Abstract
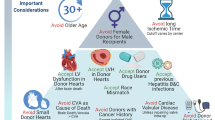
Considering the exponential increase in organ demand with a stable donor supply, cardiac donor management and selection is of utmost importance. The criteria for an acceptable donor have changed dramatically over the last 40 years and transplant teams are accepting older patients, longer ischemic times, donor substance abuse, and sometimes donor infection. Expanding the donor pool to the “increased risk” donors has enforced a more complex balance of donor and recipient components. A risk benefit ratio is commonly explored to provide the best donor to the more stable patient and accept an increased-risk donor in the patient with a shorter life expectancy. Patients with more urgent status designation include those on veno-arterial extracorporeal membrane oxygenation (VA ECMO), with surgically implanted ventricular-assist devices and nondischargeable status or life threatening ventricular arrhythmias. Recently, use of VA ECMO as a bridge to heart transplant has expanded moving patients to the top of the waiting list. The intricacy of this risk-benefit balance will be highlighted in this chapter to provide optimal cardiac transplant outcomes to as many patients as possible and use all resources to their full potential.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Since the first cardiac transplant was performed in 1967, the introduction and improvement of immunosuppressive drugs have contributed to increased success worldwide (John et al. 2000). Currently, more than 2300 cardiac transplants are performed in the United States annually. Additionally, ventricular assist devices have been established as a viable option for heart failure patients as a bridge to transplant (BTT). These accomplishments have led to a longer waiting list of recipients for cardiac transplant; however, the organ donor supply is consistent. Considering this challenge, donor selection must be performed methodically in order to achieve a favorable prognosis. The selection criteria have broadened due to increased demand for donor hearts. Now included for consideration and evaluation are hearts that are older, unstable, and susceptible to longer ischemic times (Brock et al. 2001). Using such donor hearts necessitates assessing the risk/benefit ratio associated with the cardiac transplant operation and postoperative outcomes versus the mortality and morbidity risk of the recipient remaining on the waiting list (John et al., “Donor management…” 2004).
Donor Selection Overview
Heart failure patients are eligible to become transplant candidates when they meet the criteria outlined by the United Network for Organ Sharing (UNOS). The preliminary donor assessment is performed to determine a potential donor match. The initial evaluation includes confirmation and mechanism of brain death, consent for donation, ABO type and screen, and geographic location (Kilic et al. 2014). Ideally, the cardiac donor will not have the following: penetrating cardiac trauma, known cardiac disease, prolonged cardiac arrest (>15 min), human immunodeficiency virus, or an extra cranial malignancy (Edwards et al. 2005). However, recent experience has shown that a prolonged history of cardiac arrest, up to 1 h, may not exclude young donors under the age of 30. Often with hormone therapy, such as T4 (levothyroxine) infusion, the ejection fraction (EF) of such donor hearts has improved from below 30–40% to above 55%.
ABO Compatibility and Panel Reactive Antibodies
When selecting a donor recipient match, ABO blood group compatibility is the essential first consideration. Conversely, the Rhesus blood group does not have to match. When compared to ABO-identical grafts (i.e., A-recipient with A-donor), ABO-compatible (i.e., A-recipient with O-donor) adult hearts do not result in unfavorable outcomes for graft survival and incidence of acute rejection (Jawitz et al. 2013).
All recipients on the waiting list are tested for panel reactive antibody (PRA) ) , which is repeated monthly. When there is a > 10% reactivity to the testing panel, a prospective cross-match will be requested at the time of provisional donation. Some recipients with <10% reactivity may still require cross-match due to history of pregnancy, exposure to blood products, or previous surgery. If a recipient has >10% reactivity and the donor hospital distance is too far for a prospective cross-match, a “virtual cross-match” can be performed by comparing the recipients HLA antibody specificity profile to the HLA type of the potential donor.
Donor Assessment Studies
Electrocardiogram (EKG), cardiac enzymes, echocardiogram, and often coronary catheterization will be necessary for cardiac donor evaluation. The quality of the study and interpretation can differ between institutions. It is recommended the transplant program evaluate the donor EKGs, TTE/TEEs, and request repeat examinations as needed.
A 12-lead EKG must be performed on all donors. Abnormal EKG rhythms such as bundle branch block or ST wave changes are common findings leading to declination of a donor heart (Khasati et al. 2007). However, abnormal EKG findings are often a result of brain death catecholamine surges which are uninhibited by central vagal input. This sympathetic response is known to cause acute myocardial dysfunction. Appropriate hemodynamic management can improve EKG and echocardiographic findings (Allan et al. 2014).
Cardiac enzymes should be measured on all donors since sustained elevation suggests severe myocardial injury. An initial rise in cardiac enzymes may be due to CPR-induced myocardial trauma. Transient elevations may be due to hypoxic injury to other organs. It is therefore important to correlate abnormal enzyme values with EKG, echocardiogram and, in some cases, cardiac catheterization (Cooper et al. 2007). A donor heart should not be accepted without resolution of abnormal cardiac enzymes.
It is important that the initial echocardiogram, whether transthoracic (TTE) or trans esophageal (TEE) is performed after conventional management has taken place. The volume status should be adjusted to a CVP 6–10 mmHg, the pH 7.4–7.45, Hgb >10 g/dL, and MAP>60. While an initial TTE can be used to screen for abnormalities such as poor EF, substantial left ventricular hypertrophy (LVH), and aortic insufficiency, a TEE may also be required to assess the other valves, particularly mitral, and rule out congenital lesions and regional wall abnormalities. If the EF is <45%, this may be due to catecholamine depletion after initial surge leading to severely reduced vascular resistance and myocardial shock (Kilic et al. 2014) A trial of hormonal resuscitation and evaluation with placement of a pulmonary artery catheter for hemodynamic management may help improve the EF and result in a suitable donor heart.
Coronary angiography is usually required for donors over the age of 40. It is beneficial to request catheterization in patients over the age of 30–35 years when there is history of significant hypertension, smoking, diabetes, cocaine use, or regional wall motion abnormalities on echocardiogram. If coronary catheterization is not available in the donor institution, a CT angiogram can be performed. Direct coronary palpation for evaluation during procurement is not reliable, as ostial lesions cannot be evaluated.
There should be no evidence of active infection (i.e., fever, leukocytosis, chest x-ray suggesting pneumonia, positive blood cultures). Historically, an acceptable donor must have negative serologies including Hepatitis C antibody, Hepatitis B surface antigen, HIV, and HTLV 1 & 2.
In order to expand the donor pool, Hepatitis C virus Antibody (HCVAb) positive donor hearts have recently been used for transplant to consenting recipients. Donors who have positive HCVAb undergo Nucleic Acid Testing (NAT) to test for acute HCV infection. Both HCVAb +/NAT- and HCVAb +/NAT+ donors have been used for transplant. The recipients of the HCVAb+/NAT- hearts have not shown to develop a detectable HCV viral load up to 6 months postoperatively (Patel et al. 2018). Those patients receiving HCVAb+/NAT+ hearts who acquire HCV post-heart transplant subsequently undergo direct-acting antiviral therapies (DAAs) to cure the transmitted HCV. This has been suggested as a potential approach to safely broaden the donor pool (Schlendorf et al. 2018).
Donor/Recipient Match: Standard Parameters
Gender
Recently data has shown a significantly worse outcome in donor-recipient gender mismatch. More specifically, male recipients of female hearts have the poorest long-term outcomes on a multivariate analysis (Peled et al. 2017). The International Society for Heart and Lung Transplant data for female allograft allocation to a male recipient did not affect 1-year survival but was associated with higher 5-year mortality (Costanzo et al. 2010). In addition, female recipients, regardless of donor gender, have a significantly higher risk of rejection and renal dysfunction at 1 year (Stehlik et al. 2011). Gender matching has been recommended as recent literature has shown an impact on major outcomes following heart transplant. However, many successful institutions do not use gender mismatch alone as a predictor of rejection or survival outcomes. It is recommended to place a greater emphasis on the importance of size matching.
Size
Guidelines from ISHLT are primarily based on expert opinion and it is recommend that the heart from a donor weighing <70% of the intended recipient’s body weight should not be accepted (Costanzo et al. 2010). However, expert opinions also state that body weight alone does not correlate well with true adult cardiac size on echocardiogram and should not be used as an exclusion criterion for a donor heart (Chan et al. 1991). In an attempt to expand the appropriate donor pool, rather than rely on a strict weight difference requirement, an alternative approach can be used for evaluation.
With respect to weight difference, if there is greater than 30% discrepancy, it is recommended to perform specific LVEDD measurements via TEE. In general, when matching female donors to male recipients, female donors can be accepted with LVEDD >3.8 for recipients with normal pulmonary pressures, and LVEDD >4.2 for recipients with moderately elevated pulmonary pressures.
With respect to height differences, donors who are up to 6 inches shorter may be accepted for a recipient with no prior cardiac operations. For those recipients who have had prior sternotomies and possible significant scarring that may result in shortened/contractured great vessel cuffs, the donor should be no more than 4–5 inches shorter than the intended recipient. When evaluating a donor who is >3–4 inches taller than a recipient, a CT scan can be used to measure and compare the longitudinal axis distance from pulmonary valve to the diaphragmatic edge of the right ventricle.
Recent findings suggest that predicted heart mass (PHM), which is the sum of predicted right and left ventricular mass, may provide better size matching in cardiac transplantation than total body weight (TBW). Analysis confirmed that undersizing donor hearts by PHM, but not by TBW, was predictive of moderate to severe primary graft dysfunction and 90-day post-heart transplant mortality (Gong et al. 2018; Kransdorf et al. 2017). Most programs will not accept an undersized heart with a donor to recipient ratio less than 0.8–0.85 using this formula.
Age and Ischemic Time
Multiple publications have concluded that age is not an independent variable affecting postoperative survival (Schüler et al. 1989; Alexander and Vaughn 1991; Tenderich et al. 1998). The donor age requirement varies between institutions; reasonable donor criteria includes age less than 55 years. It is recommended to exert caution with regard to ischemic time when considering an older (>40yo) donor heart. In younger donors (<40yo), it is reasonable to allow for an ischemic time greater than 4 h. In contrast, if the donor heart is older, it is appropriate to ensure an ischemic time of less than 4–1/2 hours.
Cardiac and Vasoactive Medications
Donors commonly require vasoactive and/or inotropic agents for hemodynamic stability after brain death. Inotropes have been associated with direct cardiac toxicity. A recent retrospective analysis evaluated 233 donor-recipient matches and the effects of various inotropes on myocardial necrosis and clinical outcomes. Results demonstrated that high-dose dopamine appears to have a higher tendency to result in post-transplant myonecrosis; however, there is no impact on clinical outcomes (Nixon et al. 2012).
The hormonal changes after brain stem death frequently include decreases in the level of cortisol, insulin, thyroxine (T4), and tri-iodothyronine (T3). The donor should receive steroids, insulin, levothyroxine, and vasopressors in order to maintain stability and end-organ profusion.
At the time of procurement, donors are commonly on vasopressin for brain death-related diabetes insipidus (Capatina et al. 2015). If dopamine is required for circulatory stability, doses less than 5 μg/kg/min are recommended. Hypervolemic donors may exhibit cardiac edema, evident at the time of procurement by a hypokinetic right ventricle. In these situations, the anesthesiologist can administer furosemide and possibly start low dose dobutamine for support. However, a donor that becomes inotrope-dependent to maintain hemodynamic stability, or whose right ventricle does not recover after attempts at diuresis, will be deemed unsuitable .
Substance Abuse
It has become more common to find a history of prior substance abuse in an adult cardiac donor. Donors over the age of 35 with a significant history of cigarette smoking are at greater risk of having coronary artery disease, and therefore performing a cardiac catheterization is recommended. Donor alcoholism raises concern for future alcoholic myotoxicity; when alcoholic donor transplantation outcomes were analyzed, a possible correlation was found with early graft rejection and death (Houyel et al. 1992; Freimark et al. 1996). The ISHLT guidelines state “…the use of hearts from donors with a history of alcohol abuse remains uncertain, but it should probably be considered unwise.” (ISHLT 2010). However, a 2015 meta-analysis found no difference in mortality and graft dysfunction between alcoholic and non-alcoholic donors (Jacob et al. 2015). It is reasonable to accept alcoholic donors with normal echocardiograms but coronary catheterization should be performed for donors over 35 years of age.
A history of intravenous or recreational drug abuse will classify the donor as “increased risk” and the recipient is required to be made fully aware and sign a written consent to continue with transplantation. Nonintravenous cocaine use has not shown to contribute to increased morbidity, mortality, or myocardial ischemia (Freimark et al. 1994).
Recipient Comorbidities and Condition at the Time of Transplant
Recent ISHLT guidelines for heart transplant listing carefully analyze obesity, diabetes, renal function, cerebral disease, and peripheral vascular disease. Body mass index (BMI) >35 kg/m2 is associated with a worse outcome and weight loss is recommended before listing. Diabetes with end-organ damage or HbA1c >7.5% is a relative contraindication for transplant. Insulin-diabetic patients with no evidence of significant end-organ involvement can be listed as long as they are well-controlled with a HbA1c < 7.5%. Irreversible renal dysfunction (eGFR <30 ml/min/1.73 m2) is a relative contraindication for heart transplant alone. An evaluation by the renal transplant team is recommended in order to consider listing the recipient for heart-kidney transplant. Clinically, severe symptomatic cerebrovascular disease should be considered a contraindication to transplantation unless the neurological issues are reversible. Recent institutional experience includes patients on temporary mechanical support, in-house, status post-CVA, with eventual successful transplantation 4–6 weeks later. Peripheral vascular disease may be considered a relative contraindication for transplantation when its presence limits rehabilitation or limits peripheral cannulation for cardiopulmonary bypass in redo sternotomy recipients (Mehra et al. 2016) With all stated recommendations Class IIa-b, Level of evidence C, recipient physicians are left with many “borderline” heart failure patients with comorbidities who are reasonable to consider for heart transplantation with acceptable outcomes. As is usually the case, decisions are made on a case-by-case basis. Most importantly, a potential recipient cannot be listed for heart transplant without control of any of the aforementioned comorbidities.
Many aspects of heart transplantation are changing. In the recent years, older patients are being considered for heart transplantation and the number of complex congenital heart disease (CHD) patients benefitting from heart transplant is growing (Ventura and Muhammed 2001). Additionally, there is a significant increase in recipients with previous open-heart surgeries, transplants, and mechanical circulatory support (MCS) devices as bridge to transplant (Taylor et al. 2007). This changing population creates new challenges for the transplant physicians. For example, the risk of having preformed antibodies (PRA) directed against the donor heart may increase the risk of antibody-mediated rejection and allograft vasculopathy (Kerman 2007; Valantine 2004). Plasmapheresis, intravenous immunoglobulin (IVIG), and rituximab have been used to decrease the PRA prior to transplantation with varying degrees of success (Velez and Johnson 2009). The congenital heart disease patients tend to have more complex anatomy and are also at an increased risk of perioperative bleeding and mortality because of previous operations (Hosseinpour et al. 2006). It is imperative to evaluate congenital heart disease anatomy preoperatively with TEE, contrast CT scan of the chest, catheterizations and/or angiograms in order to plan the surgical approach .
Conclusion
The incidence of heart failure is increasing, patients are living longer, and more ventricular assist devices are being placed for BTT. The UNOS waiting list continues to grow with a steady donor pool; subsequently, transplant teams are left with the formidable mission of matching as many recipient patients on the waiting list as possible with suitable donor hearts.
When an initial donor offer had been made, the recipient surgeon and cardiologist need to consider all donor and recipient inpatient studies, age, ischemic time, size, location, and specific characteristics. Most cases are not simple and the risk/benefit ratio must be carefully assessed for the proposed heart transplant procedure and potential postoperative outcomes.
References
Alexander JW, Vaughn WK (1991) The use of “marginal” donors for organ transplantation. The influence of donor age on outcome. Transplantation 51(1):135–141
Allan DK, Knechtle SJ, Larsen CP, et al (2014) Textbook of organ transplantation. In: Kirk AD, Knechtle SJ, Larsen CP, Madsen JC, Pearson TC, & Webber SA (Eds.). John Wiley & Sons. Organ procurement and allocation, p 268
Brock MV, Salazar JD, Cameron DE et al (2001) The changing profile of the cardiac donor. J Heart Lung Transplant 20:1005–1009
Capatina C, Paluzzi A, Mitchell R, Karavitaki N (2015) Diabetes insipidus after traumatic brain injury. J Clin Med 4(7):1448–1462
Chan BB, Fleischer KJ, Bergin JD et al (1991) Weight is not an accurate criterion for adult cardiac transplant size matching. Ann Thorac Surg 52(6):1230–1235. discussion 1235–6
Cooper DK, Miller LW, Patterson GA (2007) The transplantation and replacement of thoracic organs: the present status of biological and mechanical replacement of the heart and lungs, p 25
Costanzo MR, Dipchand A, Starling R et al (2010) The international society for heart and lung transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 29:914–956
Edwards NM, Chen JM, Mazzeo PA (2005) Cardiac transplantation: the Columbia University Medical Center/New York-Presbyterian Hospital Manual, Contemporary cardiology. Humana Press, Totowa, pp 19–36
Freimark D, Czer LS, Admon D et al (1994) Donors with a history of cocaine use: effect on survival and rejection frequency after heart transplantation. J Heart Lung Transplant 13(6):1138–1144
Freimark D, Aleksic I, Trento A et al (1996) Hearts from donors with chronic alcohol use: a possible risk factor for death after heart transplantation. J Heart Lung Transplant 15:150–159
Gong T, Joseph S, Lima B et al (2018) Donor predicted heart mass as predictor of primary graft dysfunction. J Heart Lung Transplant 37(7):826–835
Hosseinpour AR, Cullen S, Tsang VT (2006) Transplantation for adults with congenital heart disease. Eur J Cardiothorac Surg 30:508–551
Houyel L, Petit J, Nottin R et al (1992) Adult heart transplantation: adverse role of chronic alcoholism in donors on early graft function. J Heart Lung Transplant 11:1184–1187
Jacob KA et al (2015) Chronic alcoholic donors in heart transplantation: A mortality meta-analysis. Int J Cardiol 191:7–10
Jawitz OK, Jawitz NG, Yuh D et al (2013) Impact of ABO compatibility on outcomes after heart transplantation in a national cohort during the past decade. J Thorac Cardiovasc Surg 146(5):1239–1246
John R (2004) Donor management and selection for heart transplantation. Semin Thorac Cardiovasc Surg 16:364–369
John R, Rajasinghe HA, Chen JM et al (2000) Impact of current management practices on early and late mortality in over 500 consecutive heart transplant recipients. Ann Surg 232:302–311
Kerman RH (2007) Understanding the sensitized patient. Heart Fail Clin 3:1–9
Khasati NH, Machaal A, Barnard J, Yonan N (2007) Donor heart selection: the outcome of “unacceptable” donors. J Cardiothorac Surg 2:13–13
Kilic A, Emani S, Sai-Sudhakar CB et al (2014) Donor selection in heart transplantation. J Thorac Dis 6(8):1097–1104
Kransdorf E, Kittleson M, Patel J et al (2017) Predicted heart mass is the optimal metric for size matching in heart transplantation. J Heart Lung Transplant 36(4):S113–S114
Mehra MR et al (2016) The 2016 international society for heart lung transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 35(1):1–23
Nixon JL, Kfoury AG, Brunisholz K et al (2012) Impact of high-dose inotropic donor support on early myocardial necrosis and outcomes in cardiac transplantation. Clin Transpl 26:322–327
Patel SR, Saeed O, Sims D et al (2018) Cardiac transplantation from non-viremic hepatitis C donors. J Heart Lung Transplant 37(4):S137
Peled Y, Lavee J, Arad M et al (2017) The impact of gender mismatching on early and late outcomes following heart transplantation. Esc Heart Fail 4(1):31–39
Schlendorf KH, Zalawadiya S, Shah A et al (2018) Early outcomes using hepatitis C–positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant 37(6):763–769
Schüler S, Warnecke H, Loebe M et al (1989) Extended donor age in cardiac transplantation. Circulation 80(5 Pt 2):III133–III139
Stehlik J et al (2011) The registry of the international society for heart and lung transplantation: twenty-eighth adult heart transplant report—2011. J Heart Lung Transplant 30(10):1078–1094
Taylor DO, Edwards LB, Boucek MM et al (2007) Registry of the international society for heart and lung transplantation: twenty-fourth official adult heart transplant report—2007. J Heart Lung Transplant 26:769–781
Tenderich G, Koerner MM, Stuettgen B et al (1998) Extended donor criteria: hemodynamic follow-up of heart transplant recipients receiving a cardiac allograft from donors (over) 60 years of age. Transplantation 66(8):1109–1113
Valantine H (2004) Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant 23:S187–S193
Velez M, Johnson MR (2009) Management of allosensitized cardiac transplant candidates. Transplant Rev (Orlando, Fla) 23(4):235–247
Ventura HO, Muhammed K (2001) Historical perspectives on cardiac transplantation: the past as prologue to challenges for the 21st century. Curr Opin Cardiol 16:118–123
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Schultheis, M., Camacho, M. (2020). Matching Donor to Recipient. In: Bogar, L., Stempien-Otero, A. (eds) Contemporary Heart Transplantation. Organ and Tissue Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-58054-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-58054-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58053-1
Online ISBN: 978-3-319-58054-8
eBook Packages: MedicineReference Module Medicine