Abstract
Brain metastases occur in up to 40% of patients with cancer and their frequency has increased over time. Lung, breast and melanoma are the most common sources of brain metastases, and in 10–15% of patients the primary site remains unknown. Contrast-enhanced MRI is the gold standard for the diagnosis. Subgroups of patients with different prognosis have been identified (RPA and GPA classifications). Symptomatic therapy includes corticosteroids and anticonvulsants. Complete surgical resection allows for relief of intracranial hypertension, seizures and focal neurological deficits. Stereotatic radiosurgery, alone or in conjunction with WBRT, yields results which are comparable to those reported after surgery followed by WBRT. WBRT after surgery or radiosurgery is increasingly omitted. The response rate of brain metastases to chemotherapy is similar to the response rate of the primary tumor and extracranial metastases. Targeted agents in specific molecular subgroups are emerging.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain metastases
- Epidemiology
- Molecular pathways
- Diagnosis
- Prognostic factors
- Surgery
- Radiosurgery
- Whole-brain radiotherapy
- Chemotherapy
- Targets agents
Introduction
Brain metastases represent one of the most common neurological complications of systemic cancer, in many cases resulting in significant morbidity and mortality. The incidence has increased over time as a result of advances in detection and improvements in the treatment of primary tumor and systemic disease, which have led to an increase in survival. They currently represent the most frequent intracranial tumors, outnumbering primary brain tumors.
The majority of patients who develop brain metastases have a relatively short survival, despite the fact that initial treatment is often effective. The short survival may be the result of progressive systemic disease (in more than a half of patients) or uncontrolled neurological disease. The treatment of brain metastases includes corticosteroids, anticonvulsants, surgery, radiosurgery, radiotherapy, chemotherapy, and targeted therapies. Although for many patients effective palliation is transient or not possible, other patients with metastatic brain disease do well for prolonged periods with an aggressive therapeutic approach. Prognostic factors can help to identify subgroups of patients with differing life expectancy, and tailor therapeutic approaches.
This chapter will review the state of the art and advances in the management of patients with brain metastases.
Epidemiology, Natural History and Risk Factors
Few population estimates of brain metastasis are available. The incidence of newly diagnosed brain metastasis is estimated to be 3–10 times the number of newly diagnosed primary malignant brain tumors each year [1, 2]. Brain metastases occur in up to 40% of patients with cancer, being symptomatic during life in 60–75% or discovered incidentally on CT/MRI or autopsy [3,4,5,6,7].
In adults, lung (36–64%), breast (15–25%), and skin (melanoma) (5–20%) are the most frequent sources of brain metastases. Less frequent are cancers from colon rectum, kidney, prostate, testis, ovary, and sarcomas. In general, any malignant tumor is able to metastasize to the brain. The primary site is unknown in up to 10–15% of patients with brain metastases. The propensity of primary tumors to spread to the brain parenchyma (“neurotropism”) differs, and is high for melanoma (20–45% of patients), small-cell lung cancer, choriocarcinoma and germ cell tumors; intermediate for breast cancer, nonsmall-cell lung cancers (being more frequent in adenocarcinomas than in squamous tumors) and renal cancer; low for cancers of the prostate, gastrointestinal tract, ovary, thyroid, and sarcomas. Cerebral metastatic disease in children is less frequent than in adults (6–10%) [8]. The childhood solid tumors that more frequently metastasize to the brain are neuroblastomas and a variety of sarcomas, including rhabdomyosarcoma, Wilms’ tumor, Ewing’s sarcoma, and osteogenic sarcoma. Among children older than 15 years, germ cell tumors have the highest incidence. Brain metastases are more commonly diagnosed in patients with known systemic malignancy (metachronous presentation) and may be the first evidence of the metastatic disease. Less commonly, brain metastases are discovered in patients at the same time as the primary tumor (synchronous presentation, up to 30%) or prior to discovery of primary disease (precocious presentation).
In the CT era around 50% of brain metastases were presumed to be single, while MRI has revealed that multiple lesions exist in two-thirds to three-fourths of all cases of brain metastases [9, 10]. Brain metastases from renal and abdominopelvic tumors are often single, whereas malignant melanoma and lung tumors have a greater tendency to produce multiple cerebral lesions.
The overwhelming majority of brain metastases arise from embolization of tumor cells through the arterial circulation (hematogenous spread ). The occurrence of metastases in the different locations is roughly proportional to their relative mass and blood flow: lesions are located in the cerebral hemispheres in 80% of patients, in the cerebellum in 15%, in the brainstem in 5%, and are rare in the basal ganglia, pineal gland, and hypophisis [11]. Brain metastases are commonly found at the junction of the gray and white matter, and are overrepresented in “watershed” areas of the brain, consistent with the origin of metastases from tumor cell emboli carried to terminal arterioles. Melanoma is unusual in its predilection to metastasize to the cerebral cortex and basal ganglia rather than to the gray-white matter junction [12]. There are few circumstances in which nonspecific hematogenous spread does not explain the observed distribution of brain metastases. Pelvic and abdominal tumors have a predilection to form posterior fossa metastases far in excess of what the proportion of blood flow supply to this region would predict. Dissemination byway of Batson’s vertebral venous plexus has long been invoked to explain this phenomenon, but this hypothesis cannot explain why patients with pelvic or abdominal tumors do not a high incidence of spinal and skull metastases as well, as these structures are also drained by Batson’s plexus.
Some clinico-pathological and molecular factors are recognized as risk factors for developing brain metastases. In NSCLC an increased risk for brain metastases has been related to an advanced disease stage, large primary tumor size, non-squamous histology (mainly adenocarcinoma) and, more recently, EGFR mutational status [13, 14].
In breast cancer , younger age at first diagnosis, the presence of lung metastases and short disease-free survival are considered as major clinical risk factors for the development of brain metastases. Patients with triple-negative tumors (ER-, PR-, HER2 wild type) are at higher risk of developing brain metastases compared with the luminal or HER2-positive subtypes [15]. HER2-positive patients with metastatic disease receiving the monoclonal antibody against HER2 trastuzumab have a higher incidence of brain metastases (30–55%) than patients with HER2-negative disease [16]. It is still debated whether patients with early breast cancer receiving adjuvant trastuzumab have a significant increase in the risk of CNS relapse as well [17, 18]. CNS disease as the first site of relapse remains relatively rare, but occurs more frequently in HER2-positive compared to HER2-negative patients [19]. There are two hypotheses to explain the higher risk for brain metastases in HER2-positive patients [20]. The first one is that trastuzumab is not able to cross an intact BBB, thus being active against the systemic disease but not preventing CNS disease. The second hypothesis suggests that there is an increased propensity of the HER-2 lineage to colonize the brain.
Biology and Molecular Pathways
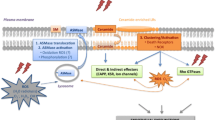
In general, metastasis of cancer cells occurs via the “metastatic cascade ,” which refers to tumor cell invasion of surrounding tissue, entry into the blood stream (intravasation), attachment to local vasculature (arrest), extravasation, and proliferation at the site of metastasis. The “soil and seed” hypothesis of metastasis formation explains why circulating tumor cells may travel throughout the body, but metastases tend to form in particular organs (as in the brain in absence of lung metastases). The metastasis formation would be the result of an interaction between the organ microenvironment (the “soil”) and the adhesive and invasive capabilities of the metastasizing tumor cells (the “seed”) [21]. Neoplastic cells with the potential to colonize the brain may express unique molecular determinants and respond to brain-derived growth factors, and thereby be able to invade, proliferate, and induce angiogenesis [22]. Moreover, the brain is a unique target organ because of the presence of the blood–brain barrier (BBB) [23] and the absence of lymphatic drainage.
Several genes that mediate the spread of the different primary tumor types to the brain have been identified. Expression profiling has revealed at least five molecular subtypes of breast cancer that differ in tropism to different organs. The luminal breast cancer subtype tends to affect pleura and bone, while the basal subtype preferentially metastasizes to lung and brain [24]. Recently, a set of specific genes was identified [25] by comparing the expression profiles of murine breast cancer cells preferentially metastasizing to brain with the profiles of breast cancer samples from patients with known cerebral metastases. A distinction was made between genes that are expressed in primary tumors with metastases, and therefore called “metastasis progression-genes,” and other genes active in the metastatic tumors but not expressed in the primary tumors. The cyclooxygenase COX2, the EGFR ligand HBEGF and the α26-sialyltransferase ST6GALNAC5 were identified as mediators of cancer cell passage through the BBB. While the EGFR ligand and COX2 are also involved in the development of lung metastases, the sialyltransferase ST6GALNAC5 is more specific to the development of brain metastases. In an invitro model COX2 was found to be crucial in the passage of the tumor cells through the BBB [25], and COX2 knock-down lowered the frequency of brain metastasis. Palmieri and colleagues [26] found hexokinase 2 (HK2) to be upregulated 1.5-fold in tumor cells in the brain and associated with poor survival in patients following craniotomy. Moreover, HER2 overexpression does not appear to affect tumor cell arrival or intravasation into the brain, but it increases brain colonization [27]. STAT3 activation has been implicated as an important driver of brain metastasis in breast cancer and its inhibition has been recently shown to suppress development of brain metastases [28]. Few studies have investigated the genomic drivers of brain metastases from lung cancer. Grinberg-Rashi and colleagues [29] reported 12 candidate genes whose overexpression was associated with brain or systemic metastasis in a large series of NSCLC primary tumors, and three genes (CDH2, KIFC1, FAL2) were able to predict prognosis. Of particular interest is the overexpression of CDH2 (N-cadherin), which is involved in multiple processes, such as invasion, migration, and adhesion. The EGFR pathway and integrins may play a role in helping promote brain metastasis in lung and breast cancers [30]. Cancer cells of neural crest origin such as melanoma and neuroendocrine carcinoma may preferentially migrate to the brain [31]. The TGF-B2 expression by murine melanoma is necessary for the establishment and growth of metastases in the brain [32].
In order to get to the brain, tumor cells must reach the cerebral microvasculature and pass through the BBB [33, 34]. Genes coding for cell surface glycoproteins are involved in the attachment of the cells to local blood vessels, and genes regulating vascular permeability are involved in passing through the vessels. Genes involved in the formation and maintenance of the astrocytic end-feet at the opposite side of the BBB have also been implicated.
In order to proliferate, metastatic tumor cells need to switch on the expression of pro-angiogenic molecules like VEGF, MMP-9, and gelatinase B, as well as molecules degrading the extracellular matrix, allowing the growth of new vessels [35]. Increased expression levels of VEGF are necessary but not sufficient for successful formation of metastases in experimental animals [34, 36]. The absence of neo-angiogenesis in metastatic tumor cell populations keep the tumor dormant, a state of increased apoptosis of tumor cells with normal proliferation [37].
Various genes that suppress the formation of metastases have been identified, including Nm23 and CD44 . Overexpression of Nm23 in breast cancer and melanoma negatively affects invasion, colonization, and motility [38], and patients with melanoma with low expression of Nm23 had increased risk of developing brain metastases [39]. The membrane glycoprotein CD44 plays a role in the adherence of circulating tumor cells to endothelium. Downregulation of the gene by DNA hypermethylation prevented the formation of metastases and, conversely, consistent expression was reported in cancers of thyroid, skin, and breast [40]. Interestingly, while the standard CD44 isoform is expressed in primary brain tumors, the CD44 splicing variant is almost exclusively found in brain metastases [41].
Clinical Presentation
The clinical presentation of brain metastases is similar to the presentation of any intracranial mass lesion. Headache is a presenting symptom in 40–50% of patients, is more common with multiple or posterior fossa metastases, and may be mild. Papilledema is associated with headache in 15–25% of patients only. Up to 40% of patients present with focal neurological deficits, and seizures occur in 15–20% of patients. Another 5–10% of patients present with acute “strokelike” symptoms due to an intratumoral hemorrhage (especially in melanoma, renal carcinoma, and choriocarcinoma). Altered mental status or impaired cognition are frequently seen in patients with multiple metastases and/or increased intracranial pressure, sometimes resembling a metabolic encephalopathy. Conversely, the symptoms and signs at presentation can be subtle. As a general rule, brain metastases should be suspected in any patient with known systemic cancer in whom new neurological findings develop.
Diagnosis by Neuroimaging
MRI is the method of choice for the assessment of brain metastasis. Contrast-enhanced MRI is more sensitive than enhanced CT (including double-dose delayed contrast) or unenhanced MRI in detecting brain metastases, particularly lesions in the posterior fossa or multiple punctate metastases [9, 10]. Although T2-weighted and FLAIR images are sensitive in showing vasogenic edema as areas of increased signal intensity, not all metastatic lesions have sufficient edema to be identified.
There are no specific features on MRI that distinguish brain metastases; however, a peripheral location, spherical shape, ring enhancement with prominent peritumoral edema and multiple lesions all suggest metastatic disease (Fig. 4.1). Differential diagnoses, including primary brain tumors (especially high-grade gliomas and lymphomas) and non-neoplastic conditions (abscesses, infections, hemorrhages) must be considered, even in patients with a history of cancer. Diffusion-weighted (DW) MR imaging may be useful in the diagnosis of ring-enhancing cerebral lesions (restricted diffusion is more typical in abscesses compared to unrestricted diffusion in necrotic glioblastomas or metastases), but the findings are not specific [42,43,44]. When employing MR perfusion imaging, there is a tendency towards lower cerebral blood volume values within the peritumoral region in brain metastases compared with glioblastomas [45, 46]. MR spectroscopy more often shows a lower choline to creatinine ratio in brain metastases than in high-grade gliomas [47, 48]. FDG-PET and18F-FET PET do not provide sufficient differentiation between metastases and high-grade glial tumors [49, 50].
Overall, advanced functional imaging techniques cannot reliably identify the histologic origin of an enhancing brain lesion and hence histopathological analysis remains the gold standard. A tissue diagnosis by biopsy should be considered in patients with either unknown primary tumor or well-controlled systemic cancer, especially if a long interval has elapsed since the initial cancer diagnosis, or (less common) in patients with active systemic cancer when the radiographic appearance is atypical. In the modern era there is seldom a justification for irradiating “presumed brain metastases” without a histological diagnosis of cancer.
Staging
A new brain mass suspected to be a metastasis in a patient with no prior history of cancer warrants additional systemic work-up for a primary malignancy. A chest CT is always recommended given the high frequency of brain metastases from lung cancer [51, 52], CT of the abdomen and ultrasound of the testis occasionally reveal the primary tumor. Additional work-up beyond this is low yield, unless the patient’s history or physical exam is suggestive of a specific primary site [53]. Whole-body fluorodeoxyglucose (FDG) PET can be useful [54], but the specificity in differentiating malignant tumors from benign or inflammatory lesions is relatively low. Notably, serial chest CTs may increase the probability of detection of lung cancer in patients with a brain metastasis from an unknown primary tumor, but for these patients early detection provides only limited if any, benefit in survival. [55]. Therefore, a costly extensive evaluation for the undetected primary during the follow-up is not appropriate until more effective cancer therapies are available [53, 55].
A CSF examination is not indicated in the work-up of brain metastases unless there are symptoms, signs or neuroimaging findings that suggest a coexistent leptomeningeal carcinomatosis.
Diagnostic Neuropathology
Routine hematoxylin-eosin stain of biopsy specimens usually reveals the neoplastic nature of the cerebral lesion, and can distinguish between metastases, malignant gliomas, meningiomas, lymphomas, and more rare entities. Immunohistochemical markers may aid in the further characterization of the tumor.
Cerebral Metastasis of Known Primary Tumors
In patients with a known primary tumor, the histology and the marker profile of the primary and the cerebral metastasis will usually show similarities. In general, histologic comparison between the specimen of primary tumor(s) and cerebral metastasis is mandatory, as not infrequently patients may harbor more than one tumor type.
Cerebral Metastasis of Unknown Primary Tumors
For the determination of the lineage of the metastatic tumor, basic morphology provides a first differentiation between carcinomas, lymphomas, or melanomas (Fig. 4.2a–c). In addition, immunohistochemical profiles of metastases may be indicative of the site and lineage of the primary tumor [56]; however, these show variable overlap, and most markers are not specific. In case of a cerebral adenocarcinoma of unknown primary, TTF-1 positivity is strongly associated with lung cancer (Fig. 4.3) and cancer of the thyroid. Negativity for CK7 and positivity for CK20 suggests colorectal cancer. Neuroendocrine differentiation is confirmed by chromogranin, synaptophysin and antibodies directed against specific hormones (insulin, gastrin, glucagon, serotonin and somatostatin). Similarly, there are immunohistochemical panels for mesenchymal tumors (vimentin, desmin, S100).
Attempts to identify unknown primary tumors from their metastases by using RNA expression profiles are ongoing. In general, few studies have focused on the comparison of primary tumors and their cerebral metastases with respect to lineage markers and biomarkers for treatment eligibility [57, 58].
Prognostic Factors
Several factors, including Karnofsky performance status (KPS) , age, primary/systemic tumor activity, neurocognitive function, number of brain metastases, primary tumor type and time from primary tumor diagnosis to the brain lesion have individual prognostic significance in patients with brain metastases [59, 60]. Of these, the KPS has consistently been shown to be the major determinant of survival. Based on the most powerful factors, prognostic indices have been developed in order to distinguish subgroups of patients with different prognosis. Utilizing recursive partitioning analysis (RPA) a three-tiered prognostic categorization (RPA Classes I, II and III) was derived from 1200 patients in the Radiation Therapy Oncology Group (RTOG) database who received WBRT [59, 61]. RPA class I represents patients with a KPS greater than 70, age younger than 65 years, controlled primary tumor and no extracranial metastases, with a median survival of 7.7 months; RPA class III represents patients with KPS less than 70, with a median survival of 2.3 months; RPA class II represents the remainder of patients with a median survival of 4.5 months.
A new prognostic index, the Graded Prognostic Assessment (GPA) , derived from an analysis of an updated RTOG database of 1960 patients, has been proposed [62]. The GPA uses four factors (age, KPS, status of extra neural disease and number of brain metastases) to subdivide patients into one of four categories with median survival ranging from 2.6 to 11 months. This new index appears equivalent to the RPA in ability to prognosticate, but is less subjective and more quantitative. Additional analysis [63] has shown that the prognostic factors for patients with brain metastases vary according to the histological diagnosis. For both nonsmall-cell and small-cell lung cancer, the significant prognostic factors were KPS, age, presence of extracranial metastases and number of brain metastases, confirming the original GPA. Conversely, for breast cancer, significant prognostic factors were KPS, age and tumor subtype (classified as HER2, estrogen receptor and progesterone receptor status), but not number of brain metastases or status of systemic disease. For melanoma and renal cell carcinoma, the significant prognostic factors were KPS and number of brain metastases. The GPA categorization has now been validated in patients with breast cancer and brain metastases [64, 65], and should be used to stratify future randomized clinical trials, estimate survival and guide the choice of management options. RPA and GPA provide group rather than individual estimates. Recently, a nomogram for the estimation of individual survival probabilities in patients with brain metastases has been proposed with use of data from RTOG database [66].
Additional prognostic scores have been developed for patients with brain metastasis undergoing radiosurgery [67, 68]. Prognosis does not differ between patients with a known and unknown primary tumor [52, 69].
Supportive Care
Corticosteroids are used to control vasogenic cerebral edema and mass effect. Two evidence-based guidelines on the role of steroids in brain metastases have been published in Europe [70] and US [71], and they substantially agree. Dexamethasone is recommended for patients who are symptomatic, with a starting dose of 4–8 mg/day, considering higher doses such as 16 mg/day or more in patients with severe symptoms. Dexamethasone is the steroid of choice because of its minimal mineralocorticoid effect and long half-life, though other corticosteroids can be effective if given in equipotent doses. A neurological improvement within 24–72 h after beginning of treatment is expected in up to 75% of patients. As monotherapy , dexamethasone can relieve symptoms for approximately one month and may slightly increase the 4–6-weeks median survival in comparison to patients who receive no treatment at all. To minimize side effects from chronic dexamethasone administration, including proximal myopathy, tapering of steroid dosing within 1 week of starting therapy and discontinuation within 2 weeks is encouraged. Asymptomatic patients do not need steroids.
The need for anticonvulsant medication is clear in patients who have experienced a seizure. There exists no evidence to support the use of prophylactic anti-epileptic drugs (AEDs) in patients with brain tumors, including metastases. Twelve studies, either randomized trials or cohort studies, investigating the ability of prophylactic AEDs (phenytoin, phenobarbital, valproic acid) to prevent the first seizure, have been examined, and none have demonstrated efficacy [72]. Subtherapeutic levels of anticonvulsants were extremely common and the severity of side effects appeared to be higher (20–40%) in brain tumor patients than in the general population receiving anticonvulsants, probably as a result of drug interactions. Phenytoin, phenobarbital, carbamazepine, and oxcarbazepine stimulate the cytochrome P450 system and accelerate the metabolism of corticosteroids and antineoplastic agents, such as nitroso ureas, paclitaxel, cyclophosphamide, topotecan, irinotecan, thiotepa, adriamycin, methotrexate, imatinib, gefitinib, erlotinib and other tyrosine kinase inhibitors (TKIs) , and thus reduce their efficacy. The role of prophylactic anticonvulsants remains to be addressed in subgroups of patients who have a higher risk of developing seizures, such as those with metastatic melanoma, hemorrhagic lesions, or multiple metastases. For patients who undergo a neurosurgical procedure the efficacy of prophylaxis has not been proven. The efficacy of novel AEDs (levetiracetam, topiramate, gabapentin, lamotrigine, lacosamide) has to date not been extensively investigated [73].
Anticoagulant therapy is the standard treatment for acute venous thromboembolism (VTE) in cancer patients. Subcutaneous low-molecular weight heparin(LMWH) is recommended for the initial 5–10 days of treatment for deep vein thrombosis and pulmonary embolism as well as for long-term secondary prophylaxis (for a minimum of 6 months) [74]. Use of novel oral anticoagulants is not currently recommended for patients with malignancy and VTE because of limited data in this patient population [75].
Prophylaxis with LMWH is required for hospitalized patients undergoing major surgery, while in the outpatient setting it is recommended only in selected high-risk patients [76, 77].
Treatment of Newly Diagnosed Brain Metastasis
Surgery
Three randomized trials in single brain metastasis have compared the efficacy of surgical resection followed by WBRT with WBRT alone [78,79,80]. See Table 4.1.
The first two studies showed a survival benefit for patients receiving the combined treatment (median survival 9–10 months vs. 3–6 months). In the Patchell study, patients who received surgery displayed a reduced rate of local relapses (20% versus 52%) and a longer time of functional independence. In the third study, which included more patients with active systemic disease and a low Karnofsky performance status, the addition of surgery to WBRT did not confer a survival benefit, suggesting that this benefit may be limited to a subgroup of patients with controlled systemic disease and good performance status.
In the majority of patients, surgical resection can alleviate symptoms of intracranial hypertension, reduce focal neurological deficits and seizures, and allow for a rapid steroid taper. It should be strongly considered for lesions ≥3 cm and/or with significant surrounding edema and/or located in the posterior fossa with mass effect and associated hydrocephalus. Gross total resection of a brain metastasis can be achieved with lower morbidity using contemporary image-guided systems, such as preoperative functional MRI, intraoperative neuronavigation, and cortical mapping [81]. An early postoperative MRI has been recommended to detect residual tumor that is present in up to 20% of patients, and can lead to an increased risk in local recurrence [82]. The same group has suggested that a supramarginal resection (i.e., a resection including a peripheral portion of normal nervous tissue) in eloquent locations could increase the rate of gross total resection [83]. The combined resection of a solitary brain metastasis and a synchronous nonsmall-cell lung carcinoma (stage I and II) yields a median survival of at least 12 months, with 10–30% of patients surviving at 5 years [84].
Leptomeningeal dissemination (LMD) can be a significant complication of the resection of metastasis, especially in case of patients with posterior fossa lesions [85, 86]. A recent retrospective study from MD Anderson Cancer Center [86] in 379 patients with posterior fossa metastases, who underwent either surgery or radiosurgery, revealed a significant increase of LMD in patients whose tumors underwent a “piecemeal” resection (13.8%) compared to en bloc resection (5–6%), and the risk of LMD after en bloc resection was comparable to that after SRS.
Surgery may be considered for patients with 2–3 surgically accessible brain metastases who are in good neurological condition, have controlled systemic disease and limited comorbidities (Fig. 4.4a–d). Complete surgical resection in this population yields results comparable to those obtained in single lesions [87].
The usefulness of carmustine wafer placement in the resection cavity in newly diagnosed brain metastasis [88] has not been proven in large series of prospective trials.
The GliaSite Radiation Therapy System is an intracavitary high-activity 125-I brachytherapy, performed with a balloon placed in the resection cavity and filled with a radioactive solution. It delivers highly localized doses of radiation to the resection margins (60 Gy to 1 cm depth). A phase II trial in resected single brain metastasis [89] has reported an overall and 1-year local control rate of 83 and 79%, respectively, with a 17% of local failures. Seventeen per cent of patients experienced radiation necrosis. A smaller retrospective study [90] reported the results of 125-I brachytherapy using low-activity permanent seeds placed in the resection cavity. Local tumor control was achieved in 96% of patients, with 4% local failure. The 1-year risk of symptomatic radiation necrosis was lower than that seen in the phase II trial [89] with high-activity seeds (8% vs. 23%).
Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) is a single, high-dose radiation treatment with precise localization of the target using stereotactic frames or image guidance. Convergence of multiple beams on the target yields a highly therapeutic effect, while the steep dose fall-off to surrounding normal structures minimizes the risk of damage. Most brain metastases represent an ideal target for SRS, owing to the small size, spheroid shape, and distinct pathologic margins [91]. The dose is inversely related to tumor diameter and volume. Maximal tolerated doses of SRS were defined in the RTOG 90-05 study [92] in previously irradiated primary brain tumors or brain metastases: 24 Gy for ≤20 mm, 18 Gy for 21–30 mm and 15 Gy for 31–40 mm in maximum diameter. As a consequence, the local tumor control decreases as the size of the metastasis increases and the dose that can be given in single fraction decreases. Recently, there has been an increasing interest in hypofractionation for larger metastases (2–5 fractions of smaller doses) with the aim to give radiobiologically higher doses to improve local control and decrease the risk of radionecrosis [93,94,95]. Comparative studies of hypofractionated SRS versus single dose SRS are awaited.
Several retrospective series have shown single dose SRS to be effective in the treatment of newly diagnosed brain metastases. One-year local control rates of 80-90% with symptom improvement and median survival of 6–12 months have been reported [96,97,98]. Patients with single lesion, controlled systemic disease and KPS of 70% or greater, have longer survival [99, 100].
Metastases from radio resistant tumors, such as melanoma and renal cell carcinoma, respond to SRS as do metastases from radiosensitive tumors [101]. Radiosurgery allows the treatment of brain metastases in almost any location, including the brainstem [102, 103]. Older patients (≥80 years of age) may respond as well as younger patients [104]. The type of radiosurgical procedure, gamma knife or linear accelerator (Linac)-based, does not impact the results. The imaging response to SRS has been reported to correlate with survival only in patients with breast cancer [105].
For patients in RPA classes 1 and 2 with 1–3 brain metastases, SRS has been reported to be more effective than WBRT alone in terms of local tumor control [106].
A randomized phase III study (RTOG95-08) in patients with 1–3 brain metastases, stratified by the RPA system, investigated the value of the addition of a SRS boost to WBRT [107], and reported better local control and performance status at 6 months in the combined therapy group. However, the survival advantage was statistically significant only in a subgroup of patients with single metastasis (6.5 months vs. 4.9 months). Recently, a secondary analysis of RTOG 95-08 that poststratified patients by the GPA classification has reported that the addition of SRS to WBRT conferred a significant survival advantage for patients with a good prognosis (GPA 3.5–4.0) regardless of whether they had 1 or 2 or 3 brain metastases [108]. Conversely, this benefit did not extend to patients with lower GPA and 2 or 3 metastases. It must be stressed that approximately two-third of patient in the trial had lung tumors. A small randomized trial, comparing WBRT alone versus WBRT + SRS in patients with 2–4 metastases, was stopped earlier due to the significant benefit in terms of local failure reduction at 1 year for patients receiving the combined treatment (8% vs. 100%) [109].
The role of SRS alone in patients with up to 3–4 metastases is discussed in the section on WBRT following surgery or radiosurgery.
The role of SRS alone, instead of WBRT, in patients with >4 brain metastases has been investigated in single arm trials. A prospective multicenter Japanese study investigated the use of SRS alone in 1194 patients with one, 2–4 or 5–10 brain metastases, and found similar overall survival (10.8 months) and treatment related toxicity rates between the groups with 2–4 and 5–10 metastases [100]. Salvage SRS was performed in 38% of patients and WBRT in 9%. Cumulative volume of metastases, rather than the number, seems to be a more significant prognostic factor [100, 110]. A consortium in the US is conducting a randomized trial in patients with ≥5 metastases to compare SRS to WBRT with neurocognitive outcome as the primary endpoint.
Acute (early) and chronic (late) complications following radiosurgery are reported in 10–40% of patients. Serious complications are rare [111]. Acute reactions (due to edema) occur more often within 2 weeks of treatment, and include headache, nausea and vomiting, worsening of preexistent neurological deficits and seizures. These reactions are generally reversible with steroids. Chronic complications include hemorrhage and radionecrosis (1–17%). Radiographically, a transient increase in the size of the irradiated lesion, with increasing edema and mass effect, with or without radionecrosis, cannot be distinguished from a tumor progression. FDG-PET, MRI spectroscopy, and MRI perfusion can provide additional information but cannot definitively distinguish between the two diagnoses [112].
Radiation necrosis can be treated with steroids, hyperbaric oxygen, anticoagulants (with risk of bleeding), and more recently with the anti-VEGF agent bevacizumab, which allows a stabilization and normalization of the damaged vascular permeability [113].
A recent review of adverse radiation effects (ARE) following SRS in a large cohort of patients with brain metastases, either radiographic or pathologic, has been published [114]. Although the incidence of ARE after SRS was overall low, the risk increased rapidly with size and volume, leveling off at 1-year cumulative incidence of 13–14%. The authors found a wide range in the time of onset and time to improvement of ARE, and at least 75% probability of improvement over time in conservatively managed AREs. With the exception of capecitabine, neither systemic therapy within 1 month of SRS norarterial hypertension or diabetes [114] appeared to increase the risk of adverse events in the brain.
Surgery Versus SRS
There is no prospective randomized study with sufficient power to compare surgery to SRS. Most comparisons [115,116,117,118], including an early terminated small randomized study [119], showed similar outcomes. The one exception is a small series by Bindal and colleagues [120], who reported a superiority of surgery over SRS in terms of OS (16.4 months vs. 7.5 months) and neurologic deaths (19% vs. 50%). However, only 80% of SRS-treated patients were deemed resectable retrospectively, and it is unclear whether this was due to the extent of systemic disease or tumor location.
In general, SRS is less invasive and can be accomplished in an outpatient setting, offering cost-effectiveness advantages over surgery. Patients with larger lesions (2–3 cm), however, may require chronic steroid administration given risk of tumor-related edema and swelling with SRS. Ultimately, the choice between surgery and SRS must be made on a case by case basis, with consideration given to tumor location, size, type of neurological symptoms, patient preference and physician expertise.
Whole Brain Radiotherapy Following Surgery or Radiosurgery
The utility of adjuvant WBRT following surgery or radiosurgery remains controversial. WBRT is believed to eradicate microscopic disease at the original tumor site and at distant intracranial locations. The risk of long-term neurotoxicity and availability of potentially effective salvage treatments [121] are the main arguments against the use of adjuvant WBRT. This needs to be weighted against potential risks of omitting treatment, including CNS progression at distant sites and resultant neurocognitive and neurological sequelae. Moreover, the effectiveness of systemic salvage therapy remains unknown [122].
There are now three phase III trials [123,124,125,126], showing that the omission of WBRT in patients with newly diagnosed brain metastases after either surgery or SRS, results in significantly inferior local and distant control, without effect on overall and functionally independent survival. An American-led trial [123] investigating the role of WBRT following surgery, and a Japanese led trial [124] investigating the role of WBRT following SRS, included patients with progressive systemic disease. Conversely, the EORTC 22952-26001 trial focused on patients with stable systemic disease, i.e., on those who could maximally benefit from the addition of early WBRT. This trial randomized 359 patients with 1–3 metastases (81% had one lesion and 19% had two-three lesions), who had previous surgery or SRS, to either WBRT or observation [125]. Adjuvant WBRT significantly reduced the risk of intracranial progression, both locally and at distant sites, by about 50%, but failed to improve functional independence and OS (median 10.9 months vs. 10.7 months). A meta-analysis of the three randomized trials assessing SRS with or without WBRT has challenged our current understanding of the effect of adjuvant WBRT [127]. The investigators reported a survival advantage for SRS alone in those patients presenting with one to four metastases, KPS of 70 or higher and age of 50 years or younger. Moreover, in the subgroup of patients with <50 years, a reduction in the risk of new brain metastases with adjuvant WBRT was not observed. Conversely, in older patients (aged >50 years), WBRT decreases the risk of new brain metastases, but did not affect survival.
A retrospective study [128] has reported that adjuvant WBRT following surgery is of particular value in reducing local and distant recurrence in the brain among patients with metastases >3 cm or with active systemic disease.
The impact of adjuvant WBRT on cognition and quality of life has been examined in several recent studies. Aoyama and coworkers [129] compared the neurocognitive function of patients who underwent either SRS alone or SRS + WBRT. More than 50% of patients experienced significant improvement in the MMSE score shortly after therapy (2–3 months), regardless of treatment, but there was evidence of neurocognitive decline in long-term survivors (up to 36 months) after WBRT. In a randomized controlled trial, Chang and coworkers [130] showed that patients treated with SRS plus WBRT were at greater risk of a significant decline in learning and memory function by 4 months compared with the group receiving SRS alone.
A recently completed randomized phase III trial (NCCTG N0574) compared SRS alone versus SRS + WBRT in patients with 1–3 brain metastases using a primary neurocognitive endpoint, defined as a decline from baseline in any six cognitive tests at 3 months [131]. Overall, the decline was significantly more frequent after SRS + WBRT versus SRS alone (88% vs. 61.9%, respectively). Specifically, there was more deterioration in the SRS + WBRT arm in immediate recall (31% vs. 8%), delayed recall (51% vs. 20%) and verbal fluency (19% vs. 2%). Intracranial tumor control at 6 and 12 months was higher in the combined arm, but not statistically significant.
Soffietti and coworkers [132] analyzed the quality of life data of the EORTC 22952-26001. They found no significant difference in the global Health-Related Quality of Life over a one-year follow-up period, but patients who underwent adjuvant WBRT had transiently lower physical functioning and lower cognitive functioning scores.
These data suggest that adjuvant WBRT after SRS in patients with a limited number of metastases (up to 3–4) improves intracranial control without improving survival, and carries a high risk of neurocognitive decline, and is therefore not unequivocally recommended. In this regard, the American Society for Radiation Oncology (ASTRO) has recently recommended in their Choose Wisely campaign SRS without adjuvant WBRT for the treatment of limited number of brain metastases [133].
The need for WBRT following surgical resection remains debated, as randomized trials [123, 125] have reported a higher rate of local relapses following surgery alone (about 60% in the EORTC trial) compared with SRS alone.
In general, omission of WBRT, following either SRS or surgery, requires close monitoring with serial imaging (every 3–4 months).
SRS Following Surgery
Postoperative SRS is an approach used to decrease local relapse and avoid the cognitive sequelae of WBRT. Several cohort studies and one phase II trial have reported local control rates of 85–95% [134,135,136]. The median survival in published studies is around 4 months (range 10–20.5 months). The improved local control and survival rates suggest that postoperative SRS may be as effective as WBRT.
Postoperative SRS is advantageous because it can be delivered within a day or two following surgery and completed in one day. WBRT cannot be initiated for at least 10 days but usually up to two weeks postoperatively to allow for adequate wound healing. For patients receiving systemic therapy, a break from chemotherapy is necessary during WBRT administration, and up to one month following its completion. With postoperative SRS, there is minimal to no interruption of systemic agents, thereby decreasing time off treatment and risk of systemic disease progression.
Despite a growing body of literature [134, 136,137,138,139], SRS to the resection cavity does not seem to be superior over WBRT in terms of local control at 1 and 2 years. Moreover, SRS may pose increased risk over WBRT of radionecrosis and other neurological complications, as well as leptomeningeal relapse.
Several questions remain regarding SRS following surgery [135, 140, 141]. The optimal dose and fractionation schedule, especially for large brain metastases (>3 cm) associated with higher risk for local failure, are unknown. The same holds true for the optimal margin around the resection cavity to be included in the treatment field. From a clinical standpoint, there remain little data about the impact of postoperative SRS on HRQOL and neurocognitive function. The timing of SRS after surgery also remains unclear. There is little evidence that SRS administered in the immediate postoperative period is more effective than when administered at the time of tumor progression.
The risk of radionecrosis following postoperative SRS [135, 141,142,143] is higher (between 9 and 17.5%) than that reported by the EORTC study with WBRT following either surgery or radiosurgery (2.6%), and could increase over time (7% at 1 year and 16% at 2 years) [141]. However, the actual incidence of pathologically proven radionecrosis is unknown, as often the values reported in the different series represent a combination of biopsy proven and MRI suspected cases of radionecrosis. Several advanced neuroimaging techniques (MRS, MRI perfusion, PET with FDG or amino acids) can aid in the diagnosis of radiation necrosis but oftentimes produce conflicting results. Treatment may include the use of bevacizumab [113], but not without significant financial costs. Alternatively, steroids can reduce symptoms and edema related to radiation effects, but their use increases risk of steroid dependency. The average frequency and duration of steroid using following postoperative SRS remains unknown.
The use of SRS to the resection cavity without the addition of WBRT may be associated with increased risk of leptomeningeal disease (LMD) . The incidence has been found to range from 8 to 13% [139, 141,142,143,144,145,146]. Patients with breast histology may be at higher risk (at 1 year 24% vs. 9%) [146]. It is unclear whether the inclusion of WBRT would decrease this risk or whether the increased risk for LMD seen in patients with brain metastases from breast is associated with the biology of this tumor type. To better characterize this risk of LMD, future reports on the use of SRS to the resection cavity should distinguish between compartments of failure: local, distant and leptomeningeal.
In conclusion, the main limitations of available studies on postoperative SRS in single brain metastasis include relatively small sample size, short follow-up, heterogeneous primary histologies, unknown disease stage, and concurrent use of chemotherapy. Given the lack of clear risk/benefit data and increased associated financial costs, additional research in this area is needed before the use of postoperative SRS becomes routine clinical practice [147].
WBRT Alone
WBRT alone is the treatment of choice for patients with any of the following: single or multiple brain metastases not amenable to surgery or radiosurgery, a low KPS (≤50) or active systemic disease. Complete and partial responses have been reported in up to 60% of patients, with a neurological improvement that probably is in part attributable to steroids. Tumor volume reduction after WBRT seems to be associated with better neurocognitive function preservation and prolonged survival [148]. Median survival following WBRT alone ranges from 3 to 6 months, with 10–15% of patients alive at 1 year. A meta-analysis of 39 trials involving 10.835 patients concluded that, in comparison to standard fractionation (30 Gy in 10 fractions or 20 Gy in 5 fractions), altered WBRT dose fractionation schemes do not improve overall survival, neurologic function or symptom control [149]. However, a recent randomized trial in patients with NSCLC not candidate for either surgery or radiosurgery, did not show any difference in overall survival and quality of life between WBRT and supportive care alone [150].
Nausea, vomiting, headache, fewer and worsening of neurological symptoms can be observed in the initial phase of therapy, requiring steroid administration for control.
Up to date, radiosensitizers have not provided any clear additional benefit over conventional treatment. Recently, the addition of motexafin gadolinium to early WBRT in patients with brain metastases from NSCLC yielded an improvement in time to neurological progression over WBRT alone (5.5 months vs. 3.7 months) [151]. Likewise, the addition of efaproxiral (RSR 13) to WBRT in patients with brain metastases from breast cancer has reduced the death rate by 46%, while improving quality of life [152].
Cognitive Dysfunctions Following WBRT: Risk Factors, Pathogenesis, and Prevention
A radiation-induced dementia with ataxia and urinary incontinence has been reported in up to 30% of patients by one year from receiving unconventional large size fractions of WBRT (6–8.5 Gy) [153]. The picture on CT/MRI is that of a leukoencephalopathy (diffuse hyperintensity of the periventricular white matter on T2-weighted and FLAIR images) with associated hydrocephalus. Ventriculo-peritoneal shunt may be of clinical value in some patients. When using more conventional size fractions (up to 3 or 4 Gy per fraction) the risk is that of milder cognitive dysfunctions, consisting mainly of deficits in learning and memory with associated white matter damage and cortical atrophy on MRI. Patients with arterial hypertension, diabetes or other vascular diseases are at higher risk of developing cognitive dysfunctions. The pathogenesis of this radiation-damage is thought to include injury to the endothelium of small vessels, resulting in accelerated atherosclerosis and ultimately in a chronic ischemia similar to small vessel disease of vascular dementia. For this reason, there is interest in investigating vascular dementia treatments to prevent or reduce radiation-induced cognitive decline. One of these approaches is using memantine in combination with WBRT. Memantine is a non-competitive, low affinity antagonist of the N-methyl-D-aspartate (NMDA) receptor, a receptor activated by the principal excitatory neurotransmitter glutamate. Memantine has the potential to block the excessive NMDA stimulation following ischemia that may lead to excitotoxic damage of the normal brain. In two placebo-controlled phase III trials memantine was well tolerated and effective in treating patients with small vessel disease [154, 155]. In a recently published randomized, double-blind, placebo-controlled phase II trial of RTOG (RTOG 0614) the use of memantine during and after WBRT resulted in better cognitive function over time, specifically delaying time to cognitive decline, and reducing the rates of decline in memory, executive function and processing speed [156].
Radiation-induced cognitive deficits may also result, at least in part, from injury to neuronal stem cells in the subgranular zone of the hippocampus [157, 158]. Stem cell neurogenesis critical in memory function, especially for encoding new episodic memories. Low-dose irradiation in rodents results in a blockade of hippocampal neurogenesis and damage of the neurogenic microenvironment, leading to significant short-term memory impairment. Sparing the hippocampus during WBRT could prevent damage to neuronal progenitor cells and improve memory function [159]. Hippocampal avoidance WBRT (HAWBRT) uses intensity modulated radiotherapy (IMRT) to conformally reduce the radiation dose to the hippocampus, while applying the usual higher dose to the whole brain. A potential concern is whether hippocampal avoidance could lead to loss of control of metastases in or around this region. However, recent studies analyzing the distribution of recurrent brain metastases have shown that the metastatic involvement of the limbic circuit is uncommon [160, 161]. The recent single arm phase II RTOG 0933 suggested that the conformal avoidance of the hippocampus during WBRT spares memory and QoL. Performance at 4 months on standardized memory tests declined 7% from baseline in patients treated with HAWBRT compared with 30% in a historical control group [162]. Importantly, the trial reported that of the patients who developed intracranial progression only 4.5% experienced progression in the hippocampal avoidance area.
Building on results of RTOG 0933 and RTOG 0614, NRGCC001 is a US National Cancer Institute approved phase III trial that will evaluate the potential combined neuroprotective effects of hippocampal avoidance in addition to memantine during WBRT for brain metastases [163]. Given the increased cost of hippocampal avoidance, the trial will also perform a comparative cost-effectiveness analysis.
Treatment of Recurrent Brain Metastasis
Re-resection can afford neurological improvement and prolongation of survival in patients with local accessible brain relapse, high performance status, stable extracranial disease, and relatively long time to recurrence (˃6 months) [81, 164,165,166]. Salvage WBRT following previous WBRT or SRS is now rarely employed. Salvage SRS after WBRT has been widely used during the initial development of SRS, and RTOG 9005 [92] has established the standard doses according to diameter. Several retrospective studies have reported reasonable local control and survival rates with SRS for salvage after WBRT [167,168,169,170]. A population-based study has suggested similar survival outcomes following either salvage SRS or boost SRS [171].
Reirradiation with SRS after local recurrence at a site treated with SRS has been employed in a limited number of patients, and the long-term risk of radionecrosis should be considered against the benefit [172].
Multiple courses of SRS for new brain metastases after an initial course of SRS, with continued deferral of WBRT, may yield high rates of local control, low risk of toxicity, and favorable duration of overall and neurologic progression-free survival [100, 173, 174]. A recent large retrospective series [175] has reported that in patients undergoing multiple courses of SRS the aggregate volume, but not the cumulative number of brain metastases, and the GPA score, as recalculated at the second course of SRS, correlate with duration of survival and help guide management.
Chemotherapy and Targeted Therapies
For many years chemotherapy has not been considered to play a major role in the treatment of patients with brain metastases, due to the presence of the blood–brain barrier (BBB) which limits the access of hydrophilic and/or large drugs into the CNS. However, the BBB is partially disrupted in many brain metastases (˃1 mm in size) allowing for many chemotherapeutic agents to reach the tumor cells. Intrinsic chemosensitivity of tumor cells is a more critical factor for the response to chemotherapy [176]. Response rates of brain metastases often reflect the sensitivity of the primary tumor: relatively high response rates in SCLC (30–80%), intermediate rates in breast cancer (30–50%) and NSCLC (10–30%), and low rates in melanoma (10–15%).Importantly even in the most chemosensitive tumors response to chemotherapy is typically equivalent to that observed with radiotherapy.
The combination of radiotherapy and chemotherapy may improve response rates compared to radiotherapy alone, but does not improve survival [177], as in the case of radiotherapy combined with temozolomide (TMZ) for brain metastases [178, 179].
At least two theories may help to explain the disappointing results with chemotherapy. First, metastatic tumor cells in the brain may be resistant to chemotherapy. Brain metastases often develop later in the course of disease after multiple rounds of prior chemotherapies, allowing for the development of resistance through the accumulation of different mutations. Second, permeability of BBB in brain metastases is likely heterogeneous, thereby preventing sufficient drug accumulation and distribution. It has been hypothesized [180] that brain metastases from primary tumors with an intrinsic low expression of P-glycoprotein (an ATP-dependent efflux pump linked to chemoresistance) may be more permeable to antineoplastic drugs.
Recent advances in the understanding of the molecular pathways of tumor growth in many solid tumors have allowed the development of agents targeting specific molecular pathways both in extracranial and intracranial disease [181, 182]. Overall, the response rates to targeted agents seem higher than those observed after conventional chemotherapy. However, for some of these agents passage across the BBB remains an issue. Most of these new compounds, similarly to the old chemotherapeutics, have been shown to be substrates of one or more active efflux transporters.
The promising activity of several TKIs in brain metastases from different primaries has led to two main avenues of clinical investigation [183]. The first is the use of targeted agents as radiosensitizers, which has led to the combination of targeted agents with radiotherapy (WBRT, SRS).The second is the upfront use of targeted agents to control micrometastases, which would allow WBRT to be withheld. Clinical and translation work is ongoing to fully characterize the potential utility of TKIs in the management of brain metastases.
The antiangiogenic drug bevacizumab , a monoclonal antibody targeting VEGF with activity in high-grade, is now being investigated in brain metastases from miscellaneous solid tumor types. Its associated risk of bleeding has been shown to be quite low [184].
Two important factors can limit the impact of targeted agents on brain metastases: a lack of molecular concordance between the primary tumor and brain metastasis and the emergence of secondary resistance.
Brain Metastases from NSCLC
Platinum compounds (cisplatin, carboplatin), alone or in combination (etoposide, vinorelbine), are the most commonly used chemotherapeutics against brain metastases from NSCLC, either upfront or after radiation at the time of recurrence [176]. Pemetrexed and temozolomide also have some activity. The role of EGFR tyrosine kinase inhibitors (gefitinib, erlotinib) in the management of brain metastases from NSCLC is emerging. A pooled analysis of published data to evaluate the efficacy of EGFR TKIs in NSCLC patients with brain metastases has been recently published [185]. Sixteen studies were included in the analysis, with a total of 464 enrolled patients. The EGFR mutational status was unknown for 362 (unselected group) and 102 had activating EGFR mutations. A higher response rate (85% vs. 45.1%), and a longer PFS (12.3 months vs. 5.9 months) and OS (16.2 months vs. 10.3 months) were observed in the EGFR mutation group compared with the unselected group. These data strongly suggest that the EGFRTKIs are an effective treatment for NSCLC patients with brain metastases, particularly for those patients harboring activating EGFR mutations. However, even in EGFR wild-type patients EGFR-TKIs represent a valuable second-line therapy with a response rate of around 10% [14].
Based on the high intracranial response rates, TKIs monotherapy may be used in lieu of WBRT in patients harboring activating EGFR mutations and asymptomatic brain metastases (i.e., not needing the palliation from WBRT) [186,187,188]. However, it is important to note that the discordance rate of EGFR mutations between the primary tumor and brain metastases can be as high as 32%, and the CSF penetration rate of gefitinib (1–10%) and erlotinib (2.5–13%) is limited. A systematic review and metanalysis of the literature has suggested that upfront cranial radiotherapy (SRS or WBRT), alone or with TKIs, may improve survival outcome relative to TKIs alone. On the other hand, the combination of erlotinib with radiation therapy (SRS or WBRT) in patient cohorts not specifically selected for target expression has failed to demonstrate superiority over radiotherapy alone [189,190,191]. To date, there are no published data on the efficacy for brain metastases of the newer EGFR TKI inhibitors (afatinib, doconitib, and icotinib).
Other “druggable” alterations seen in up to 5% of NSCLC patients include rearrangements of the “anaplastic lymphoma kinase” (ALK) gene. In particular, ALK translocations have been found in 3% of brain metastases from NSCLC, and appear to be concordant between brain metastasis and primary tumor [192]. NSCLC with ALK activating translocations has been shown to be sensitive to treatment with the ALK inhibitor crizotinib. A recent study on brain metastases from ALK-rearranged NSCLC [193] has reported that crizotinib was associated with a 55% rate of disease control within CNS at 3 months of therapy in both RT-naïve and RT-pretreated patients. Moreover, crizotinib was associated with a moderate (18–33%) RECIST-confirmed response rate.
Other multitarget ALK-TKIs, such as ceritinib and alectinib, which are active in patients with ALK-rearranged NSCLC who are either naïve or resistant to crizotinib, are now being investigated in patients with brain metastases. Veliparib, a PARP 1, 2 inhibitor, is another interesting compound currently under investigation in combination with WBRT for the treatment of brain metastases from NSCLC.
Brain Metastases from SCLC
Various combinations of etoposide , teniposide, cisplatinum or carboplatinum are active against brain metastases [176]. So far, there are no effective targeted agents available.
Brain Metastases from Breast Cancer
Chemotherapy regimens, variably combining cyclophosphamide, 5-FU, methotrexate, vincristine, cisplatin, and etoposide are active in patients with brain metastases from breast cancer [176]. Capecitabine monotherapy has activity against breast cancer brain metastases. In a retrospective review conducted at MSKCC [194], three out of four patients showed complete response, and three had stable disease, with a median overall and progression-free survival after treatment of 13 and 8 months respectively. Capecitabine, combined with TMZ, has some efficacy as well [195]. Likewise, high-dose methotrexate has activity in recurrent brain metastases [196]; however, the risk of leukoencephalopathy, especially when administered after WBRT, is a limiting factor.
The dual EGFR and HER2 tyrosine kinase inhibitor lapatinib has shown modest activity in a phase II study in HER2-positive breast cancer patients with brain metastases, following trastuzumab-based systemic chemotherapy and WBRT [197]. CNS objective responses to lapatinib were observed in 6% of patients, and 21% experienced ≥20% volumetric reduction in the CNS lesions. A recent phase II single arm study (LANDSCAPE) has shown that the combination of lapatinib and capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer yields durable responses in up to 65% of patients [198]. Based on the strength of these data, a randomized trial comparing lapatinib and capecitabine versus WBRT has been launched. In addition, trials of other HER2 directed TKIs, including neratinib and afatinib, are currently in progress.
It is not clear whether trastuzumab, with limited blood–brain barrier penetration, may be active as well [20].
Brain Metastases from Melanoma
Fotemustine (response rate of 5–25%) and temozolomide (response rate 6–10%), either as single agent or in combination with WBRT, are the most active chemotherapeutics against brain metastases from melanoma [176, 199].
BRAF V600E inhibitors are emerging as treatment options for patients with BRAF-mutant melanoma metastatic to the CNS. The activity of vemurafenib is meaningful. Both retrospective [200] and phase II trials [201] have reported an intracranial response rate of 16–50%, despite disappointing results on OS. A phase II study of dabrafenib in patients with BRAF-mutated melanoma and brain metastasis reported an overall intracranial objective response rate of 31%, with little difference between patients who progressed after prior CNS therapy and patients who were treatment naïve [202].
The activity of BRAF inhibitors on brain metastases appears to be superior to that of ipilimumab [203,204,205], a monoclonal antibody with immunomodulatory activity that has been approved for the treatment of metastatic melanoma. An open-label phase 2 multicenter trial in the US [204] showed that ipilimumab has activity in patients with asymptomatic melanoma brain metastases off steroids. Disease control (CR + PR + SD) after 12 weeks of treatment was 16% in the cohort of asymptomatic patients without steroids compared with 5% in the cohort of symptomatic patients receiving steroids. Steroid use may suppress the immune response thereby limiting the effect of immunotherapy, though this remains to be clearly established. Importantly, the investigators did not report any neurological deterioration as an effect of an inflammatory response to treatment in the CNS, even in patients who had received prior radiation therapy. There is some interest in combining ipilimumab with radiation therapy [206], and new molecular agents, such as trametinib, are being investigated.
Prophylaxis
The brain can be a sanctuary for micrometastases that can become radiologically and/or clinically evident after the primary tumor has been controlled by effective therapies. Thus, treating the subclinical disease could prevent the development of overt brain metastases. This strategy is particularly relevant for SCLC where prophylactic cranial irradiation (PCI) reduces the risk of brain metastases at 2 years and increases overall survival in patients with SCLC who achieve complete remission after upfront therapy [207, 208]. Cognitive decline in SCLC patients after PCI is relatively uncommon up to 2 years after PCI [209, 210]. There is currently insufficient evidence to support PCI in high-risk NSCLC patients [211].
In patients with metastatic HER2-positive breast cancer, PCI or newer drugs with improved penetration of an intact BBB (i.e., TMZ, lapatinib) might be useful. The combination of these agents with radiation could allow a lowering of the PCI dose.
Effective agents are needed to prophylactically treat micrometastatic disease in patients at high risk for brain metastases. Well-designed clinical trials that include detailed neuropsychological assessments are needed to both identify effective agents and establish the role for CNS prophylaxis in different systemic malignancies.
Prevention Strategies: Molecular and Clinical Data
Numerous molecular compounds have been tested in preclinical models in a prevention setting and overall the studies have shown that prevention of brain metastases is feasible [212, 213]. Experimental models have shown that bevacizumab may prevent early angiogenesis and induce prolonged dormancy of micrometastases [214]. Lapatinib, vorinostat, and pazopanib are able to prevent the formation of metastases by brain-topic breast cancer cells [215,216,217]. The selective PLK1 inhibitor GSK 46I1364A, inhibits the development of large brain metastases and prolongs the survival in a xenograft model of breast cancer brain metastases [218]. Limited clinical data have shown that prevention of brain metastases can also be achieved in clinical settings. In a metastatic breast cancer trial of lapatinib plus capecitabine versus capecitabine alone there was a significant reduction in the incidence of metastases in the brain as first site of relapse after combined treatment [219]. A retrospective review of a sub-cohort of patients with advanced EGFR-mutated NSCLC treated with gefinitib or erlotinib reported 1-year and 2-year CNS relapse rates of 6 and 13%, respectively, an improvement from historical data [220]. A retrospective analysis of the clinical trial data from sorafenib in patients with renal cell cancer(RCC) and brain metastases demonstrated a 75% prevention of brain metastases development, compared with 4% response rate for established metastases [221]. A recent review of patients enrolled in a phase III trial on RCC (TARGET trial) revealed a significantly lower incidence of brain metastases in patients who received sorafenib (3%) than in those who received placebo (12%) [222]. The protective effect of TKIs (sorafenib, sunitinib, pazopanib) on the development of brain metastases from RCC has been recently outlined [223].
A major challenge in the field has been the identification of patients at highest risk of developing brain metastases because of tumor and host factors. Up to date, only HER2-positive breast cancer patients have entered prevention trials to better define the role of lapatinib.
Challenges in Developing Trials in Brain Metastases
The design of clinical trials in brain metastases can be challenging [224,225,226]. The choice of endpoints is influenced by several factors including the patient population, primary tumor type, phase of trial and the setting (treatment of established brain metastases or prevention). The ideal measure of drug activity in the brain is the assay of target modulation within the tumor obtained after resection in patients treated preoperatively. Moreover, advanced neuroimaging techniques may provide valuable surrogate pharmacodynamic information. Objective response has been commonly used as primary endpoint for phase II trials in patients with brain metastases, being a possible surrogate for other markers of clinical benefit, such as neurological status, neurocognitive decline or neurological deterioration free survival. Unfortunately, none of the standard response criteria (RECIST, WHO, MacDonald, RANO) were designed specifically for brain metastases. There is a need to standardize MRI criteria for lesion measurement (tumor area versus volume) and the definition of response to treatment, including use of both steroids and neurological symptoms in the response criteria. Use of unique therapies such as antiangiogenic agents and immunomodulators will require specific adaptations. A clear distinction between intracranial, extracranial and overall progression-free survival is important. When the concurrent systemic disease is controlled by a standard systemic regimen, the safety (not only the efficacy) of concurrent use of an investigational agent for brain metastasis must be carefully evaluated.
As the number of experimental agents increases and available resources become limited, new trial designs should be considered. Adaptive randomization can make clinical trials more efficient in reaching endpoints with fewer patients than with conventional randomization [227].
The RANO Brain Metastasis Group has recently proposed new response criteria for clinical trials in patients with brain metastases [228]; these need validation in the next generation of studies.
Other Cranial Metastases
The topics of skull and dural metastases are covered in Chap. 23 (Neurological Complications of Breast Cancer and Its Treatment).
References
Central Brain Tumor Registry of the US. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the in 2004.2008. 2012. Available at www.cbtrus.org. Accessed 5 Mar 2012.
Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14(9):1171–7.
Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–705.
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973–2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–72.
Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14.
Fabi A, Felici A, Metro G, Mirri A, Bria E, Telera S, Moscetti L, Russillo M, Lanzetta G, Mansueto G, Pace A, Maschio M, Vidiri A, Sperduti I, Cognetti F, Carapella CM. Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res. 2011;18(30):10.
Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54.
Bouffet E, Doumi N, Thiesse P, Mottolese C, Jouvet A, Lacroze M, Carrie C, Frappaz D, Brunat-Mentigny M. Brain metastases in children with solid tumors. Cancer. 1997;79(2):403–10.
Sze G, Milano E, Johnson C, Heier L. Detection of brain metastases: comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol. 1990;11(4):785–91.
Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999;44(3):275–81.
Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45(7):741–4.
Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1(4):313–7.
Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, Bartsch R, Marosi C, Zielinski C, Mehta MP, Winkler F, Wick W, von Deimling A. Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol. 2012;123(2):205–22.
Berger LA, Riesenberg H, Bokemeyer C, Atanackovic D. CNS metastases in non-small-cell lung cancer: current role of EGFR-TKI therapy and future perspectives. Lung Cancer. 2013;80(3):242–8.
Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A, du Bois A. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45(16):2792–8.
Berghoff AS, Preusser M. Biology in prevention and treatment of brain metastases. Expert Rev Anticancer Ther. 2013;13(11):1339–48.
Yin W, Jiang Y, Shen Z, Shao Z, Lu J. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS ONE. 2011;6(6):e21030.
Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, Láng I, Wardley A, Lichinitser M, Sanchez RI, Müller V, Dodwell D, Gelber RD, Piccart-Gebhart MJ, Cameron D. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244–8.
Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, Lin NU. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525–31.
Larsen PB, Kümler I, Nielsen DL. A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Cancer Treat Rev. 2013;39(7):720–7.
Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21(2):107–12.
Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–56.
Arshad F, Wang L, Sy C, Avraham S, Avraham HK. Blood-brain barrier integrity and breast cancer metastasis to the brain. Patholog Res Int. 2010;29(2011):920509.
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–14.
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–9.
Palmieri D, Fitzgerald D, Shreeve SM, Hua E, Bronder JL, Weil RJ, Davis S, Stark AM, Merino MJ, Kurek R, Mehdorn HM, Davis G, Steinberg SM, Meltzer PS, Aldape K, Steeg PS. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res. 2009;7(9):1438–45.
Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D, Vortmeyer AO, Steinberg SM, Aldape K, Steeg PS. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–8.
Lee H-Te, Xue J, Chou P-C, Zhou A, Yang P, Conrad CA, Aldape KD, Priebe W, Patterson C, Sawaya R, Xie K, Huang S. Stat3 orchestrate interaction between endothelial and tumor cells and inhibition of Stat3 suppresses brain metastasis of breast cancer cells. Oncotarget. 2015;6(12):10016–29.
Grinberg-Rashi H, Ofek E, Perelman M, Skarda J, Yaron P, Hajdúch M, Jacob-Hirsch J, Amariglio N, Krupsky M, Simansky DA, Ram Z, Pfeffer R, Galernter I, Steinberg DM, Ben-Dov I, Rechavi G, Izraeli S. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009;15(5):1755–61.
Gril B, Evans L, Palmieri D, Steeg PS. Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. Eur J Cancer. 2010;46(7):1204–10.
Marchetti D, Denkins Y, Reiland J, Greiter-Wilke A, Galjour J, Murry B, Blust J, Roy M. Brain-metastatic melanoma: a neurotrophic perspective. Pathol Oncol Res. 2003;9(3):147–58.
Zhang C, Zhang F, Tsan R, Fidler IJ. Transforming growth factor-beta2 is a molecular determinant for site-specific melanoma metastasis in the brain. Cancer Res. 2009;69(3):828–35.
Nicolson GL, Menter DG, Herrmann JL, Yun Z, Cavanaugh P, Marchetti D. Brain metastasis: role of trophic, autocrine, and paracrine factors in tumor invasion and colonization of the central nervous system. Curr Top Microbiol Immunol. 1996;213(Pt 2):89–115.
Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Davis DW, McConkey DJ, Fidler IJ. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60(17):4959–67.
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44.
Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis. 2004;21(2):107–18.
Kirsch M, Schackert G, Black PM. Angiogenesis, metastasis, and endogenous inhibition. J Neurooncol. 2000;50(1–2):173–80.
Leone A, Flatow U, King CR, Sandeen MA, Margulies IM, Liotta LA, Steeg PS. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991;65(1):25–35.
Sarris M, Scolyer RA, Konopka M, Thompson JF, Harper CG, Lee CS. Cytoplasmic expression of nm23 predicts the potential for cerebral metastasis in patients with primary cutaneous melanoma. Melanoma Res. 2004;14(1):23–7. Erratum in: Melanoma Res. 2004;14(3):239. Lee, Soon C [corrected to Lee, C Soon].
Harabin-Słowińska M, Słowiński J, Konecki J, Mrówka R. Expression of adhesion molecule CD44 in metastatic brain tumors. Folia Neuropathol. 1998;36(3):179–84.
Li H, Liu J, Hofmann M, Hamou MF, de Tribolet N. Differential CD44 expression patterns in primary brain tumors and brain metastases. Br J Cancer. 1995;72(1):160–3.
Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteaux M. Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol. 1999;20(7):1252–7.
Hartmann M, Jansen O, Heiland S, Sommer C, Münkel K, Sartor K. Restricted diffusion within ring enhancement is not pathognomonic for brain abscess. AJNR Am J Neuroradiol. 2001;22(9):1738–42.
Duygulu G, Ovali GY, Calli C, Kitis O, Yünten N, Akalin T, Islekel S. Intracerebral metastasis showing restricted diffusion: correlation with histopathologic findings. Eur J Radiol. 2010;74(1):117–20.
Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222(3):715–21.
Bulakbasi N, Kocaoglu M, Farzaliyev A, Tayfun C, Ucoz T, Somuncu I. Assessment of diagnostic accuracy of perfusion MR imaging in primary and metastatic solitary malignant brain tumors. AJNR Am J Neuroradiol. 2005;26(9):2187–99.
Chiang IC, Kuo YT, Lu CY, Yeung KW, Lin WC, Sheu FO, Liu GC. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology. 2004;46(8):619–27.
Server A, Josefsen R, Kulle B, Maehlen J, Schellhorn T, Gadmar Ø, Kumar T, Haakonsen M, Langberg CW, Nakstad PH. Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol. 2010;51(3):316–25.
Kosaka N, Tsuchida T, Uematsu H, Kimura H, Okazawa H, Itoh H. 18F-FDG PET of common enhancing malignant brain tumors. AJR Am J Roentgenol. 2008;190(6):365–9.
Hutterer M, Nowosielski M, Putzer D, Jansen NL, Seiz M, Schocke M, McCoy M, Göbel G, la Fougère C, Virgolini IJ, Trinka E, Jacobs AH, Stockhammer G. [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15(3):341–51.
Le Chevalier T, Smith FP, Caille P, Constans JP, Rouesse JG. Sites of primary malignancies in patients presenting with cerebral metastases. A review of 120 cases. Cancer. 1985;56(4):880–2.
Merchut MP. Brain metastases from undiagnosed systemic neoplasms. Arch Intern Med. 1989;149(5):1076–80.
Van de Pol M, van Aalst VC, Wilmink JT, Twijnstra A. Brain metastases from an unknown primary tumor: which diagnostic procedures are indicated? J Neurol Neurosurg Psychiatry. 1996;61(3):321–3.
Klee B, Law I, Højgaard L, Kosteljanetz M. Detection of unknown primary tumors in patients with cerebral metastases using whole-body 18F-flouorodeoxyglucose positron emission tomography. Eur J Neurol. 2002;9(6):657–62.
Rudà R, Borgognone M, Benech F, Vasario E, Soffietti R. Brain metastases from unknown primary tumor: a prospective study. J Neurol. 2001;248(5):394–8.
Monzon FA, Koen TJ. Diagnosis of metastatic neoplasms: molecular approaches for identification of tissue of origin. Arch Pathol Lab Med. 2010;134(2):216–24.
Berghoff AS, Bartsch R, Wöhrer A, Streubel B, Birner P, Kros JM, Brastianos PK, von Deimling A, Preusser M. Predictive molecular markers in metastases to the central nervous system: recent advances and future avenues. Acta Neuropatol. 2014;128(6):879–91.
Shen Q, Sahin AA, Hess KR, Suki D, Aldape KD, Sawaya R, Ibrahim NK. Breast cancer with brain metastases: clinicopathologic features, survival, and paired biomarker analysis. Oncologist. 2015;20(5):466–73.
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–51.
Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43(4):795–803.
Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):1001–6.
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–4.
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–61.
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):2111–7.
Subbiah IM, Lei X, Weinberg JS, Sulman EP, Chavez-MacGregor M, Tripathy D, Gupta R, Varma A, Chouhan J, Guevarra RP, Valero V, Gilbert MR, Gonzalez-Angulo AM. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol. 2015;33(20):2239–45.
Barnholtz-Sloan JS, Yu C, Sloan AE, Vengoechea J, Wang M, Dignam JJ, Vogelbaum MA, Sperduto PW, Mehta MP, Machtay M, Kattan MW. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012;14(7):910–8.
Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, de Oliveira Borges SR, Wajsbrot DB. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46(5):1155–61.
Lorenzoni J, Devriendt D, Massager N, David P, Ruíz S, Vanderlinden B, Van Houtte P, Brotchi J, Levivier M. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60(1):218–24.
Nguyen LN, Maor MH, Oswald MJ. Brain metastases as the only manifestation of an undetected primary tumor. Cancer. 1998;83(10):2181–4.
Soffietti R, Cornu P, Delattre JY, Grant R, Graus F, Grisold W, Heimans J, Hildebrand J, Hoskin P, Kalljo M, Krauseneck P, Marosi C, Siegal T, Vecht C. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol. 2006;13(7):674–81.
Ryken TC, McDermott M, Robinson PD, Ammirati M, Andrews DW, Asher AL, Burri SH, Cobbs CS, Gaspar LE, Kondziolka D, Linskey ME, Loeffler JS, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Kalkanis SN. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103–14.
Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC, Grossman SA, Cairncross JG. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–93.
Rudà R, Soffietti R. What is New in the management of epilepsy in gliomas? Curr Treat Options Neurol. 2015;17(6):351.
Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Somerfield MR, Falanga A. American society of clinical oncology. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654–6.
Sardar P, Chatterjee S, Herzog E, Pekler G, Mushiyev S, Pastori LJ, Visco F, Aronow WS. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22(6):460–8.
Akl EA, Kahale L, Sperati F, Neumann I, Labedi N, Terrenato I, Barba M, Sempos EV, Muti P, Cook D, Schünemann H. Low molecular weight heparin versus unfractionated heparin for perioperative thromboprophylaxis in patients with cancer. Cochrane Database Syst Rev. 2014;6:CD009447.
Connors JM. Prophylaxis against venous thromboembolism in patients with cancer. N Engl J Med. 2014;371(13):1263–4.
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500.
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33(6):583–90.
Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, Duncan G, Skingley P, Foster G, Levine M. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78(7):1470–6.
Vogelbaum MA, Suh JH. Resectable brain metastases. J Clin Oncol. 2006;24(8):1289–94.
Kamp MA, Rapp M, Bünher J, Slotty OJ, Reichelt D, Sadat H, Dibué-Adjei M, Steiger H-J, Turowski B, Sabel M. Early postoperative magnet resonance tomography after resection of cerebral metastases. Acta Neuroch. 2015;157(9):1573–80.
Kamp MA, Rapp M, Slotty PJ, Turowski B, Sadat H, Smuga M, Dibué-Adjei M, Steiger H-J, Szelényi A, Sabel M. Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neuroch. 2015;157(6):905–10.
Kelly K, Bunn PA Jr. Is it time to reevaluate our approach to the treatment of brain metastases in patients with non-small cell lung cancer? Lung Cancer. 1998;20(2):85–91.
Norris LK, Grossman SA, Olivi A. Neoplastic meningitis following surgical resection of isolated cerebellar metastasis: a potentially preventable complication. J Neurooncol. 1997;32(3):215–23.
Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108(2):248–57.
Pollock BE, Brown PD, Foote RL, Stafford SL, Schomberg PJ. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J Neurooncol. 2003;61(1):73–80.
Ewend MG, Brem S, Gilbert M, Goodkin R, Penar PL, Varia M, Cush S, Carey LA. Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control. Clin Cancer Res. 2007;13(12):3637–41.
Rogers LR, Rock JP, Sills AK, Vogelbaum MA, Suh JH, Ellis TL, Stieber VW, Asher AL, Fraser RW, Billingsley JS, Lewis P, Schellingerhout D. Shaw EG; Brain Metastasis Study Group. Results of a phase II trial of the GliaSite Radiation Therapy System for treatment of newly diagnosed, resected single brain metastases. J Neurosurg. 2006;105(3):375–8.
Dagnew E, Kanski J, McDermott MW, Sneed PK, McPherson C, Breneman JC, Warnick RE. Management of newly diagnosed single brain metastasis using resection and permanent iodine-125 seeds without initial whole-brain radiotherapy: a two institution experience. Neurosurg Focus. 2007;22(3):E3.
Baumert BG, Rutten I, Dehing-Oberije C, Twijnstra A, Dirx MJ, Debougnoux-Huppertz RM, Lambin P, Kubat B. A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2006;66(1):187–94.
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–8.
Aoyama H, Shirato H, Onimaru R, Kagei K, Ikeda J, Ishii N, Sawamura Y, Miyasaka K. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003;56(3):793–800.
Minniti G, D’Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM, Trodella L. Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol. 2014;117(2):295–301.
Eaton BR, LaRiviere MJ, Kim S, Prabhu RS, Patel K, Kandula S, Oyesiku N, Olson J, Curran W, Shu HK, Crocker I. Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol. 2015;123(1):103–11.
Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, Mills M, Rogers CL, Souhami L. The American society for therapeutic radiology and oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63(1):37–46.7/s11060-015-1767-4. Epub 2015 Apr 11. Erratum in: J Neurooncol. 2015;123(1):113.
Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362(12):1119–27.
Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40(1):48–59.
Karlsson B, Hanssens P, Wolff R, Söderman M, Lindquist C, Beute G. Thirty years’ experience with Gamma Knife surgery for metastases to the brain. J Neurosurg. 2009;111(3):449–57.
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, Nagano O, Kenai H, Moriki A, Suzuki S, Kida Y, Iwai Y, Hayashi M, Onishi H, Gondo M, Sato M, Akimitsu T, Kubo K, Kikuchi Y, Shibasaki T, Goto T, Takanashi M, Mori Y, Takakura K, Saeki N, Kunieda E, Aoyama H, Momoshima S, Tsuchiya K. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–95.
Manon R, O’Neill A, Knisely J, Werner-Wasik M, Lazarus HM, Wagner H, Gilbert M, Mehta M. Eastern cooperative oncology group. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern cooperative oncology group study (E 6397). J Clin Oncol. 2005;23(34):8870–6.
Huang CF, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91(4):563–8.
Fuentes S, Delsanti C, Metellus P, Peragut JC, Grisoli F, Regis J. Brainstem metastases: management using gamma knife radiosurgery. Neurosurgery. 2006;58(1):37–42.
Watanabe S, Yamamoto M, Sato Y, Kawabe T, Higuchi Y, Kasuya H, Yamamoto T, Matsumura A, Barfod BE. Stereotactic radiosurgery for brain metastases: a case-matched study comparing treatment results for patients 80 years of age or older versus patients 65–79 years of age. J Neurosurg. 2014;121(5):1148–57.
Iyer A, Harrison G, Kano H, Weiner GM, Luther N, Niranjan A, Flickinger JC, Lunsford LD, Kondziolka D. Volumetric response to radiosurgery for brain metastasis varies by cell of origin. J Neurosurg. 2014;121(3):564–9.
Rades D, Pluemer A, Veninga T, Hanssens P, Dunst J, Schild SE. Whole-brain radiotherapy versus stereotactic radiosurgery for patients in recursive partitioning analysis classes 1 and 2 with 1–3 brain metastases. Cancer. 2007;110(10):2285–92.
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–72.
Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R, Bahary J-P, Souhami L, Won M, Mehta M. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1–3 metastases; poststratified by the grade prognostic assessment (GPA). Int J Radiat Oncol Biol Phys. 2014;90(3):526–31.
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427–34.
Bhatnagar AK, Flickinger JC, Kondziolka D. Lunsford LD Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64(3):898–903.
Maldaun MV, Aguiar PH, Lang F, Suki D, Wildrick D, Sawaya R. Radiosurgery in the treatment of brain metastases: critical review regarding complications. Neurosurg Rev. 2008;31(1):1–8.
Chao ST, Ahluwalia MS, Barnett GH, Stevens GH, Murphy ES, Stockham AL, Shiue K, Suh JH. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–57.
Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15(9):1257–63.
Sneed P, Mendez J, Vemer-van den Hoek JGM, Seymour ZA, Ma L, Molinaro AM, Fogh SE, Nakamura JL, McDermott MW. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factor. J Neurosurg. 2015; 123(2):373–86.
Auchter RM, Lamond JP, Alexander E, Buatti JM, Chappell R, Friedman WA, Kinsella TJ, Levin AB, Noyes WR, Schultz CJ, Loeffler JS, Mehta MP. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35(1):27–35.
Muacevic A, Kreth FW, Horstmann GA, Schmid-Elsaesser R, Wowra B, Steiger HJ, Reulen HJ. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91(1):35–43.
O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169–76.
Rades D, Kueter JD, Veninga T, Gliemroth J, Schild SE. Whole brain radiotherapy plus stereotactic radiosurgery (WBRT + SRS) versus surgery plus whole brain radiotherapy (OP + WBRT) for 1–3 brain metastases: results of a matched pair analysis. Eur J Cancer. 2009;45(3):400–4.
Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87(3):299–307.
Bindal AK, Bindal RK, Hess KR, Shiu A, Hassenbusch SJ, Shi WM, Sawaya R. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84(5):748–54.
Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappel R, Buatti JM, Regine WF, Weltman E, King VJ, Breneman JC, Sperduto PW, Mehta MP. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002; 53(3):519–526.
Regine WF, Huhn JL, Patchell RA, St Clair WH, Strottman J, Meigooni A, Sanders M, Young AB. Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: results and implications. Int J Radiat Oncol Biol Phys. 2002;52(2):333–8.
Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Merkesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–9.
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Keniyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–91.
Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–41.
Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118(9):2486–93.
Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M, Shaw P, Beyene J, Chang EL. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1–4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710–7.
McPherson CM, Suki D, Feiz-Erfan I, Mahaian A, Chang E, Sawaya R, Lang FF. Adjuvant whole-brain radiation therapy after surgical resection of single brain metastases. Neuro Oncol. 2010;12(7):711–9.
Aoyama H, Tago M, Kato N, Toyoda T, Keniyo M, Hirota S, Shioura H, Inomata T, Kunieda E, Hayakawa K, Nakagawa K, Kobashi G, Shirato H. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Iny J Radiat Oncol Biol Phys. 2007;68(5):1388–95.
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–44.
Brown PD. NCCTG N0574 (Alliance): a phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1–3 brain metastases. Paper presented at: 2015 ASCO 51st Annual Meeting 2015; Chicago, IL.
Soffietti R, Kocher M, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van der Berge D, Mueller RP, Tridello G, Collette L, Bottomley A. A European organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013; 31(1):65–72.
Hahn C, Kavanagh B, Bhatnagar A, Jacobson G, Lutz S, Patton C, Potters L, Steinberg M. Choosing wisely: the American Society for Radiation Oncology’s top 5 list. Pract Radiat Oncol. 2014;4(6):349–55.
Soltys SG, Adler JR, Lipani JD, Jackson PS, Choi CY, Puataweepong P, White S, Gibbs IC, Chang SD. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70(1):187–93.
Brennan C, Yang TJ, Hilden P, Zhang Z, Chan K, Yamada Y, Chan TA, Lymberis SC, Narayana A, Tabar V, Gutin PH, Ballangrud Å. Lis E9, Beal K. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88(1):130–6.
Amsbaugh MJ, Boling W et Woo S. Tumor bed radiosurgery: an emerging treatment for brain metastases. J Neurooncol. 2015; 123(2):197–203.
Kelly PJ, Lin YB, Yu AY, Alexander BM, Hacker F, Marcus KJ, Weiss SE. Stereotactic irradiation of the postoperative resection cavity for brain metastasis: a frameless linear accelerator-based case series and review of the technique. Int J Radiat Oncol Biol Phys. 2012;82(1):95–101.
Hartford AC, Paravati AJ, Spire WJ, Li Z, Jarvis LA, Fadul CE, Rhodes CH, Erkmen K, Friedman J, Gladstone DJ, Hug EB, Roberts DW, Simmons NE. Postoperative stereotactic radiosurgery without whole-brain radiation therapy for brain metastases: potential role of preoperative tumor size. Int J Radiat Oncol Biol Phys. 2013;85(3):650–5.
Hsieh J, Elson P, Otvos B, Rose J, Loftus C, Rahmathulla G, Angelov L, Barnett GH, Weil RJ, Vogelbaum MA. Tumor progression in patients receiving adjuvant whole-brain radiotherapy vs localized radiotherapy after surgical resection of brain metastases. Neurosugery. 2015;76(4):411–20.
Choi CY, Chang SD, Gibbs IC, Adler JR, Harsh GR 4th, Lieberson RE, Soltys SG. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: prospective evaluation of target margin on tumor control. Int J Radiat Oncol Biol Phys. 2012;84(2):336–42.
Minniti G, Esposito V, Clarke E, Scaringi C, Lanzetta G, Salvati M, Raco A, Bozzao A, Maurizi Enrici R. Multidose stereotactic radiosurgery (9 Gy x 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys. 2013;86(4):623–9.
Ling DC, Vargo JA, Wegner RE, Flickinger JC, Burton SA, Engh J, Amankulor N, Quinn AE, Ozhasoglu C, Heron DE. Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases: clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neurosurgery. 2015;76(2):150–6.
Mathieu D, Kondziolka D, Flickinger JC, Fortin D, Kenny B, Michaud K, Mongia S, Niranjan A, Lunsford LD. Tumor bed radiosurgery after resection of cerebral metastases. Neurosurgery. 2008;62(4):817–23.
Jensen CA, Chan MD, McCoy TP, Bourland JD, deGuzman AF, Ellis TL, Ekstrand KE, McMullen KP, Munley MT, Shaw EG, Urbanic JJ, Tatter SB. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg. 2011;114(6):1585–91.
Robbins JR, Ryu S, Kalkanis S, Cogan C, Rock J, Movsas B, Kim JH, Rosenblum M. Radiosurgery to the surgical cavity as adjuvant therapy for resected brain metastasis. Neurosurgery. 2012;71(5):937–43.
Atalar B, Modlin LA, Choi CY, Adler JR, Gibbs IC, Chang SD, Harsh GR 4th, Li G, Nagpal S, Hanlon A, Soltys SG. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87(4):713–8.
Roberge D, Parney I, Brown PD. Radiosurgery to the postoperative surgical cavity: who needs evidence? Int J Radiat Oncol Biol Phys. 2012;83(2):486–93.
Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260–6.
Tsao MN, Lloyd N, Wong RK, Chow E, Rakovitch E, Laperriere N, Xu W, Sahgal A. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4(6):349–55.
Mulvenna PM. The management of brain metastases in patients with non-small cell lung cancer-is it time to go back to the drawing board? Clin Oncol (R Coll Radiol). 2010;22(5):365–73.
Mehta MP, Shapiro WR, Phan SC, Gervais R, Carrie C, Chabot P, Patchell RA, Glantz MJ, Recht L, Langer C, Sur RK, Roa WH, Mahe MA, Fortin A, Meyers CA, Smith JA, Miller RA, Renschler MF. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys. 2009;73(4):1069–76.
Scott C, Suh J, Stea B, Nabid A, Hackman J. Improved survival, quality of life, and quality-adjusted survival in breast cancer patients treated with efaproxiral (Efaproxyn) plus whole-brain radiation therapy for brain metastases. Am J Clin Oncol. 2007;30(6):580–7.
DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–96.
Orgogozo JM, Rigaud AS, Stöffler A, Möbius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke. 2002;33(7):1834–9.
Wilcock GK. Memantine for the treatment of dementia. Lancet Neurol. 2003;2(8):503–5.
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D. Radiation Therapy Oncology Group (RTOG). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–37.
Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):12–34.
Gibson E, Monje M. Effect of cancer therapy on neural stem cells: implications for cognitive function. Curr Opin Oncol. 2012;24(6):672–8.
Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–6.
Ghia A, Tomé WA, Thomas S, Cannon G, Khuntia D, Kuo JS, Mehta MP. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68(4):971–7.
Marsh JC, Herskovic AM, Gielda BT, Hughes FF, Hoeppner T, Turian J, Abrams RA. Intracranial metastatic disease spares the limbic circuit: a review of 697 metastatic lesions in 107 patients. Int J Radiat Oncol Biol Phys. 2010;76(2):504–12.
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–6.
Suh JH. Hippocampal-avoidance whole-brain radiation therapy: a new standard for patients with brain metastases? J Clin Oncol. 2014;32(34):3789–91.
Sundaresan N, Sachdev VP, DiGiacinto GV, Hughes JE. Reoperation for brain metastases. J Clin Oncol. 1988;6(10):1625–9.
Arbit E, Wroński M, Burt M, Galicich JH. The treatment of patients with recurrent brain metastases. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer. 1995;76(5):765–73.
Bindal RK, Sawaya R, Leavens ME, Hess KR, Taylor SH. Reoperation for recurrent metastatic brain tumors. J Neurosurg. 1995;83(4):600–4.
Chao ST, Barnett GH, Vogelbaum MA, Angelov L, Weil RJ, Neyman G, Reuther AM, Suh JH. Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer. 2008;113(8):2198–204.
Caballero JA, Sneed PK, Lamborn KR, Ma L, Denduluri S, Nakamura JL, Barani IJ, McDermott MW. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):303–9.
Kurtz G, Zadeh G, Gingras-Hill G, Millar BA, Laperriere NJ, Bernstein M, Jiang H, Ménard C, Chung C. Salvage radiosurgery for brain metastases: prognostic factors to consider in patient selection. Int J Radiat Oncol Biol Phys. 2014;88(1):137–42.
Lucas JT Jr, Colmer HG 4th, White L, Fitzgerald N, Isom S, Bourland JD, Laxton AW, Tatter SB, Chan MD. Competing risk analysis of neurologic versus nonneurologic death in patients undergoing radiosurgical salvage after whole-brain radiation therapy failure: who actually dies of their brain metastases? Int J Radiat Oncol Biol Phys. 2015;92(5):1008–15.
Hsu F, Kouhestani P, Nguyen S, Cheung A, McKenzie M, Ma R, Toyota B, Nichol A. Population-based outcomes of boost versus salvage radiosurgery for brain metastases after whole brain radiotherapy. Radiother Oncol. 2013;108(1):128–31.
Kim DH, Schultheiss TE, Radany EH, Badie B, Pezner RD. Clinical outcomes of patients treated with a second course of stereotactic radiosurgery for locally or regionally recurrent brain metastases after prior stereotactic radiosurgery. J Neurooncol. 2013;115(1):37–43.
Chen JC, Petrovich Z, Giannotta SL, Yu C, Apuzzo ML. Radiosurgical salvage therapy for patients presenting with recurrence of metastatic disease to the brain. Neurosurgery. 2000;46(4):860–6; discussion 866–7.
Kondziolka D, Kano H, Harrison GL, Yang HC, Liew DN, Niranjan A, Brufsky AM, Flickinger JC, Lunsford LD. Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. Clinical article. J Neurosurg. 2011;114(3):792–800.
Shultz DB, Modlin LA, Jayachandran P, Von Eyben R, Gibbs IC, Choi CY, Chang SD, Harsh GR 4th, Li G, Adler JR, Hancock SL, Soltys SG. Repeat courses of stereotactic radiosurgery (SRS), deferring whole-brain irradiation, for new brain metastases after initial SRS. Int J Radiat Oncol Biol Phys. 2015;92(5):993–9.
Soffietti R, Rudà R, Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol. 2008;20(6):676–84.
Mehta MP, Paleologos NA, Mikkelsen T, Robinson PD, Ammirati M, Andrews DW, Asher AL, Burri SH, Cobbs CS, Gaspar LE, Kondziolka D, Linskey ME, Loeffler JS, McDermott M, Olson JJ, Patchell RA, Ryken TC, Kalkanis SN. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):71–83.
Liu R, Wang X, Ma B, Yang K, Zhang Q, Tian J. Concomitant or adjuvant temozolomide with whole-brain irradiation for brain metastases: a meta-analysis. Anticancer Drugs. 2010;21(1):120–8.
Cao KI, Lebas N, Gerber S, Levy C, Le Scodan R, Bourgier C, Pierga JY, Gobillion A, Savignoni A, Kirova YM. Phase II randomized study of whole-brain radiation therapy with or without concurrent temozolomide for brain metastases from breast cancer. Ann Oncol. 2015;26(1):89–94.
Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25(16):2306–12.
Soffietti R, Trevisan E, Rudà R. Targeted therapy in brain metastasis. Curr Opin Oncol. 2012;24(6):679–86.
Lin NU. Targeted therapies in brain metastases. Curr Treat Options Neurol. 2014;16(1):276.
Mehta MP. Brain metastases: the changing landscape. Oncology (Williston Park). 2015;29(4):257–60.
Besse B, Lasserre SF, Compton P, Huang J, Augustus S, Rohr UP. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16(1):269–78.
Fan Y, Xu X, Xie C. EGFR-TKI therapy for patients with brain metastases from non-small-cell lung cancer: a pooled analysis of published data. Onco Targets Ther. 2014;10(7):2075–84.
Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012;18(4):938–44.
Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. 2013;111(1):1–10.
Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40(6):716–22.
Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, Chang JY, Wefel JS, McGovern SL, Garland LL, Chen SS, Holt J, Liao Z, Brown P, Sulman E, Heymach JV, Kim ES, Stea B. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902.
Lee SM, Lewanski CR, Counsell N, Ottensmeier C, Bates A, Patel N, Wadsworth C, Ngai Y, Hackshaw A, Faivre-Finn C. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst. 2014;106(7).
Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, Souhami L, Buyyounouski MK, Khuntia D, Demas W, Shah SA, Nedzi LA, Perry G, Suh JH, Mehta MP. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1–3 brain metastases: radiation therapy oncology group 0320. Int J Radiat Oncol Biol Phys. 2013;85(5):1312–8.
Preusser M, Berghoff AS, Ilhan-Mutlu A, Magerle M, Dinhof C, Widhalm G, Dieckmann K, Marosi C, Wöhrer A, Hackl M, Zöchbauer-Müller S, von Deimling A, Schoppmann SF, Zielinski CC, Streubel B, Birner P. ALK gene translocations and amplifications in brain metastases of non-small cell lung cancer. Lung Cancer. 2013;80(3):278–83.
Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, Schnell P, Wilner KD, Wiltshire R, Camidge DR, Crinò L. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8.
Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85(2):223–7.
Rivera E, Meyers C, Groves M, Valero V, Francis D, Arun B, Broglio K, Yin G, Hortobagyi GN, Buchholz T. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107(6):1348–54.
Lassman AB, Abrey LE, Shah GD, Panageas KS, Begemann M, Malkin MG, Raizer JJ. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78(3):255–60. Erratum in: J Neurooncol. 2006;78(3):261. Shah, Gaurav G [corrected to Shah, Gaurav D].
Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, Harris GJ, Bullitt E, Van den Abbeele AD, Henson JW, Li X, Gelman R, Burstein HJ, Kasparian E, Kirsch DG, Crawford A, Hochberg F, Winer EP. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–9.
Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Gonçalves A, Leheurteur M, Domont J, Gutierrez M, Curé H, Ferrero JM, Labbe-Devilliers C. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer(LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71.
Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22(11):2101–7.
Harding JJ, Catalanotti F, Munhoz RR, Cheng DT, Yaqubie A, Kelly N, McDermott GC, Kersellius R, Merghoub T, Lacouture ME, Carvajal RD, Panageas KS, Berger MF, Rosen N, Solit DB, Chapman PB. A retrospective evaluation of vemurafenib as treatment for BRAF-mutant melanoma brain metastases. Oncologist. 2015;20(7):789–97.
Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, Veronese L, Hilfiker PR, Felderer L, Rinderknecht JD. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611–21.
Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi A, Mortier L, Tawbi H, Wilhelm T, Zimmer L, Switzky J, Swann S, Martin AM, Guckert M, Goodman V, Streit M, Kirkwood JM, Schadendorf D. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–95.
Weber JS, Amin A, Minor D, Siegel J, Berman D, O’Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21(6):530–4.
Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF, Richards J, Michener T, Balogh A, Heller KN, Hodi FS. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–65.
Queirolo P, Spagnolo F, Ascierto PA, Simeone E, Marchetti P, Scoppola A, Del Vecchio M, Di Guardo L, Maio M, Di Giacomo AM, Antonuzzo A, Cognetti F, Ferraresi V, Ridolfi L, Guidoboni M, Guida M, Pigozzo J, Chiarion Sileni V. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol. 2014;118(1):109–16.
Patel KR, Shoukat S, Oliver DE, Chowdhary M, Rizzo M, Lawson DH, Khosa F, Liu Y, Khan MK. Ipilimumab and Stereotactic Radiosurgery Versus Stereotactic Radiosurgery Alone for Newly Diagnosed Melanoma Brain Metastases. Am J Clin Oncol. 2015. [Epub ahead of print].
Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, Aisner J. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med. 1999;341(7):476–84.
Meert AP, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F, Verdebout JM, Lafitte JJ, Sculier JP. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5.
Arriagada R, Le Chevalier T, Borie F, Rivière A, Chomy P, Monnet I, Tardivon A, Viader F, Tarayre M, Benhamou S. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87(3):183–90.
Gregor A, Cull A, Stephens RJ, Kirkpatrick JA, Yarnold JR, Girling DJ, Macbeth FR, Stout R, Machin D. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer. 1997;33(11):1752–8.
Lester JF, MacBeth FR, Coles B. Prophylactic cranial irradiation for preventing brain metastases in patients undergoing radical treatment for non-small-cell lung cancer: a Cochrane Review. Int J Radiat Oncol Biol Phys. 2005;63(3):690–4.
Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–63.
Trinh VA, Hwu WJ. Chemoprevention for brain metastases. Curr Oncol Rep. 2012;14(1):63–9.
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1):116–22.
Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD, Steeg PS. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100(15):1092–103.
Palmieri D, Lockman PR, Thomas FC, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15:6148–57.
Gril B, Palmieri D, Qian Y, Smart D, Ileva L, Liewehr DJ, Steinberg SM, Steeg PS. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2 + breast cancer brain metastasis. Clin Cancer Res. 2011;17(1):142–53.
Qian Y, Hua E, Bisht K, Woditschka S, Skordos KW, Liewehr DJ, Steinberg SM, Brogi E, Akram MM, Killian JK, Edelman DC, Pineda M, Scurci S, Degenhardt YY, Laquerre S, Lampkin TA, Meltzer PS, Camphausen K, Steeg PS, Palmieri D. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clin Exp Metastasis. 2011;28(8):899–908.
Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–43.
Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–82.
Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 2010;116:1272–80.
Massard C, Zonierek J, Gross-Goupil M, et al. Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann Oncol. 2010;21:1027–31.
Verma J, Jonasch E, Allen P, Tannir N, Mahajan A. Impact of tyrosine kinase inhibitors on the incidence of brain metastasis in metastatic renal cell carcinoma. Cancer. 2011;117(21):4958–65.
Peereboom DM. Clinical trial design in brain metastasis: approaches for a unique patient population. Curr Oncol Rep. 2012;14:91–6.
Lin NU, Lee EQ, Aoyama H, Barani IJ, Baumert BG, Brown PD, Camidge DR, Chang SM, Dancey J, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Lamborn KR, Linskey ME, Macdonald DR, Margolin K, Mehta MP, Schiff D, Soffietti R, Suh JH, van den Bent MJ, Vogelbaum MA, Wefel JS, Wen PY; Response Assessment in Neuro-Oncology (RANO) group. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 2013;14(10):e396–406.
Lin NU, Wefel JS, Lee EQ, Schiff D, van den Bent MJ, Soffietti R, Suh JH, Vogelbaum MA, Mehta MP, Dancey J, Linskey ME, Camidge DR, Aoyama H, Brown PD, Chang SM, Kalkanis SN, Barani IJ, Baumert BG, Gaspar LE, Hodi FS, Macdonald DR, Wen PY; Response Assessment in Neuro-Oncology (RANO) group. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14(10):e407–16.
Berry DA. Adaptive clinical trials: the promise and the caution. J Clin Oncol. 2011;29:606–9.
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, Bendszus M, Brown PD, Camidge DR, Chang SM, Dancey J, de Vries EG, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Linskey ME, Macdonald DR, Margolin K, Mehta MP, Schiff D, Soffietti R, Suh JH, van den Bent MJ, Vogelbaum MA, Wen PY; Response Assessment in Neuro-Oncology (RANO) group. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Soffietti, R., Franchino, F., Rudà, R. (2018). Brain Metastasis as Complication of Systemic Cancers. In: Schiff, D., Arrillaga, I., Wen, P. (eds) Cancer Neurology in Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-319-57901-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-57901-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57899-6
Online ISBN: 978-3-319-57901-6
eBook Packages: MedicineMedicine (R0)