant for processing of stressful acoustic information. Thirdly, the phantom sound experienced by the tinnitus patient eventually may precipitate signs of a stress-related disorder. The chapter concludes with options to exploit cortisol action for tinnitus management.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
The stress response is nature’s tool to facilitate coping and adaptation. However, coping may fail if information is inadequate to predict outcome. Such a lack of control causes feelings of uncertainty and fear, which—if persistent—is damaging to health. The stress response is also activated during adverse conditions such as loud noise, infection, or inflammation that may cause tinnitus.
Tinnitus may be the consequence of damage to an auditory system that is insufficiently protected to unwanted stress reactions. Alternatively, tinnitus also is the cause of a prolonged state of emotional distress which in itself may aggravate the tinnitus percept (Mazurek et al. 2012). Moreover, lack of control over tinnitus may compromise adequate processing of stressful information and precipitate stress-related pathology. It is therefore of great interest that recent fMRI studies revealed a different processing of emotionally loaded acoustic information in the limbic system of the tinnitus patient (Georgiewa et al. 2016). The studies suggested a functional tinnitus neuronal network that may underlie the cortisol hyporesponsiveness to a severe psychosocial stressor (Hebert and Lupien 2007).
Tinnitus is aggravated by excessive stress reactions.
In this chapter we will focus on cortisol—the principal glucocorticoid hormone of man—and its hypothetical role in generation and/or aggravation of tinnitus. Cortisol is secreted by the adrenal cortex as end product of the hypothalamic-pituitary-adrenal (HPA) axis, which mediates the endocrine stress response. We start with the common concepts of stress, homeostasis/allostasis, and allostatic load. Next, we will discuss the functioning of the HPA axis and the cortisol under basal and stressful conditions. Cortisol action in the brain is mediated by mineralocorticoid receptors (MR) and glucocorticoid receptors (GR), which operate in a complementary manner in processing of stressful information (de Kloet 2014; de Kloet et al. 2005). This implies that also the auditory system is a target of cortisol for better or worse (Kil and Kalinec 2013; Mazurek et al. 2012; Trune and Canlon 2012). Chapter 2 is concluded by asking the question if and how corticosteroids acting through MR and GR expressed in the inner ear, the cochlear neuronal network, and the auditory-limbic system are implicated in tinnitus.
2.2 The Concept Stress, Allostasis, and the Allostatic Load
The term “stress” was coined by the Austrian-Canadian endocrinologist of Hungarian origin—János Hugo Bruno Selye (also known as Hans Selye)—in 1936. Using this term, Selye wanted to describe the “strain or tension” that is building up in the body and brain if the individual is faced with a threat. This state of stress evoked by the stressor is defined as any stimulus that causes on the organismic level a threat to the individuals’ psychological and physiological integrity and disrupts cellular homeostasis. The stress response is the array of physiological and behavioral responses aimed to restore homeostasis and integrity. Homeostasis refers to the stability of the “milieu intérieur” (Claude Bernard), e.g., electrolyte concentrations and body temperature that need to be indispensably maintained within narrow limits.
Stress is a state of nonspecific tension in living matter, which manifests itself by tangible morphologic changes in various organs and particularly in the endocrine glands which are under anterior pituitary control (Selye 1936).
The stress concept was initially based on a stimulus-response paradigm. Thus, the experimenter applies a physical stressor (pain, blood loss, ether vapor) and measures the subsequent response. By doing so, Selye highlighted stress as “the syndrome of being sick,” which actually captures a state induced by an array of psychological and physical stress reactions. Selye also distinguished three phases upon chronic exposure to a stressor: initial alarm, then resistance, and finally an exhaustion phase, the latter occurring only after several weeks (Selye 1952). This “general adaptation syndrome” had as hallmarks enlarged adrenals, increased vulnerability to infection, and stomach ulcers (both being a consequence of immune resistance being reduced by cortisol) and is considered to be fundamental for pathogenesis of stress-related diseases.
The stress concept developed further with the notion that it is not so much what happens to the individual but rather how the experience is taken or—more importantly—whether coping is successful (Lazarus 2006; McEwen 2007). Coping depends on personality, past experience, controllability (available options), and available information to predict outcome of an action. Self-esteem is important but also social support, sense of safety, and hierarchical position. Accordingly, coping with stress is characterized by a striking difference between individuals. Today’s challenge is to identify neuropsychological and biological determinants that could be used for prediction of how given individual will cope in a certain situation.
Since all physical stressors have a psychological component, this has led some researchers to restrict the definition of stress to the ability to cope. Coping depends on the sense of control and predictability of a given situation. Accordingly, stress should be restricted to conditions where an environmental demand exceeds the regulatory and adaptive capacity of an organism, in particular, in case of unpredictability and uncontrollability (Koolhaas et al. 2011). Hence, the most severe stressful condition is no information, no control, and no clue to predict upcoming events and a fearful feeling of uncertainty. This implies that appraisal and anticipation of an either real or imagined situation is in fact the most important determinant of a stressful experience. Appraisal refers to cognitive processes regulated by circuits in the limbic brain (Hermans et al. 2014; Vogel et al. 2016).
The most severe stressful condition includes:No informationNo controlNo clue to predict upcoming eventsand is characterized by a fearful feeling of uncertainty
To capture this state of readiness in the face of a presumed threat, the concept of allostasis was introduced (Sterling and Eyer 1988, 2012). It is not an easy concept primarily because of the variable definitions that are exercised in the literature. McEwen and Wingfield (McEwen and Wingfield 2010) state that allostasis is the “process of achieving stability or homeostasis through physiological and behavioral change,” although this viewpoint was also challenged by Day (2005) with the opinion that the stress concept would already sufficiently cover allostasis. In our view, allostasis describes a labile equilibrium characterized by structural and functional changes in neuronal networks in anticipation of upcoming events as opposed the homeostatic stable equilibrium as in the maintenance of the Na/K balance within narrow limits. The cost of allostasis through energy-consuming adaptations is called allostatic load (McEwen and Gianaros 2011).
Seymour Levine, American psychologist and one of the pioneers of stress research, weary of the endless discussion regarding the question “What is stress?” turned to use an operational definition:
Stress is defined as a composite multidimensional construct in which three components interact: (1) the input, when a stimulus, the stressor, is perceived and appraised, (2) the processing of stressful information, and (3) the output, or stress response. The three components interact via complex self-regulating feedback loops with the goal to restore homeostasis through behavioral and physiological adaptations. (Levine 2005)
Allostasis describes a “labile” equilibrium characterized by variable set points and changes in neuronal network structure and function (comparable with a juggler who keeps a dinner plate in delicate balance on top of a pointer).
2.3 Mediators of the Stress Response
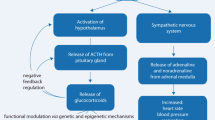
The principal mediators of the stress response are the HPA axis and the sympathetic nervous system. The latter’s workhorses are noradrenaline and adrenaline that evoke centrally a state of arousal, alertness, and vigilance and peripherally the well-known symptoms of the immediate action to deal with imminent danger, i.e., increased heart rate, elevated blood pressure, goose bumps, dry mouth, and suppression of unnecessary reproductive, consummatory, and digestive activities, all in support of Cannon’s “fight or flight” reaction (Cannon 1939). In behavioral realm, this immediate coping repertoire was extended with “fright” or “freeze,” which is the immobile position the individual assumes in the hope of not to be discovered. The catecholamines make energy substrates available for the subsequent initial defense reactions.
Slower in response is the HPA axis and its adrenal corticosteroid end products. The central conductor of the stress response is located in the parvocellular neurons of the paraventricular nucleus (PVN) in the hypothalamus. The PVN is under control of ascending aminergic neurons originating from the locus coeruleus (A6) and the nucleus tractus solitarii (A2) that mediate the effect of physical stressors. Psychological stressors are processed in higher brain regions and reach via multiple transsynaptic and inhibitory GABA-ergic network surrounding the PVN (Herman 2013; Herman et al. 2003). The hippocampus has an excitatory input to this network, which implies that stimulation of the hippocampus results in inhibition of the PVN. The amygdala input is inhibitory causing an outcome inhibition of the inhibitory network and thus excitation of the PVN.
The PVN synthetizes besides CRH also vasopressin, which after release during stress in the portal vessels potentiates CRH action on the pituitary level. CRH is responsible for the synthesis of the pro-opiomelanocortin (POMC) precursor of lipotropin (precursor of β-endorphin) and adrenocorticotropin (ACTH), while vasopressin makes intracellular calcium available to enhance in synergy with CRH the release of ACTH. At the level of the adrenal zona reticularis, the synthesis of corticosteroids from cholesterol is initiated causing with some delay the secretion of corticosteroids. The principal corticosteroid of man is cortisol which is secreted in tenfold excess over corticosterone. Rodents lack 11β-hydroxylase and secrete only corticosterone. ACTH also stimulates the growth of the adrenal cortex.
Corticosteroids act back on the body and brain, primarily to contain the initial stress reaction. Presence of corticosteroids suppresses the immune response to infection and the inflammatory reactions to tissue damage. As a result, bacterial and viral infections are not being well controlled by the immune surveillance, and the individuals exposed to chronic stress are more susceptible to infectious diseases. Also in the brain the corticosteroids act back on circuits that were initially involved in processing of stressful information. In principle, the corticosteroids act to contain initial defense (stress) reactions which are essential, but that become damaging if they are overshooting. Marius Tausk, who in the years 1927–1968 acted as a director of the pharmaceutical company Organon, used a metaphor: “glucocorticoids limit the water damage caused by the fire brigade” to explain why exogenous glucocorticoids are indicated where the endogenous cortisol is insufficient to contain inflammatory or immune disorders (de Kloet et al. 1998, 2005; Munck et al. 1984; Sapolsky et al. 2000).
Corticosteroid prevents initial stress reactions (e.g., autonomic, immune, inflammatory, metabolic, neurochemical/physiological) from overshooting and becoming damaging themselves.
Corticosteroids act on immune regulation, the gut microbiome-brain axis, the autonomic nervous system, the renin-angiotensin-aldosterone system (RAAS), and the energy metabolism axis (Dallman 2010). The action of corticosteroids is therefore extremely diverse. For an endocrinologist this may perhaps be not surprising because the task of these hormones is primarily to coordinate and to synchronize these diverse body and brain functions with the goal to promote coping with the stressor. The energy network in the brain largely overlaps with the stress responsive network, as demonstrated by fMRI studies. Corticosteroids are often indicated as glucocorticoids for their activation of gluconeogenesis by breaking down proteins and fatty acids in times of emergency to provide energy substrates. Hence, during episodes of hunger and excessive exercise, corticosteroids are mobilized: the hormones coordinate appetite and food intake with energy disposition and allocation (Dallman and Hellhammer 2011). ACTH also can promote the secretion of aldosterone from the adrenal zona glomerulosa showing how volume depletion and hemorrhage enhance both stress responses (Funder 2015; Jaisser and Farman 2016).
Corticosteroids act on immune regulation, the gut microbiome-brain axis, the autonomic nervous system, the renin-angiotensin-aldosterone system (RAAS), and the energy metabolism axis.
2.4 Pulsatility of the HPA Axis
The HPA axis has two modes of operation: the axis mediates the response to stress and coordinates the circadian activities related to, e.g., feeding, drinking, and sleep-related events. For this purpose the corticosteroids are secreted in pulsatile fashion (Lightman and Conway-Campbell 2010).
Biological rhythms:
-
CIRCADIAN—rhythm of about 24 hours
-
DIURNAL—24 hour rhythm pertained to daylight
-
ULTRADIAN—rhythm with periods shorter than the 24 h circadian cycle
-
INFRADIAN—rhythm with periods longer than the 24 h circadian cycle
About every hour cortisol and corticosterone are secreted in bursts with largest amplitude around the time physical activity starts. Thus in man the highest secretory pulse is around awakening in the morning: the cortisol awakening response. In rodents which are nighttime animals, corticosterone is highest at the beginning of the dark period. This pattern of corticosteroid pulses shows therefore a characteristic circadian variation with a peak at the beginning and a trough at the end of the activity period. In fact, high corticosteroid concentrations prevent the onset of slow wave sleep (Groch et al. 2013). The networks involved in sleep-wake overlap partly with energy and stress networks (e.g., orexin) and are targets for corticosteroids (Dallman and Hellhammer 2011).
Ultradian rhythmicity regulates tissue responsiveness to stress-induced corticosteroid action.
The hourly ultradian cycles can vary in amplitude, frequency, and organization. For instance, depression is characterized by a larger cortisol amplitude particularly at nighttime, a phenomenon that contributes to the disturbance in sleep hygiene (Groch et al. 2013). Inflammatory disorders display a higher ultradian frequency, and with aging, the pulses diminish in amplitude and become disorganized. In fact, ultradian rhythmicity provides a mechanism to maintain responsiveness of tissues and cells to corticosteroids. Indeed enhanced corticosteroid responsiveness was demonstrated when the effect of pulsatile vs constant exposure to corticosteroids was compared: responsiveness diminished in flattened circulating corticosteroid patterns (Sarabdjitsingh et al. 2010b). Such flattened patterns occur with corticosteroid replacement therapy in case of adrenal insufficiency or during pharmacotherapy with synthetic glucocorticoids (de Kloet 2014).
Stress-induced corticosteroid secretion can occur anytime during the ultradian rhythm of the hormone. If in adrenalectomized animals an hourly pulsatile rhythm was mimicked with corticosterone infusion, a stressful challenge triggered a much more profound corticosterone secretion at the ascending rather than the descending arm of the pulse (Sarabdjitsingh et al. 2010a). The dynamics of circulating corticosteroid concentrations clearly demonstrate that for the assessment of HPA axis activity only patterns matter. Single-point measurements of the hormone in either saliva or blood are useless because of the ultradian variations and stressful influences that often may occur.
2.5 Access of Corticosteroids to Brain Targets
Multidrug resistance P glycoprotein (Pgp or MDR1) is a cell membrane protein that pumps various substances from the cell to the extracellular environment. Because of its activity, Pgp is a barrier to exogenous substances. Synthetic glucocorticoids such as dexamethasone are recognized by Pgp as substrates and are removed from the cells (de Kloet 1997; Meijer et al. 1998). This has been elegantly demonstrated in vitro. That dexamethasone is a substrate for Pgp can also be demonstrated in vivo using Pgp knockout mice. Upon administration of 3H-labeled dexamethasone or 3H prednisone (Karssen et al. 2002) to these mutants, the radioactive steroids were demonstrated to accumulate in the typical target sites: hippocampus, PVN, and biogenic amine neurons. Surprisingly, the 3H-labeled cortisol that is also not retained in wildtype mice with the proper activity of Pgp pump shows profound retention in the Pgp knockouts (Karssen et al. 2001). Hence, corticosterone seems the only steroids that readily penetrate the mouse brain, since also the penetration of aldosterone is hampered. This remarkable dichotomy between cortisol and corticosterone is maintained in people. While in the blood, the ratio cortisol/corticosterone is 10:1, this ratio is decreased to 10:4 in the cerebrospinal fluid (Karssen et al. 2005).
The access of corticosteroids to its brain receptors is regulated by P-glycoprotein transporters in the blood-brain barrier and by an intracellular oxidoreductase.
There is an additional, intracellular mechanism that determines access of corticosterone, cortisol, and aldosterone to the nucleus and finally to the genome. The enzyme 11-β-hydroxysteroid dehydrogenase type 2 (HSD-2) is an oxidase that specifically inactivates cortisol and corticosterone, but not aldosterone. HSD-2 acts as gatekeeper blocking access of the naturally occurring glucocorticoids but allowing binding of bioactive aldosterone to the mineralocorticoid receptors (MR) and the genome (Edwards et al. 1988; Funder et al. 1988). In adult rodent brain, the expression of HSD-2 is discrete and restricted to some periventricular tissues where aldosterone activates circuits involved in salt appetite. Remarkably, also the solitary nucleus (NTS) near the area postrema expresses abundantly HSD-2. This group of cells has projections innervating the forebrain and provides the limbic-forebrain circuits with an aldosterone-selective mechanism which have been postulated to underlie salt appetite and preference and harbors vital cognitive functions to store spatial information on salt resources (Geerling and Loewy 2009). The HSD-1 isoform is a reductase widely present in the brain and has the capacity (e.g., in the liver) to regenerate bioactive cortisol and corticosterone. Inhibitors to the HSD-1 isoform were developed as a strategy to limit overexposure to corticosteroids (Chapman et al. 2013).
The Fukushima disaster from 2011 has significantly increased the incidence of tinnitus and Ménière syndrome in the local population.
Both HSD proteins have been found in the inner ear: HSD-2 was found in the endolymphatic sac of the inner ear of rodents (Akiyama et al. 2010), whereas HSD-1 was identified in the stria vascularis and in the outer and inner auditory hair cells (Terakado et al. 2011). Endolymphatic sac is responsible for the resorption, transport, and recirculation of ions in the entire inner ear, i.e., in the cochlea and in the vestibular system. It is believed that individuals diagnosed with the Ménière syndrome have occasionally increased hydraulic pressure within the inner ear endolymphatic system often attributed to the hyperactivity of the endolymphatic sac. This hyperactivity induces a triad of symptoms: vertigo, hearing loss, and tinnitus. Interestingly, patients with Ménière syndrome often report having an attack after being exposed to emotional stress. In the inner ear, upon stress-induced overproduction of cortisol, the decreased activity of HSD-2 or the increased activity of HSD-1 could lead to upregulated infiltration of cortisol, decreased accessibility of aldosterone, and dysregulation of ion dynamics that are responsible for the attacks. Unfortunately, no study addressed this issue in people yet, as only a postmortem study could answer open questions. Recently published systematic review revealed two facts: the first one was confirmation of the association between Meniere syndrome and posttraumatic stress disorder and health anxiety, whereas the second was a need for large, properly designed and conducted epidemiological studies (Kirby and Yardley 2008). Recent study investigating the incidence of otological conditions following the disaster in Fukushima (earthquake and subsequent nuclear accident) in the local Fukushima population demonstrated significant increase in the number of new cases of Ménière syndrome and also of tinnitus coinciding with an increased number of comorbid mental stress-related conditions, such as depression or anxiety (Hasegawa et al. 2015).
2.6 Corticosteroid Receptors
In 1968 Bruce McEwen made a landmark discovery (McEwen et al. 1968, 2015). He discovered that 3H-corticosterone given to adrenalectomized animals was not retained as expected in the hypophysiotropic region in the hypothalamus but in the hippocampus. In subsequent studies, de Kloet et al. (1975) discovered that the potent synthetic glucocorticoid dexamethasone was not retained by these receptors and also did not compete for 3H-corticosterone or 3H-aldosterone retention, suggesting the presence of two distinct receptor populations for corticosteroids. The pattern of 3H-aldosterone retention appeared to match localization of HSD-2, particularly in NTS (Geerling and Loewy 2009).
In the mid-1980s, the existence of two types of receptors for corticosteroids in the brain was demonstrated. The receptors were cloned and identified as the mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) (Evans and Arriza 1989). Around the same time, Reul and de Kloet (1985) demonstrated with binding studies that the rat hippocampus contained two receptor populations that did bind corticosterone with different affinity. The high affinity binding sites were designated type 1 receptors and later MR, the lower affinity sites—type 2 receptors—and later GR. Since in rodents and humans the principal corticosteroid (corticosterone and cortisol, respectively) circulates in a 100–1000-fold excess over aldosterone, these steroids are the predominant MR ligands in vivo. MR is abundantly expressed in limbic brain structures, e.g., hippocampus, lateral septum, and amygdala. GR binds cortisol and corticosterone with a tenfold lower affinity than MR and is widely distributed in neurons and glial cells with highest expression in PVN, limbic structures, and neocortical regions (de Kloet et al. 1998, 2005; de Kloet 1991).
The presence of MR and GR was demonstrated in the inner ear and a very distinct expression pattern of the receptors was shown (Fig. 2.1). Stria vascularis contains predominantly MR, which is in agreement with its function, namely, recycling and regulating K+ (and Na+). The spiral ganglion neurons contain predominantly GR, whereas the rest of the cells in the cochlea (auditory hair cells, supporting cells, fibrocytes, interdigital cells, and spiral limbus cells) contains both—MR and GR (Basappa et al. 2012).
Distribution of MR and GR in the cochlear tissues. OHC outer hair cells, IHC inner hair cells, DC Deiters cells, IP inner pillar cells, OP outer pillar cells, HC Hensen cells, SV stria vascularis, SL spiral ligament, SLi spiral limbus, ISC inner sulcus cells, OSC outer sulcus cells, SGN spiral ganglion neurons, SP spiral prominence, PC pillar cells (From Kil and Kalinec 2013, Reprinted with permission) (Kil and Kalinec 2013)
The implication of the difference in affinity of the MR and GR for corticosterone and cortisol is differential occupation of these two receptor types during circadian variation and after stress. This differential activation of MR and GR as a function of circulating steroid concentration provided for over 30 years the experimental basis for research on neuronal networks underlying stress coping, behavioral adaptation, and energy metabolism (Dallman 2010; de Kloet 1991, 2014, 2016; de Kloet and Reul 1987; Lupien et al. 2009; McEwen et al. 2015).
Since MR and GR are transcription factors regulating gene expression, they are expected to interact with the genome upon binding their ligand. Using chromatin immunoprecipitation (ChIP) followed by a deep sequencing (ChIP seq), Nicole Datson and Annelies Polman have made a complete inventory of all genomic binding sites for MR and GR in the hippocampal genome (Polman et al. 2013). They observed that 40% of the GR binding sites are within the genes. The experiment involved adrenalectomized animals injected with increasing doses of corticosterone. Also on the genomic level, two populations of genome binding sites for MR and GR were found. Already at a low dose, MR/corticosterone complex associated with DNA and this binding remained relatively constant up to 3 mg of administered corticosterone. GR did bind only at higher doses of corticosterone to DNA binding sites, thus reflecting the differential binding of MR and GR to corticosterone.
Binding of mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) with differential affinity to endogenous corticosteroids enables distinct responses during circadian cycle and after stress.
Moreover, using a neurophysiological approach Marian Joëls and Henk Karst discovered as yet another surprise hidden in corticosteroid receptorology. They demonstrated that pyramidal and dentate gyrus neurons of the hippocampus and neurons of basolateral amygdala harbored an MR variant that responded rapidly to corticosterone, cortisol, and aldosterone (Joels and Baram 2009; Joels and de Kloet 2012; Karst et al. 2005). This membrane MR was deleted in the MR knock animals, and the signal was maintained when the steroids were applied when penetration in the cell was prevented because of coupling of the ligand to bovine serum albumin. Activation of the receptor caused within minutes increased excitatory postsynaptic potentials (EPSP) indicating a rapidly enhanced release of the excitatory transmitter glutamate.
The membrane MR-mediated action depended on an ERK1/2 pathway (Olijslagers et al. 2008). Simultaneous with MR-induced glutamate release, the voltage dependent I(A) K current at the postsynaptic membrane was decreased. Moreover, probably as a result of increased synaptic release of glutamate, the presynaptic mGLU2/3 receptor was downregulated (Nasca et al. 2015). Also GR appeared to entertain a lower affinity GR membrane variant that mediated the release of cannabinoids for transsynaptic inhibitory action on the presynaptic release of glutamate (Di et al. 2003).
2.7 Behavioral and Neuroendocrine Feedback Action of Corticosteroids in the Brain
Corticosteroids secreted by the adrenals after stress exert a negative feedback action to suppress the enhanced HPA axis activity (Fig. 2.2). This phenomenon was demonstrated by a classical endocrine experiment in 1938 by Dwight Ingle (see Raff 2005). He was the first to show that ACTH was needed for adrenal growth and steroid secretion by administering the peptide to hypophysectomized animals. Next, Ingle showed that corticosterone given to the ACTH-treated animal did not affect adrenal weight while it suppressed adrenal weight in the intact animal. Hence, corticosterone exerted in high doses pituitary feedback on ACTH release.
Subsequent research demonstrated different levels of corticosteroid feedback operation. The first level is on the anterior pituitary level—as noted by Ingle—and mediated by GR expressed in the corticotrophs. This feedback site responds to potent synthetic glucocorticoid such as dexamethasone and very high levels of endogenous cortisol and corticosterone (de Kloet et al. 1974). The onset of suppression occurs with a delay of 30 min, and in case of dexamethasone, the suppression may last several hours, even more than 12 h as used in the dexamethasone suppression test with or without CRH (see Box 2.1). The rise and fall of the dexamethasone suppression test in endocrine psychiatry is wonderfully described in “The riddle of Melancholia” (Shorter and Fink 2010).
Box 2.1
Dexamethasone suppression test (DST): A low dose of dexamethasone is administered at 11.00 pm and plasma cortisol levels are measured the next morning at 9.00 am. In a hyperactive HPA axis—as occurs in depression—cortisol will escape from dexamethasone suppression at that time (Carroll et al. 1976).
Combined dexamethasone-CRH test: dexamethasone is administered at 11.00 pm, but in addition the next afternoon, CRH is administered, and plasma cortisol levels are measured at 15, 30, and 45 min post CRH (Heuser et al. 1994).
The second level is at higher brain regions harboring circuits that process stressful information and that communicate transsynaptically with the GABA-ergic network surrounding the PVN. The steroid feedback is complex in these circuits and operates over different time domains depending on the nature and severity of the psychological stressor. The coordinate action exerted by corticosteroids via membrane and genomic MR and GR adds to this complexity (Dallman and Hellhammer 2011; de Kloet 2014).
Figure 2.3 shows the role of MR and GR in processing of stressful information in the limbic brain with the goal to support coping and adaptation. Corticosteroids affect virtually every step from detection and perception of a salient event triggering emotional arousal and appraisal processes until coping, adaptation, and memory storage of the experience to be prepared if a similar encounter occurs in the future. Thus, first corticosteroids affect the detection threshold and perception of auditory information. Lack of steroids was found to enhance detection at the expense of perceiving the significance of the acoustic signal (Henkin and Daly 1968). Next, arousal is triggered (Pfaff et al. 2007) and is necessary for the limbic structures to function optimally in assessment of the valence of a novel experience and selection of an appropriate coping style.
Box 2.2
-
Limbic genomic MR regulates increases of the excitability of the hippocampus and its afferents to, e.g., the mesolimbic dopaminergic reward system.
-
Limbic membrane MR is involved in encoding and retrieval of information important for appraisal processes and selection of a coping response.
-
Genomic and membrane GR enhance allostatic processes, facilitate behavioral adaptation, and promote memory storage of the experience.
-
These actions mediated by MR and GR are complementary in detection, perception, and processing of sensory (e.g., auditory) information.
Using a large variety of behavioral tests, Melly Oitzl et al. (Oitzl et al. 2010; Oitzl and de Kloet 1992) have carefully dissected the role of MR and GR during stress coping and adaptation. Thus, corticosteroid appeared to rapidly promote appraisal processes of newly acquired information, retrieval of contextual information, and selection of an appropriate coping style. Since these MR-mediated actions proceed rapidly, they most likely are exerted by the membrane receptor variant regulating excitatory transmission; GR becomes activated only with high amount of corticosteroids induced by stress. GR activation is important for restoring cellular homeostasis and promoting allostatic processes, behavioral adaptation, and memory storage of the experience and coping style. By doing so the input from higher brain regions subsides resulting in attenuation and at last termination of stress-induced HPA axis activity because of adaptation (Box 2.2 and Fig. 2.3).
2.8 Role of MR and GR in Coping with Stress
Firstly, MR activation directs coping style. Lars Schwabe et al. (2010a) demonstrated that exposure to a stressful situation switches the coping style. Under resting conditions the rodent uses multiple cues in order to memorize the location of a food resource. If exposed to stress, the animal switches rapidly to a simpler stimulus response. In rodents the pathway activated chronically by a stressor switches from hippocampus toward the dorsal striatum supporting habit-like behavior (Dias-Ferreira et al. 2009). The phenomenon is also observed in humans: with fMRI it was shown that during stress the amygdala-hippocampus pathway rapidly switched to the amygdala-striatum connectivity (Schwabe et al. 2013; Vogel et al. 2015, 2016).
The switch from hippocampus to striatum was observed in males. If the same experiments were performed in females, the opposite results were obtained. Females under resting conditions were rather poor in spatial performance as compared to their male counterparts. Under stress the situation was reversed, females performed better, and these differences were eliminated in the MR forebrain knockout mice (ter Horst et al. 2012). Thus, context and sex determine the outcome of the MR-mediated functions in coping with stress. Anti-mineralocorticoids blocked the switch from hippocampus to striatum in rodents and man (Schwabe et al. 2010b, 2013; Vogel et al. 2015, 2016). Moreover, active vs passive coping style in mouse and rat lines correlates with MR expression in the hippocampus (Cabib and Puglisi-Allegra 2012; Veenema et al. 2003).
Secondly, GR activation promotes adaptation and memory storage. It appeared that GR-mediated effects on memory storage required the presence of noradrenaline (Joels et al. 2012; Roozendaal and McGaugh 2011). For this purpose GR mediates a plethora of activating and suppressive actions in discrete brain regions. Thus, in the CRH neurons of the amygdala GR stimulates the synthesis and release of CRH, while the reverse occurs in the PVN (Zalachoras et al. 2016). In the amygdala, GR promotes and extends MR-mediated glutamatergic excitation (Karst et al. 2010). In the hippocampal pyramidal neurons, MR enhances excitability, which is subsequently suppressed by subsequent stimulation of GR by higher concentrations of corticosteroids (Joels and de Kloet 1989, 1990, 1992). In addition multiple neuropeptide systems (oxytocin, vasopressin) are activated by stress which exert in specific behavioral domains their context-dependent effects on processes modulating the stress response. For instance, oxytocin stimulates bonding and social support, which facilitates coping with a stressful situation (Barrett et al. 2015; Young 2015).
Third, the limbic MR is important for the tone of the HPA axis and sympathetic nervous system. For instance, the higher the hippocampal MR expression, the lower the basal pulsatile and stress-induced HPA axis activation, and thus the average amount of corticosteroids secreted over 24 h is decreased. Under MR antagonists applied intracerebroventricularly enhance basal and stress-induced HPA axis activity (Ratka et al. 1989) and act as an anxiolytic (Korte et al. 1995) and anti-aggressive (Kruk et al. 2013) agent. MR antagonists also decrease the blood pressure response to a stressor (van den Berg et al. 1990). This effect mediated by MR appeared to depend on the condition of 30 min warming the animal which is needed to do a proper tail sphygmographic measurement of the blood pressure. Using this warming/stress condition of the indirect tail cuff method, the direct telemetric recording revealed that MR antagonist blocked autonomic outflow and, interestingly, now suppressed the stress-induced HPA axis response (de Kloet et al. 2000; Van den Berg et al. 1994).
Collectively, these observations have led to the formulation of the corticosteroid receptor balance hypothesis:
Upon imbalance in MR: GR-regulated limbic -cortical signaling pathways, the initiation and/or management of the stress response is compromised. At a certain threshold this may lead to a condition of HPA axis dysregulation and impaired behavioral adaptation, which can enhance susceptibility to stress-related neurodegeneration and mental disorders. (de Kloet 2014, 2016; de Kloet et al. 1991, 1998, 2005, 2016; de Kloet and Molendijk 2016; Holsboer 2000)
2.9 MR:GR Balance: Genetics
Genetic variants of MR, GR, and their regulatory proteins such as, e.g., FKBP5, have been identified that appeared associated with HPA axis regulation, emotional expressions, and cognitive performance. Genetic variation may alter control in the promotor and translation region and result in an altered primary structure. The GR variant N363S was found hypersensitive to cortisol and associated with an unhealthy metabolic profile, while E22/23EK is linked to steroid resistance and enhanced risk of depression. The Bcl-1 polymorphism predicts cardiovascular risk and contributes to individual differences in emotional and traumatic memories as well as PTSD symptoms after intensive care treatment (Quax et al. 2013).
In the MR gene, the rs5522 (minor allele frequency 12%) is an A/G SNP located in exon 2, which causes an amino acid change (I180V) in the N-terminal domain of the protein. Roel de Rijk discovered that this G-allele is a loss of function variant as shown by a reduced transactivation capacity in vitro (DeRijk et al. 2006). These G-allele carriers showed increased HPA axis and autonomic reactivity in response to psychological stressors. Moreover, Bogdan reported an association of MR gene variation with depressive symptoms and deficits in reward-motivated learning induced by stress and heightened stress-induced amygdala activity (Bogdan et al. 2010, 2012). Interestingly, the same G-allele is with a high odd ratio considered a risk factor in reverse remodeling in heart failure patients undergoing cardiac resynchronization therapy (De Maria et al. 2012).
Another MR SNP, rs2070951 (C/G), minor allele frequency 49.3%, is located 2 nucleotides before the translation start site. The G-allele produces less MR in vitro and is associated with increased renin and aldosterone and elevated blood pressure (van Leeuwen et al. 2010).
The rs5522 and rs2070951 are in linkage disequilibrium, and if merged, three common haplotypes can be identified. Haplotype (hap) 2 (CA, frequency 35%) is a gain of function variant as was shown from the increased transactivation capacity and increased translation of MR protein in vitro, while hap 4 (GG) is very rare and produces strongly reduced MR activity as compared to hap 1 (GA, frequency 49%) and hap 3 (CG, frequency 12%) (Hamstra et al. 2015; van Leeuwen et al. 2011).
Carriers of a “gain of function” MR C/A haplotype display dispositional optimism and effective coping styles and are protected from depression.
Hap 2 carriers had lower scores on the Trier Inventory for Chronic Stress (TICS) subscales “excessive demands at work” and “social overload.” In females, hap 2 appeared associated with dispositional optimism, optimistic risk decision-making in gambling tests, less rumination, and less feelings of hopelessness. GAIN cohort study (N = 3600) has demonstrated that hap 2 carriers are protected from depression (Hamstra et al. 2015; Joels et al. 2008; Klok et al. 2011). Further, this haplotype moderates the effect of childhood maltreatment and depressive symptoms in a population-based cohort (N = 665) and an independent clinical cohort from the Netherlands Study of Depression and Anxiety (NESDA, N = 1639) (Vinkers et al. 2015).
2.10 MR/GR Balance: Phenotype
Selye showed that a relative excess of mineralocorticoids was pro-inflammatory, while excess of glucocorticoids increased the risk for infection and expressed this view in the pendulum hypothesis. The balance hypothesis, however, is based on one single corticosteroid hormone which maintains homeostasis via two distinct and co-localized receptor types that carry the pharmacological activity of Selye’s two hormones: the MR and GR (de Kloet 1991). As was pointed previously, the MR in the brain, heart, and fat cells binds cortisol and corticosterone rather than aldosterone and does so with a tenfold higher affinity than GR.
Over the past 30 years, the MR/GR balance has been challenged using endocrine, pharmacological, and genetic approaches. The outcome of these challenges was measured on the molecular levels using genomic approaches and on the cellular level with neuroanatomical and electrophysiological techniques, and behavioral and physiological responses were recorded in a great variety of paradigms (de Kloet 2014, de Kloet and Joels 2016; de Kloet and Molendijk 2016; de Kloet et al. 2016; Joels et al. 2012).
Below are some general characteristics of MR/GR imbalance:
Genetically selected rat or mouse lines or strains that display overexpression of MR have a reduced HPA axis tone as expressed by lower basal and stress-induced levels of corticosterone (Harris et al. 2013; Veenema et al. 2003). The male animals have an active coping style if dealing with an inescapable stressor, a high sympathetic tone and reduced 5HT function (Veenema et al. 2003). They show less anxiety in the home environment and improved cognitive performance in maze learning and fear-motivated tasks. Their behavior once learned perseverates. This phenotype is mimicked in mice with forebrain MR overexpression, particularly in the face of reduced GR. It seems as if limbic overexpression of the MR facilitates during stress the switch from costly time-consuming declarative hippocampal learning and memory processes to a rapid and effective striatal habit performance as coping style. However, these dominant high MR expressing animals become prone to anxiety in novel situations where they have lost control (de Kloet et al. 2016).
Increased MR function in the hippocampus is protective to stress under conditions of high controllability and readily shifts coping from a time- and energy-consuming declarative hippocampal to a more direct striatal habit style.
Exposure to chronic stress decreases the expression of hippocampal MR. Likewise rats or mice exposed to adverse early life conditions have at later life reduced MR. Reduced hippocampal MR expression is observed at senescence and is a characteristic of the depressed patient’s hippocampus measured postmortem. Antidepressants increase the synthesis of hippocampal MR. Rats with viral overexpression of MR in the dentate gyrus showed improved short-term memory and were protected against the impairing effect of 3 weeks of corticosterone in a nonspatial object recognition paradigm (Ferguson and Sapolsky 2007). In mutant mice, forebrain MR overexpression restored impaired learning induced by chronic stress but only in a low arousing task. This behavioral change in the MR overexpression mice was paralleled by a normalization of hippocampal dentate gyrus function (Kanatsou et al. 2015).
That chronic stress affects the hippocampus is obvious from the profound neuroanatomical changes: the CA3 pyramidal neurons atrophy and dentate gyrus neurogenesis is reduced (McEwen 2016). Using microarrays it was found that the widely diverse gene patterns were reduced to only a few pathways that regulate chromatin organization, epigenetics, apoptosis, and inflammatory responses in the dentate gyrus. One highly responsive gene network revealed by this procedure is the mammalian target of rapamycin (mTOR) signaling pathway which is critical for different forms of synaptic plasticity and appears associated with depression (Datson et al. 2013; Polman et al. 2012).
2.11 Implications for Tinnitus
Tinnitus is a phantom sound indicating malfunction of the central auditory system. The causes of tinnitus include damage to the inner ear and consequent changes in the auditory system. The damage may, for instance, be due to aging, noise exposure, infections, altered vascular integrity, and inflammatory responses because of hypertension or atherosclerosis and local head or neck injuries (Knipper et al. 2013).
The auditory system comprises the neuronal cochlear circuit connected with the auditory cortex via the olive nucleus and the midbrain geniculate nucleus. This circuit enables arousal via the brainstem-midbrain reticular system and communicates with limbic circuitry (McIntosh and Gonzalez-Lima 1998; Middleton and Tzounopoulos 2012). Each acoustic stimulus received by the ear and passed via the process of auditory transduction into the central auditory pathway undergoes assessment leading to emotional reactions. The majority of acoustic signals are evaluated as neutral but part is appraised with positive or negative emotional weight. This assessment is possible due to the connectivity of auditory brainstem and auditory cortex with limbic circuits in the amygdala, hippocampus, and prefrontal cortex regions (Kraus and Canlon 2012); it is active not only during the awake phase but also during phases of the non-REM sleep (Portas et al. 2000). Thus, the connectivity between the auditory and limbic systems is involved in detecting adversity, danger, and regulation of the HPA axis (Fig. 2.4).
In patients with disturbing tinnitus, the persistent phantom sound is continuously evaluated and classified by the limbic system as adverse and thus negative (Rauschecker et al. 2010). This may in turn lead to a long-term dysregulation of the HPA axis characteristic for a condition of chronic stress (Fig. 2.5). Some of the consequences of the tinnitus-induced chronic stress effects are, for instance, insomnia, as the limbic system signals danger and keeps the victims of tinnitus awake. It would be of interest to accommodate these findings to the current knowledge of the action of corticosteroids, since previously it has been reported that tinnitus patients display hypocortisolism upon exposure to severe psychosocial stressors (Hebert and Lupien 2007). One scenario is therefore that this “hypocortisolism” provides an insufficiently large cortisol signal that is not capable to control the central stress reaction evoked by auditory adversity (see Sect. 3). This would suggest the existence a cortisol sensitive tinnitus connectome or neuronal network underlying an acoustic-induced allostatic load/chronic stress phenotype (McEwen and Wingfield 2010). Recent evidence indeed suggests tinnitus-specific connectivity of a functional limbic neuronal network involved in processing of emotionally loaded and emotionally neutral acoustic information which could be the tinnitus signature of such an altered phenotype (Georgiewa et al. 2016).
Evidence emerges for a corticosteroid-responsive functional neuronal network presenting a tinnitus signature in biological correlates.
Not all of the subjects with tinnitus are disturbed by its sound; however, those who are suffer greatly from tinnitus-related insomnia and concentration problems. In addition, about 50% of patients with tinnitus has additional mental comorbid condition(s) such as depression or anxiety (Pattyn et al. 2016; Zirke et al. 2013), and these are known to be set off or amplified by the emotional stress and MR/GR imbalance (de Kloet et al. 2016).
Tinnitus may cause emotional distress and, finally, stress-related pathology. At the same time, emotional exhaustion or the pathology accompanying posttraumatic stress disorder was suggested to be predisposing for tinnitus (Fagelson 2007; Hebert et al. 2012; Hinton et al. 2006). The argument is presented by the seminal experiments of Sylvie Hébert et al. (Hebert et al. 2012; Hebert and Lupien 2007; Mazurek et al. 2015). These authors reported a blunted cortisol response to the Trier Social Stress Test and enhanced suppression of the morning rise in cortisol by a low dose of exogenous dexamethasone administered at 11 pm on the previous day (Simoens and Hebert 2012). Collectively, these data reveal an HPA axis phenotype of tinnitus resembling that of fibromyalgia, chronic fatigue syndrome, posttraumatic stress syndrome, and atypical depression, which are all characterized by a relative underexposure to cortisol during stressful conditions (Chrousos and Gold 1992). Such a reduced cortisol secretion maybe the consequence of an overactive limbic MR conveying an enhanced inhibitory tone over the HPA axis. The recently uncovered cytokine signature of tinnitus would fit in a phenotype of an altered functional ratio of MR over GR activity (Betancur et al. 1995; de Kloet et al. 1994) causing prevalence of pro-inflammatory cytokine synthesis (Szczepek et al. 2014).
That cortisol is of relevance for auditory processing is known for a long time. Adrenally deficient patients were shown to have lower detection threshold in the frequencies 500 and 1000 Hz than the healthy controls but have a deficit in speech discrimination (Henkin and Daly 1968). This increased detection and decreased perception cannot be ameliorated by deoxycorticosterone, but the detection threshold was normalized upon ACTH, prednisolone, and fludrocortisone treatment, the latter with either dexamethasone, prednisolone, or cortisone (Henkin and Daly 1968). Corroborating this early clinical finding, recent study demonstrated that although the rats with impaired adrenal function have intact function of the outer hair cells in the inner ear, their distortion produces otoacoustic emissions (DPOAE). These adrenally deficient animals also have significantly elevated auditory brainstem responses (ABR) which are consistent with impaired tone and speech perception in people (Dogan et al. 2015) and indicative of neuronal processing rather than sensory malfunctioning. Further, in support of clinical findings, dexamethasone reversed this impairment auditory information processing. Hence, it seems that enhancing GR function contributes to reinstatement of normal auditory function. Another argument for a positive action of corticosteroids on the auditory system is the therapeutic use of synthetic corticosteroids (prednisone, dexamethasone) to treat inner ear illnesses such as sudden sensorineural hearing loss (SSHL)—a condition that is always accompanied by tinnitus (Hobson et al. 2016; Leung et al. 2016) or idiopathic tinnitus (Barreto et al. 2012; Dodson and Sismanis 2004).
Corticosteroid receptors are expressed in the inner ear and auditory networks in the brain. Several areas of the inner ear are richly endowed with MR and GR (Fig. 2.1) (Kil and Kalinec 2013; Terakado et al. 2011). The current notion is that glucocorticoids prevent the hearing loss via GR because of their anti-inflammatory and immunosuppressive action, while the aldosterone-selective MR is involved in maintenance of ion homeostasis required for optimal hearing (Meltser and Canlon 2011). Since 85% of subjects with tinnitus have some degree of hearing loss (Mazurek et al. 2010), it would be very interesting to examine whether prevention of hearing loss is connected with prevention of tinnitus. Also the cochlear neuronal network expresses differentially in discrete nuclei MR and GR. However, so far no systematic studies have been reported on the function of these brain receptors in the onset and modulation of tinnitus.
2.12 Corticosteroids-Based Treatment Options
Synthetic corticosteroids are used since decades as systemic or local therapy for tinnitus. Dexamethasone and methylprednisolone are most commonly used, and the administration routes vary from per os, intravenous injection to intratympanic injections (see Table 2.1). Recent systematic review scrutinized clinical studies that used the latter method and concluded lack of effectiveness (Lavigne et al. 2016). However, the authors also recognized that the extreme heterogeneity of the clinical protocols and the lack of long-term follow-up undermined their disappointing conclusion.
Lack of successful pharmacological treatment for tinnitus reflects lack of a clear-cut, worldwide accepted classification of tinnitus. Tinnitus is a symptom that can accompany numerous diseases. Curative treatment for tinnitus may depend on its cause. In agreement with this, a case report was published, in which the authors claim that epidural injection with triamcinolone acetonide was successfully used to cure the patient from chronic tinnitus (McCormick and Walega 2015). The patient described in that case report had somatic (or somatosensoric) tinnitus. This subtype of tinnitus is characterized by the ability of the patient to modulate the tone or volume of tinnitus by the head and neck movements. Somatic tinnitus is caused due to neuronal convergence of auditory and somatic pathways on the level of dorsal cochlear nucleus and inferior colliculus (Dehmel et al. 2008). McCormick and Walega propose the mechanism for curative action of corticosteroids in that case and attribute it to the afferental modulation of neuronal signals carried by somatic spine roots that converge with auditory pathways. More time and studies with this particular type of tinnitus subtype is needed to prove the general efficacy of synthetic corticosteroids for somatic tinnitus.
Tinnitus is a symptom that can accompany numerous diseases. Curative treatment for tinnitus may depend on its cause.
Taken the data together, after a scholarly discussion of the organization of the HPA axis and the action of its end product cortisol, we concentrated on the precipitation of tinnitus by a great variety of insults. We next discussed the potential role of stress elements in the auditory-limbic connectome with respect to the onset and progression of tinnitus. This refers in particular to the notion that prolonged acoustic adversity evoked by tinnitus would lead to inadequate cortisol containment of stress reactions in the auditory system. As a consequence this chronic stress condition would progressively cause damage to circuits underlying the central processing of auditory information resulting in further aggravation of tinnitus and enhanced vulnerability to mood disorders. The chapter is concluded with a discussion of treatment options to the benefit of the tinnitus patient that are based on corrections of stress-induced changes in the auditory system.
References
Akiyama K, Miyashita T, Matsubara A, Inamoto R, Mori T, Nishiyama A, Mori N (2010) Expression and localization of 11beta-hydroxysteroid dehydrogenase (11betaHSD) in the rat endolymphatic sac. Acta Otolaryngol 130:228–232
Araujo MF, Oliveira CA, Bahmad FM Jr (2005) Intratympanic dexamethasone injections as a treatment for severe, disabling tinnitus: does it work? Arch Otolaryngol Head Neck Surg 131(2):113–117. doi:10.1001/archotol.131.2.113
Barreto MA, Silva IB, de Oliveira CA, Bahmad F Jr (2012) Intratympanic corticotherapy and tinnitus control after sudden hearing loss. Int Tinnitus J 17:186–193
Barrett CE, Arambula SE, Young LJ (2015) The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry 5:e606
Basappa J, Graham CE, Turcan S, Vetter DE (2012) The cochlea as an independent neuroendocrine organ: expression and possible roles of a local hypothalamic-pituitary-adrenal axis-equivalent signaling system. Hear Res 288:3–18
van den Berg DT, de Kloet ER, van Dijken HH, de Jong W (1990) Differential central effects of mineralocorticoid and glucocorticoid agonists and antagonists on blood pressure. Endocrinology 126:118–124
Betancur C, Borrell J, Guaza C (1995) Cytokine regulation of corticosteroid receptors in the rat hippocampus: effects of interleukin-1, interleukin-6, tumor necrosis factor and lipopolysaccharide. Neuroendocrinology 62:47–54
Bogdan R, Perlis RH, Fagerness J, Pizzagalli DA (2010) The impact of mineralocorticoid receptor iso/val genotype (rs5522) and stress on reward learning. Genes Brain Behav 9:658–667
Bogdan R, Williamson DE, Hariri AR (2012) Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry 169:515–522
Cabib S, Puglisi-Allegra S (2012) The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev 36:79–89
Cannon WB (1939) The wisdom of the body, Rev. and enlarged edn. Norton, New York
Carroll BJ, Curtis GC, Mendels J (1976) Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry 33:1051–1058
Chapman K, Holmes M, Seckl J (2013) 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93:1139–1206
Choi SJ, Lee JB, Lim HJ, In SM, Kim JY, Bae KH, Choung YH (2013) Intratympanic dexamethasone injection for refractory tinnitus: prospective placebo-controlled study. Laryngoscope 123(11):2817–2822. doi:10.1002/lary.24126
Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–1252
Dallman MF (2010) Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21:159–165
Dallman MF, Hellhammer DH (2011) Regulation of the hypothalamo-pituitary-adrenal axis, chronic stress, and energy: the role of brain networks. Springer, New York
Datson NA, van den Oever JM, Korobko OB, Magarinos AM, de Kloet ER, McEwen BS (2013) Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology 154:3261–3272
Day TA (2005) Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuro-Psychoph 29:1195–1200
De Maria R, Landolina M, Gasparini M, Schmitz B, Campolo J, Parolini M, Sanzo A, Galimberti P, Bianchi M, Brand SM, Parodi O, Lunati M (2012) Genetic variants of the renin-angiotensin-aldosterone system and reverse remodeling after cardiac resynchronization therapy. J Card Fail 18:762–768
Dehmel S, Cui YL, Shore SE (2008) Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol 17:S193–S209
DeRijk RH, Wust S, Meijer OC, Zennaro MC, Federenko IS, Hellhammer DH, Giacchetti G, Vreugdenhil E, Zitman FG, de Kloet ER (2006) A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J Clin Endocrinol Metab 91:5083–5089
Di S, Malcher-Lopes R, Halmos KC, Tasker JG (2003) Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857
Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N (2009) Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325:621–625
Dodson KM, Sismanis A (2004) Intratympanic perfusion for the treatment of tinnitus. Otolaryngol Clin N Am 37:991–1000
Dogan R, Meric A, Gedik O, Tugrul S, Eren SB, Ozturan O (2015) Does systemic steroid deficiency affect inner ear functions? Am J Otolaryngol 36:568–574
Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, de Kloet ER, Monder C (1988) Localisation of 11 beta-hydroxysteroid dehydrogenase—tissue specific protector of the mineralocorticoid receptor. Lancet 2:986–989
Evans RM, Arriza JL (1989) A molecular framework for the actions of glucocorticoid hormones in the nervous system. Neuron 2:1105–1112
Fagelson MA (2007) The association between tinnitus and posttraumatic stress disorder. Am J Audiol 16:107–117
Ferguson D, Sapolsky R (2007) Mineralocorticoid receptor overexpression differentially modulates specific phases of spatial and nonspatial memory. J Neurosci 27:8046–8052
Funder JW (2015) Primary aldosteronism: seismic shifts. J Clin Endocrinol Metab 100:2853–2855
Funder JW, Pearce PT, Smith R, Smith AI (1988) Mineralocorticoid action—target tissue-specificity is enzyme, not receptor, mediated. Science 242:583–585
Geerling JC, Loewy AD (2009) Aldosterone in the brain. Am J Physiol Renal Physiol 297:F559–F576
Georgiewa P, Szczepek AJ, Rose M, Klapp BF, Mazurek B (2016) Cerebral processing of emotionally loaded acoustic signals by tinnitus patients. Audiol Neurootol 21:80–87
Groch S, Wilhelm I, Lange T, Born J (2013) Differential contribution of mineralocorticoid and glucocorticoid receptors to memory formation during sleep. Psychoneuroendocrinology 38:2962–2972
Hamstra DA, de Kloet ER, van Hemert AM, de Rijk RH, Van der Does AJ (2015) Mineralocorticoid receptor haplotype, oral contraceptives and emotional information processing. Neuroscience 286:412–422
Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR (2013) Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology 38:648–658
Hasegawa J, Hidaka H, Kuriyama S, Obara T, Hashimoto K, Tateda Y, Okumura Y, Kobayashi T, Katori Y (2015) Change in and long-term investigation of neuro-otologic disorders in disaster-stricken Fukushima prefecture: retrospective cohort study before and after the great East Japan earthquake. PLoS One 10:e0122631
Hebert S, Lupien SJ (2007) The sound of stress: blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neurosci Lett 411:138–142
Hebert S, Canlon B, Hasson D (2012) Emotional exhaustion as a predictor of tinnitus. Psychother Psychosom 81:324–326
Henkin RI, Daly RL (1968) Auditory detection and perception in normal man and in patients with adrenal cortical insufficiency: effect of adrenal cortical steroids. J Clin Invest 47:1269–1280
Herman JP (2013) Neural control of chronic stress adaptation. Front Behav Neurosci 7:61
Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180
Hermans EJ, Henckens MJ, Joels M, Fernandez G (2014) Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37:304–314
Heuser I, Yassouridis A, Holsboer F (1994) The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res 28:341–356
Hinton DE, Chhean D, Pich V, Hofmann SG, Barlow DH (2006) Tinnitus among cambodian refugees: relationship to PTSD severity. J Trauma Stress 19:541–546
Hobson CE, Alexander TH, Harris JP (2016) Primary treatment of idiopathic sudden sensorineural hearing loss with intratympanic dexamethasone. Curr Opin Otolaryngol Head Neck Surg 24(5):407–412
Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501
ter Horst JP, van der Mark MH, Arp M, Berger S, de Kloet ER, Oitzl MS (2012) Stress or no stress: mineralocorticoid receptors in the forebrain regulate behavioral adaptation. Neurobiol Learn Mem 98:33–40
Jaisser F, Farman N (2016) Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol Rev 68:49–75
Joels M, Baram TZ (2009) The neuro-symphony of stress. Nat Rev Neurosci 10:459–466
Joels M, de Kloet ER (1989) Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science 245:1502–1505
Joels M, de Kloet ER (1990) Mineralocorticoid receptor-mediated changes in membrane properties of rat CA1 pyramidal neurons in vitro. Proc Natl Acad Sci USA 87:4495–4498
Joels M, de Kloet ER (1992) Control of neuronal excitability by corticosteroid hormones. Trends Neurosci 15:25–30
Joels M, de Kloet ER (2012) Nothing is written in stone. Biol Psychiatry 72:432–433
Joels M, Karst H, DeRijk R, de Kloet ER (2008) The coming out of the brain mineralocorticoid receptor. Trends Neurosci 31:1–7
Joels M, Sarabdjitsingh RA, Karst H (2012) Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev 64:901–938
Kanatsou S, Fearey BC, Kuil LE, Lucassen PJ, Harris AP, Seckl JR, Krugers H, Joels M (2015) Overexpression of mineralocorticoid receptors partially prevents chronic stress-induced reductions in hippocampal memory and structural plasticity. PLoS One 10:e0142012
Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, de Kloet ER (2001) Multidrug resistance p-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 142:2686–2694
Karssen AM, Meijer OC, van der Sandt IC, De Boer AG, De Lange EC, de Kloet ER (2002) The role of the efflux transporter p-glycoprotein in brain penetration of prednisolone. J Endocrinol 175:251–260
Karssen AM, Meijer OC, Berry A, Sanjuan Pinol R, de Kloet ER (2005) Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology 146:5587–5595
Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M (2005) Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102:19204–19207
Karst H, Berger S, Erdmann G, Schutz G, Joels M (2010) Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA 107:14449–14454
Kil SH, Kalinec F (2013) Expression and dexamethasone-induced nuclear translocation of glucocorticoid and mineralocorticoid receptors in guinea pig cochlear cells. Hear Res 299:63–78
Kirby SE, Yardley L (2008) Understanding psychological distress in meniere’s disease: a systematic review. Psychol Health Med 13:257–273
de Kloet ER (1991) Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol 12:95–164
de Kloet ER (1997) Why dexamethasone poorly penetrates in brain. Stress 2:13–20
de Kloet ER (2014) From receptor balance to rational glucocorticoid therapy. Endocrinology 155:2754–2769
de Kloet ER, Reul JM (1987) Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology 12:83–105
de Kloet ER, Joels M (2016) Stress research: past, present, and future. In: Pfaff DW (ed) Neuroscience in the 21st century from basic to clinical. Springer, New York, pp 1979–2007
de Kloet ER, Molendijk ML (2016) Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast 2016:6503162
de Kloet ER, van der Vies J, de Wied D (1974) The site of the suppressive action of dexamethasone on pituitary-adrenal activity. Endocrinology 94:61–73
de Kloet R, Wallach G, McEwen BS (1975) Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology 96:598–609
de Kloet ER, Joels M, Oitzl M, Sutanto W (1991) Implication of brain corticosteroid receptor diversity for the adaptation syndrome concept. Methods Achiev Exp Pathol 14:104–132
de Kloet ER, Oitzl MS, Schobitz B (1994) Cytokines and the brain corticosteroid receptor balance: relevance to pathophysiology of neuroendocrine-immune communication. Psychoneuroendocrinology 19:121–134
de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301
de Kloet ER, Van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, de Jong W (2000) Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int 57:1329–1336
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475
de Kloet ER, Otte C, Kumsta R, Kok L, Hillegers MH, Hasselmann H, Kliegel D, Joels M (2016) Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol. doi:10.1111/jne.12379
Klok MD, Giltay EJ, Van der Does AJ, Geleijnse JM, Antypa N, Penninx BW, de Geus EJ, Willemsen G, Boomsma DI, van Leeuwen N, Zitman FG, de Kloet ER, DeRijk RH (2011) A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl Psychiatry 1:e62
Knipper M, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U (2013) Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol 111:17–33
Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E (2011) Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35:1291–1301
Korte SM, Buwalda B, Meijer O, De Kloet ER, Bohus B (1995) Socially defeated male rats display a blunted adrenocortical response to a low dose of 8-OH-DPAT. Eur J Pharmacol 272:45–50
Kraus KS, Canlon B (2012) Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288:34–46
Kruk MR, Haller J, Meelis W, de Kloet ER (2013) Mineralocorticoid receptor blockade during a rat’s first violent encounter inhibits its subsequent propensity for violence. Behav Neurosci 127:505–514
Lavigne P, Lavigne F, Saliba I (2016) Intratympanic corticosteroids injections: a systematic review of literature. Europ Arc Otorhinolaryngol 273(9):2271–2278
Lazarus RS (2006) Emotions and interpersonal relationships: toward a person-centered conceptualization of emotions and coping. J Pers 74:9–46
van Leeuwen N, Caprio M, Blaya C, Fumeron F, Sartorato P, Ronconi V, Giacchetti G, Mantero F, Fernandes-Rosa FL, Simian C, Peyrard S, Zitman FG, Penninx BW, de Kloet ER, Azizi M, Jeunemaitre X, Derijk RH, Zennaro MC (2010) The functional c.-2g>c variant of the mineralocorticoid receptor modulates blood pressure, renin, and aldosterone levels. Hypertension 56:995–1002
van Leeuwen N, Bellingrath S, de Kloet ER, Zitman FG, DeRijk RH, Kudielka BM, Wust S (2011) Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: implication for the stress response. Psychoneuroendocrinology 36:699–709
Leung MA, Flaherty A, Zhang JA, Hara J, Barber W, Burgess L (2016) Sudden sensorineural hearing loss: primary care update. Hawaii J Med Public Health 75:172–174
Levine S (2005) Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 30:939–946
Lightman SL, Conway-Campbell BL (2010) The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci 11:710–718
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
Mazurek B, Olze H, Haupt H, Szczepek AJ (2010) The more the worse: the grade of noise-induced hearing loss associates with the severity of tinnitus. Int J Environ Res Public Health 7:3071–3079
Mazurek B, Haupt H, Olze H, Szczepek AJ (2012) Stress and tinnitus-from bedside to bench and back. Front Syst Neurosci 6:47
Mazurek B, Szczepek AJ, Hebert S (2015) Stress and tinnitus. HNO 63:258–265
McCormick ZL, Walega DR (2015) Cervical epidural steroid injection for refractory somatic tinnitus. Pain Pract 15:e28–e33
McEwen BS (2007) Stress, definitions and concepts of. In: Fink G (ed) Encyclopedia of stress, vol 3, 2nd edn. Academic Press, San Diego, CA, pp 653–654
McEwen BS (2016) Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res 1645:50–54
McEwen BS, Wingfield JC (2010) What is in a name? Integrating homeostasis, allostasis and stress. Horm Behav 57:105–111
McEwen BS, Gianaros PJ (2011) Stress- and allostasis-induced brain plasticity. Annu Rev Med 62:431–445
McEwen BS, Weiss JM, Schwartz LS (1968) Selective retention of corticosterone by limbic structures in rat brain. Nature 220:911–912
McEwen BS, Gray JD, Nasca C (2015) 60 years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol 226:T67–T83
McIntosh AR, Gonzalez-Lima F (1998) Large-scale functional connectivity in associative learning: interrelations of the rat auditory, visual, and limbic systems. J Neurophysiol 80:3148–3162
Meijer OC, de Lange EC, Breimer DD, de Boer AG, Workel JO, de Kloet ER (1998) Penetration of dexamethasone into brain glucocorticoid targets is enhanced in MDR1a p-glycoprotein knockout mice. Endocrinology 139:1789–1793
Meltser I, Canlon B (2011) Protecting the auditory system with glucocorticoids. Hear Res 281:47–55
Middleton JW, Tzounopoulos T (2012) Imaging the neural correlates of tinnitus: a comparison between animal models and human studies. Front Syst Neurosci 6:35
Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5:25–44
Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nistico R, McEwen BS (2015) Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA 112:14960–14965
Oitzl MS, de Kloet ER (1992) Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci 106:62–71
Oitzl MS, Champagne DL, van der Veen R, de Kloet ER (2010) Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev 34:853–866
Olijslagers JE, de Kloet ER, Elgersma Y, van Woerden GM, Joels M, Karst H (2008) Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci 27:2542–2550
Pattyn T, Van Den Eede F, Vanneste S, Cassiers L, Veltman DJ, Van De Heyning P, Sabbe BC (2016) Tinnitus and anxiety disorders: a review. Hear Res 333:255–265
Pfaff DW, Martin EM, Ribeiro AC (2007) Relations between mechanisms of CNS arousal and mechanisms of stress. Stress 10:316–325
Polman JA, Hunter RG, Speksnijder N, van den Oever JM, Korobko OB, McEwen BS, de Kloet ER, Datson NA (2012) Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology 153:4317–4327
Polman JA, de Kloet ER, Datson NA (2013) Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome. Endocrinology 154:1832–1844
Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD (2000) Auditory processing across the sleep-wake cycle: simultaneous EEG and FMRI monitoring in humans. Neuron 28:991–999
Quax RA, Manenschijn L, Koper JW, Hazes JM, Lamberts SW, van Rossum EF, Feelders RA (2013) Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol 9:670–686
Raff H (2005) Teaching glucocorticoid negative feedback and adrenocortical regulation using a classic paper by Dr. Dwight Ingle. Adv Physiol Educ 29:141–143
Ratka A, Sutanto W, Bloemers M, de Kloet ER (1989) On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology 50:117–123
Rauschecker JP, Leaver AM, Muhlau M (2010) Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66:819–826
Reul JM, de Kloet ER (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505–2511
Roozendaal B, McGaugh JL (2011) Memory modulation. Behav Neurosci 125:797–824
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Sarabdjitsingh RA, Conway-Campbell BL, Leggett JD, Waite EJ, Meijer OC, de Kloet ER, Lightman SL (2010a) Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain-region-specific manner. Endocrinology 151:5369–5379
Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, de Kloet ER, Meijer OC (2010b) Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology 151:1177–1186
Schwabe L, Schachinger H, de Kloet ER, Oitzl MS (2010a) Corticosteroids operate as a switch between memory systems. J Cogn Neurosci 22:1362–1372
Schwabe L, Schachinger H, de Kloet ER, Oitzl MS (2010b) Stress impairs spatial but not early stimulus-response learning. Behav Brain Res 213:50–55
Schwabe L, Tegenthoff M, Hoffken O, Wolf OT (2013) Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol Psychiatry 74:801–808
Selye H (1936) A syndrome produced by diverse nocuous agents. Nature 138:32
Selye H (1952) The story of the adaptation syndrome. Acta Inc., Medical Publishers, Montreal, Canada
She W, Dai Y, Du X, Yu C, Chen F, Wang J, Qin X (2010) Hearing evaluation of intratympanic methylprednisolone perfusion for refractory sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 142(2):266–271. doi:10.1016/j.otohns.2009.10.046
Shim HJ, Song SJ, Choi AY, Hyung Lee R, Yoon SW (2011) Comparison of various treatment modalities for acute tinnitus. Laryngoscope 121(12):2619–2625. doi:10.1002/lary.22350
Shorter E, Fink M (2010) Endocrine psychiatry. Solving the riddle of melancholia. Oxford University Press, Oxford
Simoens VL, Hebert S (2012) Cortisol suppression and hearing thresholds in tinnitus after low-dose dexamethasone challenge. BMC Ear Nose Throat Disord 12:4
Sterling P (2012) Allostasis: a model of predictive regulation. Physiol Behav 106:5–15
Sterling P, Eyer J (1988) Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J (eds) Handbook of life stress, cognition, and health. Wiley, Chicester, NY
Szczepek AJ, Haupt H, Klapp BF, Olze H, Mazurek B (2014) Biological correlates of tinnitus-related distress: an exploratory study. Hear Res 318:23–30
Terakado M, Kumagami H, Takahashi H (2011) Distribution of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase isoforms in the rat inner ear. Hear Res 280:148–156
Topak M, Topak A, Sahin Yilmaz T, Ozdoganoglu HB, Yilmaz M, Ozbay M (2009) Intratympanic methylprednisolone injections for subjective tinnitus. J Laryngol Otol 123(11):1221–1225
Trune DR, Canlon B (2012) Corticosteroid therapy for hearing and balance disorders. Anat Rec 295:1928–1943
Van den Berg DT, de Jong W, de Kloet ER (1994) Mineralocorticoid antagonist inhibits stress-induced blood pressure response after repeated daily warming. Am J Phys 267:E921–E926
Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM (2003) Genetic selection for coping style predicts stressor susceptibility. J Neuroendocrinol 15:256–267
Vinkers CH, Joels M, Milaneschi Y, Gerritsen L, Kahn RS, Penninx BW, Boks MP (2015) Mineralocorticoid receptor haplotypes sex-dependently moderate depression susceptibility following childhood maltreatment. Psychoneuroendocrinology 54:90–102
Vogel S, Klumpers F, Krugers HJ, Fang Z, Oplaat KT, Oitzl MS, Joels M, Fernandez G (2015) Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology 40:947–956
Vogel S, Fernandez G, Joels M, Schwabe L (2016) Cognitive adaptation under stress: a case for the mineralocorticoid receptor. Trends Cogn Sci 20:192–203
Young LJ (2015) Oxytocin, social cognition and psychiatry. Neuropsychopharmacology 40:243–244
Zalachoras I, Verhoeve SL, Toonen LJ, van Weert LT, van Vlodrop AM, Mol IM, Meelis W, de Kloet ER, Meijer OC (2016) Isoform switching of steroid receptor co-activator-1 attenuates glucocorticoid-induced anxiogenic amygdala CRH expression. Mol Psychiatry 21(12):1733–1739
Zirke N, Seydel C, Arsoy D, Klapp BF, Haupt H, Szczepek AJ, Olze H, Goebel G, Mazurek B (2013) Analysis of mental disorders in tinnitus patients performed with composite international diagnostic interview. Qual Life Res 22:2095–2104
Acknowledgment
The support to ER de Kloet by the Royal Netherlands Academy of Arts and Sciences, COST Action ADMIRE BM1301 and STW Take-off 14095 is gratefully acknowledged.
AJ Szczepek thankfully acknowledges the research support of Dürr Foundation and the German Tinnitus Foundation.
Declaration of Interest
E R. de Kloet is on the scientific advisory Board of Dynacorts Therapeutics and Pharmaseed Ltd. and owns stock of Corcept Therapeutics. The current paper bears no relationship to these interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
de Kloet, E.R., Szczepek, A.J. (2017). Stress and Glucocorticoid Action in the Brain and Ear: Implications for Tinnitus. In: Szczepek, A., Mazurek, B. (eds) Tinnitus and Stress. Springer, Cham. https://doi.org/10.1007/978-3-319-58397-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-58397-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58396-9
Online ISBN: 978-3-319-58397-6
eBook Packages: MedicineMedicine (R0)