Abstract
Emotional stress has accompanied humans since the dawn of time and has played an essential role not only in positive selection and adaptation to an ever-changing environment, but also in the acceleration or even initiation of many illnesses. The three main somatic mechanisms induced by stress are the hypothalamus-pituitary-adrenal axis (HPA axis), the sympathetic-adreno-medullar (SAM) axis, and the immune axis. In this chapter, the stress-induced mechanisms that can affect cochlear physiology are presented and discussed in the context of tinnitus generation and auditory neurobiology. It is concluded that all of the presented mechanisms need to be further investigated. It is advised that clinical practitioners ask patients about stressful events or chronic stress preceding the tinnitus onset and measure the vital signs. Finally, taking into account that tinnitus itself acts as a stressor, the implementation of anti-stress therapies for tinnitus treatment is recommended.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Catecholamines
- Cytokines
- Glucocorticoids
- Glutamate excitotoxicity
- HPA axis
- Immune axis
- SAM axis
- The cochlea
1 Introduction
The association between emotional stress and tinnitus has long been known, appearing in medical journals for at least 200 years ago (Curtis 1841). The crosstalk between tinnitus and stress is still a subject of intense research (Szczepek and Mazurek 2017; Aydin and Searchfield 2019; Biehl et al. 2019; Brueggemann et al. 2019; Moossavi et al. 2019). An epidemiological study with 12,166 subjects demonstrated that the correlation between tinnitus incidence and stress is as strong as between tinnitus and noise exposure (Baigi et al. 2011). The authors of another study that involved 658 tinnitus patients demonstrated a direct effect of stress level on tinnitus loudness and tinnitus distress (Probst et al. 2016). However, the question of how stress affects the auditory pathway to induce tinnitus remains open.
Non-auditory health conditions strongly associated with stress include depression and anxiety (Craske and Stein 2016; Michaelides and Zis 2019). Interestingly, tinnitus patients frequently report having depressive and anxious symptoms (Zöger et al. 2006; Adoga et al. 2008; Zirke et al. 2013; Gomaa et al. 2014; Salviati et al. 2014; Conrad et al. 2015; Waechter and Brännström 2015; Bruggemann et al. 2016; Brueggemann et al. 2019). Also, tinnitus patients are significantly more likely to have symptoms of depression and anxiety when compared to age-matched control subjects (Danioth et al. 2020) and a higher incidence of anxiety (26.1%) and depressive symptoms (25.6%) as compared to age-matched persons without tinnitus (9.2% incidence of anxiety and 9.1% of depressive symptoms) (Bhatt et al. 2017). In agreement, current epidemiological studies suggest a direct correlation between tinnitus and anxiety or depression (Hébert et al. 2012).
Definitive scientific evidence demonstrating that stress causes tinnitus is still lacking. There are multiple reasons for this knowledge gap, the main being the patients’ diffuse knowledge about the time of onset of the phantom sound and a lack of medical and psychological information from that period. The other reason is that the persons who developed tinnitus may not necessarily be bothered by it. What is not lacking is the abundant clinical data demonstrating that the individuals affected by tinnitus are more likely to experience a higher level of stress than this experienced by tinnitus-free patients (Betz et al. 2017; Biehl et al. 2019; Mazurek et al. 2019). Accordingly, various therapeutic methods such as cognitive-behavioral therapy (CBT), mindfulness-based cognitive therapy (MBCT), mindfulness-based stress reduction (MBSR), brief solution-focused therapy, narrative therapy, acceptance and commitment therapy (ACT), and eye movement desensitization and reprocessing (EMDR) have been used to reduce the tinnitus-induced burden (National Guideline 2020). The success of these types of therapeutic approaches in treating tinnitus points towards the essential role that stress plays in tinnitus pathobiology. However, the outstanding questions are if and how the stress-induced responses affect the auditory pathway to produce a sensory activation without an acoustic stimulus. Before an attempt to answer that question, the mechanism evoked by stress needs to be described.
2 Stressors
Two general types of stressors are recognized: psychological and physical stressors. Psychological stressors include mental stressors (concentration tasks, memory requirements, intelligence tests) (Kirschbaum et al. 1993), social stress situations, and stressors acting throughout life (posttraumatic stress disorder after deprivation or abuse), which produce changes in stress regulation patterns over a lifetime and induce a central hyper-responsiveness (Heim and Nemeroff 2009; Lupien et al. 2009; Slavich and Shields 2018). Environmental (physical) stressors include hypo- or hyperthermia, noise, over-illumination, or overcrowding.
The effects of some physical stressors – namely noise and temperature changes – were and still are studied extensively in the auditory system (Seifert et al. 1998; El Ganzoury et al. 2012; Sliwinska-Kowalska and Davis 2012; Lie et al. 2016; Le et al. 2017). Also, the effects of psychological stressors on the auditory system have been investigated, providing insights into the pathophysiology of tinnitus and hyperacusis (Horner 2003; Mazurek et al. 2010b; Hasson et al. 2013; Mazurek et al. 2015). Interestingly, tinnitus itself is considered to be a stressor.
3 Neurobiological Mechanisms Associated with Tinnitus Induction
Tinnitus is a symptom, and the conditions associating with tinnitus are discussed in detail elsewhere in this book. In this chapter, selected processes leading to tinnitus are listed, and later, their association with stress is demonstrated.
It is well accepted that tinnitus initiation is associated with damage to the auditory periphery, whereas tinnitus maintenance correlates with the progressive changes in the central auditory system (Eggermont 1990; Eggermont and Roberts 2015; Haider et al. 2018). The cochlear structures that could be damaged include outer and inner hair cells, supporting cells, and spiral ganglion neurons. On the molecular level, the injury can be induced by glutamate excitotoxicity (Puel et al. 2002; Ryan and Bauer 2016; Kim et al. 2019), an excess of free radicals (Evans and Halliwell 1999; Huang et al. 2000; Rybak et al. 2019), and all processes leading to apoptosis (Op de Beeck et al. 2011; Gauvin et al. 2018). On the structural level, cochlear synaptopathy has been proposed to represent an important mechanism contributing to tinnitus onset (Liberman and Kujawa 2017; Altschuler et al. 2019). However, this mechanism has recently been questioned for humans (Guest et al. 2017) and animals (Pienkowski 2018).
Interestingly, accumulating evidence supports the view that the limbic system makes an essential contribution to the onset and maintenance of tinnitus percept (Jastreboff 1990; Lockwood et al. 1998; Mühlau et al. 2006; Landgrebe et al. 2009; Leaver et al. 2016; Ryan and Bauer 2016; Caspary and Llano 2017; Qu et al. 2019; Kapolowicz and Thompson 2020). The limbic system was proposed to provide negative feedback to the central auditory system and, thus, to turn off the perception of the tinnitus sound. However, the stress-affected limbic system no longer provides that negative feedback, leaving the phantom sound uncancelled (Rauschecker et al. 2010). Corroborating studies have demonstrated significant volume reduction of grey matter in the (left) parahippocampal cortex of tinnitus patients (Landgrebe et al. 2009; Besteher et al. 2019; Liu et al. 2019).
4 Stress-Induced Responses
The term stress has been in use for about a century. Physicists first introduced it in an attempt to describe a distribution of energy leading to tension. In the twenties of the last century, an American physiologist Walter Cannon used the term stress when describing fight or flight response (Cannon 1922). A few years later, a Hungarian-Canadian endocrinologist, Hans Seyle originated the research on emotional stress (Selye and Fortier 1949; Selye 1950). The experiments performed with animals led to the discovery of the hypothalamus-pituitary-adrenal axis (HPA axis) (Fortier and Selye 1949), which we will describe later in detail. He also introduced the concept of positive or negative stress and explored various states of stress reactions. The followers of Seyle’s model of stress continue to conduct research trying to understand how the emotional status may influence the functioning of cells, tissues, and the entire organism, leading in some cases to somatic pathologies.
The factors inducing stress are termed stressors and can be divided into physical and psychological stressors. The physical stressors (such as pain, heat, or cold) can cause similar but not identical effects on the organism compared to emotional stressors (Hermann et al. 2019). Also, duration of stress is an essential factor, where the outcome of acute, short-time stress differs from the chronic exposure to stress (Bryant 2018). Therefore, the result of stress differs, depending on the type of stressor, age, gender, genetics, social status, and education of the affected person (Fig. 1) (Oyola and Handa 2017).
Schematic illustration of the factors influencing the outcome of stress. The stressors involved in the stress event (physical, psychological, or both) induce effects, depending on age, gender, stress duration, and several other factors. The outcome of stress may range from staying healthy to acquiring a health condition
4.1 The Hypothalamus-Pituitary-Adrenal Axis (HPA Axis)
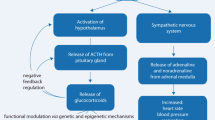
The pioneering work of Hans Seyle paved the way for understanding the somatic mechanisms induced by emotional stress (Selye 1937). The principal pathway caused by stress is the hypothalamic-pituitary-adrenal axis (HPA axis). HPA axis encompasses the following structural elements: the hypothalamus (paraventricular nucleus, PV), pituitary gland (the anterior lobe), and the adrenal cortex. The hypothalamic neurons in PV are capable of synthesizing vasopressin and corticotropin-releasing hormone (CRH). These two peptides are secreted upon stress and stimulate adrenocorticotropic hormone (ACTH) release from the pituitary gland. ACTH promotes the production and release of corticosteroids from the adrenal gland. The corticosteroids-driven negative feedback mechanism tightly regulates the HPA axis (Fig. 2). In humans, the principal corticosteroid produced is cortisol, whereas in rodents, it is corticosterone.
The schematic HPA pathway. Upon stress, the CRH is released from the hypothalamus to activate the pituitary gland and induce adrenocorticotropic hormone (ACTH) release. In response to ACTH, the adrenal gland produces and releases glucocorticoid hormones, which in turn inhibit the production of ACTH and CRH. The green color indicates the stimulatory pathway, while the red color indicates the inhibitory pathway
In response to diurnal rhythm or stress, cortisol is released to the bloodstream to act on all tissues and cells of the body, influencing the metabolism and gene transcription regulation. The metabolic effect of cortisol is associated with the de novo production of glucose (gluconeogenesis) in the liver, kidney, intestine, muscle, and brain (Yip et al. 2016). In contrast, gene transcription regulation occurs almost in each somatic cell due to glucocorticoid receptors’ ubiquitous presence (Fig. 3). The glucocorticoid-mediated transcriptional modulation is complex and comprises several types of processes involving direct binding of glucocorticoid-glucocorticoid receptor complex to specific sequences on the genomic DNA and activation/deactivation of several transcription factors through various mechanisms. Upon binding its receptor (GR) and translocation to the nucleus, GR inhibits or stimulates the expression of several genes – these genes belong to the so-called glucocorticoid-responsive genes. The glucocorticoid- responsive gene pattern differs depending on the cell type. For instance, in adipocytes, corticosteroids bind to 8,848 sites on the genomic DNA to upregulate the expression of 421 and downregulate the expression of 198 genes (Yu et al. 2010). In contrast, only 4,392 sites are bound by corticosteroids in A549 epithelial cell line carcinoma (Reddy et al. 2009). Similarly, in the neuronal cell line PC12, a unique, cell-type restricted GR specificity was described, demonstrating 1,183 genomic binding sites (Polman et al. 2012). The processes regulated by corticosteroids in the neuronal cells include neuron projection morphogenesis, neuron projection regeneration, synaptic transmission, and regulation of apoptosis, suggesting a strong influence of corticosteroids on neuronal plasticity.
The effect of corticosteroid on the cell on the molecular level. The corticosteroid attaches to the glucocorticoid receptor (GR) and translocates to the nucleus and the mitochondria, acting as a transcription factor. Upon binding to the chromosomal or mitochondrial DNA, GR influences gene transcription in both organelles, leading to altered transcription/translation (nucleus and mitochondria) and oxidative stress (mitochondria)
The nuclear and mitochondrial DNA can be expressionally regulated by stress and glucocorticoids (Hunter et al. 2016), adding to the complexity of glucocorticoid effects in the cells, tissues, and entire body. Exposure to stress results in an inhibition of mitochondrial complex I activity and an increase in reactive oxygen species (ROS) production, damaging the affected cells (Fig. 3). The physiological significance of mitochondria affected by stress was demonstrated in the animal models of anxiety-related disorders and human anxiety disorders (Misiewicz et al. 2019).
Under ideal physiological circumstances, the HPA axis can be quickly activated by stress and promptly stopped by negative feedback via corticosteroids (cortisol in humans). However, under chronic stress, the inhibitory mechanisms are either no longer in place or aberrant. In response to experimental social stress (Trier Social Stress Task (Kirschbaum et al. 1993)), healthy subjects produce free cortisol detectable in saliva 30 min later (Hébert and Lupien 2007). Tinnitus patients were shown to have delayed reactions to the same stressor, suggesting anomalous HPA axis responses.
The potential effects of the HPA axis on the cochlea and its hypothetical contribution to the onset of tinnitus are discussed below.
4.2 Potential Involvement of the Stress-Activated HPA Axis in Tinnitus Generation
4.2.1 Mitochondrial Damage and ROS Formation
The association between mitochondrial DNA (mtDNA) integrity and cochlear physiology is clearly seen in the human genetically-mediated syndromic and non-syndromic deafness, caused by the mutations in mtDNA (Kokotas et al. 2007). Moreover, some of the isoforms and mutations in mitochondrial DNA have been associated with presbyacusis in humans and the mouse model and correlated with a loss of spiral ganglion neurons (Pickles 2004; Crawley and Keithley 2011). In addition, it was shown that some specific mutations in the mitochondrial DNA, which associate with tinnitus, are ethnically distributed (Mostafa et al. 2014; Lechowicz et al. 2018). Some other mutations in the mtDNA, which targeted the 12S rRNA gene known to predispose to ototoxicity, are also associated with a sudden tinnitus onset (Fischel-Ghodsian et al. 1997).
In the cochlea of humans and animals and the organ of Corti-derived cell line, ototoxic medications such as cisplatin or gentamicin were shown to induce overproduction of reactive oxygen species leading to mitochondrial damage and finally, hair cell death (Bertolaso et al. 2001; Poirrier et al. 2010; Sheth et al. 2017; Desa et al. 2018; O’reilly et al. 2019). Collectively, this evidence strongly implies the detrimental role of damaged mitochondria and the overproduction of reactive oxygen species in cochlear pathology. Even though no studies have examined the role of the HPA axis in the generation of ROS in the cochlea, this process might still play a role in the induction of cochlear hearing loss and tinnitus.
4.2.2 Glucocorticoid-Modified Expression of Genes
Glucocorticoid- (GR) and mineralocorticoid receptors (MR) are expressed in the cochlea by various cell types such as inner and outer hair cells, spiral ganglion cells, supporting cells (ten Cate et al. 1993; Zuo et al. 1995; Kil and Kalinec 2013). Stria vascularis expresses mainly MR, whereas fibrocytes type IV mainly GR (Kil and Kalinec 2013). In an animal model, the short-term acute restrain was used to study the effect of non-auditory stress on the auditory pathway. In the spiral ganglion neurons, the restrain has induced GR’s nuclear translocation, whereas in the cochlea, it downregulated the expression of cochlear GR. The changes occurred 24 h after stress and indicated negative feedback mechanism (Tahera et al. 2006a). The GR translocation was associated with protection against noise-induced injury (Tahera et al. 2006b). In contrast to short-term stress, long-term stress was shown to be associated with an increased incidence of hearing loss and tinnitus in humans, indicating that the general dysregulation of the HPA axis might be detrimental to the auditory system (Canlon et al. 2013; Herr et al. 2018).
Many studies of gene expression in the cochlea following exposure to corticosteroid have been performed using synthetic steroid dexamethasone. These studies demonstrated, for instance, that dexamethasone modulates the expression of genes encoding apoptosis-relevant proteins in the cochlea (Hoang et al. 2009). The somewhat limited evidence obtained using the stress model is consistent with cochlear gene expression being modulated during or after stress. However, only a few genes were investigated, of which hypoxia-inducible factor 1 (Hif1) was downregulated in the cochlea of Wistar rats 7 days after mild, 24-h-long stress (Mazurek et al. 2010a).
To summarize, the influence of stress released corticosteroids on gene transcription in the cochlea should be further studied using a global approach (e.g., mRNA sequencing) and with a large-scale data.
4.2.3 Influence of HPA Axis on Glutamate Signaling
The role of cochlear glutamate-depending signaling in the generation of tinnitus has been suggested (Puel et al. 2002; Sahley et al. 2013). The model has been supported by a study using C57BL/6J mice that provided evidence of an imbalance between cochlear NMDA and AMPA receptors during a long-term administration of salicylate, associated with the induction of tinnitus (Cui et al. 2019). Consistent with this observation, salicylate-induced tinnitus could be inhibited by selective NMDA blocker memantine (Ralli et al. 2014). In primary hippocampal cultures, corticosterone was shown to increase the endocytosis of AMPAR, leading to its surface decrease (Martin et al. 2009). It remains to be determined if the HPA axis influences overexpression of NMDA or downregulation of AMPA receptors in the cochlea, therefore contributing to cochlear synaptic plasticity and eventually to a generation of tinnitus.
Stress (e.g., forced swim stress and restraint stress) has been shown to increase glutamate release in the medial prefrontal cortex, hippocampus, striatum, and nucleus accumbens in rats (Moghaddam 1993). Moreover, corticosterone application on rats hippocampal brain slices rapidly increased the glutamate release via MR (Karst et al. 2005). Although this phenomenon has not yet been investigated in the cochlea, it was demonstrated that spiral ganglion neurons (Furuta et al. 1994) and the inner hair cells express MR (Yao and Rarey 1996), making the glucocorticoid-mediated rapid glutamate release in the cochlea hypothetically possible. Such a quick release of glutamate could stimulate the auditory pathway without acoustic stimuli. Also, depending on a local concentration of freshly released glutamate, it could lead to excitotoxicity associated with peripheral deafferentation and tinnitus (Sahley and Nodar 2001).
4.3 The Sympathetic-Adreno-Medullar (SAM) Axis
The sympathetic-adreno-medullar axis is one of two stress axes, which alongside the HPA axis acts as a mediator for specific stress responses and adaptation to psychological and environmental stressors. The SAM axis mediates quick responses that activate fight or flight reaction. During that reaction, several tissues and organs necessary for survival are activated (e.g., muscles, heart, and respiratory function), whereas tissues and organs with tasks that are non-essential for survival (e.g., digestion) are suppressed at the same time. The SAM axis comprises the hypothalamus, sympathetic neurons, and catecholamines (epinephrine and norepinephrine) (Fig. 4). Similar to the HPA axis, there is a negative feedback system mediated by epinephrine, which extinguishes the SAM activation.
The schematic presentation of the sympathetic-adreno-medullar (SAM) axis. Stress activates the hypothalamus, which in turn activates sympathetic neurons. The sympathetic neurons projecting to the adrenal medulla induce epinephrine (adrenaline) release into the bloodstream. Epinephrine increases the supply of glucose and oxygen to the brain and muscles (flight or fight) and suppresses the body’s non-crisis functions (e.g., digestion). The sympathetic neurons themselves release the norepinephrine (noradrenaline) and, thus, activate all cells expressing adrenergic receptors. The effects of norepinephrine range from an increase in the blood volume pumped by the heart, expanding the respiratory pathway in the lungs, narrowing the blood vessels in non-essential organs, and pupil dilation
4.4 Potential Involvement of the Stress-Activated SAM Axis in Tinnitus Generation
4.4.1 Arterial Hypertension
Arterial hypertension has often been considered a cause of tinnitus. This causative relationship’s suggested mechanism is damage to cochlear microcirculation, induction of hearing loss, and deafferentation of the auditory periphery. Studies using spontaneously hypertensive (SH) and wild-type rats demonstrated differences in age-related hearing loss between the two rat strains (Borg and Viberg 1987). A progressive loss of the outer hair cells was observed in the SH but not wild-type rats already at the age of 3 months. A threshold shift in the high frequencies (16 and 24 kHz) was seen in the 21-month-old SH but not in wild-type rats.
Interestingly, the study scrutinizing the effect of noise on the cochlear vascular system in the SH and wild-type rats demonstrated dramatic differences between the two types of animals and suggested hypertension-induced cochlear vascular damage (Axelsson et al. 1983). In addition to vascular damage, arterial hypertension negatively affected the endocochlear potential (Mosnier et al. 2001). In agreement with that, several clinical studies have reported an association between arterial hypertension, hearing loss, and tinnitus (Figueiredo et al. 2015; Yang et al. 2015; Figueiredo et al. 2016). Also, a study with 80 tinnitus patients and 80 tinnitus-free subjects demonstrated that the nighttime systolic and diastolic blood pressure of tinnitus patients is higher than in the age-matched control subjects (Değirmenci et al. 2014), suggesting possible continuous upregulation of SAM axis.
4.4.2 Catecholamines
Catecholamines released during activation of the SAM axis mediate their effects through various receptors. One of those receptors is a G-protein coupled α2-adrenergic receptor, mediating vascular smooth muscle reaction, inhibiting the norepinephrine release, and platelets’ activation. The presence of α2-adrenergic receptors in cochlear microvasculature was verified in an animal model (gerbils), and α2-adrenergic stimulation provided experimental evidence for catecholamine-induced cochlear vasoconstriction (Carrasco et al. 1990). Moreover, vasoconstriction is associated with hypoxia or ischemia. In the inner ear, experimentally induced hypoxic and ischemic events lead to hair cell loss in an animal model (Shirane and Harrison 1987; Mazurek et al. 2003), followed by threshold shift (Sawada et al. 2001) and likely tinnitus. The expression of α2-adrenergic receptors was also demonstrated on the outer and inner hair cells and the supporting cells, the spiral ganglion neurons, stria vascularis, and all five types of fibrocytes in the cochleae of developing rats (Cai et al. 2013).
Brimonidine, an α2 adrenergic agonist (activator), protected the auditory hair cells from gentamicin-induced toxicity (Cortada et al. 2017). Surprisingly, inhibition of the α2a adrenergic receptor with istradefylline (α2 adrenergic antagonist) also protected the hair cells from glutamate excitotoxicity (Han et al. 2019). However, the above experiments were performed on an isolated organ of Corti and might not reflect the real-life situation, where the interplay of hypoxic/ischemic and toxic events and the protective mechanisms would yield an extrapolated role for α2-adrenergic receptors in the inner ear. Here too, more research should be performed to address the unanswered questions.
4.5 The Immune Axis
The immune system consists of specialized cells (neutrophils, eosinophils, basophils, lymphocytes, and monocytes) circulating in the blood, existing in primary lymphoid organs, or permanently residing in other tissues (e.g., resident macrophages). Under the steady-state condition, the immune system continually communicates with the endocrine and nervous systems, and there is a homeostatic balance between the three. During stress, the activation of the HPA and SAM axes leads to the release of mediators acting directly on the immune cells (Fig. 5), e.g., changing the number of circulating B cells (McGregor et al. 2016) or increasing the number of circulating T cells (Gupta et al. 2017). Also, chronic stress can change the activation status of immunocytes (Arranz et al. 2009) and modify cytokines’ release (Jung et al. 2019).
The mediators set free by activation of the HPA and SAM axes (corticosteroids, norepinephrine, and epinephrine) act directly on the immune cells and modify their release from the primary lymphoid organs, affect their migration and alter the cytokine expression patterns.
4.6 Potential Involvement of the Stress-Activated Immune Axis in Tinnitus Generation
4.6.1 A Direct Influence of Stress on the Immune Cells in the Cochlea
Several types of immune cells have been found in a steady-state, healthy cochlea of humans and animals (Hu et al. 2018). The cochlear immunocytes include resident macrophages (Hu et al. 2018; Liu et al. 2018; Kishimoto et al. 2019), NK cells (Iguchi et al. 1997), and T cells (Liu and Rask-Andersen 2019). Of all the immune cell types, resident macrophages’ cochlear function has been studied in the most detail. Macrophages phagocytose the damaged hair cells in Corti’s organ, thus preventing cochlear inflammation and consecutive hearing loss (Hirose et al. 2017). In addition to phagocytic features, cochlear macrophages possess still not well-understood repair abilities that enable regeneration of ribbon synapses after noise exposure (Kaur et al. 2019). It is tempting to speculate that cochlear macrophages’ activity might be impaired by the stress mediators, especially by corticosteroids. This impairment could be of a long duration during chronic stress and might result in reduced phagocytosis, migration, and a compromised spiral ganglion repair process upon acoustic injury. Macrophages also reside in the stria vascularis, where they regulate the cochlear intrastrial fluid–blood barrier. Here, long-term suppression of resident macrophages via stress hormones could result in modulation of endocochlear potential, which might induce hearing loss and tinnitus, similarly to what is observed during the aging process (Keithley 2020). Cochlear immunology is a rapidly developing field, and information is being published frequently, advancing our understanding of immune- and non-immune processes mediated by the cochlear immune cells.
4.6.2 Influence of Cytokines
Cytokines released by the immune and non-immune cells, such as interleukin 1-beta (IL-1beta), IL-6, and TNF-alpha, were shown to influence the plasticity in the brain and the peripheral nervous system (Aldskogius and Kozlova 1998; Levin and Godukhin 2017). The influence of the cytokines on the synaptic strength, plasticity, and integrity varies depending on the co-signaling molecules, the presence of other cells, and many other factors. Cytokines can act directly on neurons and different cell types (e.g., cochlear immunocytes), and the outcome ranges between cochlear regeneration and cell death (Barald et al. 2018). Studies using an animal model of noise-induced hearing loss (NIHL) indicated that NIHL induced the expression of proinflammatory cytokine tumor necrosis factor-alpha (TNF-alpha) in the cochlea (Frye et al. 2019) and was associated with the development of tinnitus (Wang et al. 2019) and that pharmacological intervention or using genetically modified mice prevented this detrimental development. Further, in an animal model of salicylate-induced tinnitus, increased expression of genes encoding TNF-alpha and IL-1-beta was observed in the cochlea and correlated with behaviorally tested tinnitus (Hwang et al. 2011). In a sample of 30 patients with chronic tinnitus, a correlation was found between TNF-alpha in serum and tinnitus loudness, total perceived stress, tension, and depression (Szczepek et al. 2014). Another study investigating tinnitus in the elderly noted a negative association between IL-10 and tinnitus loudness and duration (Haider et al. 2020). It remains to be clarified if cytokines’ systemic concentration reflects that in the cochlea and how aging, inflammation, and infections may affect this balance.
5 Summary and Conclusions
Tinnitus is a symptom that may arise as a result of various changes in the auditory system. Persons with disturbing tinnitus perceive it as an unpleasant, distressing signal that negatively affects life quality, associates with anxiety and depression, and may last a lifetime. The accepted view on the primary mechanism inducing tinnitus is that it is a consequence of a cochlear lesion, which could have been caused by noise, ototoxic medications, the physiological aging process, or other means. Here, the evidence was reviewed for the emotional stress-induced mechanisms, which could contribute to cochlear pathologies and, in consequence, to tinnitus (Fig. 6). These mechanisms involve HPA axis-induced corticosteroid action on MR and GR, possibly leading to glutamate excitotoxicity and altered gene expression in the cochlea. The immune axis of stress can also be affected by the HPA axis, leading to modulation of the resident cochlear macrophages’ function. Lastly, corticosteroids released upon HPA activation could contribute to the NMDA/AMPA disbalance. The SAM axis might increase blood pressure that induces degenerative changes in the cochlear microvasculature and changes in the organism’s immune cells’ repertoires, affecting cochlear immune cells.
Furthermore, SAM-induced vasoconstriction in the cochlea might likely cause hypoxia/ischemia, detrimental to the auditory hair cells and spiral ganglion neurons. It is concluded that all of the presented mechanisms need to be further investigated in the animal or ex vivo models. It is recommended for clinical practitioners to collect information about stressful events or chronic stress preceding the tinnitus onset. Furthermore, knowing the vital signs could add information to the stress-related status of a patient. Finally, taking into account that tinnitus itself acts as a stressor, the implementation of anti-stress therapies for tinnitus treatment is recommended.
References
Adoga AA, Adoga AS, Obindo JT (2008) Tinnitus and the prevalence of co-morbid psychological stress. Niger J Med 17:95–97
Aldskogius H, Kozlova EN (1998) Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol 55:1–26
Altschuler RA, Halsey K, Kanicki A, Martin C, Prieskorn D, Deremer S, Dolan DF (2019) Small arms fire-like noise: effects on hearing loss, gap detection and the influence of preventive treatment. Neuroscience 407:32–40
Arranz L, De Vicente A, Muñoz M, De La Fuente M (2009) Impaired immune function in a homeless population with stress-related disorders. Neuroimmunomodulation 16:251–260
Axelsson A, Borg E, Hornstrand C (1983) Noise effects on the cochlear vasculature in normotensive and spontaneously hypertensive rats. Acta Otolaryngol 96:215–225
Aydin N, Searchfield GD (2019) Changes in tinnitus and physiological biomarkers of stress in response to short-term broadband noise and sounds of nature. Complement Ther Med 46:62–68
Baigi A, Oden A, Almlid-Larsen V, Barrenäs ML, Holgers KM (2011) Tinnitus in the general population with a focus on noise and stress: a public health study. Ear Hear 32:787–789
Barald KF, Shen YC, Bianchi LM (2018) Chemokines and cytokines on the neuroimmunoaxis: inner ear neurotrophic cytokines in development and disease. Prospects for repair? Exp Neurol 301:92–99
Bertolaso L, Martini A, Bindini D, Lanzoni I, Parmeggiani A, Vitali C, Kalinec G, Kalinec F, Capitani S, Previati M (2001) Apoptosis in the OC-k3 immortalized cell line treated with different agents. Audiology 40:327–335
Besteher B, Gaser C, Ivanšić D, Guntinas-Lichius O, Dobel C, Nenadić I (2019) Chronic tinnitus and the limbic system: reappraising brain structural effects of distress and affective symptoms. Neuroimage Clin 24:101976
Betz LT, Muhlberger A, Langguth B, Schecklmann M (2017) Stress reactivity in chronic tinnitus. Sci Rep 7:41521
Bhatt JM, Bhattacharyya N, Lin HW (2017) Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 127:466–469
Biehl R, Boecking B, Brueggemann P, Grosse R, Mazurek B (2019) Personality traits, perceived stress, and tinnitus-related distress in patients with chronic tinnitus: support for a vulnerability-stress model. Front Psychol 10:3093
Borg E, Viberg A (1987) Age-related hair cell loss in spontaneously hypertensive and normotensive rats. Hear Res 30:111–118
Brueggemann P, Seydel C, Schaefer C, Szczepek AJ, Amarjargal N, Boecking B, Rose M, Mazurek B (2019) ICD-10 symptom rating questionnaire for assessment of psychological comorbidities in patients with chronic tinnitus. HNO 67:46–50
Bruggemann P, Szczepek AJ, Rose M, Mckenna L, Olze H, Mazurek B (2016) Impact of multiple factors on the degree of tinnitus distress. Front Hum Neurosci 10:341
Bryant RA (2018) The current evidence for acute stress disorder. Curr Psychiatry Rep 20:111
Cai J, Li J, Mao Y, Bai X, Xu L, Wang H (2013) Immunohistochemical localization of α2-adrenergic receptors in the neonatal rat cochlea and the vestibular labyrinth. J Mol Neurosci 51:1010–1020
Canlon B, Theorell T, Hasson D (2013) Associations between stress and hearing problems in humans. Hear Res 295:9–15
Cannon WB (1922) Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. D. Appleton
Carrasco VN, Prazma J, Faber JE, Triana RJ, Pillsbury HC (1990) Cochlear microcirculation. Effect of adrenergic agonists on arteriole diameter. Arch Otolaryngol Head Neck Surg 116:411–417
Caspary DM, Llano DA (2017) Auditory thalamic circuits and GABA(A) receptor function: putative mechanisms in tinnitus pathology. Hear Res 349:197–207
Conrad I, Kleinstauber M, Jasper K, Hiller W, Andersson G, Weise C (2015) The role of dysfunctional cognitions in patients with chronic tinnitus. Ear Hear 36
Cortada M, Levano S, Bodmer D (2017) Brimonidine protects auditory hair cells from in vitro-induced toxicity of gentamicin. Audiol Neurootol 22:125–134
Craske MG, Stein MB (2016) Anxiety. Lancet 388:3048–3059
Crawley BK, Keithley EM (2011) Effects of mitochondrial mutations on hearing and cochlear pathology with age. Hear Res 280:201–208
Cui W, Wang H, Cheng Y, Ma X, Lei Y, Ruan X, Shi L, Lv M (2019) Long-term treatment with salicylate enables NMDA receptors and impairs AMPA receptors in C57BL/6J mice inner hair cell ribbon synapse. Mol Med Rep 19:51–58
Curtis J (1841) Tinnitus aurium. Lancet 36:828–829
Danioth L, Brotschi G, Croy I, Friedrich H, Caversaccio MD, Negoias S (2020) Multisensory environmental sensitivity in patients with chronic tinnitus. J Psychosom Res 135:110155
Değirmenci H, Bakırcı EM, Salcan İ, Demirelli S, Duman H, Ceyhun G, Küçüksu Z (2014) Determination of correlation among heart rate variability, left atrium global strain, and nighttime blood pressure among patients with tinnitus. Med Sci Monit 20:1714–1719
Desa DE, Nichols MG, Smith HJ (2018) Aminoglycosides rapidly inhibit NAD(P)H metabolism increasing reactive oxygen species and cochlear cell demise. J Biomed Opt 24:1–14
Eggermont JJ (1990) On the pathophysiology of tinnitus; a review and a peripheral model. Hear Res 48:111–123
Eggermont JJ, Roberts LE (2015) Tinnitus: animal models and findings in humans. Cell Tissue Res 361:311–336
El Ganzoury MM, Kamel TB, Khalil LH, Seliem AM (2012) Cochlear dysfunction in children following cardiac bypass surgery. ISRN Pediatr 2012:375038
Evans P, Halliwell B (1999) Free radicals and hearing. Cause, consequence, and criteria. Ann N Y Acad Sci 884:19–40
Figueiredo RR, Azevedo AA, Penido NO (2016) Positive association between tinnitus and arterial hypertension. Front Neurol 7:171
Figueiredo RR, De Azevedo AA, Penido Nde O (2015) Tinnitus and arterial hypertension: a systematic review. Eur Arch Otorhinolaryngol 272:3089–3094
Fischel-Ghodsian N, Prezant TR, Chaltraw WE, Wendt KA, Nelson RA, Arnos KS, Falk RE (1997) Mitochondrial gene mutation is a significant predisposing factor in aminoglycoside ototoxicity. Am J Otolaryngol 18:173–178
Fortier C, Selye H (1949) Adrenocorticotrophic effect of stress after severance of the hypothalamo-hypophyseal pathways. Am J Physiol 159:433–439. illust
Frye MD, Ryan AF, Kurabi A (2019) Inflammation associated with noise-induced hearing loss. J Acoust Soc Am 146:4020
Furuta H, Mori N, Sato C, Hoshikawa H, Sakai S, Iwakura S, Doi K (1994) Mineralocorticoid type I receptor in the rat cochlea: mRNA identification by polymerase chain reaction (PCR) and in situ hybridization. Hear Res 78:175–180
Gauvin DV, Yoder J, Zimmermann ZJ, Tapp R (2018) Ototoxicity: the radical drum beat and rhythm of cochlear hair cell life and death. Int J Toxicol 37:195–206
Gomaa MA, Elmagd MH, Elbadry MM, Kader RM (2014) Depression, anxiety and stress scale in patients with tinnitus and hearing loss. Eur Arch Otorhinolaryngol 271:2177–2184
Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ (2017) Tinnitus with a normal audiogram: relation to noise exposure but no evidence for cochlear synaptopathy. Hear Res 344:265–274
Gupta MA, Jarosz P, Gupta AK (2017) Posttraumatic stress disorder (PTSD) and the dermatology patient. Clin Dermatol 35:260–266
Haider HF, Bojić T, Ribeiro SF, Paço J, Hall DA, Szczepek AJ (2018) Pathophysiology of subjective tinnitus: triggers and maintenance. Front Neurosci 12
Haider HF, Ribeiro SF, Martins C, Ribeiro D, Trigueiros N, Szczepek AJ, Caria H, Hoare DJ, Paço J, Borrego LM (2020) Tinnitus, hearing loss and inflammatory processes in an older portuguese population. Int J Audiol 59:323–332
Han BR, Lin SC, Espinosa K, Thorne PR, Vlajkovic SM (2019) Inhibition of the adenosine A(2A) receptor mitigates excitotoxic injury in organotypic tissue cultures of the rat cochlea. Cell 8
Hasson D, Theorell T, Bergquist J, Canlon B (2013) Acute stress induces hyperacusis in women with high levels of emotional exhaustion. PLoS One 8:e52945
Hébert S, Canlon B, Hasson D, Magnusson Hanson LL, Westerlund H, Theorell T (2012) Tinnitus severity is reduced with reduction of depressive mood – a prospective population study in Sweden. PLoS One 7:e37733
Hébert S, Lupien SJ (2007) The sound of stress: blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neurosci Lett 411:138–142
Heim C, Nemeroff CB (2009) Neurobiology of posttraumatic stress disorder. CNS Spectr 14:13–24
Hermann R, Biallas B, Predel HG, Petrowski K (2019) Physical versus psychosocial stress: effects on hormonal, autonomic, and psychological parameters in healthy young men. Stress 22:103–112
Herr RM, Bosch JA, Theorell T, Loerbroks A (2018) Bidirectional associations between psychological distress and hearing problems: an 18-year longitudinal analysis of the British household panel survey. Int J Audiol 57:816–824
Hirose K, Rutherford MA, Warchol ME (2017) Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hear Res 352:70–81
Hoang KN, Dinh CT, Bas E, Chen S, Eshraghi AA, Van De Water TR (2009) Dexamethasone treatment of naïve organ of corti explants alters the expression pattern of apoptosis-related genes. Brain Res 1301:1–8
Horner K (2003) The emotional ear in stress. Neurosci Biobehav Rev 27:437–446
Hu BH, Zhang C, Frye MD (2018) Immune cells and non-immune cells with immune function in mammalian cochleae. Hear Res 362:14–24
Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, Lefebvre PP, Malgrange B, Kopke R, Moonen G, Van De Water TR (2000) Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci 18:259–270
Hunter RG, Seligsohn M, Rubin TG, Griffiths BB, Ozdemir Y, Pfaff DW, Datson NA, Mcewen BS (2016) Stress and corticosteroids regulate rat hippocampal mitochondrial DNA gene expression via the glucocorticoid receptor. Proc Natl Acad Sci U S A 113:9099–9104
Hwang JH, Chen JC, Yang SY, Wang MF, Chan YC (2011) Expression of tumor necrosis factor-α and interleukin-1β genes in the cochlea and inferior colliculus in salicylate-induced tinnitus. J Neuroinflammation 8:30
Iguchi H, Yamane H, Konishi K, Nakagawa T, Shibata S, Takayama M, Nishimura K, Sunami K, Nakai Y (1997) Asialo GM1-positive cells in mouse cochlea. Acta Otolaryngol Suppl 528:6–9
Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:221–254
Jung YH, Shin NY, Jang JH, Lee WJ, Lee D, Choi Y, Choi SH, Kang DH (2019) Relationships among stress, emotional intelligence, cognitive intelligence, and cytokines. Medicine (Baltimore) 98:e15345
Kapolowicz MR, Thompson LT (2020) Plasticity in limbic regions at early time points in experimental models of tinnitus. Front Syst Neurosci 13
Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M (2005) Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A 102:19204–19207
Kaur T, Clayman AC, Nash AJ, Schrader AD, Warchol ME, Ohlemiller KK (2019) Lack of fractalkine receptor on macrophages impairs spontaneous recovery of ribbon synapses after moderate noise trauma in C57BL/6 mice. Front Neurosci 13:620
Keithley EM (2020) Pathology and mechanisms of cochlear aging. J Neurosci Res 98(9):1674–1684. https://doi.org/10.1002/jnr.24439. Epub 2019 May 7
Kil SH, Kalinec F (2013) Expression and dexamethasone-induced nuclear translocation of glucocorticoid and mineralocorticoid receptors in guinea pig cochlear cells. Hear Res 299:63–78
Kim KX, Payne S, Yang-Hood A, Li SZ, Davis B, Carlquist J, V-Ghaffari B, Gantz JA, Kallogjeri D, Fitzpatrick JAJ, Ohlemiller KK, Hirose K, Rutherford MA (2019) Vesicular glutamatergic transmission in noise-induced loss and repair of cochlear ribbon synapses. J Neurosci 39:4434–4447
Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
Kishimoto I, Okano T, Nishimura K, Motohashi T, Omori K (2019) Early development of resident macrophages in the mouse cochlea depends on Yolk Sac hematopoiesis. Front Neurol 10:1115
Kokotas H, Petersen MB, Willems PJ (2007) Mitochondrial deafness. Clin Genet 71:379–391
Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, De Ridder D, Hajak G (2009) Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage 46:213–218
Le TN, Straatman LV, Lea J, Westerberg B (2017) Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg 46:41
Leaver AM, Seydell-Greenwald A, Rauschecker JP (2016) Auditory-limbic interactions in chronic tinnitus: challenges for neuroimaging research. Hear Res 334:49–57
Lechowicz U, Pollak A, Raj-Koziak D, Dziendziel B, Skarżyński PH, Skarżyński H, Ołdak M (2018) Tinnitus in patients with hearing loss due to mitochondrial DNA pathogenic variants. Eur Arch Otorhinolaryngol 275:1979–1985
Levin SG, Godukhin OV (2017) Modulating effect of cytokines on mechanisms of synaptic plasticity in the brain. Biochemistry (Mosc) 82:264–274
Liberman MC, Kujawa SG (2017) Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res 349:138–147
Lie A, Skogstad M, Johannessen HA, Tynes T, Mehlum IS, Nordby KC, Engdahl B, Tambs K (2016) Occupational noise exposure and hearing: a systematic review. Int Arch Occup Environ Health 89:351–372
Liu W, Molnar M, Garnham C, Benav H, Rask-Andersen H (2018) Macrophages in the human cochlea: saviors or predators – a study using super-resolution immunohistochemistry. Front Immunol 9:223
Liu W, Rask-Andersen H (2019) Super-resolution immunohistochemistry study on CD4 and CD8 cells and the relation to macrophages in human cochlea. J Otol 14:1–5
Liu Y, Niu H, Zhu J, Zhao P, Yin H, Ding H, Gong S, Yang Z, Lv H, Wang Z (2019) Morphological neuroimaging biomarkers for tinnitus: evidence obtained by applying machine learning. Neural Plast 2019:1712342
Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW (1998) The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50:114–120
Lupien SJ, Mcewen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
Martin S, Henley JM, Holman D, Zhou M, Wiegert O, Van Spronsen M, Joëls M, Hoogenraad CC, Krugers HJ (2009) Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One 4:e4714
Mazurek B, Boecking B, Brueggemann P (2019) Association between stress and tinnitus-new aspects. Otol Neurotol 40:e467–e473
Mazurek B, Haupt H, Joachim R, Klapp BF, Stöver T, Szczepek AJ (2010a) Stress induces transient auditory hypersensitivity in rats. Hear Res 259:55–63
Mazurek B, Stöver T, Haupt H, Klapp B, Adli M, Gross J, Szczepek A (2010b) The significance of stress: its role in the auditory system and the pathogenesis of tinnitus. HNO 58:162–172
Mazurek B, Szczepek A, Hebert S (2015) Stress and tinnitus. HNO 63:258–265
Mazurek B, Winter E, Fuchs J, Haupt H, Gross J (2003) Susceptibility of the hair cells of the newborn rat cochlea to hypoxia and ischemia. Hear Res 182:2–8
Mcgregor BA, Murphy KM, Albano DL, Ceballos RM (2016) Stress, cortisol, and B lymphocytes: a novel approach to understanding academic stress and immune function. Stress 19:185–191
Michaelides A, Zis P (2019) Depression, anxiety and acute pain: links and management challenges. Postgrad Med 131:438–444
Misiewicz Z, Iurato S, Kulesskaya N, Salminen L, Rodrigues L, Maccarrone G, Martins J, Czamara D, Laine MA, Sokolowska E, Trontti K, Rewerts C, Novak B, Volk N, Park DI, Jokitalo E, Paulin L, Auvinen P, Voikar V, Chen A, Erhardt A, Turck CW, Hovatta I (2019) Multi-omics analysis identifies mitochondrial pathways associated with anxiety-related behavior. PLoS Genet 15:e1008358
Moghaddam B (1993) Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem 60:1650–1657
Moossavi A, Sadeghijam M, Akbari M (2019) The hypothetical relation between the degree of stress and auditory cortical evoked potentials in tinnitus sufferers. Med Hypotheses 130:109266
Mosnier I, Teixeira M, Loiseau A, Fernandes I, Sterkers O, Amiel C, Ferrary E (2001) Effects of acute and chronic hypertension on the labyrinthine barriers in rat. Hear Res 151(1–2):227–236. https://doi.org/10.1016/s0378-5955(00)00229-x
Mostafa H, Saad M, El-Attar A, Ahmed G, Berrettini S, Forli F, Siciliano G, Mancuso M (2014) Mitochondrial DNA (mtDNA) haplotypes and dysfunctions in presbyacusis. Acta Otorhinolaryngol Ital 34:54–61
Mühlau M, Rauschecker JP, Oestreicher E, Gaser C, Röttinger M, Wohlschläger AM, Simon F, Etgen T, Conrad B, Sander D (2006) Structural brain changes in tinnitus. Cereb Cortex 16:1283–1288
National Guideline (2020) Evidence review for psychological therapies: tinnitus: assessment and management: evidence review L. National Institute for Health and Care Excellence (UK), London. Copyright © NICE 2020
O’reilly M, Young L, Kirkwood NK, Richardson GP, Kros CJ, Moore AL (2019) Gentamicin affects the bioenergetics of isolated mitochondria and collapses the mitochondrial membrane potential in cochlear sensory hair cells. Front Cell Neurosci 13:416
Op De Beeck K, Schacht J, Van Camp G (2011) Apoptosis in acquired and genetic hearing impairment: the programmed death of the hair cell. Hear Res 281:18–27
Oyola MG, Handa RJ (2017) Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress 20:476–494
Pickles JO (2004) Mutation in mitochondrial DNA as a cause of presbyacusis. Audiol Neurootol 9:23–33
Pienkowski M (2018) Prolonged exposure of CBA/Ca mice to moderately loud noise can cause cochlear synaptopathy but not tinnitus or hyperacusis as assessed with the acoustic startle reflex. Trends Hear 22:2331216518758109
Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B (2010) Oxidative stress in the cochlea: an update. Curr Med Chem 17:3591–3604
Polman JAE, Welten JE, Bosch DS, De Jonge RT, Balog J, Van Der Maarel SM, De Kloet ER, Datson NA (2012) A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci 13:118
Probst T, Pryss R, Langguth B, Schlee W (2016) Emotional states as mediators between tinnitus loudness and tinnitus distress in daily life: results from the “TrackYourTinnitus” application. Sci Rep 6:20382
Puel JL, Ruel J, Guitton M, Wang J, Pujol R (2002) The inner hair cell synaptic complex: physiology, pharmacology and new therapeutic strategies. Audiol Neurootol 7:49–54
Qu T, Qi Y, Yu S, Du Z, Wei W, Cai A, Wang J, Nie B, Liu K, Gong S (2019) Dynamic changes of functional neuronal activities between the auditory pathway and limbic systems contribute to noise-induced tinnitus with a normal audiogram. Neuroscience 408:31–45
Ralli M, Troiani D, Podda MV, Paciello F, Eramo SL, De Corso E, Salvi R, Paludetti G, Fetoni AR (2014) The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta Otorhinolaryngol Ital 34:198–204
Rauschecker JP, Leaver AM, Mühlau M (2010) Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66:819–826
Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM (2009) Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res 19:2163–2171
Ryan D, Bauer CA (2016) Neuroscience of tinnitus. Neuroimaging Clin N Am 26:187–196
Rybak LP, Mukherjea D, Ramkumar V (2019) Mechanisms of cisplatin-induced ototoxicity and prevention. Semin Hear 40:197–204
Sahley TL, Hammonds MD, Musiek FE (2013) Endogenous dynorphins, glutamate and N-methyl-d-aspartate (NMDA) receptors may participate in a stress-mediated type-I auditory neural exacerbation of tinnitus. Brain Res 1499:80–108
Sahley TL, Nodar RH (2001) A biochemical model of peripheral tinnitus. Hear Res 152:43–54
Salviati M, Bersani FS, Terlizzi S, Melcore C, Panico R, Romano GF, Valeriani G, Macri F, Altissimi G, Mazzei F, Testugini V, Latini L, Delle Chiaie R, Biondi M, Cianfrone G (2014) Tinnitus: clinical experience of the psychosomatic connection. Neuropsychiatr Dis Treat 10:267–275
Sawada S, Mori N, Mount RJ, Harrison RV (2001) Differential vulnerability of inner and outer hair cell systems to chronic mild hypoxia and glutamate ototoxicity: insights into the cause of auditory neuropathy. J Otolaryngol 30:106–114
Seifert E, Lamprecht-Dinnesen A, Asfour B, Rotering H, Bone HG, Scheld HH (1998) The influence of body temperature on transient evoked otoacoustic emissions. Br J Audiol 32:387–398
Selye H (1937) The significance of the adrenals for adaptation. Science 85:247–248
Selye H (1950) Stress and the general adaptation syndrome. Br Med J 1:1383–1392
Selye H, Fortier C (1949) Adaptive reactions to stress. Res Publ Assoc Res Nerv Ment Dis 29:3–18
Sheth S, Mukherjea D, Rybak LP, Ramkumar V (2017) Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci 11:338
Shirane M, Harrison RV (1987) The effects of hypoxia on sensory cells of the cochlea in chinchilla. Scanning Microsc 1:1175–1183
Slavich GM, Shields GS (2018) Assessing lifetime stress exposure using the stress and adversity inventory for adults (adult STRAIN): an overview and initial validation. Psychosom Med 80:17–27
Sliwinska-Kowalska M, Davis A (2012) Noise-induced hearing loss. Noise Health 14:274–280
Szczepek A, Mazurek B (2017) Tinnitus and stress. Springer, Cham
Szczepek AJ, Haupt H, Klapp BF, Olze H, Mazurek B (2014) Biological correlates of tinnitus-related distress: an exploratory study. Hear Res 318:23–30
Tahera Y, Meltser I, Johansson P, Canlon B (2006a) Restraint stress modulates glucocorticoid receptors and nuclear factor kappa B in the cochlea. Neuroreport 17:879–882
Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B (2006b) Glucocorticoid receptor and nuclear factor-kappa B interactions in restraint stress-mediated protection against acoustic trauma. Endocrinology 147:4430–4437
Ten Cate WJ, Curtis LM, Small GM, Rarey KE (1993) Localization of glucocorticoid receptors and glucocorticoid receptor mRNAs in the rat cochlea. Laryngoscope 103:865–871
Waechter S, Brännström KJ (2015) The impact of tinnitus on cognitive performance in normal-hearing individuals. Int J Audiol 54(11):845–851
Wang W, Zhang LS, Zinsmaier AK, Patterson G, Leptich EJ, Shoemaker SL, Yatskievych TA, Gibboni R, Pace E, Luo H, Zhang J, Yang S, Bao S (2019) Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol 17:e3000307
Yang P, Ma W, Zheng Y, Yang H, Lin H (2015) A systematic review and meta-analysis on the association between hypertension and tinnitus. Int J Hypertens 2015:583493
Yao X, Rarey KE (1996) Localization of the mineralocorticoid receptor in rat cochlear tissue. Acta Otolaryngol 116:493–496
Yip J, Geng X, Shen J, Ding Y (2016) Cerebral gluconeogenesis and diseases. Front Pharmacol 7:521
Yu C-Y, Mayba O, Lee JV, Tran J, Harris C, Speed TP, Wang J-C (2010) Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One 5:e15188
Zirke N, Seydel C, Arsoy D, Klapp BF, Haupt H, Szczepek AJ, Olze H, Goebel G, Mazurek B (2013) Analysis of mental disorders in tinnitus patients performed with composite international diagnostic interview. Qual Life Res 22:2095–2104
Zöger S, Svedlund J, Holgers KM (2006) Relationship between tinnitus severity and psychiatric disorders. Psychosomatics 47:282–288
Zuo J, Curtis LM, Yao X, Ten Cate WJ, Bagger-Sjöbäck D, Hultcrantz M, Rarey KE (1995) Glucocorticoid receptor expression in the postnatal rat cochlea. Hear Res 87:220–227
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Szczepek, A.J., Mazurek, B. (2021). Neurobiology of Stress-Induced Tinnitus. In: Searchfield, G.D., Zhang, J. (eds) The Behavioral Neuroscience of Tinnitus. Current Topics in Behavioral Neurosciences, vol 51. Springer, Cham. https://doi.org/10.1007/7854_2020_215
Download citation
DOI: https://doi.org/10.1007/7854_2020_215
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85502-4
Online ISBN: 978-3-030-85503-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)