Abstract
Metastases to the central nervous system (CNS) are common in several cancer types. For most primary tumors that commonly metastasize to the CNS, molecular biomarker analyses are recommended in the clinical setting for selection of appropriate targeted therapies. Therapeutic efficacy of some of these agents has been documented in patients with brain metastases, and molecular testing of CNS metastases should be considered in the clinical setting. Here, we summarize the clinically relevant biomarker tests that should be considered in neurosurgical specimens based on the current recommendations of the European Society of Medical Oncology (ESMO) or the National Comprehensive Cancer Network (NCCN) for the most relevant primary tumor types: lung cancer (EGFR mutations, ALK rearrangement, BRAF mutations), breast cancer (HER2 amplification, steroid receptor overexpression), melanoma (BRAF mutations), and colorectal cancer (RAS mutations). Furthermore, we discuss emerging therapeutic targets including novel oncogenic alterations (ROS1 rearrangements, FGFR1 amplifications, CMET amplifications, and others) and molecular features of the tumor microenvironment (including immune-checkpoint molecules such as CTLA4 and PD-1/PD-L1). We also discuss the potential role of advanced biomarker tests such as next-generation sequencing and “liquid biopsies” for patients with CNS metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastases to the central nervous system (CNS) are common in cancer patients and most frequently affect the parenchyma of the brain (~80 % supratentorial, 20 % infratentorial) via hematogenous spread, while the spinal cord is less frequently involved [66]. In some cases, the CNS is directly infiltrated by growth of metastases or primary tumors of the surrounding soft tissues or bones. The cerebrospinal fluid (CSF) or the leptomeninges (pia and arachnoid) are involved by metastatic spread in ~4–15 % of patients with cancer [17].
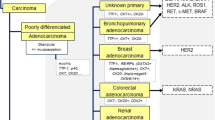
Population-based investigations report incidences of CNS metastases ranging from 8.3 to 14.3 per 100.000 people [55]. Autopsy studies demonstrated that brain metastases can be detected in up to 25 % of all cancer patients [66, 86]. The most common primary tumors to metastasize to the brain are lung cancer (39–56 % of cases), breast cancer (13–30 %), melanoma (6–11 %), renal cell carcinoma (2–4 %) and colorectal cancer (3–8 %). In up to 15 % of patients presenting with brain metastases, no primary tumor is identified despite extensive diagnostic investigation (“cancer of unknown primary”, CUP) [28, 72].
Conventional therapeutic approaches for CNS metastases comprise local treatments such as neurosurgical resection, stereotactic neurosurgery, and whole-brain radiotherapy [58]. Cytotoxic chemotherapy has a limited role. However, several targeted agents approved for the treatment of primary tumor types commonly associated with CNS involvement have shown clinically relevant efficacy in brain metastases [68, 70, 72]. Examples include the human epidermal growth factor receptor 2 (HER2) tyrosine kinase inhibitor (TKI) lapatinib, the epidermal growth factor receptor (EGFR) TKIs erlotinib and gefitinib, the anaplastic lymphoma kinase (ALK) TKI crizotinib, and the v-RAF murine sarcoma viral oncogene homolog B1 (BRAF) TKIs vemurafenib and dabrafenib [3, 26, 41, 47, 93]. A large number of novel targeted agents aimed at oncogenic intratumoral signaling molecules or the tumor microenvironment are in clinical development and are expected to significantly expand the therapeutic options for many cancer types in the near future. Thus, in selected patients with CNS metastases, therapy with specific inhibitors of molecular aberrations is increasingly considered in the clinical setting. Patient selection for such therapies relies on molecular biomarker analyses of tumor tissues. Patient outcome may be optimized using molecular information on a case-by-case basis. Recommendations for clinical biomarker analyses for most primary tumor types have been published and are regularly updated by the European Society of Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN) [54]. However, so far no evidence-based recommendations for clinical biomarker analyses of CNS metastases exist. We provide here a summary of ESMO and NCCN recommendations on clinical biomarker analyses for the tumor types most commonly causing CNS metastases: lung cancer, breast cancer, melanoma, renal cell cancer, and colorectal cancer. Furthermore, we discuss potentially emerging candidate biomarkers and advanced test methods (e.g. next-generation sequencing, “liquid biopsies”) that may become clinically relevant in the future.
Lung cancer
Non-small-cell lung cancer (NSCLC)
At present, two genetic alterations directly influence therapeutic decision making in patients with metastatic NSCLC and should be determined at diagnostic work-up in patients that qualify for systemic therapy. There are a large number of experimental targeted agents under clinical development in metastatic NSCLC.
Epidermal growth factor receptor (EGFR) gene mutations
Activating mutations of the EGFR gene occur in ~10–15 % of Caucasians and 40 % of East-Asian NSCLC patients with a higher prevalence in non- or light smokers, adenocarcinomas and women [8, 81]. EGFR mutations predict response to therapy with the TKIs gefitinib and erlotinib and therapeutic activity of these agents has been documented in patients with CNS metastases [10, 15, 93]. EGFR mutation testing is recommended in metastatic NSCLC with non-squamous histology [63]. Routine testing is not recommended in squamous cell carcinoma, except in patients with no or light smoking history, or patients with SCLC. The recommended method for EGFR testing is genetic sequencing.
Small case series have investigated the concordance of EGFR mutation status between primary tumors and matched CNS metastases. Discordance rates between 0 and 32 % have been reported [34, 49, 84]. Variability of the EGFR status has also been demonstrated between different extra-CNS tumor sites with most studies comparing primary tumors and lymph node metastases where discordance rates of 4.5–36 % have been observed [10]. Although no evidence from adequately designed clinical studies on this issue is available, the relatively high discordance rates support re-testing of the EGFR status in neurosurgical NSCLC specimens in cases with known EGFR status in extra-CNS lesions.
ALK rearrangements
The EML4-ALK fusion gene is detectable in <5 % of NSCLC with a higher prevalence in non- or light smokers, younger patients, and adenocarcinomas [82]. ALK translocations appear to occur at similar frequencies in brain metastases as compared to other disease sites [71]. The discordance rate for ALK translocation between primary tumor and matched CNS metastases was shown to be 0 %, while ALK amplification was more frequently observed in CNS metastases than in the matched primary tumors at a discordance rate of 12.5 % [71]. ALK rearrangements are predictive of response to the TKI crizotinib and responses to this drug have been seen in patients with brain metastases [41, 61]. ALK testing is recommended particularly in cases with non-squamous histology and never/former light smokers and in cases without EGFR mutations [63]. The recommended method for ALK translocation testing is fluorescent in situ hybridization (FISH). Immunohistochemistry (IHC) for ALK has been shown to correlate with ALK gene status and can be used as a screening tool with confirmation by FISH [20]. Advantages of IHC include faster turnaround time and cost effectiveness and wider availability of immunohistochemistry in relation to FISH [20, 38, 96]. Several inter-observer and inter-laboratory studies have reported high analytical performance of this method, but it must be noted that its reliability is highly dependent on the staining protocol, the use of appropriate quality control measures (such as strict use of positive and negative controls) and the training of the scorer [23, 39, 96, 97]. Due to the difficult interpretation of FISH results in some cases, this method should preferably be performed in centralized laboratories with a high level of expertise [20].
Candidate biomarkers
A large number of promising molecular candidate biomarkers such as alterations of ROS1, RET, BRAF, HER2, fibroblast growth factor receptor 1 (FGFR1), cellular mesenchymal epithelial transition (CMET), KRAS or programmed death ligand 1 (PD-L1) are being investigated in ongoing studies of metastatic NSCLC [73, 74, 89]. Routine testing for these biomarkers in CNS metastases of NSCLC is not generally recommended at the current time, but it is likely that some of these biomarkers may become relevant for clinical decision making in the near future, as more targeted agents become available.
BRAF mutations are found in ~3 % of NSCLC cases with a significant association with adenocarcinomatous histology [18]. A recent case report documented regression of both visceral and brain metastases induced by treatment with the BRAF inhibitor vemurafenib in a patient with BRAF V600E-mutated NSCLC. However, the BRAF V600E mutation was detected in only 1/355 (0.3 %) of NSCLC brain metastases in another study [12].
Based on dramatic responses seen in ROS1-altered lung adenocarcinomas treated with inhibitors such as crizotinib, some institutions regularly test for ROS1 in the clinical setting [19]. Screening using ROS1 immunohistochemistry and subsequent confirmation of positive cases using FISH analysis has been suggested as a feasible algorithm, although this approach requires further investigation [16]. However, ROS1 alterations have been detected at a very low frequency of 1.3 % in NSCLC brain metastases [74]. A recent study reported frequent protein overexpression (44.4 %) and gene amplification (21.6 %) of CMET in brain metastases of NSCLC and there was evidence that CMET alterations may be acquired during the metastatic process [75]. Since inhibitors of the CMET/hepatocyte growth factor (HGF) axis with good penetration of the blood–brain/blood–tumor barrier are being developed for brain tumors, these molecular alterations may open avenues for targeted therapy of NSCLC CNS metastases that should be explored in clinical trials.
Recent studies reported FGFR1 amplifications in a significant fraction of NSCLC brain metastases [73]. In squamous cell carcinomas, 19 % of cases had FGFR1 amplifications consistent with the frequency reported for primary tumors. Interestingly however, brain metastases of NSCLC adenocarcinoma had a significantly higher rate of FGFR1 amplifications (15 %) than expected from the prevalence in primary tumors (3 %). These data seem to imply a role of FGFR1 activation in metastasis formation and may be of relevance for targeted therapy or prevention of CNS metastases in NSCLC. As such, FGFR1 inhibitors are under clinical investigation.
One of the most promising novel therapeutic strategies for NSCLC (and other tumors such as melanoma, renal cell cancer, and others) is immunomodulation using immune-checkpoint inhibitors of molecules such as CTLA-4, PD-1, and PD-L1 [85]. These agents inhibit tumor-associated immunosuppression generated by co-inhibitory T cell receptors. A growing number of immune-checkpoint inhibitors are found in various stages of clinical development and initial results demonstrate tolerable toxicity profiles and prolonged and significant clinical responses. Many of these studies are investigating reliable molecular biomarkers of response such as expression of PD-L1 or the density of tumor-infiltrating lymphocytes. Investigation of the inflammatory microenvironment and its therapeutic value in NSCLC CNS metastases is highly promising given the paucity of treatment options and potential synergistic or ‘abscopal’ effects seen in conjunction with radiotherapy. The abscopal effect refers to the regression of non-irradiated metastatic lesions distant from the primary tumor site directly subjected to irradiation.
Small-cell lung cancer (SCLC)
Although SCLC frequently metastasizes to the brain, there are no predictive molecular markers or any candidate biomarkers and promising therapeutic targets [30]. More research on the pathobiology and potential molecular alterations in SCLC is needed.
Breast cancer
Subtypes
Breast cancer is a heterogeneous disease not only with regard to its histopathological presentation but also on a molecular level. There are several disease subtypes with significant variability in biology, clinical behavior, treatment response, and prognosis [62]. In the clinical setting, breast cancer is classified as belonging to one of the following four different subtypes [33]:
-
Luminal A high expression of estrogen receptor (ER) and progesterone receptor (PR); HER2 negative; low proliferation rate (Ki67 <20 %).
-
Luminal B expression of ER, PR low or negative; high proliferation rate (Ki67 ≥20 %) and/or HER2 positive.
-
HER2 non-luminal HER2 positive; lack of steroid receptor expression.
-
Triple-negative HER2 negative; lack of steroid receptor expression.
This classification provides the standard approach for clinical decision making in the adjuvant and metastatic setting including in CNS metastases [14]. Therefore, routine analysis of ER, PR, and HER2 status in CNS metastases is recommended.
Steroid hormone receptors
Approximately two-thirds of all breast cancers are steroid receptor positive [1], predicting response to endocrine treatment in both the adjuvant and palliative settings. Routine testing of ER and PR expression in primary breast cancers is performed using immunohistochemistry (Fig. 1); a re-biopsy should be considered in the setting of metastatic disease as receptor status may change in 10–17 % of all cases [2, 22, 88]. Little is known about the concordance rate of steroid receptor expression between primary tumors and CNS metastases, but a loss of hormone receptor expression in 30–50 % of all patients was reported [4, 24]. Therefore, ER and PR staining should be routinely performed on resected CNS metastases, as tamoxifen, a selective estrogen receptor modulator, has potential activity in the CNS [44]. In patients with multiple metastases, discordance of the hormone receptor expression status between tumor sites may lead to mixed responses to endocrine therapies. Prediction and monitoring of such heterogeneous responses may be achieved by molecular imaging techniques such as 16α-[18F]-fluoro-17β-oestradiol (18F-FES) positron emission tomography (PET) [91]. Currently, there is little evidence to demonstrate activity of other endocrine treatments such as aromatase inhibitors or fulvestrant in CNS metastases, but novel agents are under development (e.g. RAD1901).
a Example of a HER2 3+++ positive breast cancer brain metastasis with HER2 immunostaining showing complete, intense, membranous, specific immunohistochemical signal on all tumor cells (anti-HER2, original magnification ×400). b FISH for HER2 gene (red signals) and centromere chromosome 17 (CEP17). Note the large clusters of red signals, indicating amplification of HER2 gene with a HER2/CEP17 ratio >2) (original magnification ×1,000). c Prominent expression of estrogen receptor (ER) in tumor cell nuclei of a breast cancer brain metastasis (ant-ER, original magnification ×400). d Melanoma brain metastasis with prominent cytoplasmic expression in all tumor cells of BRAF V600E protein (anti-BRAF V600E antibody VE1, original magnification ×400)
HER2
HER2 protein overexpression and/or HER2 gene amplification occurs in ~15 % of all breast cancer cases [67]. Several targeted treatment options are currently available in HER2-positive breast cancer and adjuvant therapy with trastuzumab has dramatically improved outcomes in HER2-positive breast cancer [65, 78].
Only patients with strong HER2 overexpression (immunohistochemistry 3+) or HER2 gene amplification may benefit from anti-HER2 therapy, indicating the need for standardized high-quality HER2 testing (Fig. 1). Furthermore, HER2 discordance between primary tumors and metastases has been reported in up to 24 % of cases, and this discordance is associated with decreased survival [2, 56]. Re-testing of CNS metastases is recommended, as a change of the HER2 receptor status was reported in up to 14 % of patients in either direction, from negative to positive and from positive to negative [24].
Of note, response to HER2-targeted agents such as trastuzumab, lapatinib, and T-DM1 has been documented in patients with brain metastases as well, rendering these drug-attractive systemic treatment options [3, 6, 7, 43]. Due to the low number of patients (2–5 % of patients with HER2-positive breast cancer) presenting with leptomeningeal carcinomatosis, very few clinical trials have been carried out to guide clinical management of such patients. Thus, treatment strategies should be discussed on a case-to-case basis in the context of a multidisciplinary tumor board. Of note, high concordance rates of HER2 status between primary tumors and tumor cells in the CSF have been reported [60], although discordance has been observed in some cases [45]. Intrathecal administration of trastuzumab may be a feasible treatment approach in selected patients with leptomeningeal carcinomatosis of HER2-positive breast cancer presenting with no or limited extra-CNS disease [99].
To ensure accurate high-quality HER2 testing, strict adherence to the following algorithm is recommended according to the American Society of Clinical Oncology (ASCO)/CAP guidelines (Fig. 2) [95]. It is important to note that the pre-analytical tissue processing is a major determinant of the test performance. According to the ASCO/CAP recommendations, the pathologist should ensure that any specimen used for HER2 testing (cytological specimens, needle biopsies, or resection specimens) begins the fixation process quickly (time to fixative within 1 h) and is fixed in 10 % neutral buffered formalin for 6–72 h, and that routine processing as well as staining or probing are done according to the standardized analytically validated protocols.
Algorithm for HER2 testing in breast cancer. Adapted from [95]
Ki67 tumor proliferation index
Ki67 is a proliferation marker which is used to differentiate the less aggressive luminal A from the more aggressive luminal B/HER2-negative subtypes of steroid receptor-positive breast cancer [33]. Therefore, Ki67 is an important decision-making tool in early breast cancer patients; however, its role in CNS metastases remains undefined.
Candidate biomarkers
Candidate biomarkers in breast cancer diagnostics that warrant further clinical validation include the following: androgen receptor, ER-ß, BRCA-1 methylation, EGFR, HER2 mutations, p95 HER2, HER3, IGF-1, phosphatidyl-inositol-3-kinase (PI3K) mutations, PTEN loss, and TP53 status. More research is needed before we know how promising some of these biomarkers are in breast cancer CNS metastases. A major unresolved need is the identification of drugable targets in the group of patients with triple-negative breast cancer. CNS metastases are frequent in these patients and are associated with poor median overall survival times of ~5 months [57]. Recent studies have found PD-L1 expression in approximately 20 % of cases, thus introducing specific immune-checkpoint inhibitors as a potential opportunity for clinical trials [52].
Melanoma
BRAF mutations
BRAF mutations, most commonly V600E, occur in approximately half of metastatic melanomas and the prevalence is similar between CNS metastases and other disease sites [12]. BRAF mutations predict response to BRAF inhibitors such as vemurafenib and dabrafenib and these drugs also have activity in patients with brain metastases [26, 47]. The addition of a MEK inhibitor like trametinib improves activity and is currently being investigated in clinical trials in brain metastases [29].
BRAF testing is recommended in all patients with metastatic melanoma [25, 64]. Very high concordance of the BRAF status between primary tumors and different metastatic sites including brain metastases has been reported [12, 51]. Thus, testing of specimens from more than one tumor site is not necessary to determine BRAF status for treatment planning in metastatic melanoma.
BRAF testing can be performed by genetic sequencing, immunohistochemistry or using the United Stated Food and Drug Administration (FDA)-approved DNA-based COBAS BRAF V600 (Roche) and THxIDTM-BRAF (bioMérieux Inc) tests. Immunohistochemistry using the monoclonal antibody VE1 has been shown to accurately distinguish cases with or without the BRAF V600E point mutation, which constitutes approximately >95 % of all BRAF mutations (Fig. 1). Compared to most DNA-based methods, immunohistochemistry using VE1 has the advantages of working more reliably in formalin-fixed and paraffin-embedded tissue samples, having a lower false-negative rate in samples with low tumor cell content, being easier to implement in standard pathology laboratories, and being cheaper and taking less time [11, 13, 46]. However, less frequent mutation types that are also associated with response to therapeutic BRAF inhibition like BRAF V600K are not picked up by VE1 immunohistochemistry [50]. Thus, a sequential testing strategy using VE1 immunohistochemistry first and subsequent DNA-based analysis in VE1-negative cases is a reasonable approach for treatment stratification of patients with metastatic melanoma.
Candidate biomarkers
Testing for other important oncogenic mutations such as NRAS and CKIT are not unequivocally recommended due to the lack of a direct impact on clinical decision making, especially in patients with brain metastases [25, 48, 64, 80]. Several immunohistochemical signatures including expression of immune-checkpoint molecules such as PD-1 and its ligands may emerge as predictive biomarkers for response to immunomodulatory agents [94]. Frequent expression of PD-1 and PD-L1 was shown in melanoma brain metastases [9].
Mutations of GNAQ and GNA11 are typically detected in primary melanocytic CNS tumors and not in metastatic lesions and may therefore aid differential diagnosis in some cases [32, 42, 53]. In addition, mutation-related activation of the MAPK pathway may influence the response to specific inhibitors and may therefore emerge as a useful biomarker.
Renal cell carcinoma
In recent years, considerable insight into the pathobiology of renal cell cancer has been gained. A wealth of targeted therapies including TKI (sunitinib, sorafenib), immuno-modulators (interleukin-2, interferon-alpha), and antiangiogenic agents (bevacizumab) have shown clinically meaningful efficacy and are prescribed in standard practice. However, although some correlation of molecular factors with response to targeted therapies has been observed, so far no reliable predictive molecular markers have been identified [27, 77]. Forthcoming experimental therapies of particular interest are agents that inhibit the interaction between PD-1 receptor and its ligand PD-L1 [59]. Several monoclonal antibodies are in clinical development and show promising results. Expression of PD-L1 or the density of tumor-infiltrating lymphocytes may be relevant biomarkers of response and investigation of these factors in brain metastases of renal cell carcinoma is certainly of interest.
Colorectal cancer
KRAS and NRAS
RAS mutations occur in 40 % of metastatic colorectal cancers and are associated with lack of sensitivity to the EGFR inhibitors cetuximab or panitumumab [31, 40]. Testing for KRAS and NRAS mutations is recommended for all cases of colorectal cancer and can be performed by genetic sequencing [79]. It must be noted that the therapeutic activity of anti-EGFR monoclonal antibodies against brain metastases has not been studied, although cetuximab may be able to cross the blood–brain/blood–tumor barrier [76]. However, RAS mutation testing may still be relevant in selected cases, e.g. in patients with resected CNS lesions and progressive extra-cerebral disease. No data on the concordance of RAS mutations between primary tumor and brain metastases exist.
Candidate biomarkers
In patients with colorectal cancer, BRAF V600 mutations seem to be associated with unfavorable prognosis. Furthermore, BRAF mutations may influence the efficacy of EGFR inhibitors, although more data are needed to substantiate this correlation.
Rare brain metastases and special clinical situations
In brain metastases from cancer types that do not typically metastasize to the CNS (e.g. gastroesophageal cancer) or cancers of unknown primary (CUP), testing for specific molecular alterations with proven predictive value for response to specific inhibitors in metastatic disease may be considered.
Gastroesophageal cancer
In patients with CNS metastases of gastroesophageal cancers, HER2 testing by immunohistochemistry and/or FISH may be considered irrespective of histological subtype [90]. HER2 overexpression/amplifications occur in ~15 % of gastroesophageal adenocarcinomas (but only very rarely in squamous cell carcinomas) and are predictive for response to therapy with trastuzumab [5, 83, 92]. HER2 overexpression is also found in a fraction of CNS metastases of gastroesophageal cancers in concordance with the HER2 status of the matched primary tumor [69]. However, therapeutic efficacy of HER2 antagonists in patients with brain metastases of gastroesophageal cancers has not been studied. Of note, HER2 testing algorithms differ between breast cancer and gastroesophageal cancers [95].
Cancer of unknown primary (CUP)
In patients with CNS metastases without a detectable primary tumor, molecular testing for characteristic molecular aberrations such as EGFR mutations, ALK rearrangements, HER2 overexpression/amplifications, or BRAF mutations may be reasonable, both for information about the possible origin of the tumor and for possible therapeutic targets. However, it must be noted that the administration of targeted therapies in such cases remains purely experimental and should be reserved for situations in which no other options are deemed appropriate. Another point of concern is the significant costs of multiple molecular tests. Molecular profiling using novel multiplex assays may become a cost-effective and clinically useful alternative to determine the tissue of origin and allow rational treatment planning in the future. This notion is corroborated by the results of a recent large prospective clinical trial showing that molecular tumor profiling identified the primary tumor site in most patients with CUP and allowed assay-directed site-specific therapy [35, 36].
Advanced molecular testing methods on the horizon
The portfolio of targetable molecular alterations in cancer is rapidly expanding and recent technical advances may offer the opportunity to apply comprehensive high-throughput assays that cover a multitude of therapy-relevant (epi-)genetic aberrations within one analytical step to clinical biomarker investigations. Indeed, emerging technologies such as next-generation sequencing or genome-wide methylation assays may allow the quick and costeffective screening for known predictive factors in a way that is compatible with clinical practice. Furthermore, such approaches may support clinical drug development by allowing efficient screening of patients for inclusion into clinical trials. Collaborative research groups are setting up centralized platforms that enable prospective molecular profiling of biologic patient materials for selection and allocation of patients to studies with specific targeted agents; for example, the European Organization for Research and Treatment of Cancer (EORTC) recently launched the Screening Patients for Efficient Clinical Trial Access (SPECTA) program that applies multiplex molecular testing to tumor tissue samples of patients with lung cancer, colorectal cancer and brain tumors to enroll them into appropriate clinical trials based on the individual molecular tumor profile.
In some patients, no or only insufficient amounts of tumor tissue are available for comprehensive molecular analyses. In such cases, biomarker testing from circulating tumor cells, cell-free DNA, or membranous extracellular vesicles in the blood may be useful for non-invasive biomarker screening (“liquid biopsy”). Several recent studies established multiplex assays covering hotspot mutation regions of relevant genes that are able to detect tumor-associated mutations in the blood of cancer patients with high sensitivity and specificity [21, 37, 87, 98]. Importantly, molecular alterations detected in the blood may be amenable to sequential monitoring during therapy and may thus allow effective treatment modulation during follow-up. The liquid biopsy approach may be of particular interest for treatment planning of brain metastases, as biopsy or resection of brain metastases is indicated only under specific circumstances (e.g. <2 to 3 metastases, accessible location in non-eloquent CNS areas) and is associated with relatively high procedural risks (e.g. bleeding, neurological complications).
In sum, novel technologies show promising utility for biomarker testing in the clinical setting and for patient stratification for clinical trials, and may be of particular interest for patients with brain metastases. Ongoing studies need to carefully validate the multitude of emerging methods to provide sufficiently reliable and accurate techniques.
Summary
Systemic therapy of patients with CNS metastases with novel targeted agents is increasingly being considered and has been shown to be effective in selected patient populations [58]. Patient selection for most targeted agents requires adequate clinical biomarker analysis. At present, small numbers of biomarkers are potentially clinically meaningful in brain metastases and should be considered on a case-to-case basis in the clinical setting to optimize individual patient outcome (Table 1). A large number of promising candidate biomarkers is being evaluated in current studies and may soon become relevant for clinical practice.
References
Ali S, Coombes RC (2002) Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2(2):101–112. doi:10.1038/nrc721
Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, Oldfield M, Dranitsaris G, Tomlinson G, Laupacis A, Tannock IF, Clemons M (2012) Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol 30(6):587–592. doi:10.1200/JCO.2010.33.5232
Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Goncalves A, Leheurteur M, Domont J, Gutierrez M, Cure H, Ferrero JM, Labbe-Devilliers C (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14(1):64–71. doi:10.1016/S1470-2045(12)70432-1
Bachmann C, Grischke EM, Staebler A, Schittenhelm J, Wallwiener D (2013) Receptor change-clinicopathologic analysis of matched pairs of primary and cerebral metastatic breast cancer. J Cancer Res Clin Oncol 139(11):1909–1916. doi:10.1007/s00432-013-1511-4
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
Bartsch R, Berghoff AS, Preusser M (2013) Optimal management of brain metastases from breast cancer. Issues and considerations. CNS Drugs 27(2):121–134. doi:10.1007/s40263-012-0024-z
Bartsch R, Berghoff AS, Preusser M (2014) Breast cancer brain metastases responding to primary systemic therapy with T-DM1. J Neurooncol 116(1):205–206. doi:10.1007/s11060-013-1257-5
Bauml J, Mick R, Zhang Y, Watt CD, Vachani A, Aggarwal C, Evans T, Langer C (2013) Frequency of EGFR and KRAS mutations in patients with non small cell lung cancer by racial background: do disparities exist? Lung Cancer 81(3):347–353. doi:10.1016/j.lungcan.2013.05.011
Berghoff A, Ricken G, Widhalm G, Rajky O, Dieckmann K, Birner P, Bartsch R, Höller C, Preusser M (2014) Tumor infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology (in press)
Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F (2013) EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol 111(1):1–10. doi:10.1007/s11060-012-0990-5
Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, Pusch S, Mechtersheimer G, Zentgraf H, von Deimling A (2011) Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 122(1):11–19. doi:10.1007/s00401-011-0841-z
Capper D, Berghoff AS, Magerle M, Ilhan A, Wohrer A, Hackl M, Pichler J, Pusch S, Meyer J, Habel A, Petzelbauer P, Birner P, von Deimling A, Preusser M (2012) Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol 123(2):223–233. doi:10.1007/s00401-011-0887-y
Capper D, Berghoff AS, von Deimling A, Preusser M (2012) Clinical neuropathology practice news 2-2012: bRAF V600E testing. Clin Neuropathol 31(2):64–66
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E (2012) Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):711–719. doi:10.1093/annonc/mds232
Ceresoli GL (2012) Role of EGFR inhibitors in the treatment of central nervous system metastases from non-small cell lung cancer. Curr Cancer Drug Targets 12(3):237–246
Cha YJ, Lee JS, Kim HR, Lim SM, Cho BC, Lee CY, Shim HS (2014) Screening of ROS1 rearrangements in lung adenocarcinoma by immunohistochemistry and comparison with ALK rearrangements. PLoS One 9(7):e103333. doi:10.1371/journal.pone.0103333
Chamberlain M, Soffietti R, Raizer J, Ruda R, Brandsma D, Boogerd W, Taillibert S, Groves MD, Le Rhun E, Junck L, van den Bent M, Wen PY, Jaeckle KA (2014) Leptomeningeal metastasis: a response assessment in neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol 16(9):1176–1185. doi:10.1093/neuonc/nou089
Chen D, Zhang LQ, Huang JF, Liu K, Chuai ZR, Yang Z, Wang YX, Shi DC, Liu Q, Huang Q, Fu WL (2014) BRAF mutations in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 9(6):e101354. doi:10.1371/journal.pone.0101354
Chiari R, Buttitta F, Iacono D, Bennati C, Metro G, Di Lorito A, Iezzi M, Tiseo M, Mazzoni F, Cappuzzo F, Marchetti A, Crino L (2014) Dramatic response to crizotinib in ROS1 fluorescent in situ hybridization- and immunohistochemistry-positive lung adenocarcinoma: a case series. Clin Lung Cancer. doi:10.1016/j.cllc.2014.06.004
Cooper W, Fox S, O’Toole S, Morey A, Frances G, Pavlakis N, O’Byrne K, Dettrick A, Leong T, Rathi V, Spagnolo D, Hemmings C, Singh M, Moffat D, Tsao MS, Wilner K, Buller R, Pitman Lowenthal S, Arifeen S, Binko J, Alam M (2014) National Working Group Meeting on ALK diagnostics in lung cancer. Asia Pac J Clin Oncol 10(Suppl 2):11–17. doi:10.1111/ajco.12190
Couraud S, Vaca Paniagua F, Villar S, Oliver J, Schuster T, Blanche H, Girard N, Tredaniel J, Guilleminault L, Gervais R, Prim N, Vincent M, Margery J, Larive S, Foucher P, Duvert B, Vallee M, Le Calvez-Kelm F, McKay J, Missy P, Morin F, Zalcman G, Olivier M, Souquet PJ (2014) Non-invasive diagnosis of actionable mutations by deep sequencing of circulating-free DNA in non-small cell lung cancer: findings from BioCAST/IFCT-1002. Clin Cancer Res 20(17):4613–4624. doi:10.1158/1078-0432.CCR-13-3063
Curtit E, Nerich V, Mansi L, Chaigneau L, Cals L, Villanueva C, Bazan F, Montcuquet P, Meneveau N, Perrin S, Algros MP, Pivot X (2013) Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. Oncologist 18(6):667–674. doi:10.1634/theoncologist.2012-0350
Cutz JC, Craddock KJ, Torlakovic E, Brandao G, Carter RF, Bigras G, Deschenes J, Izevbaye I, Xu Z, Greer W, Yatabe Y, Ionescu D, Karsan A, Jung S, Fraser RS, Blumenkrantz M, Lavoie J, Fortin F, Bojarski A, Cote GB, van den Berghe JA, Rashid-Kolvear F, Trotter M, Sekhon HS, Albadine R, Tran-Thanh D, Gorska I, Knoll JH, Xu J, Blencowe B, Iafrate AJ, Hwang DM, Pintilie M, Gaspo R, Couture C, Tsao MS (2014) Canadian anaplastic lymphoma kinase study: a model for multicenter standardization and optimization of ALK testing in lung cancer. J Thorac Oncol 9(9):1255–1263. doi:10.1097/JTO.0000000000000239
Duchnowska R, Dziadziuszko R, Trojanowski T, Mandat T, Och W, Czartoryska-Arlukowicz B, Radecka B, Olszewski W, Szubstarski F, Kozlowski W, Jarosz B, Rogowski W, Kowalczyk A, Limon J, Biernat W, Jassem J (2012) Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res 14(4):R119. doi:10.1186/bcr3244
Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G (2012) Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):786–791. doi:10.1093/annonc/mds229
Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, Veronese L, Hilfiker PR, Felderer L, Rinderknecht JD (2014) Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer 50(3):611–621. doi:10.1016/j.ejca.2013.11.002
Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, Mulders P, Kataja V (2012) Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):765–771. doi:10.1093/annonc/mds227
Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G (2011) Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 22(Suppl 6):664–668. doi:10.1093/annonc/mdr389
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA 3rd, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367(18):1694–1703. doi:10.1056/NEJMoa1210093
Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E (2013) Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi99–vi105. doi:10.1093/annonc/mdt178
Gavin PG, Colangelo LH, Fumagalli D, Tanaka N, Remillard MY, Yothers G, Kim C, Taniyama Y, Kim SI, Choi HJ, Blackmon NL, Lipchik C, Petrelli NJ, O’Connell MJ, Wolmark N, Paik S, Pogue-Geile KL (2012) Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res 18(23):6531–6541. doi:10.1158/1078-0432.CCR-12-0605
Gessi M, Hammes J, Lauriola L, Dorner E, Kirfel J, Kristiansen G, zur Muehlen A, Denkhaus D, Waha A, Pietsch T (2013) GNA11 and N-RAS mutations: alternatives for MAPK pathway activating GNAQ mutations in primary melanocytic tumours of the central nervous system. Neuropathol Appl Neurobiol 39(4):417–425. doi:10.1111/j.1365-2990.2012.01288.x
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. doi:10.1093/annonc/mdt303
Gow CH, Chang YL, Hsu YC, Tsai MF, Wu CT, Yu CJ, Yang CH, Lee YC, Yang PC, Shih JY (2009) Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol 20(4):696–702. doi:10.1093/annonc/mdn679
Hainsworth JD, Rubin MS, Spigel DR, Boccia RV, Raby S, Quinn R, Greco FA (2013) Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol 31(2):217–223. doi:10.1200/JCO.2012.43.3755
Hechtman JF, Polydorides AD (2012) HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Arch Pathol Lab Med 136(6):691–697. doi:10.5858/arpa.2011-0168-RS
Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, Polanski R, Burt DJ, Simpson KL, Morris K, Pepper SD, Nonaka D, Greystoke A, Kelly P, Bola B, Krebs MG, Antonello J, Ayub M, Faulkner S, Priest L, Carter L, Tate C, Miller CJ, Blackhall F, Brady G, Dive C (2014) Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 20(8):897–903. doi:10.1038/nm.3600
Houang M, Toon CW, Clarkson A, Sioson L, Watson N, Farzin M, Selinger CI, Chou A, Morey AL, Cooper WA, O’Toole SA, Gill AJ (2014) Reflex ALK immunohistochemistry is feasible and highly specific for ALK gene rearrangements in lung cancer. Pathology 46(5):383–388. doi:10.1097/PAT.0000000000000114
Hutarew G, Hauser-Kronberger C, Strasser F, Llenos IC, Dietze O (2014) Immunohistochemistry as a screening tool for ALK rearrangement in NSCLC. Histopathology. doi:10.1111/his.12399
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357(20):2040–2048. doi:10.1056/NEJMoa071834
Kaneda H, Okamoto I, Nakagawa K (2013) Rapid response of brain metastasis to crizotinib in a patient with ALK rearrangement-positive non-small-cell lung cancer. J Thorac Oncol 8(4):e32–e33. doi:10.1097/JTO.0b013e3182843771
Kusters-Vandevelde HV, Klaasen A, Kusters B, Groenen PJ, van Engen-van Grunsven IA, van Dijk MR, Reifenberger G, Wesseling P, Blokx WA (2010) Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol 119(3):317–323. doi:10.1007/s00401-009-0611-3
Larsen PB, Kumler I, Nielsen DL (2013) A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Cancer Treat Rev 39(7):720–727. doi:10.1016/j.ctrv.2013.01.006
Lien EA, Wester K, Lonning PE, Solheim E, Ueland PM (1991) Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer 63(4):641–645
Lin NU (2010) Concordance of HER2 in primary tumor and leptomeningeal metastases: now what? Breast Cancer Res Treat 123(1):129–131. doi:10.1007/s10549-009-0720-7
Long GV, Wilmott JS, Capper D, Preusser M, Zhang YE, Thompson JF, Kefford RF, von Deimling A, Scolyer RA (2013) Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol 37(1):61–65. doi:10.1097/PAS.0b013e31826485c0
Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi A, Mortier L, Tawbi H, Wilhelm T, Zimmer L, Switzky J, Swann S, Martin AM, Guckert M, Goodman V, Streit M, Kirkwood JM, Schadendorf D (2012) Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 13(11):1087–1095. doi:10.1016/S1470-2045(12)70431-X
Martin-Algarra S, Fernandez-Figueras MT, Lopez-Martin JA, Santos-Briz A, Arance A, Lozano MD, Berrocal A, Rios-Martin JJ, Espinosa E, Rodriguez-Peralto JL (2013) Guidelines for biomarker testing in metastatic melanoma: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol 16(4):362–373. doi:10.1007/s12094-013-1090-5
Matsumoto S, Takahashi K, Iwakawa R, Matsuno Y, Nakanishi Y, Kohno T, Shimizu E, Yokota J (2006) Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer 119(6):1491–1494. doi:10.1002/ijc.21940
McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, Garbe C, Testori A, Maio M, Lorigan P, Lebbe C, Jouary T, Schadendorf D, O’Day SJ, Kirkwood JM, Eggermont AM, Dreno B, Sosman JA, Flaherty KT, Yin M, Caro I, Cheng S, Trunzer K, Hauschild A (2014) Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 15(3):323–332. doi:10.1016/S1470-2045(14)70012-9
Menzies AM, Lum T, Wilmott JS, Hyman J, Kefford RF, Thompson JF, O’Toole S, Long GV, Scolyer RA (2014) Intrapatient homogeneity of BRAFV600E expression in melanoma. Am J Surg Pathol 38(3):377–382. doi:10.1097/PAS.0000000000000136
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G (2014) PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2(4):361–370. doi:10.1158/2326-6066.CIR-13-0127
Murali R, Wiesner T, Rosenblum MK, Bastian BC (2012) GNAQ and GNA11 mutations in melanocytomas of the central nervous system. Acta Neuropathol 123(3):457–459. doi:10.1007/s00401-012-0948-x
Nabors LB, Ammirati M, Bierman PJ, Brem H, Butowski N, Chamberlain MC, DeAngelis LM, Fenstermaker RA, Friedman A, Gilbert MR, Hesser D, Holdhoff M, Junck L, Lawson R, Loeffler JS, Maor MH, Moots PL, Morrison T, Mrugala MM, Newton HB, Portnow J, Raizer JJ, Recht L, Shrieve DC, Sills AK Jr, Tran D, Tran N, Vrionis FD, Wen PY, McMillian N, Ho M (2013) Central nervous system cancers. J Natl Compr Cancer Netw 11(9):1114–1151
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1):48–54. doi:10.1007/s11912-011-0203-y
Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN, Ueno NT (2012) Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol 30(6):593–599. doi:10.1200/JCO.2010.33.8889
Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Tokuda Y, Saji S, Iwata H (2014) Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat 147(1):103–112. doi:10.1007/s10549-014-3090-8
Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, Kalkanis SN, Whitsett TG, Salhia B, Tran NL, Ryken T, Moore MK, Egan KM, Olson JJ (2014) Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11(4):203–222. doi:10.1038/nrclinonc.2014.25
Pal SK, Hu A, Chang M, Figlin RA (2014) Programmed death-1 inhibition in renal cell carcinoma: clinical insights and future directions. Clin Adv Hematol Oncol 12(2):90–99
Park IH, Kwon Y, Ro JY, Lee KS, Ro J (2010) Concordant HER2 status between metastatic breast cancer cells in CSF and primary breast cancer tissue. Breast Cancer Res Treat 123(1):125–128. doi:10.1007/s10549-009-0627-3
Peled N, Zach L, Liran O, Ilouze M, Bunn PA Jr, Hirsch FR (2013) Effective crizotinib schedule for brain metastases in ALK rearrangement metastatic non-small-cell lung cancer. J Thorac Oncol 8(12):e112–e113. doi:10.1097/JTO.0000000000000038
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E (2012) Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):756–764. doi:10.1093/annonc/mds226
Pflugfelder A, Kochs C, Blum A, Capellaro M, Czeschik C, Dettenborn T, Dill D, Dippel E, Eigentler T, Feyer P, Follmann M, Frerich B, Ganten MK, Gartner J, Gutzmer R, Hassel J, Hauschild A, Hohenberger P, Hubner J, Kaatz M, Kleeberg UR, Kolbl O, Kortmann RD, Krause-Bergmann A, Kurschat P, Leiter U, Link H, Loquai C, Loser C, Mackensen A, Meier F, Mohr P, Mohrle M, Nashan D, Reske S, Rose C, Sander C, Satzger I, Schiller M, Schlemmer HP, Strittmatter G, Sunderkotter C, Swoboda L, Trefzer U, Voltz R, Vordermark D, Weichenthal M, Werner A, Wesselmann S, Weyergraf AJ, Wick W, Garbe C, Schadendorf D (2013) Malignant melanoma S3-guideline “diagnosis, therapy and follow-up of melanoma”. J der Deutschen Dermatol Ges 11(Suppl 6):1–116, 111–126. doi:10.1111/ddg.12113_suppl
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672. doi:10.1056/NEJMoa052306
Pickren J, Lopez G, Tsukada Y, Lane W (1983) Brain metastases: an autopsy study. Cancer Treat Symp 2:295–313
Pinhel I, Hills M, Drury S, Salter J, Sumo G, A’Hern R, Bliss JM, Sestak I, Cuzick J, Barrett-Lee P, Harris A, Dowsett M (2012) ER and HER2 expression are positively correlated in HER2 non-overexpressing breast cancer. Breast Cancer Res 14(2):R46. doi:10.1186/bcr3145
Preusser M, Winkler F, Collette L, Haller S, Marreaud S, Soffietti R, Klein M, Reijneveld JC, Tonn JC, Baumert BG, Mulvenna P, Schadendorf D, Duchnowska R, Berghoff AS, Lin N, Cameron DA, Belkacemi Y, Jassem J, Weber DC (2012) Trial design on prophylaxis and treatment of brain metastases: lessons learned from the EORTC Brain Metastases Strategic Meeting 2012. Eur J Cancer 48(18):3439–3447. doi:10.1016/j.ejca.2012.07.002
Preusser M, Berghoff AS, Ilhan-Mutlu A, Dinhof C, Magerle M, Marosi C, Hejna M, Capper D, VOND A, Schoppmann SF, Birner P (2013) Brain metastases of gastro-oesophageal cancer: evaluation of molecules with relevance for targeted therapies. Anticancer Res 33(3):1065–1071
Preusser M, Berghoff AS, Schadendorf D, Lin NU, Stupp R (2012) Brain metastasis: opportunity for drug development? Curr Opin Neurol 25(6):786–794. doi:10.1097/WCO.0b013e328359320d
Preusser M, Berghoff AS, Ilhan-Mutlu A, Magerle M, Dinhof C, Widhalm G, Dieckmann K, Marosi C, Wohrer A, Hackl M, Zochbauer-Muller S, von Deimling A, Schoppmann SF, Zielinski CC, Streubel B, Birner P (2013) ALK gene translocations and amplifications in brain metastases of non-small cell lung cancer. Lung Cancer 80(3):278–283. doi:10.1016/j.lungcan.2013.01.019
Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, Bartsch R, Marosi C, Zielinski C, Mehta MP, Winkler F, Wick W, von Deimling A (2012) Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol 123(2):205–222. doi:10.1007/s00401-011-0933-9
Preusser M, Berghoff AS, Berger W, Ilhan-Mutlu A, Dinhof C, Widhalm G, Dieckmann K, Wohrer A, Hackl M, von Deimling A, Streubel B, Birner P (2014) High rate of FGFR1 amplifications in brain metastases of squamous and non-squamous lung cancer. Lung Cancer 83(1):83–89. doi:10.1016/j.lungcan.2013.10.004
Preusser M, Streubel B, Birner P (2014) ROS1 translocations and amplifications in lung cancer brain metastases. J Neurooncol 118(2):425–426. doi:10.1007/s11060-014-1446-x
Preusser M, Streubel B, Berghoff AS, Hainfellner JA, von Deimling A, Widhalm G, Dieckmann K, Wohrer A, Hackl M, Zielinski C, Birner P (2014) Amplification and overexpression of CMET is a common event in brain metastases of non-small cell lung cancer. Histopathology. doi:10.1111/his.12475
Rades D, Nadrowitz R, Buchmann I, Hunold P, Noack F, Schild SE, Meller B (2010) Radiolabeled cetuximab plus whole-brain irradiation (WBI) for the treatment of brain metastases from non-small cell lung cancer (NSCLC). Strahlenther Onkol 186(8):458–462. doi:10.1007/s00066-010-2153-y
Reuter VE, Argani P, Zhou M, Delahunt B, Members of the IIiDUPG (2014) Best practices recommendations in the application of immunohistochemistry in the kidney tumors: report from the international society of urologic pathology consensus conference. Am J Surg Pathol 38(8):e35–e49. doi:10.1097/PAS.0000000000000258
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. doi:10.1056/NEJMoa052122
Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Kohne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A (2012) ESMO consensus guidelines for management of patients with colon and rectal cancer A personalized approach to clinical decision making. Ann Oncol 23(10):2479–2516. doi:10.1093/annonc/mds236
Scolyer RA, Judge MJ, Evans A, Frishberg DP, Prieto VG, Thompson JF, Trotter MJ, Walsh MY, Walsh NM, Ellis DW (2013) Data set for pathology reporting of cutaneous invasive melanoma: recommendations from the international collaboration on cancer reporting (ICCR). Am J Surg Pathol 37(12):1797–1814. doi:10.1097/PAS.0b013e31829d7f35
Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC (2014) A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 9(2):154–162. doi:10.1097/JTO.0000000000000033
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448(7153):561–566. doi:10.1038/nature05945
Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D (2013) Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):651–656. doi:10.1093/annonc/mdt342
Sun M, Behrens C, Feng L, Ozburn N, Tang X, Yin G, Komaki R, Varella-Garcia M, Hong WK, Aldape KD, Wistuba II (2009) HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res 15(15):4829–4837. doi:10.1158/1078-0432.CCR-08-2921
Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA (2014) Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 85(2):101–109. doi:10.1016/j.lungcan.2014.05.005
Takakura K, Sano K, Hojo S, Hirano A (1982) Metastatic tumors of the central nervous system. Igaku-Shoin, Tokyo
Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, Gillet B, Gongora C, Dechelotte P, Robert B, Del Rio M, Lamy PJ, Bibeau F, Nouaille M, Loriot V, Jarrousse AS, Molina F, Mathonnet M, Pezet D, Ychou M (2014) Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med 20(4):430–435. doi:10.1038/nm.3511
Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie CA (2010) Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the breast recurrence in tissues study (BRITS). Breast Cancer Res 12(6):R92. doi:10.1186/bcr2771
Ulivi P, Zoli W, Capelli L, Chiadini E, Calistri D, Amadori D (2013) Target therapy in NSCLC patients: relevant clinical agents and tumour molecular characterisation. Mol Clin Oncol 1(4):575–581. doi:10.3892/mco.2013.100
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, Lopez-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Ruschoff J (2014) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. doi:10.1007/s10120-014-0402-y
van Kruchten M, de Vries EG, Brown M, de Vries EF, Glaudemans AW, Dierckx RA, Schroder CP, Hospers GA (2013) PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol 14(11):e465–e475. doi:10.1016/S1470-2045(13)70292-4
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D (2014) Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol 110(1):189–194. doi:10.1016/j.radonc.2013.09.015
Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, Chang JY, Wefel JS, McGovern SL, Garland LL, Chen SS, Holt J, Liao Z, Brown P, Sulman E, Heymach JV, Kim ES, Stea B (2013) Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 31(7):895–902. doi:10.1200/JCO.2011.40.1174
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133. doi:10.1056/NEJMoa1302369
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
Wynes MW, Sholl LM, Dietel M, Schuuring E, Tsao MS, Yatabe Y, Tubbs RR, Hirsch FR (2014) An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol 9(5):631–638. doi:10.1097/JTO.0000000000000115
Yi ES, Boland JM, Maleszewski JJ, Roden AC, Oliveira AM, Aubry MC, Erickson-Johnson MR, Caron BL, Li Y, Tang H, Stoddard S, Wampfler J, Kulig K, Yang P (2011) Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 6(3):459–465. doi:10.1097/JTO.0b013e318209edb9
Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, Furuta K, Nakajima T, Hayashi H, Sugisaki H, Higashimoto H, Kato T, Takeshita F, Ochiya T (2014) Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun 5:3591. doi:10.1038/ncomms4591
Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E, Dimopoulos MA, Preusser M (2013) Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat 139(1):13–22. doi:10.1007/s10549-013-2525-y
Conflict of interest
None of the authors report a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berghoff, A.S., Bartsch, R., Wöhrer, A. et al. Predictive molecular markers in metastases to the central nervous system: recent advances and future avenues. Acta Neuropathol 128, 879–891 (2014). https://doi.org/10.1007/s00401-014-1350-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1350-7